Open Access Article
Open Access ArticleTuning viscoelastic properties of supramolecular peptide gels via dynamic covalent crosslinking†
Mohammad Aref
Khalily
,
Melis
Goktas
and
Mustafa O.
Guler
*
Institute of Materials Science and Nanotechnology, National Nanotechnology Research Center (UNAM), Bilkent University, Ankara, Turkey 06800. E-mail: moguler@unam.bilkent.edu.tr; Fax: +90 312 266 4365; Tel: +90 312 290 3552
First published on 19th December 2014
Abstract
A dynamic covalent crosslinking approach is used to crosslink supramolecular peptide gels. This novel approach facilitates tuning viscoelastic properties of the gel and enhances mechanical stability (storage modulus exceeding 105 Pa) of the peptide gels.
Supramolecular peptide gels are a class of supramolecular polymers, which are capable of encapsulating a large amount of water, up to 100 times of their dry weight. These soft materials are produced by molecular self assembly of naturally occurring short peptide sequences.1 Beside their inherent biocompatibility, biodegradability and bio-functionality peptides can be straightforwardly designed with a desired sequence, function and molecular weight.2 In soft materials research, versatile peptide gels have attracted a large amount of attention and they have been explored comprehensively.3 Supramolecular peptide gels have been exploited as scaffolds for wound healing, sustained release of drugs and biomolecules, cell culture media and tissue engineering,3b,c,4 templates for nanofabrication,5 catalysts for organic reactions,6 and light harvesting devices.7 Despite the versatility of peptide hydrogels, they suffer from low mechanical stability with storage moduli of 100–5000 Pa.8 There has been extensive research to tune and enhance the mechanical stability of peptide gels. Light,8 metal ions,9 disulfide,10 catalytic,11 and enzymatic12 crosslinking mechanisms are main approaches to improve the mechanical stability of peptide hydrogels.
Here, we have utilized a novel dynamic imine covalent bonding approach to crosslink the supramolecular peptide hydrogels to tune and enhance their viscoelastic characteristics. This approach is quite simple and straightforward. The crosslinking takes place between amine and aldehyde groups in aqueous medium at room temperature and forming a dynamic covalent bond, imine.13
This novel crosslinking system has many advantages over other crosslinking strategies; it does not require any catalyst, enzyme, photocrosslinker, metal ions and any other crosslinking agent. Moreover, there is no need for purification of crosslinked peptide gels since water is the only side product from the reaction of amine and aldehyde. The reversibility of the imine bond is crucial for suitable self-assembly of peptide molecules to conserve the nanofibrous morphology and self-healing properties.
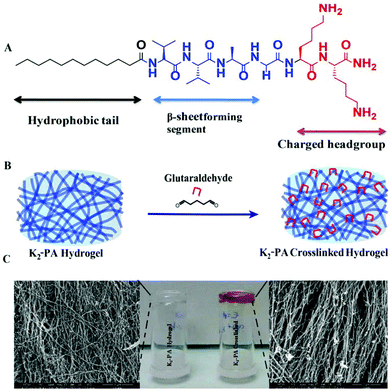
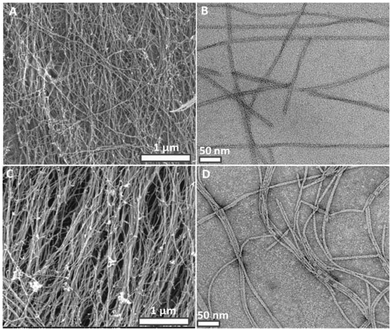
In this study, we have designed and synthesized a peptide amphiphile (PA) molecule (Lauryl-VVAGKK-Am, K2-PA) (Fig. 1A). At pH > 7, the PA molecules self-assemble into nanofibers with a diameter of ca. 10 nm (Fig. 5B, and S5†) directed by β-sheet structures. A three-dimensional network of the PA nanofibers forms a self-supporting gel at concentrations of 1 wt% (Fig. 1C and S3a†) or even lower (0.1 wt%. Fig. S11†).
In this design, the lauryl group promotes hydrophobic interactions. Meanwhile, β-sheet forming amino acids (VVA) facilitate hydrogen-bonding. These two segments are required for self-assembly of peptide molecules in aqueous medium4 (Fig. 1C). Two lysine residues have significant roles in assisting solubility of the peptide molecules in water and then reacting with glutaraldehyde molecules to crosslink the peptide nanofibers (Fig. 1B).
We investigated the formation of the imine bond and its effect on peptide self-assembly and the β-sheet structure using UV–Vis and circular dichroism (CD) spectroscopy. Although glutaraldehyde is widely used for crosslinking of biomaterials, its chemistry is still not well understood.14 The reaction between amines and glutaraldehyde in aqueous solution is quite complex and many studies have been conducted to determine the products.14 After peptide gel formation at pH 7.5, 10 μL of glutaraldehyde (25% solution) was added and then imine formation was followed by UV–Vis spectroscopy. Interestingly, the crosslinked supramolecular peptide gels showed absorbance at 265 nm (Fig. 2A), which proves the presence of the imine bond.15
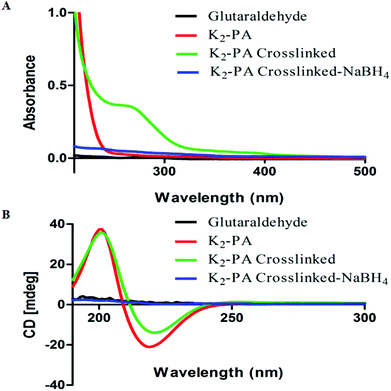 | ||
| Fig. 2 UV–Vis spectroscopy (A) and circular dichroism (B) analysis of peptide gels and crosslinked peptide gels at pH 7.5. | ||
We further investigated the effect of the crosslinking on peptide self-assembly by circular dichroism (CD). Dilute solution of peptide gels showed the β-sheet structure (a positive signal at 195 nm and a negative signal at 220 nm) and glutaraldehyde crosslinking did not affect the β-sheet structure (Fig. 2B). This could be due to the nature of dynamic and reversible imine bond formation in aqueous media, which does not disturb the peptide self-assembly. The imine can be reduced to amine by reductive amination using a reducing agent.13 Therefore, NaBH4 solution was added (2 equiv. with respect to glutaraldehyde) to crosslinked peptide hydrogels.
As soon as the reducing agent was added into the gel, the gel collapsed immediately. The reduced peptide gels showed no absorbance at 265 nm (Fig. 2A) and no β-sheet structure was observed (Fig. 2B). Reduction of imines to amines locked the system by the formation of covalent bonds instead of dynamic covalent bonds, therefore, the peptide self-assembly was disturbed and the peptides lost their β-sheet structure and the gel formation was prevented.
We further investigated the degree of deprotonated side chain amines by zeta potential analysis because only deprotonated amines can react with glutaraldehyde. Therefore, zeta potentials were measured at different pH values by titration of the peptide solution with 0.1 M NaOH (Fig. S7†). Despite the positive zeta potential (∼15 mV), peptide molecules can form gel at around pH 7.5. The positive zeta potential indicates that amines are not fully neutralized (Fig. S7†).
The complete neutralization takes place when pH is above 8. Therefore, we investigated the gelation kinetics at minimum pH of gelation (7.5) and at pH 10 (Fig. S8 and S9†) to ensure the entire deprotonation of amines.
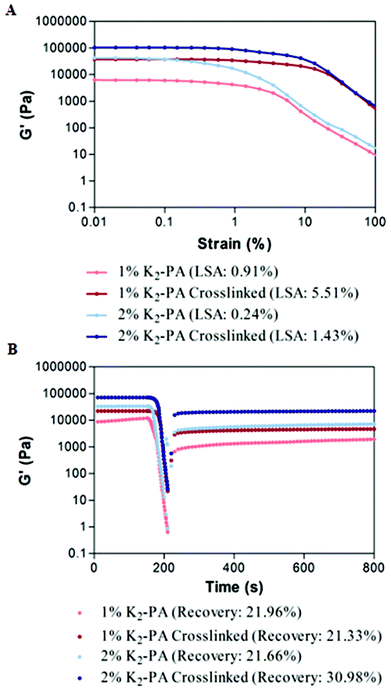
Gelation kinetics and viscoelastic properties are important material characteristics indicating the potential use of a hydrogel. Oscillatory rheology was used to monitor the mechanical stiffness and elasticity of the gel systems. Gelation kinetics was determined by a time-sweep test within the linear viscoelastic range. Within 75 min, storage moduli, the energy stored during deformation, of all groups reached a plateau demonstrating the complete gelation (Fig. 3A, S8a†). However, storage moduli of non-covalently self-assembled peptide gels showed a greater ramp at pH 7.5 (Fig. 3A) when compared to pH 10 (Fig. S8a†), which reached a stationary phase at the earlier periods. This result indicated that the self-assembly process was completed at an increased rate under basic conditions. In addition, covalent crosslinking through glutaraldehyde addition resulted in rapid completion of the gelation process by locking the mobile PA molecules within the self-assembled system both at pH 7.5 and pH 10 (Fig. S8a†) when compared to regular PA gel systems.
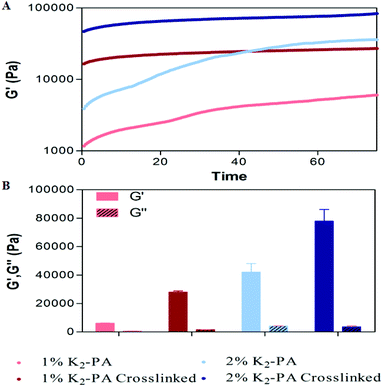 | ||
| Fig. 3 Rheological characterization of the gels. (A) Gelation kinetics and (B) equilibrium moduli at pH 7.5. | ||
For all the groups, storage moduli (G′) were greater than loss moduli (G′′) confirming the gel character of the resulting networks (Fig. 3B, S8b†). Storage modulus of glutaraldehyde crosslinked samples was significantly higher for all groups when compared to their self-assembled PA controls showing that the crosslinking resulted in the formation of stiffer gels (Fig. 3B, S8b†). Another important point to elucidate is the effect of crosslinking on the mechanical properties (G′′/G′ ratio or loss (damping) factor). Gelation takes place when the G′′/G′ value is below 1 and a smaller damping factor refers to a more pronounced elastic character against a viscous character.
Storage moduli of glutaraldehyde crosslinked samples were significantly higher than non-covalently self-assembled PA controls while their loss moduli were comparable. Covalent crosslinking resulted in a smaller damping factor indicating the formation of stronger gels. Furthermore, the peptide formed stronger gels at pH 10 (Fig. S10†) than at pH 7.5 (Fig. S10†). The zeta potential analysis (Fig. S7†) at pH 7.5 indicated the presence of partially protonated amines. The positively charged amines cause repulsion among the peptide molecules and therefore weakening of the gel formation. In the case of glutaraldehyde crosslinking at pH 7.5, the protonated amines lose their nucleophilicity and therefore cannot attack the aldehyde. We can conclude that the degree of crosslinking at pH 7.5 is lesser than at pH 10.
To investigate the viscoelastic properties of the resulting gels, we performed an amplitude sweep test showing the relationship between the storage modulus and strain amplitudes. Within the linear viscoelastic (LVE) range, gels maintained their storage moduli at constant values independent of increasing strain values. When a certain limit of LVE (limiting strain amplitude (LSA)) was exceeded, the gels showed a transition from linear to nonlinear viscoelastic behavior and the storage values started to decrease under increased strain values (Fig. 4A, S9a†). For all the groups, the limiting strain amplitude of glutaraldehyde crosslinked samples was approximately six times higher than that of self-assembled peptide nanofiber controls. This increase in LSA showed that compared to noncovalent networks, glutaraldehyde crosslinked PA gels can withstand six times higher shear strain. Covalent crosslinking supported the increased resistance to deformation and elasticity of the resulting networks.
To investigate the self-healing ability, in other words recovery after high shear loads, we performed a thixotrophic test under increasing strain amplitudes far beyond the linear viscoelastic range up to 1000%. At this range, all noncovalent interactions were expected to be broken to completely deform the network. When the shear load was removed, recovery of the noncovalent bonds was measured. The recovery of the glutaraldehyde crosslinked samples was comparable to their noncovalently self-assembled PA controls and no significant decrease was observed in the self-healing ability of the gels after covalent crosslinking (Fig. 4B, S9b†). After deformation, noncovalent interactions driving the self-assembly of PA molecules were similarly restored both for noncovalently self-assembled PA gels and glutaraldehyde crosslinked PA gels. Therefore, these results indicate that the glutaraldehyde crosslinking increased the mechanical stability of PA gels, support the load bearing capacity and elasticity of the resulting networks, and also as an additional advantage, did not harm the self-healing ability of noncovalent interactions within the self-assembled network.
Interestingly, the dynamic crosslinking process did not affect the nanofiber morphology, porosity and fibrous structure of the peptide gels (Fig. 5C, D and S4, S5, S6†). SEM (Fig. 5A, C) and TEM (Fig. 5B, D) images show no difference in width and length of the peptide nanofibers. Crosslinking at the molecular level is observed by UV spectroscopy (Fig. 2A) but we could not observe distinct morphological changes at the nanometer level. This could be due to dominant intrafibrillar crosslinking rather than interfibrillar crosslinking. UV–Vis, CD, SEM, TEM characterizations and self-healing ability of the crosslinked peptide gels prove the presence of a dynamic covalent imine bonding.
Conclusion
In this work, we have developed a novel supramolecular peptide nanostructure system, which could be crosslinked in a dynamic manner. This approach facilitates tuning and enhancing the viscoelastic characteristics of the self-assembled peptide gels. Besides improved mechanical stability, the peptide gels also conserved their porous, fibrous structures and self-healing characteristics. The viscoelastic properties of the peptide gels could further be tuned by addition of changing ratios of glutaraldehyde or addition of shorter or longer length dialdehydes.Notes and references
- H. Cui, M. J. Webber and S. I. Stupp, Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials, Biopolymers, 2010, 94(1), 1–18 CrossRef CAS PubMed.
- R. V. Ulijn and A. M. Smith, Designing peptide based nanomaterials, Chem. Soc. Rev., 2008, 37(4), 664–675 RSC.
- (a) A. Dasgupta, J. H. Mondal and D. Das, Peptide hydrogels, RSC Adv., 2013, 3(24), 9117–9149 RSC; (b) H. Hosseinkhani, P. D. Hong and D. S. Yu, Self-Assembled Proteins and Peptides for Regenerative Medicine, Chem. Rev., 2013, 113(7), 4837–4861 CrossRef CAS PubMed; (c) A. M. Jonker, D. W. P. M. Lowik and J. C. M. van Hest, Peptide- and Protein-Based Hydrogels, Chem. Mater., 2012, 24(5), 759–773 CrossRef CAS; (d) S. Bhattacharjee and S. Bhattacharya, Phthalate mediated hydrogelation of a pyrene based system: a novel scaffold for shape-persistent, self-healing luminescent soft material, J. Mater. Chem. A, 2014, 2(42), 17889–17898 RSC; (e) P. Moitra, K. Kumar, P. Kondaiah and S. Bhattacharya, Efficacious Anticancer Drug Delivery Mediated by a pH-Sensitive Self-Assembly of a Conserved Tripeptide Derived from Tyrosine Kinase NGF Receptor, Angew. Chem., Int. Ed., 2014, 53(4), 1113–1117 CrossRef CAS PubMed.
- X. Zhao, F. Pan, H. Xu, M. Yaseen, H. Shan, C. A. Hauser, S. Zhang and J. R. Lu, Molecular self-assembly and applications of designer peptide amphiphiles, Chem. Soc. Rev., 2010, 39(9), 3480–3498 RSC.
- M. A. Khalily, O. Ustahuseyin, R. Garifullin, R. Genc and M. O. Guler, A supramolecular peptide nanofiber templated Pd nanocatalyst for efficient Suzuki coupling reactions under aqueous conditions, Chem. Commun., 2012, 48(92), 11358–11360 RSC.
- M. O. Guler and S. I. Stupp, A self-assembled nanofiber catalyst for ester hydrolysis, J. Am. Chem. Soc., 2007, 129(40), 12082–12083 CrossRef CAS PubMed.
- S. K. M. Nalluri, C. Berdugo, N. Javid, P. W. J. M. Frederix and R. V. Ulijn, Biocatalytic Self-Assembly of Supramolecular Charge-Transfer Nanostructures Based on n-Type Semiconductor-Appended Peptides, Angew. Chem., Int. Ed., 2014, 53(23), 5882–5887 CrossRef CAS PubMed.
- Y. Ding, Y. Li, M. Qin, Y. Cao and W. Wang, Photo-Cross-Linking Approach to Engineering Small Tyrosine-Containing Peptide Hydrogels with Enhanced Mechanical Stability, Langmuir, 2013, 29(43), 13299–13306 CrossRef CAS PubMed.
- J. C. Stendahl, M. S. Rao, M. O. Guler and S. I. Stupp, Intermolecular forces in the self-assembly of peptide amphiphile nanofibers, Adv. Funct. Mater., 2006, 16(4), 499–508 CrossRef CAS.
- W. Y. Seow and C. A. E. Hauser, Tunable Mechanical Properties of Ultrasmall Peptide Hydrogels by Crosslinking and Functionalization to Achieve the 3D Distribution of Cells, Adv. Healthcare Mater., 2013, 2(9), 1219–1223 CrossRef CAS PubMed.
- J. Boekhoven, J. M. Poolman, C. Maity, F. Li, L. van der Mee, C. B. Minkenberg, E. Mendes, J. H. van Esch and R. Eelkema, Catalytic control over supramolecular gel formation, Nat. Chem., 2013, 5(5), 433–437 CrossRef CAS PubMed.
- Y. Li, Y. Ding, M. Qin, Y. Cao and W. Wang, An enzyme-assisted nanoparticle crosslinking approach to enhance the mechanical strength of peptide-based supramolecular hydrogels, Chem. Commun., 2013, 49(77), 8653–8655 RSC.
- M. E. Belowich and J. F. Stoddart, Dynamic imine chemistry, Chem. Soc. Rev., 2012, 41(6), 2003–2024 RSC.
- (a) L. H. H. O. Damink, P. J. Dijkstra, M. J. A. Vanluyn, P. B. Vanwachem, P. Nieuwenhuis and J. Feijen, Glutaraldehyde as a Cross-Linking Agent for Collagen-Based Biomaterials, J. Mater. Sci. Mater. Med., 1995, 6(8), 460–472 Search PubMed; (b) I. Migneault, C. Dartiguenave, M. J. Bertrand and K. C. Waldron, Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking, Biotechniques, 2004, 37(5), 790–796 CAS.
- J. H. Bowes and C. W. Cater, The interaction of aldehydes with collagen, Biochim. Biophys. Acta, 1968, 168(2), 341–352 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional details are provided in the ESI. See DOI: 10.1039/c4ob02217c |
| This journal is © The Royal Society of Chemistry 2015 |