Carbon quantum dots and their applications
Shi Ying
Lim
,
Wei
Shen
and
Zhiqiang
Gao
*
Department of Chemistry, National University of Singapore, Singapore 117543. E-mail: chmgaoz@nus.edu.sg; Fax: +65 6779-1691; Tel: +65 6516-3887
First published on 15th October 2014
Abstract
Fluorescent carbon nanoparticles or carbon quantum dots (CQDs) are a new class of carbon nanomaterials that have emerged recently and have garnered much interest as potential competitors to conventional semiconductor quantum dots. In addition to their comparable optical properties, CQDs have the desired advantages of low toxicity, environmental friendliness low cost and simple synthetic routes. Moreover, surface passivation and functionalization of CQDs allow for the control of their physicochemical properties. Since their discovery, CQDs have found many applications in the fields of chemical sensing, biosensing, bioimaging, nanomedicine, photocatalysis and electrocatalysis. This article reviews the progress in the research and development of CQDs with an emphasis on their synthesis, functionalization and technical applications along with some discussion on challenges and perspectives in this exciting and promising field.
1. Introduction
The unique properties of carbonic nanomaterials such as nanodiamonds, fullerenes, carbon nanotubes, graphene sheets and fluorescent carbon nanoparticles or carbon quantum dots (CQDs) have inspired extensive studies on them due to their great potential for a wide variety of technical applications. Among the electronic and physicochemical characteristics of CQDs, their optical properties and their fluorescence emissions in particular have attracted increasing interest in recent years. For many years, semiconductor quantum dots have been extensively investigated for their strong and tunable fluorescence emission properties, which enable their applications in biosensing and bioimaging. However, semiconductor quantum dots possess certain limitations such as high toxicity due to the use of heavy metals in their production.1–3 It is known that heavy metals are highly toxic even at relatively low levels, which may prove prohibitive to any clinical studies.4,5 This prompted the creation of CQDs to replace semiconductor quantum dots due to their low toxicity, biocompatibility, low cost and chemical inertness in addition to having similar fluorescence properties.The accidental discovery of CQDs during the separation and purification of single-walled carbon nanotubes (SWCNTs) by Xu et al. in 2004 triggered subsequent studies to exploit the fluorescence properties of CQDs and create a new class of viable fluorescent nanomaterials.6 Fluorescent carbon nanoparticles received their name “carbon quantum dots” in 2006 from Sun et al.7 who proposed a synthetic route to produce CQDs with much enhanced fluorescence emissions via surface passivation. CQDs are synthesised by two routes, namely the top-down route7–9 and the bottom-up route.3,10–12 CQDs are typically quasi-spherical nanoparticles comprising amorphous to nanocrystalline cores with predominantly graphitic or turbostratic carbon (sp2 carbon) or graphene and graphene oxide sheets fused by diamond-like sp3 hybridised carbon insertions (Fig. 1).13–16 Oxidised CQDs contain considerable amounts of carboxyl moieties at their surface. Depending on the synthetic route, the oxygen content in the oxidised CQDs ranges from 5 to 50% (weight).14 As shown in Fig. 1, there are many carboxyl moieties on the CQD surface, which impart excellent water solubility and suitable chemically reactive groups for further functionalization and surface passivation with various organic, polymeric, inorganic or biological materials to CQDs. Upon surface passivation, the fluorescence properties of CQDs are enhanced. Surface functionalization also modifies their physical properties, like their solubility in aqueous and non-aqueous solvents.
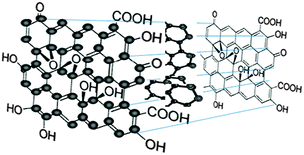 | ||
| Fig. 1 Chemical structure of CQDs. (Reproduced with permission from ref. 13.) | ||
As a group of newly emerged fluorescent nanomaterials, CQDs have shown tremendous potential as versatile nanomaterials for a wide range of applications, including chemical sensing, biosensing, bioimaging, drug delivery, photodynamic therapy, photocatalysis and electrocatalysis. Compared to conventional semiconductor quantum dots, the unique attributes of CQDs, for example their benign chemical composition, tunable fluorescence emissions, facile functionalization and excellent physicochemical and photochemical stability (non-photobleaching or non-photoblinking), render them very attractive for technical applications. Together with other advantages such as low cost and ease of synthesis,17 CQDs are in a favourable position for achieving unprecedented performance. On the other hand, complex procedures for their separation, purification and functionalization, their generally low quantum yields, and ambiguity in their geometry, composition and structure are some of the issues that need to be tackled before they can truly outperform their semiconductor quantum dot counterparts in areas like bioimaging, biosensing and nanomedicine. This article reviews the progress in the research and development of CQDs and their technical applications. We first examine the fluorescence properties of CQDs and their tunable emissions. Then, we discuss the various synthetic approaches for their production and possible surface passivation and functionalization routes to impart desired properties to CQDs. Finally, we discuss in great detail the applications of CQDs in chemical sensing, biosensing, bioimaging, nanomedicine, photocatalysis and electrocatalysis, especially the advantages they could bring to these fields. In view of several excellent review articles focusing on different aspects of CQDs, such as their synthesis and physicochemical properties,14,18 surface functionalization,19 bioimaging and biosensing19,20 and photocatalysis and optoelectronics,18 it is hoped that this article will provide a comprehensive overview of the current status of CQD research and open new perspectives toward the research and development of CQDs with much improved physicochemical properties.
2. Fluorescence properties of CQDs
CQDs are a new class of nanomaterials that have attracted significant attention in the past decade. Two classes of fluorescence emission mechanisms have been proposed for CQDs even though the exact origins of their fluorescence emissions remain debatable and more research is needed in order to paint a clearer picture of the mechanisms of their fluorescence emissions. The first class of fluorescence emission mechanism is that of bandgap transitions caused by conjugated π-domains, while the second class involves more intricate origins associated with surface defects in CQDs.2.1 Fluorescence emissions from bandgap transitions of conjugated π-domains
For the first class of fluorescence mechanism, bandgap transitions arise from conjugated π-domains. These π-domains are isolated by creating sp2 hybridised islands rich in π-electrons through the reduction of graphene oxides obtained by using Hummers method of oxidising and exfoliating graphite flakes.21 They are created in a way that there are no π-connections between the sp2 islands, because any π-connections between the sp2 islands would lead to interisland quenching of desired fluorescence emissions.22,23In this type of bandgap transitions, single-layer graphene sheets have to be used to prevent interlayer quenching.24 The single-layer graphene sheets are used as precursors for electronically slicing into isolated π-conjugated domains, which resemble large aromatic molecules with extended π-conjugation of specific electronic energy bandgap for optical absorption and fluorescence emissions.25 Such electronic transitions display strong absorption in the ultraviolet (UV) region, but weak or no fluorescence emissions (Fig. 2A). The strong absorption is likely due to light absorption by a large amount of high density π-electrons in the sp2 hybridised islands, which form excitonic states while the weak emissions are possibly a result of quenching via radiationless relaxations to the ground state during exciton migration to energy traps.13
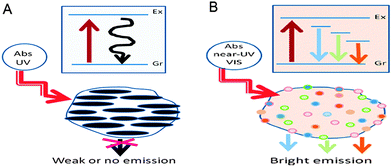 | ||
| Fig. 2 (A) CQDs with strong absorption in the UV region and weak emissions and (B) CQDs with weak absorption in the near UV-vis region but strong multicolour emissions in the visible region. (Reproduced with permission from ref. 13.) | ||
2.2 Fluorescence emissions of surface defect-derived origins
The second class of the fluorescence mechanism arises from surface-related defective sites – generally any sites that have non-perfect sp2 domains will result in surface energy traps. Both sp2 and sp3 hybridised carbons and other functionalised surface defects,26,27 such as carbonyl-related localised electronic states,24,28 present in CQDs contribute to their multicolour emissions that are concentrated in the blue and green regions of the visible light spectrum. These surface defects behave like aromatic molecules that are individually incorporated into solid hosts, exhibiting multicolour emissions due to the existence of multiple surface defects with different excitation and emission properties.13,25Robertson and O'Reilly suggested that the optical properties of carbon nanomaterials which contain both sp2 and sp3 bonds are determined by the π-states of the sp2 sites.29 Thus, the bright surface defect-derived fluorescence of CQDs is due to the recombination of electron–hole pairs in the strongly localised π and π* electronic levels of the sp2 sites. These sites lie between the bandgap of the σ and σ* states of the sp3 matrix,30,31 leading to strong visible emissions. Such electronic transitions exhibit weak absorption in the near ultraviolet-visible (UV-vis) region but strong emissions in the visible region as shown in Fig. 2B. In addition, upon surface passivation or functionalization, the surface defects become more stable to facilitate more effective radiative recombination of surface-confined electrons and holes, thus achieving brighter fluorescence emissions.25
2.3 Tunable fluorescence emissions of CQDs
One unique property of CQDs is their tunable fluorescence emissions. Generally, CQDs possess tunable emissions even without any surface passivation, but they usually have very low quantum yields due to unstable surface defects leading to reduced radiative recombination.Upon surface passivation with organic or polymeric materials, such as poly(propionyl ethyleneimine-co-ethyleneimine) (PPEI-EI) attached to the CQD surface, surface defects are stabilised and strong fluorescence emissions both in solution-like suspension and in solid state were detected. The emissions of such passivated CQDs covered a broad range of the visible region and extended into the near-infrared (NIR) region as shown in Fig. 3.7
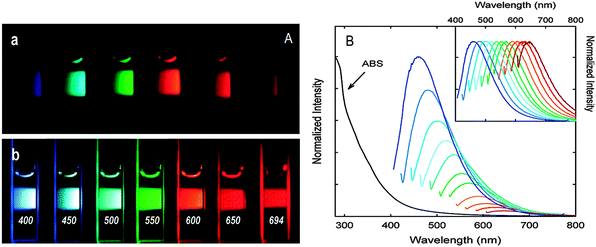 | ||
| Fig. 3 (A) Aqueous solutions of PEG1500N-passivated CQDs (a) excited at 400 nm and (b) excited at the indicated wavelengths; (B) absorption and emission spectra of PPEI-EI passivated CQDs in water with increasing λex from 400 nm on the left with 20 nm increments. Inset: emission intensities normalized to quantum yields. (Reproduced with permission from ref. 7.) | ||
It should be noted that the surface passivation agents used were not emissive in the visible and NIR regions, thus any fluorescence emissions observed must have originated from the surface-passivated CQDs. The tunable emission property of CQDs is clearly demonstrated in Fig. 3. From the fluorescence spectra of PPEI-EI-passivated CQDs, it is evident that the emissions are broad and excitation wavelength-dependent.7,26 The tunable emissions of the surface-passivated CQDs could be a result of varied fluorescence characteristics of particles of different sizes of the CQDs and the distribution of different emissive sites on the surface of the CQDs. However, the exact mechanism accounting for the excitation wavelength-dependent emission remains to be established and the requirement for surface passivation in order to produce fluorescence emissions is poorly understood. Experimental observations are not helpful since controversial results are often observed. Moreover, the optical properties of CQDs are closely associated with the synthetic routes used in their preparation. For example, Sun and co-workers attributed the fluorescence emissions to the radiative recombination of excitons of surface energy traps of CQDs. Upon surface passivation, these energy traps are stabilised and therefore become emissive – a phenomenon that has been observed in semiconductor quantum dots.32 They postulated that there must be a quantum confinement effect of emissive energy traps on the surface of the CQDs. Nonetheless, such tunable fluorescence emission property of CQDs provides an added advantage in the selection of different emission wavelengths with different excitation wavelengths that can be applied to optical labelling and fluorescence imaging.
In addition to the excitation wavelength-dependent emission, several reports have indicated that the fluorescence emissions of CQDs are pH-dependent.10,33,34 Liu and colleagues noticed that the fluorescence intensity of their CQDs decreases when the pH of the solution is shifted from the optimal value of 7.0 regardless of whether pH is increased or decreased.10 In another report, it was observed that the fluorescence intensity of CQDs only shows a slight decrease of ∼3% when the pH of the solution is changed from 5 to 9.33 Jia et al. reported that CQDs prepared from direct heating of ascorbic acid solution show a practically linear dependence on the pH of the solution in the range of 4.0 to 8.0. The fluorescence intensity decreased by as much as 90% from pH 4.0 to 8.0. Varying the pH of the solution from 4.0 to 8.0, corresponding to the deprotonation of the carboxyl groups on the surface of the CQDs, might cause electrostatic doping/charging to the CQDs and shift the Fermi level.35 As seen above, the significantly large variation of fluorescence intensity with the pH of the solution and the synthetic routes used in the preparation of the CQDs adds additional complexity in finding out a widely accepted fluorescence emission mechanism.
2.4 Up-conversion fluorescence
In addition to conventional down-conversion fluorescence emissions, reports have also shown that certain CQDs have up-conversion fluorescence emission properties.1,26,34,36–39 Up-conversion fluorescence emission is an optical phenomenon wherein the fluorescence emission wavelength is shorter than the used excitation wavelength, which is particularly attractive for in vivo bioimaging since bioimaging at longer wavelengths especially in the NIR region is usually preferred owing to the improved photon tissue penetration and reduced background auto-fluorescence. Cao's team first observed that their CQDs emit visible light when excited by a 800 nm femtosecond pulsed laser,1 thereby suggesting that such CQDs possess up-conversion properties. Later, several other groups also observed up-conversion fluorescence emissions from CQDs prepared by drastically different synthetic routes.26,34,36–39 For instance, Jia et al. demonstrated that CQDs prepared by directly heating ascorbic acid solution at 90 °C exhibit excellent up-conversion fluorescence emission properties in addition to their normal down-conversion fluorescence emission.34 Upon excitation in the NIR region of 800–1000 nm, green light with a peak wavelength of 540 nm was emitted from their CQDs. The up-conversion florescence emissions likely originated from a multi-photon excitation process – an essential feature of up-conversion fluorescence emission.This is, however, not without dispute. In view of the practically constant energy difference of ∼1.1 eV between the excitation light and emission light, Shen and colleagues argued that the multi-photon excitation is inadequate to account for the up-conversion fluorescence emission properties of CQDs.26 They postulated that the up-conversion fluorescence emission originates from the relaxation of electrons from a higher energy state of the π orbital (LUMO) to the σ orbital since some electrons would inevitably transit to the LUMO when a large number of low-energy photons excite the electrons in the π orbital. Of course, the electrons in the σ orbital can also be excited, but they only emit conventional down-conversion light.
On the other hand, Wen et al. believed that some of the apparent up-conversion fluorescence emissions are artefacts originating from the conventional down-conversion emissions which have been excited by the leaking component from the second diffraction in the monochromator of the spectrofluorometer.40 By simply inserting a long pass filter into the excitation pathway the leaking component can be removed. Thus, great care must be taken when interpreting the fluorescence emissions of CQDs and further clarification is necessary to explain up-conversion CQDs.
3. Synthesis of CQDs
3.1 Top-down synthetic route
Synthetic approaches for CQDs are generally classified into two categories – “top-down” and “bottom-up”. The former involves breaking down larger carbon structures, such as nanodiamonds,41 graphite,7 carbon nanotubes,42 carbon soot,10 activated carbon43 and graphite oxide44 by methods like arc discharge, laser ablation and electrochemical oxidation. Xu et al. discovered the first example of fluorescent CQDs when they were purifying SWCNTs from arc-discharged soot.6 The arc-discharged soot was oxidised with nitric acid, extracted using sodium hydroxide solution and the black suspension from the extract was then subjected to gel electrophoresis to obtain CQDs. Subsequently, Sun and colleagues pioneered the synthesis of CQDs by laser ablation of a carbon material using argon as a carrier gas in the presence of water vapour.7 Nanosized carbon particles in aggregates of different sizes were formed but these CQDs gave no obvious fluorescence emissions. Therefore, the sample was given an acid oxidative treatment followed by surface passivation in order to observe bright fluorescence. Zhou's group first described an electrochemical method to prepare CQDs.42 It involved growing multi-walled carbon nanotubes on a carbon paper, which was then inserted into an electrochemical cell that contained degassed acetonitrile with 0.1 M tetrabutyl ammonium perchlorate as the supporting electrolyte. To significantly cut down the cost of the starting materials and increase the scale of CQD production, a one-step electrochemical approach utilising low-cost and readily available graphite as a carbon source was recently proposed by Ming et al.45 The prepared CQDs were predominantly multi-layer graphene oxide exhibiting both down- and up-conversion fluorescence emissions.3.2 Bottom-up synthetic route
On the other hand, the “bottom-up” approaches synthesise CQDs from molecular precursors such as citrate,46 carbohydrates47,48 and polymer–silica nanocomposites3 through combustion/thermal treatments, and supported synthetic and microwave synthetic routes. For instance, Liu's team reported a synthetic method based on the use of modified silica spheres as carriers and resols as carbon precursors (Fig. 4).3 Bourlinos et al. described an easy, one-step thermal decomposition method of obtaining fluorescent CQDs from ammonium citrate.46 Zhu and co-workers showed that CQDs are readily formed by heating a solution of poly(ethylene glycol) (PEG) and saccharide in a 500 W microwave oven for 2 to 10 min.49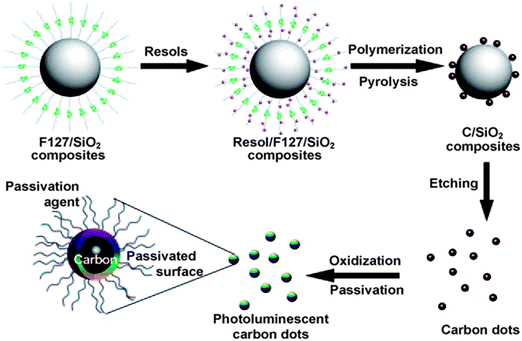 | ||
| Fig. 4 Supported-synthesis of CQDs using modified silica spheres as carriers and resols as carbon precursors. (Reproduced with permission from ref. 3.) | ||
Cost is one of the important determinants of CQDs becoming viable competitors to semiconductor quantum dots. However, the above-described approaches require either costly precursors, complex instrumental set-ups or post-treatments to synthesise fluorescent CQDs. Recently, green synthetic approaches were introduced. With these green routes, CQDs were produced in one step without the need for expensive materials and elaborate experimental set-ups. Li's team was one of the first groups to introduce the concept of preparing fluorescent CQDs using a simple and green route.50 Their approach only involved a single electrochemical treatment step of ethanol with sodium hydroxide. This approach is less costly and easier to manage, and the CQDs produced were found to possess desirable fluorescence properties along with high water solubility, stability and sensitivity to pH. Thereafter, the use of other inexpensive and biocompatible starting materials, like ethanol,50 citrate,51 glucosamine,52 ascorbic acid,53,54 saccharides,49,55,56 candle soot,57 watermelon peels,58 pomelo peels,59 orange juice,60 strawberry juice,61 sugar cane juice,62 chicken eggs,63 chitosan,64,65 organogel66 and gelatine,67 were developed. Many of these “green” CQDs displayed excellent performance in cell imaging and chemical sensing applications. Table 1 summarises the representative examples of the green synthetic routes developed for the preparation of CQDs.
| Precursor | Synthetic method | Quantum yield (%) | Application | Ref. |
|---|---|---|---|---|
| Phenol/formaldehyde resin, silica particle | Carbonisation at 900 °C, NaOH etching | 14.7 | Bioimaging | 3 |
| Ascorbic acid | Heat treatment at 90 °C | 3.22 | pH sensing | 34 |
| Citrate | Carbonisation in air at 300 °C or hydrothermal treatment at 300 °C | 3 | — | 46 |
| Carbohydrate | H2SO4, HNO3 treatment, amine passivation | 13 | — | 47 |
| Carbohydrate (glucose) | Alkali- or acid-assisted ultrasonic synthesis | 7 | — | 48 |
| poly(ethylene glycol) and saccharide | Microwave treatment (500 W) | 3.1–6.3 | — | 49 |
| Ethanol in NaOH solution | Electrochemical treatment (25–40 V) | 4 | — | 50 |
| Citrate | Hydrothermal treatment at 180 °C | 68 | Hg2+ sensing | 51 |
| Glucosamine–HCl | Hydrothermal treatment at 140 °C | — | — | 52 |
| Ascorbic acid | Hydrothermal treatment at 140 °C | 5.7 | Bioimaging, pH sensing | 53 |
| Ascorbic acid | Hydrothermal treatment at 180 °C | 6 | — | 54 |
| Glucose | Hydrothermal treatment at 200 °C | 1.1–2.4 | Bioimaging | 55 |
| Sucrose | Microwave oven at 100 W | — | Bioimaging | 56 |
| Candle soot | HNO3 oxidation | 3 | Bioimaging | 57 |
| Watermelon peels | Carbonisation at 220 °C | 7.1 | Bioimaging | 58 |
| Pomelo peels | Hydrothermal treatment at 200 °C | 6.9 | Hg2+ sensing | 59 |
| Orange juice | Hydrothermal treatment at 120 °C | 26 | Bioimaging | 60 |
| Strawberry juice | Hydrothermal treatment at 120 °C | 6.3 | Hg2+ sensing | 61 |
| Sugar cane juice | Hydrothermal treatment at 120 °C | 5.76 | Bioimaging | 62 |
| Chicken egg | Plasma irradiation (50 V, 2.4 A) | 6.8 | Printing | 63 |
| Chitosan | Hydrothermal treatment at 180 °C | 43 | Bioimaging | 64 |
| Chitosan | Microwave oven | — | — | 65 |
| Organogel | Topochemical polymerisation | — | — | 66 |
| Gelatine | Hydrothermal treatment at 200 °C | 31.6 | Bioimaging | 67 |
| Hair fibre | H2SO4 treatment | 11.1 | Bioimaging | 68 |
| Ionic liquids | Microwave oven (700 W) | 1.65–5.14 | Quercetin sensing | 69 |
| 3-(3,4-Dihydroxyphenyl)-L-alanine, L-histidine, and L-arginine | Carbonisation at 300 °C | — | Bioimaging | 70 |
| Citric acid and ethylenediamine | Hydrothermal treatment at 150–300 °C | 80 | Fe3+ sensing, printing | 71 |
| Acetic acid | Carbonisation with P2O5 | — | Bioimaging | 72 |
| Grass | Hydrothermal treatment at 150–200 °C | 2.5–6.2 | Cu2+ sensing | 73 |
In addition to acid-oxidative treatment and surface passivation, other methods have been explored in a bid to increase quantum yield and obtain CQDs with better fluorescence properties. One approach is to dope newly-produced and surface-passivated CQDs with inorganic compounds such as ZnS and ZnO.74 An aqueous suspension of CQDs and Zn(CH3COO)2 was hydrolysed with NaOH or precipitated with Na2S to obtain ZnO- and ZnS-doped CQDs, respectively. In the case of doping with ZnO, an additional thermal annealing step was required to transform Zn(OH)2 to ZnO. The respective quantum yields of the ZnS- and ZnO-doped CQDs in aqueous solutions were above 50% and around 45% with the former being very close to the quantum yield of commercially available semiconductor CdSe/ZnS quantum dots.55 It was speculated by the authors that doping might have reinforced the surface passivation effect or even served as an additional form of surface passivation mode together with the existing organic passivating agents. These doped CQDs were shown to have strong multi-photon fluorescence properties,55 giving them great potential in the field of one- and two-photon excitation imaging applications.
3.3 Surface passivation and functionalization
Surfaces of CQDs possess high sensitivity to contaminants in their environment, such that their properties are easily affected by tiny levels of contaminants. In order to alleviate this problem, surface passivation of CQDs is performed to reduce the detrimental effect of surface contamination to their optical properties.75 Surface passivation is usually attained by the formation of a thin insulating layer, usually by the attachment of polymeric materials, such as oligomeric PEG, PEG1500N, on an acid-treated CQD surface.7 It was shown that effective surface passivation is an essential step in order to produce CQDs with high fluorescence intensities. Furthermore, Dong and co-workers reported that electrochemiluminescence activities are partly responsive to surface passivation, where the surface-passivated CQDs exhibited weak electrochemiluminescence activities but strong fluorescence.43 In contrast, CQDs whose surfaces are unpassivated or “naked” might emit colourful fluorescence, but their quantum yields are generally low,9,10,42,57,76 except for a recent report by Shen's group.77 After reductive treatment with NaBH4, they demonstrated that hydrothermally treated CQDs have quantum yields as high as 40.5%.58 These “bare” CQDs might serve as good alternatives to acid-treated CQDs since their surfaces are prone to oxidative erosion during chemical oxidation using strong acids. However, this method of synthesising bare CQDs with high quantum yields does not seem to be highly efficient and reproducible. Therefore, many still relied on the acid treatment and surface passivation to enhance the quantum yield of CQDs. Wang et al. prepared CQDs with quantum yields as high as 60%,78 which puts them on par with the best commercial CdSe/ZnS semiconductor quantum dots in aqueous solutions. Precursor CQDs were first synthesised using the method described by Sun et al. and surface passivated with PEG1500N.7 To form acyl chlorides on their surface, these precursor CQDs were then treated with thionyl chloride. Following that, the acyl chlorides reacted with PEG1500N when the CQDs were kept at 110 °C. The sample of the treated CQDs was subsequently loaded onto an aqueous Sephadex G-100 gel column and eluted with water. At the end of the column, individual fractions were collected. While all fractions produced similar fluorescence spectra, the quantum yield was found to be higher for the later eluted fractions. A quantum yield between 55 and 60% was reached in the last fraction,59 which probably consisted of smaller and better-passivated CQDs.Anilkumar's group introduced the concept of crosslinking surface passivated CQDs for better optical performance.79 They showed that crosslinking of the passivating PEG1500N on the surface of CQDs results in the formation of fluorescent particles that contain multiple CQDs in covalently-bound clusters (Fig. 5). Interestingly, it was observed that the fluorescence properties of the CQDs in each particle are additive and up to seven CQDs exist in a single particle for maximum brightness when one particle is subjected to fluorescence imaging. It was suggested that crosslinking most likely results in the stabilisation of surface functionalization by reinforcing the structure of the soft shell of PEG1500N molecules that surround the hard fluorescent CQD cores, thus achieving improved fluorescence emissions.
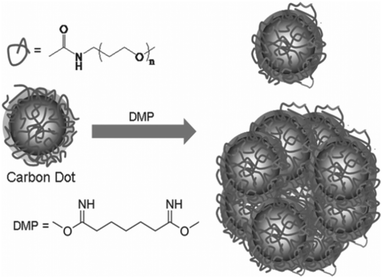 | ||
| Fig. 5 Crosslinking of PEG1500N-functionalised CQDs by reaction with dimethyl pimelimidate in a pH 8 phosphate buffer. (Reproduced with permission from ref. 79.) | ||
Functionalization of CQDs is important because the introduction of functional groups, such as amines and carboxyls, can impose different defects on the CQD surface. These defects work as excitation energy traps and lead to large variations in fluorescence emissions.63,64 In fact, oxidative treatment using strong acids is a simple and very effective means to introduce carbonyl and carboxyl groups on the surface of CQDs, imparting greater water solubility to the CQDs.14 It has been shown by Liu's group that an oxidant is a necessary component to produce visible fluorescence emissions.10 His group proposed that an additional function of this acid oxidative treatment is to possibly break down carbon aggregates into smaller nanoparticles. Therefore, an oxidative acid like nitric acid was often added to the reaction mixture in the synthesis of CQDs.
Very often, surface passivating agents act as functionalising agents as well, where the physical properties of the CQDs are modified together with their fluorescence properties. Thus, there is no need for additional modification steps in the later stage of synthesis. For example, Dong and colleagues used branched polyethylenimine (b-PEI) as both the surface passivating and functionalising agent, where the polyamines passivate the CQD surface and the free amine groups allow for the functionalization of the passivated CQDs.80 In another report, surface passivation was achieved using diamine-terminated oligomeric PEG, thereby achieving bright luminescence and surface functionalization of CQDs simultaneously.81
As a class of novel fluorescent nanoparticles with environmental and biological benign composition and high biocompatibility, major technical applications of CQDs would naturally be in the fields of bioimaging and biosensing. In order to rival their organic dye and semiconductor quantum dot counterparts, a high quantum yield of CQDs is vital for these applications. Consequently, extensive research efforts have been devoted to engineering CQDs to improve their quantum yields. A wide variety of synthetic routes for the preparation of CQDs have been developed. Although a quantum yield as high as ∼80% has been obtained,71 the majority of the CQDs synthesised so far have quantum yields below 10% (Table 1). In addition to surface-passivation, doping with heteroatoms and nitrogen in particular has shown great potential to significantly enhance the quantum yield of CQDs. As demonstrated by Zhu et al.,71 nitrogen-doped CQDs (N-CQDs) exhibit a quantum yield of 80%, comparable to most organic dyes and semiconductor quantum dots. Leveraging on the synergistic effect of N- and Mg-doping, a quantum yield of 83% was recently reported.82 Nonetheless, unlike organic dyes and semiconductor quantum dots of which both composition and structure are well-defined, considerable ambiguities in the composition and structure of CQDs are likely the cause of their low and often varied quantum yields. Therefore, in addition to significantly enhancing the quantum yields of CQDs, synthetic approaches with high reproducibility and scalability that are capable of producing geometrically, compositionally and structurally well-defined CQDs with high reproducibility and scalability are urgently needed.
4. Applications of CQDs
4.1 Chemical sensing
An interesting application of CQDs is in the field of chemical sensing. The detection of heavy metals such as Hg2+ is of utmost importance because of their hazardous effect on the environment and human health. CQDs were used for chemical sensing due to their low toxicity, water solubility, high photostability and superior chemical stability.One of the first attempts of utilising CQDs in chemical sensing is the selective detection of Hg2+ in aqueous solutions51,61,68–87 and live cells.87 Goncalves and colleagues demonstrated that the fluorescence emissions of both CQD solution and CQDs immobilized in sol–gel are sensitive to the presence of Hg2+,84,85 In their study, laser-ablated and NH2-PEG200 and N-acetyl-L-cysteine-passivated CQDs were used as fluorescent probes. It was observed that the fluorescence intensity of the CQDs is efficiently quenched by micromolars of Hg2+ with a Stern–Volmer constant of 1.3 × 105 M−1. Therefore, judging from the relatively large magnitude of the Stern–Volmer constant,88 the quenching provoked by Hg2+ is probably due to static quenching arising from the formation of a stable non-fluorescent complex between CQD and Hg2+. A substantial improvement in the sensitivity down to nanomolars was later realised by replacing the laser-ablated CQDs with N-CQDs. Again, static quenching is thought to be responsible for the quenching of fluorescence but with a much larger Stern–Volmer constant of 1.4 × 107 M−1, two orders of magnitude higher than that of the previous system.85 It was suggested that the presence of nitrogen element in the N-CQDs, most probably –CN groups on the N-CQD surface, is responsible for the much improved performance of Hg2+ sensing.
More recently, Yan and co-workers adopted the Hg2+–CQD system for selective detection of Hg2+ in aqueous solution as well as in live cells.87 The authors reported the synthesis of two types of CQDs using citric acid with 1,2-ethyldiamine (CQD-1) and N-(b-aminoethyl)-g-aminopropyl (CQD-2) that possess high quantum yields of 65.5 and 55.4%, respectively. They studied the effective and selective quenching of fluorescence emissions of CQD-1 and CQD-2 by Hg2+. Both CQDs acted as selective and sensitive fluorescent probes for the detection of traces of Hg2+ in both aqueous solutions and live cells. Upon the addition of 20 μM of Hg2+, the fluorescence intensity of CQD-1 was rapidly quenched by 80%, while that of CQD-2 was quenched by 55%, and both remained stable after 1 h of observation (Fig. 6). This substantiates the viability of using CQD-1 and CQD-2 as chemical sensing probes for Hg2+. The selectivity of CQD-1 and CQD-2 toward Hg2+ was then assessed by comparing the extent of fluorescence quenching of CQD-1 and CQD-2 by the addition of 20 μM of different metal ions. As shown in Fig. 6, among all metal ions tested, Hg2+ quenched both the fluorescence of CQD-1 and CQD-2 to the largest extent. Such quenched fluorescence was reversible and could be recovered by adding a strong chelating agent such as EDTA, making these CQDs reversible fluorescent probes. Furthermore, successful attempts were made in detecting Hg2+ in cultured cells.87
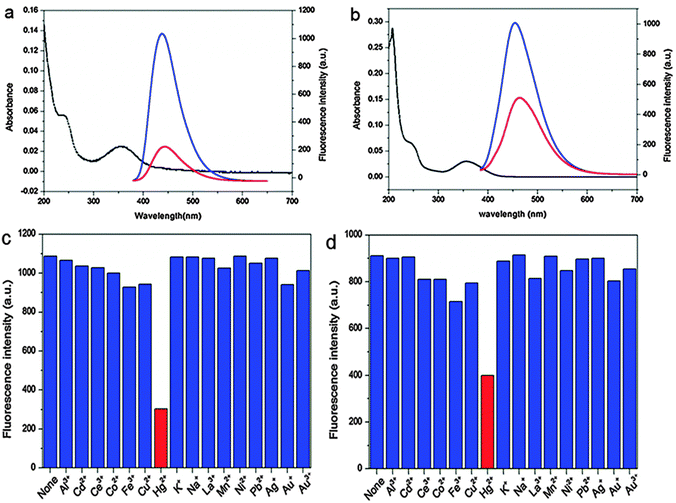 | ||
| Fig. 6 UV-vis (black lines) and fluorescence spectra of (a) CQD-1 and (b) CQD-2 aqueous solutions in the absence (blue lines) and presence (red lines) of Hg2+; fluorescence responses of (c) CQD-1 and (d) CQD-2 aqueous solutions in the presence of 20 μM of different metal ions. (λex = 360 nm). (Reproduced with permission from ref. 87.) | ||
Other applications of CQDs in chemical sensing included the detections of Cu2+,89–96 Fe3+,97,98 Pb2+,99 Cr(VI)100 and Ag+.93 Similar to the Hg2+ sensing, most of the procedures proposed are based on the above-mentioned fluorescence quenching by the metal ions. For example, Liu et al. reported a procedure for selective detection of Cu2+ in aqueous samples such as tap water by CQDs modified with lysine and bovine serum albumin (CQDs-BSA-Lys).89 Highly sensitive detection of Cu2+ was achieved by making use of the coordination reaction of Cu2+ with both the –COOH and –NH2 groups of the CQDs-BSA-Lys. To improve the sensitivity of the assays, apart from coating CQDs with various polymeric materials, metal–organic frameworks (ZIF-8 – zinc imidazolate frameworks)94 and silica nanoparticles95 were employed in the configuration of more sensitive CQD-based fluorescent probes. For instance, Lin and co-workers showed that highly sensitive nanocomposite fluorescent probes can be readily prepared by encapsulating b-PEI-coated CQDs into ZIF-8. Leveraging on the synergistic effect of the strong fluorescence of the encapsulated CQDs and the selective accumulation effect of the ZIF-8 host, as low as 80 pM Cu2+ was detected by the CQD-ZIF-8 nanocomposite probes.74 It is envisioned that the same strategy can be extended to the preparation of other CQD–metal–organic framework probes for highly sensitive and selective detections of many other analytes.
Along with the development of sensitive metal ion assays, CQDs have also found niche applications in the detections of pH,93,101 C2O42−,96 PO43−,102 CN−,103 F−,104 S2−,105 ClO−,106 I−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85,107 and NO2 gas.108 Contrary to the metal ion assays which leverage on the fluorescence quenching mechanism, many of the anion assays are based on the fluorescence enhancement (fluorescence recovery) of the already quenched CQD–metal complexes. For example, in the I− assay, the fluorescence of the CQDs was recovered due to the formation of more stable complexes between I− and the metal ions, displacing the CQDs in the CQD–metal complexes.107 Furthermore, assays for small organic compounds including ascorbic acid100via fluorescence enhancement; and 4-nitrophenol,109 quercetin,69 2,4-dinitrophenol and 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxalin110 through fluorescence quenching have been reported.
85,107 and NO2 gas.108 Contrary to the metal ion assays which leverage on the fluorescence quenching mechanism, many of the anion assays are based on the fluorescence enhancement (fluorescence recovery) of the already quenched CQD–metal complexes. For example, in the I− assay, the fluorescence of the CQDs was recovered due to the formation of more stable complexes between I− and the metal ions, displacing the CQDs in the CQD–metal complexes.107 Furthermore, assays for small organic compounds including ascorbic acid100via fluorescence enhancement; and 4-nitrophenol,109 quercetin,69 2,4-dinitrophenol and 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxalin110 through fluorescence quenching have been reported.
Apart from utilising the fluorescence of CQDs as an analytical signal, recent studies have revealed that CQDs exhibit good chemiluminescence111 and electrochemiluminescence.112 Therefore, several groups have developed chemiluminescent assays for NO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 113 and Co2+;114 and electrochemiluminescent assays for traces of pentachlorophenol115 and Cu2+.116
113 and Co2+;114 and electrochemiluminescent assays for traces of pentachlorophenol115 and Cu2+.116
So far, a wide variety of procedures and a large number of starting materials have been used in the preparation of CQDs for the assays, thus leading to substantial batch-to-batch and lab-to-lab variations. Such variations in the synthesis of CQDs have serious consequences since studies have suggested that the fluorescence characteristics are strongly dependent on the composition of CQDs and residue chemical groups on their surface; and different starting materials and procedures inevitably produce CQDs with rather different physicochemical properties and optical properties in particular. Standardisation is therefore urgently needed in the preparation of CQDs and the assessment of the performance of CQDs.
4.2 Biosensing
CQDs were also used in biosensing based on the use of antibodies and their gene-recombinant fragments. In this scheme, CQDs are mainly applied in immunoassays as fluorescent labels. This was shown in an example by Posthuma-Trumpie et al.117 with an emphasis of the use of CQDs in lateral flow and microarray immunoassays. CQDs are less costly, more stable and more sensitive, hence they were chosen over other commonly used fluorescent labels for this study. CQDs were shown to have higher sensitivity as labels in lateral flow assays (LFAs) in comparison to gold or latex nanoparticles.118 It was claimed that CQDs exhibit sensitivity in the picomolar range.13 In a general example of nucleic acid LFA (NALFA) (Fig. 7), the discriminating tags on the amplicons are recognised by their respective antibodies and fluorescence signals are provided by the attached CQDs.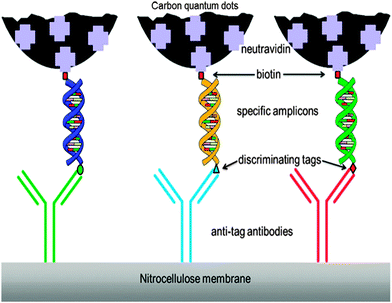 | ||
| Fig. 7 Schematic illustration of NALFIA. (Reproduced with permission from ref. 117.) | ||
Employing the principles of Förster resonance energy transfer (FRET) and homogenous immunoassay, Bu and co-workers119 proposed an immunosensor for quick and specific detection of 4,4′-dibrominated biphenyl (PBB15) – a persistent organic pollutant that disturbs the endocrine system.120 This immunosensor comprised of gold nanoparticle (AuNP)-functionalised anti-PBB15 antibodies and CQD-labelled PBB15 antigens that function as the fluorescence acceptors and donors, respectively. As a result of FRET, the fluorescence of the CQDs was effectively quenched by the AuNPs. Upon the addition of PBB15 to the solution, the CQD-labelled antigens were released from the AuNP surface due to competitive immunoreaction and fluorescence recovery occurred. This immunosensor serves as a good example for future development of immunoassays to detect other analytes with suitable antibodies and antigens.
The concept of fluorescence quenching was also applied to the detection of nucleic acids, where the level of selectivity was so high that even a single-base mismatch could be identified.121 First, a single-stranded DNA (ss-DNA) labelled with a fluorescent dye adsorbed onto a CQD, effectively quenching the fluorescent dye. When the ss-DNA hybridised with its complementary target to form a double-stranded DNA (ds-DNA), the newly-formed ds-DNA desorbed from the CQD surface and the fluorescence recovered. This application showed great prospects in the detection of single-nucleotide polymorphisms based on the fluorescence intensity changes.
Another application of CQDs was based on the exploitation of aptamers, which are target binders selected from a large nucleic acid library.13 The aptamers are usually coupled with a conformational change induced by the target and could possibly result in a detectable change in radiometric response.122 Such an application was realised by Xu's group,123 who demonstrated that thrombin induces aptamer-functionalised CQDs to form a sandwich-structure with aptamer-functionalised silica nanoparticles through specific thrombin–aptamer interaction. This assay was highly specific to thrombin with a detection limit of 1.0 nM, making it one of the more sensitive fluorescent assays for thrombin. Stable fluorescent CQDs prepared using a single-step microwave pyrolysis method were used for the detection of proteins after they were separated by gel electrophoresis.124 Staining of proteins using these CQDs turned out to give comparable or even better sensitivity as compared to conventional staining agents such as Coomassie Brilliant Blue and Ag+. Water-soluble CQDs, prepared from thermal combustion of rice straws in a furnace under insufficient air flow, were used for rapid detection and counting of bacteria cells in sewage water.125 These CQDs selectively interacted with the receptors on the bacterial cell membrane only.
In addition to macrobiomolecules, CQDs have also shown promise as fluorescent probes in the detection of small bioanalytes like anti-bacterial drugs. One such example was shown by the experiments performed by Niu and Gao.126 First, fluorescent N-CQDs were produced from glutamic acid through a one-step pyrolysis method. These N-CQDs were subsequently used for the detection of amoxicillin. Amoxicillin is a common drug used to treat bacterial infections. The authors found that amoxicillin molecules effectively separate the N-CQDs from each other, thus lowering the frequency of non-radiative transitions that ultimately leads to a rise in fluorescence intensity. CQDs were also applied to the detections of other small bioanalytes such as dopamine,98,127,128 ascorbic acid100 and glucose.129 For instance, Qu's group synthesised highly fluorescent CQDs using dopamine as carbon source and applied them to label-free detection of dopamine. Similar to the anion sensing mentioned earlier, dopamine effectively recovered the fluorescence of the already quenched Fe3+–CQD complex. It was observed that the enhancement of the fluorescence is proportional to the dopamine concentration in the range of 0.1–10 μM with a detection limit of 68 nM.98 However, dopamine was believed to be an efficient quencher in another report.128 This controversy likely originates from the different carbon sources and synthetic routes used in the preparation of the CQDs. Therefore, caution must be taken when interpreting the data and standardisation in CQD synthesis is urgently needed.
4.3 Bioimaging
As previously discussed, CQDs have multiple advantages over semiconductor quantum dots, including comparable optical properties and good chemical and photochemical stability. Most importantly, carbon is largely non-toxic and environmentally friendly. These traits make CQDs very desirable as alternatives to semiconductor quantum dots to visualise biological systems both in vitro and in vivo.62 In general, the carbon cores of CQDs themselves are not toxic and any cytotoxicity of CQDs is primarily due to surface passivating agents on the CQD surface.130 It has been demonstrated that surface passivating agents of low cytotoxicity can be used safely at high concentrations for in vivo imaging. For example, PEGylated CQDs showed no noticeable toxic effects in vivo up to 28 days when intravenously injected 8–40 mg kg−1 (CQD/bodyweight) of the PEGylated CQDs into mice for toxicity evaluation. Physiological indicators were all at similar levels for mice exposed to different dosages of CQDs and the NaCl control, thereby suggesting the non-toxicity of CQDs at exposure levels and times beyond those typically used in in vivo imaging studies. No abnormalities were observed in harvested organs although the amounts of CQDs found in liver and spleen were higher than those found in other organs.16 Moreover, cell viability was measured after cells had been treated with different amounts of CQDs. It was found that the average cell viability is greater than 95% at CQD concentrations up to 1.8 mg ml−1. These results clearly demonstrated that CQDs are much more biocompatible than semiconductor quantum dots.14,131 Even if agents with high cytotoxicity profiles are used to coat CQDs, CQDs modified with these agents can still be used for in vivo applications if these agents are maintained at low concentrations and/or the incubation time is kept relatively short.It was revealed that organic dye-conjugated CQDs are effective fluorescent probes for H2S. A FRET process took place in the presence of trace amounts of H2S, turning the blue emission of the organic dye-conjugated CQDs to green.132 Previous work has proved that H2S can penetrate cell membrane by simple diffusion.133 Using a fluorescence microscope, the ability of the organic dye-conjugated CQDs to visualise changes in physiologically relevant levels of H2S in live cells was evaluated. Fig. 8 displays the fluorescence images of HeLa and L929 cells incubated with the organic dye-conjugated CQDs before and after being treated with H2S. As seen in Fig. 8, the intracellular fluorescence of the organic dye-conjugated CQD-stained cells exposed to H2S for 30 min at 37 °C turned green, clearly indicating that the organic dye-conjugated CQDs are promising fluorescent probes to track H2S level change in live cells.132 In another example, CQDs synthesised using N-(β-aminoethyl)-γ-aminopropyl methyl-dimethoxysilane as a carbon source selectively interacted with Cu2+ because of the residue ethylenediamine groups on their surface.95 Additionally, dual-emission probes for Cu2+ were prepared by coating such CQDs on the surface of Rhodamine B (RhB)-doped silica nanoparticles. The fluorescence of the CQDs was efficiently quenched by Cu2+, while that of RhB was negligibly affected. Utilising these probes, successful attempts were made in in vivo imaging of Cu2+ in live cells.95
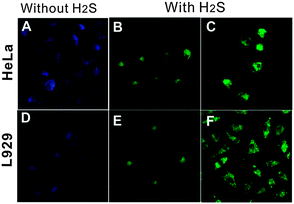 | ||
| Fig. 8 Fluorescence images of the organic dye-conjugated CQDs loaded live cells before (A, D) and after incubating with 30 (B, E) and 100 mM H2S (C, F). (Reproduced with permission from ref. 132.) | ||
Cell images obtained by Hsu et al. showed that CQDs are mostly localised in the cytoplasm and cell membrane.134 It was also shown that water-soluble CQDs passivated with PPEI-EI could label the cell membrane and cytoplasm of MCF-7 cells and they do not reach the nucleus.1 Furthermore, CQDs synthesised from activated carbon selectively labelled the cell membrane and cytoplasm of COS-7 cells.135 On the other hand, cells incubated with silica-encapsulated CQDs were found to only exhibit bright fluorescence in the cytoplasmic area.136 Fowley and colleagues prepared CQDs that were enclosed in an amphiphilic biocompatible polymer, which were subsequently found to travel across Chinese hamster ovary cell membrane and settle in the cytosol.137 Hu's team prepared b-PEI-coated CQDs with a quantum yield of 54.3% and these CQDs were observed to distribute uniformly throughout the cytoplasm.138 These examples clearly show that the localisation of CQD varies, depending on the choice of the surface passivating agents and the mode of surface passivation.
Before imaging, cells are usually incubated with CQDs so that the CQDs can be internalised by the cells. This ability of cells to take in CQDs was revealed to be dependent on temperature, where no CQDs were found to internalise into the cells at 48 °C.1 It was proposed that CQDs likely translocate into cells by endocytosis. Also, it was suggested that the uptake of CQDs may be enhanced by coupling CQDs with membrane translocation peptides, so as to facilitate this translocation procedure by overcoming the cell membrane barrier.139,140
As mentioned above, CQDs are able to exhibit multicolour emissions, which is a huge advantage that sets them apart from the majority of labelling agents. This allows researchers to control and choose the excitation and emission wavelengths.141 As illustrated in Fig. 9, the property of tunable emissions of CQDs was clearly visible,134 where light at different wavelengths was emitted upon excitation at different wavelengths. This property was also seen in HepG2 cells incubated with 4,7,10-trioxa-1,13-tridecanediamine-passivated CQDs, which portrayed multicolour emissions when excited at different wavelengths.142 “Green” CQDs prepared from sugar cane juice also exhibited multicolour emissions upon different excitation modes in bacteria and yeast cells.62 Notably, Ray et al. revealed that surface passivation might not be necessary to achieve a high level of fluorescence intensity required for cell imaging.57 CQDs prepared from the thermal combustion of soot and treated with acid were able to translocate into Ehrlich ascites carcinoma cells successfully even though they were not coated with any surface passivating agents.57
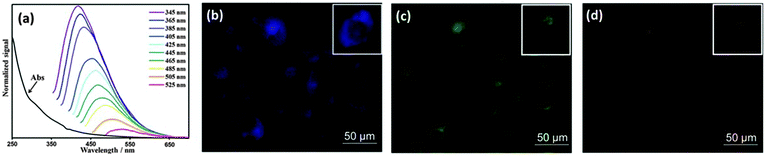 | ||
| Fig. 9 (a) Emission spectra of CQDs at different excitation wavelengths; fluorescence images of MCF-10A cells treated with CQDs upon excitation with (b) UV, (c) blue and (d) green light. (Reproduced with permission from ref. 134.) | ||
If the excitation wavelength is red-shifted enough, CQDs could emit in the NIR region. Although the emission in the NIR region is relatively weak, CQDs have great potential for in vivo fluorescence tracking studies141 because the animal body is practically transparent in the NIR region.143 Yang's group was the first to explore the viability of CQDs as contrast agents in live mice.15 In their experiments, PEG1500N-passivated CQDs were injected subcutaneously into mice and bright fluorescence emissions were observed, only fading away 24 h after the injection. If the CQDs were injected intravenously, emissions were only observed in the bladder region, thereby suggesting that urine extraction is the main exiting route for intravenously introduced CQDs. The same group also successfully tracked the migration in lymph vessels using ZnS-doped CQDs (Fig. 10).15
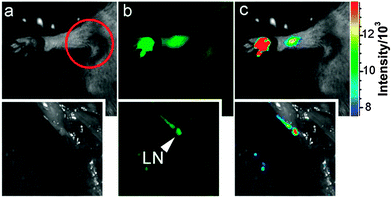 | ||
| Fig. 10 Tracking of migration through lymph vessels using ZnS-doped CQDs. (Reproduced with permission from ref. 15.) | ||
In another report, Cao et al. employed the ZnS-doped CQDs for in vivo imaging in mice, which showed comparable brightness with the well-established CdSe/ZnS quantum dots.144 However, after intradermal injections of the CQDs and CdSe/ZnS quantum dots into mice, it was observed that though both types of quantum dots moved to the axillary lymph nodes, the former moved at a much slower rate than the latter.36 This was ascribed to the PEG molecules on the CQD surface, which reduce the interaction between the CQDs and the lymph cells. Of particular interest was the observed competitive performance of the CQDs in vivo to that of the CdSe/ZnS quantum dots. The above results suggest that CQDs may be further developed into a new class of high-performance yet non-toxic agents for bioimaging.144
The exact mechanism of CQD uptake by cells remains to be elucidated, but increasing experimental evidence suggested that CQDs are likely internalised by the cell through endocytosis without significant infiltration to the cell nucleus.1,14,18,19 In addition, the interaction between protective/functional coatings on CQDs is believed to play an important role in selective cell targeting. In future, better targeting ability of CQDs to cells and perhaps even the nuclei may be achieved by conjugating CQDs with facilitating proteins or peptides, enabling the CQDs cross the cell membrane barrier more readily.
4.4 Nanomedicine
CQDs, being small fluorescent nanoparticles which can be synthesised quickly via many inexpensive and simple synthetic routes, serve as good alternatives to other fluorescent nanomaterials. CQDs are also very attractive in nanomedicine because they do not show any visible signs of toxicity in animals and thus can be used for in vivo studies.145 This is exemplified by in vivo toxicity studies in mice, where mice were intravenously injected with CQDs and tested after four weeks. It was concluded that their organs and internal functions are hardly affected. The high biocompatibility of CQDs was also supported by prothrombin time assays in plasma samples. The results indicated that CQDs do not limit the activity of thrombin and do not lead to any blood coagulation.134The work by Bechet and co-workers showed that CQDs can be used for photodynamic therapy. Photodynamic therapy is a clinical treatment mainly for superficial tumours.146 It involves the localisation and accumulation of photosensitizers in the tumour tissue, following which they are irradiated with a specific wavelength, triggering the formation of singlet oxygen species that result in cell death. It has been validated that CQDs have high inhibition effect on MCF-7 and MDA-MB-231 cancer cells.134 This phenomenon was attributed to CQDs being able to generate more reactive oxygen species, making them promising photosensitizers. It was also noted that the circulation and uptake of CQDs in the body is dependent on their surface coating and the route of administration.147 Huang et al. investigated the effect of the injection route on the distribution, clearance and tumour uptake of CQDs.81 It was learnt that CQDs are quickly and effectively excreted from the body when intravenous, intramuscular and subcutaneous injection routes are used. Additionally, the high tumour-to-background fluorescence contrast and low fluorescence levels in other tissues and organs demonstrated the suitability of CQDs to act as photosensitizers as they are able to localise selectively into tumours (Fig. 11).
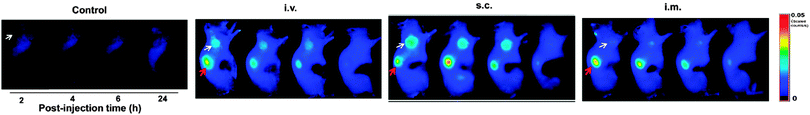 | ||
| Fig. 11 Fluorescence images of tumour-bearing mice. (Reproduced with permission from ref. 81.) | ||
Juzenas and colleagues also explored the use of CQDs as photosensitizers in photodynamic therapy to destroy cancer cells.148 Small CQDs functionalised with PPEI-EI (PPEI-EI-CQDs) were prepared. Upon irradiation with UV light, these CQDs displayed substantial photodynamic effect in Du145 and PC3 cells. It was proposed that photo-induced generation of singlet oxygen (Type II mechanism) and other reactive oxygen species and radicals (Type I mechanism) is responsible for the observed photodynamic effect. TiO2 is one of the most popular semiconductor photocatalysts, but it can only be excited with UV light due to the size of its bandgap.149 Unfortunately, UV radiation has a low penetration power and can only penetrate slightly into the skin tissue, thus resulting in poor photodynamic efficiency in deep tissues.150 Compared to TiO2, PPEI-EI-CQDs have the additional advantage of tunable bandgap, where their bandgap can be made smaller to allow excitation at longer wavelengths. This would allow for the destruction of buried tumours because light at longer wavelengths can penetrate deeper into tissues. By attaching a photosensitizer (chlorin e6) to CQDs, a synergistic photodynamic therapy platform was developed.151 It was shown that the CQDs can indirectly excite the photosensitizer by FRET mechanism. Moreover, the capability of up-conversion fluorescence emissions of CQDs is more attractive in photodynamic therapy. As demonstrated by Fowley et al.,152 the up-conversion property of CQDs is potentially useful in the treatment of deep-seated tumours in photodynamic therapy. In their study, a conventional photosensitizer (protoporphyrin IX) was first conjugated to CQDs. The photosensitizer was indirectly excited via FRET by the up-conversion fluorescence emission of the CQDs upon excitation at 800 nm. The excitation light of 800 nm is in the phototherapeutic window and can penetrate human tissue four times deeper than the 630 nm light used in clinical photodynamic therapy.
In addition to phototherapy, CQDs can be used for radiotherapy. As described in a report by Andrius et al.,153 PEG-CQDs coated with a silver shell (C-Ag-PEG CQDs) could be used as radiosensitizers in Du145 cells. When irradiated with low energy X-rays, electrons were ejected from the C-Ag-PEG CQDs, which in turn generates free radicals and damages the cancer cells surrounding the CQDs, reducing the damage of normal cells and increasing therapeutic selectivity.
In the field of nanomedicine, a fusion of nanotechnology and medicine, CQDs could function as nano-carriers for tracking and delivery of drugs or genes. In particular, CQDs with fluorescence in the red or NIR region would be the most desirable because background illumination from endogeneous fluorophores can be avoided during imaging.154 Demonstrated by Hu's team, bPEI-coated CQDs (bPEI-CQDs) displayed great potential in the application of gene delivery.138 These CQDs have a large number of amino groups on their surface, which could condense DNA to aid in their function as gene carriers. In order to check for the viability of these CQDs as gene carriers, the authors carried out additional transfection experiments using enhanced green fluorescent protein (EGFP) gene, which served as the reporter gene. As presented in Fig. 12, the overlay of the three images revealed that the pPEI-CQDs exhibit high fluorescence intensity and transfection efficiency comparable to EGFR. Evidently, the gene carried by the bPEI-CQDs was successfully brought into the cells. Because of its significant positive charge density and proton-sponge effect, bPEI spontaneously attracts and condenses gene (polyanionic DNA strands) to form toroidal complexes that are readily taken up by cells through endocytosis. The un-protonated amine moieties in the complexes are thought to buffer endolysosomal pH, thereby allowing cytoplasmic release of gene.155
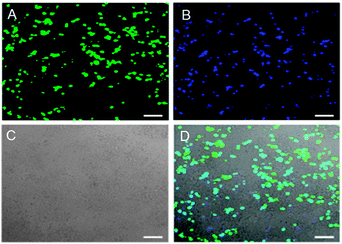 | ||
| Fig. 12 Fluorescence images of 293T cells transfected with (A) EGFR and (B) DNA–bPEI-CQD complexes; (C) the bright field image (D) the overlay of the three images. (Reproduced with permission from ref. 138.) | ||
Drug delivery systems (DDSs) based on nanotechnology are increasingly developed in recent years. The most widely investigated DDSs are based on AuNPs, but the issue of toxicity limits their applications in clinical therapy.156 AuNPs also require thiol groups for drug loading through Au–thiol interaction, which imposes a further limitation of drug choice.154 In addition, AuNPs are difficult to track in in vivo systems because they cause the quenching of fluorophores.157 Therefore, CQDs serve as good alternatives to AuNPs since different functionalizations could result in many possibilities for conjugation with drug molecules in combination with targeting agents, expanding the drug choices for delivery.154
Zheng and co-workers conjugated a platinum(VI)-based anti-cancer pro-drug – oxidised oxaliplatin (Oxa(VI)–COOH) on the surface of CQDs through chemical coupling.158 The pro-drug-conjugated CQDs were taken up by cancer cells through endocytosis and the drug was released upon the reduction of Oxa(IV)–COOH to oxaliplatin(II) because of the highly reducing environment in cancer cells. It was also demonstrated that the distribution of the pro-drug-conjugated CQDs can be closely tracked by monitoring the fluorescence signal of the CQDs, thereby offering great help in the customisation of the injection time and dosage of the medicine. To engineer a controlled-release mechanism, Karthik et al. introduced a photosensitive molecule (quinolone) to the CQD-based drug delivery system.159 The strong fluorescent properties of the CQDs serve as a conventional means to precisely track the distribution of the drug–CQD conjugates and the quinoline molecules on the surface of the CQDs serve as triggers for photo-regulated drug release. Other drug-releasing mechanism like pH-triggered drug release has also been tested.160,161 In an attempt to enhance the loading capacity of CQDs, Mewada and co-workers tested the drug carrying and folic acid-mediated delivering capacities of highly fluorescent swarming CQDs.162 Folic acid on the CQD surface was used as a navigational molecule thanks to its widespread association with most types of cancer cells.163 The drug loading capacity for an anti-cancer drug doxorubicin (DOX) was estimated to be ∼86% and the release of DOX from the DOX-loaded CQDs followed first order release kinetics at physiological pH – an ideal drug release profile. More interestingly, due to the better targeting ability of the folic acid molecule, the DOX-loaded CQDs showed a higher killing rate of cancer cells than free DOX and were less toxic to normal cells.162
Besides serving as drug carriers and fluorescent tracers, CQDs were found to be able to control drug release. An example was given by Lai and colleagues where CQDs loaded with DOX could control the release of DOX in HeLa cells.164 Any pre-release of DOX before its uptake by the target cells was reduced, thus drastically increasing the anti-cancer efficacy of this drug. Moreover, CQDs functionalised with PEG oligomers allowed for a longer circulation time in the physiological systems before they targeted the selected tissues to achieve localised therapy.165 However, thus far, there have not been any reports of CQDs that can specifically target a disease state,81 thus severely limiting their uses in therapeutics.
4.5 Photocatalysis
In recent years, photocatalytic processes have gained tremendous momentum as greener alternatives in organic synthesis.166–169 Interest in photocatalysis has been motivated in part by the realisation that sunlight is effectively an inexhaustible energy source. However, the high energy of UV and short wavelength visible light may adversely damage organic compounds.166 The demonstrated capability of harnessing long wavelength light and energy exchange with solution species of CQDs offers an excellent opportunity for their use as photocatalysts in organic synthesis. Indeed, a recent study has indicated that smaller CQDs (1–4 nm) are effective NIR light-driven photocatalysts for selective oxidation of alcohols to benzaldehydes with good conversion efficiency (92%) and selectivity (100%), due to their excellent catalytic activity for H2O2 decomposition and NIR light driven electron transfer property.170 Further studies suggested that the photocatalytic activity of CQDs can be effectively modulated by doping the CQDs171 and by tailoring the surface groups.172,173 On the other hand, larger CQDs (5–10 nm) synthesised by electrochemical ablation of graphite showed light-induced proton properties in solution, which can be used as acid catalysts to catalyse a series of organic transformations in aqueous media under visible light.174 More recently, Liu et al. reported the utilisation of the photochemical properties of AuNP–CQD nanocomposites for high-efficiency and high-selectivity photocatalytic systems for green oxidation of cyclohexane.175 The AuNP–CQD nanocomposites yielded 63.8% conversion efficiency and 99.9% selectivity for the oxidation of cyclohexane to cyclohexanone using H2O2 as the oxidant under visible light at room temperature.175 Given its versatility in the design of the nanocomposite photocatalysts, this approach deserves more attention since it may provide an exciting solution for the development of high-performance photocatalysts and green synthetic routes for the chemical industry.As one of the most popular photocatalysts, TiO2 has been used in the removal of organic pollutants and in the generation of H2 through water splitting.176 However, a major drawback in its photocatalytic efficiency resides in its ineffective utilisation of visible light as the irradiation source. Because the bandgap of bulk TiO2 lies in the UV region (3.0–3.2 eV), only less than 5% of sunlight is utilised by TiO2. Therefore, bandgap engineering by possible modification of TiO2-based materials is one of the plausible approaches to enhance the performance of TiO2 photocatalysts. In view of their attractive optical properties and up-conversion in particular, a nanocomposite of CQDs and TiO2 is expected to realise the efficient usage of the full spectrum of sunlight. Using methylene blue (MB) as a model compound, Li et al. showed that TiO2–CQD nanocomposites are able to completely degrade MB (50 mg mL−1) within 25 min under visible light irradiation, where only <5% of MB is degraded when pure TiO2 is used as the photocatalyst.36
Apart from harvesting visible light and converting it to shorter wavelength light through up-conversion (Fig. 13), which in turn excites TiO2 to form electron–hole pairs,36,177–179 it is believed that the CQDs in the nanocomposites facilitate the transfer of electrons from TiO2 and the electrons can be shuttled freely along the conducting paths of the CQDs, allowing charge separation, stabilisation and hindering recombination, thereby generating long-lived holes on the TiO2 surface.180 The longer-lived holes then account for the much enhanced photocatalytic activity of the TiO2–CQD nanocomposites. Likewise, similar behaviour was observed with CQD–TiO2 nanotube composites in the photocatalytic degradation of MB181 and CQD–TiO2 nanosheet nanocomposites in the photocatalytic degradation of RhB.182 Further to the above TiO2–CQD-based photocatalysts, other metal oxide nanoparticle–CQD nanocomposites like Fe2O3–CQD,183,184 ZnQ–CQD185 and Cu2O–CQD;186 and metal phosphate–CQD composite (Ag3PO4–CQD),187 were also used to harness the full spectrum of sunlight in their respective photocatalytic systems. Moreover, Kang and colleagues reported SiO2–CQD nanocomposites in the photocatalytic degradation of MB36 and selective hydrocarbon oxidation.188 Nonetheless, more work is needed to improve the lifespan of the above-mentioned photocatalysts before they can be employed in practical scenarios. Such work will be worthwhile because the photocatalytic activities of the nanocomposites are much greater than that of the well-known TiO2.
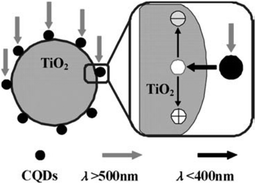 | ||
| Fig. 13 Possible catalytic mechanism of theTiO2–CQD nanocomposites under visible light. (Reproduced with permission from ref. 36.) | ||
In recent years, researchers have succeeded in utilising TiO2–CQD nanocomposites as photocatalysts to generate H2 through water splitting. One of the first reports was authored by Zhang and co-workers.189 They noted that TiO2 nanotube arrays loaded with CQDs are capable of producing H2 from water through photocatalysis under visible light. As schematically depicted in Fig. 14, the TiO2 nanotubes function as active sites for the photochemical reduction of water into H2 and the CQDs as photosensitizers. The presence of the CQDs effectively extends the light harvesting range of the TiO2 nanotube arrays to the visible and NIR regions. As a result, a four-fold enhancement in photocurrent density and a hydrogen evolution rate of 14.1 mmol h−1 at 0 V (vs. Ag/AgCl) were obtained.189 To further improve the photocatalytic efficiency, the TiO2–CQD nanocomposites were modified by adding narrow bandgap semiconductor quantum dots (CdSe) to them to leverage on the up-conversion property of the CQDs and the synergistic effect between the CdSe quantum dots and the CQDs.190 Similar to the TiO2 nanotube array–CQD nanocomposites, photochemical production of H2 from water under irradiation from visible light was observed. The nanocomposites were stable in the catalytic reaction and the photocurrent density was dependent on the size of the CQDs. The highest current density of 0.9 mA cm−2 was obtained with 3.8 nm CQDs, which is four times higher than that of pristine TiO2.191
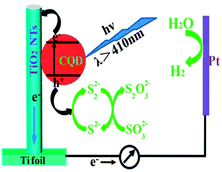 | ||
| Fig. 14 Illustration of the sensitization mechanism of CQDs. (Reproduced with permission from ref. 189.) | ||
In addition to being used as photocatalysts, CQDs have been capturing the attention of researchers as potential photosensitizers in solar cells. For example, a CQD–RhB–TiO2 system showed that the CQDs effectively bridge the RhB molecules to the TiO2 substrate by acting as a one-way electron transfer intermediary. Comparing to the RhB–TiO2 system, the presence of CQDs significantly enhanced the photoelectric conversion efficiency by as much as seven times.192 In another report, a CQD/TiO2 electrode was employed in a solar cell. The photocurrent density was 2.7 times larger than that of pristine TiO2 electrode under visible light illumination.181 Enhanced performance of a solar cell was also obtained when N-CQDs were used as photosensitizers.193 Although the photo-to-electricity conversion efficiency of the above-mentioned solar cells is far from satisfactory, these findings definitely encourage more research in the application of CQDs in photovoltaic devices.
4.6 Electrocatalysis
For technological relevance in clear energy production like fuel cells and clear fuel production, oxygen reduction reaction (ORR) and its reverse reaction – oxygen evolution reaction (OER) are at the centre of intensive research. Because of the sluggish kinetics of ORR,194 electrocatalysts are usually used to improve the kinetics of ORR and of which platinum is the “state-of-the-art”. Unfortunately, the formidably high cost of platinum-based electrocatalysts has prompted researchers to look for non-platinum-based electrocatalysts for ORR, aiming at achieving comparable or even better electrocatalytic efficiency than that of platinum-based electrocatalysts.The ultra-small size of CQDs along with their high stability and good electrical conductivity makes them interesting contenders as electrocatalytic materials for ORR. Previous investigations on graphene have indicated that doped nitrogen atoms in carbon materials, especially in the form of pyridinium moieties, play a critical role in enhancing their electrocatalytic activities toward ORR.195 One of the pioneering reports on the use of CQDs as electrocatalysts for ORR was by Li and co-workers.196 They demonstrated that N-CQDs with oxygen-rich functional groups prepared via an electrochemical procedure are electrocatalytically active toward electrochemical reduction of oxygen. The onset potential of ORR was found to be −0.16 V (vs. Ag/AgCl), which is close to that of commercial platinum-based electrocatalysts (Fig. 15). Similar results were later obtained by Yan and co-workers197 and Liu et al.198 with N-CQDs synthesised by totally different procedures. A comparison between nitrogen-free CQDs and the N-CQDs suggested that the electrocatalytic activity of the N-CQDs is indeed closely associated with the N-doping effect. In addition, the N-CQDs exhibited excellent tolerance to a possible crossover effect from methanol. First-principles investigations of the N-CQDs suggested that pyridinic and graphitic nitrogen are responsible for the observed electrocatalytic activity.199 In another report, Zhu and colleagues investigated the electrocatalytic activity of CQDs prepared from natural biomass – soy milk.200 Similar to the N-CQDs, a much enhanced electrochemical reduction profile of oxygen was obtained.
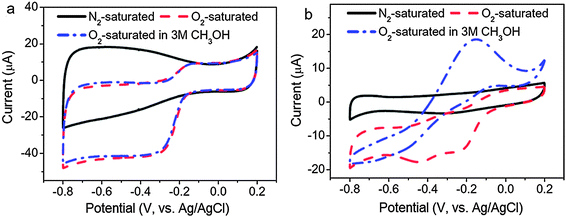 | ||
| Fig. 15 Cyclic voltammograms of (a) N-CQD/graphene and (b) commercial Pt/C on a GC electrode in N2-saturated 0.1 M KOH, O2-saturated 0.1 M KOH and O2-saturated 3 M CH3OH solutions. (Reproduced with permission from ref. 196.) | ||
Likewise, OER also suffers from sluggish kinetics and a high over-potential is required in order to drive OER at a reasonably high rate. Currently, the best electrocatalysts for OER are ruthenium- and iridium-based materials. Again, the formidably high cost of these materials has urged researchers to search for alternative electrocatalysts that can offer high efficiency in OER and yet readily available at low cost. Unfortunately, reasonably high electrocatalytic activity of CQDs toward OER has yet to be reported. On the other hand, CQD-based hybrid materials such as CQD–NiFe-layered double-hydroxide composites have shown some promise in OER.201 It was reported that the composites exhibit high electrocatalytic activity toward the oxidation of water at a relatively low over-potential of ∼235 mV in 1 M KOH at a current density of 10 mA cm−2, which is comparable to those of the most active perovskite-type electrocatalysts. It was suggested that the synergistic effect arising from strong association of the CQDs with the NiFe hydroxide greatly facilitates charge transport, thereby improving the catalytic activity of the NiFe hydroxide. It is likely that this kind of CQD composites will offer new opportunities for OER as well as many other electrocatalytic applications.
5. Conclusion and outlook
Since the discovery of CQDs in 2004, a number of simple, low-cost and efficient routes for the synthesis of CQDs have been developed. This is in drastic contrast to the much more laborious and expensive chemistry that is required to synthesise other fluorescent nanoparticles, for example noble metal nanoparticles and semiconductor quantum dots. Growing evidence has suggested that the defects in CQDs play a critical role in their observed fluorescence emissions. Consequently, approaches and techniques that are capable of facially manipulating the population and location of the defects in CQDs are both fundamentally and technically critical in the synthesis of CQDs. The physicochemical properties of CQDs, including their fluorescence properties without photobleaching and photoblinking, their facile emission tuning and their chemical stability, enable the development of highly sensitive sensing platforms. It is believed that CQDs will play an increasing role in analytical and bioanalytical science in the near future. Unfortunately, CQDs with high quantum yields still remain rare. Future research efforts should be directed to significantly enhancing the quantum yield of CQDs in addition to the preparation of geometrically, compositionally and structurally well-defined CQDs. Meanwhile, research in the applications of CQDs in sensing and bioimaging should focus on enhancing the sensitivity, selectivity and robustness of the sensing and bioimaging platforms and on the use of delayed fluorescence techniques to minimise the background and to offload the interference of auto-fluorescence of biological samples.The advent of CQD research has heralded a new chapter in biomedicine. The proof-of-concept experiments mentioned above have confirmed that CQDs are able to contribute to the advances of biomedicine via applications in bioimaging and nanomedicine. The excellent chemical and photochemical stability of CQDs together with their chemically non-toxic composition give a clear advantage in the context of in vivo biomedical applications. With these attractive features, CQDs have clearly become a legitimate competitor to the conventional semiconductor quantum dots as bioimaging agents with comparable or even better performance due to their excellent biocompatibility, high optical performance without photoblinking and the ability to be functionalised with various moieties. More importantly, by conjugating targeting moieties and therapeutic components, CQDs will enable “theranostics” which holds the potential to address the challenges of cancer heterogeneity and adaptation. Therefore, CQDs have the technical capability that will enable the development of new diagnostics, therapeutics and preventives, which can cause a paradigm shift in the way we diagnose, treat and prevent cancer. Nonetheless, in drug delivery and controlled release, a more fundamental question that should be probed is why CQDs would be efficacious at all. What are the advantages of CQDs for drug delivery compared with monoclonal antibodies in conjugation with anticancer drugs,202 and nanospheres made of biodegradable polymers?203 The applications of CQDs in nanomedicine should then be to first determine the tasks for which CQDs are particularly valuable although preliminary results are encouraging. Further research is required in the development of CQDs with better targeting ability to target specific cell types and specific cell compartments. It is also essential that CQDs to be used in bioimaging use relatively low-energy excitation sources, preferably red or NIR sources, thus making them more effective in tissue penetration and reducing the interference from background fluorescence. Consequently, a major challenge in CQD research is the synthesis of CQDs with brighter fluorescence emissions excitable by red or NIR sources. Also, more research is desired on the synthesis of CQDs with controlled shape, size and functionalities. Although significant findings have been reported with regard to the applications of CQDs, their exact mechanism of cellular uptake and precise toxicological effect remain to be elucidated due to the fact that the pharmacokinetics and bio-distribution of CQDs are dependent upon multiple factors such as size, shape, surface chemistry and so on. Through rational design and optimisation of these factors, non-specific uptake of CQDs can be significantly reduced and blood circulation can be extended, thereby conferring CQDs enhanced abilities in targeting specific tissues in the human body. First, surface functionalization is the most important factor to alter and fine-tune the pharmacological properties of CQDs. For example, CQDs with oligomeric PEG coatings exhibited much lower toxicity, longer blood circulation half-life and better tumour targeting efficiency in vivo than CQDs without appropriate surface coatings.81 Moreover, the right shape and size of CQDs can result in reduced non-specific capture by macrophages, which can also affect their excretion route, circulation half-life, as well as how they will interact with different tissues in vivo. Therefore, there is a need for reliable techniques to produce CQDs with controlled and consistent properties. Considerably more research must be carried out before the viability of CQDs can be fully realised and the development of CQDs for potential biomedical uses should proceed in parallel with a thorough evaluation of the cytotoxic effect of CQDs.
The general strategy for the adoption of environmentally benign CQDs as photocatalysts in synthetic chemistry represents an attractive approach in the development of green chemistry, which may eventually lessen the burden of energy consumption, product clean-up and waste disposal. The immediate goal in this emerging area should be geared toward the discovery of photochemical solutions for increasingly ambitious synthetic goals. The long-term goals should be to improve efficiency and synthetic utility and to eventually perform chemical synthesis under sunlight.
Compared to other applications of CQDs, there have been fewer studies in the usability of CQDs as electrocatalysts for ORR and OER. In-depth theoretical and experimental studies are needed to delicately design CQD-based electrocatalysts with desirable electrocatalytic activity and long-term operation stability. The combination of CQD doping and CQD-based nanocomposites with other nanomaterials may open up new avenues to systematically study the effect of structural parameters and chemical compositions on the catalytic performance of the electrocatalysts, thus leading to fundamental insights and practical applications.
Although still in the midst of development, CQDs have already shown immense potential to play a big role in nanotechnology for the development of assays, sensors, bioimaging agents, drug carriers, phototherapy, photocatalysis and electrocatalysis. Despite the fact that many optical and electronic properties of CQDs are not well understood yet, there is no doubt that CQDs will play a huge role in bioimaging and biomedical research in the near future upon further development. Being highly versatile, CQDs have the propensity to be rationally modified according to different needs. Applications of CQDs in niche areas such as photocatalysis exemplify the versatility of CQDs in the most unexpected areas. It is heartening to witness the diversion of research interest in CQDs away from traditional fields such as bioimaging, and into more urgent and pressing needs such as green chemistry and clean energy production. The fact that the advantages of CQDs are being recognised by researchers with interest in areas as diverse as materials science, synthetic chemistry, drug delivery, nanomedicine and clean energy suggests that research on CQDs will continue to grow in synergistic relationship with intellectually adjacent fields. It seems clear that the future of CQDs remains promising.
Acknowledgements
This work is supported by Ministry of Education and the A*STAR-ANR Program.References
- L. Cao, X. Wang, M. J. Meziani, F. S. Lu, H. F. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wise and W. W. Webb, Science, 2003, 300, 1434–1436 CrossRef CAS PubMed.
- R. L. Liu, D. Q. Wu, S. H. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS PubMed.
- J. Geys, A. Nemmar, E. Verbeken, E. Smolders, M. Ratoi, M. F. Hoylaerts, B. Nemery and P. H. M. Hoet, Environ. Health Perspect., 2008, 116, 1607–1613 CrossRef CAS PubMed.
- P. Lin, J.-W. Chen, L. W. Chang, J.-P. Wu, L. Redding, H. Chang, T.-K. Yeh, C. S. Yang, M.-H. Tsai, H.-J. Wang, Y.-C. Kuo and R. S. H. Yang, Environ. Sci. Technol., 2008, 42, 6264–6270 CrossRef CAS.
- X. Y. Xu, R. Ray, Y. L. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. G. Luo, H. Yang, M. E. Kose, B. L. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- L. Y. Zheng, Y. W. Chi, Y. Q. Dong, J. P. Lin and B. B. Wang, J. Am. Chem. Soc., 2009, 131, 4564–4565 CrossRef CAS PubMed.
- X. Y. Li, H. Q. Wang, Y. Shimizu, A. Pyatenko, K. Kawaguchi and N. Koshizaki, Chem. Commun., 2011, 47, 932–934 RSC.
- H. P. Liu, T. Ye and C. D. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS PubMed.
- F. Wang, S. P. Pang, L. Wang, Q. Li, M. Kreiter and C.-Y. Liu, Chem. Mater., 2010, 22, 4528–4530 CrossRef CAS.
- X. H. Wang, K. G. Qu, B. L. Xu, J. S. Ren and X. G. Qu, J. Mater. Chem., 2011, 21, 2445–2450 RSC.
- P. Demchenko and M. O. Dekaliuk, Methods Appl. Fluoresc., 2013, 1, 042001 CrossRef.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- S.-T. Yang, L. Cao, P. G. Luo, F. S. Lu, X. Wang, H. F. Wang, M. J. Meziani, Y. F. Liu, G. Qi and Y.-P. Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS PubMed.
- S.-T. Yang, X. Wang, H. F. Wang, F. S. Lu, P. G. Luo, L. Cao, M. J. Meziani, J.-H. Liu, Y. F. Liu, M. Chen, Y. P. Huang and Y.-P. Sun, J. Phys. Chem. C, 2009, 113, 18110–18114 CAS.
- R. Q. Ye, C. S. Xiang, J. Lin, Z. W. Peng, K. W. Huang, Z. Yan, N. P. Cook, E. L. G. Samuel, C. C. Hwang, G. D. Ruan, G. Ceriotti, A. R. O. Raji, A. A. Marti and J. M. Tour, Nat. Commun., 2013, 4, 2943 Search PubMed.
- H. T. Li, Z. H. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- C. Q. Ding, A. W. Zhu and Y. Tian, Acc. Chem. Res., 2014, 47, 20–30 CrossRef CAS PubMed.
- J. C. G. E. da Silva and H. M. R. Goncalves, Trends Anal. Chem., 2011, 30, 1327–1336 CrossRef PubMed.
- W. S. Hummers, Jr. and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- G. Eda, Y.-Y. Lin, C. Mattevi, H. Yamaguchi, H.-A. Chen, I.-S. Chen, C.-W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505–509 CrossRef CAS PubMed.
- K. Krishnamoorthy, M. Veerapandian, R. Mohan and S.-J. Kim, Appl. Phys. A: Mater. Sci. Process., 2012, 106, 501–506 CrossRef CAS.
- T. Gokus, R. R. Nair, A. Bonetti, M. Bohmler, A. Lombardo, K. S. Novoselov, A. K. Geim, A. C. Ferrari and A. Hartschuh, ACS Nano, 2009, 3, 3963–3968 CrossRef CAS PubMed.
- L. Cao, M. J. Meziani, S. Sahu and Y.-P. Sun, Acc. Chem. Res., 2013, 46, 171–180 CrossRef CAS PubMed.
- J. H. Shen, Y. H. Zhu, C. Chen, X. L. Yang and C. Z. Li, Chem. Commun., 2011, 47, 2580–2582 RSC.
- J. H. Shen, Y. H. Zhu, X. L. Yang, J. Zong, J. M. Zhang and C. Z. Li, New J. Chem., 2012, 36, 97–101 RSC.
- A. Nourbakhsh, M. Cantoro, T. Vosch, G. Pourtois, F. Clemente, M. H. van der Veen, J. Hofkens, M. M. Heyns, S. De Gendt and B. F. Sels, Nanotechnology, 2010, 21, 435203–435211 CrossRef PubMed.
- J. Robertson and E. P. O'Reilly, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 2946–2957 CrossRef CAS.
- C. Mathioudakis, G. Kopidakis, P. C. Kelires, P. Patsalas, M. Gioti and S. Logothetidis, Thin Solid Films, 2005, 482, 151–155 CrossRef CAS PubMed.
- C. W. Chen and J. Robertson, J. Non-Cryst. Solids, 1998, 227–230, 602–606 CrossRef CAS.
- W. L. Wilson, P. F. Szajowski and L. E. Brus, Science, 1993, 262, 1242–1244 CAS.
- R. L. Liu, D. Q. Wu, S. H. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS PubMed.
- X. F. Jia, J. Lia and E. K. Wang, Nanoscale, 2012, 4, 5572–5575 RSC.
- W. Zhao, C. Song and P. E. Pehrsson, J. Am. Chem. Soc., 2002, 124, 12418–12419 CrossRef CAS PubMed.
- H. T. Li, X. D. He, Z. H. Kang, H. Huang, Y. Liu, J. L. Liu, S. Y. Lian, C. H. A. Tsang, X. B. Yang and S.-T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed.
- J. Zong, Y. H. Zhu, X. L. Yang, J. H. Shen and C. Z. Li, Chem. Commun., 2011, 47, 764–766 RSC.
- S. J. Zhu, J. H. Zhang, X. Liu, B. Li, X. F. Wang, S. J. Tang, Q. N. Meng, Y. F. Li, C. Shi, R. Hu and B. Yang, RSC Adv., 2012, 2, 2717–2720 RSC.
- C. F. Wang, X. Wu, X. P. Li, W. T. Wang, L. Z. Wang, M. Gu and Q. Li, J. Mater. Chem., 2012, 22, 15522–15525 RSC.
- X. M. Wen, P. Yu, Y.-R. Toh, X. Q. Ma and J. Tang, Chem. Commun., 2014, 50, 4703–4706 RSC.
- S. J. Yu, M. W. Kang, H. C. Chang, K. M. Chen and Y. C. Yu, J. Am. Chem. Soc., 2005, 127, 17604–17605 CrossRef CAS PubMed.
- J. G. Zhou, C. Booker, R. Y. Li, X. T. Zhou, T.-K. Sham, X. L. Sun and Z. F. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed.
- Y. Q. Dong, N. N. Zhou, X. M. Lin, J. P. Lin, Y. W. Chi and G. N. Chen, Chem. Mater., 2010, 22, 5895–5899 CrossRef CAS.
- Q. L. Wang, H. Z. Zheng, Y. J. Long, L. Y. Zhang, M. Gao and W. J. Bai, Carbon, 2011, 49, 3134–3140 CrossRef CAS PubMed.
- H. Ming, Z. Ma, Y. Liu, K. M. Pan, H. Yu, F. Wang and Z. H. Kang, Dalton Trans., 2012, 41, 9526–9531 RSC.
- B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455–458 CrossRef PubMed.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563–5565 CrossRef CAS.
- H. T. Li, X. D. He, Y. Liu, H. Huang, S. Y. Lian, S.-T. Lee and Z. H. Kang, Carbon, 2010, 49, 605–609 CrossRef PubMed.
- H. Zhu, X. L. Wang, Y. L. Li, Z. J. Wang, F. Yang and X. R. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- H. T. Li, H. Ming, Y. Liu, H. Yu, X. D. He, H. Huang, K. M. Pan, Z. H. Kang and S.-T. Lee, New J. Chem., 2011, 35, 2666–2670 RSC.
- Y. M. Guo, Z. Wang, H. W. Shao and X. Y. Jiang, Carbon, 2013, 52, 583–589 CrossRef CAS PubMed.
- Z.-C. Yang, X. Li and J. Wang, Carbon, 2011, 49, 5207–5212 CrossRef CAS PubMed.
- H. Y. Wu, C. C. Mi, H. Q. Huang, B. F. Han, J. Li and S. K. Xu, J. Lumin., 2012, 132, 1603–1607 CrossRef CAS PubMed.
- B. Zhang, C.-Y. Liu and Y. Liu, Eur. J. Inorg. Chem., 2010, 4411–4414 CrossRef CAS.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
- S. Chandra, P. Das, S. Bag, D. Laha and P. Pramanik, Nanoscale, 2011, 3, 1533–1540 RSC.
- S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546–18551 CAS.
- J. J. Zhou, Z. H. Sheng, H. Y. Han, M. Q. Zou and C. X. Li, Mater. Lett., 2012, 66, 222–224 CrossRef CAS PubMed.
- W. B. Lu, X. Y. Qin, S. Liu, G. H. Chang, Y. W. Zhang, Y. L. Luo, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, Anal. Chem., 2012, 84, 5351–5357 CrossRef CAS PubMed.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- H. Huang, J.-J. Lv, D.-L. Zhou, N. Bao, Y. Xu, A.-J. Wang and J.-J. Feng, RSC Adv., 2013, 3, 21691–21696 RSC.
- V. N. Mehta, S. Jha and S. K. Kailasa, Mater. Sci. Eng., C, 2014, 38, 20–27 CrossRef CAS PubMed.
- J. Wang, C.-F. Wang and S. Chen, Angew. Chem., Int. Ed., 2012, 51, 9297–9301 CrossRef CAS PubMed.
- Y. H. Yang, J. H. Cui, M. T. Zheng, C. F. Hu, S. Z. Tan, Y. Xiao, Q. Yang and Y. L. Liu, Chem. Commun., 2012, 48, 380–382 RSC.
- D. Chowdhury, N. Gogoi and G. Majumdar, RSC Adv., 2012, 2, 12156–12159 RSC.
- J. R. Neabo, C. Vigier-Carriere, S. Rondeau-Gagne and J. F. Morin, Chem. Commun., 2012, 48, 10144–10146 RSC.
- Q. H. Liang, W. J. Ma, Y. Shi, Z. Li and X. M. Yang, Carbon, 2013, 60, 421–428 CrossRef CAS PubMed.
- D. Sun, R. Ban, P.-H. Zhang, G.-H. Wu, J.-R. Zhang and J.-J. Zhu, Carbon, 2013, 64, 424–434 CrossRef CAS PubMed.
- D. L. Xiao, D. H. Yuan and H. He, J. Lumin., 2013, 140, 120–125 CrossRef CAS PubMed.
- Y. Xu, M. Wu, Y. Liu, X.-Z. Feng, X.-B. Yin, X.-W. He and Y.-K. Zhang, Chem. – Eur. J., 2013, 19, 2276–2283 CrossRef CAS PubMed.
- S. J. Zhu, Q. N. Meng, L. Wang, J. H. Zhang, Y. B. Song, H. Jin, K. Zhang, H. C. Sun, H. Y. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- Y. X. Fang, S. J. Guo, D. Li, C. Z. Zhu, W. Ren, S. J. Dong and E. K. Wang, ACS Nano, 2012, 6, 400–409 CrossRef CAS PubMed.
- S. Liu, J. Q. Tian, L. Wang, Y. W. Zhang, X. Y. Qin, Y. L. Luo, A. M. Asiri, A. O. Al Youbi and X. P. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed.
- Y. P. Sun, X. Wang, F. Lu, L. Cao, M. J. Meziani, P. G. Luo, L. Gu and L. M. Veca, J. Phys. Chem. C, 2008, 112, 18295–18298 CAS.
- E. H. Nicollian, J. Vac. Sci. Technol., 1971, 8, S39–S49 CrossRef CAS.
- L. Cao, P. Anilkumar, X. Wang, J.-H. Liu, S. Sahu, M. J. Meziani, E. Myers and Y.-P. Sun, Can. J. Chem., 2011, 89, 104–109 CrossRef CAS PubMed.
- R. Shen, K. Song, H. R. Liu, Y. S. Li and H. W. Liu, ChemPhysChem, 2012, 13, 3549–3555 CrossRef CAS PubMed.
- X. Wang, L. Cao, S. T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y.-P. Sun, Angew. Chem., Int. Ed., 2010, 49, 5310–5314 CrossRef CAS PubMed.
- P. Anilkumar, L. Cao, J. J. Yu, K. N. Tackett 2nd, P. Wang, M. J. Meziani and Y.-P. Sun, Small, 2013, 9, 545–551 CrossRef CAS PubMed.
- Y. Q. Dong, R. X. Wang, H. Li, J. W. Shao, Y. W. Chi, X. M. Lin and G. N. Chen, Carbon, 2012, 50, 2810–2815 CrossRef CAS PubMed.
- X. L. Huang, F. Zhang, L. Zhu, K. Y. Choi, N. Guo, J. X. Guo, K. Tackett, P. Anilkumar, G. Liu, Q. M. Quan, H. S. Choi, G. Niu, Y.-P. Sun, S. Lee and X. Y. Chen, ACS Nano, 2013, 7, 5684–5693 CrossRef CAS PubMed.
- F. Li, C. J. Liu, J. Yang, Z. Wang, W. G. Liu and F. Tian, RSC Adv., 2014, 4, 3201–3205 RSC.
- H. Goncalves, P. A. S. Jorge, J. R. A. Fernandes and J. C. G. Esteves da Silva, Sens. Actuators, B, 2010, 145, 702–707 CrossRef CAS PubMed.
- H. M. Goncalves, A. J. Duarte and J. C. E. da Silva, Biosens. Bioelectron., 2010, 26, 1302–1306 CrossRef CAS PubMed.
- S. Barman and M. Sadhukhan, J. Mater. Chem., 2012, 22, 21832–21837 RSC.
- Y. Liu, C.-Y. Liu and Z.-y. Zhang, Appl. Surf. Sci., 2012, 263, 481–485 CrossRef CAS PubMed.
- F. Y. Yan, Y. Zou, M. Wang, X. L. Mu, N. Yang and L. Chen, Sens. Actuators, B, 2014, 192, 488–495 CrossRef CAS PubMed.
- L. D. Lu, R. Helgeson, R. M. Jones, D. McBranch and D. Whitten, J. Am. Chem. Soc., 2002, 124, 483–488 CrossRef CAS PubMed.
- J. M. Liu, L. P. Lin, X. X. Wang, S. Q. Lin, W. L. Cai, L. H. Zhang and Z. Y. Zheng, Analyst, 2012, 137, 2637–2642 RSC.
- Y. Q. Dong, R. X. Wang, G. L. Li, C. Q. Chen, Y. W. Chi and G. N. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- A. Salinas-Castillo, M. Ariza-Avidad, C. Pritz, M. Camprubi-Robles, B. Fernandez, M. J. Ruedas-Rama, A. Megia-Fernandez, A. Lapresta-Fernandez, F. Santoyo-Gonzalez, A. Schrott-Fischer and L. F. Capitan-Vallvey, Chem. Commun., 2013, 49, 1103–1105 RSC.
- D. Zhao, C. Q. Zhao, M. Li, J. S. Ren and X. G. Qu, Anal. Chim. Acta, 2014, 809, 128–133 CrossRef PubMed.
- Z. S. Qian, J. J. Ma, X. Y. Shan, H. Feng, L. X. Shao and J. R. Chen, Chem. – Eur. J., 2014, 20, 2254–2263 CrossRef CAS PubMed.
- X. M. Lin, G. M. Gao, L. Y. Zheng, Y. W. Chi and G. N. Chen, Anal. Chem., 2014, 86, 1223–1228 CrossRef CAS PubMed.
- X. J. Liu, N. Zhang, T. Bing and D. H. Shangguan, Anal. Chem., 2014, 86, 2289–2296 CrossRef CAS PubMed.
- S. R. Zhang, Q. Wang, G. H. Tian and H. G. Ge, Mater. Lett., 2014, 115, 233–236 CrossRef CAS PubMed.
- Y.-L. Zhang, L. Wang, H.-C. Zhang, Y. Liu, H.-Y. Wang, Z.-H. Kang and S.-T. Lee, RSC Adv., 2013, 3, 3733–3738 RSC.
- K. G. Qu, J. S. Wang, J. S. Ren and X. G. Qu, Chem. – Eur. J., 2013, 19, 7243–7249 CrossRef CAS PubMed.
- S. S. Wee, Y. H. Ng and S. M. Ng, Talanta, 2013, 116, 71–76 CrossRef CAS PubMed.
- M. Zheng, Z. G. Xie, D. Qu, D. Li, P. Du, X. B. Jing and Z. C. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 13242–13247 CAS.
- F. K. Du, Y. H. Ming, F. Zeng, C. M. Yu and S. Z. Wu, Nanotechnology, 2013, 24, 365101–365109 CrossRef PubMed.
- H. X. Zhao, L. Q. Liu, Z. D. Liu, Y. Wang, X. J. Zhao and C. Z. Huang, Chem. Commun., 2011, 47, 2604–2606 RSC.
- Y. Q. Dong, R. X. Wang, W. R. Tian, Y. W. Chi and G. N. Chen, RSC Adv., 2014, 4, 3701–3705 RSC.
- J. M. Liu, L. P. Lin, X. X. Wang, L. Jiao, M. L. Cui, S. L. Jiang, W. L. Cai, L. H. Zhang and Z. Y. Zheng, Analyst, 2013, 138, 278–283 RSC.
- X. F. Hou, F. Zeng, F. K. Du and S. Z. Wu, Nanotechnology, 2013, 24, 335502–335510 CrossRef PubMed.
- D. Yin, J. H. Deng, X. Peng, Q. Long, J. N. Zhao, Q. J. Lu, Q. Chen, H. T. Li, H. Tang, Y. Y. Zhang and S. Z. Yao, Analyst, 2013, 138, 6551–6557 RSC.
- F. K. Du, F. Zeng, Y. H. Ming and S. Z. Wu, Microchim. Acta, 2013, 180, 453–460 CrossRef CAS.
- R. X. Wang, G. L. Li, Y. Q. Dong, Y. W. Chi and G. N. Chen, Anal. Chem., 2013, 85, 8065–8069 CrossRef CAS PubMed.
- Y. Zhou, Z. B. Qu, Y. B. Zeng, T. S. Zhou and G. Y. Shi, Biosens. Bioelectron., 2014, 52, 317–323 CrossRef CAS PubMed.
- A. Cayuela, M. L. Soriano and M. Valcarcel, Anal. Chim. Acta, 2013, 804, 246–251 CrossRef CAS PubMed.
- Z. Lin, W. Xue, H. Chen and J.-M. Lin, Chem. Commun., 2012, 48, 1051–1053 RSC.
- Y. Q. Dong, C. Q. Chen, J. P. Lin, N. N. Zhou, Y. W. Chi and G. N. Chen, Carbon, 2013, 56, 12–17 CrossRef CAS PubMed.
- Z. Lin, W. Xue, H. Chen and J.-M. Lin, Anal. Chem., 2011, 83, 8245–8251 CrossRef CAS PubMed.
- J. X. Shi, C. Lu, D. Yan and L. N. Ma, Biosens. Bioelectron., 2013, 45, 58–64 CrossRef CAS PubMed.
- J. Z. Li, N. Y. Wang, T. T. T. Thanh, C. A. Huang, L. Chen, L. J. Yuan, L. P. Zhou, R. Shen and Q. Y. Cai, Analyst, 2013, 138, 2038–2043 RSC.
- Y. Xu, M. Wu, X.-Z. Feng, X.-B. Yin, X.-W. He and Y.-K. Zhang, Chem. – Eur. J., 2013, 19, 6282–6288 CrossRef CAS PubMed.
- G. A. Posthuma-Trumpie, J. H. Wichers, M. Koets, L. B. J. M. Berendsen and A. van Amerongen, Anal. Bioanal. Chem., 2012, 402, 593–600 CrossRef CAS PubMed.
- J. Gordon and G. Michel, Clin. Chem., 2008, 54, 1250–1251 CAS.
- D. Bu, H. S. Zhuang, G. X. Yang and X. X. Ping, Sens. Actuators, B, 2014, 195, 540–548 CrossRef CAS PubMed.
- T. Hyotylainen and K. Hartonen, Trends Anal. Chem., 2002, 21, 13–30 CrossRef CAS.
- H. L. Li, Y. W. Zhang, L. Wang, J. Q. Tian and X. P. Sun, Chem. Commun., 2011, 47, 961–963 RSC.
- P. Demchenko, J. Fluoresc., 2010, 20, 1099–1128 CrossRef PubMed.
- L. Xu, C. Q. Zhao, W. L. Wei, J. S. Ren, D. Miyoshi, N. Sugimoto and X. G. Qu, Analyst, 2012, 137, 5483–5486 RSC.
- N. Na, T. T. Liu, S. H. Xu, Y. Zhang, D. C. He, L. Y. Huang and J. Ouyang, J. Mater. Chem. B, 2013, 1, 787–792 RSC.
- T. K. Mandal and N. Parvin, J. Biomed. Nanotechnol., 2011, 7, 846–848 CrossRef CAS PubMed.
- J. J. Niu and H. Gao, J. Lumin., 2014, 149, 159–162 CrossRef CAS PubMed.
- Q. T. Huang, S. R. Hu, H. Q. Zhang, J. H. Chen, Y. S. He, F. M. Li, W. Weng, J. C. Ni, X. X. Bao and Y. Lin, Analyst, 2013, 138, 5417–5423 RSC.
- Y. Mao, Y. Bao, D. X. Han, F. H. Li and L. Niu, Biosens. Bioelectron., 2012, 38, 55–60 CrossRef CAS PubMed.
- S. Liu, J. Q. Tian, L. Wang, Y. L. Luo and X. P. Sun, RSC Adv., 2012, 2, 411–413 RSC.
- Y. Wang, P. Anilkumar, L. Cao, J.-H. Liu, P. G. Luo, K. N. Tackett, II, S. Sahu, P. Wang, X. Wang and Y.-P. Sun, Exp. Biol. Med., 2011, 236, 1231–1238 CrossRef CAS PubMed.
- B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, V. Georgakilas and E. P. Giannelis, Chem. Mater., 2008, 20, 4539–4541 CrossRef.
- M. Yu, X. Z. Li, F. Zeng, F. Y. Zheng and S. Z. Wu, Chem. Commun., 2013, 49, 403–405 RSC.
- S. Chen, Z.-J. Chen, W. Ren and H.-W. Ai, J. Am. Chem. Soc., 2012, 134, 9589–9592 CrossRef CAS PubMed.
- P.-C. Hsu, P.-C. Chen, C.-M. Ou, H.-Y. Chang and H.-T. Chang, J. Mater. Chem. B, 2013, 1, 1774–1781 RSC.
- Z.-A. Qiao, Y. F. Wang, Y. Gao, H. W. Li, T. Y. Dai, Y. L. Liu and Q. S. Huo, Chem. Commun., 2010, 46, 8812–8814 RSC.
- F. Wang, Z. Xie, H. Zhang, C.-Y. Liu and Y.-G. Zhang, Adv. Funct. Mater., 2011, 21, 1027–1031 CrossRef CAS.
- C. Fowley, B. McCaughan, A. Devlin, I. Yildiz, F. M. Raymo and J. F. Callan, Chem. Commun., 2012, 48, 9361–9363 RSC.
- L. M. Hu, Y. Sun, S. L. Li, X. L. Wang, K. L. Hu, L. R. Wang, X.-J. Liang and Y. Wu, Carbon, 2014, 67, 508–513 CrossRef CAS PubMed.
- S. Santra, H. Yang, J. T. Stanley, P. H. Holloway, B. M. Moudgil, G. Walter and R. A. Mericle, Chem. Commun., 2005, 3144–3146 RSC.
- M. Stroh, J. P. Zimmer, D. G. Duda, T. S. Levchenko, K. S. Cohen, E. B. Brown, D. T. Scadden, V. P. Torchilin, M. G. Bawendi, D. Fukumura and R. K. Jain, Nat. Med., 2005, 11, 678–682 CrossRef CAS PubMed.
- C. J. Liu, P. Zhang, X. Y. Zhai, F. Tian, W. C. Li, J. H. Yang, Y. Liu, H. B. Wang, W. Wang and W. G. Liu, Biomaterials, 2012, 33, 3604–3613 CrossRef CAS PubMed.
- C. J. Liu, P. Zhang, F. Tian, W. C. Li, F. Li and W. G. Liu, J. Mater. Chem., 2011, 21, 13163–13167 RSC.
- H. Ding, L.-W. Cheng, Y.-Y. Ma, J.-L. Kong and H.-M. Xiong, New J. Chem., 2013, 37, 2515–2520 RSC.
- L. Cao, S.-T. Yang, X. Wang, P. G. Luo, J.-H. Liu, S. Sahu, Y. M. Liu and Y.-P. Sun, Theranostics, 2012, 2, 295–301 CrossRef CAS PubMed.
- H. Q. Tao, K. Yang, Z. Ma, J. M. Wan, Y. J. Zhang, Z. H. Kang and Z. Liu, Small, 2012, 8, 281–290 CrossRef CAS PubMed.
- D. Bechet, P. Couleaud, C. Frochot, M.-L. Viriot, F. Guillemin and M. Barberi-Heyob, Trends Biotechnol., 2008, 26, 612–621 CrossRef CAS PubMed.
- K. Yang, H. Gong, X. Z. Shi, J. M. Wan, Y. J. Zhang and Z. Liu, Biomaterials, 2013, 34, 2787–2795 CrossRef CAS PubMed.
- P. Juzenas, A. Kleinauskas, P. G. Luo and Y.-P. Sun, Appl. Phys. Lett., 2013, 103, 063701 CrossRef PubMed.
- S. N. Frank and A. J. Bard, J. Phys. Chem., 1977, 81, 1484–1488 CrossRef CAS.
- K. P. Nielsen, A. Juzeniene, P. Juzenas, K. Stamnes, J. J. Stamnes and J. Moan, Photochem. Photobiol., 2005, 81, 1190–1194 CrossRef CAS PubMed.
- P. Huang, J. Lin, X. S. Wang, Z. Wang, C. L. Zhang, M. He, K. Wang, F. Chen, Z. M. Li, G. X. Shen, D. X. Cui and X. Y. Chen, Adv. Mater., 2012, 24, 5104–5110 CrossRef CAS PubMed.
- C. Fowley, N. Nomikou, A. P. McHale, B. McCaughana and J. F. Callan, Chem. Commun., 2013, 49, 8934–8936 RSC.
- A. Kleinauskas, S. Rocha, S. Sahu, Y.-P. Sun and P. Juzenas, Nanotechnology, 2013, 24, 325103–325112 CrossRef CAS PubMed.
- V. Kumar, G. Toffoli and F. Rizzolio, ACS Med. Chem. Lett., 2013, 4, 1012–1013 CrossRef CAS PubMed.
- W. T. Godbey, K. K. Wu and A. G. Mikos, J. Controlled Release, 1999, 60, 149–160 CrossRef CAS.
- A. Alkilany and C. J. Murphy, J. Nanopart. Res., 2010, 12, 2313–2333 CrossRef CAS PubMed.
- E. Dulkeith, A. C. Morteani, T. Niedereichholz, T. A. Klar, J. Feldmann, S. A. Levi, F. C. J. M. van Veggel, D. N. Reinhoudt, M. Moller and D. I. Gittins, Phys. Rev. Lett., 2002, 89, 203002 CrossRef CAS.
- M. Zheng, S. Liu, J. Li, D. Qu, H. F. Zhao, X. G. Guan, X. L. Hu, Z. G. Xie, X. B. Jing and Z. C. Sun, Adv. Mater., 2014, 26, 3554–3560 CrossRef CAS PubMed.
- S. Karthik, B. Saha, S. K. Ghosh and N. D. P. Singh, Chem. Commun., 2013, 49, 10471–10473 RSC.
- N. Gogoi and D. Chowdhury, J. Mater. Chem. B, 2014, 2, 4089–4099 RSC.
- L. Zhou, Z. H. Li, Z. Liu, J. S. Ren and X. G. Qu, Langmuir, 2013, 29, 6396–6403 CrossRef CAS PubMed.
- A. Mewada, S. Pandey, M. Thakur, D. Jadhav and M. Sharon, J. Mater. Chem. B, 2014, 2, 698–705 RSC.
- A. Kamen and A. Capdevila, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 5983–5987 CrossRef.
- C.-W. Lai, Y.-H. Hsiao, Y.-K. Peng and P.-T. Chou, J. Mater. Chem., 2012, 22, 14403–14409 RSC.
- T. T. Wang, F. Chai, Q. Fu, L. Y. Zhang, H. Y. Liu, L. Li, Y. Liao, Z. M. Su, C. G. Wang, B. Y. Duan and D. X. Ren, J. Mater. Chem., 2011, 21, 5299–5306 RSC.
- S. Kumar, T. Ye, T. Takami, B. C. Yu, A. K. Flatt, J. M. Tour and P. S. Weiss, Nano Lett., 2008, 8, 1644–1648 CrossRef PubMed.
- M. Zhang, Q. Wang, C. C. Chen, L. Zang, W. H. Ma and J. C. Zhao, Angew. Chem., Int. Ed., 2009, 48, 6081–6084 CrossRef CAS PubMed.
- Q. Wang, M. Zhang, C. C. Chen, W. H. Ma and J. C. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7976–7979 CrossRef CAS PubMed.
- Z.-R. Tang, Y. H. Zhang and Y.-J. Xu, ACS Appl. Mater. Interfaces, 2012, 4, 1512–1520 CAS.
- H. T. Li, R. H. Liu, S. Y. Lian, Y. Liu, H. Huang and Z. H. Kang, Nanoscale, 2013, 5, 3289–3297 RSC.
- Z. Ma, H. Ming, H. Huang, Y. Liu and Z. H. Kang, New J. Chem., 2012, 36, 861–864 RSC.
- S. L. Hu, R. X. Tian, Y. G. Dong, J. L. Yang, J. Liu and Q. Chang, Nanoscale, 2013, 5, 11665–11671 RSC.
- S. L. Hu, R. X. Tian, L. L. Wu, Q. Zhao, J. L. Yang, J. Liu and S. R. Cao, Chem. – Asian J., 2013, 8, 1035–1041 CrossRef CAS PubMed.
- H. T. Li, R. H. Liu, W. Q. Kong, J. Liu, Y. Liu, L. Zhou, X. Zhang, S.-T. Lee and Z. H. Kang, Nanoscale, 2014, 6, 867–873 RSC.
- R. H. Liu, H. Huang, H. T. Li, Y. Liu, J. Zhong, Y. Y. Li, S. Zhang and Z. H. Kang, ACS Catal., 2014, 4, 328–336 CrossRef CAS.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- Z. H. Kang, Y. Liu, C. H. A. Tsang, D. D. D. Ma, X. Fan, N.-B. Wong and S.-T. Lee, Adv. Mater., 2009, 21, 661–664 CrossRef CAS.
- Z. H. Kang, C. H. A. Tsang, Z. D. Zhang, M. L. Zhang, N.-B. Wong, J. A. Zapien, Y. Y. Shan and S.-T. Lee, J. Am. Chem. Soc., 2007, 129, 5326–5527 CrossRef CAS PubMed.
- Z. H. Kang, C. H. A. Tsang, N.-B. Wong, Z. D. Zhang and S.-T. Lee, J. Am. Chem. Soc., 2007, 129, 12090–12091 CrossRef CAS PubMed.
- Y. Yao, G. H. Li, S. Ciston, R. M. Lueptow and K. A. Gray, Environ. Sci. Technol., 2008, 42, 4952–4957 CrossRef CAS.
- M. X. Sun, X. Q. Ma, X. Chen, Y. J. Sun, X. L. Cui and Y. H. Lin, RSC Adv., 2014, 4, 1120–1127 RSC.
- X. J. Yu, J. J. Liu, Y. C. Yu, S. L. Zuo and B. S. Li, Carbon, 2014, 68, 718–724 CrossRef CAS PubMed.
- H. C. Zhang, H. Ming, S. Y. Lian, H. Huang, H. T. Li, L. L. Zhang, Y. Liu, Z. H. Kang and S.-T. Lee, Dalton Trans., 2011, 40, 10822–10825 RSC.
- B. Y. Yu and S.-Y. Kwak, J. Mater. Chem., 2012, 22, 8345–8353 RSC.
- H. Yu, H. C. Zhang, H. Huang, Y. Liu, H. T. Li, H. Ming and Z. H. Kang, New J. Chem., 2012, 36, 1031–1035 RSC.
- H. T. Li, R. H. Liu, Y. Liu, H. Huang, H. Yu, H. Ming, S. Y. Lian, S.-T. Lee and Z. H. Kang, J. Mater. Chem., 2012, 22, 17470–17475 RSC.
- H. C. Zhang, H. Huang, H. Ming, H. T. Li, L. L. Zhang, Y. Liu and Z. K. Kang, J. Mater. Chem., 2012, 22, 10501–10506 RSC.
- X. Han, Y. Z. Han, H. Huang, H. C. Zhang, X. Zhang, R. H. Liu, Y. Liu and Z. H. Kang, Dalton Trans., 2013, 42, 10380–10383 RSC.
- X. Zhang, F. Wang, H. Huang, H. T. Li, X. Han, Y. Liu and Z. H. Kang, Nanoscale, 2013, 5, 2274–2278 RSC.
- X. Zhang, H. Huang, J. Liu, Y. Liu and Z. H. Kang, J. Mater. Chem. A, 2013, 1, 11529–11533 CAS.
- X. L. Yu, R. J. Liu, G. J. Zhang and H. B. Cao, Nanotechnology, 2013, 24, 335401–335407 CrossRef PubMed.
- Z. Ma, Y.-L. Zhang, L. Wang, H. Ming, H. T. Li, X. Zhang, F. Wang, Y. Liu, Z. H. Kang and S.-T. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 5080–5084 CAS.
- Y.-Q. Zhang, D.-K. Ma, Y.-G. Zhang, W. Chen and S.-M. Huang, Nano Energy, 2013, 2, 545–552 CrossRef CAS PubMed.
- Morozan and F. Jaouen, Energy Environ. Sci., 2012, 5, 9269–9290 CAS.
- S. B. Yang, X. L. Feng, X. C. Wang and K. Müllen, Angew. Chem., Int. Ed., 2011, 50, 5339–5343 CrossRef CAS PubMed.
- Y. Li, Y. Zhao, H. H. Cheng, Y. Hu, G. Q. Shi, L. M. Dai and L. T. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- R. Yan, H. Wu, Q. Zheng, J. Y. Wang, J. L. Huang, K. J. Ding, Q. G. Guo and J. Z. Wang, RSC Adv., 2014, 4, 23097–23106 RSC.
- Y. Liu and P. Y. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 3362–3369 CAS.
- W. A. Saidi, J. Phys. Chem. Lett., 2013, 4, 4160–4165 CrossRef CAS.
- C. Z. Zhu, J. F. Zhai and S. J. Dong, Chem. Commun., 2012, 48, 9367–9369 RSC.
- D. Tang, J. Liu, X. Y. Wu, R. H. Liu, X. Han, Y. Z. Han, H. Huang, Y. Liu and Z. H. Kang, ACS Appl. Mater. Interfaces, 2014, 6, 7918–7925 CAS.
- T. A. Waldmann, Nat. Med., 2003, 9, 269–277 CrossRef CAS PubMed.
- N. Murthy, Y. X. Thng, S. Schuck, M. C. Xu and J. M. J. Frechet, J. Am. Chem. Soc., 2002, 124, 12398–12399 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |