Multivalent, bifunctional dendrimers prepared by click chemistry†
Peng
Wu
a,
Michael
Malkoch
b,
Jasmine N.
Hunt
b,
Robert
Vestberg
b,
Eiton
Kaltgrad
a,
M. G.
Finn
*a,
Valery V.
Fokin
*a,
K. Barry
Sharpless
*a and
Craig J.
Hawker
*b
aThe Scripps Research Institute, Department of Chemistry, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037, USA. E-mail: sharples@scripps.edu; Fax: +1 858 784 7562; Tel: +1 858 784 7515
bMaterials Research Laboratories, University of California, Santa Barbara, CA 93106, USA. E-mail: hawker@mrl.ucsb.edu; Tel: +1 805 893 7161
First published on 4th November 2005
Abstract
Unsymmetrical dendrimers, containing both mannose binding units and coumarin fluorescent units, have been prepared using click chemistry and shown to be highly efficient, dual-purpose recognition/detection agents for the inhibition of hemagglutination.
The high functional group density at the chain ends of dendrimers, coupled with unprecedented control over molecular structure, makes these synthetic materials extremely attractive candidates for a variety of surface active applications.1 One of the most promising is to exploit the numerous chain end groups as multivalent binding sites2 for interaction with biological receptors and cell surfaces in the construction of targeted drug delivery systems.3 This concept has been exploited by Cloninger et al.4 in a series of pioneering studies designed to alter and control the strength of lectin binding by varying the generation number of a series of mannose functionalized PAMAM dendrimers. For future practical applications, at least 3 functional units are required: a targeting moiety, a medicinally active agent (drug), and a diagnostic label such as a fluorescent dye, each attached at a specific position within the nanostructure. While a number of approaches have been reported for combining all of these elements into a single system,5 the structural control and monodispersity of dendrimer-based macromolecules promise superior performance.6 A general strategy for the facile synthesis of chemically differentiated dendrimers which allows for the introduction of functional groups at defined locations has however not been reported. To address this challenge, the synthesis of dendritic block copolymers7 in which two distinct clusters of functionality (targeting and detection) are placed at the chain ends in a controlled fashion is described. The key chemical transformation which allows simple and facile preparation of these dual-purpose, multifunctional materials is the copper(I)-catalyzed azide–alkyne cycloaddition, a premier “click” reaction.8 The high efficiency of this process is exploited to couple the dendritic blocks together, while its tolerance of a wide variety of functional groups allows the introduction of reactive units at the periphery without the use of protecting groups.9
The synthetic approach selected is based on 2,2-bis(hydroxymethyl)propionic acid (bis-MPA) as a biocompatible building block and the resulting anhydride 1 provides easy access to both the alkyne 2 and the azide 3 by condensation with the appropriate alcohol.10 Removal of the acetonide protecting groups and subsequent acylation with the anhydride 1 allows for facile generation growth of the dendritic blocks containing either a single acetylene 4 or azide group 5 at the focal point. The resulting dendrons can be rendered reactive and hydrophilic by deprotection or kept protected and hydrophobic by retention of the acetonide caps.
To facilitate these studies and allow preparation of a library of structures, two series of dendrons up to the 4th generation were synthesized in high yield and purity (Scheme 1). Coupling of the differentiated dendritic blocks containing a variety of chain end functional groups proceeded smoothly under the copper(I)-catalysis conditions. Thus, reaction of (HO)8–[G-3]–Az, 6, and (An)4–[G-3]–Acet, 7, in dry THF in the presence of catalytic [Cu(PPh3)3Br] and N,N-diisopropylethylamine furnished the diblock dendrimer 8 in 92% yield after purification (Scheme 2). The orthogonality of this process is demonstrated by the ability to employ both the hydroxy and the acetonide terminated bis-MPA dendrons with no unwanted side reactions occurring at the numerous chain ends. The efficiency of this polymer coupling reaction was further proved by GPC, NMR and MALDI analysis, the latter showing a single peak at 1985 (MH+) for 8.11 Using the same methodology, a series of amphiphilic dendrimers from generation 1 to 4 were prepared from dendrons of varied sizes. For example, the asymmetrical structure 9, in which both the size and functionality of the dendron is varied, was assembled from a hydroxy functionalized [G-1]–azide and an acetonide functionalized [G-4]–acetylene.
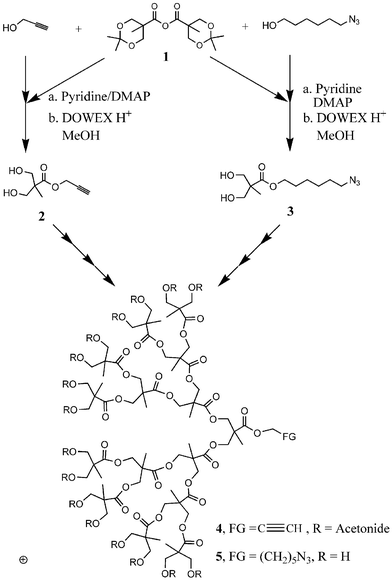 | ||
| Scheme 1 Synthesis of hydrophilic and hydrophobic dendrons with either acetylene 4 or azide 5 groups at the focal point. | ||
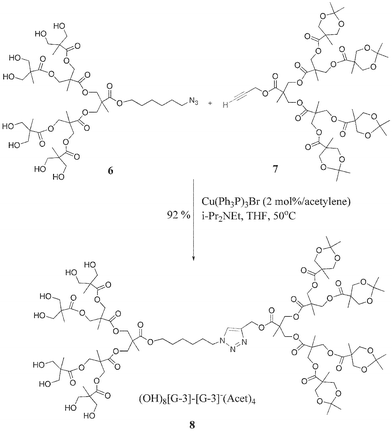 | ||
| Scheme 2 Synthesis of unsymmetrical dendrimer 8 containing a chemically differentiated surface. | ||
The modular nature of this synthetic strategy and the chemical stability of triazoles, azides and acetylenes allows for the efficient introduction of functional groups at different stages of the process. For example, the asymmetrical dendrimer (An)16–[G-4]–[G-1]–(OH)2, 9, was first decorated with alkyne groups by esterification of the two free hydroxyls with pent-4-ynoic anhydride (Scheme 3). Removal of the acetonide protecting groups on the 4th generation dendritic block of 10 revealed 16 reactive hydroxy groups (11) followed by attachment of the two 7-diethylaminocoumarin dyes12 to the alkyne units resulting in 12. After introduction of the 16 alkynes (via esterification with the anhydride of pentynoic acid), the resulting dendrimer 13 was directly coupled with the unprotected 2-azidoethyl α-D-mannopyranoside in THF–water to furnish the desired asymmetrical, dual functionalized dendrimer, 14. Complete characterization of the asymmetric dendrimers by GPC, NMR and MALDI spectroscopy showed essentially monodisperse materials with quantitative functionalization of the chain ends after every step. For example, the acetylene functionalized, fluorescently labelled dendrimer 13 showed a single molecular ion (MH+ = 4184; MNa+ = 4206) in the MALDI spectrum which correlates with 2 coumarin and 16 acetylene chain end groups (Fig. 1). Designed to bear peripheral groups for polyvalent binding (mannose) and fluorescent dyes (coumarin) for visualization/diagnostic purposes, this macromolecular structure is an example of the sophisticated, multifunctional nanomaterials that can be constructed in a stepwise, yet facile manner using Click methodology.13
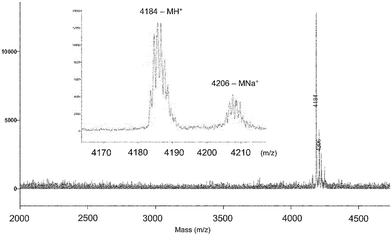 | ||
| Fig. 1 MALDI mass spectrum of the differentiated, dye-labelled dendrimer 13 showing efficient functionalization and monodispersity. | ||
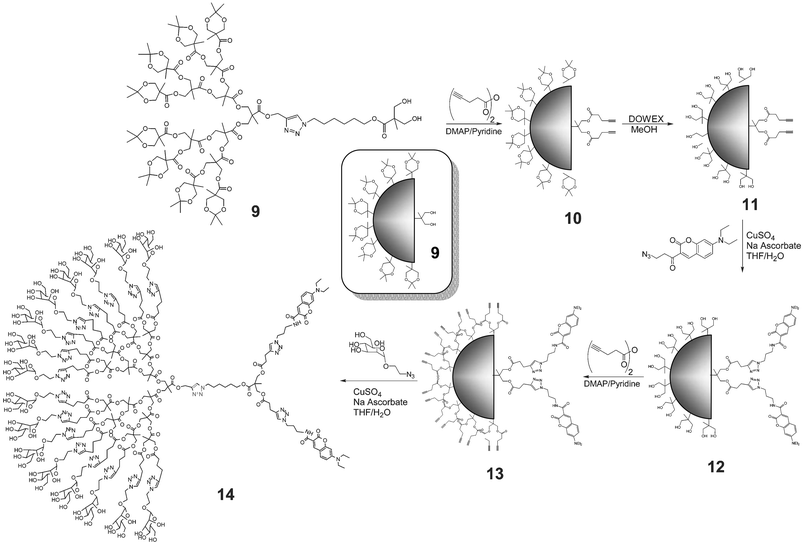 | ||
| Scheme 3 Synthesis of multivalent, asymmetrical dendrimer 14 containing 16 mannose units and 2 coumarin chromophores. | ||
The performance of the mannosylated dendrimer was evaluated in the standard hemagglutination assay using the mannose binding protein concanvalin A and rabbit red blood cells.14 Dendrimer 14 exhibited 240-fold greater potency than monomeric mannose, corresponding to a relative activity of 15 per sugar moiety when compared to mannose (activity = 1). This demonstrates the synergistic benefit provided by the multivalent, dendritic array of receptor groups. A complete study of polyvalent affinity vs. dendrimer size and generation number is underway and will be described in the future.
In summary, copper(I)-catalyzed azide–acetylene cycloaddition15 has proven to be a powerful tool for both the preparation of unsymmetric diblock dendrimers and for efficient differentiation of the dendritic chain end groups. By preparing dendrons with unique acetylenic and azide groups at the focal point, highly efficient coupling of these blocks was achieved through the formation of a stable [1,2,3]-triazole linkage. Quantitative modification and sequential differentiation of the chain ends by the introduction of mannose and coumarin unit derivatives to the periphery of individual blocks allowed preparation of agents with dual function, recognition and detection, which may prove useful in identification and treatment of pathological conditions via multivalent interactions.
Financial support of this work by the National Institutes of Health through a Program of Excellence in Nanotechnology Grant (1 U01 HL080729-01) and the National Institute of General Medical Sciences (GM 28384), National Science Foundation (UCSB MRSEC, DMR-0520415, DMR-0301833, CHE-0514031), the W. M. Keck Foundation, the Skaggs Institute for Chemical Biology, Skaggs predoctoral fellowship (P.W.), and the Sweden-American Foundation (M.M.) is gratefully acknowledged. For synthetic help and fruitful discussions, thanks to D. Thayer and S. Narayan.
Notes and references
- C. S. Gudipati, C. M. Greenlief, J. A. Johnson, P. Prayongpan and K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 6193–6208 CrossRef CAS; P. Y. W. Dankers, M. C. Harmsen, L. A. Brouwer, M. J. A. Van Luyn and E. W. Meijer, Nat. Mater., 2005, 4, 568–574 CrossRef CAS; S. Fuchs, T. Kapp, H. Otto, T. Schoneberg, P. Franke, R. Gust and A. D. Schluter, Chem.–Eur. J., 2004, 10, 1167–1192 CrossRef CAS; C. J. Hawker and K. L. Wooley, Science, 2005, 309, 1200–1203 CrossRef CAS.
- M. Mammen, S. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754–2794 CrossRef.
- M. Liu and J. M. J. Fréchet, Pharm. Sci. Technol. Today, 1999, 2, 393 CrossRef CAS; R. Esfand and D. A. Tomalia, Drug Discovery Today, 2001, 6, 427 CrossRef CAS.
- E. K. Woller, E. D. Walter, J. R. Morgan, D. J. Singel and M. J. Cloninger, J. Am. Chem. Soc., 2003, 125, 8820–8826 CrossRef CAS; E. K. Woller and M. J. Cloninger, Org. Lett., 2002, 4, 7–10 CrossRef CAS; E. K. Woller and M. J. Cloninger, Biomacromolecules, 2001, 2, 1052–1054 CrossRef CAS.
- E. R. Gillies, T. B. Jonsson and J. M. J. Fréchet, J. Am. Chem. Soc., 2004, 126, 11936 CrossRef CAS.
- E. R. Gillies and J. M. J. Fréchet, Drug Discovery Today, 2005, 10, 35 CrossRef CAS.
- Stefan Hecht, J. Polym. Sci., Sect A: Polym. Chem., 2003, 41, 1047–1058 Search PubMed; H. Baigude, K. Katsuraya, K. Okuyama, K. Hatanaka, E. Ikeda, N. Shibata and T. Uryu, J. Polym. Sci., Sect. A: Polym. Chem., 2004, 42, 1400–1414 Search PubMed; C. J. Hawker, K. L. Wooley and J. M. J. Fréchet, J. Chem. Soc., Perkin Trans. 1, 1993, 1287–1297 RSC.
- V. V. Rostovsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS.
- J. A. Opsteen and J. C. M. Van Hest, Chem. Commun., 2005, 57–59 RSC; M. Malkoch, K. Schleicher, E. Drockenmuller, C. J. Hawker, T. P. Russell, P. Wu and V. V. Fokin, Macromolecules, 2005, 38, 3663–3678 CrossRef CAS; N. V. Tsarevsky, B. S. Sumerlin and K. Matyjaszewski, Macromolecules, 2005, 38, 3558–3561 CrossRef CAS; B. Parrish, R. B. Breitenkamp and T. Emrick, J. Am. Chem. Soc., 2005, 127, 7404–7410 CrossRef CAS.
- M. Malkoch, E. Malmström and A. Hult, Macromolecules, 2002, 35, 8307–8314 CrossRef CAS.
- See supporting information.
- L. Zhu, V. M. Lynch and E. V. Anslyn, Tetrahedron, 2004, 60, 7267 CrossRef CAS.
- D. D. Díaz, S. Punna, P. Holzer, A. K. McPherson, K. B. Sharpless, V. V. Fokin and M. G. Finn, J. Polym. Sci., Sect A: Polym. Chem., 2004, 42, 4392–4403 Search PubMed.
- (a) L. L. So and I. J. Goldstein, J. Biol. Chem., 1968, 243, 2003–2007 CAS; (b) T. Osawa and I. Matsumoto, Methods Enzymol., 1972, 28, 323–327 CrossRef.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of dendritic macromolecules. See DOI: 10.1039/b512021g |
| This journal is © The Royal Society of Chemistry 2005 |
