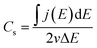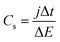Open Access Article
Open Access ArticleAmorphous molybdenum sulfide on graphene–carbon nanotube hybrids as supercapacitor electrode materials
Kien-Cuong Pham ab,
David S. McPhailc,
Andrew T. S. Weead and
Daniel H. C. Chua*e
ab,
David S. McPhailc,
Andrew T. S. Weead and
Daniel H. C. Chua*e
aNUS Graduate School for Integrative Sciences and Engineering (NGS), National University of Singapore, 28 Medical Drive, Singapore 117456, Singapore. E-mail: phamkiencuong@u.nus.edu
bDepartment of Materials, Imperial College London, Exhibition Road, London SW7 2AZ, UK
cDepartment of Chemistry and Biochemistry, University of Texas at Dallas, TX 75080-3021, USA. E-mail: dsm160330@utdallas.edu
dDepartment of Physics, National University of Singapore, 2 Science Drive 3, Singapore 117542, Singapore. E-mail: phyweets@nus.edu.sg
eDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore 117576, Singapore. E-mail: msechcd@nus.edu.sg; Fax: +65-6776-3604; Tel: +65-6516-8933
First published on 20th January 2017
Abstract
Herein, we report the application of amorphous molybdenum sulfide (MoSx, x ≈ 3) as the main active material for supercapacitor electrodes. MoSx was deposited at room temperature onto a high specific surface area electrode made of graphene–carbon nanotube hybrids directly grown on carbon paper (GCNT/CP), using an electrochemical deposition method. The MoSx/GCNT/CP electrode showed high specific capacitance. A gravimetric specific capacitance of 414 F g−1 was demonstrated at a constant discharge rate of 0.67 A g−1. The deposition of MoSx onto a conductive, high surface area support played a crucial role for a high specific capacitance. An up to 4.5-fold enhancement in specific capacitance was demonstrated when MoSx was deposited on GCNT/CP as compared to MoSx deposited on a simple carbon paper support. The MoSx/GCNT/CP electrode is suggested to be a novel candidate for supercapacitor applications.
1. Introduction
Renewable energy solutions are being very actively sought, due to energy security and environmental concerns. Among these, supercapacitors have recently attracted much research attention for their high power density, and quick charge/discharge capability.1–3 Supercapacitors frequently make use of high surface area carbon-based materials, such as activated carbon,4 graphene2,5 and carbon nanotubes,6 as electrode materials. However, the reported specific capacitances of carbon-based electrodes have been relatively low. Recently, other electrode materials have been actively investigated for supercapacitor applications, for example, metal oxides,7,8 metal hydroxides,9,10 metal sulfides,11,12 to name a few. Transitional metal chalcogenides such as WS2, MoS2, Ni3S4, MoSe2, and CuS have recently emerged as promising supercapacitor electrode materials.3,11–23 One notable example in this family of materials is molybdenum disulfide (MoS2). MoS2 is used as a supercapacitor electrode material due to its higher ionic conductivity than metal oxides and its higher specific capacitance than graphite.3,17,18 While much research attention has been paid to crystalline MoS2,3,12,15–18 little attention has been paid to its amorphous form, except for one recent study reporting the use of amorphous MoS2 as an encapsulating material for Ni3S4.19Amorphous molybdenum sulfide (MoSx) possesses many advantageous merits that can warrant further attention. In supercapacitors, charge storage in crystalline MoS2 can occur via the double layer charging on the external surface of the material or via the diffusion of ions into the inter-layer space of MoS2.14,17,18 The diffusion of ions into the inter-layer space occurs through the open edge sites of MoS2, due to the anisotropic layered structure of crystalline MoS2. Naturally occurring crystalline MoS2 has a layered crystal structure similar to graphite. To enhance this diffusion process, Soon and Loh strategically oriented the open edge planes of MoS2 to maximize the availability of these “entry” sites.14 In contrast, the amorphous form of materials generally has more open and isotropic atomic structures.24 The diffusion of ions in MoSx is therefore not limited by the availability of edge sites. The diffusion of ions may happen throughout the entire material surface. The diffusion coefficient of ions (e.g. lithium) in MoSx was found to be several orders of magnitude higher than that in crystalline MoS2.24,25 The electrical conductivity of MoSx was also found to be two orders of magnitude higher than that of crystalline MoS2.24 The higher electronic conductivity of MoSx is beneficial for a better supercapacitive performance. Furthermore, in layered materials such as graphene and MoS2, it was suggested that edge sites and defect sites in the material structures were more electro-active than the basal plane atoms and can enhance electrode capacitance.26 MoSx intrinsically contains abundance of defect sites. The contribution of defect sites in MoSx to the total capacitance is non-trivial and deserves further attention. As an additional advantage, MoSx syntheses can be routinely done at ambient temperature using wet chemical syntheses27,28 or electrodeposition methods,29,30 in contrast to the higher temperature syntheses of crystalline MoS2. Herein, we investigate molybdenum sulfide in its amorphous form as the main active supercapacitor material.
Although molybdenum sulfide materials potentially have higher specific capacitance than graphite, they are generally less electronically conductive than graphitic materials.3 The poor electronic conductivity of molybdenum sulfide could result in low material utilization and poor high-discharge-rate performance. Depositing MoSx on a high surface area conductive support can be an effective approach to enhance the electron transport between the active material and the current collector, and therefore maximize the electrode capacitance.
In our previous studies, we reported a hierarchical electrode structure of graphene–carbon nanotube hybrids grown directly on carbon paper (GCNT/CP).31–35 In this structure, CNTs were firstly grown on Toray carbon paper, and free-standing graphene was then grown directly on CNT scaffolds. The electrode had an integrated, binder-free, high specific surface area, and conductive structure. MoSx was also successfully deposited on the GCNT/CP support for the Hydrogen Evolution Reaction (HER) application in our previous study, reporting one of the most active molybdenum sulfide-based HER catalysts to date.33 Herein, the supercapacitive properties of MoSx and specific capacitance enhancement effects of the MoSx/GCNT/CP electrode will be reported.
2. Experimental
2.1. Growth of the GCNT hybrids
The GCNT hybrids were grown on carbon paper using the Chemical Vapor Deposition (CVD) methods in a two-step procedure. The details of growth procedure and the hybrid structures have been previously reported elsewhere.31,32,34 To summarize, CNTs were firstly grown on a carbon paper substrate using the thermal CVD technique. Subsequently, free-standing graphene was grown on the CNT scaffolds using the catalyst-free radio frequency plasma enhanced CVD.2.2. Deposition of MoSx on the GCNT/CP support
MoSx was deposited onto the GCNT/CP electrode using an electrodeposition method as previously reported.33 To summarize, MoSx was firstly electrodeposited on the GCNT/CP support from a 20 mM aqueous solution of ammonium tetrathiomolybdate ((NH4)2MoS4 99.97%, Sigma-Aldrich), performed in a three-electrode electrochemical cell. The deposition was carried out by holding the GCNT/CP working electrode at 0.7 V vs. Standard Hydrogen Electrode (SHE) potential. Subsequently, the MoSx-deposited GCNT/CP electrode underwent 10 reductive potential sweeps from 0.2 to −0.5 V vs. SHE in a 0.5 M H2SO4 electrolyte solution. In a typical experiment, the final MoSx loading was approximately 3 mg cm−2. In all subsequent discussions, MoSx/GCNT/CP refers to this final condition of the electrode. For comparisons, MoSx was also deposited onto a carbon paper substrate (MoSx/carbon paper) using the same aforementioned procedure and MoSx loading of 3 mg cm−2.2.3. Material characterization
The morphological characterization of the GCNT/CP and MoSx/GCNT/CP electrodes was performed with Field Emission Scanning Electron Microscopy (FE-SEM, JEOL JSM-6700F). The chemical analyses were characterized with X-ray Photoelectron Spectroscopy (XPS) (Omicron 7-channeltron analyzer, Al Kα X-ray source of 1486.7 eV photon energy, Omicron X-ray twin anode source). Constant analyzer energy (CAE) mode of 20 eV pass energy was used for high resolution scans. The amorphous nature of MoSx was assessed by Raman spectroscopy (Renishaw inVia Raman microscope, 532 nm laser) and X-ray diffraction (XRD) (Philips X'Pert, Cu Kα (λ = 1.54 Å) source).2.4. Electrochemical measurements
Supercapacitive properties of the MoSx/GCNT/CP electrode were measured in a three-electrode cell configuration. The MoSx/GCNT/CP electrode was used as working electrode. A platinum rod, a Ag/AgCl (3 M KCl) electrode and a 1 M Na2SO4 solution were used as counter electrode, reference electrode and electrolyte, respectively. The neutral 1 M Na2SO4 electrolyte was used instead of acidic H2SO4 electrolyte to avoid the hydrogen evolution process. The MoSx/GCNT/CP electrode has been shown to be highly active towards HER in acidic electrolyte in our previous study.33 All electrodepositions and electrochemical measurements were carried out with an Autolab PGSTAT302N potentiostat/galvanostat (Metrohm), fitted with a FRA2.V10 frequency response analyzer and a SCANGEN analog scan generator. All potentials in the following contexts were reported against SHE. Cyclic voltammetry and galvanostatic charge/discharge experiments were carried out in a potential window of between −0.5 and 0.3 V vs. SHE. Electrochemical Impedance Spectroscopy (EIS) was measured at −0.1 V vs. SHE in a frequency range of between 10 kHz and 0.01 Hz with an AC perturbation voltage of 5 mV. Stability testing was performed using 500 galvanostatic charge/discharge cycles at a constant charge/discharge rate of 5 A g−1.3. Results and discussion
3.1. Physical properties of the GCNT hybrids and the MoSx/GCNT/CP electrode
The morphological study with FE-SEM of the GCNT/CP and MoSx/GCNT/CP electrodes is provided in Fig. 1. Fig. 1a shows an SEM micrograph of the GCNT/CP support. The porous and high surface area structure of the GCNT/CP support was clearly observed. The GCNT hybrids possessed a unique morphology with high density of free-standing graphene deposited on the fibrous CNT scaffolds. The CNT scaffolds have two main roles in the GCNT/CP electrode structure. Firstly, as opposed to regular two-dimensional graphene sheets which have tendency to restack and block the mass transport pathways, CNTs provide a porous overall electrode structure. Such a porous electrode structure could better facilitate the permeation of electrolyte solution towards the active material of MoSx. Secondly, the direct growth of CNTs on carbon paper can provide better mechanical integrity and more direct electrical conducting paths. The porous and high surface area structure of the GCNT hybrids could facilitate the deposition of MoSx with good material dispersion, good electron and electrolytic ion transport. Further characterization of the GCNT/CP support with TEM, Raman spectroscopy, and XPS can be found in our previous study.31 Fig. 1b shows an SEM micrograph of the MoSx/GCNT/CP electrode. MoSx was successfully deposited on the high specific surface area GCNT/CP support. MoSx was found to coat and disperse well on the conductive support, which is highly beneficial for the enhanced electron transport towards the active material MoSx. A schematic illustration of the MoSx/GCNT/CP electrode structure is shown in Fig. 1c.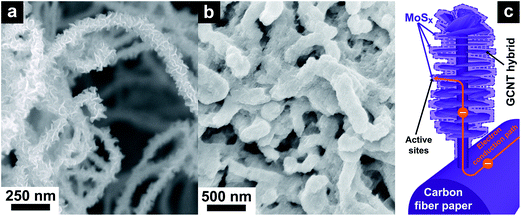 | ||
| Fig. 1 Morphological characterization with FE-SEM of (a) the GCNT/CP support, and (b) the MoSx/GCNT/CP electrode. (c) Schematic illustration of the MoSx/GCNT/CP electrode structure. | ||
The chemical state of MoSx was assessed with XPS. Fig. 2a shows the high resolution XPS scan in the S 2p region of spectrum. The spectrum was deconvoluted into two doublets. The S(A) 2p doublet at Binding Energy (BE) of 162.9–164.1 eV was assigned to apical S2− or bridging S22−.36 The S(B) 2p doublet at BE of 161.4–162.6 eV was assigned to basal plane S2− or terminal S22−.36 Fig. 2b shows the high resolution XPS scan in the Mo 3d–S 2s region. The spectrum was deconvoluted into four peak sets, including two S 2s peaks at BE of 227.3 and 226.1 eV, corresponding to the respective S(A) and S(B) chemical states, a Mo(A) 3d doublet at BE of 231.85–235.0 eV, and a Mo(B) 3d doublet at BE of 229.3–232.45 eV. The Mo(A) doublet was attributed to Mo5+ chemical state28 or Mo4+ as in molybdenum oxysulfide MoSxOy.30,37 The Mo(B) doublet was attributed to Mo4+ as in MoS2 or MoS3.37 Both Mo and S appeared in mixed chemical states and in good agreement with reported XPS spectra of amorphous MoS3.36 As opposed to the XPS spectra of MoSx/GCNT/CP, typical XPS spectra of crystalline MoS2 only show Mo(B) and S(B) chemical states. Using XPS spectra of crystalline MoS2 as references, the stoichiometry of MoSx was found as Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.09.
3.09.
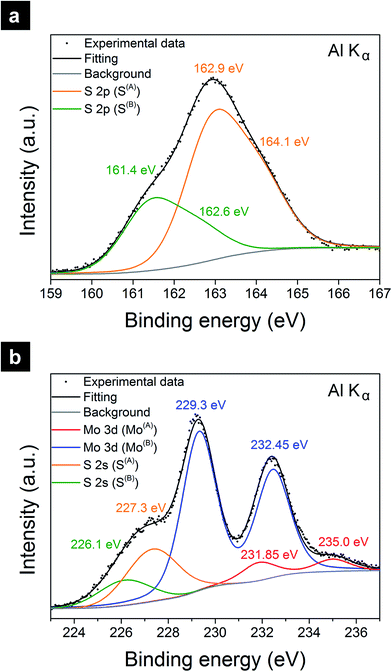 | ||
| Fig. 2 Chemical analysis of the MoSx/GCNT/CP electrode with XPS in (a) S 2p, and (b) Mo 3d–S 2s regions. | ||
The amorphous nature of MoSx was further examined with Raman spectroscopy and XRD. Fig. 3a shows the Raman spectrum of the MoSx/GCNT/CP electrode. As opposed to crystalline MoS2 of which the Raman spectrum typically shows characteristic peaks E12g and A1g, the Raman spectrum of the as-deposited MoSx showed broad peaks with Raman shift of between 200 and 1000 cm−1. The spectrum was in good agreement with previously reported Raman spectra of amorphous molybdenum sulfide materials.36,38,39 The Raman peaks at ∼550 and ∼520 cm−1 were assigned to the vibration modes of bridging S22− (ν(S–S)br) and terminal S22− (ν(S–S)t), respectively.39 The Raman peaks at ∼270–380 cm−1 and ∼445 cm−1 were assigned to molybdenum sulfide vibration modes of ν(Mo–S) and ν(Mo3–μ3S), respectively.39 The pronounced vibration modes of bridging and terminal S22− as well as apical sulfide center were characteristic for the amorphous nature of the as-deposited MoSx. The Raman peaks at ∼800–950 cm−1 was assigned to molybdenum oxide defects,39 suggesting the possible presence of molybdenum oxysulfide MoSxOy. The possible presence of MoSxOy was also evident by the Mo(A) state in the XPS analysis. To further probe the amorphous nature of MoSx, XRD measurements were performed on the GCNT/CP and MoSx/GCNT/CP electrodes. The resulting XRD spectra are shown in Fig. 3b. Both spectra showed sharp and strong characteristic peaks of graphitic carbon (002) plane, and therefore indicated the high crystallinity of the GCNT/CP support. The XRD spectrum of MoSx/GCNT/CP did not show any strong additional characteristic peaks that could otherwise suggest the presence of crystalline MoS2, except very weak and broad diffraction bands of 2θ from 10° to 20° and from 30° to 40°. The XRD result further indicated the amorphous nature of the deposited MoSx.
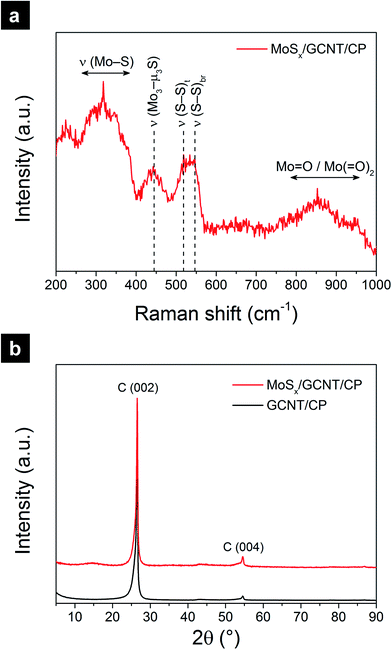 | ||
| Fig. 3 Crystallinity analyses of the MoSx/GCNT/CP electrode with (a) Raman spectroscopy, and (b) XRD. | ||
3.2. Supercapacitive properties of the MoSx/GCNT/CP electrode
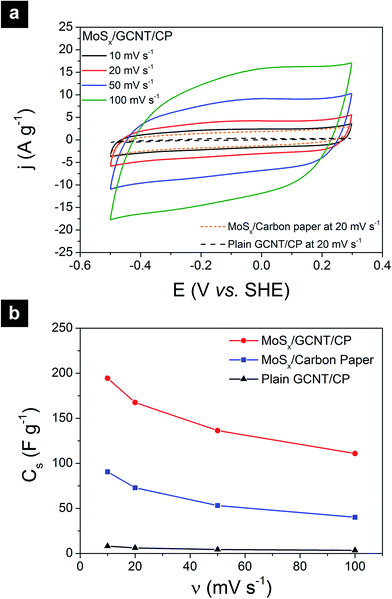
The supercapacitive properties of MoSx/GCNT/CP electrode were assessed with cyclic voltammetry and galvanostatic charge/discharge. Fig. 4a shows the cyclic voltammograms of MoSx/GCNT/CP electrode obtained at scan rates of 10, 20, 50, and 100 mV s−1. For reference purposes, the cyclic voltammograms of bare GCNT/CP support and of MoSx/carbon paper were also recorded. However, for a clearer presentation, only the cyclic voltammograms obtained at the scan rate of 20 mV s−1 were plotted for these two reference samples. It should be noted that, while the bare GCNT/CP sample did not contain any MoSx, in order to present the contribution of the GCNT/CP support to the overall capacitance of MoSx/GCNT/CP, the cyclic voltammograms of the bare GCNT/CP were also normalized for a hypothetical MoSx loading of 3 mg cm−2. The cyclic voltammograms of MoSx/GCNT/CP appeared quasi-rectangular. The quasi-rectangular shape was retained even at a high scan rate of 100 mV s−1, which suggested a good high-scan-rate capacitive performance of the MoSx/GCNT/CP electrode. The MoSx/GCNT/CP electrode showed much higher current density j than that of the GCNT/CP support and of the MoSx/carbon paper electrode.The gravimetric specific capacitance of electrodes was calculated as following:
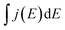 is the integral area enclosed by the cyclic voltammograms.
is the integral area enclosed by the cyclic voltammograms.
The calculated Cs of electrodes based on cyclic voltammetry are summarized in Fig. 4b. The MoSx/GCNT/CP electrode demonstrated a capacitance of 194 F g−1 at the scan rate of 10 mV s−1 and retained a capacitance of 111 F g−1 at a high scan rate of 100 mV s−1. The capacitive contribution from the GCNT/CP support to the total capacitance of the MoSx/GCNT/CP electrode was minimal (e.g. 8.2 F g−1 at the scan rate of 10 mV s−1). MoSx/carbon paper showed much lower capacitance than that of the MoSx/GCNT/CP electrode. MoSx/carbon paper showed a capacitance of 91 F g−1 at the scan rate of 10 mV s−1 and a capacitance of 40 F g−1 at the scan rate of 100 mV s−1. The reported capacitance values were 2–3 times lower than those of the MoSx deposited on the high surface area GCNT/CP support. In this work, both MoSx/GCNT/CP and MoSx/carbon paper samples were prepared using the same MoSx deposition protocol. As such, the specific capacitance enhancement in the MoSx/GCNT/CP as compared to MoSx/carbon paper was attributed to the enhanced dispersion of MoSx, better access to the electrolyte, and more efficient electron transport, resulted from a much higher specific surface area of the GCNT/CP support. Comparisons of the specific surface area of GCNT/CP and carbon paper supports have been reported in detail in our previous work.33 As reported in our previous work, the GCNT/CP support had ≈50 times higher roughness factor (the ratio between the microscopic extended surface area and the electrode projected surface area) compared to the simple carbon paper support.33 The deposition of MoSx on a high surface area conductive support demonstrated this to be an effective method to enhance the electrode capacitance.
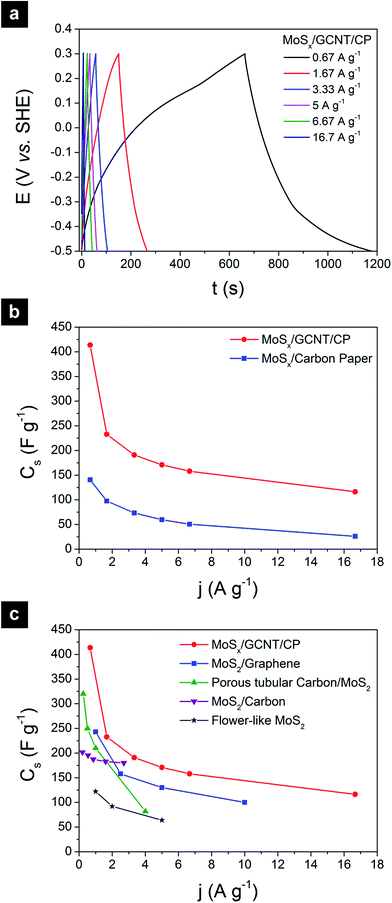
The specific capacitance of MoSx can also be characterized with galvanostatic charge/discharge curves, as shown in Fig. 5. Fig. 5a shows the galvanostatic charge/discharge curves of the MoSx/GCNT/CP electrode, measured at different charge/discharge rates from 0.67 to 16.7 A g−1. The charge/discharge curves were quasi-triangular at the low charge/discharge rate of 0.67 A g−1 and became more symmetric and triangular at higher charge/discharge rates. The gravimetric specific discharge capacitance of MoSx/GCNT/CP based on the charge/discharge curves was calculated as following:
The calculated Cs of electrodes based on galvanostatic charge/discharge curves are summarized in Fig. 5b. The MoSx/GCNT/CP electrode demonstrated a remarkable discharge capacitance of 414 F g−1 at the discharge rate of 0.67 A g−1. The MoSx/GCNT/CP electrode retained a high discharge capacitance of 116 F g−1 even at a high discharge rate of 16.7 A g−1. Similar to the cyclic voltammetry experiments, the MoSx/carbon paper electrode once again showed much lower specific capacitance than that of the MoSx/GCNT/CP electrode, with Cs of 140 and 26 F g−1 at discharge rates of 0.67 and 16.7 A g−1, respectively. A 3–4.5 fold enhancement factor in specific capacitance was demonstrated by MoSx/GCNT/CP as compared to MoSx/carbon paper. The strongly enhanced specific capacitance of MoSx when being deposited on a high surface area support was once again demonstrated through the galvanostatic charge/discharge experiments.
With the specific capacitance of 414 F g−1 obtained at the discharge rate of 0.67 A g−1, the MoSx/GCNT/CP electrode demonstrated a significant improvement in specific capacitance over that of carbon-based supercapacitor electrode materials.40,41 Although the demonstrated capacitance was lower than the best-in-class capacitance demonstrated by hydrous ruthenium oxide (RuO2·xH2O),42,43 MoSx has the advantages of low material cost and simple room-temperature synthesis. The high cost of precious metal in RuO2 has been preventing the material from practical applications despite the high supercapacitive performance. Fig. 5c shows a comparison of discharge capacitance of the MoSx/GCNT/CP electrode with examples of crystalline MoS2-based electrode materials reported in the literature, including MoS2/graphene,17 porous tubular carbon/MoS2,13 MoS2/carbon composite,44 and flower-like MoS2 nanospheres.45 The MoSx/GCNT/CP electrode consistently showed better high-discharge-rate capacitance than the crystalline MoS2 materials, in which their capacitances decreased rapidly at higher discharge current densities. The higher capacitance at high discharge rates of the MoSx/GCNT/CP electrode could be due to the faster ion diffusion in MoSx as compared to crystalline MoS2 and/or the good electron and electrolytic ion transport in the MoSx/GCNT/CP electrode.
Table 1 provides a comparison for the specific capacitance of the MoSx/GCNT/CP electrode against other representative supercapacitor materials reported in the literature. Due to the large variety of specific material approaches, the specific capacitance of materials other than MoSx and MoS2-based materials were presented by the typical range of specific capacitance reported in the literature.
| Electrode material | Typical range of specific capacitance (F g−1) | References |
|---|---|---|
| Activated carbon | 115–340 | 46–48 |
| Purified CNTs | 20–80 | 49 and 50 |
| Graphene, reduced graphene oxide | 135–205 | 2 and 51 |
| Hydrous RuO2 | 720–1300 | 42 and 52–54 |
| MnO2 | 195–350 | 55–57 |
| Conductive polymers | 230–1030 | 58–63 |
| MoSx/GCNT/CP | 414 (at 0.67 A g−1) | This study |
| MoS2/graphene | 243 (at 1 A g−1) | 17 |
| Porous tubular carbon/MoS2 | 250 (at 0.5 A g−1) | 13 |
| MoS2/carbon | 195 (at 0.54 A g−1) | 44 |
| Flower-like MoS2 | 122 (at 1 A g−1) | 45 |
| 1T-MoS2 | ∼100 (at 20 mV s−1) | 12 |
Coulombic efficiency during charge/discharge cycles of the MoSx/GCNT/CP electrode started at relatively modest values of between 77% and 87%—depending on the charge/discharge rate—in the first charge/discharge cycle. However, the coulombic efficiency of the electrode quickly reached above 97% after 10 charge/discharge cycles, and above 99% after 25 charge/discharge cycles at 5 A g−1.
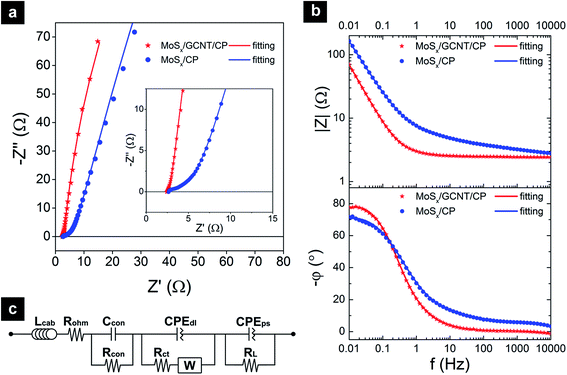
For a better understanding of the electrode interfacial properties, EIS was performed at −0.1 V vs. SHE. EIS spectra of the MoSx/GCNT/CP and MoSx/carbon paper electrodes were analyzed with equivalent circuit fitting. The EIS spectra and fitting curves of the electrodes are shown in Fig. 6. Fig. 6a and b show the EIS spectra and fitting curves presented in Nyquist plot and Bode plots, respectively. The Nyquist plots of the MoSx/GCNT/CP and MoSx/carbon paper electrodes showed nearly vertical curves, which suggested nearly idealistic capacitive behaviors of the electrodes. The equivalent circuit used for all fittings is presented in Fig. 6c. In this circuit, Lcab represents a parasitic inductance due to connecting cables or instruments. A minor inductive response was observed at the high frequency of ∼104 Hz. Rohm represents the ohmic resistance contributed by the uncompensated electrolyte resistance and internal resistance of electrode. Rcon and Ccon represent contact resistance and contact capacitance, respectively. The contact resistance and contact capacitance account for the small arc at high frequency range of ∼103 Hz. Rct, W, and CPEdl represent the charge transfer resistance, Warburg diffusion impedance, and electrical double layer capacitance constant phase element, respectively. RL and CPEps are leak resistance and pseudo-capacitance constant phase element, respectively. Equivalent circuit fitting results showed that MoSx/GCNT/CP demonstrated a much faster charge transfer kinetic (Rct = 0.352 Ω cm2) as compared to MoSx deposited on carbon paper (Rct = 4.850 Ω cm2). The enhancement in charge transfer kinetic (13.8 times smaller Rct) was most likely attributed to the effective material dispersion and efficient electron transport towards MoSx provided by the GCNT/CP support. Similar enhancement in kinetic was also demonstrated in the HER application of these materials.33 The equivalent pseudo-capacitance CPEps of MoSx/GCNT/CP (157 F g−1) was almost twice that of MoSx/carbon paper (80 F g−1).
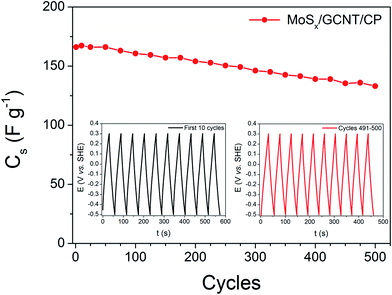
Beside a high capacitance, the electrochemical stability of supercapacitor electrode materials is also of great interest. The stability of the MoSx/GCNT/CP electrode was evaluated by a degradation test performed with 500 galvanostatic charge/discharge cycles at a high charge/discharge current density of 5 A g−1. The degradation of the electrode discharge capacitance during the degradation test is reported in Fig. 7. Fig. 7 shows that the discharge capacitance of the electrode slowly degraded during the test with an 80% retention of capacitance after 500 charge/discharge cycles. It should be noted that the degradation test was performed at a high charge/discharge rate of 5 A g−1. A high charge/discharge current can potentially degrade the electrode faster than a slow charge/discharge rate due to the quick ion insertion into and extraction from the electrode active materials. Capacitance degradation is commonly found in pseudo-capacitive active materials. Although further optimizations of the electrode will need to be pursued, we consider an 80% capacitance retention at a high charge/discharge rate of 5 A g−1 a good electrochemical stability demonstrated by the MoSx/GCNT/CP electrode.
4. Conclusions
In this work, we report the application of MoSx as the main active material for supercapacitor electrodes. MoSx was deposited onto a high surface area conductive electrode of GCNT/CP at room temperature. MoSx was successfully deposited on the GCNT hybrids and formed intimate contacts with the conductive GCNT/CP support. The MoSx/GCNT/CP composite electrode showed high specific capacitance even at high discharge rates. The deposition of MoSx on a conductive and high specific surface area support demonstrated this to be an effective method to enhance the supercapacitive performance of MoSx. Together with the good electrochemical stability, the MoSx/GCNT/CP electrode was a promising electrode material candidate for supercapacitor applications.Acknowledgements
K. C. P., D. H. C. C. and A. T. S. W. acknowledge the support from National University of Singapore (NUS) (Grants number: R284-000-142-112, R284-000-120-281, and R-144-000-321-112). K. C. P. acknowledges NUS Graduate School for Integrative Sciences and Engineering for the NGS Scholarship. K. C. P. acknowledges Mr Wong How Kwong, Dr Zheng Minrui and Ms Pang Teng Jar for their assistance with XPS, Raman spectroscopy and XRD, respectively.References
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- Y. Wang, Z. Shi, Y. Huang, Y. Ma, C. Wang, M. Chen and Y. Chen, J. Phys. Chem. C, 2009, 113, 13103–13107 CAS.
- K.-J. Huang, L. Wang, J.-Z. Zhang, L.-L. Wang and Y.-P. Mo, Energy, 2014, 67, 234–240 CrossRef CAS.
- J. Gamby, P. L. Taberna, P. Simon, J. F. Fauvarque and M. Chesneau, J. Power Sources, 2001, 101, 109–116 CrossRef CAS.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- E. Frackowiak, K. Metenier, V. Bertagna and F. Beguin, Appl. Phys. Lett., 2000, 77, 2421–2423 CrossRef CAS.
- Y. Hou, Y. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727–2733 CrossRef CAS PubMed.
- H. Jiang, J. Ma and C. Li, Adv. Mater., 2012, 24, 4197–4202 CrossRef CAS PubMed.
- R. R. Salunkhe, K. Jang, S.-w. Lee, S. Yu and H. Ahn, J. Mater. Chem., 2012, 22, 21630–21635 RSC.
- H. Jiang, T. Zhao, C. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818–3823 RSC.
- S. Ratha and C. S. Rout, ACS Appl. Mater. Interfaces, 2013, 5, 11427–11433 CAS.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- B. Hu, X. Qin, A. M. Asiri, K. A. Alamry, A. O. Al-Youbi and X. Sun, Electrochim. Acta, 2013, 100, 24–28 CrossRef CAS.
- J. M. Soon and K. P. Loh, Electrochem. Solid-State Lett., 2007, 10, A250–A254 CrossRef CAS.
- E. G. da Silveira Firmiano, A. C. Rabelo, C. J. Dalmaschio, A. N. Pinheiro, E. C. Pereira, W. H. Schreiner and E. R. Leite, Adv. Energy Mater., 2014, 4 DOI:10.1002/aenm.201301380.
- G. Sun, X. Zhang, R. Lin, J. Yang, H. Zhang and P. Chen, Angew. Chem., Int. Ed., 2015, 54, 4651–4656 CrossRef CAS PubMed.
- K.-J. Huang, L. Wang, Y.-J. Liu, Y.-M. Liu, H.-B. Wang, T. Gan and L.-L. Wang, Int. J. Hydrogen Energy, 2013, 38, 14027–14034 CrossRef CAS.
- A. Ramadoss, T. Kim, G.-S. Kim and S. J. Kim, New J. Chem., 2014, 38, 2379–2385 RSC.
- Y. Zhang, W. Sun, X. Rui, B. Li, H. T. Tan, G. Guo, S. Madhavi, Y. Zong and Q. Yan, Small, 2015, 11, 3694–3702 CrossRef CAS PubMed.
- K.-J. Huang, J.-Z. Zhang and J.-L. Cai, Electrochim. Acta, 2015, 180, 770–777 CrossRef CAS.
- X. Liu, J.-Z. Zhang, K.-J. Huang and P. Hao, Chem. Eng. J., 2016, 302, 437–445 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang, Y.-L. Jia, K. Xing and Y.-M. Liu, J. Alloys Compd., 2015, 641, 119–126 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang and Y. Fan, J. Alloys Compd., 2015, 625, 158–163 CrossRef CAS.
- Y. Miki, D. Nakazato, H. Ikuta, T. Uchida and M. Wakihara, J. Power Sources, 1995, 54, 508–510 CrossRef CAS.
- M. Bouroushian, Electrochemistry of Metal Chalcogenides, Springer-Verlag Berlin Heidelberg, Berlin Heidelberg, 1st edn, 2010 Search PubMed.
- M. Pumera, Z. Sofer and A. Ambrosi, J. Mater. Chem. A, 2014, 2, 8981–8987 CAS.
- D. J. Li, U. N. Maiti, J. Lim, D. S. Choi, W. J. Lee, Y. Oh, G. Y. Lee and S. O. Kim, Nano Lett., 2014, 14, 1228–1233 CrossRef CAS PubMed.
- J. D. Benck, Z. Chen, L. Y. Kuritzky, A. J. Forman and T. F. Jaramillo, ACS Catal., 2012, 2, 1916–1923 CrossRef CAS.
- D. Merki, S. Fierro, H. Vrubel and X. Hu, Chem. Sci., 2011, 2, 1262–1267 RSC.
- C. G. Morales-Guio and X. Hu, Acc. Chem. Res., 2014, 47, 2671–2681 CrossRef CAS PubMed.
- K.-C. Pham, D. H. C. Chua, D. S. McPhail and A. T. S. Wee, ECS Electrochem. Lett., 2014, 3, F37–F40 CrossRef CAS.
- K.-C. Pham, D. S. McPhail, C. Mattevi, A. T. S. Wee and D. H. C. Chua, J. Electrochem. Soc., 2016, 163, F255–F263 CrossRef CAS.
- K.-C. Pham, Y.-H. Chang, D. S. McPhail, C. Mattevi, A. T. S. Wee and D. H. C. Chua, ACS Appl. Mater. Interfaces, 2016, 8, 5961–5971 CAS.
- K. C. Pham, NUS-ICL Joint Ph.D. (NGS) thesis, National University of Singapore, 2016.
- T. A. J. Loh, Y. Hu, K. C. Pham, Z. Tan and D. H. C. Chua, IEEE 16th International Conference on Nanotechnology (IEEE-NANO), 2016, pp. 899–900 Search PubMed.
- T. Weber, J. C. Muijsers and J. W. Niemantsverdriet, J. Phys. Chem., 1995, 99, 9194–9200 CrossRef CAS.
- H. Vrubel and X. Hu, ACS Catal., 2013, 3, 2002–2011 CrossRef CAS.
- C. H. Chang and S. S. Chan, J. Catal., 1981, 72, 139–148 CrossRef CAS.
- P. D. Tran, T. V. Tran, M. Orio, S. Torelli, Q. D. Truong, K. Nayuki, Y. Sasaki, S. Y. Chiam, R. Yi, I. Honma, J. Barber and V. Artero, Nat. Mater., 2016, 15, 640–646 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- L. L. Zhang, R. Zhou and X. S. Zhao, J. Mater. Chem., 2010, 20, 5983–5992 RSC.
- C.-C. Hu, K.-H. Chang, M.-C. Lin and Y.-T. Wu, Nano Lett., 2006, 6, 2690–2695 CrossRef CAS PubMed.
- R. K. Das, B. Liu, J. R. Reynolds and A. G. Rinzler, Nano Lett., 2009, 9, 677–683 CrossRef CAS PubMed.
- L.-Q. Fan, G.-J. Liu, C.-Y. Zhang, J.-H. Wu and Y.-L. Wei, Int. J. Hydrogen Energy, 2015, 40, 10150–10157 CrossRef CAS.
- X. Zhou, B. Xu, Z. Lin, D. Shu and L. Ma, J. Nanosci. Nanotechnol., 2014, 14, 7250–7254 CrossRef CAS PubMed.
- J. Jiang, L. Zhang, X. Wang, N. Holm, K. Rajagopalan, F. Chen and S. Ma, Electrochim. Acta, 2013, 113, 481–489 CrossRef CAS.
- X. Du, W. Zhao, Y. Wang, C. Wang, M. Chen, T. Qi, C. Hua and M. Ma, Bioresour. Technol., 2013, 149, 31–37 CrossRef CAS PubMed.
- A. Thambidurai, J. K. Lourdusamy, J. V. John and S. Ganesan, Korean J. Chem. Eng., 2014, 31, 268–275 CrossRef CAS.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS.
- S. Talapatra, S. Kar, S. K. Pal, R. Vajtai, L. Ci, P. Victor, M. M. Shaijumon, S. Kaur, O. Nalamasu and P. M. Ajayan, Nat. Nanotechnol., 2006, 1, 112–116 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- J. P. Zheng, P. J. Cygan and T. R. Jow, J. Electrochem. Soc., 1995, 142, 2699–2703 CrossRef CAS.
- Y.-Z. Zheng, H.-Y. Ding and M.-L. Zhang, Thin Solid Films, 2008, 516, 7381–7385 CrossRef CAS.
- J. P. Zheng and T. R. Jow, J. Electrochem. Soc., 1995, 142, L6–L8 CrossRef CAS.
- C.-C. Hu and T.-W. Tsou, Electrochem. Commun., 2002, 4, 105–109 CrossRef CAS.
- A. Zolfaghari, F. Ataherian, M. Ghaemi and A. Gholami, Electrochim. Acta, 2007, 52, 2806–2814 CrossRef CAS.
- M. Ghaemi, F. Ataherian, A. Zolfaghari and S. M. Jafari, Electrochim. Acta, 2008, 53, 4607–4614 CrossRef CAS.
- V. Khomenko, E. Frackowiak and F. Béguin, Electrochim. Acta, 2005, 50, 2499–2506 CrossRef CAS.
- J. Wang, Y. Xu, X. Chen and X. Du, J. Power Sources, 2007, 163, 1120–1125 CrossRef CAS.
- H. An, Y. Wang, X. Wang, L. Zheng, X. Wang, L. Yi, L. Bai and X. Zhang, J. Power Sources, 2010, 195, 6964–6969 CrossRef CAS.
- Y. Fang, J. Liu, D. J. Yu, J. P. Wicksted, K. Kalkan, C. O. Topal, B. N. Flanders, J. Wu and J. Li, J. Power Sources, 2010, 195, 674–679 CrossRef CAS.
- S. H. Mujawar, S. B. Ambade, T. Battumur, R. B. Ambade and S.-H. Lee, Electrochim. Acta, 2011, 56, 4462–4466 CrossRef CAS.
- H. Zhang, G. Cao, Z. Wang, Y. Yang, Z. Shi and Z. Gu, Electrochem. Commun., 2008, 10, 1056–1059 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |