Biologically active isoquinoline alkaloids with drug-like properties from the genus Corydalis†
M. Iranshahy
a,
R. J. Quinn
b and
M. Iranshahi
*a
aBiotechnology Research Center and School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. E-mail: Iranshahim@mums.ac.ir; Fax: +98-511-8823251; Tel: +98-511-8823255
bEskitis Institute, Griffith University, Brisbane QLD 4111, Australia
First published on 12th March 2014
Abstract
The genus Corydalis (Papaveraceae), comprising more than 400 species in Eurasia and North America, is a rich source of isoquinoline alkaloids with various biological properties including acetylcholinesterase inhibitory effects, anti-proliferative activities, antiviral activities and antiplasmodial activities. Traditionally, some Corydalis species have long been used to treat gastric and duodenal ulcers, dysmenorrhoea, rheumatism and cardiac arrhythmia disease and this traditional background so far has proven pharmacological activities resulting in isolation of more than 100 isoquinoline alkaloids from this genus. This overview aims to inform medicinal chemists of the good drug-like properties and versatile biological activities of Corydalis alkaloids to stimulate further medicinal chemistry research. Mechanisms of action and structure–activity relationship of Corydalis alkaloids are also included.
1. Introduction
Native to the temperate Northern Hemisphere, the genus Corydalis consists of more than 400 species in Eurasia and North America.1 These annual and perennial herbaceous plants with beautiful colorful flowers are a rich source of alkaloids, particularly isoquinoline alkaloids with various biological properties. Traditionally, some Corydalis species have long been used for the treatment of different ailments in China, Korea, Japan and other Eastern Asian countries. For example, C. yanhusuo, a traditional Chinese medicine, has been used to treat gastric and duodenal ulcers, dysmenorrhoea, rheumatism and cardiac arrhythmia disease.2,3 Every year new alkaloid structures, together with their biological activities, are reported from the plants of this genus. Some of these alkaloids possess promising biological/pharmacological properties for the treatment of important diseases including cancer, Alzheimer's and microbial infections.To date, there is no comprehensive review on Corydalis alkaloids (CA) and their biological activities. This review deals with chemistry, drug-like features, and biological properties of CA.
Although isoquinoline alkaloids have been reported from many plants, and even other biological sources, however, the diversity of structures in CA has particularly been attractive to natural product researchers.
In today's era of drug development, many bioactive compounds are discovered from nature or combinatorial synthesis by high throughput screening, but only a few bioactive compounds reach the clinical trials phases, often because of the lack of bioavailability. In recent years, several parameters have been introduced for the prediction of drug-like physicochemical properties of drug candidates including intestinal absorption, bioavailability and cell penetration.4,5 In this regard, the most well-known rule is “rule of five” or Lipinski's rule. According to this rule, an oral drug-like molecule should have an octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) of <5, <5 hydrogen bond donors (HBD), <10 hydrogen bond acceptors (HBA) and a molecular weight of <500 Da.6 Two more requirements for drug-like molecules, proposed by Veber and co-workers, include NROT (number of rotatable bonds) of <10 and a PSA (polar surface area) of <140 Å2.7 Considering this description, we evaluated the above parameters for CA in this review to see the eligibility of biologically active CA for being future drugs. Interestingly, most of CA satisfied these criteria for lead-like compounds.
P) of <5, <5 hydrogen bond donors (HBD), <10 hydrogen bond acceptors (HBA) and a molecular weight of <500 Da.6 Two more requirements for drug-like molecules, proposed by Veber and co-workers, include NROT (number of rotatable bonds) of <10 and a PSA (polar surface area) of <140 Å2.7 Considering this description, we evaluated the above parameters for CA in this review to see the eligibility of biologically active CA for being future drugs. Interestingly, most of CA satisfied these criteria for lead-like compounds.
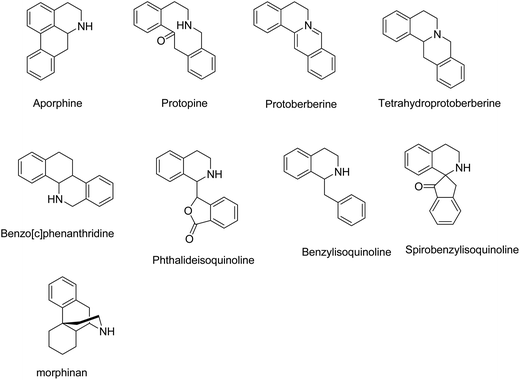
Up to now, different classes of isoquinoline alkaloids have been found in Corydalis species including aporphine, protopine, protoberberine, tetrahydroprotoberberine, benzo[c]phenanthridine, phthalideisoquinoline, benzylisoquinoline, morphinan, and spirobenzylisoquinoline (Fig. 1). On the basis of these scaffolds, in this review, we identified structure–activity relationships for CA, in order to stimulate further medicinal chemistry researches. Given the generally good drug-like properties, medicinal chemistry programs need to conserve the physic-chemical properties in analogous development.
2. Biological properties
2.1. Anti-proliferative/anti-cancer activity
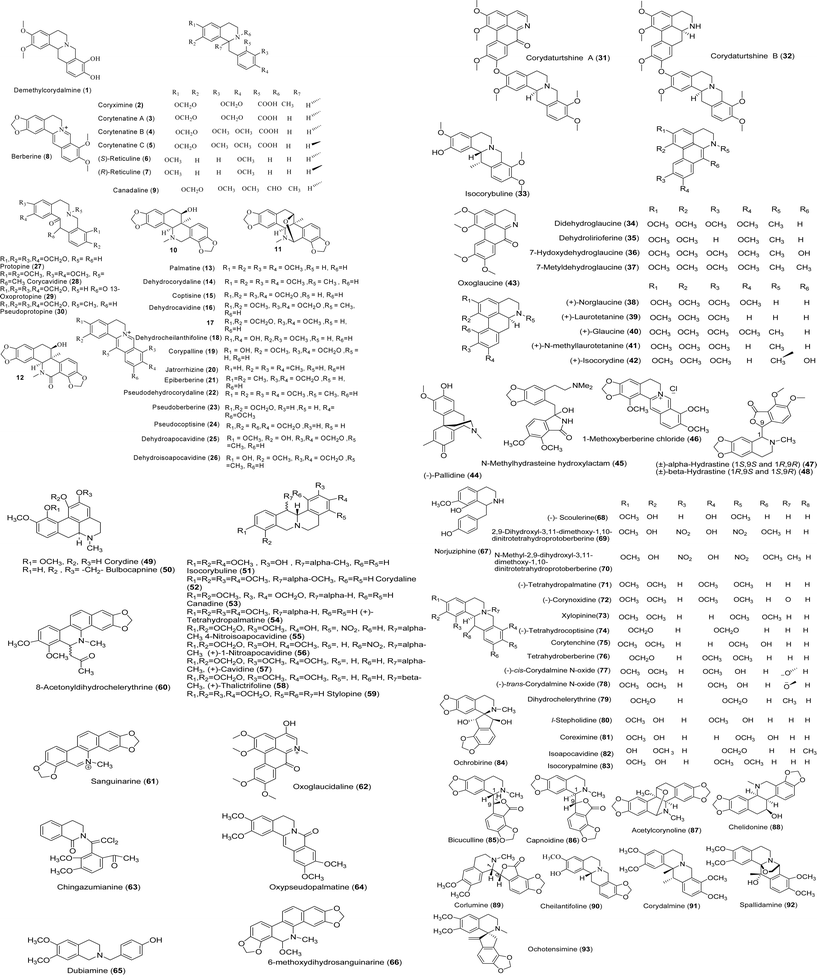
Kim et al. studied the cytotoxicity of fourteen alkaloids from the tubers C. ternata on several tumor cell lines such as A549, SK-OV-3, SK-MEL-2, and HCT-15. They found that demethylcorydalmine (1, Fig. 2) had significant cytotoxicity against A549, SK-OV-3, SK-MEL-2, and HCT-15 cell lines with IC50 values of 8.34, 5.14, 7.87, and 2.86 µM, respectively.8 In addition, they found that the benzylisoquinoline alkaloids of C. ternata such as epi-coryximine (2), coryternatines A–C (3–5), (S)-reticuline (6) and (R)-reticuline (7) were selectively cytotoxic against HCT-15 with IC50 values ranging from 23 to 29 µM. Their findings revealed that protoberberine-type alkaloids such as compound 1 and berberine (8) showed cytotoxicity against human cell lines (IC50 values ranging from 6.27 to 16.59 µM), yet the protopine-type alkaloid such as protopine (27) was inactive.8 In another study, Kim et al. found that berberine (8) obtained from C. pallida possessed cytotoxic activity against HT-1080 (human fibrosarcoma) and SNU-638 (human stomach adenocarcinoma), with IC50 values of 3.2 and 3.4 µg ml−1, respectively. The other constituents of this plant were inactive.9 In 2007, Choi and coworkers showed cytotoxic activity of the benzo[c]phenanthridine-type alkaloid corynoline (10) from C. incisa against A549, SK-OV-3, SK-MEL-2, and HCT-15 with ED50 values ranging from 5.27 to 6.14 µM. Compounds 11 and 12 from this plant with benzo[c]phenanthridine-type structures exhibited weaker cytotoxicity with ED50 values greater than 30 µM.10As shown in Table 1, the strongest cytotoxic activities were observed in the protoberberine and aporphine classes. One of cytotoxicity mechanisms of CA (protoberberine class) have been studied by Cheng et al. in 2008. They found that berberine (8) and its analogues (13, 16–19) possessed topoisomerase I inhibitory activities. However, among the tested compounds, compound 44, a morphinadienone alkaloid, and compound 68 showed the best topoisomerase I inhibitory activity.11
| No. | Chemical class | IC50/ED50 (cancer cell line) | Reference |
|---|---|---|---|
| 1 | Protoberberine | 8.34 µM (A549), 5.14 µM (SK-OV-3), 7.87 µM (SK-MEL-2), 2.86 µM (HCT-15) | 8 |
| 2 | Benzylisoquinoline | 27.75 µM HCT-15 | 8 |
| 3 | Benzylisoquinoline | 23.29 µM HCT-15 | 8 |
| 4 | Benzylisoquinoline | 24.58 µM HCT-15 | 8 |
| 5 | Benzylisoquinoline | 27.63 µM HCT-15 | 8 |
| 6 | Benzylisoquinoline | 29.44 µM HCT-15 | 8 |
| 7 | Benzylisoquinoline | 26.83 µM HCT-15 | 8 |
| 8 | Protoberberine | 6.27 µM (A549), 16.44 µM (SK-OV-3), 13.76 µM (SK-MEL-2), 16.59 µM (HCT-15), 3.2 µg ml (HT-1080), 3.4 µg ml−1 (SNU-638), 3.1 µg ml−1 (HepG2), 15.2 µg ml−1 (Hep3B), 3.3 µg ml−1 (SK-Hep1), 13.9 µg ml−1 (PLC/PRF/5), 14.1 µg ml−1 (K562), 9.0 µg ml−1 (U937), 7.9 µg ml−1 (P3H1), 0.6 µg ml−1 (Raji), 20.0 µM (HL-60), 28.7 µM (HEp-2), 19.6 µM (MCF-7), 0.0149 µg ml−1 (B16) | 8, 9 and 14–17 |
| 10 | Benzo[c]phenanthridine | 5.27 µM (A549), 5.82 µM (SK-OV-3), 5.59 µM (SK-MEL-2), 6.14 µM (HCT-15) | 10 |
| 11 | Benzo[c]phenanthridine | 17.05 µM (SK-MEL-2) | 10 |
| 13 | Protoberberine | 4 µg ml−1 (hydrogen iodide salt, KB) | 18 |
| 14 | Protoberberine | 4.5 µg ml−1 (P388), 4 µg ml−1 (L1210), 28.76 µM (SK-MEL-2) | 19 and 20 |
| 15 | Protoberberine | 2.97 µg ml−1 (P388), 0.36 µg ml−1 (HCT-8), 0.32 µg ml−1 (A549), 3.5 µg ml−1 (HepG2), 5.4 µg ml−1 (Hep3B), 1.4 µg ml−1 (SK-Hep1), 6.6 µg ml−1 (PLC/PRF/5), 7.2 µg ml−1 (K562), 1.5 µg ml−1 (U937), 10.9 µg ml−1 (P3H1), 0.6 µg ml−1 (Raji) | 14 and 19 |
| 31 | Aporphine + tetrahydroprotoberberine | 12.9 µM (A549), 8.75 µM (SK-OV-3), 12.28 µM (SK-MEL-2), 14.21 µM (HCT-15) | 20 |
| 32 | Aporphine + protoberberine | 10.08 µM (SK-MEL-2), 29.31 µM (HCT-15) | 20 |
| 33 | Tetrahydroprotoberberine | 22.43 µM (SK-OV-3), 29.61 µM (SK-MEL-2), 15.55 µM (HCT-15) | 20 |
| 34 | Aporphine | 4.51 µM (A549), 4.53 µM (SK-OV-3), 4.21 µM (SK-MEL-2), 4.76 µM (HCT-15) | 20 |
| 35 | Aporphine | 12.9 µM (A549), 4.53 µM (SK-OV-3), 4.21 µM (SK-MEL-2), 4.76 µM (HCT-15) | 20 |
| 36 | Aporphine | 15.94 µM (A549), 12.29 µM (SK-OV-3), 5.99 µM (SK-MEL-2), 15.15 µM (HCT-15) | 20 |
| 37 | Aporphine | 15.90 µM (SK-OV-3), 20.86 µM (SK-MEL-2), 27.70 µM (HCT-15) | 20 |
| 38 | Aporphine | 19.38 µM (SK-OV-3), 21.97 µM (HCT-15) | 20 |
| 39 | Aporphine | 26.19 µM (SK-OV-3), 26.64 µM (SK-MEL-2), 18.60 µM (HCT-15) | 20 |
| 40 | Aporphine | 26.76 µM (A549), 21.57 µM (SK-OV-3), 20.39 µM (SK-MEL-2), 18.63 µM (HCT-15) | 20 |
| 41 | Aporphine | 12.61 µM (A549), 13.64 µM (SK-OV-3), 11.21 µM (SK-MEL-2), 15.06 µM (HCT-15) | 20 |
| 43 | Aporphine | 12.21 µM (A549), 7.19 µM (SK-OV-3), 15.40 µM (SK-MEL-2), 13.10 µM (HCT-15) | 20 |
In Table 1, IC50 values of CA were summarized. The IC50 values less than 30 µM (or µg ml−1) were considered in the table as significant cytotoxic activities. It should be pointed out, however, inter-lab variation on the IC50 values is probable, and should be considered for a better comparison.
In total, aporphine, benzylisoquinoline and protoberberine scaffolds of CA showed a higher cytotoxicity rather than other scaffolds. A typical example is berberine (8) with a protoberberine skeleton that showed a remarkable cytotoxicity on a wide range of cancer cell lines (Table 1). Many papers have been published regarding the anticancer properties of berberine (8) and its molecular targets. But the biological activities of berberine were not limited to anticancer activity. This compound had a large number of biological targets in the body and showed various biological properties. Because of limits on the length of this review, the subject of the biological/pharmacological activities of berberine (8) is not expounded, and readers are referred to two recently published reviews on the biological properties of berberine (8).11-13
Multidrug resistance is also a major cause of failure in cancer chemotherapy regimens and finding new scaffolds of natural and synthetic compounds is a hot topic in current multidrug resistance research.21 Recently, some of isoquinoline alkaloids showed promising activity against MDR.22,23
Glaucine (40), an aporphine-type alkaloid from C. yanhusuo, competitively inhibited two transporters involved in MDR named P-glycoprotein (P-gp) and multidrug resistance-associate protein 1 (MRP1). In MCF-7/ADR cancer cell line, 12.5 µM of glaucine (40) reduced the IC50 values of adriamycin and mitoxantrone from 41 and 24.19 µg ml−1 to 12.6 and 2.11 µg ml−1, respectively.22
Chelidonine (88), a benzo[c]phenanthridine-type alkaloid from Chelidonium majus, inhibited P-gp/MDR1 activity in Caco-2 cell line. An alkaloid extract of this plant showed similar results and further identification of alkaloids exhibited berberine (8), coptisine (15), stylopine (59) and protopine (27) as major components of alkaloid extract. Mechanistic studies revealed that chelidonine (88) and the alkaloid extract (50 µg ml−1) significantly decrease the mRNA levels of P-gp/MDR1, MRP1 and breast cancer resistance protein (BCRP).23
2.2. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory effects
Acetylcholine (ACh) as a neurotransmitter plays an important role in the peripheral and central nervous systems. According to the cholinergic hypothesis, Alzheimer's disease (AD) as the most predominant cause of dementia in the elderly, is a result of decreased levels of ACh in the cerebral cortex. To date, inhibition of AChE and BChE (the enzymes involved in the breakdown of acetylcholine) is the most common approach for treatment of AD. Galantamine from Galanthus wornowii is among the first FDA approved drugs for treatment of AD and acts as an AChE inhibitor (AChEI). Natural products are one of the most valuable sources for treatment of AD.24,25 In Danish and Chinese folk medicines many Corydalis species have been used as memory enhancers26,27 and modern pharmacological experiments have confirmed this effect.In 2004, Orhan et al. demonstrated that chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (1
methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) extract of Corydalis solida subsp. solida inhibited AChE with 87.56% inhibitory effect at 1 mg ml−1.28 In 2006, Adsersen et al. reported AChEI properties of aqueous and methanolic extract of 11 plants, used in Danish folk medicine as memory enhancers and Corydalis species showed the most potent AChE inhibitory effects.26 Methanolic extracts of tubers of C. solida (L.) Swartz ssp. slivenensis and C. intermedia (L.) Merat at concentrations of 0.1 mg ml−1 resulted in 97% of the enzyme inhibition. In addition, methanolic extracts of tubers of C. solida (L.) Swartz ssp. laxa and C. cava (L.) Schw. et K. at concentration of 0.1 mg ml−1 showed 96 and 92% of the enzyme inhibition, respectively.26 Regarding this ethnobotanical relevance, AChE inhibitory effects of isolated alkaloids from several Corydalis species have vastly been investigated. Among these alkaloids, dehydrocorydaline (14), coptisine (15), berberine (8), jatrorrhizine (20), and palmatine (13), from C. yanhusuo, showed potent AChE inhibitory effects with IC50 values of 0.62, 1.01, 0.47, 2.08 and 0.74 µM, respectively.29 Recently, a review on the potential of berberine (8) to combat AD was published.30 Berberine (8) combats AD by inhibition of AChE, BChE and monoamine oxidase, by reduction of amyloid-β peptide level, and by lowering cholesterol level.30 (+)-Canadaline (9), canadine (53), bulbocapnine (50) and corydaline (52) from C. cava31,32 showed moderate activity against AChE, while tetrahydropalmatine (54) and protopine (27) from C. yanhusuo were inactive.29 More information about alkaloids from Corydalis species with AChE and BChE inhibitory effects are summarized in Table 3.
1) extract of Corydalis solida subsp. solida inhibited AChE with 87.56% inhibitory effect at 1 mg ml−1.28 In 2006, Adsersen et al. reported AChEI properties of aqueous and methanolic extract of 11 plants, used in Danish folk medicine as memory enhancers and Corydalis species showed the most potent AChE inhibitory effects.26 Methanolic extracts of tubers of C. solida (L.) Swartz ssp. slivenensis and C. intermedia (L.) Merat at concentrations of 0.1 mg ml−1 resulted in 97% of the enzyme inhibition. In addition, methanolic extracts of tubers of C. solida (L.) Swartz ssp. laxa and C. cava (L.) Schw. et K. at concentration of 0.1 mg ml−1 showed 96 and 92% of the enzyme inhibition, respectively.26 Regarding this ethnobotanical relevance, AChE inhibitory effects of isolated alkaloids from several Corydalis species have vastly been investigated. Among these alkaloids, dehydrocorydaline (14), coptisine (15), berberine (8), jatrorrhizine (20), and palmatine (13), from C. yanhusuo, showed potent AChE inhibitory effects with IC50 values of 0.62, 1.01, 0.47, 2.08 and 0.74 µM, respectively.29 Recently, a review on the potential of berberine (8) to combat AD was published.30 Berberine (8) combats AD by inhibition of AChE, BChE and monoamine oxidase, by reduction of amyloid-β peptide level, and by lowering cholesterol level.30 (+)-Canadaline (9), canadine (53), bulbocapnine (50) and corydaline (52) from C. cava31,32 showed moderate activity against AChE, while tetrahydropalmatine (54) and protopine (27) from C. yanhusuo were inactive.29 More information about alkaloids from Corydalis species with AChE and BChE inhibitory effects are summarized in Table 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, PSA and MW should less than 10, 10, 5, 5, 140 and 500, respectively. log
P, PSA and MW should less than 10, 10, 5, 5, 140 and 500, respectively. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D5.5 is defined as log
D5.5 is defined as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P at pH 5.5 (pH of the small intestine) and is a more appropriate measure than log
P at pH 5.5 (pH of the small intestine) and is a more appropriate measure than log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P where oral drug absorption occurs. All physiochemical parameters used for the prediction of drug-likeness were calculated using Marvin Sketch (version 5.11, academic package, Chem Axon)
P where oral drug absorption occurs. All physiochemical parameters used for the prediction of drug-likeness were calculated using Marvin Sketch (version 5.11, academic package, Chem Axon)
| No. | MW | NROT | HBA | HBD | PSA | c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D5.5 D5.5 |
Bioavailability prediction |
|---|---|---|---|---|---|---|---|---|
| 1 | 327 | 2 | 5 | 2 | 62.18 | 2.86 | 1.98 | ✓ |
| 2 | 369 | 3 | 7 | 1 | 77.46 | 2.93 | 0.20 | ✓ |
| 3 | 355 | 3 | 7 | 2 | 86.25 | 2.55 | 0.08 | ✓ |
| 4 | 371 | 5 | 7 | 2 | 86.25 | 2.61 | 0.14 | ✓ |
| 5 | 371 | 5 | 7 | 2 | 86.25 | 2.61 | 0.14 | ✓ |
| 6 | 283 | 4 | 3 | 1 | 30.49 | 3.33 | 0.17 | ✓ |
| 7 | 283 | 4 | 3 | 1 | 30.49 | 3.33 | 0.17 | ✓ |
| 8 | 336 | 2 | 4 | 0 | 40.80 | −1.28 | −1.28 | ✓ |
| 9 | 369 | 5 | 6 | 0 | 57.23 | 3.05 | 1.39 | ✓ |
| 31 | 660 | 8 | 10 | 0 | 97.81 | 5.62 | 5.33 | ✗ |
| Compound | IC50 (µM) ± SD | Plant source | Reference | |
|---|---|---|---|---|
| AChE | BChE | |||
| Canadine (53) | 12.4 ± 0.9 | C. cava | 31 | |
| Protopine (27) | 16.1 | ND | C. speciosa | 35 |
| 14.5 ± 0.5 | C. turtschaninovii | 34 | ||
| 50 | C. ternata | 33 | ||
| Berberine (8) | 0.47 ± 0.01 | ND | C. yanhusuo | 29 |
| 1.87 ± 0.48 | >200 µM | C. saxicola | 36 | |
| 3.3 | C. speciosa | 35 | ||
| 4.7 ± 0.2 | ||||
| Palmatine (13) | 0.74 ± 0.06 | ND | C. yanhusuo | 29 |
| 2.20 ± 0.46 | >200 µM | C. saxicola | 36 | |
| 5.8 | C. speciosa | 35 | ||
| 10.4 ± 0.4 | C. turtschaninovii | 34 | ||
| Jatrorrhizine (20) | 2.08 ± 0.09 | ND | C. yanhusuo | 29 |
| Coptisine (15) | 1.01 ± 0.03 | ND | C. yanhusuo | 29 |
| Dehydrocorydaline (14) | 0.62 ± 0.05 | ND | C. yanhusuo | 29 |
| Dehydrocavidine (16) | 9.92 ± 0.23 | >200 µM | C. saxicola | 36 |
| 2,9-Dihydroxyl-3,11-dimethoxy-1,10-dinitrotetrahydroprotoberberine (69) | 8.77 ± 0.20 | >200 µM | C. saxicola | 36 |
| (+)-1-Nitroapocavidine (56) | 1.70 ± 0.31 | >200 µM | C. saxicola | 36 |
| (+)-Thalictrifoline (58) | 14.50 ± 0.20 | >200 µM | C. saxicola | 36 |
| Sanguinarine (61) | 1.93 ± 0.01 | >200 µM | C. saxicola | 36 |
| Stylopine (59) | 15.8 ± 1.2 | ND | C. turtschaninovii | 34 |
| Epiberberine (21) | 6.5 ± 0.5 | ND | C. turtschaninovii | 34 |
| Pseudodehydrocorydaline (22) | 8.4 ± 0.5 | ND | C. turtschaninovii | 34 |
| Pseudocoptisine (24) | 4.3 ± 0.3 | ND | C. turtschaninovii | 34 |
| Pseudoberberine (23) | 4.5 ± 0.2 | ND | C. turtschaninovii | 34 |
| Tacrine | 0.16 ± 0.01 | ND | ||
| Galantamine | 4.0 ± 1.4 | 1.4 ± 0.2 | ||
In spite of extensive in vitro studies on AChE inhibitory effects of CA, in vivo studies are limited. Kim et al. (1999) found that protopine (27) from C. ternata alleviates scopolamine-induced memory impairment in mice.33 In 2008, Bae et al. screened plants used in Korean folk medicine as memory enhancers and they found that C. turtschaninovii significantly inhibited AChE activity. Further studies led to the isolation and characterization of 16 alkaloids. Pseudoberberine (23) was tested for anti-amnesic activity in mice. The study revealed that pseudoberberine (23) (5.0 mg kg−1) significantly reversed cognitive impairments induced by scopolamine (1.0 mg kg−1, i.p.) in mice.34
2.3. Anti-platelet aggregation
Platelets are blood cells that play a crucial rule in haemostasis, but some pathological conditions including thrombosis and inflammation are attributed to these cells. These pathological conditions lead to cardiovascular disease. Hence, targeting platelets is an effective approach for prevention and treatment of some cardiovascular diseases. Although current anti-platelet aggregation (PA) drugs show serious side effects including dangerous bleeding, finding new drugs in this field is still necessary.38 Interestingly, some Corydalis species have traditionally been used for the treatment of thromboembolism and can be used for finding PA inhibitors.39In 1989, Wu et al. found that 100 µg ml−1 of protopine (27) isolated from C. tashiroi and C. pallida inhibited platelet aggregation caused by 20 µM of adenosine 5′-diphosphate (ADP), 100 µM of arachidonic acid (AA), 2 ng ml−1 of platelet activation factor (PAF) and 10 µg ml−1 of collagen, however, was unable to inhibit platelet aggregation caused by 0.1 U ml−1 of thrombin in rabbit platelet suspension.39 In 1994, Wei et al. investigated anti-platelet aggregation of tetrahydroberberine (76) isolated from C. ambigua. Tetrahydroberberine (76) (0.50 mg ml−1) showed more than 80% inhibition in PA induced by AA (0.78 mmol l−1), ADP (2 µmol l−1) and collagen (30 µg ml−1).40 In 2000, Lin et al. isolated three known alkaloids with one novel chlorinated alkaloid, chingazumianine (63), from C. koidzumiana and tested them for inhibition of human PA induced by ADP (20 µM), collagen (10 µg ml−1) and adrenaline (5 µM). In that study, 300 µM of protopine (27) completely inhibited PA induced by ADP, collagen and adrenaline. Tetrahydropalmatine (71) (300 µM) showed 10% inhibition of PA induced by ADP and collagen, and (83.9%) inhibition of PA caused by adrenaline. Almost similar results were observed for palmatine (13). In a lower concentration of 75 µM, these three alkaloids inhibited the secondary-phase aggregation, but not the primary-phase aggregation caused by adrenaline. These findings revealed that inhibition of PA by these alkaloids may be due to inhibition of thromboxane A2 formation.41 In 2001, Chen et al. evaluated anti-platelet aggregation activity of fourteen alkaloids isolated from C. tashiroi. 6-Methoxydihydrosanguinarine (66) (50 µg ml−1) and norjuziphine (67) (100 µg ml−1) were the most potent PA inhibitors and completely inhibited PA caused by AA (100 µM), collagen (10 µg ml−1) and PAF (2 ng ml−1). (−)-cis-Corydalmine N-oxide (77) and (−)-trans-corydalmine N-oxide (78) (100 µg ml−1) also showed 90% inhibitory activities.42
Wu and coworkers studied forty-one isoquinoline alkaloids with different structures for their PA inhibitory activities, however, they could not find any consistency between the structures and PA inhibitory activities of CA.
Overall, some of CA showed potent PA inhibitory activity. For example, 6-methoxydihydrosanguinarine (66) showed more potent PA inhibition than aspirin.42 Moreover, aspirin can only inhibit PA induced by AA, while CA inhibit PA caused by different inducers. Regarding the drug-likeness of CA, it seems that anti-platelet Corydalis alkaloids have a good potential for being future anti-platelet drugs.
2.4. Anti-inflammatory properties
Many natural products, particularly alkaloids and terpenes, possessed anti-inflammatory properties. Anti-inflammatory natural products have been fully reviewed by Gautam and Jachak.43 Barbosa-Filho et al. reviewed anti-inflammatory alkaloids isolated in the period of 1907 to 2000.44 They reviewed 171 alkaloids, of which, 137 alkaloids showed activity. Isoquinoline alkaloids were the most studied chemical structures.44 The first report on anti-inflammatory alkaloids from Corydalis species dates back to 1994, a study of anti-inflammatory activities of methanolic extract and alkaloids from C. turtschaninovii.45 Oral administration of 200 or 500 mg kg−1 of methanolic extract from C. turtschaninovii in rats inhibited increasing vascular permeability induced by acetic acid, and reduced acute paw edema induced by compound 40/80 (condensation product of N-methyl-p-methoxyphenethylamine with formaldehyde) and carrageenin. Adjuvant-induced edema in arthritis rats was suppressed by methanolic extract. Also, methanolic extract of C. turtschaninovii which contains dehydrocorydaline (14), D-glaucine (40) and L-tetrahydrocoptisine (74) inhibited histamine release from mast cells in rats.45 The same group investigated anti-inflammatory activities of dehydrocorydaline (14) from C. turtschaninovii on acute inflammatory models. Orally administered dehydrocorydaline (14) (0.125 mmol kg−1) inhibited increasing vascular permeability induced by acetic acid, inflammation induced by serotonin and bradykinin, ear edema induced by AA, prostaglandine E2 and leukotriene C4, and acute paw edema induced by carrageenin. However, dehydrocorydaline (14) was unable to inhibit the edema induced by histamine.46 Tetrahydropalmatine (71), an isoquinoline alkaloid from C. yanhusuo and many other plants, possess anti-inflammatory properties. Oh et al. studied effects of this compound on lipopolysaccharide-induced interleukin (IL)-8 production in the human monocytic cell line THP-1. Pretreatment of THP-1 cells with 0.2, 1 or 2 mM of tetrahydropalmatine (71) significantly inhibited IL-8 secretion induced by lipopolysaccharide. Their studies revealed the inhibitory mechanism of tetrahydropalmatine (71) on IL-8 formation via the mitogen-activated protein kinases (MAPKs) and the NF-κB signaling pathway inhibition.47 Pseudocoptisine (24) another isoquinoline alkaloid isolated from C. turtschaninovii inhibited pro-inflammatory mediators in lipopolysaccharide-stimulated murine macrophage RAW 264.7 cells. Pseudocoptisine (24) (30, 60 and 90 µM) significantly inhibited the production of IL-6 and TNF-α and reduced the levels of nitric oxide (NO) and cyclooxygenase 2. The mechanism of action of pseudocoptisine (24) involved in suppression of the production and mRNA expressions of inflammatory cytokines and inhibition of NF-κB.48 In another study, effects of 11 alkaloids isolated from C. impatiens on inhibition of TNF-α and NO production have been evaluated. Ochotensimine (93) showed the most potent TNF-α and NO inhibition with IC50 values of 1.64 and 1.14 µM, respectively.49 Overall, it seems that anti-inflammatory activity of CA is mainly due to inhibition of mediators and enzymes involving in inflammation process.2.5. Antiviral activities
In 2006, Orhan et al. studied antiviral activity of 33 isoquinoline alkaloids belonging to Fumaria and Corydalis on Herpes simplex (HSV) and Parainfluenza (PI-3) viruses. Protopine (27) was the most active alkaloid against PI-3 with minimum cytophatogenic effect inhibitory concentration of 1 µg ml−1 and maximum of 32 µg ml−1 in Vero cells which was comparable with oseltamivir as the reference with minimum and maximum concentrations of 0.25 and 32 µg ml−1, respectively.50 However, (−)-stylopine (59) and berberine (8) were inactive against both viruses. Also, (+)-bulbocapnine (50), palmatine (13), dehydrocorydaline (14) and dehydrocavidine (16) were totally inactive against HSV.Traditional background of C. saxicola in treatment of hepatitis has attracted scientists' attention to study anti-hepatitis B virus (HBV) activity of this plant. A study on antiviral activity of this plant was conducted by Wu and co-workers. Dihydrochelerythrine (79) exhibited the most potent inhibitory activities against hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) secretion with an IC50 value < 0.05 µM in Hep G 2.2.15 cell line while lamivudine as positive control showed IC50 values of 15.37 and 44.85 µM against HBsAg and HBeAg secretion, respectively.51 Dihydrochelerythrine (79) gave hope for finding new anti-HBV drugs in the future.52 Similar experiments were conducted by Li et al. Dehydrocavidine (16), dehydroapocavidine (25) and dehydroisoapocavidine (26) (15.6–250 µg ml−1) showed anti-HBsAg activity with average inhibition percentage of 53%, 59% and 54% and anti-HBeAg activity with average inhibition percentage of 41%, 43% and 43%, respectively.53 Recently, Zeng et al. evaluated anti-HBV virus activity of dehydrocheilanthifoline (18). After six days of treatment, dehydrocheilanthifoline (18) showed an inhibitory effect on HBsAg and HBeAg secretion from Hep G 2.2.15 cell line with IC50 values of 15.84 and 17.12 µM, respectively. In addition, they found that dehydrocheilanthifoline (18) acted through inhibition of extracellular DNA, intracellular DNA and covalently closed circular DNA.54 However, further studies are needed to understand the exact mechanism of action of this alkaloid.
Reviewing alkaloids isolated from C. saxicola revealed that protoberberine-type alkaloids are the most active alkaloid against HBV. The study showed that the introduction of quaternary nitrogen increased the anti-HBV activity. Interestingly, the presence of methyl group at C13 position of protoberberine-type alkaloids is vital for the activity (Fig. 4). For example, dehydroisoapocavidine (26) and berberine (8) have similar structures, however, dehydroisoapocavidine (26) had an extra methyl group at C13 position and exhibited potent anti-HBV activity, while berberine (8) was inactive.53
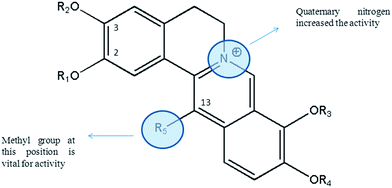 | ||
| Fig. 4 Structure–antiviral activity relationship of protoberberine alkaloids. Protoberberine structure was the best scaffold for anti-HBV properties. | ||
Further studies are necessary to unravel more aspects of structure–antiviral activity relationship of isoquinoline alkaloids, particularly protopine- and protoberberine-type scaffold.
2.6. Cardiovascular activities
In traditional medicine of China, Korea and Japan, C. yanhusuo has been used in patients suffered from cardiovascular diseases.62 The first report on beneficial effects of C. yanhusuo in heart failure after myocardial infarction dates back to 2006.62 Coronary artery ligated-rats were treated 8 weeks by orally administered ethanolic extract of C. yanhusuo. At doses 100 and 200 mg kg−1, C. yanhusuo significantly decreased left ventricular end-diastolic pressure from 19 ± 5 mmHg to 12 ± 2 and 9 ± 3 mmHg, respectively, as well as reduction in the plasma concentration of atrial natriuretic peptide. MI leads to hypertrophy of ventricles and increasing in weights of both ventricles and lungs. Treatment with 200 mg kg−1 of C. yanhusuo significantly reduced the weights of ventricles and lungs as well as infarct size in rats.62 The molecular mechanism of action of C. yanhusuo was provided by Ling and co-workers. They suggested that C. yanhusuo acted by the inhibition of myocardial apoptosis through modulation of the Bcl-2 family and this may provide the basis for novel therapeutic strategy in MI.63 Another study concerned the salutary effects of C. yanhusuo extract on cardiac hypertrophy due to pressure overload induced by transverse abdominal aorta constriction in rats.64 200 mg kg−1 of C. yanhusuo significantly reduced cardiac hypertrophy and heart rate in rats compared with vehicle-treated animals.64 The study was not led to the isolation and purification of bioactive alkaloids.
Further studies on L-tetrahydropalmatine (71), an active constituent of C. yanhusuo, showed cardioprotective activity in myocardial ischaemia-reperfusion injury induced by 30 min occlusion of the left anterior descending coronary artery and 6 h reperfusion in rats.65 L-Tetrahydropalmatine (71) (20 and 40 mg kg−1) reduced the infarct area/risk area ratio from 36.3 ± 3.9% in case of vehicle to 20.5 ± 2.2 and 18.6 ± 2.2%, respectively, while 10 mg kg−1 of this alkaloid was not effective. L-Tetrahydropalmatine (71) at doses of 10, 20 and 40 mg kg−1 significantly increased ejection fraction and fractional shortening when compared with sham-operated group. Moreover, mechanism of action of L-tetrahydropalmatine (71) involved in the reduction of iNOS-mediated NO biosynthesis and TNF-α.65
2.7. Antiplasmodial activities
In Bhutanese traditional medicine, some of Corydalis species have been used as febrifuge and antimalarial drugs including C. bhutanica, C. crispa and C. dubia.66 Recent efforts led to the isolation of some isoquinoline alkaloids from these Corydalis species with highly significant antimalarial activities. The extract of C. bhutanica did not show antiplasmodial activity even at the highest concentration of 25 µg ml−1. However, the chloroform extract of C. crispa showed significant activity against Plasmodium falciparum strains TM4/8.2 (a wild type chloroquine and antifolate sensitive strain) and K1CB1 (multidrug resistance strain) with IC50 values of 2.11 µg ml−1 and 1.89 µg ml−1, respectively. Similarly, the chloroform extract of C. dubia exhibited strong activity against TM4/8.2 and K1CB1 strains with IC50 values of 2.21 µg ml−1 and 2.84 µg ml−1, respectively.66 Further investigations on C. dubia resulted in the isolation of eight alkaloids. Among them, scoulerine (68), cheilanthifoline (63) and protopine (27) showed remarkable antiplasmodial activities against TM4/8.2 strain with IC50 values of 1.78, 0.9 and 1.45 µg ml−1, respectively.68 Almost similar results were observed for K1CB1 strain, however, scoulerine (68) was the most active alkaloid with an IC50 value of 1.04 µg ml−1. Chloroquine, which is a well-known antimalarial drug, exhibited IC50 values of 0.010 and 0.089 µg ml−1 against TM4/8.2 and K1CB1 strains, respectively.66Based upon the results from previous studies,66 Wangchuk and co-workers isolated nine isoquinoline alkaloids from C. crispa. Among the tested alkaloids coreximine (81) exhibited a potent antimalarial activity against TM4/8.2 and K1CB1 strains of P. falciparum with IC50 values of 5.56 and 6.87 µg ml−1, respectively.69 For more information please see Table 4.
| Samples | Antiplasmodial IC50 (µg ml−1) | Plant source(s) | |
|---|---|---|---|
| TM4/8.2 | K1CB1 | ||
| MeOH extract | 11.53 | 13.24 | C. crispa |
| 6.38 | 9.50 | C. dubia | |
| 1.00 | 2.56 | C. calliantha | |
| >6.25 | >6.25 | C. bhutanica | |
| CH2Cl2 extract | 12.09 | 11.27 | C. crispa |
| >6.25 | >6.25 | C. bhutanica | |
| Hexane extract | 14.57 | 15.82 | C. crispa |
| >1.56 | >1.56 | C. bhutanica | |
| CHCl3 extract | 2.11 | 1.89 | C. crispa |
| 2.21 | 2.84 | C. dubia | |
| >6.25 | >6.25 | C. bhutanica | |
| H2O extract | >25 | >25 | C. bhutanica, C. dubia and C. crispa |
| Crude alkaloid extract | 0.33 | 0.63 | C. calliantha |
| Protopine (27) | 1.45 | 1.38 | C. crispa |
| 13-Oxoprotopine (29) | >4.6 | >4.6 | C. crispa |
| Stylopine (59) | >4.0 | >4.0 | C. crispa |
| Coreximine (81) | 5.56 | 6.87 | C. crispa |
| Scoulerine (68) | 1.78 | 1.04 | C. dubia |
| Cheilanthifoline (90) | 0.90 | 1.22 | C. calliantha |
2.8. Miscellaneous activities
In animal studies, pure alkaloids of C. cava showed sedative properties,76,77 and further studies showed that this effect was induced by interaction between protoberberine alkaloids and GABAergic system.78 Protoberberine type alkaloids influenced the binding behavior of GABAA receptor, exhibited positive cooperation in binding of [3H] bicuculline (85) methochloride ([3H] BMC) and increased the specific binding of about 21–49%. Corydaline (52), isoapocavidine (82), isocorypalmine (83), scoulerine (68) and tetrahydropalmatine (54) significantly increased specific binding of [3H] BMC when compared with diazepam, and isoapocavidine (82) exhibited the most potent activity.77,78
Park and co-workers evaluated insecticidal activity of alkaloids from C. turtschaninovii against Aphis gossypii, which is a widely distributed pest of a variety of agricultural crops.85 At concentration of 1000 ppm, (+)-stylopine (59), (+)-corydaline (52), demethylcorydalmine (1), isocorypalmine (83), glaucine (40), and pseudoprotopine (30) exhibited significant insecticidal activity with inhibition values of 69.7, 46.9, 68.5, 75.5, 80.2, and 78.9%, respectively.85
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture against spore germination of some plant pathogenic fungi, including Alternaria, Curvularia, Colletotrichum, Helminthosporium and Ustilago.87 Also, (±)-α-hydrastine (47) and (±)-β-hydrastine (48) from C. longipes exhibited remarkable activity against Helminthosporium echinoclova.88
1 mixture against spore germination of some plant pathogenic fungi, including Alternaria, Curvularia, Colletotrichum, Helminthosporium and Ustilago.87 Also, (±)-α-hydrastine (47) and (±)-β-hydrastine (48) from C. longipes exhibited remarkable activity against Helminthosporium echinoclova.88Berberine (8) showed potent antifungal activity with MIC values of 5, 7.81, and 5.89 µg ml−1 against Candida albicans, C. tropicalis, and C. glabrata, respectively.89,90 Berberine (8) and its derivatives have attracted considerable attention as antifungal and antibacterial agents. The authors encourage the reader to review the articles provided in this regard.91–93
Protopine (27), (+)-bulbocapnine (50), (−)-canadine (53), corydalmine (91) and corydaline (52) from Corydalis species showed potent antifungal activity against Candida albicans with an MIC value of 4 µg ml−1 as compared with ketoconazole as a standard compound (MIC value = 2 µg ml−1).50
The petroleum ether extract of C. bungeana, a constituent of Chinese traditional medicine Zi Hua Di Ding, was tested against Bacillus subtilis and Pseudomonas syringae and at a concentration of 6.25 µg ml−1 exhibited a significant activity against B. subtilis.95 However, no alkaloids isolated from C. bungeana showed antibacterial activity against B. subtilis.95
A more comprehensive study on antibacterial activity of CA was conducted by Orhan and co-workers (2007).50 Thirty three isoquinoline alkaloids from Fumaria and Corydalis species were tested against Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, Acinetobacter baumannii, Staphylococcus aureus and Bacillus subtilis. Some of them showed a moderate activity (MIC = 8 µg ml−1) against K. pneumonia and A. baumannii, while the other alkaloids showed a weak activity (MIC ≥ 32 µg ml−1) against the other bacteria.50
Other biological activities have sporadically been reported from CA includes antiallergic activity,96 hepatoprotective activity,97,98 attenuation of cocaine self-administration and cocaine-induced reinstatement in rats,99 antiangiogenic activity,100 hypothermic effects,101 enhancement of gastrointestinal motor function102 and cancer chemopreventive activity.103 The results and mechanism of action are summarized in Table 5.
| Alkaloid | Biological activity | Result(s) | Mechanism of action(s) |
|---|---|---|---|
| Dehydrocavidine (16) (0.5 and 1 mg kg−1) | Hepatoprotective activity | Significantly reversed the elevating serum enzymes (ALT, AST, LDH and ALP) induced by CCl4 | Free radical scavenging activity, promoting collagenolysis and regulating fibrosis-related genes |
| Dehydrocorydaline (14) (0.5 mmol kg−1 p.o.) | Antiallergic activity | Inhibited 48 h homologous passive cutaneous anaphylaxis (type 1 allergic model) | Inhibition of antibody-mediated and cell-mediated allergic reactions |
| DL-Tetrahydropalmatine (54) (10–50 mg kg−1 i.p.) | Hypothermic activity | Induced hypothermia in rats | Acts through serotoninergic mechanisms to induce body temperature |
| C. yanhusuo alkaloids and berberine (8) | Anti-angiogenic activity | Showed powerful anti-angiogenic activity | Suppressed the upregulation of matrix metalloproteinase 2 induced by VEGF at mRNA and protein levels |
| L-Tetrahydropalmatine (71) 3.75, and 7.5 mg kg−1, i.p. | Treatment of cocaine addiction | Blocking both cocaine-induced reinforcing effects and cocaine craving | Acts on dopamine receptors |
| Tetrahydroberberine (76) | Enhances gastrointestinal motor function | Significant acceleration of gastric emptying | Acts on dopamine D2 and/or serotonin 5-HT1A receptors |
| Spallidamine (92) and 17 other alkaloids | Cancer chemopreventive activity | Strong cancer chemopreventive activity comparable with β-carotene as positive standard | Not reported |
3. Drug-like properties of CA
According to “rule of five” or Lipinski's rule an oral drug-like molecule should have an octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) of <5, <5 hydrogen bond donors (HBD), <10 hydrogen bond acceptors (HBA) and a molecular weight of <500 Da.6 Later on, two more requirements for drug-like molecules, proposed by Veber and co-workers, include NROT (number of rotatable bonds) of <10 and a PSA (polar surface area) of <140 Å2.7 This rule describes physico-chemical properties crucial for a drug's pharmacokinetics in the human body, including their absorption, distribution, metabolism, and excretion (ADME). However, the rule does not predict pharmacological activity/potency of a compound. Compounds do not violate more than two criteria are considered as drug-like compounds. For example, compounds 69 and 70 violated PSA criteria, however, they are still considered as drug-like compounds (Table 2).
P) of <5, <5 hydrogen bond donors (HBD), <10 hydrogen bond acceptors (HBA) and a molecular weight of <500 Da.6 Later on, two more requirements for drug-like molecules, proposed by Veber and co-workers, include NROT (number of rotatable bonds) of <10 and a PSA (polar surface area) of <140 Å2.7 This rule describes physico-chemical properties crucial for a drug's pharmacokinetics in the human body, including their absorption, distribution, metabolism, and excretion (ADME). However, the rule does not predict pharmacological activity/potency of a compound. Compounds do not violate more than two criteria are considered as drug-like compounds. For example, compounds 69 and 70 violated PSA criteria, however, they are still considered as drug-like compounds (Table 2).
All CA (except compound 31) interestingly satisfied the drug-like criteria (For more information about drug-like properties of other alkaloids, please see Table 2 in ESI†).
It is important to know, the world of drug-like compounds is quite limited, and filters for differentiating nondrug and drug-like compounds could certainly assist in drug discovery programs. The drug-like properties of CA ensure that CA will show acceptable ADME and translate effectively into clinical trials. The importance of drug-like properties encouraged researchers to design protocols that front-loaded of the desired physico-chemical properties at the first stage of isolation of bioactive natural products.104
In conclusion, drug-like properties of CA accompanied by their various biological activities make CA promising lead compounds in medicinal chemistry field.
4. Conclusion and future perspectives
Many natural drugs are discovered from medicinal plants that have a background of medicinal applications in the past. Corydalis species (CA) consists of more than 400 species in Eurasia and North America. Some Corydalis species such as C. yanhusuo has been traditionally used for the treatment of different diseases. Corydalis species are rich source of alkaloids, particularly isoquinoline alkaloids, with various biological activities including anti-cancer, acetylcholinesterase inhibitory, antimalarial, antiplatelet and antimicrobial activities. To date, different classes of isoquinoline alkaloids have been identified from this genus including aporphine, protopine, protoberberine, tetrahydroprotoberberine, benzo[c]phenanthridine, phthalideisoquinoline, benzylisoquinoline, morphinan (with a rearrangement in isoquinoline scaffold) and spirobenzylisoquinoline.As we mentioned in this paper, most of CA satisfied the criteria for lead-like compounds. This means, if we would find a bioactive CA that it could show an acceptable efficacy in in vivo studies, and would not have a remarkable toxicity, the compound has a great chance to translate effectively into clinically meaningful activity in patients. For instance, in response to the growing mass of in vitro and in vivo evidence for berberine's antihyperglycemic and antihyperlipidemic efficacy, a number of clinical trials have recently addressed the pharmacokinetics, safety, and efficacy of berberine in humans.105 In the United States, several randomized phase II/III/IV trials are investigating berberine's effects on a range of metabolic disorders. Five ongoing phase III/IV trials are studying berberine's effects on lipid profile. Three phase IV trials are investigating berberine's therapeutic effects in metabolic syndrome. Two phase IV trials are investigating berberine's therapeutic effects in polycystic ovary syndrome. Antihyperglycemic effects of berberine in diabetes mellitus type 2 are being investigated in two phase II/III trials. One phase II trial is studying berberine's therapeutic effects in non-alcoholic fatty liver disease. It seems that other CA can likewise translate into clinical trial if they show safety/efficacy in in vivo studies.
It is ultimately recommended that regarding the versatile biological properties of CA, these compounds may have even a broader range of biological applications in the future.
Acknowledgements
The authors would like to thank Dr Seyed Ahmad Emami for his scientific help.References
- K. Kubitzki, The families and Genera of vascular plants, Springer, 1993, vol. 2, pp. 315–316 Search PubMed.
- B. Liu, B. Shen, F. Guo and Y. Chang, Sep. Purif. Technol., 2008, 64, 242–246 CrossRef CAS.
- S. Tong, J. Yan and J. Lou, J. Liq. Chromatogr. Relat. Technol., 2005, 28, 2979–2989 CrossRef CAS.
- M. M. Hann and T. I. Oprea, Curr. Opin. Chem. Biol., 2004, 8, 255–263 CrossRef CAS PubMed.
- R. J. Quinn, A. R. Carroll, N. B. Pham, P. Baron, M. E. Palframan, L. Suraweera, G. K. Pierens and S. Muresan, J. Nat. Prod., 2008, 71, 464–468 CrossRef CAS PubMed.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS.
- D. F. Veber, S. R. Johnson, H.-Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615–2623 CrossRef CAS PubMed.
- K. H. Kim, I. K. Lee, C. J. Piao, S. U. Choi, J. H. Lee, Y. S. Kim and K. R. Lee, Bioorg. Med. Chem. Lett., 2010, 20, 4487–4490 CrossRef CAS PubMed.
- H. R. Kim, H. Y. Min, Y. H. Jeong, S. K. Lee, N. S. Lee and E.-K. Seo, Arch. Pharmacal Res., 2005, 28, 1224–1227 CrossRef CAS.
- S. U. Choi, N. l. Baek, S.-H. Kim, J. H. Yang, J. S. Eun, T. Y. Shin, J. P. Lim, J. H. Lee, H. Jeon and M.-Y. Yun, Arch. Pharmacal Res., 2007, 30, 151–154 CrossRef CAS.
- X. Cheng, D. Wang, L. Jiang and D. Yang, Chem. Biodiversity, 2008, 5, 1335–1344 CAS.
- I. P. Singh and S. Mahajan, Expert Opin. Ther. Pat., 2013, 23, 215–231 CrossRef CAS PubMed.
- Y. Sun, K. Xun, Y. Wang and X. Chen, Anticancer Drugs, 2009, 20, 757–769 CrossRef CAS PubMed.
- C. C. Lin, L. T. Ng, F. F. Hsu, D. E. Shieh and L. C. Chiang, Clin. Exp. Pharmacol. Physiol., 2004, 31, 65–69 CrossRef CAS PubMed.
- J. Kluza, B. Baldeyrou, P. Colson, P. Rasoanaivo, L. Mambu, F. Frappier and C. Bailly, Eur. J. Pharm. Sci., 2003, 20, 383–391 CrossRef CAS PubMed.
- C. Cordero, S. Gómez-González, C. León-Acosta, S. Morantes-Medina and F. Aristizabal, Fitoterapia, 2004, 75, 225–227 CrossRef CAS PubMed.
- S. Letašiová, S. Jantová and M. Múčková, Cancer Lett., 2006, 239, 254–262 CrossRef PubMed.
- Y. C. Wu, T. Yamagishi and K.-H. Lee, The Kaohsiung journal of medical sciences, 1989, 5, 409 CAS.
- T. S. Wu, Y. L. Leu, C. S. Kuoh, S. D. Jiang, C. F. Chen and K. H. Lee, J. Chin. Chem. Soc., 1997, 44, 357–359 CrossRef CAS.
- K. H. Kim, C. J. Piao, S. U. Choi, M. W. Son and K. R. Lee, Planta Med., 2010, 76, 1732–1738 CrossRef CAS PubMed.
- Z. Binkhathlan and A. Lavasanifar, Curr. Cancer Drug Targets, 2013, 13, 326–346 CrossRef CAS PubMed.
- Y. Lei, J. Tan, M. Wink, Y. Ma, N. Li and G. Su, Food Chem., 2013, 136, 1117–1121 CrossRef CAS PubMed.
- M. Z. El-Readi, S. Eid, M. L. Ashour, A. Tahrani and M. Wink, Phytomedicine, 2013, 20, 282–294 CrossRef CAS PubMed.
- S. Y. Park, Arch. Pharmacal Res., 2010, 33, 1589–1609 CrossRef CAS PubMed.
- P. Williams, A. Sorribas and M.-J. R. Howes, Nat. Prod. Rep., 2011, 28, 48–77 RSC.
- A. Adsersen, B. Gauguin, L. Gudiksen and A. K. Jäger, J. Ethnopharmacol., 2006, 104, 418–422 CrossRef PubMed.
- Z. Yang, D. Zhang, J. Ren, M. Yang and S. Li, Med. Chem. Res., 2012, 21, 734–738 CrossRef CAS.
- I. Orhan, B. Şener, M. I. Choudhary and A. Khalid, J. Ethnopharmacol., 2004, 91, 57–60 CrossRef CAS PubMed.
- H. T. Xiao, J. Peng, Y. Liang, J. Yang, X. Bai, X. Y. Hao, F. M. Yang and Q. Y. Sun, Nat. Prod. Res., 2011, 25, 1418–1422 CrossRef CAS PubMed.
- H.-F. Ji and L. Shen, Molecules, 2011, 16, 6732–6740 CrossRef CAS PubMed.
- J. Chlebek, K. Macakova, L. Cahlikovi, M. Kurfurst, J. Kunes and L. Opletal, Nat. Prod. Commun., 2011, 6, 607–610 CAS.
- A. Adsersen, A. Kjolbye, O. Dall and A. K. Jager, J. Ethnopharmacol., 2007, 113, 179–182 CrossRef CAS PubMed.
- S. R. Kim, S. Y. Hwang, Y. P. Jang, M. J. Park, G. J. Markelonis, T. H. Oh and Y. C. Kim, Planta Med., 1999, 65, 218–221 CrossRef CAS PubMed.
- T. M. Hung, M. Na, N. T. Dat, T. M. Ngoc, U. Youn, H. J. Kim, B. S. Min, J. Lee and K. Bae, J. Ethnopharmacol., 2008, 119, 74–80 CrossRef CAS PubMed.
- D. K. Kim, K. T. Lee, N. I. Baek, S. H. Kim, H. W. Park, J. P. Lim, T. Y. Shin, D. O. Eom, J. H. Yang and J. S. Eun, Arch. Pharmacal Res., 2004, 27, 1127–1131 CrossRef CAS.
- Q. Q. Huang, J. L. Bi, Q. Y. Sun, F. M. Yang, Y. H. Wang, G. H. Tang, F. W. Zhao, H. Wang, J. J. Xu, E. J. Kennelly, C. L. Long and G. F. Yin, Planta Med., 2012, 78, 65–70 CrossRef CAS PubMed.
- Z. Novak, J. Chlebek, L. Opletal, P. Jiros, K. Macakova, J. Kunes and L. Cahlikova, Nat. Prod. Commun., 2012, 7, 859–860 CAS.
- N. E. Barrett, L. Holbrook, S. Jones, W. J. Kaiser, L. A. Moraes, R. Rana, T. Sage, R. G. Stanley, K. L. Tucker, B. Wright and J. M. Gibbins, Br. J. Pharmacol., 2008, 154, 918–939 CrossRef CAS PubMed.
- F. N. Ko, T. S. Wu, S. T. Lu, Y. C. Wu, T. F. Huang and C. M. Teng, Thromb. Res., 1989, 56, 289–298 CrossRef CAS PubMed.
- B. Xuan, W. Wang and D. Li, Acta Pharmacol. Sin., 1994, 15, 133 CAS.
- S. C. Chan, M. I. Chung, M. H. Yen, G. H. Lien, C. N. Lin and M. Y. Chiang, Helv. Chim. Acta, 2000, 83, 2993–2999 CrossRef CAS.
- J. J. Chen, Y. L. Chang, C. M. Teng, W. Y. Lin, Y. C. Chen and I. S. Chen, Planta Med., 2001, 67, 423–427 CrossRef CAS PubMed.
- R. Gautam and S. M. Jachak, Med. Res. Rev., 2009, 29, 767–820 CrossRef CAS PubMed.
- J. M. Barbosa-Filho, M. R. Piuvezam, M. D. Moura, M. S. Silva, K. V. B. Lima, E. V. L. da-Cunha, I. M. Fechine and O. S. Takemura, Rev. Bras. Farmacogn., 2006, 16, 109–139 CrossRef CAS.
- M. Kubo, H. Matsuda, K. Tokuoka, S. Ma and H. Shiomoto, Biol. Pharm. Bull., 1994, 17, 262 CAS.
- H. Matsuda, K. Tokuoka, J. Wi and H. Shiomoto, Nat. Med., 1997, 51, 293–297 Search PubMed.
- Y. C. Oh, J. G. Choi, Y. S. Lee, O. O. Brice, S. C. Lee, H. S. Kwak, Y. H. Byun, O. H. Kang, J. R. Rho and D. W. Shin, J. Med. Food, 2010, 13, 1125–1132 CrossRef CAS PubMed.
- K. J. Yun, J. S. Shin, J. H. Choi, N. I. Back, H. G. Chung and K. T. Lee, Int. Immunopharmacol., 2009, 9, 1323–1331 CrossRef CAS PubMed.
- X.-F. Niu, H.-B. Xu, X. Liu, T. Fan and L. Qi, Chem. Nat. Compd., 2013, 49, 187–189 CrossRef CAS.
- I. Orhan, B. Ozcelik, T. Karaoglu and B. Sener, Z. Naturforsch., C: J. Biosci., 2007, 62, 19 CAS.
- Y.-R. Wu, Y.-B. Ma, Y.-X. Zhao, S.-Y. Yao, J. Zhou, Y. Zhou and J.-J. Chen, Planta Med., 2007, 73, 787–791 CrossRef CAS PubMed.
- C. Wohlfarth and T. Efferth, Acta Pharmacol. Sin., 2008, 30, 25–30 CrossRef PubMed.
- H. L. Li, T. Han, R. H. Liu, C. Zhang, H. S. Chen and W. D. Zhang, Chem. Biodiversity, 2008, 5, 777–783 CAS.
- F. L. Zeng, Y. F. Xiang, Z. R. Liang, X. Wang, D. E. Huang, S. N. Zhu, M. M. Li, D. P. Yang, D. M. Wang and Y. F. Wang, Am. J. Chin. Med., 2013, 41, 119–130 CrossRef CAS PubMed.
- J. K. Lee, J. G. Cho, M. C. Song, J. S. Yoo, D. Y. Lee, H. J. Yang, K. M. Han, D. H. Kim, Y. J. Oh and T. S. Jeong, J. Korean Soc. Appl. Biol. Chem., 2009, 52, 646–654 CrossRef CAS.
- S. Saeidnia and M. Abdollahi, Toxicol. Appl. Pharmacol., 2013, 271, 49–63 CrossRef CAS PubMed.
- L. H. Chu, F. L. Hsu, F. Y. Chueh, C. S. Niu and J. T. Cheng, J. Chin. Chem. Soc., 1996, 43, 489–492 CrossRef CAS.
- M. Lin, F. Chueh, M. Hsieh and C. Chen, Clin. Exp. Pharmacol. Physiol., 1996, 23, 738–745 CrossRef CAS PubMed.
- T. D. Hou, A. p. Liu, J. Zhang, F. Cheng and Y. Q. Gong, Xibei Shifan Daxue Xuebao, Ziran Kexueban, 2004, 40, 71–73 CAS.
- P. Chan, W. T. Chiu, Y. J. Chen, P. J. Wu and J. T. Cheng, Planta Med., 1999, 65, 340–342 CrossRef CAS PubMed.
- B. Xuan, D. Li and W. Wang, Acta Pharmacol. Sin., 1992, 13, 167 CAS.
- L. Wu, H. Ling, L. Li, L. Jiang and M. He, J. Pharm. Pharmacol., 2007, 59, 695–701 CrossRef CAS PubMed.
- H. Ling, L. Wu and L. Li, Phytother. Res., 2006, 20, 448–453 CrossRef CAS PubMed.
- C. Wen, L. Wu, H. Ling and L. Li, J. Pharm. Pharmacol., 2007, 59, 1159–1165 CrossRef CAS PubMed.
- Y. Han, W. Zhang, Y. Tang, W. Bai, F. Yang, L. Xie, X. Li, S. Zhou, S. Pan and Q. Chen, PLoS One, 2012, 7, e38627 CAS.
- P. Wangchuk, P. A. Keller, S. G. Pyne, M. Taweechotipatr, A. Tonsomboon, R. Rattanajak and S. Kamchonwongpaisan, J. Ethnopharmacol., 2011, 137, 730–742 CrossRef PubMed.
- P. Wangchuk, J. B. Bremner, R. Rattanajak and S. Kamchonwongpaisan, Phytother. Res., 2010, 24, 481–485 CrossRef CAS PubMed.
- P. Wangchuk, P. A. Keller, S. G. Pyne, A. C. Willis and S. Kamchonwongpaisan, J. Ethnopharmacol., 2012, 143, 310–313 CrossRef CAS PubMed.
- P. Wangchuk, P. A. Keller, S. G. Pyne, T. Sastraruji, M. Taweechotipatr, R. Rattanajak, A. Tonsomboon and S. Kamchonwongpaisan, Nat. Prod. Commun., 2012, 7, 575–580 CAS.
- G. Lin, S. S. K. Chan, H. S. Chung and S. L. Li, in Studies in Natural Products Chemistry, ed. R. Attaur, Elsevier, 2005, vol. 32, part L, pp. 611–669 Search PubMed.
- S. Piredda, C. R. Lim and K. Gale, Life Sci., 1985, 36, 1295–1298 CrossRef CAS PubMed.
- N. Chernevskaja, O. Krishtal and A. Valeyev, Toxicon, 1990, 28, 727–730 CrossRef CAS PubMed.
- C. K. Chang and M. T. Lin, Neurosci. Lett., 2001, 307, 163–166 CrossRef CAS PubMed.
- T. Chu and M. Lin, Neurosci. Lett., 1996, 218, 169–172 CrossRef CAS PubMed.
- M. T. Lin, J. J. Wang and M. S. Young, Neurosci. Lett., 2002, 320, 113–116 CrossRef CAS PubMed.
- M. T. Hsieh, S. H. Su, H. Y. Tsai, W. H. Peng, C. C. Hsieh and C. F. Chen, Jpn. J. Pharmacol., 1993, 61, 1 CrossRef CAS PubMed.
- H. Schäfer, H. Schäfer, W. Schneider and E. Elstner, Arzneim.-Forsch., Beih., 1995, 45, 124–126 Search PubMed.
- C. Halbsguth, O. Meißner and H. Häberlein, Planta Med., 2003, 69, 305–309 CrossRef CAS PubMed.
- C. Wang, S. Wang, G. Fan and H. Zou, Anal. Bioanal. Chem., 2010, 396, 1731–1740 CrossRef CAS PubMed.
- J. G. Choi, S. Y. Kang, J. M. Kim, D. H. Roh, S. Y. Yoon, J. B. Park, J. H. Lee and H. W. Kim, The Korean Journal of Physiology & Pharmacology, 2012, 16, 387–392 Search PubMed.
- F. L. Cao, G. W. Shang, Y. Wang, F. Yang, C. L. Li and J. Chen, Pharmacol., Biochem. Behav., 2011, 100, 199–204 CrossRef CAS PubMed.
- H. Chu, G. Jin, E. Friedman and X. Zhen, Cell. Mol. Neurobiol., 2008, 28, 491–499 CrossRef CAS PubMed.
- M. Miyazawa, K. Yoshio, Y. Ishikawa and H. Kameoka, J. Agric. Food Chem., 1998, 46, 1914–1919 CrossRef CAS.
- M. Miyazawa, K. Yoshio, Y. Ishikawa and H. Kameoka, Nat. Prod. Lett., 1996, 8, 299–302 CrossRef CAS.
- H. J. Park, M. Y. Baek, J. G. Cho, K. H. Seo, G. Y. Lee, S. J. Moon, E.-M. Ahn and N. I. Baek, J. Korean Soc. Appl. Biol. Chem., 2011, 54, 345–352 CrossRef CAS.
- W. G. Ma, Y. Fukushima and S. Tahara, Fitoterapia, 1999, 70, 358–365 CrossRef.
- N. Singh, S. Azmi, S. Maurya, U. Singh, R. Jha and V. Pandey, Folia Microbiol., 2003, 48, 605–609 CrossRef CAS.
- M. Goel, U. Singh, R. Jha, V. Pandey and M. Pandey, Folia Microbiol., 2003, 48, 363–368 CrossRef CAS.
- Y. Zhao, D. Yan, J. Wang, P. Zhang and X. Xiao, J. Therm. Anal. Calorim., 2010, 102, 49–55 CrossRef CAS.
- G.-X. Wei, X. Xu and C. D. Wu, Arch. Oral Biol., 2011, 56, 565–572 CrossRef CAS PubMed.
- B. Fang, C. H. Zhou and X. D. Zhou, Journal of International Pharmaceutical Research, 2010, 37, 105–109 CAS.
- I. P. Singh and S. Mahajan, Expert Opin. Ther. Pat., 2013, 23, 215–231 CrossRef CAS PubMed.
- G. Nair, S. Narasimhan, S. Shiburaj and T. Abraham, Fitoterapia, 2005, 76, 585–587 CrossRef CAS PubMed.
- Y. Li, C. Xu, Q. Zhang, J. Y. Liu and R. X. Tan, J. Ethnopharmacol., 2005, 98, 329–333 CrossRef PubMed.
- C. Xie, T. Kokubun, P. J. Houghton and M. S. Simmonds, Phytother. Res., 2004, 18, 497–500 CrossRef CAS PubMed.
- H. Matsuda, K. Tokuoka, J. Wu, H. Shiomoto and M. Kubo, Biol. Pharm. Bull., 1997, 20, 431 CAS.
- T. Wang, N. L. Sun, W.-D. Zhang, H. L. Li, G. C. Lu, B. J. Yuan, H. Jiang, J. H. She and C. Zhang, J. Ethnopharmacol., 2008, 117, 300–308 CrossRef CAS PubMed.
- T. Wang, L. J. Zhao, P. Li, H. Jiang, G. C. Lu, W. D. Zhang, H. L. Li and B. J. Yuan, J. Ethnopharmacol., 2011, 138, 76–84 CrossRef CAS PubMed.
- J. R. Mantsch, S. J. Li, R. Risinger, S. Awad, E. Katz, D. A. Baker and Z. Yang, Psychopharmacology, 2007, 192, 581–591 CrossRef CAS PubMed.
- J. L. Gao, J. M. Shi, S. M. Y. Lee, Q. W. Zhang and Y. T. Wang, Oncol. Res., 2009, 17, 11–12 CrossRef.
- M. T. Lin, F. Y. Chueh and M. T. Hsieh, Neurosci. Lett., 2001, 315, 53–56 CrossRef CAS PubMed.
- T. H. Lee, K. H. Kim, S. O. Lee, L. R. Lee, M. Son and M. Jin, J. Pharmacol. Exp. Ther., 2011, 338, 917–924 CrossRef CAS PubMed.
- C. Ito, M. Itoigawa, H. Tokuda, M. Kuchide, H. Nishino and H. Furukawa, Planta Med., 2001, 67, 473–475 CrossRef CAS PubMed.
- D. Camp, R. A. Davis, M. Campitelli, J. Ebdon and R. J. Quinn, J. Nat. Prod., 2011, 75, 72–81 CrossRef PubMed.
- Available from: http://clinicaltrials.gov/ct2/results?term=berberine%26Search=Search.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47944g |
| This journal is © The Royal Society of Chemistry 2014 |