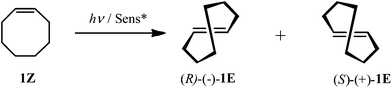
Solvent- and phase-controlled photochirogenesis. Enantiodifferentiating photoisomerization of (Z)-cyclooctene sensitized by cyclic nigerosylnigerose-based nanosponges crosslinked by pyromellitate†
Xueqin
Wei
a,
Wenting
Liang
b,
Wanhua
Wu
a,
Cheng
Yang
*a,
Francesco
Trotta
*c,
Fabrizio
Caldera
c,
Andrea
Mele
d,
Tomoyuki
Nishimoto
e and
Yoshihisa
Inoue
*f
aCollege of Chemistry, State Key Laboratory of Biotherapy, West China Medical School and State Key Laboratory of Polymer Materials Engineering, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: yangchengyc@scu.edu.cn
bInstitute of Environmental Sciences, Shanxi University, Taiyuan 030006, China
cDepartment of Chemistry and University of Torino, Via P. Giuria 7, 10125 Torino, Italy. E-mail: francesco.trotta@unito.it
dDepartment of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, Piazza L. Da Vinci 32, 20133 Milano, Italy
eHayashibara Co., 675-1 Fujisaki, Naka-ku, Okayama 702-8006, Japan
fDepartment of Applied Chemistry, Osaka University, 2-1 Yamada-oka, Suita 565-0871, Japan. E-mail: inoue@chem.eng.osaka-u.ac.jp
First published on 22nd December 2014
Abstract
Cyclic nigerosylnigerose (CNN), a saucer-shaped cyclic tetrasaccharide with a shallow concave surface, was reacted with pyromellitic dianhydride in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios to give two CNN-based polymers of different degrees of crosslinking, both of which swelled upon soaking in water, acting as a ‘nanosponge’ (NS). These NSs evolved several phases from isotropic solution to flowing and rigid gels via suspension by gradually increasing the concentration in water. The CNN-NSs thus prepared effectively mediated the enantiodifferentiating photoisomerization of (Z)-cyclooctene (1Z) to chiral (E)-isomer (1E). The enantiomeric excess (ee) of 1E obtained was a critical function of the solvent composition and the phase evolved at different CNN-NS concentrations in water. In isotropic solution, the enantioselectivity was generally low (−4% to +6% ee) but the chiral sense of 1E was inverted by increasing the methanol content. Interestingly, the product's ee was controlled more dramatically by the phase evolved, as was the case with the cyclodextrin-based nanosponge (CD-NS) reported previously. Thus, the ee of 1E was low in solution and suspension, but suddenly leaped at the phase border of flowing gel and rigid gel to give the highest ee of 22–24%, which are much higher than those obtained with CD-NSs (6–12% ee), revealing the positive roles of the chiral void space formed upon gelation of the crosslinked saccharide polymer.
4 ratios to give two CNN-based polymers of different degrees of crosslinking, both of which swelled upon soaking in water, acting as a ‘nanosponge’ (NS). These NSs evolved several phases from isotropic solution to flowing and rigid gels via suspension by gradually increasing the concentration in water. The CNN-NSs thus prepared effectively mediated the enantiodifferentiating photoisomerization of (Z)-cyclooctene (1Z) to chiral (E)-isomer (1E). The enantiomeric excess (ee) of 1E obtained was a critical function of the solvent composition and the phase evolved at different CNN-NS concentrations in water. In isotropic solution, the enantioselectivity was generally low (−4% to +6% ee) but the chiral sense of 1E was inverted by increasing the methanol content. Interestingly, the product's ee was controlled more dramatically by the phase evolved, as was the case with the cyclodextrin-based nanosponge (CD-NS) reported previously. Thus, the ee of 1E was low in solution and suspension, but suddenly leaped at the phase border of flowing gel and rigid gel to give the highest ee of 22–24%, which are much higher than those obtained with CD-NSs (6–12% ee), revealing the positive roles of the chiral void space formed upon gelation of the crosslinked saccharide polymer.
Introduction
Catalytic photochirogenesis is one of the crucial challenging topics in current photochemistry.1–4 Catalytic asymmetric synthesis in photochemistry has been realized mostly using chiral photosensitization, where the chirality transfer takes place in a diastereomeric exciplex intermediate formed between a chiral sensitizer and a prochiral substrate. Despite a considerable number of studies devoted to chiral photosensitization since the pioneering work reported by Hammond and Cole in 1965,5 to achieve high enantioselectivity in chiral photosensitization is still a nontrivial task, as can be recognized from the limited number and type of photoreactions that afford satisfactory results.6–11 This is primarily due to the weak, short-lived, and less-defined chiral environment realized in the exciplex intermediates formed in conventional chiral photosensitization systems. For a better photochirogenic performance, supramolecular photochirogenesis2 has been proposed as a new strategy, wherein a robust, long-lasting, and well-defined chiral environment is attained not only in the ground state but also in the excited state. A variety of chiral supramolecular hosts, including cyclodextrins (CDs),12–18 chirally modified zeolite supercages,19–21 biomolecules,22,23 and chiral hydrogen-bonding templates,24–26 has been explored to achieve modest to excellent enantioselectivities. More recently, chiral gels27,28 and liquid crystals29 have also been employed as an effective chiral supramolecular environment for photochirogenesis.We have recently reported the enantiodifferentiating photoisomerizations of (Z)-cyclooctene (1Z) (Scheme 1) and (Z,Z)-1,3-cyclooctadiene (2ZZ) sensitized by β- and γ-CD polymers crosslinked with pyromellitate (3–5) (Scheme 2),30 which were named ‘nanosponges’ after their swelling nature upon soaking in water and some organic solvents. These CD-based nanosponges (CD-NSs), incorporating pyromellitate linkers, not only accommodate 1Z and 2ZZ but also sensitize the geometrical isomerization to chiral 1E and 2EZ upon photoirradiation. The enantioselectivities of 1E and 2EZ obtained critically depended on the phase conditions of CD-NS to afford the highest enantiomeric excesses (ee's) at the border of the flowing and rigid gel states. Such behavior had never been observed with non-polymeric CD-based sensitizers reported earlier.31–35
A progressive aggregation mechanism has been proposed in order to rationalize the phase-dependent photochirogenic behavior. The inter-particle complexation of the pyromellitate moiety by the CD cavity exposed at the surface of CD-NS particles is considered to drive the progressive aggregations, leading to the phase transitions, upon the gradual increase of the CD-NS concentration. Simultaneously, the chiral environment available for the enantiodifferentiating photosensitization is switched from the CD cavity in isotropic media to the void space surrounded by the exterior walls of CD in gel states with accompanying changes in ee.30
To elucidate the role of aggregation in the supramolecular photochirogenesis and to gain further insights into the chiral sensitization mechanism in gel systems, we synthesized new pyromellitate-crosslinked saccharide polymers based on cyclic nigerosylnigerose (CNN) in this study. CNN differs from CD in the number and connectivity of the building units, being composed of only four D-glucopyranoses connected by alternating α-(1→3)- and α-(1→6)-linkages.36–40 As a cyclic tetrasaccharide, CNN is much smaller in ring size than CDs and does not bear a confining hydrophobic cavity but a shallow concave which is not sufficient to accommodate a cyclooctene molecule. Therefore, CNN serves as an ideal saccharide building block for investigating the effects of the aggregation of NS and the void space derived therefrom on photochirogenic behaviors, without the influence of cavity binding. Two crosslinked CNN nanosponges (CNN-NSs) 6 and 7 (Scheme 2) were prepared by crosslinking CNN with pyromellitic dianhydride (PDA) at different CNN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDA ratios of 1
PDA ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively.
4, respectively.
Results and discussion
Photosensitization in solution
In order to evaluate the chiral environment around the pyromellitate linker, the circular dichroism spectra of CNN-NSs were measured in aqueous solutions. As can be seen from Fig. 1 and 2 (red lines), both CNN-NSs 6 and 7 exhibit the induced Cotton effect signals at the main band of pyromellitate, which are however much weaker than those observed for CD-NSs 3–5 reported previously.30 This seems reasonable as the shallow concave of CNN is not sufficient in size to fully accommodate the pyromellitate linker, allowing only weak hydrophobic interaction. Furthermore, the addition of 1Z to an isotropic CNN-NS solution did not cause any appreciable changes in circular dichroism, probably due to the lack of a confining cavity and the weak interaction in the ground state. Unlike β-CD that possesses a hydrophobic cavity to bind 1Z with a relatively high affinity,32,33 saucer-shaped CNN does not have sufficient space for accommodating the 1Z molecule. Nevertheless, the concave is considered to be hydrophobic enough to weakly interact with 1Z in aqueous solutions at least in the excited state. Indeed, we have shown that the optical yield of 1E obtained in the photosensitized isomerization of 1Z with a sensitizer-appended CNN monomer in aqueous solution is appreciably higher than those obtained with α-CD-based sensitizers, indicating a positive role of the CNN concave upon photosensitization.38 This previous result also encouraged us to investigate the photoisomerization of 1Z sensitized by CNN-NS in aqueous solutions, despite the apparently negative result obtained upon CD spectral titration.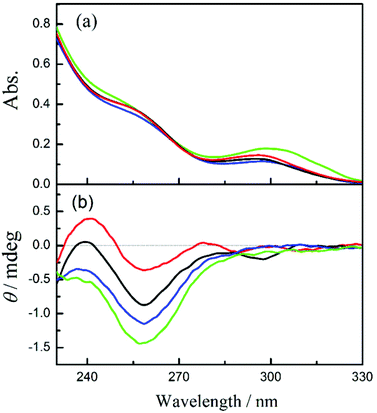 | ||
| Fig. 1 (a) UV-Vis and (b) circular dichroism spectra of CNN-NS 6 (0.2 mg mL−1) in aqueous solutions containing 0% (red), 10% (black), 50% (blue), and 80% methanol (green). | ||
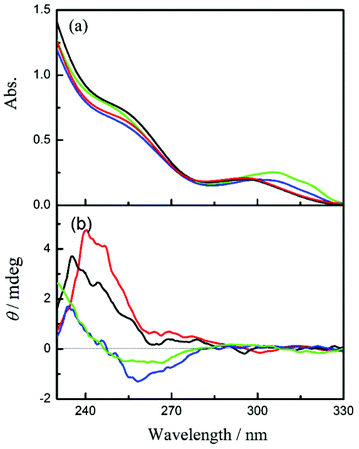 | ||
| Fig. 2 (a) UV-Vis and (b) circular dichroism spectra of CNN-NS 7 (0.1 mg mL−1) in aqueous solutions containing 0% (red), 10% (black), 50% (blue), and 80% methanol (green). | ||
The enantiodifferentiating photoisomerization of 1Z sensitized by CNN-NSs 6 and 7 was first performed in isotropic aqueous solutions containing 0–80% methanol. As shown in Table 1, the solution-phase photoisomerization of 1Z sensitized by CNN-NS gave 1E in a modest yield of −4% to +6% ee. Somewhat unexpectedly, the photosensitizations with 6versus7 afforded the opposite enantiomers of 1E under comparable conditions. Thus, in aqueous solution at 0.5 °C, less crosslinked CNN-NS 6 afforded (R)-1E in 3.9% ee, while more crosslinked CNN-NS 7 gave antipodal (S)-1E in 4.4% ee. Intriguingly, the enantioselectivity obtained with 6 gradually deteriorated by raising the methanol content from 10% to 50% and was eventually inverted in sign (affording the antipode) to reach the highest ee of 6% in 80% methanol. It is to be noted that 6 and 7 share the same chiral CNN monomer unit and achiral pyromellitate linker, but differ in the degree of crosslinking and the framework rigidity. The above results reveal that the CNN-NS's chiral environment for the sensitized photoisomerization of 1Z is readily tunable by adjusting the monomer/linker ratio or the solvent composition. Since CNN has only a shallow concave insufficient to fully include a cyclooctene molecule, the enantioselectivity achieved upon photoisomerization should rely more on the chiral environment formed by crosslinked CNN molecules, which is manipulatable by external factors such as temperature and solvent properties.
| Host | Phase | Methanol content/% | T/°C | [CNN-NS]/mg mL−1 | Irradiation time/min | 1E/1Z ratio | eeb/% |
|---|---|---|---|---|---|---|---|
| a Photoirradiation of 1Z (1 mM) with 6 (0.2–150 mg mL−1) or 7 (0.2–500 mg mL−1) performed at 254 nm in a quartz cell under a nitrogen atmosphere. b Enantiomeric excess of 1E determined by chiral GC (error in ee < 0.5%); positive/negative ee values indicate the preferred formation of (S)/(R)-enantiomers, respectively. | |||||||
| 6 | Sol | 0 | 0.5 | 0.2 | 10 | 0.03 | −3.9 |
| 10 | 0.5 | 0.2 | 15 | 0.05 | −2.7 | ||
| 25 | 0.2 | 10 | 0.01 | −2.5 | |||
| 50 | 0.5 | 0.2 | 10 | 0.02 | 1.5 | ||
| 80 | 0.5 | 0.2 | 20 | 0.02 | 5.6 | ||
| 30 | 0.02 | 6.1 | |||||
| Suspension | 0 | 0.5 | 1 | 60 | 0.07 | −2.3 | |
| 3 | 60 | 0.01 | 1.0 | ||||
| 0.5 | 10 | 60 | 0.15 | 0.1 | |||
| Flowing gel | 0 | 0.5 | 30 | 60 | 0.07 | 4.5 | |
| 100 | 60 | 0.15 | 24.3 | ||||
| Gel | 0 | 0.5 | 150 | 60 | 0.01 | 6.1 | |
| 7 | Sol | 0 | 0.5 | 0.2 | 10 | 0.07 | 4.4 |
| 25 | 0.2 | 10 | 0.06 | 1.0 | |||
| 30 | 0.13 | 1.0 | |||||
| 10 | 25 | 0.2 | 30 | 0.04 | −1.0 | ||
| 50 | 0.5 | 0.2 | 10 | 0.03 | −3.7 | ||
| 20 | 0.05 | −3.3 | |||||
| 25 | 0.2 | 10 | 0.05 | −3.6 | |||
| 80 | 0.5 | 0.2 | 5 | 0.02 | −1.6 | ||
| Suspension | 0 | 0.5 | 1 | 60 | 0.01 | 0.8 | |
| 5 | 60 | 0.06 | 0.8 | ||||
| 20 | 60 | 0.06 | 0.4 | ||||
| Flowing gel | 0 | 0.5 | 100 | 60 | 0.05 | 5.9 | |
| 200 | 60 | 0.07 | 7.0 | ||||
| Gel | 0 | 0.5 | 350 | 60 | 0.01 | 21.9 | |
| 500 | 60 | 0.01 | 1.6 | ||||
To elucidate the origin of the switching of product chirality by solvent composition, the UV-Vis and circular dichroism spectra of 6 and 7 were examined in aqueous solutions of varying methanol contents. In pure water, both 6 and 7 showed the 1La and 1Lb bands of pyromellitate at 258 nm and 295 nm, respectively; see Fig. 1a and 2a. By increasing the methanol content, the 1Lb band of 6 exhibited gradual bathochromic shifts with initial hypochromic effects up to 50% methanol, and then a hyperchromic effect at 80% methanol, for which discontinuous changes in solvent structure and/or solvation mode would be responsible. Similar solvent-dependent shifts were observed also for 7 (Fig. 2a). This is presumably due to the change in microenvironmental polarity experienced by the pyromellitate chromophore. Interestingly, the circular dichroism spectra of 6 and 7 were more significantly affected by the methanol content. Thus, 6 showed a small positive Cotton effect peak at 241 nm and a negative one at 259 nm in pure water. By increasing the methanol content, the whole circular dichroism signals became more negative to give a single negative extremum of −1.5 mdeg ellipticity at 258 nm in 80% methanol (Fig. 1b). The UV-Vis and circular dichroism spectra of 7 showed similar trends in aqueous methanol solutions (Fig. 2). These UV-Vis and circular dichroism spectral changes indicate that increasing the methanol content alters not only the microenvironmental polarity around the pyromellitate chromophore but also the conformation of the pyromellitate linker, which inevitably affects the photochirogenic behavior of CNN-NS as observed.
Thus, the solvent-induced switching of product chirality is phenomenologically well correlated with the circular dichroism spectral changes in aqueous solutions of different methanol contents. Although elucidating the more detailed mechanism of the chirality inversion, including the excited-state interaction of a cyclooctene substrate with a pyromellitate sensitizer, would not be readily feasible, the present methodology may be employed as a convenient tool for obtaining both enantiomers upon supramolecular photochirogenesis without preparing the antipodal host, which is often unpractical when naturally occurring supramolecular hosts, such as cyclodextrin and protein, are used.
Photosensitization in gels
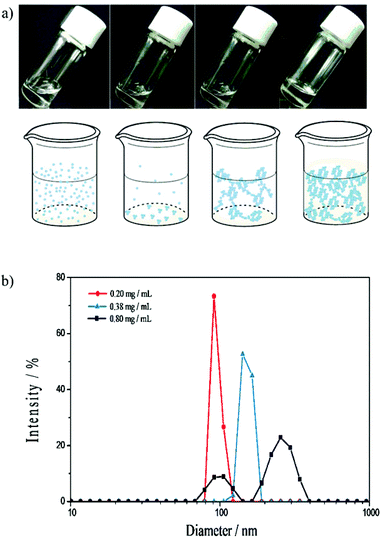
In our previous study on the enantiodifferentiating photoisomerization of cyclooctenes 1Z and 2ZZ sensitized by CD-NSs 3–5,30 we have demonstrated that the ee of 1E and 2EZ produced is highly sensitive to the phase of CD-NS evolved in water, which led us to a tentative conclusion that the photosensitization occurs not in the CD cavity but in the void space of NS surrounded by the exterior walls of CD. In this context, the saucer-shaped CNN, lacking enough space for fully accommodating a cyclooctene molecule, is an ideal cyclic oligosaccharide to substitute CD for proving or disproving our hypothesis, and hence we prepared CNN-NSs 6 and 7 by crosslinking CNN with PDA under the comparable conditions employed for the synthesis of CD-NSs.30The phase-transition behavior of CNN-NS was examined by gradually increasing its concentration in water at ambient temperature. As crosslinked polymers of a cyclic oligosaccharide, both 6 and 7 exhibited a stepwise phase-evolution from a transparent sol to a suspension, a flowing gel and finally a rigid gel, a behavior similar to CD-NS. Thus, CNN-NS 6 gave a homogeneous solution up to 0.4 mg mL−1 concentration, where transparent precipitates began to appear (Fig. 3a). When the concentration was increased to 20 mg mL−1, a gel-like binary phase composed of liquid and gel, or a ‘flowing gel’ state, emerged, which was eventually converted to rigid gel at a critical gelation concentration (CGC) of 120 mg mL−1.
Since the CNN concave is too shallow to strongly hold the linker moiety (i.e. pyromellitate), the host–guest complexation is not likely to play a major role in the aggregation and phase evolution of CNN-NS. As an alternative mechanism, we propose that the inter-particle hydrogen-bonding interactions between CNN moieties and the hydrophobic interaction of the linker moiety with the CNN's inside/outside walls are jointly responsible for the progressive aggregation of CNN-NS nanoparticles. CNN-NS 6 showed a phase transition from solution to suspension at a 0.4 mg mL−1 concentration, which is lower than those of β-CD-NSs 3 and 4 but slightly higher than that of γ-CD-NS 5, while the CGC of 6 is lower than those of 4 and 5. These results suggest that the inter-particle interaction of CNN-NS does not greatly differ from that of CD-NS. Taking into account the fact that both CD-NS and CNN-NS show relatively high CGC values, we deduce that the inter-particle interaction is relatively weak for these two nanosponges, probably due to the shallow penetration of the pyromellitate moiety to the CD cavity or the CNN concave, as well as the relatively weak hydrogen-bonding interaction in water. Dynamic light scattering (DLS) studies confirmed a gradual growth of the particle size upon increasing the concentration of CNN-NS. As illustrated in Fig. 3b, the DLS examination revealed that 6 forms aggregates of ca. 90, 140 and >250 nm diameter at 0.2, 0.38 and 0.8 mg mL−1 concentrations, respectively, while visible precipitates were formed at a 0.4 mg mL−1 concentration.
The more crosslinked CNN-NS 7 showed a similar stepwise phase-evolution behavior in water, but the phase transitions from a solution to a suspension, to a flowing gel and then to a rigid gel occurred at concentrations of 0.4, 50 and 350 mg mL−1, respectively, each of which is higher than the corresponding value observed for the less crosslinked 6, suggesting weaker inter-particle interactions for 7. In 7, there should be a lower number of CNN units exposed on the surface, compared to 6, which may discourage the inter-particle hydrogen-bonding interactions, leading to the higher phase transition concentrations. Nevertheless, CNN-NS 7 formed suspension at lower concentrations and the suspension state was kept over a wider range of concentrations, compared to CD-NSs 3 and 4.
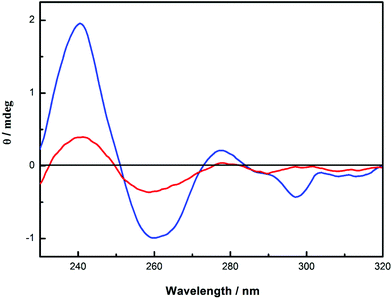
In this regard, it is be to noted that the circular dichroism spectrum of 6 does not show any essential changes in shape in solution (0.2 mg mL−1) and in flowing gel (120 mg mL−1), except the small negative Cotton effect at 297 nm, as can be seen from Fig. 5. This result indicates that CNN-NS nanoparticles aggregate at higher concentrations through the inter-particle interactions on the surface, while the chiral environment around the pyromellitate inside the particle is not significantly altered.
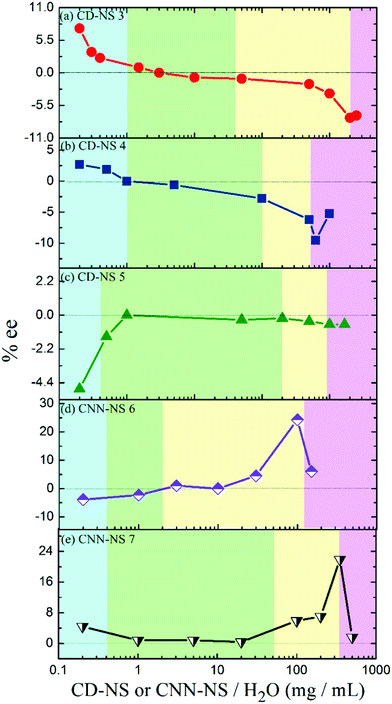
Photoisomerization of 1Z was sensitized by 6 and 7 in various phases prepared by changing the CNN-NS concentration. The E/Z ratio obtained at a relatively early stage of photoisomerization (Table 1) was significantly phase-dependent, varying from 0.01 in rigid gel to 0.15 in suspension and flowing gel. The particularly low E/Z ratio of 0.01 in rigid gel may be attributed to the less-efficient photosensitization due to the slow diffusion of the substrate to the excited sensitizer moiety within its lifetime. The enantioselectivity of 1E produced was also a critical function of the phase of the media; see Table 1 and Fig. 4. For instance, in the photoisomerization of 1Z sensitized by 7 (Fig. 4e), the ee of 1E decreased from 4.4% to almost zero by increasing the CNN-NS concentration from 0.2 to 1 mg mL−1, with an accompanying phase transition from sol to suspension, and stayed low throughout the suspension region (0.4–50 mg mL−1), but jumped to 21.9% at the border of the flowing and rigid gel regions. A similar phase-dependent ee profile was observed upon sensitization with 6 (Fig. 4d), affording an even higher ee value of 24.3% near the border of the flowing and rigid gel regions. These ee profiles are consistent in general with those obtained with CD-NSs 3 and 430 (Fig. 4a and b), but significantly differ from the ee profile obtained with γ-CD-NS 5 (Fig. 4c), which possesses a much larger CD cavity than 3 and 4 and gives a nearly racemic product in all the anisotropic phases.30 These resembling photochirogenic behaviors suggest the operation of conceptually the same enantiodifferentiation mechanism in both CNN-NS and CD-NS photosensitizations.
It is noteworthy that CNN-NSs, possessing no confining cavity but a shallow concave, afford 1E in such good ee's as 22–24% in gel states, which far exceed the ee values of 6–14% obtained with CD-NSs30 under comparable conditions. This result reinforces our previous claim that not the individual host cavity but the polymer void surrounded by the host's exterior walls is responsible for the chiral induction in the supramolecular photosensitization in crosslinked CD and CNN gels.30 In the present case, the concave shape of CNN may provide a better chiral environment for the effective enantiodifferentiating photoisomerization of 1Z sensitized by a pyromellitate crosslinker located nearby. It is interesting that CNN's concave itself is not sufficient to tightly hold the substrate molecule and indeed only a poor ee was obtained upon enantiodifferentiating photosensitization in isotropic solution,32 whereas CNN aggregates formed in polymer gel show much better photochirogenic performance. Thus, the originally poor chirality-transfer ability of CNN-NS was significantly improved by supramolecular aggregation, for which the closer contacts with the CNN’ inside and/or outside walls as well as the well-ordered but less dynamic nature of the gel are likely to be jointly responsible.
Conclusion
In this work, we have employed cyclic tetrasaccharide CNN as a ‘pseudo’ or ‘hemi’ host that lacks a confining cavity to fully accommodate a guest substrate. Our immediate task was to assess and compare the photochirogenic performances of the CNN's shallow concave at the molecular level in isotropic solution and at the supramolecular level in aggregates. A more crucial task was to critically examine our previous claim that the chiral polymer void formed upon aggregation of cyclic oligosaccharide hosts plays major roles in determining the stereochemical outcomes of supramolecular photochirogenic reactions.For these purposes, we synthesized two CNN-based nanosponges of different degrees of crosslinking (6 and 7) and employed them as chiral supramolecular sensitizers for mediating the enantiodifferentiating photoisomerization of 1Z to 1E in aqueous solutions containing 0–80% methanol. The enantioselectivity of 1E obtained was highly sensitive to the solvent composition and the degree of crosslinking, revealing a switching of the product chirality, which provides us with a convenient tool for manipulating the photochirogenic outcomes. Both 6 and 7 showed the phase evolution from sol to rigid gel by increasing the CNN-NS concentration in water. Interestingly, the product's ee was a critical function of the phase of CNN-NS to achieve the highest enantioselectivity at the phase border of flowing and rigid gels. This result unambiguously reveals that the polymer void surrounded by the exterior walls of the host is the source of chirality transfer upon photosensitization with CD- and CNN-NSs. The concept and methodology developed in this study are not restricted to the present system, but expandable to other substrates and photochirogenic reactions by choosing suitable sensitizing crosslinkers and chiral building blocks.
Experimental section
Instruments
UV-Vis and circular dichroism spectra were obtained on JASCO V-650 and JASCO J-A500-150 spectrometers, respectively. DLS measurements were performed using a Zetasizer Nano S90 (Malvern Instruments, Worcestershire, UK) instrument.Materials
CNN was obtained from Hayashibara Co., Inc. Cyclooctene was purchased from Tokyo Chemical Industries and used after fractional distillation. Other chemicals were commercially available and used without further purification.CNN-NSs 6 and 7 were synthesized and purified according to essentially the same procedures as that employed for the preparation of CD-NSs 3–5.30 Thus, 2.0 g of CNN (dried at 100 °C in an oven, until a constant weight was reached) were dissolved in 8 mL of DMSO with continuous stirring, to which were added 2 mL of triethylamine and, 5 min later, 1.345 g (for 6) or 2.691 g (for 7) of pyromellitic dianhydride. The mixture was allowed to react at room temperature, while the gelation began in a few seconds after the addition of the crosslinker. After 24 h, the crosslinking appeared to be complete, affording a monolithic block, which was ground in a mortar, washed with deionized water and rinsed with acetone in a Buchner funnel with an aspirator. The polymer residue was air-dried and extracted with acetone for 14 h in a Soxhlet apparatus to give CNN-NS 6 (4.3 g, 97% yield) or 7 (5.8 g, 96% yield) as a white powder. These samples, which were practically insoluble (but swelled) in water and many organic solvents, gave the satisfactory elemental analyses, ATR-FTIR spectra, and thermogravimetric analyses shown in ESI.†
Photolysis
A sample solution containing 1Z and CNN-NS in a quartz cell was placed on a UNISOKU CoolSpek USP-203CD cryostat, purged with nitrogen gas, and irradiated at a given temperature at 254 nm with a 20 W low pressure mercury lamp (UVL20PH-6). No appreciable phase change or transition was observed after irradiation. The photolyzed sample was added to 10% aqueous KOH solution (2 mL), and the resulting mixture was extracted with pentane (1 mL). An aliquot of the pentane extract was analyzed by gas chromatography (GC) on a Shimadzu CBP-20 (PEG) column for the E/Z ratio. To determine the ee value, an aqueous silver nitrate solution (2 mL) was added to the organic extract at 0 °C to give a stable Ag+ complex with 1E. The aqueous solution of the [Ag+1E] complex was washed twice with pentane (1 mL) and added to a 28% aqueous ammonia solution at 0 °C to liberate 1E. Finally, the liberated cyclooctene was extracted with pentane (0.3 mL), and the pentane extract was analyzed by GC on a Supelco β-DEX 225 column.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21372165 and 21321061), State Key Laboratory of Polymer Materials Engineering, grant no. sklpme2014-2-06, Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University and Japan Society for the Promotion of Science (no. 21245011 and 26620030).Notes and references
- Y. Inoue and V. Ramamurthy, Chiral Photochemistry, Dekker, New York, 2004 Search PubMed.
- C. Yang and Y. Inoue, Chem. Soc. Rev., 2014, 43, 4123 RSC.
- A. G. Griesbeck and U. J. Meierhenrich, Angew. Chem., Int. Ed., 2002, 41, 3147 CrossRef.
- C. Müller and T. Bach, Aust. J. Chem., 2008, 61, 557 CrossRef.
- G. S. Hammond and R. S. Cole, J. Am. Chem. Soc., 1965, 87, 3256 CrossRef CAS.
- Y. Inoue, T. Yokoyama, N. Yamasaki and A. Tai, Nature, 1989, 341, 225 CrossRef CAS.
- Y. Inoue, H. Ikeda, M. Kaneda, T. Sumimura, S. R. L. Everitt and T. Wada, J. Am. Chem. Soc., 2000, 122, 406 CrossRef CAS.
- A. Bauer, F. Westkaemper, S. Grimme and T. Bach, Nature, 2005, 436, 1139 CrossRef CAS PubMed.
- R. Brimioulle and T. Bach, Science, 2013, 342, 840 CrossRef CAS PubMed.
- J. Sivaguru, A. Natarajan, L. S. Kaanumalle, J. Shailaja, S. Uppili, A. Joy and V. Ramamurthy, Acc. Chem. Res., 2003, 36, 509 CrossRef CAS PubMed.
- H. Saito, T. Mori, T. Wada and Y. Inoue, J. Am. Chem. Soc., 2004, 126, 1900 CrossRef CAS PubMed.
- C. Yang, Chin. Chem. Lett., 2013, 24, 437 CrossRef PubMed.
- (a) Y. Inoue, S. F. Dong, K. Yamamoto, L.-H. Tong, H. Tsuneishi, T. Hakushi and A. Tai, J. Am. Chem. Soc., 1995, 113, 2793 Search PubMed; (b) C. Yang, T. Mori, Y. Origane, Y. H. Ko, N. Selvapalam, K. Kim and Y. Inoue, J. Am. Chem. Soc., 2008, 130, 8574 CrossRef CAS PubMed.
- C. Yang, C. Ke, W. Liang, G. Fukuhara, T. Mori, Y. Liu and Y. Inoue, J. Am. Chem. Soc., 2011, 133, 13786 CrossRef CAS PubMed.
- J. Yao, Z. Yan, W. Wu, C. Yang, M. Nishijima, G. Fukuhara, T. Mori and Y. Inoue, J. Am. Chem. Soc., 2014, 136, 6916 CrossRef CAS PubMed.
- Q. Wang, C. Yang, C. Ke, G. Fukuhara, T. Mori, Y. Liu and Y. Inoue, Chem. Commun., 2011, 47, 6849 RSC.
- G.-H. Liao, L. Luo, H.-X. Xu, X.-L. Wu, L. Lei, C.-H. Tung and L.-Z. Wu, J. Org. Chem., 2008, 73, 7345 CrossRef CAS PubMed.
- L. Luo, S.-F. Cheng, B. Chen, C.-H. Tung and L.-Z. Wu, Langmuir, 2010, 26, 782 CrossRef CAS PubMed.
- A. Joy, S. Uppili, M. R. Netherton, J. R. Scheffer and V. Ramamurthy, J. Am. Chem. Soc., 2000, 122, 728 CrossRef CAS.
- J. Sivaguru, T. Shichi and V. Ramamurthy, Org. Lett., 2002, 4, 4221 CrossRef CAS PubMed.
- J. Sivaguru, S. Jockusch, N. J. Turro and V. Ramamurthy, Photochem. Photobiol. Sci., 2003, 2, 1101 CAS.
- S. Asaoka, T. Wada and Y. Inoue, J. Am. Chem. Soc., 2003, 125, 3008 CrossRef CAS PubMed.
- M. Nishijima, T. Wada, T. Mori, T. C. S. Pace, C. Bohne and Y. Inoue, J. Am. Chem. Soc., 2007, 129, 3478 CrossRef CAS PubMed.
- J. Mizoguchi, Y. Kawanami, T. Wada, K. Kodama, K. Anzai, T. Yanagi and Y. Inoue, Org. Lett., 2006, 8, 6051 CrossRef CAS PubMed.
- T. Bach, H. Bergmann, B. Grosch and K. Harms, J. Am. Chem. Soc., 2002, 124, 7982 CrossRef CAS PubMed.
- C. Müller, A. Bauer, M. M. Maturi, M. C. Cuquerella, M. A. Miranda and T. Bach, J. Am. Chem. Soc., 2011, 133, 16689 CrossRef PubMed.
- A. Dawn, T. Shiraki, S. Haraguchi, H. Sato, K. Sada and S. Shinkai, Chem. – Eur. J., 2010, 16, 3676 CrossRef CAS PubMed.
- M. Shirakawa, N. Fujita, T. Tani, K. Kaneko and S. Shinkai, Chem. Commun., 2005, 4149 RSC.
- Y. Ishida, Y. Kai, S.-y. Kato, A. Misawa, S. Amano, Y. Matsuoka and K. Saigo, Angew. Chem., Int. Ed., 2008, 47, 8241 CrossRef CAS PubMed.
- W. Liang, C. Yang, D. Zhou, H. Haneoka, M. Nishijima, G. Fukuhara, T. Mori, F. Castiglione, A. Mele, F. Caldera, F. Trotta and Y. Inoue, Chem. Commun., 2013, 49, 3510 RSC.
- C. Yang, T. Mori, T. Wada and Y. Inoue, New J. Chem., 2007, 31, 697 RSC.
- W. Liang, C. Yang, M. Nishijima, G. Fukuhara, T. Mori, A. Mele, F. Castiglione, F. Caldera, F. Trotta and Y. Inoue, Beilstein J. Org. Chem., 2012, 8, 1305 CrossRef CAS PubMed.
- R. Lu, C. Yang, Y. Cao, Z. Wang, T. Wada, W. Jiao, T. Mori and Y. Inoue, J. Org. Chem., 2008, 7695 CrossRef CAS PubMed.
- R. Lu, C. Yang, Y. Cao, Z. Wang, T. Wada, W. Jiao, T. Mori and Y. Inoue, Chem. Commun., 2008, 374 RSC.
- G. Fukuhara, T. Mori, T. Wada and Y. Inoue, Chem. Commun., 2005, 4199 RSC.
- P. Biely, G. L. Cote and A. Burgess-Cassler, Eur. J. Biochem., 1994, 226, 633 CrossRef CAS PubMed.
- G. L. Cote and P. Biely, Eur. J. Biochem., 1994, 226, 641 CrossRef CAS PubMed.
- T. Nishimoto, H. Aga, K. Mukai, T. Hashimoto, H. Watanabe, M. Kubota, S. Fukuda, M. Kurimoto and Y. Tsujisaka, Biosci. Biotech. Biochem., 2002, 66, 1806 CrossRef CAS PubMed.
- H. Aga, T. Nishimoto, M. Kuniyoshi, K. Maruta, H. Yamashita, T. Higashiyama, T. Nakada, M. Kubota, S. Fukuda, M. Kurimoto and Y. Tsujisaka;, J. Biosci. Bioeng., 2003, 95, 215 CrossRef CAS.
- C. Yang, W. Liang, M. Nishijima, G. Fukuhara, T. Mori, H. Hiramatsu, Y. Dan-oh, K. Tsujimoto and Y. Inoue, Chirality, 2012, 24, 921 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Elemental analysis, ATR-FTIR spectral and thermogravimetric analysis data for CNN-NSs 6 and 7. See DOI: 10.1039/c4ob02390k |
| This journal is © The Royal Society of Chemistry 2015 |