Enhanced p-nitrophenol removal in a membrane-free bio-contact coupled bioelectrochemical system†
Shuai Lou‡
,
Xinbai Jiang‡,
Dan Chen,
Jinyou Shen*,
Weiqing Han,
Xiuyun Sun,
Jiansheng Li and
Lianjun Wang*
Jiangsu Key Laboratory for Chemical Pollution Control and Resources Reuse, School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu Province, China. E-mail: shenjinyou@mail.njust.edu.cn; wanglj@mail.njust.edu.cn; Fax: +86 25 84303965; Fax: +86 25 84315941; Tel: +86 25 84303965 Tel: +86 25 84315941
First published on 11th March 2015
Abstract
In this study, a membrane-free bio-contact coupled bioelectrochemical system (BC-BES) was established for the enhanced reductive transformation of p-nitrophenol (PNP). The results showed that the electric field played a key role in both PNP reduction and p-aminophenol (PAP) formation. The vast majority of PNP was reductively transformed to PAP in the biocathode of BC-BES. At a cathode potential of −1000 mV vs. Ag/AgCl and hydraulic retention time (HRT) of 8.9 h, PNP removal rate as high as 18.95 ± 0.10 mol per m3 per day could be achieved in the BC-BES with acetate as the electron donor. High PNP removal and PAP formation could be achieved at low acetate dosage, high initial PNP concentration and short HRT, indicating the strong ability of the BC-BES to resist shock loading. Furthermore, partial mineralization of PAP was observed in the anode of the BC-BES, which was beneficial for the further polishing of the BC-BES effluent. Considering the advantages of high loading rate, low acetate consumption and high system stability, it is hoped that the application of this BC-BES will enhance the reductive removal of nitrophenols from wastewaters.
1. Introduction
p-Nitrophenol (PNP), as the common industry intermediate in the synthesis of explosives, dyes, pesticides and pharmaceuticals, has been abundantly released into the environment, causing serious environmental problems.1,2 Due to its severe damage to some important organs of animals and human beings, it has been classified as a priority pollutant by the US Environmental Protection Agency (USEPA).3 Therefore, the removal of PNP from the environment has been of special concern.To date, various physicochemical technologies such as sonolysis,4 adsorption,5 Fenton oxidation,6 nickel catalysts7 and electrochemical oxidation8 have been used for the remediation of PNP pollution. However, all these methods have significant defects such as high cost or the formation of the secondary pollution during the treatment process.9,10 Biological process is regarded as cost-effective and efficient for PNP degradation.11 However, due to the strong electro-withdrawing effect of nitro group in the PNP molecular structure, PNP is difficult to oxidize in the aerobic bioprocess.12,13 Anaerobic process, where PNP can be reductively transformed to less toxic p-aminophenol (PAP) by co-metabolism, is more appropriate for PNP removal. However, anaerobic reduction process has the inherent shortcomings, such as low degradation rate, long hydraulic retention time (HRT) and poor system stability, especially for the treatment of wastewater containing high strength PNP waste.13,14
Bioelectrochemical system (BES) is a neoteric creation which has gained much attention across the globe in the past two decades due to its high versatility in the field of wastewater treatment.15,16 In BES, microorganisms are used as catalysts for the electrochemical reactions in an anode or cathode.17 The non-conservative substances such as glucose, sodium acetate, methanol, etc., are oxidized in the anode and then the generated electrons and protons transfer from the anode to the cathode though external circuit and membrane, respectively. The inorganic or organic substances in the cathode gain electrons and protons, which initiate the reduction reaction. Attempts in terms of developing BES reactors with abiotic cathode for the reduction of oxidative compounds have been made.10,15 Compared with the traditional anaerobic biodegradation processes, it has been demonstrated that the reductive transformation of pollutants as the electron acceptor in BES was significantly reinforced.18–20 In addition, it has been suggested that the reduction reaction in the BES cathode at the presence of microbial catalyst could be enhanced, with the economic viability increased compared with the abiotic cathode.21–23 Microorganisms can not only take up electrons from cathode surface and utilize them for the subsequent electrochemical reactions, but also directly degrade organics through co-metabolic reaction.
In most of the previous studies, the BES reactors were separated into anode and cathode chambers by ion/proton exchange membrane. Due to the presence of the ion/proton exchange membrane, the internal resistance of the two-chamber BES was relatively high, which could be a serious bottleneck for energy losses.24 The cost and operational maintenance of the ion/proton exchange membrane hindered their practical applications as well. In addition, the installation of membrane could cause pH gradient especially during the long-term operation for wastewater treatment.25,26 Recently, in order to overcome these shortages, membrane-free BES has been suggested.25,27 Several studies have demonstrated that the internal resistance could be reduced and the power density could be further enhanced in the membrane-free BES.28 In addition, its adaptability to various recalcitrant compounds had got confirmed.29,30 Therefore, for the efficient treatment of PNP containing wastewater, membrane-free BES could be a favorite alternative.30
In this study, a membrane-free bio-contact coupled bioelectrochemical system (BC-BES) was designed for the enhanced reduction of PNP. The effect of acetate dosage, influent PNP concentration, hydraulic retention time (HRT) on PNP removal and PAP formation was investigated. The ability of the BC-BES to resist shock loading was assessed. Additionally, the mineralization of PNP reduction product, i.e., PAP, in the anode of the BC-BES was evaluated.
2. Experimental
2.1 Construction of the membrane less BC-BES reactor
The schematic diagram of the BC-BES is illustrated in Fig. 1. A bench-scale reactor consisted of a PVC column with the dimension of 60 mm inner diameter × 400 mm height was used in this study. The temperature of the BC-BES system was maintained at 35 ± 2 °C with water jacket throughout the experimental period. The total empty volume of reactor was 995 mL and the total effective volume was reduced to 785 mL after the installation of the anode and cathode. Graphite granules filled in the bottom of the reactor was used as cathode and the working electrode. Before use, the graphite granules were treated according to the procedure described by Mu et al.15 Graphite felt (Chemshine Carbon Co., China) was rolled up to a cylinder and was placed at the upper portion of the reactor as the anode material. Two graphite rods (5 mm diameter) were inserted into both the anodic and cathodic compartments to connect the two electrodes to the external circuit. Both the anode and cathode zones are 150 mm height. To allow even distribution of the influent, two plates with evenly spaced holes were installed at the bottom of the cathode and anode, respectively. The anode was connected to the cathode through a potentiostat (Bio-Logic Science Instruments, France) for current/potential control. An Ag/AgCl reference electrode (assumed +0.197 V vs. SHE) was placed between the anode and cathode for the measurement of half potentials. Both cathodic and anodic half-cell potentials were reported with respect to the Ag/AgCl reference electrode in this study. Two samples ports, i.e., sampling port above the anode zone and sampling port above the cathode zone, were used for sampling the anode effluent and cathode effluent, respectively.2.2 Reactor startup and operation
The influent composed of the BC-BES was as follows: KH2PO4 0.76 g L−1, Na2HPO4 3.06 g L−1, MgCl2 0.21 g L−1, NH4Cl 0.3 g L−1, CaCl2 0.02 g L−1 and trace element solution 10 mL L−1. Sodium acetate and PNP was added at desired concentrations, which served as the electron donor and electron acceptor, respectively. The trace element solution was prepared according to our previous study.31 Before use, the influent was autoclaved in an autoclave at 120 °C for 30 min and sparged with nitrogen for 20 min to remove oxygen.The sludge collected from an anaerobic baffled reactor treating a real wastewater containing nitroaromatic compounds was used as the inoculum of the BC-BES system. The initial mixed liquid suspended solid (MLSS) concentration in BC-BES was 8.5 g L−1. Before inoculation, the seed sludge was initially acclimatized to the influent containing 0.72 mM PNP and 9.75 mM acetate in a 10 L sequencing batch reactor.
The experimental period was divided into four phases, as shown in Table 1. At the first phase, i.e., start-up stage, the BC-BES reactor was operated in open circuit mode firstly. The influent was pumped into the bottom of the reactor continuously at HRT of 8.9 h and initial PNP concentration of 0.72 mM, resulting in a low PNP loading rate of 1.94 mol per m3 per day. The purpose of this control experiment was to evaluate the PNP removal through direct anaerobic reduction. Ten days later, the circuit of BC-BES was closed. In order to improve the PNP reduction performance and confirm the positive role of current density, the cathode potential was adjusted to −1000 mV gradually, with the PNP removal and PAP formation performance evaluated. Then, in order to further increase the PNP removal capacity in BC-BES, PNP concentration increased step by step from 0.72 mM to 3.23 mM with the corresponding PNP loading rate increased from 1.94 mol per m3 per day to 8.72 mol per m3 per day. The influent acetate dosage was remained at 9.75 mM regardless of the PNP loading rate in the influent.
| Phase | Days | PNP concentration (mM) | Acetate dosage (mM) | HRT (h) | PNP loading rate (mol per m3 per day) | Cathode potential (mV) |
|---|---|---|---|---|---|---|
| Phase 1 | 1–10 | 0.72 | 9.75 | 8.9 | 1.94 | Open circuit |
| Phase 1 | 11–96 | 0.72–3.23 | 9.75 | 8.9 | 1.94–8.72 | −50 to −1000 |
| Phase 2 | 97–158 | 2.52 | 14.63–1.83 | 8.9 | 6.78 | −1000 |
| Phase 3 | 159–205 | 3.23–7.19 | 4.68–10.43 | 8.9 | 8.72–19.38 | −1000 |
| Phase 4 | 206–250 | 2.52 | 9.75 | 8.9–2.5 | 6.78–23.22 | −1000 |
In phase 2, in order to investigate the effect of acetate dosage on the performance of PNP reduction and PAP formation, and to assess the electron donor requirement in BC-BES, the influent acetate dosage decreased from 14.63 to 1.83 mM at influent PNP concentration of 2.52 mM and HRT of 8.9 h. BC-BES was operated at cathode potential of −1000 mV at this stage.
In phase 3, in order to evaluate the effect of the high PNP dosage on PNP removal and PAP formation, the influent PNP concentration increased step by step from 3.23 mM to 7.19 mM, while HRT was controlled at 8.9 h, resulting to the increase of PNP loading rate from 8.72 mol per m3 per day to 19.38 mol per m3 per day. The acetate was added according to the molar ratio of 1.45 mol acetate per mol PNP. The cathode potential was controlled at −1000 mV at this stage.
In phase 4, in order to test the performance of the BC-BES at low HRTs, the HRT decreased step by step from 8.9 to 2.5 h with the influent PNP concentration at 2.52 mM and the acetate dosage at 9.75 mM, resulting to the increase of PNP loading rate from 6.78 mol per m3 per day to 23.22 mol per m3 per day. BC-BES was operated at cathode potential of −1000 mV at this stage.
Each experiment lasted at least 4 days to ensure that the reactor reached a steady state, judging from the slight variation of PNP removal efficiency as well as anode and cathode potentials.
2.3 Analytical methods
Before analysis, sample taken from the reactor were filtered through a 0.22 μm filter. PNP and PAP were quantified using a HPLC (Waters 2996, Waters Incorporation, USA) equipped with a RP18 column (5 mm, 4.6 × 250 mm, Waters Co., USA) and a UV-Vis detector. The mobile phase was methanol/water with a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for PNP and 8
4 for PNP and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for PAP respectively and pumped at a flow rate of 1.00 mL min−1. The analysis was performed at 254 nm with a column temperature of 35 °C. NH4+–N were analyzed according to China NEPA standard methods (1997). Acetate dosage was determined through an ion chromatograph (ICS-2100, DIONEX) using Ion Pac® As11-HC (4 × 250 mm) column and a suppressed conductivity detector. The 30 mM NaOH eluent was pumped at a flow rate of 1.5 mL min−1. Electrochemical monitoring and calculations were carried out according to our previous study,20 including anode and cathode potentials, PNP removal efficiency, PAP formation efficiency, PNP removal rate, PAP formation rate, acetate removal efficiency, cosubstrate usage ratio and energy consumption.
2 for PAP respectively and pumped at a flow rate of 1.00 mL min−1. The analysis was performed at 254 nm with a column temperature of 35 °C. NH4+–N were analyzed according to China NEPA standard methods (1997). Acetate dosage was determined through an ion chromatograph (ICS-2100, DIONEX) using Ion Pac® As11-HC (4 × 250 mm) column and a suppressed conductivity detector. The 30 mM NaOH eluent was pumped at a flow rate of 1.5 mL min−1. Electrochemical monitoring and calculations were carried out according to our previous study,20 including anode and cathode potentials, PNP removal efficiency, PAP formation efficiency, PNP removal rate, PAP formation rate, acetate removal efficiency, cosubstrate usage ratio and energy consumption.
3. Results and discussion
3.1 Startup the BC-BES
After 10 days' operation in an open circuit, the BC-BES circuit was closed with the cathode potential gradually adjusted from −50 mV on day 11 to −1000 mV on day 30. As shown in Fig. 2, PNP removal efficiency and PAP formation efficiency increased from 17.40% and 14.39% on day 10 to 99.91% and 83.48% on day 30, respectively. The stable PNP removal and the high PAP formation at the end of the first month indicated the success of the startup of the BC-BES. More negative cathode potential often resulted in higher anode potential and higher current. During this period, with the decrease of the cathode potential from −50 mV on day 11 to −1000 mV on day 30, the anode potential was well below −400 mV (Fig. S1†). The relatively low anode potential at cathode potential of −1000 mV indicated that the electrochemically active microorganisms were enriched on the anode for acetate oxidation and were capable of supplying electrons to the cathode for PNP reduction at this cathode potential. Previous study has shown that the reductive potential of PNP was usually lower than −700 mV, moreover, a more negative cathode potential benefit the reduction of PNP.10,20,30 Thus the cathode potential in the following experiment was normally controlled at −1000 mV, considering that the anode potential could be well maintained below −200 mV at this cathode potential.40 days later, in order to further improve the reactor performance, PNP loading rate increased gradually from 1.94 on day 40 to 8.72 mol per m3 per day on day 96. As was indicated in Fig. 2, PNP removal and PAP formation were always kept at high levels within the PNP loading range of 1.94 to 8.72 mol per m3 per day. In the BC-BES effluent, almost 100% PNP could be removed while higher than 90% of the total PNP could be transformed into PAP, confirming that PNP could be efficiently reduced into PAP in the BC-BES. Meanwhile, current density increased from 1.96 ± 0.13 A m−3 at PNP loading rate of 1.94 mol per m3 per day to 6.72 ± 0.01 A m−3 at PNP loading rate of 8.72 mol per m3 per day, due to the high availability of the electron acceptor at high PNP loading rate. In order to confirm the key role of the electric field in PNP reduction, the electric field was removed at the 55th day. What is interesting is that, PNP removal efficiency sharply decrease from 100% to 76.27%, and correspondingly, PAP formation efficiency declined from 99.56% to 56.53%. After the cathode potential was reduced back to the low level, both PNP removal efficiency and PAP formation efficiency recovered to the previous level. This phenomenon showed that the electric field played a key role in both PNP reduction and PAP formation.
For the reduction process of nitroaromatic compounds such as PNP, three steps has been proposed, with the nitroso aromatic compounds and hydroxylamine aromatic compounds as intermediates, and with aminoaromatic compounds as the end products.10 In this study, both the UV-Vis spectrum and the HPLC chromatogram confirmed that PNP could be majorly converted into the final product p-aminophenol (PAP) in the BC-BES (Fig. S2 and S3†). The reduction intermediates of PNP, such as p-nitrosophenol and p-hydroxylaminophenol, were not detectable, probably due to the more negative cathode potential adopted (as low as −1000 mV), which was beneficial for both PNP reduction and PAP formation.30 Another reason could be the inoculation of the BC-BES cathode in this study. PNP reduction and PAP formation could be enhanced at the presence of the inoculated bacteria as biocatalysts.
After the successful startup of the BC-BES, from day 30 to day 40, the PNP removal efficiency and PAP formation efficiency in the anode effluent and cathode effluent were 100% and 83.48%, 100% and 75.54%, respectively. The PNP removal efficiency and PAP formation efficiency determined in the anode effluent and in the cathode effluent during this period were nearly the same, demonstrating that PNP removal and PAP formation dominantly occurred in the cathode zone of the BC-BES.
3.2 Effect of acetate dosage on PNP removal and PAP formation
The dosage of the electron donor, i.e. acetate, played a key role in both PNP reduction and PAP formation. Theoretically, only 1.5 mol COD or 0.75 mol acetate was required for complete conversion of one mole PNP to PAP, i.e., the cosubstrate usage ratio was 1.5 mol-COD or 0.75 mol-acetate per mol-PNP removed, as shown in eqn (1).| 4HO–Ar–NO2 + 3CH3COO− + 4H2O → 4HO–Ar–NH2 + 6HCO3− + 3H+ | (1) |
In order to investigate the effect of acetate dosage on PNP reduction and PAP formation, different acetate dosage conditions in BC-BES was tested. As indicated in Fig. 3a, when the acetate dosage decreased from 14.63 to 3.66 mM, the PNP removal efficiency in BC-BES was maintained at the level as high as 100%, while PAP formation efficiency decreased slightly from 98.29 ± 3.60% to 89.39 ± 2.76%. However, further decrease of acetate dosage resulted in sharp decrease of both PNP removal and PAP formation. As acetate dosage decreased from 3.66 mM to 1.83 mM, the PNP removal efficiency in BC-BES decreased sharply from 100% to 82.71 ± 5.32%. At the meanwhile the PAP formation efficiency decreased sharply from 89.39 ± 2.76% to 68.43 ± 6.18%. In addition, at acetate dosage of 1.83 mM, the anode potential was sharply increased to above 0 mV, which suggested that the electrochemically active microorganisms on the anode might be seriously suppressed (Fig. S4†). Thereafter, acetate dosage was increased back to a high level for stable reactor operation.
 | ||
| Fig. 3 Effect of influent acetate dosage on PNP reduction and PAP formation (a) and acetate removal efficiency and cosubstrate usage ratio (b) in BC-BES. | ||
The effect of acetate dosage on acetate removal efficiency and cosubstrate usage ratio in BC-BES was shown in Fig. 3b. The cosubstrate usage ratio was significantly influenced by acetate dosage. The cosubstrate usage ratio was as high as 11.63 ± 0.40 mol COD per mol PNP when the acetate dosage was 14.63 mM, but it was reduced to 2.90 ± 0.13 mol COD per mol PNP when the acetate dosage was 3.66 mM. The acetate removal efficiency remained higher than 98% during the whole period, indicating that excessive acetate could be almost completely consumed in the BC-BES system. However, the minimal electron donor dosage of 2.90 mol-COD per mol-PNP in BC-BES was rather low, compared with conventional anaerobic process for PNP reduction, where the electron donor dosage was often higher than 20 mol COD per mol PNP.11,31,32 The result indicated that BC-BES had the advantage in terms of low requirement for electron donor, which would significantly reduce the operational cost. What's more, the acetate consumption in this BC-BES was also lower than that in double-chamber BES, where acetate consumption varied in the range of 5–8 mol COD per mol PNP.10,20,30
3.3 Effect of the influent PNP concentration on PNP removal and PAP formation
For biological process, influent PNP concentration has significant effect on the reactor performance, as PNP exhibits inhibitory and recalcitrant nature. At HRT of 8.9 h, as the influent PNP concentration increased from 3.23 mM to 7.19 mM, the corresponding PNP loading rate increased from 8.72 to 19.38 mol per m3 per day. As shown in Fig. 4a, with the influent PNP concentration below 6.47 mM, PNP removal efficiency was always as high as 100%. PNP removal rate and PAP formation rate increased progressively with the influent PNP concentration increased from 2.88 mM to 6.47 mM (Fig. 4b). However, further increase of the influent PNP concentration from 6.47 to 7.19 mM resulted into the slight decrease of PNP removal efficiency from 100% to 97.79 ± 1.59%. Corresponding, PNP removal rate increased from 17.45 ± 0.08 to 18.95 ± 0.10 mol per m3 per day. However, at influent PNP concentration of 7.19 mM, PAP formation efficiency and PAP formation rate decreased to 91.56 ± 5.64% and 17.74 ± 1.03 mol per m3 per day, respectively, probably due to the increased formation of the other reductive intermediates during PNP reduction, such as p-nitrosophenol and p-hydroxylaminophenol.30 In addition, with the further increase of influent PNP concentration, the anode potential increased from −290.75 ± 40.78 mV at influent PNP concentration of 6.47 mM to above 0 mV at influent PNP concentration of 7.19 mM, probably due to the suppression of the high strength PNP towards the electrochemically active microorganisms. | ||
| Fig. 4 PNP removal and PAP formation efficiency (a) and rate (b) at various influent PNP concentrations in BC-BES. | ||
Both the PNP removal rate and PNP loading rate of 18.95 ± 0.10 and 17.74 ± 1.03 mol per m3 per day in this study were rather high compared to those in most anaerobic systems and BESs treating PNP containing wastewater, where the influent PNP concentration and PNP loading rate was often below 5.03 mM and 6.33 ± 0.11 mol per m3 per day, respectively.10,11,32,33 Moreover, at high influent PNP concentration and high PNP loading rate, both PNP removal and PAP formation was always high, although slight decrease was observed. The strong ability to resist shock loading in BC-BES could be attributed to the cathode potential as low as −1000 mV,34 which was much more negative than that in the open circuit conditions. The strong ability to resist shock loading in BC-BES further added up to the application attractiveness of such a system and enabled better sustainability of the BC-BES system.
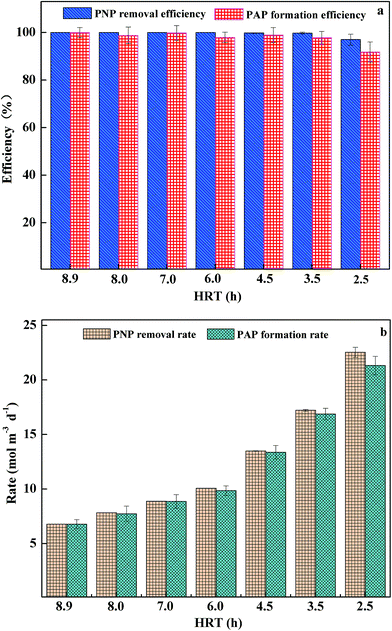
3.4 Effect of HRT on PNP removal and PAP formation
HRT is an important parameter which greatly influences the BC-BES performance. The efficiency would decrease and the construction cost would increase if HRT was too long. At influent PNP concentration of 2.52 mM, as HRT decreased from 8.9 h to 2.5 h, the corresponding PNP loading rate increased from 6.78 to 23.22 mol per m3 per day. As shown in Fig. 5a, at HRT higher than 3.5 h, PNP removal efficiency and PAP formation efficiency were well above 99% and 93%, respectively, indicating that PNP could be efficiently reduced and the BC-BES reactor was rather stable. Correspondingly, PNP removal rate and PAP formation rate increased from 6.78 ± 0.00 and 6.77 ± 0.42 mol per m3 per day to 17.21 ± 0.10 and 16.87 ± 0.53 mol per m3 per day when the HRT increased from 8.9 to 3.5 h (Fig. 5b). In addition, PNP removal efficiency and PAP formation efficiency, as well as PNP removal rate and PAP formation rate, were relatively close, demonstrating that PAP was the dominant reduction product, even at low HRTs. Further decrease in HRT from 3.5 h to 2.5 h caused decline of PNP removal efficiency and PAP formation efficiency from 99.76 ± 0.41% and 97.80 ± 2.66% to 97.02 ± 2.29% and 91.83 ± 1.20%, respectively. Corresponding, PNP removal rate and PAP formation rate increased from 17.21 ± 0.08 and 16.87 ± 0.53 mol per m3 per day to 22.53 ± 0.46 and 21.32 ± 0.84 mol per m3 per day. At this HRT, the effluent quality deteriorated, with relatively high chromaticity observed, implying that BC-BES reactor could not work well at HRT as short as 2.5 h. However, compared with most anaerobic systems and BESs treating PNP containing wastewater, the minimal HRT of 3.5 h was much lower in BC-BES, indicating the excellent performance of the BC-BES reactor.11,32,333.5 Mineralization of PAP in the anode
During the PNP reduction process, PAP was identified as the dominant product, which was much less toxic than PNP.20 Subsequent oxidation process, such as aerobic biodegradation, was often required for the polishing of the effluent containing PAP. Theoretically, the organic compounds, e.g., PAP, could be biodegraded in the anode of the BES, providing the electron for cathodic reduction. However, in an up-flow biocatalyzed electrolysis reactor (UBER) developed by Wang et al.,35 the reduction product of nitrobenzene, i.e., aniline, was not oxidized further in the anode zone. Thus the fate of the reduction product PAP in the BC-BES anode zone was a main concern of this study.As indicated in Fig. 6, PAP concentration in the anode effluent of the BC-BES was always lower than that in the cathode effluent when acetate dosage varied from 9.75 to 3.66 mM. In addition, the NH4+–N concentration in the anode effluent of BC-BES was always higher than that in the cathode effluent. As was reported by other researchers, nitrogen in the PAP structure was often transformed into NH4+ during PAP biodegradation.20 Therefore, the slight increase of NH4+–N concentration in the anode effluent was a key evidence for the PAP biodegradation in BC-BES anode. In addition, when acetate dosage was as low as 3.66 mM, 100% removal of acetate in the cathode zone was observed, however, the anode potential was well below −300 mV, indicating that the electrochemically active microorganisms on the anode did not lose their functions of electron transfer, although acetate was unavailable in the anode. This result indicated that in the anode zone there are alternative electron donors, such as PAP. However, PAP removal efficiency in the anode of the BC-BES was a bit low. Therefore, further work will be focusing on how to accelerate the bioelectrochemical oxidation of PAP in the anode zone of the BC-BES.
3.6 Implication
At PNP loading rate lower than 11.63 mol per m3 per day, PNP removal efficiency and PAP formation efficiency determined in the anode effluent and in the cathode effluent during this period were nearly the same. However, at high PNP loading rate, with the increase of the PNP concentration in cathode effluent, only minor part of PNP could be removed in the anode. Thus, PNP reduction in the anode or cathode was largely dependent on the PNP loading rate. In addition, the anode potential during the experiment was well above −400 vs. Ag/AgCl, which was unfavorable for the PNP reduction in the anode.10,20,30 These results indicated that PNP removal and PAP formation dominantly occurred in the cathode zone of the BC-BES.The BC-BES could be a favorable alternative for PNP removal compared with conventional anaerobic reduction processes and conventional double-chamber BESs. As shown in Table 2, the maximum PNP removal rate in the BC-BES were above 18.95 ± 0.10 mol per m3 per day, which is much higher than those of conventional anaerobic systems,11,32,33 double chamber BES10 and UASB-BES coupling system,30 demonstrating the high efficiency of BC-BES for PNP removal from wastewater. Compared with conventional anaerobic systems, the more negative cathode potential in BC-BES was beneficial for PNP reduction and PAP formation. Compared with the double chamber BES using abiotic cathode, the bacteria attached on cathode of BC-BES could significantly contribute to the enhanced PNP reduction and PAP formation. Biocathode played an important role in increasing the ability of BC-BES to resist shock loading. In addition, the biofilm formed on the graphite granules in the cathode zone might play a key role in PNP reduction, judge from the much more excellent performance of BC-BES than that of UASB-BES coupling system, where suspended anaerobic sludge was dominant.
| Reactor | Electron donor | Maximum RRPNP (mol per m3 per day) | COD usage (mol COD per mol PNP) | Reference |
|---|---|---|---|---|
| a Cathode potential −1000 mV, HRT 8.9 h.b Current density 4.71 A m−3, HRT 9.0 h.c Cathodic potential −500 mV vs. SHE, HRT 2.6 h.d Anaerobic migrating blanket reactor.e Anaerobic baffled reactor.f Upflow anaerobic sludge blanket. | ||||
| BC-BESa | Acetate | 18.95 ± 0.10 | 2.90 ± 0.09 | This study |
| UASB-BESb | Acetate | 6.77 ± 0.00 | 2.41 ± 0.10 | 30 |
| BESc | Acetate | 6.33 ± 0.11 | 7.81 ± 0.56 | 10 |
| AMBRd | Glucose | <0.07 | >120 | 11 |
| ABRe | Glucose | <0.64 | >20 | 32 |
| UASBf | VFA mixture | 5.49 | 64 | 33 |
The low organic cosubstrate consumption of 2.90 ± 0.09 mol COD per mol PNP was another advantage of this BC-BES, probably due to the significant suppression of the biogas production with the supply of the power.30 What's more important, the energy consumption in the BC-BES was well below 0.02 kW h mol−1 PNP in the BC-BES. The low energy consumption in BC-BES was much lower than that in pure electrochemical system, which typically higher than 2 kW h mol−1 PNP.36,37 In addition, the energy consumption in BC-BES was comparable with that in the conventional double-chamber BES, which typically ranged between 0.01 and 0.10 kW h mol−1 PNP.10,20 The low energy consumption in BC-BES would significantly reduce the operational costs, exhibiting the prospect of cost-effectiveness. In addition, any expensive proton/ion exchange membrane, which was usually considered as the main costly component for BES, was not adopted in the BC-BES. This further added up to the economical attractiveness of such a system and enabled better sustainability of the BC-BES.
4. Conclusion
In this study, stable and effective removal of PNP was achieved in BC-BES, due to the key role of the electric field applied. High PNP removal and PAP formation could be achieved at low acetate dosage, high initial PNP concentration and short HRT, indicating the strong ability of the BC-BES to resist shock loading. Partial PAP mineralization in the anode of the BC-BES system was observed, which was beneficial for the further polishing of the BC-BES effluent. The BC-BES system has been proven to show a great potential for the treatment of nitrophenol-containing wastewater, especially with high strength nitrophenols and without adequate organic cosubstrates inside.Acknowledgements
This research is financed by National Natural Science Foundation of China (51208258 and 51378261), Major Project of Water Pollution Control and Management Technology of P. R. China (2012ZX07101-003-001), Zijin Intelligent Program of NJUST (2013-ZJ-02-19), Research Innovation Grant for Graduate of Jiangsu Common High School (CXZZ12-0219), and Research Innovation & Practice Program for Graduate Students of NJUST.References
- V. Uberoi and S. K. Bhattacharya, Water Environ. Res., 1997, 69, 146–156 CrossRef CAS.
- B. Bagchi, P. Thakur, A. Kool, S. Das and P. Nandy, RSC Adv., 2014, 4, 61114–61123 RSC.
- L. H. Keith and W. A. Telliard, Environ. Sci. Technol., 1979, 13, 416–423 CrossRef.
- A. Tauber, H. P. Schuchmann and C. Sonntag, Ultrason. Sonochem., 2000, 7, 45–52 CrossRef CAS.
- J. M. Chern and Y. W. Chien, Water Res., 2002, 3, 647–655 CrossRef.
- J. G. Mahy, L. Tasseroul, A. Zubiaur, J. Geens, M. Brisbois, M. Herlitschke, R. Hermann, B. Heinrichs and S. D. Lambert, Microporous Mesoporous Mater., 2014, 197, 164–173 CrossRef CAS PubMed.
- R. Chen, Q. Wang, Y. Du, W. Xing and N. Xu, Chem. Eng. J., 2009, 145, 371–376 CrossRef CAS PubMed.
- C. Borras, T. Laredo and B. R. Scharifker, Electrochim. Acta, 2003, 48, 2775–2780 CrossRef CAS.
- M. B. Kasiri, H. Aleboyeh and A. Aleboyeh, Appl. Catal., B, 2008, 84, 9–15 CrossRef CAS PubMed.
- J. Shen, Y. Zhang, X. Xu, C. Hua, X. Sun, J. Li, Y. Mu and L. Wang, Water Res., 2013, 47, 5511–5519 CrossRef CAS PubMed.
- D. T. Sponza and Ö. S. Kuşçu, Process Biochem., 2005, 40, 1679–1691 CrossRef CAS PubMed.
- X. P. Zhu, S. Y. Shi, J. J. Wei, F. X. Lv, H. Z. Zhao, J. T. Kong, Q. He and J. R. Ni, Environ. Sci. Technol., 2007, 41, 6541–6546 CrossRef CAS.
- Z. I. Bhatti, H. Toda and K. Furukawa, Water Res., 2002, 36, 1135–1142 CrossRef CAS.
- Z. Liang and Z. Hu, J. Membr. Sci., 2012, 415–416, 93–100 CrossRef CAS PubMed.
- Y. Mu, R. A. Rozendal, K. Rabaey and J. Keller, Environ. Sci. Technol., 2009, 43, 8690–8695 CrossRef CAS PubMed.
- D. Pant, A. Singh, G. Van Bogaert, Y. A. Gallego, L. Diels and K. Vanbroekhoven, Renewable Sustainable Energy Rev., 2011, 15, 1305–1313 CrossRef CAS PubMed.
- T. H. Pham, P. Aelterman and W. Verstraete, Trends Biotechnol., 2009, 27, 168–178 CrossRef CAS PubMed.
- F. Y. Kong, A. J. Wang and H. Y. Ren, Bioresour. Technol., 2014, 158, 32–38 CrossRef CAS PubMed.
- Y. Mu, K. Rabaey, R. A. Rozendal, Z. Yuan and J. Keller, Environ. Sci. Technol., 2009, 43, 5137–5143 CrossRef CAS.
- J. Shen, C. Feng, Y. Zhang, F. Jia, X. Sun, J. Li, W. Han, L. Wang and Y. Mu, J. Hazard. Mater., 2012, 209–210, 516–519 CrossRef CAS PubMed.
- L. Huang, J. M. Regan and X. Quan, Bioresour. Technol., 2011, 102, 316–323 CrossRef CAS PubMed.
- G. D. Zhang, Q. L. Zhao, Y. Jiao, J. N. Zhang, J. Q. Jiang, N. Ren and B. H. Kim, J. Power Sources, 2011, 196, 6036–6041 CrossRef CAS PubMed.
- R. Rozendal, A. Jeremiasse, H. M. Hamelers and C. N. Buisman, Environ. Sci. Technol., 2008, 42, 629–634 CrossRef CAS.
- T. H. J. A. Sleutels, H. V. M. Hamelers, R. A. Rozendal and C. J. N. Buisman, Int. J. Hydrogen Energy, 2009, 34, 3612–3620 CrossRef CAS PubMed.
- H. Hu, Y. Fan and H. Liu, Water Res., 2008, 42, 4172–4178 CrossRef CAS PubMed.
- D. Call and B. E. Logan, Environ. Sci. Technol., 2008, 42, 3401–3406 CrossRef CAS.
- A. Aldrovandi, E. Marsili, L. Stante, P. Paganin, S. Tabacchioni and A. Giordano, Bioresour. Technol., 2009, 100, 3252–3260 CrossRef CAS PubMed.
- A. J. Wang, D. Cui, H. Y. Cheng, Y. Q. Guo, F. Y. Kong, N. Q. Ren and W. M. Wu, J. Hazard. Mater., 2012, 199–200, 401–409 CrossRef CAS PubMed.
- D. Cui, Y. Q. Guo, H. Y. Cheng, B. Liang, F. Y. Kong, H. S. Lee and A. J. Wang, J. Hazard. Mater., 2012, 239–240, 257–264 CrossRef CAS PubMed.
- J. Shen, X. Xu, X. Jiang, C. Hua, L. Zhang, X. Sun, J. Li, Y. Mu and L. Wang, Water Res., 2014, 67, 11–18 CrossRef CAS PubMed.
- J. Shen, R. He, H. Yu, L. Wang, J. Zhang, X. Sun, J. Li, W. Han and L. Xu, Bioresour. Technol., 2009, 6, 1922–1930 CrossRef PubMed.
- Ö. S. Kuşçu and D. T. Sponza, Enzyme Microb. Technol., 2005, 36, 888–895 CrossRef PubMed.
- B. A. Donlon, E. Razo-Flores, G. Lettinga and J. A. Field, Biotechnol. Bioeng., 1996, 51, 439–449 CrossRef CAS.
- M. C. F. Oliveira, Electrochim. Acta, 2003, 48, 1829–1835 CrossRef CAS.
- A. J. Wang, D. Cui, H. Y. Cheng, Y. Q. Guo, F. Y. Kong, N. Q. Ren and W. M. Wu, J. Hazard. Mater., 2012, 199–200, 401–409 CrossRef CAS.
- S. Yuan, M. Tian, Y. Cui, L. Lin and X. Lu, J. Hazard. Mater., 2006, B137, 573–580 CrossRef PubMed.
- P. Jiang, J. Zhou, A. Zhang and Y. Zhong, J. Environ. Sci., 2010, 22, 500–506 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra17218c |
| ‡ These authors contributed to the paper equally. |
| This journal is © The Royal Society of Chemistry 2015 |