Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective cis-β-lactam synthesis by intramolecular C–H functionalization from enoldiazoacetamides and derivative donor–acceptor cyclopropenes†
Xinfang
Xu
*a,
Yongming
Deng
b,
David N.
Yim
b,
Peter Y.
Zavalij
b and
Michael P.
Doyle
*b
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: xinfangxu@suda.edu.cn; Tel: +86 0512 65883612
bDepartment of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, USA. E-mail: mdoyle3@umd.edu
First published on 28th January 2015
Abstract
β-Lactam derivatives are produced through intermediate donor–acceptor cyclopropene intermediates in high yield, exclusive cis-diastereoselectivity, and high enantiocontrol in a chiral dirhodium carboxylate catalyzed intramolecular C–H functionalization reaction of enoldiazoacetamides.
Introduction
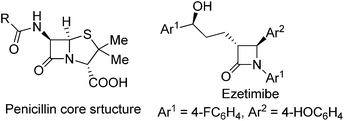
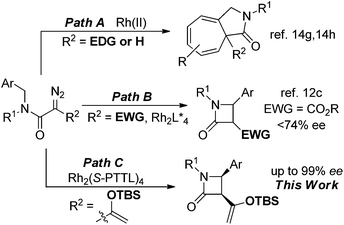
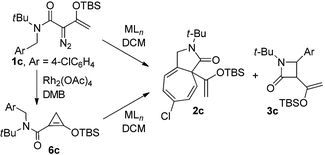
The importance of β-lactam compounds (2-azetidinones) in biology and medicine is undisputed since the discovery of the antibiotic activity of the bicyclic penicillin in 1928,1,2 and monocyclic β-lactams that include the plasma cholesterol-lowering Ezetimibe3 also show biological activity.4 Considerable effort has been focused on chemical catalysis for the construction of β-lactams, including those through catalytic intramolecular amide N–H insertion reactions of diazo compounds,5 and asymmetric synthesis has been a primary focus.2,6 However, the same C–H functionalization methodology of diazo compounds that has provided exceptional selectivities in intermolecular reactions7 and intramolecular syntheses of γ-lactones and γ-lactams8 has been limited in efforts to synthesize β-lactams.Direct intramolecular C–H functionalization of diazoacetamides catalyzed by transition metal catalysts is straightforward.9 The amide nitrogen activates the adjacent C–H bond for insertion. Although β-lactam formation by photocatalysis was described fifty years ago,10 and the first enantioselective process was reported in 1992,2c,11 there have only been a few examples that have overcome a majority of the electronic, steric, and conformational factors which control the selectivity of this process.12,13 Competing reactions include intramolecular cycloaddition to an aromatic ring of an aryl- or heterocycle attachment (Buchner reaction), addition to a carbon–carbon multiple bond of an allylic or propargylic system, or regioselective formation of a γ-lactam from C–H functionalization.14 Product selectivity is highly dependent on the diazo compound that is employed;12,14 for example, acceptor or donor–acceptor diazoamides form aromatic cycloaddition products when catalyzed by dirhodium catalysts (Scheme 1, Path A, R2 = H or EDG), but acceptor–acceptor diazoamides produce β-lactam products by a C–H functionalization reaction (Path B, R2 = EWG).
To achieve high selectivity in C–H functionalization reactions that form β-lactam products, acceptor–acceptor diazoamides (R2 = EWG) have been constructed to avoid side reactions, but high regio- and enantiocontrol (>90% ee) has only been demonstrated in constrained cyclic systems in which the aliphatic γ-position has been made inaccessible for insertion.12a,b We have developed enoldiazoacetates as a new class of stable donor–acceptor diazo compounds with extensive applications for cycloaddition reactions.15 An attractive feature of this vinyldiazo compound in catalytic reactions with dirhodium(II) carboxylates is the apparent donor–acceptor cyclopropene intermediate that serves as a resting state for the apparent reactive metal carbene intermediate.16 Could enoldiazoacetamides form intermediate donor–acceptor cyclopropenes and be precursors to donor–acceptor metal carbene intermediates on the pathway to C–H functionalization? Earlier work by Müller suggested that enantioselectivity in cyclopropanation reactions of styrene with an enoldiazoacetate is significantly greater than that with the corresponding diazoacetoacetate.17 We wish to report that asymmetric catalysis with donor–acceptor N-benzyldiazoamides proceeds through donor–acceptor cyclopropene intermediates to form cis-disubstituted β-lactams by intramolecular C–H functionalization in high yields and stereoselectivities (Path C).
Results and discussion
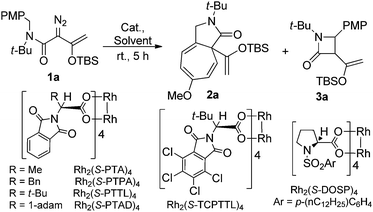
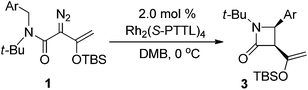
In initial studies we selected the N-tert-butyl-N-(p-methoxy-benzyl)enoldiazoacetamide 1a as a model substrate since previous studies have shown that the tert-butyl group fixed the reactant in the conformation in which the benzyl and diazo functional groups are syn to each other.13 In this conformation C–H insertion into the tert-butyl group is prevented, but aromatic cycloaddition into the anisyl group could be competitive with C–H insertion into its benzyl group, and indeed this competition was observed (Table 1). The Buchner product 2a was dominant in reactions catalyzed by sterically unencumbered Rh2(OAc)4 or the electrophilic Rh2(pfb)4, but with the sterically restrictive Rh2(tpa)4 or Rh2(esp)2 the sole product was the cis-disubstituted β-lactam 3a, formed by C–H insertion into the benzylic position. With asymmetric catalysts similar steric influences were operative so that increasing the steric bulk of the chiral Hashimoto dirhodium(II) carboxylate catalyst ligand increased the 3a/2a ratio and, also, enhanced enantioselectivity (entries 5–9) for both products. Both Rh2(S-PTTL)4 and Rh2(S-PTAD)4 gave β-lactam 3a as the only product in high yield with 64% ee (entries 8 and 9). We chose Rh2(S-PTTL)4 for further optimization and, after screening solvents and reaction temperatures, the reaction carried out at 0 °C in 2,2-dimethylbutane (DMB) gave the optimum result with 85% isolated yield and 92% ee of 3a (entry 17).18 The more Lewis acidic Rh2(S-TCPTTL)4 gave 3a in higher yield but slightly lower % ee. Chiral dirhodium carboxamidates were not effective as catalysts for this transformation, but the more reactive Rh2(S-DOSP)4 produced a mixture of 2a and 3a with low enantioselectivities (entry 5).| Entry | Rh(II) | Solvent |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ae 3ae |
Yieldf, (%) 2a + 3a | eeg (%) 2a/3a |
|---|---|---|---|---|---|
| a Reactions were carried out at room temperature on a 0.2 mmol scale in 1.0 mL solvent with 2.0 mol% dirhodium catalyst in 5 hours. b Reactions were carried out at room temperature on a 0.2 mmol scale in 0.5 mL DCCl3 with 2.0 mol% dirhodium catalyst in an NMR tube. c The reaction was carried out at 0 °C in 3 hours. d The reaction was carried out at −20 °C overnight. e The ratio was determined by integration of characteristic 1H NMR absorptions from the spectrum of the reaction mixture. f Isolated yield after chromatography. g Enantioselectivity was determined by chiral HPLC analysis, see ESI for details. TBME = tert-butyl methyl ether; DMB = 2,2-dimethylbutane. | |||||
| 1b | Rh2(OAc)4 | DCCl3 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
87 | —/— |
| 2b | Rh2(pfb)4 | DCCl3 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
89 | —/— |
| 3b | Rh2(tpa)4 | DCCl3 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
92 | —/— |
| 4b | Rh2(esp)2 | DCCl3 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
87 | —/— |
| 5b | Rh2(S-DOSP)4 | DCCl3 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
90 | 27/10 |
| 6b | Rh2(S-PTA)4 | DCCl3 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
92 | 35/30 |
| 7b | Rh2(S-PTPA)4 | DCCl3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
90 | 53/41 |
| 8b | Rh2(S-PTTL)4 | DCCl3 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
93 | —/64 |
| 9b | Rh2(S-PTAD)4 | DCCl3 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
93 | —/64 |
| 10 | Rh2(S-PTTL)4 | DCM | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
89 | —/65 |
| 11 | Rh2(S-PTTL)4 | DCE | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
88 | —/68 |
| 12 | Rh2(S-PTTL)4 | Toluene | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
76 | —/75 |
| 13 | Rh2(S-PTTL)4 | CF3Ph | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
72 | —/67 |
| 14 | Rh2(S-PTTL)4 | Cyclohexane | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
90 | —/82 |
| 15 | Rh2(S-PTTL)4 | TBME | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
91 | —/71 |
| 16 | Rh2(S-PTTL)4 | DMB | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
83 | —/88 |
| 17c | Rh2(S-PTTL)4 | DMB | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
85 | —/92 |
| 18d | Rh2(S-PTTL)4 | DMB | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
82 | —/91 |
| 19c | Rh2(S-TCPTTL)4 | DMB | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
88 | —/89 |
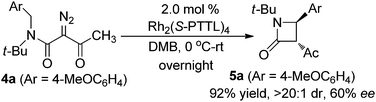
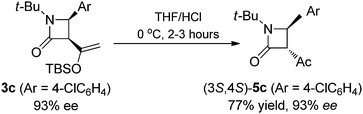
The observed high enantioselectivity obtained from catalytic intramolecular C–H insertion of 1a was not observed with the corresponding diazoacetamide. β-Lactam 5a was obtained in only 60% ee for when diazoacetamide 4a was reacted under the same conditions (eqn (1)) and, in contrast to the exclusive cis-selectivity observed in the formation of β-lactam 3a, 5a was formed with exclusive trans-selectivity.19 The trans β-lactam product was also obtained in high yield in reactions catalyzed by achiral [RuCl2(p-cymene)]2 reported by Chi.14f The reason for this difference in diastereoselectivity is probably isomerization of the β-ketoamide and, indeed, cis-3c is converted to trans-5c upon hydrolysis (eqn (2)) without loss of enantioselectivity.
 | (1) |
 | (2) |
The scope of the C–H functionalization reaction of representative diazoacetamides 1 was investigated with Rh2(S-PTTL)4 catalysis under the optimum conditions established for 1a (Table 2). In all examples, cis-β-lactam 3 was generated exclusively and in high yield (80–92%) and with high enantioselectivities (83–99% ee). The % ee of β-lactam 3 was lower when strong electron-withdrawing substituents were on the aromatic ring, and these reactions also required longer times for completion than did enoldiazoacetamides with electron-donating substituents (entries 6 and 7). Lower enantioselectivities were obtained with substrates having m- or o-substituents, and the lowered % ee was independent of a second substituent at the para position (entries 10 and 11) or of the size of the ortho substituent (entries 12 and 13). Using the N,N-diisopropylamide instead of the benzyl-t-butylamide also resulted in a β-lactam with lower enantioselectivity but good yield. Rh2(S-NTTL)4 gave higher enantiocontrol by 7% ee in the reaction of unsubstituted enoldiazo acetamide 1b (entry 2), but the same or lower enantioselectivities were observed in reactions with 1a, 1f, 1g, and 1k. Product mixtures were obtained when Ar = the heteroaryl 3-furanyl group and included products from [3 + 4]-cycloaddition.7 It is noteworthy that β-lactam 3i with the p-dimethylamino substituent was obtained with 99% ee in 80% yield (entry 9). And TIPS protected substrate 1c′ gave similar results as 1c (entry 3 vs. entry 15). When an N-aryl substituent was used instead of the t-butyl group diazo compound 1o, the reaction gave both Buchner reaction and C–H functionalization products in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio with 78% total yield, and β-lactam 3o was formed with 71% ee (eqn (3)).
2 ratio with 78% total yield, and β-lactam 3o was formed with 71% ee (eqn (3)).
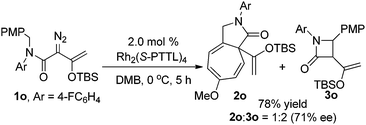 | (3) |
| Entry | Ar (1) | t (hours) | 3 | Yieldc (%) | eed (%) |
|---|---|---|---|---|---|
| a Reactions were carried out on a 0.2 mmol scale in 1.0 mL DMB with 2.0 mol% Rh2(S-PTTL)4. b Results in parentheses was catalyzed by Rh2(S-NTTL)4. c Isolated yield. d Enantioselectivity was determined by chiral HPLC analysis, see ESI for details. e N,N-Diisopropyl instead of N-tert-butyl-N-benzyl diazoamide was used. f TIPS protection instead of TBS was used. | |||||
| 1 | 4-MeO6H4 (1a) | 3 | 3a | 85 | 92 |
| 2 | C6H5(1b) | 12 | 3b | 82 (81)b | 80 (87)b |
| 3 | 4-ClC6H4 (1c) | 5 | 3c | 88 | 93 |
| 4 | 4-MeC6H4 (1d) | 5 | 3d | 92 | 93 |
| 5 | 4-FC6H4 (1e) | 5 | 3e | 88 | 91 |
| 6 | 4-BrC6H4 (1f) | 12 | 3f | 89 | 89 |
| 7 | 4-NO2C6H4 (1g) | 12 | 3g | 88 | 83 |
| 8 | 4-PhC6H4 (1h) | 5 | 3h | 89 | 91 |
| 9 | 4-Me2NC6H4 (1i) | 5 | 3i | 80 | 99 |
| 10 | 3,4-(MeO)2C6H3 (1j) | 5 | 3j | 81 | 77 |
| 11 | 3-MeOC6H4 (1k) | 5 | 3k | 92 | 78 |
| 12 | 2-MeOC6H4 (1l) | 12 | 3l | 85 | 25 |
| 13 | 1-Naphthyl (1m) | 5 | 3m | 85 | 24 |
| 14e | N,N-Diisopropyl (1n) | 5 | 3n | 81 | 67 |
| 15f | 4-ClC6H4 (1c′) | 5 | 3c′ | 84 | 92 |
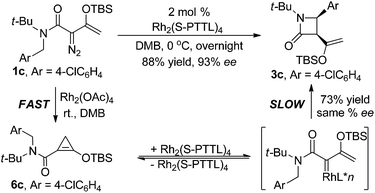
As is suggested by the reaction conditions, these reactions are relatively rapid. However, close spectroscopic inspection of reactions with 1c revealed that donor–acceptor cyclopropene 6c was formed at a much faster rate than was the product from C–H insertion. To determine if the donor–acceptor cyclopropene is a precursor to the donor–acceptor chiral metal carbene intermediate whose intramolecular C–H insertion produces highly enantiomerically enriched β-lactam, reaction of 1c was performed in DMB with 2.0 mol% Rh2(OAc)4 and at 5 min was filtered through Celite to remove the Rh2(OAc)4. Spectral analysis of the residue showed <1% 1c, 8 ± 1% 3c, and 92 ± 2% donor–acceptor cyclopropene 6c. This mixture was then submitted to the same reaction conditions as reported in Table 2 with Rh2(S-PTTL)4 catalysis to produce 3c in 73% isolated yield and 83 ± 3% ee. Subtracting racemic product formed from Rh2(OAc)4 gives 3c formed from donor–acceptor cyclopropene 6c with the same selectivity as that formed from 1c (Scheme 2).
That donor–acceptor cyclopropene 6 can serve as a precursor to an intermediate metal carbene of dirhodium(II) which undergoes C–H insertion prompted us to investigate if other transition metals, particularly those of copper(I) and silver(I), could undergo the same transformation. Although both of these catalytically active metal ions are known to form metal carbenes directly from diazo compounds,7,8,20 they undergo Lewis acid catalyzed reactions with enoldiazoacetates,15 and they are distinctly different from dirhodium(II) carboxylates in cycloaddition reactions with nitrones.18c Since C–H insertion is notably unique to metal carbenes, reactions of metal catalysts with enoldiazoacetamides or their derivative cyclopropenes would be a demonstration of metal carbene involvement with these catalysts. To undertake this investigation, reactions were performed on both enoldiazoacetamide 1c and cyclopropene 6c that was prepared from 1c by treatment with Rh2(OAc)4 in DMB as previously described, and these results are described in Table 3. The copper(I) and silver(I) catalysts are distinctly different from each other and from Rh2(OAc)4 in their reactions with enoldiazoacetamide 1c: aromatic cycloaddition is favored over C–H-insertion in reactions of 1c in the catalyst order: Ag(SbF6) > Cu(MeCN)4PF6 > Rh2(OAc)4 (entries 1 and 2); and this difference is also reflected in the results from reactions with cyclopropene 6c. Surprisingly, the Cu(MeCN)4PF6 and AgSbF6 catalyzed reactions with enoldiazoacetamide 1c provide more of the aromatic cycloaddition product, which is reported to be due to a more electrophilic metal carbene intermediate,13 than do their reactions with donor–acceptor cyclopropene 6c. The observed differences in the 2c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c ratios from reactions with 1c and 6c suggest that there may be some dependence on the carbene source among the catalysts employed for aromatic cycloaddition and C–H insertion, and that donor–acceptor cyclopropene 6c and enoldiazoacetate 1c may not form the same conformationally identical metal carbene intermediate. Use of a box ligand effectively inhibits dinitrogen extrusion from enoldiazoacetamide 1c, but metal carbene formation from cyclopropene 6c occurs without this limitation (entries 3 and 5). Other Lewis acids or under the thermal condition did not give any Buchner reaction or C–H insertion product, and only slowly decomposition of 6c was observed (entries 8–11).
3c ratios from reactions with 1c and 6c suggest that there may be some dependence on the carbene source among the catalysts employed for aromatic cycloaddition and C–H insertion, and that donor–acceptor cyclopropene 6c and enoldiazoacetate 1c may not form the same conformationally identical metal carbene intermediate. Use of a box ligand effectively inhibits dinitrogen extrusion from enoldiazoacetamide 1c, but metal carbene formation from cyclopropene 6c occurs without this limitation (entries 3 and 5). Other Lewis acids or under the thermal condition did not give any Buchner reaction or C–H insertion product, and only slowly decomposition of 6c was observed (entries 8–11).
| Entry | Catalyst | Reactant | t (hours) |
2c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c 3c |
Yieldd (%) 2c + 3c | eee (%) 3c |
|---|---|---|---|---|---|---|
| a Reactions were carried out on a 0.2 mmol scale in 1.0 mL DCM. b Reactions were carried out with 10 mol% Lewis acid catalyst. c Reactions were carried out with 10 mol% Lewis acid catalyst and 12 mol% ligand. d Isolated yield. e The enantioselectivity was determined by chiral HPLC analysis, see ESI for details. f Neither 2c nor 3c was observed, and only slowly decomposition of 6c was observed. g The reaction was carried out in 70 °C. | ||||||
| 1b | AgSbF6 | 1c | 12 | >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
87 | — |
| 2b | Cu(MeCN)4PF6 | 1c | 12 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
85 | — |
| 3c | Cu(MeCN)4PF6/(S)-t-BuBox | 1c | 48 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
10 | 28 |
| 4b | Cu(MeCN)4PF6 | 6c | 12 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
87 | — |
| 5c | Cu(MeCN)4PF6/(S)-t-BuBox | 6c | 12 | <5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
89 | 30 |
| 6b | AgSbF6 | 6c | 12 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
82 | — |
| 7c | AgSbF6/(S)-t-BuBox | 6c | 12 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
91 | 24 |
| 8 | Sc(OTf)3 | 6c | 12 | — | NRf | — |
| 9 | La(OTf)3 | 6c | 12 | — | NRf | — |
| 10 | BF3·Et2O | 6c | 12 | — | NRf | — |
| 11g | (—) | 6c | 12 | — | NRf | — |
The assignment of relative stereochemistry for β-lactam 3 was made from 1H NMR coupling constants,21 and the observed cis-configuration is consistent with a steric influence of the catalyst attachments and rhodium surface on the aryl substituent of the intermediate metal carbene. The chiral Rh2(S-PTTL)4 catalyst was reported to exist in a crown conformation by Fox,22 which means all of the ligands in an “all-up” orientation, and the bulky t-butyl and TBS substituents of the carbene are enclosed within the crown. This crowded transition state defines conformational preference for the aryl group to approach the carbene center for C–H insertion and the Buchner reaction. The (3S,4R)-configuration of the generated chiral centers in β-lactam 3 was confirmed by single-crystal X-ray diffraction analysis of 3i, and the configurations of other compounds were assigned by analogy.23
Conclusions
In conclusion, we have discovered a highly selective asymmetric intramolecular C–H functionalization reaction of enoldiazoacetamides catalyzed by Rh2(S-PTTL)4 that occur via an intermediate donor–acceptor cyclopropene. The Buchner reaction was totally excluded in reactions catalysed by steric bulky dirhodium carboxylate catalyst, and β-lactam derivatives are obtained from intramolecular C–H insertion as one diastereoisomer in high yield with up to 99% enantiomeric excess. Furthermore, reactions of enoldiazoacetamides and their corresponding donor–acceptor cyclopropenes performed with copper(I) and silver(I) catalysts validate their formation of metal carbene intermediates, but they show differences in reactivity and selectivity. The high enantiocontrol that is achieved relies on both electronic and steric influences of the unique silylenol group in these metal carbene transformation.Acknowledgements
Support to M.P.D. from the National Institutes of Health (GM 46503) and from the National Science Foundation (CHE-1212446) is gratefully acknowledged. Michael Mandler provided spectral information. X.F.X. thanks the startup funding from Soochow University and Key Laboratory of Organic Synthesis of Jiangsu Province.Notes and references
- A. Fleming, Rev. Infect. Dis., 1980, 2, 129 CrossRef PubMed.
- (a) E. Raviña, The Evolution of Drug Discovery, Wiley-VCH, Weinheim, Germany, 2011, pp. 254–272 Search PubMed; (b) P. A. Magriotis, Eur. J. Org. Chem., 2014, 2647 CrossRef; (c) C. R. Pitts and T. Lectka, Chem. Rev., 2014, 114, 7930 CrossRef PubMed; (d) S. France, A. Weatherwax, A. E. Taggi and T. Lectka, Acc. Chem. Res., 2004, 37, 592 CrossRef PubMed.
- (a) J. W. Clader, J. Med. Chem., 2004, 47, 1 CrossRef PubMed; (b) M. A. Blazing, R. P. Giugliano, C. P. Cannon, T. A. Musliner, A. M. Tershakovec, J. A. White, C. Reist, A. McCagg, E. Braunwald and R. M. Califf, Am. Heart J., 2014, 168, 205 CrossRef PubMed; (c) S. D. Turley and J. M. Dietschy, Curr. Opin. Lipidol., 2003, 14, 233 CrossRef PubMed.
- For reviews: (a) P. Galletti and D. Giacomini, Curr. Med. Chem., 2011, 18, 4265 CrossRef CAS; (b) O. A. Pierrat, K. Strisovsky, Y. Christova, L. J. Jonathan, K. Ansell, N. Bouloc, E. Smiljanic and M. Freeman, ACS Chem. Biol., 2011, 6, 325 CrossRef PubMed; (c) P. A. Mann, et al. , ACS Chem. Biol., 2013, 8, 2442 CrossRef PubMed; (d) S. Vandekerckhove and M. D'hooghe, Bioorg. Med. Chem., 2013, 21, 3643 CrossRef PubMed.
- (a) F. A. Davis, B. Yang and J. Deng, J. Org. Chem., 2003, 68, 5147 CrossRef PubMed; (b) R. Ratcliffe, T. Salzmann and B. Christensen, Tetrahedron Lett., 1980, 21, 31 CrossRef , intermolecular amide N–H insertion reactions: ; (c) E. C. Lee and G. C. Fu, J. Am. Chem. Soc., 2007, 129, 12066 CrossRef PubMed; (d) B. Xu, S. Zhu, X. Xie, J. Shen and Q. Zhou, Angew. Chem., Int. Ed., 2011, 50, 11483 CrossRef PubMed; (e) S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, J. Am. Chem. Soc., 2014, 136, 195 CrossRef PubMed.
- Recent advances in asymmetric catalytic syntheses of β-lactam: (a) J. Pedroni, M. Boghi, T. Saget and N. Cramer, Angew. Chem., Int. Ed., 2014, 53, 9064 CrossRef PubMed; (b) S. R. Smith, J. Douglas, H. Prevet, P. Shapland, A. M. Z. Slawin and A. D. Smith, J. Org. Chem., 2014, 79, 1626 CrossRef PubMed; (c) Z. Chen, L. Lin, M. Wang, X. Liu and X. Feng, Chem.–Eur. J., 2013, 19, 7561 CrossRef PubMed; (d) L. Huang, W. Zhao, R. J. Staples and W. D. Wulff, Chem. Sci., 2013, 4, 622 RSC; (e) S. Chen, E. C. Salo, K. A. Wheeler and N. J. Kerrigan, Org. Lett., 2012, 14, 1784 CrossRef PubMed; (f) Y. S. M. Vaske, M. E. Mahoney, J. P. Konopelski, D. L. Rogow and W. J. McDonald, J. Am. Chem. Soc., 2010, 132, 11379 CrossRef PubMed.
- (a) H. M. L. Davies and D. Morton, Chem. Soc. Rev., 2011, 40, 1857 RSC; (b) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417 CrossRef PubMed; (c) X. Cui, X. Xu, L. Jin, L. Wojtas and X. P. Zhang, Chem. Sci., 2015, 6, 1219 RSC; (d) D. N. Tran, C. Battilocchio, S. Lou, J. M. Hawkins and S. V. Ley, Chem. Sci., 2015, 6, 1120 RSC; (e) N. A. McGrath, K. A. Andersen, A. K. F. Davis, J. E. Lomax and R. T. Raines, Chem. Sci., 2015, 6, 752 RSC; (f) J. H. Hansen and H. M. L. Davies, Chem. Sci., 2011, 2, 457 RSC.
- For review: (a) M. P. Doyle, Y. Liu and M. Ratnikov, Org. React., 2013, 80, 1 Search PubMed; (b) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704 CrossRef PubMed; (c) M. P. Doyle, M. Ratnikov and Y. Liu, Org. Biomol. Chem., 2011, 9, 4007 RSC.
- For reviews: (a) M. P. Doyle and D. C. Forbes, Chem. Rev., 1998, 98, 911 CrossRef PubMed; (b) P. M. P. Gois and C. A. M. Afonso, Eur. J. Org. Chem., 2004, 3773 CrossRef.
- (a) E. J. Corey and A. M. Felix, J. Am. Chem. Soc., 1965, 87, 2518 CrossRef; (b) S. D. Burke and P. A. Grieco, Org. React., 1979, 26, 361 Search PubMed.
- M. P. Doyle, M. N. Protopopova, W. R. Winchester and K. L. Daniel, Tetrahedron Lett., 1992, 33, 7819 CrossRef.
- (a) M. P. Doyle and A. V. Kalinin, Synlett, 1995, 1075 CrossRef PubMed; (b) M. Anada, N. Watanabe and S. Hashimoto, Chem. Commun., 1998, 1517 RSC; (c) N. R. Candeias, C. Carias, L. F. R. Gomes, V. André, M. T. Duarte, P. M. P. Gois and C. A. M. Afonso, Adv. Synth. Catal., 2012, 354, 2921 CrossRef.
- A. Padwa, D. J. Austin, A. T. Price, M. A. Semones, M. P. Doyle, M. N. Protopopova, W. R. Winchester and A. Tran, J. Am. Chem. Soc., 1993, 115, 8669 CrossRef.
- Advances in regioselectivity: (a) V. K. Lo, Z. Guo, M. K. Choi, W. Yu, J. Huang and C. Che, J. Am. Chem. Soc., 2012, 134, 7588 CrossRef PubMed; (b) J. P. Snyder, A. Padwa, T. Stengel, A. J. Arduengo, A. Jockisch and H. Kim, J. Am. Chem. Soc., 2001, 123, 11318 CrossRef; (c) P. M. P. Gois and C. A. M. Afonso, Eur. J. Org. Chem., 2003, 3798 CrossRef; (d) C. H. Yoon, A. Nagle, C. Chen, D. Gandhi and K. W. Jung, Org. Lett., 2003, 5, 2259 CrossRef PubMed; (e) M. P. Doyle, M. S. Shanklin, S. Oon, H. Q. Pho, F. R. van der Heidet and W. R. Veal, J. Org. Chem., 1988, 53, 3384 CrossRef; (f) M. K. Choi, W. Yu and C. Che, Org. Lett., 2005, 7, 1081 CrossRef PubMed; (g) C. J. Moody, S. Miah, A. M. Z. Slawin, D. J. Mansfield and I. C. Richards, J. Chem. Soc., Perkin Trans. 1, 1998, 4067 RSC; (h) Z. Qu, W. Shi, X. Jin and J. Wang, Chin. J. Org. Chem., 2003, 23, 988 Search PubMed; (i) L. A. Clarke, A. Ring, A. Ford, A. S. Sinha, S. E. Lawrencea and A. R. Maguire, Org. Biomol. Chem., 2014, 12, 7612 RSC.
- For reviews: (a) X. Xu and M. P. Doyle, Acc. Chem. Res., 2014, 47, 1396 CrossRef PubMed; (b) X. Xu and M. P. Doyle, Aust. J. Chem., 2014, 67, 365 CrossRef , recent advances: ; (c) X. Xu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2013, 52, 12664 CrossRef PubMed; (d) X. Xu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2012, 51, 9829 CrossRef PubMed; (e) Y. Qian, X. Xu, X. Wang, P. Y. Zavalij, W. Hu and M. P. Doyle, Angew. Chem., Int. Ed., 2012, 51, 5900 CrossRef PubMed.
- (a) X. Xu, P. Y. Zavalij and M. P. Doyle, J. Am. Chem. Soc., 2013, 135, 12439 CrossRef PubMed; (b) X. Xu, P. Y. Zavalij and M. P. Doyle, Chem. Commun., 2013, 49, 10287 RSC.
- (a) P. Müller, Y. F. Allenbach and S. Grass, Tetrahedron: Asymmetry, 2005, 16, 2007 CrossRef PubMed; (b) P. Müller, G. Bernardinelli, Y. F. Allenbach, M. Ferri and S. Grass, Synlett, 2005, 1397 CrossRef PubMed; (c) P. Müller, G. Bernardinelli, Y. F. Allenbach, M. Ferry and H. D. Flack, Org. Lett., 2004, 6, 1725 CrossRef PubMed.
- Nonpolar solvents provide a significant increase in the enantioselectivity in some metal carbene reactions, for example, 2,2-DMB and TBME have previously been demonstrated to be an optimal solvent for enantioselective C–H functionalization and other metal carbene transformations: (a) H. Wang, G. Li, K. M. Engle, J. Yu and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6774 CrossRef PubMed; (b) A. G. Smith and H. M. L. Davies, J. Am. Chem. Soc., 2012, 134, 18241 CrossRef CAS PubMed; (c) X. Wang, X. Xu, P. Y. Zavalij and M. P. Doyle, J. Am. Chem. Soc., 2011, 133, 16402 CrossRef CAS PubMed.
- The cis isomer was not detected, even at short reaction times..
- M. Díaz-Requejo and P. J. Pérez, Chem. Rev., 2008, 108, 3379 CrossRef PubMed.
- The relative chemistry could be distinguished by 1H NMR coupling constants, see ref. 14e: generally cis = 5–8 Hz; trans = 0–2 Hz..
- (a) A. DeAngelis, O. Dmitrenko, G. P. A. Yap and J. M. Fox, J. Am. Chem. Soc., 2009, 131, 7230 CrossRef CAS PubMed; (b) S. Hashimoto, N. Watanabe, T. Sato, M. Shiro and S. Ikegami, Tetrahedron Lett., 1993, 34, 5109 CrossRef CAS; (c) D. T. Boruta, O. Dmitrenko, G. P. A. Yap and J. M. Fox, Chem. Sci., 2012, 3, 1589 RSC.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of substrates preparation, catalytic experiments, identification of the products and crystal data for 3i. CCDC 1018978. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc03991b |
| This journal is © The Royal Society of Chemistry 2015 |