Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications
Carlos M.
Costa
a,
Maria M.
Silva
b and
S.
Lanceros-Méndez
*ac
aCentro/Departamento de Física, Universidade do Minho, 4710-057 Braga, Portugal. E-mail: lanceros@fisica.uminho.pt; Fax: +351 253 604 061; Tel: + 351 253 604 073
bCentro/Departamento de Química, Universidade do Minho, 4710-057 Braga, Portugal
cINL—International Iberian Nanotechnology Laboratory, 4715-330 Braga, Portugal. E-mail: senentxu.lanceros.us@inl.int
First published on 3rd April 2013
Abstract
Poly(vinylidene fluoride), PVDF, and its copolymers exhibit interesting properties for use as separator membranes in lithium-ion battery applications. This review presents the developments and summarizes the main characteristics of these materials for battery separator membranes. The review is divided into three categories regarding the composition of the polymer membranes: single polymers, polymer composites and polymer blends. For each category, the main characteristics for battery separator membranes and the ion transport behaviour is presented. Finally, guidelines for further investigations are outlined.
 Carlos M. Costa | Carlos Miguel Costa graduated in Physics in 2005 and obtained his Master Degree in Materials Engineering in 2007. Currently, is a 3rd year PhD student in the Department of Physics at the University of Minho and is member of the Research Group in Electroactive Smart Materials at same university. His recent research focuses on the development of polymer based porous membranes for energy storage applications. |
 Maria M. Silva | Maria Manuela Silva obtained a Research Assistant position in the Department of Chemistry of the University of Minho (Braga, Portugal) in 1991. She received her PhD in Chemistry from the University of Minho in 1999 and was promoted to Assistant Professor at the same University. Her research activities focus on the synthesis and characterisation of solid polymer electrolytes (SPE) and their application in the domains of solid state electrochemistry (solid state batteries and electrochromic devices). She is also interested in the chemistry (synthesis via sol–gel) of silica-based organic-inorganic hybrid materials. |
 S. Lanceros-Méndez | S. Lanceros-Mendez graduated in physics at the University of the Basque Country, Leioa, Spain, in 1991. He obtained his Ph.D. degree in 1996 at the Institute of Physics of the Julius-Maximilians-Universität Würzburg, Germany. He was Research Scholar at Montana State University, Bozeman, MT, from 1996 to 1998 and visiting scientist at the A. F. Ioffe Physico-Technical Institute, St. Petersburg, Russia (1995), Pennsylvania State University, USA (2007) and University of Potsdam (2008). Since September 1998 he has been at the Physics Department of the University of Minho, Portugal, where he is associate professor and since 2012 he is also associate researcher at the INL-International Iberian Nanotechnology Laboratory. His work is focused in polymer based smart materials for sensors and actuators, energy and biomedical applications. |
1 Introduction
After Pike Research Consulting, the market of portable batteries will reach $30.5 billion dollars in 2015 with an annual growth rate of 8.5%.1 The most used type of portable batteries are lithium-ion batteries as they are light, cheap, show high energy density, low charge lost, no memory effect, prolonged service-life and high number of charge/discharge cycles. The market for lithium-ion (Li-ion) cells is mainly focused in portable electronic devices such as notebook computers and mobile phones. The first Li-ion batteries were commercialized 1991.2,3 This commercialization was preceded by several scientific achievements, including the pioneering work of Yazami4 regarding the use of lithium-graphite as a negative electrode. A Li-ion battery is an electrochemical cell that converts chemical energy into electrical energy.5,6 The basic constituents of an electrochemical cell are the anode, cathode and the separator, as illustrated in Fig. 1.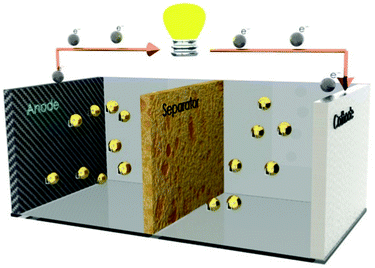 | ||
| Fig. 1 Schematic representation of the main components of a lithium-ion battery. | ||
The separator membrane separates the anode and the cathode and it is essential in all electrochemical devices.7,8 The role of the separators is to the serve as the medium for the transfer of the lithium ions between both electrodes and to control the number of lithium ions and their mobility.9 The separator is constituted by a polymer matrix soaked by the electrolyte solution, i.e., a liquid electrolyte where salts are dissolved in a solvent, water or organic molecules. Most commonly, the liquid electrolyte solution is composed by a lithium salt in a mixture of one or more solvents. The solvents present in the electrolyte solution must meet a combination of requirements for battery applications, which are, in some cases, not easy to achieve, as for example, high fluidity vs. high dielectric constant.10 The characteristics of an ideal solvent are high dielectric constant, for dissolving high salt concentrations; low viscosity, for improving ion transportation; to be inert to all cell components and to be in the liquid state in a wide temperature range. The nonaqueous solvents most used in electrolyte solutions belong to organic esters and ethers classes.11 In both classes, the most used solvents are ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), diethyl carbonate (DEC) and ethyl methyl carbonate (EMC). Other possibility for the fabrication of polymer electrolyte separators is by incorporating the lithium salts directly into the polymer matrix.12
A large diversity of requirements determine the performance of separator membranes for battery applications, such as low ionic strength, mechanical and dimensional stability, physical strength to allow easy handling, resistance to thermal and chemical degradation by electrolyte impurities and chemical reagents, to be easily wetted by liquid electrolytes and to show uniform thickness.9,12,13Table 1 summarizes the typical values and the relevance of the main requirements of lithium-ion battery separators, adapted from.12,13
| Parameter | Ideal Value | Relevance |
|---|---|---|
| Thickness (μm) | <25 | Determines the mechanical strength of the membrane and the risk of inner battery electrical shorting. |
| Electrical resistance (MacMullin no.) | <8 | Describes the relative contribution of a separator to cell resistance. |
| Gurley (s) | ∼25/mil | Expresses the time necessary for a specific amount of air to pass through a specific area of the separator with a specific pressure. |
| Porosity (%)/Pore Size (μm) | 40/<1 | Determines the permeability required for battery separators. |
| Shrinkage (%) | <5% in both MD and TD | Dimensional stability. The separator should not shrink when exposed to the electrolyte solution. |
| Tensile strength (%) | <2% offset at 1000 psi | The separator should stand mechanical stress between the electrodes. |
| Shutdown T (°C) | 130 | The temperature safety range of the battery that is provided by the separator. |
| High-temperature melt integrity | >150 | Separators with good mechanical properties at high temperatures may provide a larger safety margin for batteries |
| Skew (mm m−1) | <0.2 | When a separator is laid out, the separator should be straight and not bowed or skewed. |
The materials used as separators for batteries are mainly polymers or polymer composites with dispersed fillers of various types. The most used polymers are poly(ethylene) (PE),14,15 poly(propylene) (PP),16 poly(ethylene oxide) (PEO).17–19 poly(acrylonitrile) (PAN)20–22 and poly(vinylidene fluoride) (PVDF) and its copolymers.23–27 The most used fillers incorporated into the polymer hosts are inert oxide ceramics (Al2O3, SiO2, TiO2), molecular sieves (zeolites), ferroelectric materials (BaTiO3) and carbonaceous fillers, among others, with the main function of increasing the mechanical stability and/or ionic conductivity of the separator.28
Fig. 2 illustrates the increasing number of published scientific articles related to lithium ions battery separators and polymer electrolytes.
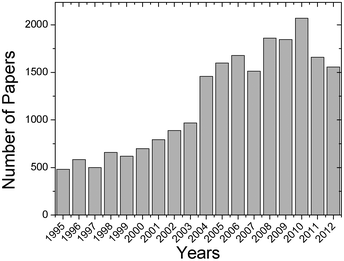 | ||
| Fig. 2 Research articles published on battery separators and polymer electrolytes for lithium ion battery applications. Search performed in Scopus database with the keywords “battery separators” and “polymer electrolytes”. | ||
The strong growth of work in this field in the past decade results from the development of new materials and processing techniques, which allows rapid and efficient technology transfer of the novel developed materials.
PVDF is semi-crystalline polymer in which the amorphous chains are embedded between the lamellar crystalline structures with a degree of crystallinity ranging from 40% to 60%. It exhibits four polymorphs called α, β, γ, δ.29,30 The most common and important polymorphs of PVDF are the α- and β-phases. The α-phase is non-polar, it is the phase thermodynamically more stable when the material is obtained from the melt and when the solvent is evaporated at temperatures above 80 °C.31 The β-phase is the most interesting phase for technological applications due to its electroactive properties: piezoelectric, pyroelectric and ferroelectric.32 The β-phase is obtained with a porous microstructure directly by solution at crystallization temperatures below 70 °C33 or by mechanical stretching of the α-phase at temperatures between 70 °C and 100 °C.34 The dielectric constant of the β-phase ranges between 10 at 13 and the conformational repeating unit (planar zigzag, all-trans) has a dipolar moment of 7 × 10−30 Cm.35
The semi-crystalline copolymer poly(vinylidene fluoride-co-trifluoroethylene), P(VDF-TrFE), shows, for specific molar ratios of VDF and TrFE, a polar ferroelectric transplanar chain conformation similar to the one of the β-phase of PVDF.36 P(VDF-TrFE) exhibits the ferroelectric (FE)-paraelectric (PE) phase transition at a Curie temperature, Tc, below the melting temperature, Tm. Both temperatures depend on the crystallization conditions and molar ratio of VDF and TrFE.37–39 The copolymer poly(vinylidene fluoride-co-hexafluoropropene), P(VDF-HFP), is also a semi-crystalline polymer with a degree of crystallinity significantly reduced due to the addition of hexafluoropropylene (HFP).40 Therefore, it shows high flexibility as compared to PVDF41 and a dielectric constant of 8.4.
In the copolymer poly(vinylidene fluoride-co-chlorotrifluoroethylene), P(VDF-CTFE), the amount of chlorotrifluoroethylene, CTFE is essential for determining properties and applications.42 For 25–70% mol of VDF, the P(VDF-CTFE) is amorphous43 being for the remaining concentrations a semicrystalline copolymer with a hexagonal structure.44 The dielectric constant of P(VDF-CTFE) is 1345 and shows high electromechanical response for 9 and 12 mol% CTFE content.46
PVDF and its copolymers poly(vinylidene fluoride-co-trifluoroethylene), PVDF-TrFE, poly(vinylidene fluoride-co-hexafluoropropylene), PVDF-HFP, and poly(vinylidene fluoride-co-chlorotrifluoroethylene), PVDF-CTFE show strong advantages for their use as separator membranes in comparison to polyolefins47 and other used materials due to their strong polarity (high dipolar moment) and high dielectric constant for a polymer material, which can assist ionization of lithium salts. It is also possible to control the porosity of the materials through binary and ternary polymer/solvent systems. Further, they are wetted by organic solvents, chemically inert, show good contact between electrode and electrolyte and are stable in cathodic environment.48–56 Different processing techniques, such as solvent casting, electrospinning and hot-press have been used for the development for battery separators from these materials.57–62
This review focused on battery separators and polymer electrolytes based on PVDF and its copolymers, PVDF-HFP, PVDF-TrFE and PVDF-CTFE, for lithium-ion battery application due to the recent advances and their large potential for energy storage applications. A summary of the obtained results will allow establishing the maturity of these materials for the intended purpose as well as to reflect on the future steps to be taken both in research and technology transfer.
This review is structured in three sections devoted to the state of the art in single polymers, composites and polymer blends, respectively. For each section, the materials and electrolyte solutions will be presented as well as the main characteristics of the materials, such as porosity, ionic conductivity and related properties. Some remarks on the electrodes of batteries based on the aforementioned separators will be provided and, finally, the main problems and future directions will be presented.
2 Polymer electrolytes based on poly(vinylidene fluoride) and its copolymers
2.1 Single polymer and copolymers
Fluorinated polymers such as PVDF and its copolymer show advantages when compared to commercial polyolefine separators (PE) due to their high polarity and dielectric permittivity, which provides larger affinity with polar liquid electrolytes. The characteristics of the developed PVDF and copolymer membranes are summarized in Table 2 as achieved in chronological order.| Material | Electrolyte solution/lithium ions | Porosity (%)/Fiber Diameter* (electrospun) (nm) | Uptake (%) | σ i/(S cm−1) at 25 °C | Ref. |
|---|---|---|---|---|---|
| PVDF-HFP | 1 M LiPF6 in EC/PC | — | 60 | 0.8 × 10−3 | 68 |
| PVDF | 1 M LiPF6 + PC/EC/3DMC | 70 | 65 | 3.7 × 10−3 | 63 |
| PVDF | 1 M LiTFSI in EC/DEC (2/3 in volume ratio) | — | — | 6.7 × 10−3 | 27 |
| PVDF | 10% LiBF4 in EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 3.4 × 10−4 | 69 |
| PVDF | 10% LiPF6 in EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 4.7 × 10−4 | 69 |
| PVDF | 10% LiAsF6 in EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 6.6 × 10−4 | 69 |
| PVDF | 1 M LiTFSI in EC/DEC | 0 | 20 | 5.6 × 10−8 | 72 |
| PVDF | 1 M LiTFSI in EC/DEC | 23 | 32 | 2.7 × 10−6 | 72 |
| PVDF | 1 M LiTFSI in EC/DEC | 30 | 41 | 1.0 × 10−6 | 72 |
| PVDF | 1 M LiTFSI in EC/DEC | 70 | 60 | 9.8 × 10−5 | 72 |
| PVDF | 1 M LiTFSI in EC/DEC | 75 | 65 | 1.3 × 10−4 | 72 |
| PVDF-HFP | 1 M LiPF6 in 1/1 w/w (EC/DEC) | — | — | 1.5–2.0 × 10−3 | 73 |
| PVDF | 1 M LiPF6 in 1/1 w/w (EC/DEC) | 23 | 33 | 2.2 × 10−5 | 74,75 |
| PVDF | 1 M LiPF6 in 1/1 w/w (EC/DEC) | 30 | 39 | 2.4 × 10−5 | 74,75 |
| PVDF | 1 M LiPF6 in 1/1 w/w (EC/DEC) | 38 | 45 | 1.5 × 10−4 | 74,75 |
| PVDF | 1 M LiPF6 in 1/1 w/w (EC/DEC) | 71 | 77 | 1.0 × 10−3 | 74,75 |
| PVDF-HFP | 1 M LiClO4–EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
83 | 220 | 1.5 × 10−3 | 76 |
| PVDF | 1 M LiPF6–EC/PC | — | — | 2.0 × 10−3 | 77 |
| PVDF | 1 M LiPF6–EC/DMC/DEC (2/2/1) | 70 | 142 | 5.0 × 10−2 | 78 |
| PVDF-HFP | 1 M LiPF6–EC/DMC (1/1) | 23 | 76.4 | 0.3 × 10−3 | 79 |
| PVDF | 1 M LiTFSI in distilled water | 100–800* | 50–73 | 1.6–2.0 × 10−3 | 64 |
| PVDF-HFP | 1 M LiBF4 in 1/3 w/w (EC/GBL) | — | 120 | 3.4 × 10−3 | 80 |
| PVDF | 1 M LiPF6–EC/DMC/DEC (2/2/1) | — | — | 3.5 × 10−3 | 81 |
| PVDF | 20 wt% LiClO4 | — | — | 8.7 × 10−4 | 82 |
| PVDF | 1 M LiPF6–EC/DMC/DEC (2/2/1) | 70 | — | 3.1 × 10−3 | 83 |
| PVDF-HFP | 1 M LiPF6–EC/DEC (1/1) | 70–90 | — | 1.2 × 10−3 | 84 |
| PVDF | LiBF4–PC:EC | — | — | 1.0 × 10−3 | 85 |
| PVDF | EC/PC/LiPF6 = 43/43/7 (in wt%) | — | — | 1.0 × 10−3 | 86 |
| PVDF | 15 wt% of LiFePO4 | — | — | 6.7 × 10−6 | 87 |
| PVDF | 1 M LiPF6-EC/DMC/DEC (1/1/1). | 750–1630* | 300–400 | 6.7 × 10−2 | 88 |
| PVDF-HFP | 0.5 M LiTFSI in BMITFSI | <1000* | 750 | 2.3 × 10−3 | 65 |
| PVDF-HFP | 0.5 M LiBF4 in BMIBF4 | <1000* | 600 | 2.3 × 10−3 | 65 |
| PVDF-HFP | 1 M LiPF6 in EC/DMC | 59 | 165 | 9.1 × 10−2 | 89 |
| PVDF-HFP | 1 M LiCF3SO3 in TEGDME | 59 | 210 | 1.8 × 10−2 | 89 |
| PVDF | 1 M LiPF6-EC/DMC/EMC (1/1/1) | 70 | 230 | 1.4 × 10−3 | 50 |
| PVDF | 1 M LiCF3SO3 in TEGDME/DIOX (1/1) | — | 250 | 0.6 × 10−3 | 90 |
| PVDF-HFP | 1 M LiPF6-EC/DMC/EMC (1/1/1) | — | — | 1.8 × 10−3 | 91 |
| PVDF-HFP | 1 M LiPF6-EC/DMC (1/1) | 78 | 321 | 3.36 × 10−4 | 92 |
| PVDF-HFP | 1 M LiPF6-EC/DMC/DEC (1/1/1) | 70 | — | 1.4 × 10−3 | 66 |
| PVDF | 1 M LiPF6-EC/DMC/EMC (1/1/1) | — | 230 | 4.8 × 10−3 | 67 |
| PVDF-HFP | 0.3 M Mg(CF3SO3)2 in EMITf | — | — | 4.8 × 10−3 | 93 |
| PVDF | 1 M LiPF6-EC/DMC (1/1) | 77 | — | 1.9 × 10−3 | 56 |
| PVDF-CTFE | 1 M LiPF6-EC/DMC (1/1) | 230* | 800 | 2.0 × 10−3 | 94 |
| PVDF | 50 wt% LiTFS | — | — | 1.7 × 10−2 | 95 |
| PVDF-HFP | 40 wt% LiTf | — | — | 7.8 × 10−5 | 96 |
| PVDF-HFP | 0.8 M LiTFSI in 1 g 13TFSI | — | 670 | 3.2 × 10−4 | 97 |
| PVDF-HFP | LiTFSI-PC (0.15/0.3 wt%) | — | — | 1.0 × 10−5 | 98 |
| PVDF | 1 M TEABF4 in AN | 80 | 117 | 1.8 × 10−3 | 53 |
| PVDF | 1 M LiPF6-EC/DEC (4/6) | 48 | 142 | — | 99 |
| PVDF-TrFE | 1 M LiClO4-PC | 72 | 255 | 2.7 × 10−7 | 23,100 |
| PVDF-TrFE | LiClO4·3H2O (n = 1.5) | 67 | — | 3.5 × 10−11 | 101 |
The porous battery separators of fluorinated polymers are most commonly obtained by phase inversion processes such as thermal induced phase separation (TIPS), using solvent and non-solvent system and electrospinning.27,63–65
The achieved porosity of the battery separators ranges between 0 to 90% and the pore size from 0.5 μm to 16 μm.23,63 Porous membranes with controlled porosity and pore sizes of 2 μm66 and 1 μm67 were also obtained by adding urea and salicylic acid, respectively, as foaming agents for PVDF or PVDF-HFP.
In 1996, Tarascon et al. produced the first Li-ion battery with a fluorinated polymer (PVDF-HFP) as battery separator.68 The performance of such a battery compares favourably in terms of gravimetric or volumetric energy density, life cycle, power rate and self-discharge with its liquid counterparts, while having enhanced safety characteristics, larger shape flexibility and scale ability. One of the main advantages of fluorinated polymers is their ability to be tailored in different geometries, including very thin cells.
Kataoka et al.27 showed that the ionic conductivity depends on the immersion time of the polymer membrane in the electrolyte solution and on the aging time after removal from the solution. PVDF for polymer electrolytes is optimized with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EC
1 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC plasticizer in salts such as LiAsF6 (lithium hexafluoroarsenate), LiPF6 (Lithium hexafluorophosphate) and LiBF4 (Lithium tetrafluoroborate). Nevertheless, LiAsF6 gives better results for ionic conductivity than LiBF4 and LiPF6, irrespective of the nature of the polymer and the amount of plasticizer.69 Salts with a polarizing cation and a large anion with a well delocalized charge, and therefore with low lattice energy, are the most suitable for polymer electrolytes.70
PC plasticizer in salts such as LiAsF6 (lithium hexafluoroarsenate), LiPF6 (Lithium hexafluorophosphate) and LiBF4 (Lithium tetrafluoroborate). Nevertheless, LiAsF6 gives better results for ionic conductivity than LiBF4 and LiPF6, irrespective of the nature of the polymer and the amount of plasticizer.69 Salts with a polarizing cation and a large anion with a well delocalized charge, and therefore with low lattice energy, are the most suitable for polymer electrolytes.70
Modifications of the properties of PVDF have been achieved by radiation grafting for improving adhesion to electrodes, leading to good rate performance and stable cycle life.71
From Table 2 it is observed that PVDF and PVDF-HFP with LiPF6 and LiCF3SO3 in different organic solvents lead to the best values of ionic conductivity (1.8 − 5 × 10−2 S cm−1).
PVDF polymer as battery separator was found to be effective in enhancing the lithium transport number due to selective interactions with the anion. The ionic conductivity of PVDF is associated to the total solution uptake, which depends on the gelation process related to porosity and pore size. The solution introduced in the polymer is stored in the pores and then penetrates into the polymer, swelling the polymer network.72 Other possibility for obtaining polymer electrolytes taking advantage of the properties of PVDF is by coating a microporous polyolefin membrane with a fluorinated polymer.73 The cells with these polymer electrolytes showed good electrochemical and rate performance during cycling. Ideal membranes for porous polymer electrolytes based on PVDF for battery applications should present high porosity and small pore diameters with a narrow distribution. Experimental results show that porosity should be >80% and pore diameter should be <1 μm.76 This porous structure has been also achieved with electrospun nanofiber webs.64
The effect of the liquid organic solvents in PVDF microporous membranes was studied by Saunier et al.81 It was observed that the affinity of PVDF for the liquid electrolyte may affect its mechanical strength and compromise battery safety. This indicates that the thermal and mechanical stability are affected when too much solvent is incorporated into the polymer. The reversible modifications can also affect the membrane properties, as the glass transition and melting temperature are lowered.81
The ionic conductivity of the PVDF microporous membranes is also affected by solvent/polymer and solvent/salt interactions, ionic dissociation and tortuosity value.83 It was proven also that interactions between PVDF and PC mainly occur in the surface area of the PVDF crystalline phase, whereas interactions between PC, PVDF, and lithium salt mainly occur in the amorphous area.49
It was also observed that ionic conductivity decreases in the order EC/DEC > EC/EMC > EC/DMC among the electrospun PVDF fiber-based polymer electrolytes with the same weight fraction of EC.88
For PVDF-HFP-based solid polymer electrolytes, lithium triflate salt effectively reduces the degree of crystallinity of the polymer and increases the ionic conductivity of the membrane.96 Costa et al. demonstrated which parameters-porosity, treatment of lithium ion and processing technique-influence at most the electrical performance of PVDF-TrFE membranes for battery separators. The membrane that exhibits the highest ionic conductivity is a porous membrane prepared by the uptake technique. The performance of the membrane for battery applications are, therefore, strongly influenced both by porosity and processing technique due to its influence in the specific surface available for lithium ion adsorption and trapping.102
The ionic conductivity depends not only on the characteristics of the electrolyte solution but also on the properties of the membrane—porosity and pore size—as shown in Fig. 3.
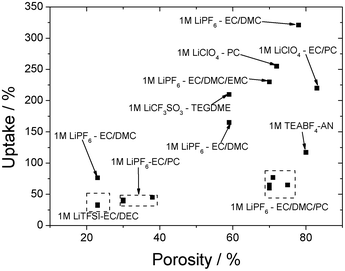 | ||
| Fig. 3 Porosity vs. uptake for various electrolyte solutions incorporated into PVDF membranes. | ||
In Fig. 3, it is observed that for the same porosity are obtained different uptake ratios and ionic conductivities (Table 2) due to the interactions with the cations and anions produced from the Li salts by the solvation process. The viscosity of the solvent also influences the transport and the transference numbers of the ions.103,104
Therefore, the main problem still to be optimized for battery separators persists: to obtain a combination of good ionic conductivity with high uptake ratio and excellent mechanical properties without deterioration of the ionic conductivity in the temperature range of the lithium-ion battery operation.
PVDF was proven to battery separator by Yamamoto et al. in a 4.4 V Li-ion polymer battery. The discharge capacity reached 520 Wh l−1 and the capacity retention ratio was 91.4% at 3C.86
2.2 Polymer and copolymer composites
To solve some of the problems existing in single polymer membranes, battery separators have been developed by the incorporation of suitable fillers into the host polymer for improving mechanical strength, thermal stability and ionic conductivity. Among these fillers are oxide ceramic, zeolites, ferroelectric ceramics, carbon, etc.28,105 These fillers can be divided into two groups: the fillers that participate in the ionic conduction process and the fillers that are not involved in the lithium transport process.28The characteristics of PVDF and copolymer composites for separator membranes are summarized in Table 3 in chronological order.
| Material | Fillers | Electrolyte solution/lithium ions | Porosity (%) | Uptake (%) | σ i/(S cm−1) at 25 °C for maximum amount | Ref. |
|---|---|---|---|---|---|---|
| PVDF-HFP | SiO2 | 1 M LiPF6 in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 100–250 | 0.87–3.1 × 10−3 | 106 |
| PVDF-HFP | MgO | 1 M LiPF6 in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 40 | 4.0 × 10−4 | 107 |
| PVDF | SiO2 | 1 MLiPF6 in EC/PC (1/1) | — | — | — | 112 |
| PVDF | SiO2 | 1 M LiClO4 in EC-PC (1/1) | — | — | — | 112 |
| PVDF | SiO2 | 1 M LiPF6 in EC-PC (1/1) | — | — | 3.5 × 10−2 | 113 |
| PVDF-HFP | MMT | LiCF3SO3 in PC | — | — | 1.0 × 10−3 | 108 |
| PVDF-HFP | SiO2 | 1 M LiTFSI in EC/DEC (1/1) | 77 | — | 2.7 × 10−2 | 114 |
| PVDF-HFP | SBA-15 | 1 M LiPF6 in EC/DMC/EMC (1/1/1) | 59 | 76 | 0.8 × 10−3 | 111 |
| PVDF-HFP | MCM-41 | 1 M LiPF6 in EC/DMC/EMC (1/1/1) | 14 | 30 | 4.6 × 10−2 | 111 |
| PVDF-HFP | NaY | 1 M LiPF6 in EC/DMC/EMC (1/1/1) | 9 | 39 | 3.0 × 10−3 | 111 |
| PVDF-HFP | TiO2 | DMBITFSI/LiPF6 | — | — | 1.3 × 10−3 | 115 |
| PVDF-HFP | AlO[OH]n | 5 wt% of LiN(CF3SO2)2 | — | — | 1.1 × 10−2 | 116 |
| PVDF-HFP | TiO2 | LiClO4 in EC/PC | 26 | 110 | 4.1 × 10−2 | 117 |
| PVDF-HFP | MgO | LiClO4 in EC/PC | 27 | 62 | 3.7 × 10−2 | 117 |
| PVDF-HFP | ZnO | LiClO4 in EC/PC | 23 | 61 | 5.5 × 10−2 | 117 |
| PVDF-HFP | MCM-41 | LiClO4 in EC/PC | 42 | 93 | 6.1 × 10−2 | 117 |
| PVDF-HFP | SBA-41 | LiClO4 in EC/PC | 52 | 82 | 5.0 × 10−2 | 117 |
| PVDF-HFP | MMT | 1 M LiPF6 in EC:DMC (1/1) | — | 40 | 2.5 × 10−3 | 118 |
| PVDF-HFP | LiAlO2 | 1 M LiClO4 in EC:DEC (1/1) | 87 | 121 | 8.1 × 10−3 | 119 |
| PVDF-HFP | ZrO2 | 1 M LiClO4 in EC:DEC (1/1) | 86 | 91 | 11 × 10−3 | 120 |
| PVDF-HFP | TiO2 | 1 M LiPF6 in EC/DMC/DEC (1/1/1) | 67 | — | 0.9 × 10−3 | 121 |
| PVDF-HFP | SiO2 | 1 M LiClO4 in EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 4.3 × 10−3 | 122 |
| PVDF-HFP | SiO2 | LiClO4 + PC + DEC | — | — | 1.0 × 10−2 | 123 |
| PVDF-HFP | TiO2 | 1 M LiPF6 in EC/DMC (1/1) | — | 125 | 1.0 × 10−3 | 124 |
| PVDF-HFP | TiO2 | 1 M LiPF6 in EC/DMC (1/1) | 60 | 359 | 1.7 × 10−3 | 92 |
| PVDF-HFP | MgO | 1 M Mg(ClO4)2 in EC/PC (1/1) | — | — | 8.0 × 10−3 | 125 |
| PVDF-HFP | DMOImPF6 | 0.5 M NH4PF6 | — | — | 3.0 × 10−5 | 126 |
| PVDF | SiO2 | — | 136 | — | — | 127 |
| PVDF-HFP | BaTiO3 | LiBETI + EC + PC | — | — | 0.8 × 10−3 | 128 |
| PVDF-HFP | Al2O3 | 1 M LiPF6 in EC/DEC (1/1) | — | — | — | 129 |
| PVDF-HFP | Effervescent disintegrant | 1 M LiPF6 in DMC/EC/EMC (1/1/1) | 55 | — | 1.2 × 10−3 | 130 |
| PVDF-HFP | α-MnO2 | 1 M LiTFSI-PMMITFSI | — | — | 1.3 × 10−3 | 131 |
| PVDF | MCM−41 + SO42−/ZrO2 | 1 M LiPF6 in EC/DMC/DEC (1/1/1) | 62 | 161 | 1.0 × 10−3 | 132 |
| PVDF-HFP | SiO2 | 1 M LiPF6 in EC/DEC (1/1) | 68 | — | 0.61 | 133 |
| PVDF | Fe2O3, SnO2 and CoO | 1 M LiPF6 in EC/DMC (2/1) | — | — | — | 134 |
| PVDF | Organic clays | — | 75 | — | — | 135 |
| PVDF | MMT | 1 M LiClO4 in PC/DEC (1/1) | — | 177 | 2.3 × 10−3 | 109 |
| PVDF | TiO2 | 1 M LiPF6 in EC/DMC (1/1) | 65–79 | — | — | 136 |
| PVDF-HFP | SiO2 | 1 M NaTf in EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 4.1 × 10−3 | 137 |
| PVDF | SiO2 | 1 M LiPF6 in EC/DMC (1/1) | 75 | — | 1.4 × 10−3 | 138 |
| PVDF-HFP | SiO2 | 1 M LiPF6 in EC/DEC (1/1) | 61 | — | 0.9 × 10−3 | 139 |
| PVDF-HFP | Cellulose | 1 M LiTFSI in BMPyrTFSI | 58 | 712 | 4.0 × 10−4 | 140 |
| PVDF-TrFE | MMT | 1 M LiClO4·3H2O-PC | 90 | 335 | 8.0 × 10−7 | 141 |
| PVDF-TrFE | NaY | 1 M LiClO4·3H2O-PC | 36 | 233 | 2.0 × 10−6 | 142 |
From Table 3 it is observed that separator membranes with the different fillers increase the ionic conductivity with respect to the pristine polymer matrix (Table 2), the characteristics/properties of fillers playing an important role in the conduction mechanism of separator membranes.
Du Pasquier et al. showed that the combination of a phase-inversion process and the presence of finely divided silica in the separator results in the formation of a stable porous structure, in which the pores are mechanically reinforced by the silica particles at their inner surface and the ionic conductivity of the PVDF-HFP membrane increases.106
The addition of MgO fillers increases the compatibility between separator and electrodes (anode and cathode) and batteries with these membranes exhibit high power density (at 3C rate was > 280 W kg−1).107
Some authors verified that the presence of Montmorillonite (MMT) fillers have an effect on the nano-scale microenvironment for composite materials and a positive increment of the charge carriers and its mobility, the membranes exhibiting high electrochemical characteristics for Li-ion battery applications.108 Further, this filler is adequate for battery separators as it enhances the uptake of liquid electrolyte due to the excellent affinity of clays towards electrolyte molecules.109
The effect of powder particle size on battery separator was studied by Takemura et al. It was observed that composites with 0.01 μm ceramic powders (Al2O3) showed excellent cycling properties.110
The addition of molecular sieves has expanded the electrochemical stability window of polymer electrolytes, enhanced the interfacial stability of polymer electrolyte with lithium electrodes, and inhibited the crystallization of the PVDF-HFP matrix.111
Stephan et al. verified that the incorporation of inert fillers reduces the crystallinity of the polymer host, acts as ‘solid plasticizer' capable of enhancing the transport properties and provides better interfacial properties towards lithium metal anodes.116
The uptake of electrolyte solution is not related directly to the surface area or dielectric constant of the oxides. It may be due to the affinity of the metal oxide toward the electrolyte solution.117 The incorporation of fillers such as SiO2 and Al2O3 in the PVDF membrane promotes amorphicity, explaining the conductivity enhancement in PVDF-based electrolytes.143
PVDF-HFP with SiO2 nanoparticles has been prepared for Na/S batteries with a first discharge capacity of 165 mA h g−1.137
Galvanostatic cycling experiments of PVDF membranes with SiO2 showed that these membranes have a behaviour similar to the corresponding liquid electrolyte, without significant differences in capacity.112
Miao et al. showed that TiO2 added to the composite electrolyte membranes helps to improve mechanical strength, electrolyte uptake, ionic conductivity, and the electrode/electrolyte interfacial stability.92
Composite polymer electrolytes containing ionic liquids have been found to be thermally stable up to 300 °C and show results adequate to be used as battery separators.126
The nature of the filler and the filler content play therefore a very delicate role in the ionic conductivity of the composite materials.128 The maximum amount of fillers found in the different works was 32 wt%. The ionic conductivity of the composite materials as battery separators depends on the nature of the fillers, the characteristics of the membrane (porosity) and the electrolyte solution type (lithium salts and solvent). For ionic conductivity improvement, the Lewis acid–base interactions between filler surface groups, polymer matrix and cations/anions play an essential role.
Different fillers also incorporate complementary characteristics to the separator membranes. The molecular sieves produce a specific conducting pathway on the membranes and improve mechanical strength.132,142 The MMT particles do not affect the morphology of the polymer matrix and increase of electrochemical behaviour of the battery separator.108,118,141 The inert oxide ceramics (Al2O3 TiO2 ZrO2) reduce the degree of crystallinity and promotes of Li+ transport at the boundaries of the filler particles.144 Ferroelectric ceramic fillers (BaTiO3) increase the polarity of the battery separator due of the high dielectric constant of the fillers and due to the charge separation.28 The interfacial stability between electrodes and battery separators as well as the ionic conductivity are improved with fillers based of carbon (CNT, CNF).145
Fig. 4 shows the best ionic conductivity of the composite materials obtained with the different fillers.
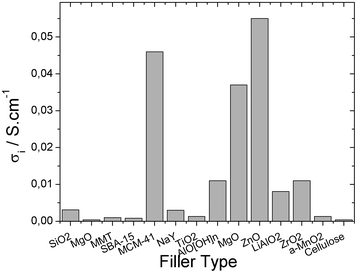 | ||
| Fig. 4 Ionic conductivity for different filler types. | ||
Fig. 4 shows that the best ionic conductivities are achieved for MgO, ZnO and MCM-41 fillers. The MgO and ZnO are inert oxide ceramics that change the dynamics of the polymer chains and MCM-41 are molecular sieves with strong Lewis acid centers in their frameworks and increase the Li+ transference number
2.3 Poly(vinylidene fluoride) and copolymer based polymer blends
Another strategy for enhancing the ionic conductivity and other relevant properties of battery separator membranes such as mechanical and thermal properties is the fabrication of polymer blends. In the polymer blends for battery separators the strategy has been the following: one polymer should show a very good affinity with the liquid electrolyte and the other polymer must show excellent mechanical properties. The dimensional and electrochemical stability are also necessary requirements for polymer blends.The developed PVDF and copolymers based polymer blend membranes are summarized in Table 4 in chronological order.
| Material | Blends | Electrolyte solution/lithium ions | Porosity (%) | Uptake (%) | σ i/(S cm−1) at 25 °C | Ref. |
|---|---|---|---|---|---|---|
| PVDF-HFP | PAN | 1 M LiPF6 in EC/DMC (1/1) | 76 | 82 | 1.9 × 10−3 | 146 |
| PVDF-HFP | PAN | 1 M LiBF4 in EC/DMC (1/1) | 76 | 80 | 1.2 × 10−3 | 146 |
| PVDF-HFP | PE | 1 M LiClO4/PC + EC | — | — | 0.2 × 10−3 | 147 |
| PVDF | PMMA | 10 mol% LiClO4 | — | — | 3.1 × 10−5 | 148 |
| PVDF-HFP | PVP | 1 M LiBF4 in EC/DMC (1/1) | — | 62 | 0.4 × 10−3 | 152 |
| PVDF | PAN | LiClO4–PC-EC | — | — | — | 153 |
| PVDF-HFP | PEG | LiTFSI | — | — | 1.0 × 10−5 | 154 |
| PVDF-HFP | PMAML | 1 M LiBF4 in EC/DMC (1/1) | 76 | 75 | 2.6 × 10−3 | 149 |
| PVDF | PMMA-PEGDA | 1 M LiPF6 in EC/DMC/EMC (1/1/1) | — | 600 | 4.5 × 10−3 | 155 |
| PVDF-HFP | PEG-PEGDMA | 1 M LiPF6 in EC/DEC (1/1) | 15 | 98 | 1.0 × 10−3 | 156 |
| PVDF-HFP | PVK | 1.5 M LiBF4 in EC | — | — | 0.7 × 10−3 | 150 |
| PVDF | PEGDA-PMMA | LiPF6/LiCF3SO3 in EC/DMC/EMC (1/1/1) | — | — | 1.0 × 10−3 | 157 |
| PVDF | PE | 1 M LiPF6 in EC/DEC/PC (35/60/5, w/w/w) | 48 | 302 | 1.1 × 10−3 | 151 |
| PVDF | PE | 1 M LiPF6 in EC/DEC/PC (35/60/5, w/w/w) | 53 | 290 | 8.9 × 10−4 | 158 |
| PVDF-HFP | PEO | 1 M LiTFSI in EC/PC | — | — | — | 159 |
| PVDF-HFP | PEO | 1 M LiTFSI in EC/PC (1/1) | — | — | — | 160 |
| PVDF | PEO | 1 M LiClO4 in PC | 84 | 210 | 2.0 × 10−3 | 161 |
| PVDF-HFP | PAN | 1 M LiClO4 in EC/DEC (1/1) | — | — | 3.4 × 10−3 | 162 |
| PVDF-HFP | PVP-PEG | 1 M LiPF6 in DMC/EMC/EC (1/1/1) | 49 | 125 | 0.5 × 10−3 | 163 |
| PVDF-HFP | P(EO-EC) | LiCF3SO3 | 65 | 61 | 3.7 × 10−5 | 164 |
| PVDF | PMMA | 1 M LiPF6 in DMC/EMC/EC (1/1/1) | — | — | — | 165 |
| PVDF-HFP | PEG | 1 M LiPF6 in DEC/EC (1/1) | 90 | 100 | 1.0 × 10−4 | 166 |
| PVDF | PVC | NaClO4 + PC | — | — | 1.5 × 10−4 | 167 |
| PVDF-HFP | PVA | 1 M LiClO4 in EC/DEC (1/1) | 86 | 90 | 7.9 × 10−3 | 119 |
| PVDF | PVC | LiClO4 + EC/PC | — | — | 3.7 × 10−3 | 168 |
| PVDF | PAN | 1 M LiClO4 in PC | 85 | 300 | 7.8 × 10−3 | 20 |
| PVDF-HFP | PAN | 1 M LiPF6 in EC:EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
83 | — | 6.7 × 10−3 | 169 |
| PVDF | PMMA | 1 M LiPF6 in EC:DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 260 | 7.9 × 10−3 | 55 |
| PVDF | PDPA | 1 M LiClO4 in PC | — | 280 | 3.6 × 10−3 | 24 |
| PVDF-HFP | PEGDMA | 1 M LiClO4 in EC/DEC | — | 125 | 3.8 × 10−4 | 170 |
| PVDF | PEGDA-PEO-PPO-PEO | 1 M LiClO4 in EC/PC (1/1) | 32 | 63 | 1.9 × 10−3 | 171 |
| PVDF | PMMA | 1 M LiClO4 in EC/PC (1/1) | — | 292 | 1.9 × 10−3 | 172 |
| PVDF-HFP | SN | LiClO4 | — | — | 1.0 × 10−3 | 173 |
| PVDF-HFP | PE | 1 M LiPF6 in EC/ DEC (1/1) | — | — | 0.8–1.2 × 10−3 | 174 |
| PVDF-HFP | PMMA | 1 M LiPF6 in EC:DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 377 | 2.0 × 10−3 | 175 |
| PVDF-HFP | PET | 1 M LiPF6 in EC:DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 0.8 × 10−3 | 176 |
| PVDF-HFP | PVA | 8 wt% LiBF4 + 67 wt% EC | — | — | 1.2 × 10−3 | 177 |
| PVDF | PDMS | 1 M LiPF6 in EC:DMC:EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
55 | 250 | 1.2 × 10−3 | 178 |
| PVDF-HFP | PPG-PEG-PEG | 1 M LiClO4 in EC/PC (1/1) | — | 259 | 1.3 × 10−2 | 179 |
| PVDF-HFP | PMMA | 1 M LiClO4 in EC/DEC (1/1) | 50 | 403 | 1.7 × 10−3 | 180 |
| PVDF-TrFE | PEO | 1 M LiClO4·3H2O-PC | 74 | 92 | 3.0 × 10−4 | 181 |
Table 4 shows that the polymer blends show high ionic conductivity and the polymers more used with PVDF and its copolymers are PMMA and PEO due to the increased adhesion of electrodes and battery separators as well as to the ability to solvate a wide variety of salts, respectively.
PVDF-HFP/PAN polymer blend membranes were prepared by Kim et al. and high ionic conductivity and good mechanical properties were observed for the gel polymer electrolytes.146
PVDF-HFP/PE blend membranes show that PE particles dispersed in PVDF-HFP form a continuous film with 23 wt% of PE. The continuous PE film exhibits the ability to cut off the ion diffusion between cathode and anode and induces high ionic conductivity and good mechanical strength.147
Rajendran et al. determined that the resulting ionic conductivity of the blend membranes is determined by the overall mobility of ion and polymer, which depends on the free volume around the polymer chain.148 In the PMMA/PVDF (25–75) polymer blend with LiClO4 an ionic conductivity of 3.14 × 10−5 S cm−1 was obtained at room temperature.
PMAML/PVDF-HFP is a promising electrolyte candidate for rechargeable lithium ion polymer batteries as it shows high ionic conductivity (2.6 mS cm−1 at room temperature and electrochemical window around 4.6 V) and good electrochemical stability.149
Michael et al. demonstrated that PVdF-HFP/PVK with LiBF4 offers the room temperature ionic conductivity of 0.72 mS cm−1 with an ionic transference number of 0.49.150
A new type of separator was introduced by Lee et al.151 by coating poly(vinyl alcohol) (PVAc) on the surface of a PVDF/PE non-woven matrix. The coated separator exhibits smoother surface morphology and better adhesion properties toward electrodes.
Sannier et al., produced a polymer blend of PVDF-HFP/PEO and also highlighted the role of the macroscopic blend interfaces toward dendrite in bi-layered separators.160
For PVDF/PEO or PVDF-HFP/PEG blends, the addition of PEO or PEG in the PVDF matrix improves the pore configuration (connectivity) of the PVDF microporous membranes and increases ionic conductivity.161,166
Electrospun membranes based on PVDF were prepared and modified via pre-irradiation grafting with PMMA. PMMA possesses good affinity for the liquid electrolyte and gelled PMMA could substitute nonconductive PVDF for being in contact with the electrodes.55
Sohn et al. prepared a PVDF-HFP/PEGDMA coated PE separator for lithium ion battery applications by electron beam irradiation (EB). The EB treatment of the blend membranes containing PEGDMA was found to strongly improve the thermal shrinkage of the separators by the formation of crosslinked networks, enhancing also electrolyte uptake and ionic conductivity.170
The ionic conductivity of the polymer blends for battery separators depends on the affinity between polymers and the characteristics of the membrane (e.g. porosity, crystallinity, etc.), which also depends on the processing technique such as thermal induced phase separation (TIPS). Fig. 5 shows the best ionic conductivity for each developed polymer blend type.
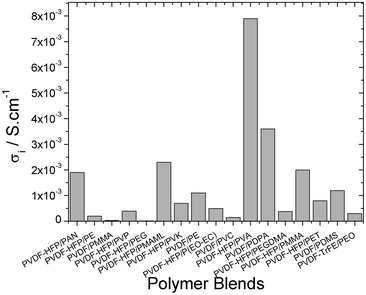 | ||
| Fig. 5 Best ionic conductivity for the different polymer blends. | ||
The common element for the polymer blends with the best ionic conductivity is the presence of PVDF-HFP (Fig. 5) due to the lower degree of crystallinity, its dielectric constant, ε = 8.4, and strong electron withdrawing functional groups (–C–F–).
3. Anode and cathode electrodes used with PVDF based separators
The two different types of electrodes, anode and cathode, immersed in the electrolyte solution create the electrical potential, i.e. the electrochemical cell.During charging process, electrons move from the cathode to the anode (Fig. 6, left) and during discharge the electrons move from the anode to the cathode (Fig. 6, right).182
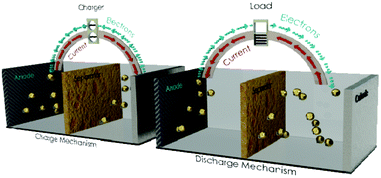 | ||
| Fig. 6 Representation of the charge and discharge modes of the electrochemical cell. | ||
The anode is the negative active material. It is commonly based on carbonaceous materials and non carbon alloys where reversion reaction occurs.183 Examples of carbonaceous materials used as anode materials are graphites, carbon nanotubes (CNT), carbon nanofibres (CNF) and lithium titanium oxides (Li4Ti5O12). Carbonaceous materials show the largest potential for improving the lithium ion cells and versatile, strong and highly conductive electrodes have been obtained to be used as anodes in batteries systems.184
The cathode is the positive active material. It is based on transition metal oxides and it is the main responsible for the cell capacity and cycle life.
Lithium cobalt oxide (LiCoO2), lithium manganese dioxide (LiMnO2), Lithium nickel oxide (LiNiO2) and lithium iron phosphate (LiFePO4) are some examples of materials used as cathodes.
For batteries with separator membranes based on PVDF and copolymers, the most used materials for the anode electrodes are Sn nanoparticles within a carbon matrix (Sn–C), graphite and lithium foil and for the cathode electrode are LiFePO4, LiCoO2 and lithium nickel manganese oxide (LiNi0.5Mn0.5O4).67,94,97
The above-mentioned electrodes are of general use for different separator membranes and some work still remains to be developed in this area in order to optimize electrodes for PVDF based separators. Further, it is to notice that electrodes are typically formed by an active material, additives and a polymer binder. The polymer binder used both as anode and cathode for lithium-ion batteries can be also based on PVDF polymer due to its electrochemical, thermal and chemical stability as well as its easy processing.
4 Conclusions and further work
This review presents an overview on PVDF and its copolymers for separator membranes in lithium-ion batteries applications. The battery separator is a critical element for improving lithium-ion battery performance. The review reports the research and developments in this field in the last decade. The correlation between properties and the fabrication methods is fundamental in order to achieve adequate battery separators for applications. It is essential the knowledge and control of their structure, stability and ionic conductivity in order to increase performance of the materials as battery separators. The review is divided into three different categories due of its characteristics, i.e., single polymers, composites and polymeric blends. Each category presents advantages and disadvantages:For single polymers, PVDF and PVDF-HFP with LiPF6 and LiCF3SO3 in different organic solvents provide the best values of the ionic conductivity (1.8 − 5 × 10−2 S cm−1), and a large range of porosities and pore sizes can be obtained allowing tailoring membrane properties for the specific battery application. The ideal membrane for porous polymer electrolytes based on PVDF for battery applications should have high porosity and small pore diameters with narrow distribution. The experiments show that porosity should be >80% and pore diameter should be <1 μm. These characteristics are also obtained with up-scalable methods such as electrospinning.
The thermal and mechanical stability of single polymer membranes can be improved both by including specific fillers and by the development of polymer blends.
Fillers have been used that contribute to the ionic conduction process and fillers that are not involved in the lithium transport process. Particularly interesting are the fillers, such as MgO, that increase the compatibility between the separator and the electrodes. Also interesting are the molecular sieves that have proven to extend the electrochemical stability window of polymer electrolytes, enhanced the interfacial stability of the polymer electrolyte with the lithium electrode and inhibited the crystallization of the polymer matrix.
With respect to the polymer blends, one of the components should show a very good affinity to the liquid electrolyte and the other polymer must show excellent mechanical properties. The best blend membranes have been obtained with PVDF-HFP. Polymer blends also improve thermal shrinkage of the separators by the formation of cross linked networks and enhance electrolyte uptake and ionic conductivity.
With respect to the future trends, single polymers and co-polymers have to be achieved with similar large degrees of porosity (∼80%) but with hierarchical pore size structures down to pore sizes below 1 μm in an up-scalable way. This will allow to improve uptake without compromising mechanical properties and to obtain larger batch productions.
The incorporation of ionic liquids in the single polymer membranes is a promising field for more environmental friendly battery separators with high ionic conductivity at room temperature and wider electrochemical windows.
In composite materials, some work is needed, e.g. through surface functionalization, to improve compatibility between fillers and polymer matrix and therefore to improve the stability of the membrane.
Polymer blends are one of the most promising ways to improve PVDF based separator membranes. The progress in this category involves the fabrication of multilayers or hierarchical pore structures to enhance the thermal, electrical, mechanical and electrochemical properties of the battery separators and to improve compatibility with electrodes.
In conclusion, PVDF based polymer electrolytes offer broad engineering possibilities for membranes preparation with tailored microstructure and properties, showing therefore large potential for a new generation of more efficient battery separator membranes.
List of symbols and abbreviations
| 13TFSI | N-methyl-N-propylpiperidinium Bis(trifluoromethanesulfonyl) Amide |
| Al2O3 | Aluminum Oxide |
| AlO[OH]n | Aluminum Oxyhydroxide |
| AN | Acetonitrile |
| BaTiO3 | Barium Titanate |
| BMIBF4 | 1-Butyl-3-Methylimidazolium Tetrafluoroborate |
| BMITFSI | 1-butyl-3-Methylimidazolium bis(trifluoromethanesulfonyl)imide |
| BMPyrTFSI | 1-Butyl-3-Methypyrrolidinium Bis (trifluoromethanesulfonyl)imide |
| CNF | Carbon Nanofibres |
| CNT | Carbon Nanotubes |
| CoO | Cobalt Oxide |
| CTFE | Chlorotrifluoroethylene |
| DEC | Diethyl Carbonate |
| DIOX | 1,3-Dioxolane |
| DMBITFSI | 1,2-dimethyl-3-n-butylimidazolium-bis-trifluoromethanesulfonylimide |
| DMC | Dimethyl Carbonate |
| DMOImPF6 | 2,3-Dimethyl-1-octylimidazolium Hexafluorophosphate |
| EC | Ethylene Carbonate |
| EMC | Ethyl Methyl Carbonate |
| EMITf | 1-ethyl-3-methylimidazolium trifluoromethanesulfonate |
| Fe2O3 | Iron Oxide |
| GBL | γ-butyrolactone |
| HFP | Hexafluoropropene |
| ILs | Ionic Liquid |
| LiAlO2 | Lithium Aluminate |
| LiAsF6 | Lithium Hexafluoroarsenate |
| LiBF4 | Lithium Tetrafluoroborate) |
| LiBETI | Lithium Bis(perfluoroethanesulfonyl)imide |
| LiCF3SO3 | Lithium Trifluoromethanesulfonate |
| LiClO4 | Lithium Perchlorate |
| LiClO4·3H2O | Lithium Perchlorate Trihydrat |
| LiCoO2 | Lithium Cobalt Oxide |
| LiFePO4 | Lithium Iron Phosphate |
| LiPF6 | Lithium Hexafluorophosphate |
| LiMnO2 | Lithium Manganese Dioxide |
| LiNiO2 | Lithium Nickel Oxide |
| LiNi0.5Mn0.5O4 | Lithium Nickel Manganese Oxide |
| LiTFSI | Lithium Bis(Trifluoromethanesulfonyl)Imide |
| Li4Ti5O12 | Lithium Titanium Oxides |
| MCM-41 | Molecular Sieves |
| Mg(CF3SO3)2 | Magnesium Triflate |
| Mg(ClO4)2 | Magnesium Perchlorate |
| MgO | Magnesium Oxide |
| NH4PF6 | Ammonium Hexafluorophosphate |
| MMT | Montmorillonite |
| MnO2 | Manganese Dioxide |
| NaClO4 | Sodium Salt |
| NaTf | Sodium Triflate |
| NaY | Molecular Sieves |
| PAN | Poly(acrylonitrile) |
| PC | Propylene Carbonate |
| PDPA | Polydiphenylamine |
| PDMS | Poly(dimethylsiloxane) |
| PE | Poly(ethylene) |
| PEG | Poly(ethylene glycol) |
| PEGDA | Poly(ethylene glycol diacrylate) |
| PEGDMA | Poly(ethylene glycol dimethacrylate) |
| PEO | Poly(ethylene oxide) |
| P(EO-EC) | Poly(ethylene oxide-co-ethylene carbonate) |
| PEO-PPO-PEO | Polyethylene oxide-co-polypropylene oxide-co-polyethylene oxide |
| PET | Poly(ethylene terephthalate) |
| PMAML | Poly(methyl methacrylate-co-acrylonitrile-co-lithium methacrylate) |
| PMMA | Poly(methyl methacrylate) |
| PMMITFSI | 1,2-dimethyl-3-propylimidazolium bis(trifluoromethanesufonyl)imide |
| PP | Poly(propylene) |
| PPG-PEG-PPG | Poly(propylene glycol)-co-poly(ethylene glycol)-co-poly(propylene glycol) |
| PVA | Poly(vinyl alcohol) |
| PVC | Poly(vinyl chloride) |
| PVDF | Poly(vinylidene fluoride) |
| P(VDF-CTFE) | Poly(vinylidene fluoride-co-chlorotrifluoroethylene |
| P(VDF-HFP) | Poly(vinylidene fluoride-co-hexafluoropropene |
| P(VDF-TrFE) | Poly(vinylidene fluoride-co-trifluoroethylene) |
| PVK | Poly(N-vinylcarbazole) |
| PVP | Poly(vinyl pyrrolidone) |
| SBA-15 | Molecular Sieves |
| SiO2 | Silicon Dioxide |
| SN | Succinonitrile |
| Sn–C | Sn nanoparticles within a carbon matrix |
| SnO2 | Tin Dioxide |
| TEABF4 | Tetraethylammonium Tetrafluoroborate |
| TEGDMA | Tetraethylene Glycol Dimethyl Ether |
| TEGDME | Tetra(ethylene glycol) Dimethyl Ether |
| TiO2 | Titanium Dioxide |
| TrFE | Trifluoroethylene |
| VDF | Vinylidene Fluoride |
| ZnO | Zinc Oxide |
| ZrO2 | Zirconium Dioxide |
Acknowledgements
This work is funded by FEDER funds through the “Programa Operacional Factores de Competitividade-COMPETE” and by national funds from FCT-Fundação para a Ciência e a Tecnologia, in the framework of the strategic project Strategic Project PEST-C/FIS/UI607/2011and the projects PTDC/CTM/69316/2006, project no. F-COMP-01-0124-FEDER-022716 (refª FCT PEst-C/QUI/UI0686/2011) and NANO/NMed-SD/0156/2007, and grant SFRH/BD/68499/2010 (C.M.C.) The authors thank Celgard LLC, Timcal, Solvay, Arkema and Clariant for kindly supplying their high quality membranes and excellent materials, respectively. The authors also thank support from the COST Action MP1003, “European Scientific Network for Artificial Muscles” and COST action MP0902, “Composites of Inorganic Nanotubes and Polymers (COINAPO)”.References
- P. R. Consulting, Advanced Batteries for Portable Power Applications, 2011 Search PubMed.
- T. Nagaura and K. Tozawa, Progress in Batteries and Solar Cells, 1990, 209 Search PubMed.
- V. A. Colin, Solid State Ionics, 2000, 134, 159–167 CrossRef.
- R. Yazami and P. Touzain, J. Power Sources, 1983, 9, 365–371 CrossRef CAS.
- J. Li, C. Daniel and D. Wood, J. Power Sources, 2011, 196, 2452–2460 CrossRef CAS.
- J. O. Besenhard, ed., Handbook of Battery Materials, Wilcy-VCH, 1999 Search PubMed.
- P. B. Balbuena and Y. Wang, ed., Lithium-Ion Batteries: Solid-Electrolyte Interphase, Imperial College Press, London, 2004 Search PubMed.
- R. A. M. Hikmet, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, W. C. Robert, C. F. Merton, I. Bernard, J. K. Edward, M. Subhash and V. Patrick, Elsevier, Oxford, 2001, pp. 6534–6545 Search PubMed.
- M. S. Gregori, Eur. Polym. J., 2006, 42, 21–42 CrossRef.
- K. Xu, Chem. Rev., 2004, 104, 4303–4418 CrossRef CAS.
- G. J. Janz and R. P. T. Tomkins, Nonaqueous Electrolytes Handbook, Academic Press, 1972 Search PubMed.
- P. Arora and Z. Zhang, Chem. Rev., 2004, 104, 4419–4462 CrossRef CAS.
- X. Huang, J. Solid State Electrochem., 2011, 15, 649–662 CrossRef CAS.
- Y. S. Chung, S. H. Yoo and C. K. Kim, Ind. Eng. Chem. Res., 2009, 48, 4346–4351 CrossRef CAS.
- K. Gao, X. Hu, T. Yi and C. Dai, Electrochim. Acta, 2006, 52, 443–449 CrossRef CAS.
- G. Venugopal, J. Moore, J. Howard and S. Pendalwar, J. Power Sources, 1999, 77, 34–41 CrossRef CAS.
- B. K. Choi, K. H. Shin and Y. W. Kim, Solid State Ionics, 1998, 113–115, 123–127 CrossRef CAS.
- Y. Kang, H. J. Kim, E. Kim, B. Oh and J. H. Cho, J. Power Sources, 2001, 92, 255–259 CrossRef CAS.
- B. Laik, L. Legrand, A. Chausse and R. Messina, Electrochim. Acta, 1998, 44, 773–780 CrossRef CAS.
- A. I. Gopalan, P. Santhosh, K. M. Manesh, J. H. Nho, S. H. Kim, C.-G. Hwang and K.-P. Lee, J. Membr. Sci., 2008, 325, 683–690 CrossRef CAS.
- B. Huang, Z. Wang, G. Li, H. Huang, R. Xue, L. Chen and F. Wang, Solid State Ionics, 1996, 85, 79–84 CrossRef CAS.
- S. S. Sekhon, N. Arora and S. A. Agnihotry, Solid State Ionics, 2000, 136–137, 1201–1204 CrossRef CAS.
- C. M. Costa, L. C. Rodrigues, V. Sencadas, M. M. Silva, G. Rocha and S. Lanceros-Méndez, Journal of Membrane Science, 2012, 407–408, 8 Search PubMed.
- A. I. Gopalan, K.-P. Lee, K. M. Manesh and P. Santhosh, J. Membr. Sci., 2008, 318, 422–428 CrossRef CAS.
- J. R. Kim, S. W. Choi, S. M. Jo, W. S. Lee and B. C. Kim, Electrochim. Acta, 2004, 50, 69–75 CrossRef CAS.
- A. Manuel Stephan and Y. Saito, Solid State Ionics, 2002, 148, 475–481 CrossRef CAS.
- H. Kataoka, Y. Saito, T. Sakai, E. Quartarone and P. Mustarelli, J. Phys. Chem. B, 2000, 104, 11460–11464 CrossRef CAS.
- S. Jung, D. W. Kim, S. D. Lee, M. Cheong, D. Q. Nguyen, B. W. Cho and H. S. Kim, Bull. Korean Chem. Soc, 2009, 30 Search PubMed.
- H. S. Nalwa, J. Macromol. Sci., Part C, 1991, 31, 341–432 CrossRef.
- D. C. Bassett, Developments in Crystalline Polymers, Applied Science Publishers, 1982 Search PubMed.
- J. R. Gregorio and M. Cestari, J. Polym. Sci., Part B: Polym. Phys., 1994, 32, 859–870 CrossRef.
- M. P. Silva, C. M. Costa, V. Sencadas, A. J. Paleo and S. Lanceros-Méndez, J. Polym. Res., 2011, 18, 1451–1457 CrossRef CAS.
- R. Magalhães, N. Durães, M. Silva, J. Silva, V. Sencadas, G. Botelho, J. L. Gómez Ribelles and S. Lanceros-Méndez, Soft Mater., 2010, 9, 1–14 CrossRef.
- V. Sencadas, R. Gregorio and S. Lanceros-Méndez, J. Macromol. Sci., Part B: Phys., 2009, 48, 514–525 CrossRef CAS.
- V. Sencadas, C. M. Costa, V. Moreira, J. Monteiro, S. K. Mendiratta, J. F. Mano and S. Lanceros-Méndez, e-Polymers, 2005, 2, 12 Search PubMed.
- H. S. Nalwa, Ferroelectric Polymers: Chemistry, Physics, and Applications, Marcel Dekker, 1995 Search PubMed.
- A. J. Lovinger, T. Furukawa, G. T. Davis and M. G. Broadhurst, Polymer, 1983, 24, 1233–1239 CrossRef CAS.
- T. Furukawa, G. E. Johnson, H. E. Bair, Y. Tajitsu, A. Chiba and E. Fukada, Ferroelectrics, 1981, 32, 61–67 CrossRef CAS.
- W. Li, Y. Zhu, D. Hua, P. Wang, X. Chen and J. Shen, Appl. Surf. Sci., 2008, 254, 7321–7325 CrossRef CAS.
- S. Abbrent, J. Plestil, D. Hlavata, J. Lindgren, J. Tegenfeldt and Å. Wendsjö, Polymer, 2001, 42, 1407–1416 CrossRef CAS.
- Z. Jiang, B. Carroll and K. M. Abraham, Electrochim. Acta, 1997, 42, 2667–2677 CrossRef CAS.
- B. Ameduri, Chem. Rev., 2009, 109, 6632–6686 CrossRef CAS.
- Z. Wang, Z. Zhang and T. C. M. Chung, Macromolecules, 2006, 39, 4268–4271 CrossRef CAS.
- N. K. Kalfoglou and H. L. Williams, J. Appl. Polym. Sci., 1973, 17, 3367–3373 CrossRef CAS.
- B. Chu, X. Zhou, K. Ren, B. Neese, M. Lin, Q. Wang, F. Bauer and Q. M. Zhang, Science, 2006, 313, 334–336 CrossRef CAS.
- Z. Li, Y. Wang and Z.-Y. Cheng, Appl. Phys. Lett., 2006, 88, 062904 CrossRef.
- T. Sarada, L. C. Sawyer and M. I. Ostler, J. Membr. Sci., 1983, 15, 97–113 CrossRef CAS.
- M. B. Yoshio, J. Ralph and A. Kozawa, Lithium-Ion Batteries: Science and Technologies, Springer, 2009 Search PubMed.
- L.-y. Tian, X.-b. Huang and X.-z. Tang, J. Appl. Polym. Sci., 2004, 92, 3839–3842 CrossRef CAS.
- G.-L. Ji, B.-K. Zhu, Z.-Y. Cui, C.-F. Zhang and Y.-Y. Xu, Polymer, 2007, 48, 6415–6425 CrossRef CAS.
- Y. Su, C. Chen, Y. Li and J. Li, Journal of Macromolecular Science, Part A, 2007, 44, 99–104 CAS.
- L.-P. Cheng, D.-J. Lin, C.-H. Shih, A.-H. Dwan and C. C. Gryte, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 2079–2092 CrossRef CAS.
- D. Karabelli, J. C. Leprêtre, F. Alloin and J. Y. Sanchez, Electrochim. Acta, 2011, 57, 98–103 CrossRef CAS.
- K. M. Kim, J.-C. Kim and K. S. Ryu, Macromol. Chem. Phys., 2007, 208, 887–895 CrossRef CAS.
- Z. Li, F. Shan, J. Wei, J. Yang, X. Li and X. Wang, J. Solid State Electrochem., 2008, 12, 1629–1635 CrossRef CAS.
- D. Djian, F. Alloin, S. Martinet and H. Lignier, J. Power Sources, 2009, 187, 575–580 CrossRef CAS.
- S. S. Kim and D. R. Lloyd, J. Membr. Sci., 1991, 64, 13–29 CrossRef CAS.
- P. Raghavan, J. Manuel, X. Zhao, D.-S. Kim, J.-H. Ahn and C. Nah, J. Power Sources, 2011, 196, 6742–6749 CrossRef CAS.
- M. M. E. Jacob, S. R. S. Prabaharan and S. Radhakrishna, Solid State Ionics, 1997, 104, 267–276 CrossRef CAS.
- A. Chandra, R. C. Agrawal and Y. K. Mahipal, J. Phys. D: Appl. Phys., 2009, 42, 135107 CrossRef.
- M. Rao, X. Geng, Y. Liao, S. Hu and W. Li, J. Membr. Sci., 2012, 399–400, 37–42 CrossRef CAS.
- A. Kumar and M. Deka, J. Solid State Electrochem., 2012, 16, 35–44 CrossRef CAS.
- F. Boudin, X. Andrieu, C. Jehoulet and I. I. Olsen, J. Power Sources, 1999, 81–82, 804–807 CrossRef CAS.
- S.-S. Choi, Y. S. Lee, C. W. Joo, S. G. Lee, J. K. Park and K.-S. Han, Electrochim. Acta, 2004, 50, 339–343 CrossRef CAS.
- G. Cheruvally, J.-K. Kim, J.-W. Choi, J.-H. Ahn, Y.-J. Shin, J. Manuel, P. Raghavan, K.-W. Kim, H.-J. Ahn, D. S. Choi and C. E. Song, J. Power Sources, 2007, 172, 863–869 CrossRef CAS.
- Z. H. Li, C. Cheng, X. Y. Zhan, Y. P. Wu and X. D. Zhou, Electrochim. Acta, 2009, 54, 4403–4407 CrossRef CAS.
- H. P. Zhang, P. Zhang, G. C. Li, Y. P. Wu and D. L. Sun, J. Power Sources, 2009, 189, 594–598 CrossRef CAS.
- J. M. Tarascon, A. S. Gozdz, C. Schmutz, F. Shokoohi and P. C. Warren, Solid State Ionics, 1996, 86–88, Part 1, 49–54 CrossRef.
- N. Muniyandi, N. Kalaiselvi, P. Periyasamy, R. Thirunakaran, B. Ramesh babu, S. Gopukumar, T. Premkumar, N. G. Renganathan and M. Raghavan, J. Power Sources, 2001, 96, 14–19 CrossRef CAS.
- C. A. Vincent, Prog. Solid State Chem., 1987, 17, 145–261 CrossRef CAS.
- C. R. Jarvis, W. J. Macklin, A. J. Macklin, N. J. Mattingley and E. Kronfli, J. Power Sources, 2001, 97–98, 664–666 CrossRef CAS.
- Y. Saito, H. Kataoka, E. Quartarone and P. Mustarelli, J. Phys. Chem. B, 2002, 106, 7200–7204 CrossRef CAS.
- Y. Wang, J. Travas-Sejdic and R. Steiner, Solid State Ionics, 2002, 148, 443–449 CrossRef CAS.
- A. Magistris, E. Quartarone, P. Mustarelli, Y. Saito and H. Kataoka, Solid State Ionics, 2002, 152–153, 347–354 CrossRef CAS.
- E. Quartarone, P. Mustarelli and A. Magistris, J. Phys. Chem. B, 2002, 106, 10828–10833 CrossRef CAS.
- Q. Shi, M. Yu, X. Zhou, Y. Yan and C. Wan, J. Power Sources, 2002, 103, 286–292 CrossRef CAS.
- F. Croce, A. D'Epifanio, J. Hassoun, P. Reale and B. Scrosati, J. Power Sources, 2003, 119–121, 399–402 CrossRef CAS.
- J. Saunier, F. Alloin, J. Y. Sanchez and G. Caillon, J. Power Sources, 2003, 119–121, 454–459 CrossRef CAS.
- J. M. Song, H. R. Kang, S. W. Kim, W. M. Lee and H. T. Kim, Electrochim. Acta, 2003, 48, 1339–1346 CrossRef CAS.
- S. S. Zhang, K. Xu, D. L. Foster, M. H. Ervin and T. R. Jow, J. Power Sources, 2004, 125, 114–118 CrossRef CAS.
- J. Saunier, F. Alloin, J. Y. Sanchez and L. Maniguet, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 2308–2317 CrossRef CAS.
- Y. J. Shen, M. J. Reddy and P. P. Chu, Solid State Ionics, 2004, 175, 747–750 CrossRef CAS.
- J. Saunier, W. Gorecki, F. Alloin and J. Y. Sanchez, J. Phys. Chem. B, 2005, 109, 2487–2492 CrossRef CAS.
- W. Pu, X. He, L. Wang, C. Jiang and C. Wan, J. Membr. Sci., 2006, 272, 11–14 CrossRef CAS.
- I. M. Ward, H. V. S. A. Hubbard, S. C. Wellings, G. P. Thompson, J. Kaschmitter and H. Wang, J. Power Sources, 2006, 162, 818–822 CrossRef CAS.
- T. Yamamoto, T. Hara, K. Segawa, K. Honda and H. Akashi, J. Power Sources, 2007, 174, 1036–1040 CrossRef CAS.
- C. Subba Reddy, M. Chen, W. Jin, Q. Zhu, W. Chen and S.-i. Mho, J. Appl. Electrochem., 2007, 37, 637–642 CrossRef.
- S. W. Choi, J. R. Kim, Y. R. Ahn, S. M. Jo and E. J. Cairns, Chem. Mater., 2006, 19, 104–115 CrossRef.
- X. Li, G. Cheruvally, J.-K. Kim, J.-W. Choi, J.-H. Ahn, K.-W. Kim and H.-J. Ahn, J. Power Sources, 2007, 167, 491–498 CrossRef CAS.
- J.-W. Choi, J.-K. Kim, G. Cheruvally, J.-H. Ahn, H.-J. Ahn and K.-W. Kim, Electrochim. Acta, 2007, 52, 2075–2082 CrossRef CAS.
- G. C. Li, P. Zhang, H. P. Zhang, L. C. Yang and Y. P. Wu, Electrochem. Commun., 2008, 10, 1883–1885 CrossRef CAS.
- R. Miao, B. Liu, Z. Zhu, Y. Liu, J. Li, X. Wang and Q. Li, J. Power Sources, 2008, 184, 420–426 CrossRef CAS.
- G. P. Pandey and S. A. Hashmi, J. Power Sources, 2009, 187, 627–634 CrossRef CAS.
- F. Croce, M. L. Focarete, J. Hassoun, I. Meschini and B. Scrosati, Energy Environ. Sci., 2011, 4, 921–927 CAS.
- J. Zhou, J. Cai, S. Cai, X. Zhou and A. N. Mansour, J. Power Sources, 2011, 196, 10479–10483 CrossRef CAS.
- S. Ramesh and S.-C. Lu, J. Mol. Struct., 2011, 994, 403–409 CrossRef CAS.
- M. Li, L. Yang, S. Fang, S. Dong, Y. Jin, S.-i. Hirano and K. Tachibana, J. Power Sources, 2011, 196, 6502–6506 CrossRef CAS.
- H. J. Alcock, O. C. White, G. Jegelevicius, M. R. Roberts and J. R. Owen, J. Power Sources, 2011, 196, 3355–3359 CrossRef CAS.
- K. Hwang, B. Kwon and H. Byun, J. Membr. Sci., 2011, 378, 111–116 CrossRef CAS.
- C. M. Costa, A. California, V. F. Cardoso, V. Sencadas, L. C. Rodrigues, M. M. Silva and S. Lanceros-Méndez, Ferroelectrics, 2012, 430, 103–107 CrossRef CAS.
- C. M. Costa, L. C. Rodrigues, V. Sencadas, M. M. Silva and S. Lanceros-Méndez, Solid State Ionics, 2012, 217, 19–26 CrossRef CAS.
- C. M. Costa, V. Sencadas, G. J. Rocha, M. M. Silva and S. Lanceros-Méndez, J. Solid State Electrochem., 2013, 17, 861–870 CrossRef CAS.
- A. Chagnes, B. Carré, P. Willmann and D. Lemordant, J. Power Sources, 2002, 109, 203–213 CrossRef CAS.
- K. Hayamizu and Y. Aihara, Electrochim. Acta, 2004, 49, 3397–3402 CrossRef CAS.
- J. E. Weston and B. C. H. Steele, Solid State Ionics, 1982, 7, 75–79 CrossRef CAS.
- A. Du Pasquier, P. C. Warren, D. Culver, A. S. Gozdz, G. G. Amatucci and J. M. Tarascon, Solid State Ionics, 2000, 135, 249–257 CrossRef CAS.
- P. P. Prosini, P. Villano and M. Carewska, Electrochim. Acta, 2002, 48, 227–233 CrossRef CAS.
- M. Wang, F. Zhao, Z. Guo and S. Dong, Electrochim. Acta, 2004, 49, 3595–3602 CrossRef CAS.
- M. Deka and A. Kumar, J. Power Sources, 2011, 196, 1358–1364 CrossRef CAS.
- D. Takemura, S. Aihara, K. Hamano, M. Kise, T. Nishimura, H. Urushibata and H. Yoshiyasu, J. Power Sources, 2005, 146, 779–783 CrossRef CAS.
- Y.-X. Jiang, Z.-F. Chen, Q.-C. Zhuang, J.-M. Xu, Q.-F. Dong, L. Huang and S.-G. Sun, J. Power Sources, 2006, 160, 1320–1328 CrossRef CAS.
- M. Wachtler, D. Ostrovskii, P. Jacobsson and B. Scrosati, Electrochim. Acta, 2004, 50, 357–361 CrossRef CAS.
- F. Ciuffa, F. Croce, A. D'Epifanio, S. Panero and B. Scrosati, J. Power Sources, 2004, 127, 53–57 CrossRef CAS.
- X. He, Q. Shi, X. Zhou, C. Wan and C. Jiang, Electrochim. Acta, 2005, 51, 1069–1075 CrossRef CAS.
- M. Ollinger, H. Kim, T. Sutto and A. Piqué, Appl. Surf. Sci., 2006, 252, 8212–8216 CrossRef CAS.
- A. M. Stephan, K. S. Nahm, M. Anbu Kulandainathan, G. Ravi and J. Wilson, Eur. Polym. J., 2006, 42, 1728–1734 CrossRef CAS.
- C.-G. Wu, M.-I. Lu, C.-C. Tsai and H.-J. Chuang, J. Power Sources, 2006, 159, 295–300 CrossRef CAS.
- M. Wang and S. Dong, J. Power Sources, 2007, 170, 425–432 CrossRef CAS.
- N. T. K. Sundaram and A. Subramania, J. Membr. Sci., 2007, 289, 1–6 CrossRef CAS.
- A. Subramania, N. T. Kalyana Sundaram, A. R. Sathiya Priya and G. Vijaya Kumar, J. Membr. Sci., 2007, 294, 8–15 CrossRef CAS.
- Z. H. Li, H. P. Zhang, P. Zhang, G. C. Li, Y. P. Wu and X. D. Zhou, J. Membr. Sci., 2008, 322, 416–422 CrossRef CAS.
- X.-L. Wang, Q. Cai, L.-Z. Fan, T. Hua, Y.-H. Lin and C.-W. Nan, Electrochim. Acta, 2008, 53, 8001–8007 CrossRef CAS.
- D. Saikia, Y. W. Chen-Yang, Y. T. Chen, Y. K. Li and S. I. Lin, Desalination, 2008, 234, 24–32 CrossRef CAS.
- Z. H. Li, H. P. Zhang, P. Zhang, Y. P. Wu and X. D. Zhou, J. Power Sources, 2008, 184, 562–565 CrossRef CAS.
- G. P. Pandey, R. C. Agrawal and S. A. Hashmi, J. Power Sources, 2009, 190, 563–572 CrossRef CAS.
- B. Lalia, K. Yamada, M. Hundal, J.-S. Park, G.-G. Park, W.-Y. Lee, C.-S. Kim and S. Sekhon, Appl. Phys. A: Mater. Sci. Process., 2009, 96, 661–670 CrossRef CAS.
- C. Yang, Z. Jia, Z. Guan and L. Wang, J. Power Sources, 2009, 189, 716–720 CrossRef CAS.
- P. Vickraman and V. Senthilkumar, Ionics, 2010, 16, 763–768 CrossRef CAS.
- H.-S. Jeong, D.-W. Kim, Y. U. Jeong and S.-Y. Lee, J. Power Sources, 2010, 195, 6116–6121 CrossRef CAS.
- P. Zhang, L. C. Yang, L. Li, Q. T. Qu, Y. P. Wu and M. Shimizu, J. Membr. Sci., 2010, 362, 113–118 CrossRef CAS.
- D. Zhang, R. Li, T. Huang and A. Yu, J. Power Sources, 2010, 195, 1202–1206 CrossRef CAS.
- Y. Zhang, G. Zhang, T. Du and L. Zhang, Electrochim. Acta, 2010, 55, 5793–5797 CrossRef CAS.
- H.-S. Jeong and S.-Y. Lee, J. Power Sources, 2011, 196, 6716–6722 CrossRef CAS.
- M. Valvo, E. García-Tamayo, U. Lafont and E. M. Kelder, J. Power Sources, 2011, 196, 10191–10200 CrossRef CAS.
- H.-Y. Hwang, D.-J. Kim, H.-J. Kim, Y.-T. Hong and S.-Y. Nam, Trans. Nonferrous Met. Soc. China, 2011, 21, Supplement 1, s141–s147 CrossRef.
- M. Mancini, F. Nobili, R. Tossici, M. Wohlfahrt-Mehrens and R. Marassi, J. Power Sources, 2011, 196, 9665–9671 CrossRef CAS.
- D. Kumar, M. Suleman and S. A. Hashmi, Solid State Ionics, 2011, 202, 45–53 CrossRef CAS.
- X. Huang, Journal of Solid State Electrochemistry, 1-7 Search PubMed.
- H.-S. Jeong, E.-S. Choi, S.-Y. Lee and J. H. Kim, J. Membr. Sci., 2012, 415–416, 513–519 CrossRef CAS.
- B. Lalia, Y. Samad and R. Hashaikeh, Journal of Solid State Electrochemistry, 1-7 Search PubMed.
- J. Nunes-Pereira, A. C. Lopes, C. M. Costa, R. Leones, M. M. Silva and S. Lanceros-Méndez, Electroanalysis, 2012, 24, 2147–2156 CrossRef CAS.
- J. Nunes-Pereira, A. C. Lopes, C. M. Costa, L. C. Rodrigues, M. M. Silva and S. Lanceros-Méndez, J. Electroanal. Chem., 2013, 689, 223–232 CrossRef CAS.
- V. Gentili, S. Panero, P. Reale and B. Scrosati, J. Power Sources, 2007, 170, 185–190 CrossRef CAS.
- F. Croce, R. Curini, A. Martinelli, L. Persi, F. Ronci, B. Scrosati and R. Caminiti, J. Phys. Chem. B, 1999, 103, 10632–10638 CrossRef CAS.
- G. B. Appetecchi and S. Passerini, Electrochim. Acta, 2000, 45, 2139–2145 CrossRef CAS.
- D.-W. Kim and Y.-K. Sun, J. Power Sources, 2001, 102, 41–45 CrossRef CAS.
- X. Liu, H. Kusawake and S. Kuwajima, J. Power Sources, 2001, 97–98, 661–663 CrossRef CAS.
- S. Rajendran, O. Mahendran and R. Kannan, Fuel, 2002, 81, 1077–1081 CrossRef CAS.
- Z.-l. Wang and Z.-y. Tang, Electrochim. Acta, 2004, 49, 1063–1068 CrossRef CAS.
- M. S. Michael and S. R. S. Prabaharan, J. Power Sources, 2004, 136, 408–415 CrossRef CAS.
- Y. M. Lee, N.-S. Choi, J. A. Lee, W.-H. Seol, K.-Y. Cho, H.-Y. Jung, J.-W. Kim and J.-K. Park, J. Power Sources, 2005, 146, 431–435 CrossRef CAS.
- Z. Wang and Z. Tang, Mater. Chem. Phys., 2003, 82, 16–20 CrossRef CAS.
- J.-U. Kim and H.-B. Gu, J. Power Sources, 2003, 119–121, 766–769 CrossRef CAS.
- A. Munch Elmer, B. Wesslen, P. Sommer-Larsen, K. West, H. Hassander and P. Jannasch, J. Mater. Chem., 2003, 13, 2168–2176 RSC.
- M.-K. Song, Y.-T. Kim, J.-Y. Cho, B. W. Cho, B. N. Popov and H.-W. Rhee, J. Power Sources, 2004, 125, 10–16 CrossRef CAS.
- C. L. Cheng, C. C. Wan and Y. Y. Wang, J. Power Sources, 2004, 134, 202–210 CrossRef CAS.
- K.-S. Kum, M.-K. Song, Y.-T. Kim, H.-S. Kim, B.-W. Cho and H.-W. Rhee, Electrochim. Acta, 2004, 50, 285–288 CrossRef CAS.
- Y. M. Lee, J.-W. Kim, N.-S. Choi, J. A. Lee, W.-H. Seol and J.-K. Park, J. Power Sources, 2005, 139, 235–241 CrossRef CAS.
- L. Sannier, R. Bouchet, S. Grugeon, E. Naudin, E. Vidal and J. M. Tarascon, J. Power Sources, 2005, 144, 231–237 CrossRef CAS.
- L. Sannier, R. Bouchet, M. Rosso and J. M. Tarascon, J. Power Sources, 2006, 158, 564–570 CrossRef CAS.
- J. Xi, X. Qiu, J. Li, X. Tang, W. Zhu and L. Chen, J. Power Sources, 2006, 157, 501–506 CrossRef CAS.
- A. Subramania, N. T. K. Sundaram and G. V. Kumar, J. Power Sources, 2006, 153, 177–182 CrossRef CAS.
- J.-H. Cao, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2006, 281, 446–453 CrossRef CAS.
- J.-D. Jeon and S.-Y. Kwak, Macromolecules, 2006, 39, 8027–8034 CrossRef CAS.
- H. P. Zhang, P. Zhang, Z. H. Li, M. Sun, Y. P. Wu and H. Q. Wu, Electrochem. Commun., 2007, 9, 1700–1703 CrossRef CAS.
- Y. J. Hwang, S. K. Jeong, K. S. Nahm and A. Manuel Stephan, Eur. Polym. J., 2007, 43, 65–71 CrossRef CAS.
- C. Reddy, Q.-Y. Zhu, L.-Q. Mai and W. Chen, J. Solid State Electrochem., 2007, 11, 543–548 CrossRef CAS.
- S. Rajendran and P. Sivakumar, Phys. B, 2008, 403, 509–516 CrossRef CAS.
- D. Bansal, B. Meyer and M. Salomon, J. Power Sources, 2008, 178, 848–851 CrossRef CAS.
- J.-Y. Sohn, J. S. Im, S.-J. Gwon, J.-H. Choi, J. Shin and Y.-C. Nho, Radiat. Phys. Chem., 2009, 78, 505–508 CrossRef CAS.
- Y.-J. Wang and D. Kim, J. Membr. Sci., 2008, 312, 76–83 CrossRef CAS.
- Q. Xiao, Z. Li, D. Gao and H. Zhang, J. Membr. Sci., 2009, 326, 260–264 CrossRef CAS.
- L.-Z. Fan, X.-L. Wang and F. Long, J. Power Sources, 2009, 189, 775–778 CrossRef CAS.
- S.-M. Eo, E. Cha and D.-W. Kim, J. Power Sources, 2009, 189, 766–770 CrossRef CAS.
- Y. Ding, P. Zhang, Z. Long, Y. Jiang, F. Xu and W. Di, J. Membr. Sci., 2009, 329, 56–59 CrossRef CAS.
- H.-S. Jeong, J. H. Kim and S.-Y. Lee, J. Mater. Chem., 2010, 20, 9180–9186 RSC.
- M. Ulaganathan and S. Rajendran, Journal of Applied Polymer Science, 2010, 118, 646–651 CAS.
- H. Li, Y.-M. Chen, X.-T. Ma, J.-L. Shi, B.-K. Zhu and L.-P. Zhu, J. Membr. Sci., 2011, 379, 397–402 CrossRef CAS.
- D. Saikia, H.-Y. Wu, Y.-C. Pan, C.-P. Lin, K.-P. Huang, K.-N. Chen, G. T. K. Fey and H.-M. Kao, J. Power Sources, 2011, 196, 2826–2834 CrossRef CAS.
- J.-Y. Sohn, J.-S. Im, J. Shin and Y.-C. Nho, J. Solid State Electrochem., 2012, 16, 551–556 CrossRef CAS.
- C. M. Costa, J. Nunes-Pereira, L. C. Rodrigues, M. M. Silva, J. L. G. Ribelles and S. Lanceros-Méndez, Electrochim. Acta, 2013, 88, 473–476 CrossRef CAS.
- J. K. Park, Principles and Applications of Lithium Secondary Batteries, Wiley, 2012 Search PubMed.
- M. Park, X. Zhang, M. Chung, G. B. Less and A. M. Sastry, J. Power Sources, 2010, 195, 7904–7929 CrossRef CAS.
- C. de las Casas and W. Li, J. Power Sources, 2012, 208, 74–85 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
