Role of zinc and copper metal ions in amyloid β-peptides Aβ1–40 and Aβ1–42 aggregation†
Subramaniam Boopathi and
Ponmalai Kolandaivel*
Department of Physics, Bharathiar University, Coimbatore-46, India. E-mail: Ponkvel@hotmail.com; Fax: +91-422-2422387
First published on 14th August 2014
Abstract
The aggregation of amyloid β-peptides (Aβ) plays a key role in the central pathological pathways in Alzheimer's disease. The interaction of zinc and copper metal ions with Aβ-peptide gives considerable understanding to fight this incurable disease. The different conformations of native and metal interacting Aβ-peptides were investigated using molecular dynamics simulations for 50 ns. Aβ1–40–Zn2+ peptide adopts a β-hairpin structure, which was promoted by the turn region (Val24-Asp27) and increases the hydrophobic contact between the central hydrophobic (CHC) (Leu17-Ala21) and c-terminal (Met35-Val40). The turn region was stabilized by forming a salt-bridge between Asp23-Lys28 and Glu22-Lys28 residues. The structure of the Aβ1–42–Zn2+ peptide was destabilized by the β-hairpin structure due to the absence of the salt-bridge, and reduces the hydrophobic contact between CHC and the c-terminal, which results in Aβ1–42 monomer disaggregation. The Aβ1–40–Cu2+ peptide has three bend regions, which are separated by a coil segment. These segments reduce the hydrophobic contact between CHC and the c-terminal, and promote the formation of a salt-bridge which in turn destabilizes the turn region of the peptide. The hairpin conformation of the Aβ1–42–Cu2+ structure was stabilized by increasing the hydrophobic contact and the salt-bridge which is facilitated for aggregation.
1. Introduction
Aggregation of amyloid β-peptide (Aβ) is considered as a hallmark for the progression of Alzheimer's disease (AD) which is a debilitating neurodegenerative disorder. It affects about 5.4 million Americans and 25 million people worldwide, and these numbers are expected to increase dramatically.1 Epidemiological studies have found that the incident of AD among people in their 70s was about 4 to 5 times lesser in India than in US.2,3 The patient with AD, lose memory, slow in communication, and experience problems with visual-spatial search, along with other side effects.4–6 Amyloid β-peptide (Aβ) is derived from the proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretase enzymes. Aβ-peptide contains 40, and 42 amino acid residues whose progression appeared from a unstructured monomer to hairpin structure. The fibrillar aggregate associated with onset to Alzheimer's disease.4,5 Recent investigations have reported that besides the amyloid plaques with characteristic cross β-pattern structure, neurotoxicity can be induced by formation of oligomers of the Aβ-peptides.7–10 In AD brains, accumulation of misfolded Aβ peptides and ptau proteins were observed.1 It is still not clear, how these misfolded aggregates are involved in the onset and progression of disease. Kodali et al. reported that the five Aβ1–40 peptide structures have formed the fibril with β-sheet.11 Solid state NMR measurement on Aβ1–40 and Aβ1–42 amyloid fibrils reveals the arrangement of parallel β-sheet within the protofilaments.8 The major difference between the Aβ1–40 and Aβ1–42 peptides is the absence or presence of c-terminal dipeptides (e.g. two hydrophobic residues Ile41-Ala42). The Aβ peptides have the following primary structure:| DAEFR5HDSGY10EVHHQ15KLVFF20AEDVG25SNKGA30IIGLM35VGGVV40IA42 |
The previous studies suggest that three distinct regions of the Aβ monomer, the central hydrophobic core (Leu17-Ala21), a turn region (Val24-Asp27), and second hydrophobic region (Gly29-Met35) play an important role in aggregation process.9–15 Fluorescence resonance energy transfer study indicates that Aβ1–40 forms the stable monomer, dimmer, trimmer and tetramer, where as Aβ1–42 forms the stable pentamer, hexamer and other larger assemblies.16,17 Cryo-electron microscopy studies have shown that the complex polymorphism of Aβ fibrils characterised by size, cross section and width.18,19 The salt bridge between Asp23 and Lys28 stabilizes the β-turn and, therefore, the entire β-hairpin structure promotes the nucleation for fibril growth.20 Experimental and computational studies have addressed the mechanism of folding and oligomerization for Aβ1–40 and Aβ1–42 peptides.7–10,21–28 Even though these studies offer precise information on the average structure, the atomic level knowledge on Aβ1–40 and Aβ1–42 monomers are essential to understand the aggregation process since they are the building blocks for larger oligomers. Normally, Aβ-peptide exists as a α-helix along with some random coil structure, but can be misfold into a β-sheet, which aggregates into amyloid oligomers leading to insoluble amyloid fibrils. The mechanism of this misfolding of Aβ peptide has not yet been understood properly. Many research groups have put their efforts to study the interaction of metal ions with monomeric Aβ peptide, and they try to correlate this with amyloid plaque formation.29–36
The structure of Aβ–Zn2+,37,38 and Aβ–Cu2+ have been investigated in detail by NMR,30–33 X-ray absorption spectroscopy,34,35 and Fourier transform infrared spectroscopy.36 Solid state NMR results help to identify the Zn2+ binding sites to characterise the critical structural changes induced by Zn2+ binding to the pathologically relevant Aβ1–42 aggregates.31,33 Mithu et al. have identified that the Zn2+ binding breaks the salt-bridge between Asp23 and Lys28 residues by driving these residues into non salt-bridge conformation, where the unperturbed cross β-structure of Aβ1–42 aggregates and increased the toxicity.33 Hane et al.39 have concluded that Cu and Cu2+ ions play a significant role to increase the binding between the two single Aβ-peptides. Nair et al.40 have found that copper binds with His residue with greater affinity than zinc, which significantly stabilizes the Aβ aggregation. High resolution structural studies of amyloid peptide by NMR experiment confirm the zinc binding with His14, which induces the localized disruption of the secondary structure of amyloid peptide.32 In AD, the concentration of Cu and Zn ions increased in plaques and reaches to 400 μM and 1 mM, respectively compared with the region outside the plaque.41 The metal–Aβ interactions have been used to test therapeutic agents, and these efforts have been made to understand the molecular details of Cu2+ and Zn2+ binding with Aβ.42,43 However, the role of metal ions in Aβ-peptide aggregation process still remains elusive. Detailed structural information would be highly valuable for revealing still unknown mechanisms of the Aβ aggregation.
Molecular dynamics (MD) simulation is a powerful tool to investigate the secondary structure of Aβ-peptides. Even though, the number of studies have been performed to investigate the structural insight of Aβ-peptides aggregation in the presence of metal ions, the process of aggregation of monomeric form of Aβ-peptide is not well understood. So, the main aim of the present study is to investigate the aggregation process of Aβ-peptide in the presence of metal ions by considering three different secondary structure regions (1) central hydrophobic core region (Leu17-Ala21), (2) loop region (Asp23-Lys28), (3) second hydrophobic region (Gly29-Met35) which are playing a major role for the aggregation. The hydrophobic interaction between the central hydrophobic core region and c-terminal, the formation of salt-bridge between Asp23(Glu22) and Lys28 residues play a critical role for the aggregation process. The metal interactions induce the significant changes in the hydrophobic interaction and salt-bridge. Further the change of hydrophobic, and hydrophilic characters of the residues promote the bend structure in Aβ peptide due to the interaction with water molecules. The dynamics of the hydrophobic interaction and salt-bridge of the Aβ peptide have been mapped by the classical molecular dynamics simulation in the presence of metal ions. The experimental observation of these informations are very difficult and sometimes impossible. So the present study helps to understand much better way the aggregation process of Aβ-peptide, which is missing in the literature.
2. Computational procedure
2.1 Molecular dynamics simulation
The initial structures of Aβ1–40 and Aβ1–42 monomers were taken from NMR study (Pdb ID: 1BA4,44 and 1IYT45). Metals (Cu2+, Zn2+) were allowed to interact with N-terminal of their monomers (Aβ1–40–Cu2+, Aβ1–40–Zn2+, Aβ1–42–Cu2+ and Aβ1–42–Zn2+). These monomers and their metal interacted complex were solvated in a water box separately. The solvent molecules were used with TIP3P water model.46,47 Each solvated structures were optimized by the molecular dynamics (MD) simulation package GROMACS 4.5.6 version utilizing the OPLSAA force field.48,49 Each structure was placed in the center of a box using three dimensional (3D) periodic boundary condition. Steepest descent minimization was performed for 500 steps prior to and after MD refinement. Equilibration was performed into two phases. In the first phase, a NVT ensemble applied for 50 ps using Berendsen weak coupling to quickly heated to 300 K.50 In the second phase, a NPT ensemble employed for 50 ps to maintain the constant temperature (300 K) and pressure (1 atm), where all atoms were allowed to move. The MD simulation was carried out under NPT ensemble condition and it ran for 50 ns and the structures were saved at each picosecond for analysis. The long range electrostatic interactions were calculated by particle mesh Ewald method.51 All the bond lengths were constrained using the leapfrog algorithm, allowing an integration time step of 2 fs. A non bonded cut off 1.0 nm was employed in the simulation and updated every 10 fs and all the short range non bonded interaction cut off at 1.4 nm.52 PyMol and Chimera were used to visualize the molecular structure and additional analyses were assisted by origin60, GNU image manipulation program.53,54 The trajectory files were analyzed through g_rmsd tool in GROMACS utilities to obtain root mean square deviation (RMSD). The secondary structure analyses were performed employing the secondary structures of protein (DSSP) protocol, which interfaces with GROMACS through do_dssp tool.55 To analyze the hydrogen bonds, a cutoff distance 0.35 nm has been fixed. The solvent accessible surface area (SASA) per residue was calculated to identify the hydrophobic and hydrophilic nature of each residue of monomers. The contact maps used for a pair of amino acid side chain considered to be formed when a minimal distance between any pair of their atoms is less than 1.5 nm. Independent simulation has been performed with different initial conformation for the Aβ-peptides in the presence of metal ions to verify the convergence of trajectories (Fig. S7†).3. Results and discussion
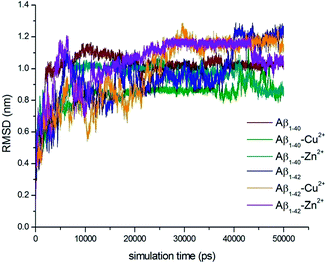
Full length of Aβ1–40 and Aβ1–42 monomers and their complex structures (Aβ1–40–Cu2+, Aβ1–40–Zn2+ and Aβ1–42–Cu2+, Aβ1–42–Zn2+) have been considered to explore the conformational dynamics of these peptides in aqueous solution for 50 ns MD simulation. The previous studies suggest that the simulation time should be sufficient to elucidate structural features of these peptides.56–59 The loop region Asp23-Lys28, the central hydrophobic core Leu17-Ala21 and second hydrophobic domain Gly29-Met35 of Aβ peptide are playing critical role in aggregation mechanisms.9–15,26 Which is the main thrust of the present study.Molecular dynamics simulations pay the way for the indepth analysis of the structural changes upon metal ion interactions with N-terminal of Aβ-peptides. The deviation between Aβ monomer and their complex structures were evaluated by root mean square deviation (RMSD) values, which have calculated for six trajectories of Aβ1–40, Aβ1–40–Cu2+, Aβ1–40–Zn2+, Aβ1–42, Aβ1–42–Cu2+ and Aβ1–42–Zn2+ as a function of time (Fig. 1). For all the six structures, considerable structural changes have been observed during the initial 500 ps lead to a RMSD 0.4–0.75 nm. When it reaches a stable value around 0.9–1.1 nm in Aβ1–40, the value of the trajectory decreases to 0.75–0.85 nm during the interaction of Cu2+. The Aβ1–40–Zn2+ structure attained the stable value around 0.85–1.0 nm during the simulation (0.5–50 ns). The final RMSD values have been observed around 0.9–1.2 nm for three structures i.e. Aβ1–42, Aβ1–42–Cu2+ and Aβ1–42–Zn2+. The above RMSD values help to understand the deviation of the minimum energy structure from the native state due to the interaction of metal ions (Aβ1–40–Cu2+, Aβ1–42–Zn2+).
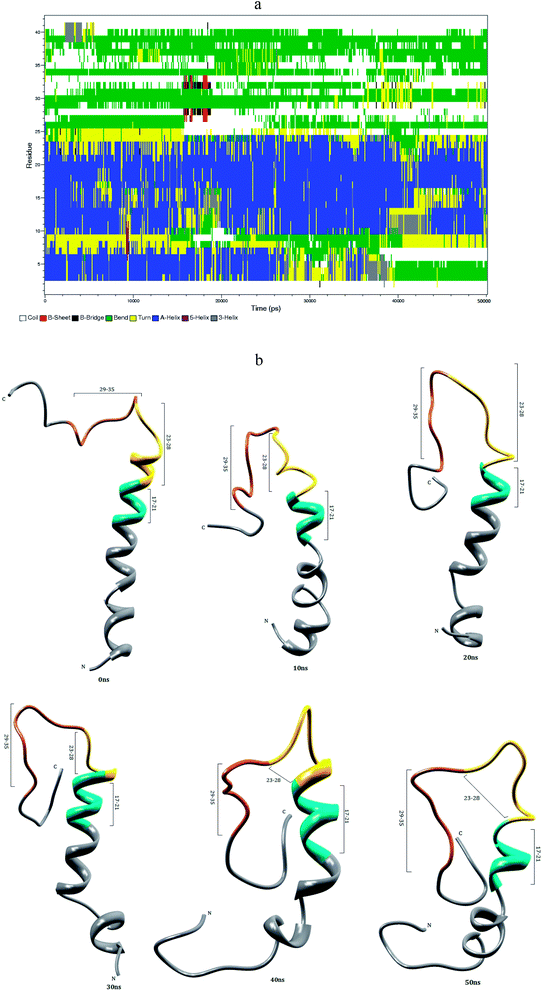
3.1 Aβ1–40 monomer conformational dynamics in aqueous solution
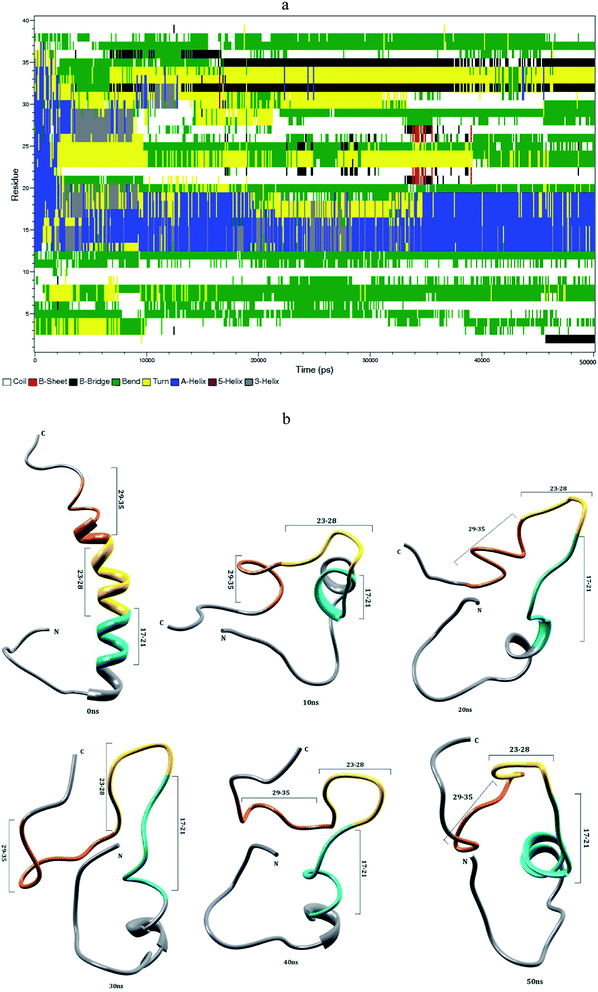
The helical structure of Aβ1–40,44 and its conformational changes have been monitored in aqueous solution using chimera visual software for every 10 ns (Fig. 2). The contact map (Fig. 8a) represents the interaction of His6-Gly9, Asp7-Gly9, Ala2-Arg5 and Tyr10-Gln15 residues. During the course of simulation, the Val12-Leu17 residues adopt alpha helical structure, His6 and Gly9 residues lead to the bend region (Fig. 8a), which promotes the first 12 residues adopt hairpin structure, tail region extended with Asp1-Arg5 and Tyr10-Val12 residues. The above interactions help to stabilize the β-hairpin structure in the region Asp1-Gln15 residues.Another twenty residues (Lys16-Met35) have been architectured by three important regions, the central hydrophobic core region (Leu17-Ala21), the loop region (Asp23-Lys28) and second hydrophobic region (Gly29-Met35). The central hydrophobic core (Leu17-Ala21) was completely transformed into β-bend structure during the 20–50 nanosecond simulation. We have performed a time dependent analysis for the salt-bridge to probe its effect on the stability of Aβ-peptide (Fig. S1a†). The salt-bridge distance was calculated as the average distance between the CO2− moiety of Asp23 (or Glu22) and the NH3+ in Lys28 residue in the Aβ peptide. The direct-salt-bridge appeared around 4.3 Å, whereas indirect or water mediated salt-bridge was appeared between 4.3 and 7.0 Å.56 We observed the higher distance between Asp23 and Lys28 residues which do not has a direct salt-bridge but the indirect salt-bridge was formed during the first twenty nanosecond simulation. Consequently, the direct salt-bridge was appeared between Glu22 and Lys28 residues during the 20–50 nanosecond simulation. The salt-bridge of Glu22-Lys28 residues is stronger than the indirect salt-bridge Asp23-Lys28 residues. This is due to the longer intra distance (4.3–8.5 Å) between the carboxyl group of Asp23 and amine group of Lys28 compared with solid state NMR model in which the distance is much shorter.20 These results are in contrast to the previous MD simulation, where the probability of forming an intra molecular salt-bridge between Asp23 and Lys28 (42%) is higher than that of the salt-bridge between Glu22 and Lys28 (36%).14 These interactions in the region Glu22-Lys28 maintains turn structure throughout the simulation.
The interaction between the hydrophobic part of the Lys28 side chain and Val24 residue stabilizes turn conformation, which occurs at a distance of 4.5 and 7.5 Å between Val24 and Asn27 residue. This conformation allows the interaction between the central hydrophobic core (Leu17-Ala21) and hydrophobic c-terminal. Consequently, the distance (Val24)-(Asn27) increases to 7.5–10.0 Å during 10–50 nanosecond simulation and the hydrophobic contact between the c-terminal and Leu17-Ala21 fragment is reduced (Fig. S3a†). The force between the electrostatic Asp23-Lys28 and hydrophobic Val24-Lys28 stabilize the turn region Val24-Asn27. The stable turn in the Val24-Asn27 domain favours hydrophobic contact between the hydrophobic core (Leu17-Ala21) and the c-terminal. These results agree with the Lazo et al.,60,61 who have studied the hydrophobic contact within the decapeptide Aβ21–30 using NMR study and MD simulation. The turn region plays a significant role in facilitating the formation of the bend in the Aβ peptide, which has been started from fifth nanosecond to the end of the simulation period. After 12 ns, the Phe19-Asp23 region and second hydrophobic region (Gly29-Met35) were converted into the turn and beta bridge structures (Fig. 2a). The bend region (Val24-Asn27) is necessary to facilitate the parallel architecture for long Aβ peptide, which agrees with the results of Tycko et al.8,22 who have measured NMR spectra for Aβ1–40. They have shown that the first β-sheet appeared after tenth residue. The contact map shows the significant interaction between the Phe4-Leu34, Phe4-Gly9, Gln15-Val18 and Ile31-Met35 residues. During the course of the simulation, the c-terminal (Met35-Val40) residues have been changed between coil and bend, and turn conformations. Our simulation result reveals that Aβ1–40 is composed of coil components (34%), helical content (18%), turn and bend conformation (43%) and β-structure (4%) in aqueous solution (Table 1).
| Aβ1–40 | Aβ1–40–Cu2+ | Aβ1–40–Zn2+ | Aβ1–42 | Aβ1–42–Cu2+ | Aβ1–42–Zn2+ | |
|---|---|---|---|---|---|---|
| Coil | 34 | 27 | 34 | 25 | 23 | 24 |
| Beta | 4 | 5 | 1 | — | 2 | 1 |
| Bend | 28 | 20 | 25 | 21 | 23 | 25 |
| Turn | 15 | 31 | 12 | 8 | 24 | 15 |
| Helix | 18 | 17 | 28 | 46 | 27 | 36 |
3.2 Interaction of Cu2+ with Aβ1–40 monomer in aqueous solution
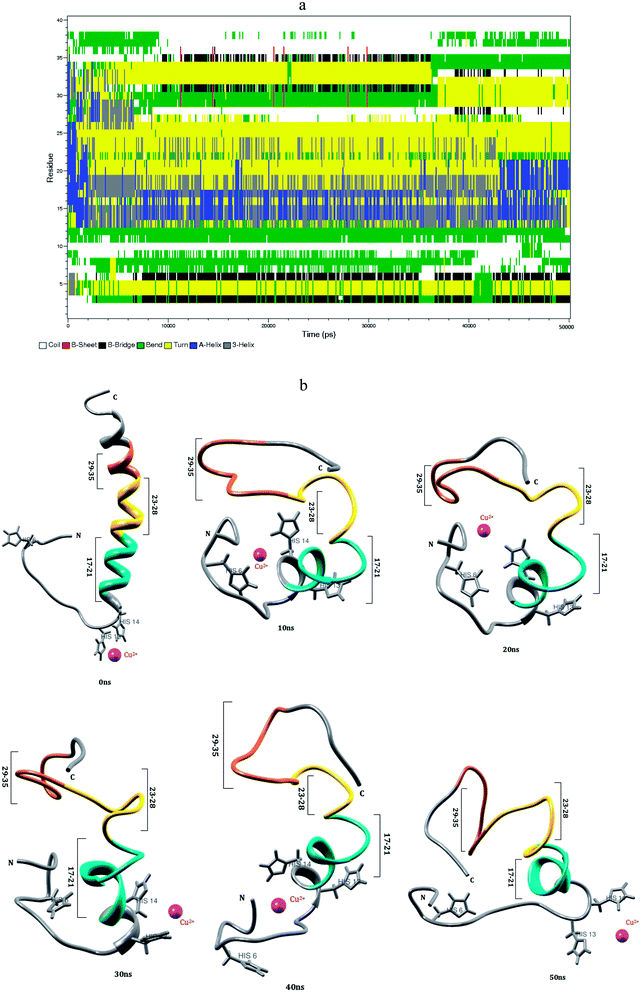
In human blood plasma, more than 98% of the amino acid bound with Cu2+ ion, which occurs in histidine complex.62 Cu2+ ion has higher affinity with three histidine residues of Aβ1–40 monomer.38 The conformational changes of Aβ1–40–Cu2+ structure have been monitored in the explicit water. The conformational structures for every 10 ns are displayed in Fig. 3. During the course of simulation, a significant difference has been noticed in Aβ1–40–Cu2+ structure. The NH of Glu3 residue and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of backbone form the β-sheet hydrogen bond with C
O group of backbone form the β-sheet hydrogen bond with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH groups of His6 residue and its distance changed from 0.2 to 0.25 nm. This interaction helps for the formation of first bend structure which appears in the Glu3-His6 region. The strong interaction has been observed between Ala2-His6 and Asp1-Asp7 residues, where the distances are varying between 0.15 and 0.25 nm (Fig. 8b). These interactions have played a significant role to stabilize the bend structure (Glu3-His6) which are helping to form a β-hairpin structure in the Asp1-Asp7 region.
O and NH groups of His6 residue and its distance changed from 0.2 to 0.25 nm. This interaction helps for the formation of first bend structure which appears in the Glu3-His6 region. The strong interaction has been observed between Ala2-His6 and Asp1-Asp7 residues, where the distances are varying between 0.15 and 0.25 nm (Fig. 8b). These interactions have played a significant role to stabilize the bend structure (Glu3-His6) which are helping to form a β-hairpin structure in the Asp1-Asp7 region.
The turn structure appears in the Asp23-Asp27 region (Fig. 3a), was stabilized by the strong hydrogen bond (0.2–0.3 nm) between Glu22 and Ser26 residues. The turn structure has attributed to the second bend structure in the Glu22-Lys28 region of Aβ1–40–Cu2+ structure. The region (Val18-Lys28) exhibits helical conformation in the first 2 ns, but after that, it completely transformed into turn structure during 2–45 ns simulation. The helical structure exhibited in the Val18-Gly25 region of the Aβ1–40–Zn2+ structure. The (Val24)-(Asn27) distance has been changed between 0.55 and 0.75 nm, and this interaction allows to increase the hydrophobic contact between the central hydrophobic core (Leu17-Ala21) and the c-terminal (Met35-Val40) region (Fig. S3a†). The salt-bridge distance calculated between Asp23 and Lys28 residues is around 0.2–0.8 nm during the first 10 ns simulation. The salt-bridge disappeared after 10 ns simulation, but the indirect salt-bridge has been formed between Glu22 and Lys28 residues. The absence of salt-bridge between Asp23 and Lys28 residues destabilize the turn structure, and contributed to depromote the β-hairpin conformation of Aβ1–40–Cu2+ structure, which agrees with previous MD simulation of Aβ1–40–Met35(O) monomer.60
The third bend region appears in the second hydrophobic region (Gly29-Met35). Gly29-Ile32 region undergoes a conformational change between turn and bend structures, which was stabilized by the interaction between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of Ile32 and NH group of Lys28 residues and the distance is around 0.3–0.35 nm. Subsequently, the c-terminal region (Met35-Val40) exhibits the coil structure throughout the simulation. The calculated SASA values (Fig. S4†) reflect the increase in the hydrophobic character of Phe4, Leu34 and Met35 residues in Aβ1–40–Cu2+ structure as compared with Aβ1–40–Zn2+ and Aβ1–40 structures. The differences in the SASA values between Aβ1–40–Cu2+ and Aβ1–40 structures (Fig. S5†), the positive and negative values represent the increase and decrease in the hydrophobic character. The difference in SASA value per residue indicate the increase of the hydrophobicity of Phe4, Leu34 and Met35 residues and increase the hydrophilic character of His6, Asp23, Lys28 and Ile32 residues. Among these, His6 residue is more exposed to the interaction with water which may be the reason to form first bend structure in the N-terminal of monomeric structure. The influence of hydrophobic/hydrophilic character on loop and SHC region, promote the bend structure during the interaction of Aβ1–40–Cu2+ in aqueous solution. However, due to the hydrogen bonds between the side chains of Asp23 and Lys28 residues, and the surrounding water molecules, the salt-bridge between Asp23-Lys28 residues get disrupted. In comparison with Aβ1–40–Zn2+, the Aβ1–40–Cu2+ structure possesses 11% less helical conformation, 7% less coil conformation, 5% less bend conformation, 4% more beta structure and 19% more turn conformation (Table 1). This shows that the helical structures were reduced, turn structure was destabilized and hydrophobic contact was reduced between SHC and C-terminal region of Aβ1–40–Cu2+ structure.
O group of Ile32 and NH group of Lys28 residues and the distance is around 0.3–0.35 nm. Subsequently, the c-terminal region (Met35-Val40) exhibits the coil structure throughout the simulation. The calculated SASA values (Fig. S4†) reflect the increase in the hydrophobic character of Phe4, Leu34 and Met35 residues in Aβ1–40–Cu2+ structure as compared with Aβ1–40–Zn2+ and Aβ1–40 structures. The differences in the SASA values between Aβ1–40–Cu2+ and Aβ1–40 structures (Fig. S5†), the positive and negative values represent the increase and decrease in the hydrophobic character. The difference in SASA value per residue indicate the increase of the hydrophobicity of Phe4, Leu34 and Met35 residues and increase the hydrophilic character of His6, Asp23, Lys28 and Ile32 residues. Among these, His6 residue is more exposed to the interaction with water which may be the reason to form first bend structure in the N-terminal of monomeric structure. The influence of hydrophobic/hydrophilic character on loop and SHC region, promote the bend structure during the interaction of Aβ1–40–Cu2+ in aqueous solution. However, due to the hydrogen bonds between the side chains of Asp23 and Lys28 residues, and the surrounding water molecules, the salt-bridge between Asp23-Lys28 residues get disrupted. In comparison with Aβ1–40–Zn2+, the Aβ1–40–Cu2+ structure possesses 11% less helical conformation, 7% less coil conformation, 5% less bend conformation, 4% more beta structure and 19% more turn conformation (Table 1). This shows that the helical structures were reduced, turn structure was destabilized and hydrophobic contact was reduced between SHC and C-terminal region of Aβ1–40–Cu2+ structure.
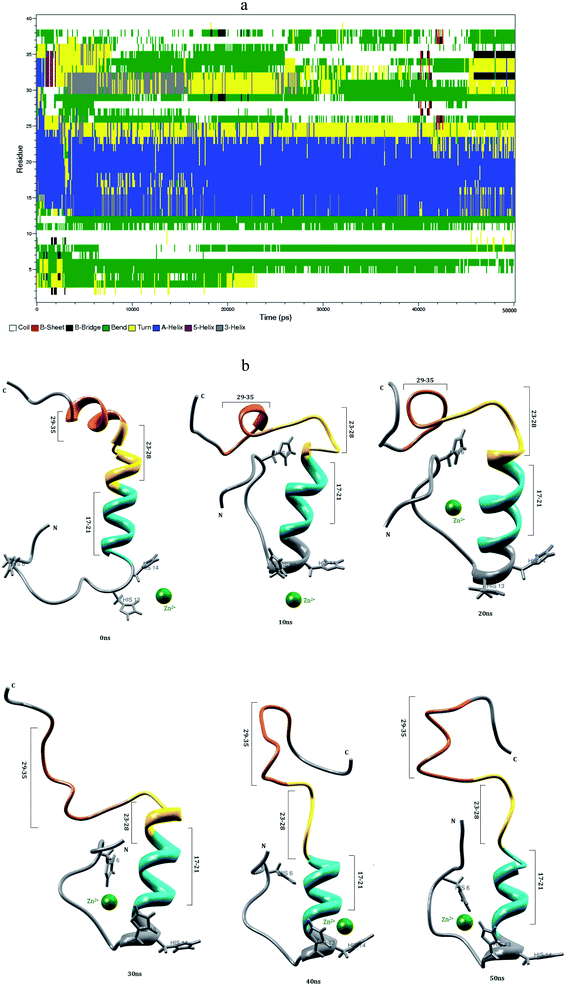
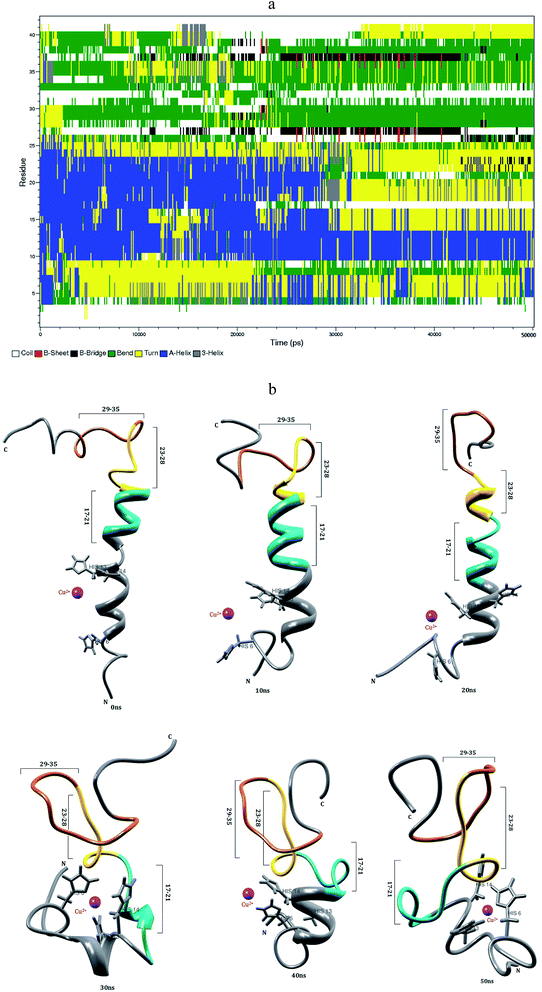
3.3 Interaction of Zn2+ with Aβ1–40 monomer in aqueous solution
Mithu et al.33 have found that more than 90% of total Zn2+ was bound to Aβ aggregates. We have made molecular dynamics simulation for Aβ1–40–Zn2+ structure in explicit water. This monomer exhibits two coil connected by the helical structure (Fig. 4b). The first eleven residues adopt β-hairpin structure with β-bend in the Phe4-Lys6 residues, the tail structure extended with Asp1-Phe4 and Asp7-Glu11 residues, during the 24–50 ns (Fig. 4a). The alpha helical structure of Val12-Gln15 residues did not changed during the interaction of Zn2+ ion. The central hydrophobic residues (Leu17-Ala21) were transformed to alpha helical structure due to the strong electrostatic interaction between the i and i + 3 residues which is represented in the contact map (Fig. 8c).The intra-chain salt bridge was observed between Glu22 and Lys28 residue, which persists throughout the simulation (5–40 ns). The indirect salt bridge appeared between Asp23 and Lys28 residues (Fig. S1a†) during the first 40 ns simulation and subsequently direct salt-bridge was observed for the remaining simulation. The hydrophobic contact increases between the hydrophobic residues (Leu17-Ala21) and c-terminal in the first 5 ns and the remaining simulation period, the hydrophobic contact decreased due the increase in distance 0.7–1.0 nm between Cα (Val24) and Cα (Asn27) residues (Fig. S3a†).
In Aβ1–40, the central hydrophobic core region (Leu17-Ala21) undergoes a conformational change between helix and turn structures which exhibits stable helix in Aβ1–40–Zn2+ structure. In the second hydrophobic region (Gly29-Met35), the residues Gly33-Met35 form turn structure throughout simulation in Aβ1–40, the same structure transformed from helix to coil in Aβ1–40–Zn2+. The above results show, the formation β-hairpin like structure is less probable during the Zn2+ ion interaction with Aβ1–40 monomer. The solvent accessible surface area (SASA) analyzed to find the hydrophobic character of individual residue of Aβ1–40 monomer. The difference in SASA values per residue between the Aβ1–40–Zn2+ and reduced Aβ1–40 structures is shown in Fig. S6,† where the positive and negative values indicate the increase and decrease in hydrophobic character. The differences in the SASA values per residue indicate the enhancement of the hydrophobicity of Gln15, Val18, Ile32 and Met35 residues and subsequently increase the hydrophilic character of Lys16, Phe20, Asp23 and Lys28 residues.
When compare with Aβ1–40 structure, the Aβ1–40–Zn2+ structure has 10% more helical and 6% less turn and bend structures and 3% less β-sheet structures (Table 1). The distance calculated between CO2−(Asp23) and NH3+(Lys28) is greater than 0.8 nm during 5–40 ns simulation. During the same period, the distance between CO2−(Glu22) and NH3+(Lys28) residues has been observed around 0.22–0.45 nm. This is due to the presence of salt-bridge. The Glu22-Lys28 region promotes stronger stabilization in the turn structure, when compared with Asp23-Lys28 region of Aβ1–40–Cu2+ structure. The hairpin like structure was stabilized by stronger salt-bridge formation between Glu22-Lys28 and Asp23-Lys28 residues during the Zn2+ ion interaction and it allows to form the greater rate of aggregation compared with Cu2+ ion interaction with N-region of Aβ1–40 monomer. This result agrees Noy et al. NMR study that Zn2+ interaction with Aβ, causes rapid aggregation into nonfibrillar species.63
3.4 Aβ1–42 monomer conformational dynamics in aqueous solution
The Aβ1–40 and Aβ1–42 are the most abundant in neuritic amyloid plaques, the presence of two hydrophobic residues, Ile41 and Ala42 at the c-terminal leads to distinct oligomeric distribution during fibrillization in in vitro. We investigated the structural differences between Aβ1–40 and Aβ1–42 monomers (Table 1), where the Aβ1–42 possesses 28% more helical, 9% less coil, 7% less bend and 7% less turn structure than the Aβ1–40. The distance calculated between Asp23 and Lys28 residues is greater than 0.9 nm, which indicate the absence of salt-bridge throughout the simulation period of Aβ1–42 monomer, whereas Aβ1–40 monomer has direct salt bridge (<0.43 nm) during the last 30 ns simulation. However the salt-bridge was observed between Glu22 and Lys28 residues of Aβ1–42 monomer (Fig. S2b†), where the interaction distance changes between 0.2 and 0.7 nm during 22–50 ns simulation. The turn structure (Val24-Asn27) was stabilized by salt-bridge of Glu22-Lys28 residues of both monomers, but the shape of the β-hairpin like structure in the Aβ1–42 monomer located in the middle region (Asp23-Asn27) has a larger curvature than that observed in Aβ1–40 monomer.Fig. S3b† shows, the distance between Cα (Val24) and Cα (Asn27) residues is around 0.4–0.65 nm, which increases the hydrophobic interaction between the central hydrophobic region (Leu17-Ala21) and c-terminal region during the first 10 ns. The Aβ1–42 monomer was formed by stronger hydrophobic interaction between the central hydrophobic region and c-terminal. Among the first ten residues (Asp1-Tyr10), Glu3-His6 residues form helical structure during the first 27 ns simulation and during further simulation (27–40 ns), it fluctuates between turn and helical conformations (Fig. 5). In contrast to the Aβ1–40 monomer, the region (Asp1-Tyr10) adopts bend structure during the simulation period. The 28% more helical structure was observed in the first twenty three residues (Asp1-Asp23) because of the presence of hydrophobic dipeptide in the c-terminal region.
The differences in the SASA values of Aβ1–40 and Aβ1–42 monomers (Fig. 9a), indicate the presence of (Ile41-Ala42) dipeptide, significantly enhances the hydrophobicity of Phe4, Gln15, Phe19 residues and subsequently increases the hydrophilic character of His13, Leu17, Val40 residues. These results indicate that the hydrophobic and hydrophilic characters help to maintain the helical structure in the Try10-Asp23 residues of the Aβ1–42 monomer in aqueous solution. The overall result suggested that the β hairpin like structure was stabilized by the salt-bridge between Glu22-Lys28 residues. This result agrees with the experimental result of Aβ1–42 monomers, where it aggregates faster than Aβ1–40 monomers.16,17,22,29
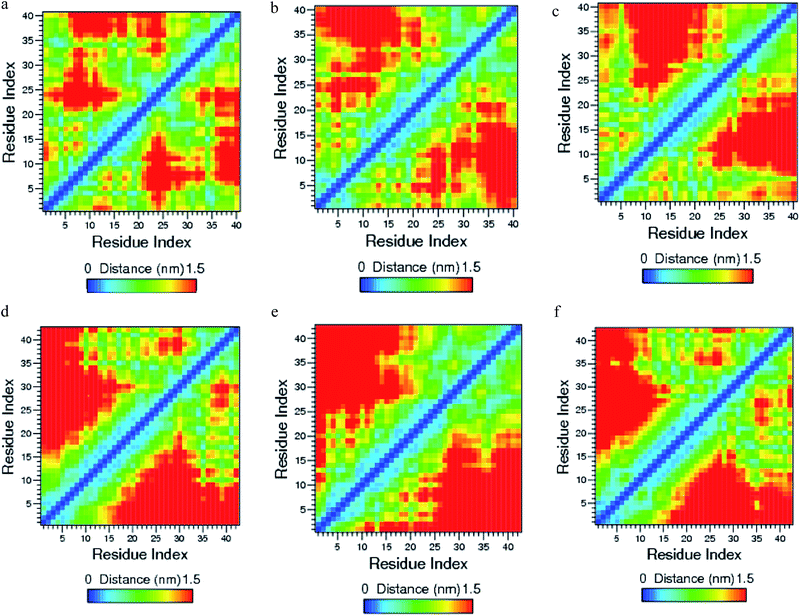 | ||
| Fig. 8 Contact map for (a) Aβ1–40, (b) Aβ1–40–Cu2+, (c) Aβ1–40–Zn2+, (d) Aβ1–42, (e) Aβ1–42–Cu and (f) Aβ1–42–Zn2+ structures. | ||
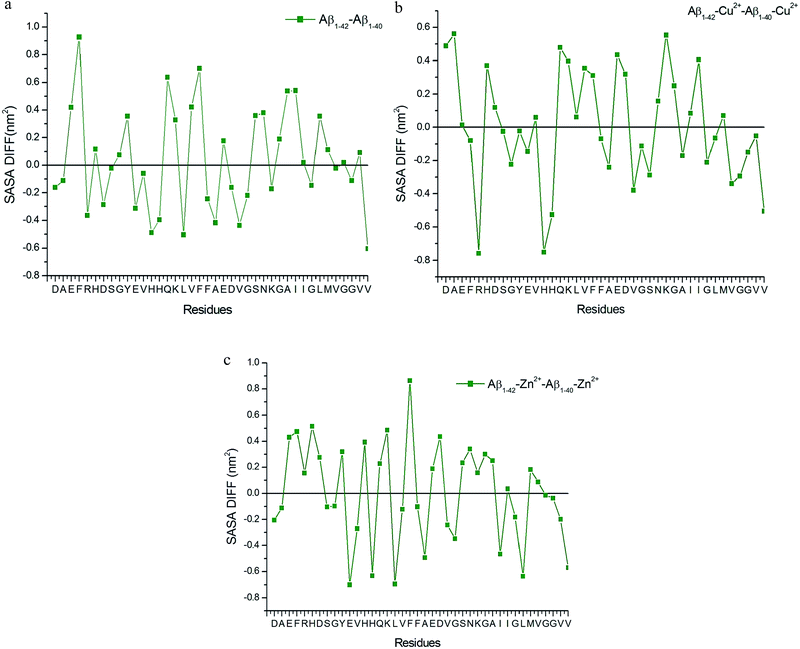 | ||
| Fig. 9 SASA values difference between (a) Aβ1–40 and Aβ1–42 monomer, (b) Aβ1–40–Cu2+ and Aβ1–42–Cu2+ structure and (c) Aβ1–40–Zn2+ and Aβ1–42–Zn2+ structure. | ||
3.5 Interaction of Cu2+ with the Aβ1–42 monomer in aqueous solution
The structural changes of Aβ1–42 monomer induced by Cu2+ ion were studied by X-ray diffraction, NMR, absorption spectroscopy, atomic force spectroscopy and Fourier transform infrared spectroscopy.22,29,31,34–36,39 In the first ten residues of Aβ1–42–Cu2+ structure, Asp1-Phe4 segment shows coil, Arg5-Tyr10 segments adopt turn and bend structures. The stronger interaction has been noticed between Phe4-Ser8 and Arg5-Tyr10 residues (Fig. 8e), which transforms the alpha helix to turn and bend conformational structures, but this was completely missing in Aβ1–40–Cu2+ structure. The Cu2+ ion change the alpha helix to the turn conformation in the His13-Asp23 region of Aβ1–42 monomer after 30 ns simulation period. The Aβ1–42–Cu2+ structure has 10% more helical structure than the Aβ1–40–Cu2+ in the first twenty four residues (Fig. 6a and Table 1).We found the stronger hydrogen bond between N–H group of Asn27 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of Gly37 residue in Aβ1–42–Cu2+ structure. The Gly33 residue has interacted with Gly37 and Ser26 residues in Aβ1–42–Cu2+ structure, whereas in the Aβ1–40–Cu2+ structure, these interactions are completely missing (Fig. 8e). The above interactions have attributed to the formation of β-hairpin like structure in the Lys28-Ala42 region. Some significant differences have been noticed in the loop region of Asp23-Lys28 residues during the interaction of Cu2+ ion in the Aβ1–42 monomer. The salt-bridge formed between Glu22 and Lys28 residues of Aβ1–42 monomer (Fig. S2b†), converted into the indirect salt-bridge when Cu2+ ion interacted with monomer. However in the Aβ1–40–Cu2+ structure, the indirect salt-bridge formed between the same residues.
O group of Gly37 residue in Aβ1–42–Cu2+ structure. The Gly33 residue has interacted with Gly37 and Ser26 residues in Aβ1–42–Cu2+ structure, whereas in the Aβ1–40–Cu2+ structure, these interactions are completely missing (Fig. 8e). The above interactions have attributed to the formation of β-hairpin like structure in the Lys28-Ala42 region. Some significant differences have been noticed in the loop region of Asp23-Lys28 residues during the interaction of Cu2+ ion in the Aβ1–42 monomer. The salt-bridge formed between Glu22 and Lys28 residues of Aβ1–42 monomer (Fig. S2b†), converted into the indirect salt-bridge when Cu2+ ion interacted with monomer. However in the Aβ1–40–Cu2+ structure, the indirect salt-bridge formed between the same residues.
The observed hydrophobic contact increased between central hydrophobic region (Leu17-Ala21) and N-terminal, when Cu2+ ion interacted with monomer, the same contact decreased due to the increasing distance around 0.45–0.65 nm between Cα (Val24)–Cα (Asn27) residues during the first 25 ns (Fig. S3b†). In the Aβ1–40–Cu2+ structure, the calculated distance between them is greater than 0.6 nm, so the hydrophobic contact get reduced throughout the simulation. The hydrophobic contact between CHC and c-terminal is stronger in Aβ1–42–Cu2+ compared with Aβ1–40–Cu2+ structure. The differences in the SASA values per residue (Fig. 9b) indicate that the presence of the hydrophobic dipeptides which significantly enhances the hydrophilic character of Arg5, His13 and increases the hydrophobic character of Asp1, Ala2 and Lys28 residues. If the Cu2+ ion interacts with His13, His14 and His6 residues, it promotes the stabilization in the β-hairpin structure. This was realized by the parallel sheet arrangement, in which Cu2+ coordinated with the N-terminal. Experimental results show that Cu2+ ions were bridged by two His13 (or His14) rings of each two single β-peptide.39 Our results indicate that the hairpin like conformation was stabilized by the presence of salt-bridge between Asp23-Lys28, Glu22-Lys28 residues, and hydrophobic interaction between CHC and c-terminal.
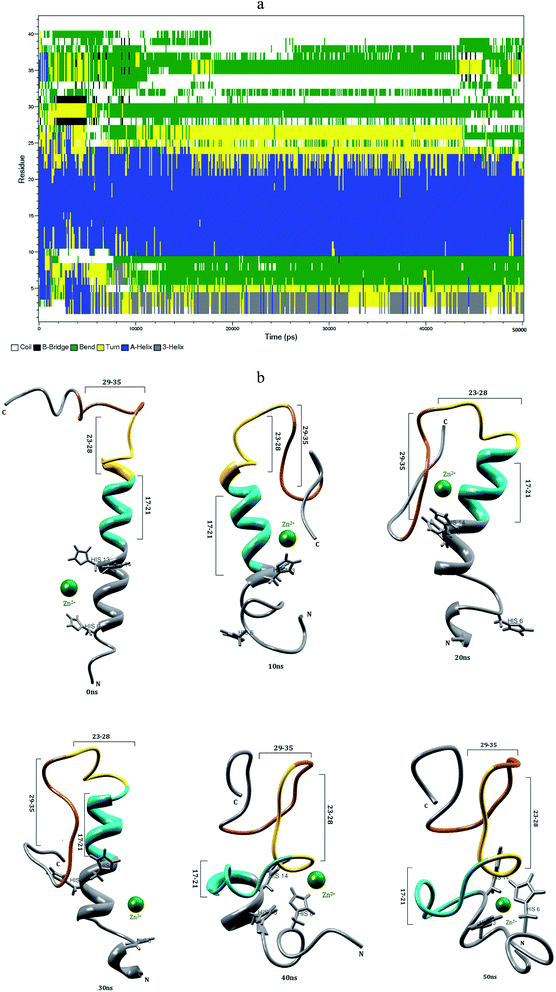
3.6 Interaction of Zn2+ with the Aβ1–42 monomer in aqueous solution
The first ten residues of Aβ1–42–Zn2+ structure forms the 310-helical structure, the N–H group of Ala2 makes hydrogen bond with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of Arg5 residue (Fig. 8f). This interaction was absent in the Aβ1–42–Cu2+ and Aβ1–40–Zn2+ structures. The next five residues (His6-Tyr10) exhibit bend and turn conformations in Aβ1–42–Zn2+ structure. Helix has been formed by the His13-Glu22, and Gly9-Glu22 residues of Aβ1–40–Zn2+. The differences in SASA value per residue between Aβ1–42–Zn2+ and Aβ1–40–Zn2+ structures (Fig. 9c) increases the hydrophobicity of Phe19 residue and enhances the hydrophilic character of Glu11, His14 and Leu17 residues. The Aβ1–42–Zn2+ structure possesses 8% more helical and 10% less coil structures than the Aβ1–40–Zn2+ structure (Fig. 7a).
O group of Arg5 residue (Fig. 8f). This interaction was absent in the Aβ1–42–Cu2+ and Aβ1–40–Zn2+ structures. The next five residues (His6-Tyr10) exhibit bend and turn conformations in Aβ1–42–Zn2+ structure. Helix has been formed by the His13-Glu22, and Gly9-Glu22 residues of Aβ1–40–Zn2+. The differences in SASA value per residue between Aβ1–42–Zn2+ and Aβ1–40–Zn2+ structures (Fig. 9c) increases the hydrophobicity of Phe19 residue and enhances the hydrophilic character of Glu11, His14 and Leu17 residues. The Aβ1–42–Zn2+ structure possesses 8% more helical and 10% less coil structures than the Aβ1–40–Zn2+ structure (Fig. 7a).
The Zn2+ interaction has promotes the significant structural changes in the N-terminal loop region connecting the two hydrophobic domain. The major change was, the disappearance of the salt-bridge between Asp23 and Lys28 residues. The hydrophobic contact between central hydrophobic core and c-terminal decreases in the Aβ1–42–Zn2+ structure than that of Aβ1–42–Cu2+ and Aβ1–40–Zn2+ structures. These results suggest that the rate of aggregation increases in the Aβ1–42 monomer due to the interaction of Cu2+ than Zn2+.
4. Conclusion
Molecular dynamics simulation has been performed to investigate the conformational and structural changes in Aβ1–40 and Aβ1–42 monomers with and without interaction of metal ions (Cu2+, Zn2+) in explicit water for 50 ns simulation. This simulation has elucidated the atomic level calculation for three important regions (central hydrophobic domain (Leu17-Ala21), loop region (Asp23-Lys28) and second hydrophobic region (Gly29-Met35)) of the monomers. The presence of hydrophobic dipeptide in Aβ1–42 monomer, the significant structural changes have been observed in the secondary structure. For instance, the CHC (Leu17-Ala21) region contains Tyr10-Asp23 residues exhibits alpha helix, whereas in Aβ1–40, region adopts helix and bend conformations. The Aβ1–42 monomer possesses 28% more helical structure between Asp1-Lys28 residues as compared with Aβ1–40 monomer. However in the Aβ1–42 monomer, the loop region (Asp23-Lys28) and salt-bridge disappeared, where as in Aβ1–40 monomer salt-bridge often forms during the course of simulation. The hydrophobic contact between C-terminal and CHC region increases and a small curvature forms in the turn region (Val24-Asn27) in Aβ1–42 monomer. In the second hydrophobic region, bend conformation has been observed in Aβ1–42, whereas in the Aβ1–40, this exists in turn and beta bridge regions. Aforementioned processes have stabilized the β-hairpin like structure in Aβ1–42 monomer and it leads to provide the chance to attach the new β-hairpin monomer.In the Aβ1–42–Zn2+ structure, Asp1-Tyr10 residues form the 310-helix and coil conformation, but these are transformed to bend conformations in Aβ1–40 monomer. The CHC (Leu17-Ala21) region contains Tyr10-Val24 residues of Aβ1–42–Zn2+ which exists in the helical conformation throughout the simulation and this region transforms into bend (Tyr10-His13) and helical (His13-Val24) conformations in Aβ1–40–Zn2+ structure. Surprisingly in the Aβ1–42–Zn2+ structure, salt-bridge was disappeared in Asp23-Lys28 residues but it often forms in Aβ1–40–Zn2+ structure throughout the simulation period. The turn (Val24-Asp27) region was stabilized due to the salt-bridge between Glu22-Lys28 residue in Aβ1–42–Zn2+ structure and where as Aβ1–40–Zn2+ structure was stabilized by salt-bridge between Asp23-Lys28 and Glu22-Lys28 residues. The second hydrophobic region (Gly29-Met35) forms bend and coil conformations in Aβ1–42–Zn2+, whereas in Aβ1–40–Zn2+ structure, this region exhibits the helix and bend conformations. The hydrophobic contact between c-terminal (Met35-Val40) and CHC was decreased in the Aβ1–42–Zn2+ structure than in the Aβ1–40–Zn2+. This structural difference has prevent to attach the new incoming β-hairpin monomer when the interaction of Zn2+ with Aβ1–42 monomer.
In Aβ1–42–Cu2+ structure, the CHC (Leu17-Ala21) residues become alpha helical and turn conformers, whereas in Aβ1–40–Cu2+ structure, this region adopts turn conformation. However the loop region (Asp23-Lys28) and turn region, Val24-Asn27 residues stabilized by salt-bridge formation between Asp23-Lys28 and Glu22-Lys28 residues in Aβ1–42–Cu2+ as compared with the Aβ1–40–Cu2+. The Aβ1–40–Cu2+ structure divided into three bend structures separated by coil segment. The interaction distance between Cα (Val24) and Cα (Asn27) residues is responsible for increasing the hydrophobic contact between CHC and c-terminal (Met35-Val40) in Aβ1–42–Cu2+ structure. The second hydrophobic region has bend conformation in Aβ1–42–Cu2+ structure, whereas in Aβ1–40–Cu2+, this region transforms to the turn and β-bridge conformations. This structural differences lead to stabilize the β-hairpin structure of Aβ1–42–Cu2+ structure and it gives chance to form the fast fibrillization of amyloid protein. Further, it has been found that the interaction of Cu2+ and Zn2+ with Aβ1–40 and Aβ1–42 is maximum at the beginning of the simulation, and fluctuates around 1–2 nm during the simulation period.
Acknowledgements
The authors are thankful to DST (Department of Science and Technology) for the financial support in the form of project (SR/CSI/31/2011G dated 07-03-2012) under Cognitive Science Initiative (CSI) scheme.References
- G. Ciprani, C. Dolciotti, L. Pichi and V. Bonucelli, J. Neurol. Sci., 2011, 32, 260–279 Search PubMed.
- T. P. Ng, P. C. Chiam, T. Lee, H. C. Chau, L. Lim and E. H. Kau, Am. J. Epidemiol., 2006, 164, 898–906 CrossRef PubMed.
- R. Pandav, S. H. Bells and S. T. Dekosky, Arch. Neurol., 2005, 60, 824 Search PubMed.
- K. Blennow, M. J. De Leon and H. Zetterberg, Lancet, 2006, 368, 387 CrossRef CAS.
- M. P. Mattson, Nature, 2004, 430, 631 CrossRef CAS PubMed.
- G. M. Mckhann, D. S. Knopman, H. Chertkow, B. T. Hyman, C. R. Jack, Jr, C. H. Kawas, W. E. Klunk, W. J. Koroshertz, J. J. Manly, R. Mayeux and R. Mohs, Alzheimer's Dis., 2011, 7, 268 Search PubMed.
- J. D. Pham, N. Chim, C. W. Gouldinng and J. S. Nowick, J. Am. Chem. Soc., 2013, 135, 12460–12467 CrossRef CAS PubMed.
- R. Tycko and R. B. Wickner, Acc. Chem. Res., 2013, 46, 1487–1496 CrossRef CAS PubMed.
- W. M. Berhanu and U. H. E. Hansmann, PLoS One, 2012, 7, e41479 CAS.
- A. T. Petkova, R. D. Leapman, Z. Guo, W. Yau, M. P. Mattson and R. Tycko, Science, 2005, 307, 262–265 CrossRef CAS PubMed.
- R. Kodali, A. D. Williams, S. Chemura and R. Wetzel, J. Mol. Biol., 2010, 401, 503–517 CrossRef CAS PubMed.
- U. Anand and M. Mukherjee, Langmuir, 2013, 29, 2713–2721 CrossRef CAS PubMed.
- B. Tarus, J. E. Straub and D. Thirumalai, J. Am. Chem. Soc., 2006, 128, 16162–16168 CrossRef PubMed.
- A. Melquiond, X. Dong, N. Mousseau and P. Derreumaux, Curr. Alzheimer Res., 2008, 5, 244–250 CrossRef CAS.
- K. Ono, M. M. Condron and D. B. Teplow, J. Biol. Chem., 2010, 285, 23186–23197 CrossRef CAS PubMed.
- W. Garzon-Rodriguez, A. Vega, M. Sepulveda-Becerra, S. Melton, D. A. Johnson and A. K. Yatsimirsky, et al., J. Biol. Chem., 2000, 275, 22645–26649 CrossRef CAS PubMed.
- Y. R. Chen and C. G. Glave, J. Biol. Chem., 2006, 281, 24414–24422 CrossRef CAS PubMed.
- C. Sachse, M. Fandrich and N. Grigorieff, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7462–7466 CrossRef CAS PubMed.
- M. Schmidt, C. Sachse, W. Richter, C. Xu and M. Fandrich, et al., Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 19813–19818 CrossRef CAS PubMed.
- A. T. Petkova, W. M. Yau and R. Tycko, Biochemistry, 2006, 45, 498–512 CrossRef CAS PubMed.
- D. B. Teplow, N. D. Lazo, G. Bitan, S. Bernstein, T. Wyttenbach, M. T. Bowers, A. Baunketner, J. Shea, B. Urbnc and L. Cruz, et al., Acc. Chem. Res., 2006, 39, 635–645 CrossRef CAS PubMed.
- I. W. Hamley, Chem. Rev., 2012, 112, 5147–5192 CrossRef CAS PubMed.
- C. Ritter, M. I. Maddelein, A. B. Siemer, T. Luhrs, M. Ernst, B. H. Meier, S. J. Saupe and R. Riek, Nature, 2005, 435, 844–848 CrossRef CAS PubMed.
- R. Nelson, M. R. Sawaya, M. Balbrinie, A. O. Madsen, C. Rickel, R. Grothe and D. Eisenberg, Nature, 2005, 435, 773–778 CrossRef CAS PubMed.
- B. Urbanc, L. Cruz, D. B. Teplow and H. E. Stanley, Curr. Alzheimer Res., 2006, 3, 493–504 CrossRef CAS.
- J. A. Lemkul and D. R. Bevan, J. Phys. Chem. B, 2010, 114, 1652–1660 CrossRef CAS PubMed.
- S. Boopathi and P. Kolandaivel, J. Mol. Model., 2014, 20(3), 2109 CrossRef PubMed.
- T. Luhrs, C. Ritter, M. Adrian, D. Rick-Loher, B. Bohrmann, H. Dobeli, D. Schubert and R. Riek, Proc. Natl. Acad. Sci. U. S. A., 2005, 29, 17342–17347 CrossRef PubMed.
- K. P. Kepp, Chem. Rev., 2012, 112, 5193–5239 CrossRef CAS PubMed.
- E. Gaggelli, A. Janicka-Klos, E. Jankowska, H. Kozlowski, C. Miglionni, E. Molteni, D. Valensin and E. Wieczerzak, J. Phys. Chem. B, 2008, 112, 100 CrossRef CAS PubMed.
- S. Parthasarathy, F. Long, Y. Miller, Y. Xiao, D. Mc Elheny, K. Thurber, B. Ma, R. Nassinov and Y. Ishii, J. Am. Chem. Soc., 2011, 133, 3390–3400 CrossRef CAS PubMed.
- J. R. Brender, K. Hartman, R. P. Reddy Nanga, V. Popovych, R. D. L. Salud Bea, S. Vivekanandhan, E. N. G. Marsh and A. Ramamoorthy, J. Am. Chem. Soc., 2010, 132, 8973–8983 CrossRef CAS PubMed.
- V. S. Mithu, B. Sarkar, D. Bhowmik, M. Chandrakesan, S. Maiti and P. K. Madhu, Biophys. J., 2011, 101, 2825–2832 CrossRef CAS PubMed.
- S. Morante, Curr. Alzheimer Res., 2008, 5, 508 CrossRef CAS.
- P. Faller and C. Hureau, Actual. Chim., 2011, 88, 356–360 Search PubMed.
- Y. El Khoury, P. Dorlet, P. Faller and P. Hellwig, J. Phys. Chem. B, 2011, 115, 14812 CrossRef PubMed.
- A. K. Sharma, S. T. Pavlova, D. Finkelstein, N. J. Hawco, N. P. Rath, J. Kim and L. M. Mirica, J. Am. Chem. Soc., 2012, 134, 6625–6636 CrossRef CAS PubMed.
- A. Asandei, I. Schiopu, S. Iftemi, L. Mereuta and T. Luchian, Langmuir, 2013, 29, 15634–15642 CrossRef CAS PubMed.
- F. Hane, G. Trans, S. J. Attwood and Z. Leonenko, PLoS One., 2013, 8, e62005 Search PubMed.
- N. G. Nair, G. Perry, M. A. Smith and V. P. Reddy, J. Alzheimer's Dis., 2010, 20, 60–66 Search PubMed.
- M. Lovell, J. Robertson, W. Teesdale, J. Campbell and W. Markesbery, J. Neurol. Sci., 1998, 161, 47–52 CrossRef.
- A. I. Bush, J. Alzheimer's Dis., 2008, 15, 223–240 CAS.
- J. S. Choi, J. J. Braymer, R. P. R. Nanga, A. Ramamoorthy and M. H. Lim, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 21990–21995 CrossRef CAS PubMed.
- M. Coles, W. Bicknell, A. A. Watson, D. P. Fairlie and D. J. Craik, Biochemistry, 1998, 37, 11064–11077 CrossRef CAS PubMed.
- O. Crescenzi, S. Tomaselli, R. Guerrini, S. Salvatori, A. M. D'ursi, P. A. Temussi and D. Picone, Eur. J. Biochem., 2002, 269, 5642–5648 CrossRef CAS.
- J. W. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Imprey and M. L. Klein, J. Chem. Phys., 1983, 76, 926 CrossRef PubMed.
- S. Boopathi and P. Kolandaivel, J. Biomol. Struct. Dyn., 2013, 31, 161–173 Search PubMed.
- B. Hess, C. Kutzner, D. Van der Spoel and E. Lindahl, J. Chem. Theory Comput., 2008, 4, 435–444 CrossRef CAS.
- G. A. Kaminski and R. A. Friesner, J. Phys. Chem. B, 2001, 105, 6474–6487 CrossRef CAS.
- H. J. C. Berendsen, J. P. M. Postma, W. F. Van Gunsteren, A. Di Nola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684–3690 CrossRef CAS PubMed.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089–10092 CrossRef CAS PubMed.
- R. W. Hockney, S. P. Goel and J. Eastwood, J. Comput. Phys., 1974, 14, 148–161 CrossRef.
- W. L. De Lano, The PyMol Molecular graphics system. Version 1.1, LLC, 2008 Search PubMed.
- T. D. Goddard, C. C. Huang and T. E. Ferrin, Structure, 2005, 13, 473–482 CrossRef CAS PubMed.
- W. Kebsch and C. Sander, Biopolymers, 1983, 22, 2607–2673 Search PubMed.
- J. Dzubiella, J. Am. Chem. Soc., 2008, 130, 14000–14007 CrossRef CAS PubMed.
- L. Triguero, R. Singh and R. Prabhakar, J. Phys. Chem. B, 2008, 112, 7123–7131 CrossRef CAS PubMed.
- J. Zheng, H. Jang and R. Nussinov, Biochemistry, 2008, 47, 2497 CrossRef CAS PubMed.
- L. Shen, H. F. Ji and H. Y. Zhang, J. Phys. Chem. B, 2008, 112, 3164 CrossRef CAS PubMed.
- N. D. Lazo, M. A. Grant, M. C. Condron, A. C. Rigby and D. B. Teplow, Protein Sci., 2005, 14, 1611–1626 Search PubMed.
- J. M. Borreguero, B. Urbane, N. D. Lazo, S. V. Buldrev, D. B. Teplow and H. E. Stanley, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 6015–6020 CrossRef CAS PubMed.
- P. S. Hallman, D. D. Perrin and A. E. Watt, Biochem. J., 1971, 121, 549–555 CAS.
- D. Noy, I. Solomonov, O. Sinkevich, T. Arad, K. Kjaer and I. Saqi, J. Am. Chem. Soc., 2008, 130, 1376–1383 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The salt-bridge was formed between the CO2− moiety of Asp23 (or Glu22) and the NH3+ in Lys28 residue as a function of time, which is shown in Fig. S1–S2. Distance between Cα (Val24) and Cα (Asn27) as a function of time is shown in Fig. S3. Solvent accessible surface area analysis of the Aβ1–40, Aβ1–40–Cu2+ and Aβ1–40–Zn2+ structures are shown in Fig. S4–S6. Convergence of trajectories for different conformation are given in Fig. S7. See DOI: 10.1039/c4ra05390g |
| This journal is © The Royal Society of Chemistry 2014 |