Recent advances in the polymerization of elemental sulphur, inverse vulcanization and methods to obtain functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs)
Yueyan
Zhang
 a,
Richard S.
Glass
a,
Kookheon
Char
a,
Richard S.
Glass
a,
Kookheon
Char
 b and
Jeffrey
Pyun
b and
Jeffrey
Pyun
 *ab
*ab
aDepartment of Chemistry and Biochemistry, University of Arizona, 1306 East University Boulevard, Tucson, AZ 85721, USA. E-mail: jpyun@email.arizona.edu
bSchool of Chemical and Biological Engineering, Program for Chemical Convergence for Energy & Environment, The National Creative Research Initiative Center for Intelligent Hybrids, Seoul 151-744, Korea. E-mail: khchar@snu.ac.kr
First published on 20th June 2019
Abstract
Recent developments in the polymerization of elemental sulfur and the preparation of functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs) are reviewed. CHIPs represent a class of polymers synthesized from elemental sulfur with the incorporation of inorganic chalcogenide components (S, Se, Te) into the organic polymeric backbones. Novel CHIP materials exhibit interesting optical, electrochemical and mechanical properties that lead to applications in thermal imaging, energy storage, self-healable materials and separation science. The emphasis of this review is on the key advances in the synthetic approaches to prepare functional polymeric sulfur-rich materials, with recent developments in synthesis, characterization, and application milestones being highlighted.
1. Introduction
Sulfur is the tenth most abundant element on Earth and has been recognized as a valuable chemical agent since antiquity. It has been used in medicine, fabric bleaching, construction of lamp wicks, gun powder formulation and then more recently, in the vulcanization of latex.1,2 In modern petroleum refining processes, the production of elemental sulfur (S8) is achieved through the removal of highly toxic hydrogen sulfide (H2S) gas from crude oil and recovered through a thermal expansion process.3 With approximately 70 million tons of by-product sulfur produced each year, refining of petroleum via hydrodesulfurization is currently the largest source of sulfur production globally.4 A significant portion of sulfur is used in the industrial production of sulfuric acid, rubber, and fertilizers;5 in addition, modern synthetic chemistry has also benefitted from the versatile chemistry of elemental sulfur.6 However, there is still a net excess of sulfur that offers few economic uses,7 thus, finding large-scale uses for this sulfur, such as conversion to useful materials, would be an important development.Numerous advances have been made in the last few decades concerning the synthesis of new types of sulfur-containing polymers and modification of the properties of some important classes of polymers by adding sulfur or polysulfide fragments to the polymer compositions.8,9 In many cases, these new synthetic methods have yielded valuable polymeric materials and have received extensive interest in this area, as indicated by the numerous studies on the sulfur-containing polymers.10–14 Recent research, both academic and applied, has been focused on enhancing the properties and processing characteristics of these materials by introducing sulfur groups into the main-chain to broaden the scope of technological applications of these materials.15 Different sulfur-containing polymers (illustrated in Fig. 1) have been previously summarized in various reviews.9,15–17
Since 2011, Pyun et al. have advanced this field by demonstrating the preparation of sulfur-containing polymers via the direct polymerization of elemental sulfur with organic comonomers. This process was termed inverse vulcanization in 2013 and the materials derived from this methodology were generally referred to in the pioneering literature on this work as “sulfur-containing (co)polymers” or polysulfides. However, since then, there have been numerous attempts to rename this class of polymers derived from elemental sulfur. These include “inverse vulcanized polymers” as first seen in 2014,18 “organically modified chalcogenides” in 2017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 and more recently “thiopolymers” in 2019.20 The complication and redundancy are that the aforementioned terms, all refer to the same class of inorganic/organic hybrid polymers derived from the polymerization of elemental sulfur. Regardless of the subjective preferences, or biases presented to name this class of materials, it is important to establish a clear and rational rubric for naming these types of polymers. This confusion is already compounded by the nomenclature similarities and ambiguities of organo-polysulfides, inorganic polysulfides, or sulfur allotropes. For the purpose of distinguishing how polymers derived from the polymerization of elemental sulphur are structurally distinctive from other sulfur containing polymers, we briefly summarize the classical terms used to describe these types of materials.
19 and more recently “thiopolymers” in 2019.20 The complication and redundancy are that the aforementioned terms, all refer to the same class of inorganic/organic hybrid polymers derived from the polymerization of elemental sulfur. Regardless of the subjective preferences, or biases presented to name this class of materials, it is important to establish a clear and rational rubric for naming these types of polymers. This confusion is already compounded by the nomenclature similarities and ambiguities of organo-polysulfides, inorganic polysulfides, or sulfur allotropes. For the purpose of distinguishing how polymers derived from the polymerization of elemental sulphur are structurally distinctive from other sulfur containing polymers, we briefly summarize the classical terms used to describe these types of materials.
Historically, the term “polysulfide” referred to both inorganic forms of sulfur-containing covalent solids of sulfur chains (–Sn–), ionic compounds (Sn2−, n > 2) and organosulfur compounds in which the sulfur chain was terminated by organic substituents R (R–Sn–R). To address this issue, a nomenclature based on sulfanes and polysulfanes was provided in a comprehensive review by Steudel in 2002.21 According to this nomenclature, the name polysulfide was established to describe inorganic ionic compounds.22,23 The term “organic polysulfanes” was used to describe compounds of the type R–Sn–R with n > 2 in which the organic residues (alkyl or aryl) are linked to the sulfur chain via carbon atoms. The polymeric materials containing multiple sulfur units and hydrocarbons in the backbones were often referred to as polysulfide polymers.8,21,24–30 Penczek et al. was among the first to comprehensively review organosulfur based polymers, the most common of which include polyalkylene sulphides, polyarylene sulphides, polythioureas, polysulfoxides and polysulfones.9 From a chemical structural perspective, all of these aforementioned sulphur containing polymers shown in Fig. 1 are composed of a single C–S moiety per repeating unit. Organo-disulphide copolymers have also been widely explored and contain an S–S moiety per repeating unit.24,31,32 In a recent review, Theato et al. have elegantly discussed recent advances in the preparation of polymers containing thioesters, thiocarbonates, thiocarbamates and thioamides, which contain multiple C–S moieties per repeating unit as incorporated through thiocarbonyl-based functional groups.15
With the advent of inverse vulcanization and other methods for the polymerization of elemental sulfur, it is apparent that a new subset of sulfur-containing polymers or polysulfides has been created that warrants classification in comparison to these known materials. In our 2016 review, we focused on classes of sulfur-containing polymers and synthetic methods to prepare these materials, however, we did not address this descriptive nomenclature issue. In particular, polymers derived from the polymerization of elemental sulfur have developed into a distinctive class of sulfur-containing polymers, which we recommend to be termed, Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). From a chemical structure perspective, CHIPs derived from the polymerization of elemental sulfur will contain a statistical/random sequence & composition of –Sx– units bonded to organic comonomer units. These sulphur units may be mono, di-, or multiple S–S bonds in the (co)polymer backbone, which is the distinctive structural feature of CHIPs made from S8 polymerization (Fig. 1). To date, either free radical inverse vulcanization or anionic polymerization processes have been the primary methods used to prepare polymeric materials from elemental sulfur. We propose that sulfur-containing polysulfides prepared from the polymerization of elemental sulfur with organic comonomers be generally referred to as CHIPs and appropriately categorized as a class of organo-polysulfides consistent with the original Penczek formalism.9 However, regardless of whether CHIPs, or the other variations referred to previously are used, we recommend that a consistent rubric be used to distinguish these polymers from other sulfur-containing polymers.
The focus of this review is to discuss the recent advances in the polymerization of elemental sulfur to prepare CHIPs, with an emphasis on new synthetic advances since our original comprehensive review on this subject in 2016.12 In particular, we discuss recent work on free radical and inverse vulcanization processes, along with new anionic polymerization methods to prepare CHIP materials. Furthermore, we review for the first time synthetic methods to introduce functionality onto monomers and polymers derived from elemental sulphur.
2. Direct polymerization with elemental sulfur
2.1 Inverse vulcanization with elemental sulfur
In the following section, we revisit the homopolymerization of S8, with an emphasis on the ramifications of low-temperature processing, along with new comonomers for polymerization with liquid sulfur. After a brief overview of elemental sulphur, we broadly discuss new comonomers that have been developed since our last comprehensive review of this literature in 2016. To aid the reader, these new comonomer advances are grouped into how chemical modifications to monomer structure can affect the thermomechanical, optical, electrochemical or metal scavenging properties of CHIP materials. Finally, advances into the accelerated or “catalytic” inverse vulcanization process are discussed.The free radical copolymerization of unsaturated organic comonomers with liquid sulfur was studied over sixty years ago but was limited to the production of high sulfur content lubricants.49,51–53 The free radical copolymerization of S8 with vinylic comonomers, such as, acrylates,54 vinyl acetates,55 tetrafluoro-ethylene,56–58 2-chloroprene,59–64 1,3-butadiene,65 cyclododeca-1,5,9-triene,66 cyclohepta-1,3,5-triene,67 cycloocta-1,3-diene,66 cyclohexene,68 1-methylcyclohexene,68 norbornene,69 dicyclopentadiene,66,70–73 tricyclopentadiene,66,72 limonene,66 6-dimethylocta-2,4,6-triene,66 7-methyl-3-methyleneocta-1,6-diene,66 3,7-dimethylocta-1,6-diene68 and 2,6-dimethylhepta-1,5-diene68 has been investigated in the 1960s and 1970′s, which was comprehensively reviewed by Pyun in 2016.12 Other classical uses of sulphur for material uses include the production of ebonite74 or sulphur cements.12
The first demonstration of utilizing elemental sulphur as the reactive medium for polymerization was reported by Pyun et al. in 2011 where Au nanoparticles were formed in the sulphur matrix.75 In 2013, Pyun and co-workers developed a new process, termed, inverse vulcanization, which enabled the bulk copolymerization of molten elemental sulphur with vinylic comonomers without the need for an externally added initiator or organic solvent.76 In this pioneering report, 1,3-diisopropylbenzene (DIB) was used as the organic comonomer for bulk polymerization. In the inverse vulcanization reaction, elemental sulphur was heated to above Tf to initiate ring-opening polymerization followed by the addition of DIB (typically at a feed ratio of 10–50 wt%) resulting in copolymerization and vitrification within minutes. The resulting red polymeric glass formed was a statistical copolymer named poly(sulfur-random-1,3-diisopropenylbenzene) or poly(S-r-DIB). Because of the high sulfur content (50–90 wt% S) and the polysulfide structure of the backbone of the polymer, poly(S-r-DIB) was found to exhibit novel electrochemical, mechanical, and optical properties.49,53,76–79 The inverse vulcanization process has since been extended into a wider class of organic comonomers, which now include alkenes,20,50,75,76,80–101 alkynes,102–105 naturally occurring olefins,90,95,106–113 allyl ethers,114–116 oleyamine,117,118 maleimide,119 and benzoxazines.120,121 The inverse vulcanization of sulfur and other unsaturated compounds such as thiourea aldehyde resin (TAR)122 has been reported; however, extensive structural characterization of these materials remains unexplored. The reactive medium of liquid sulfur also enables the incorporation of other chalcogenide elements such as selenium into CHIPs to improve the optical or electrochemical properties of these materials.19,123–125 This inverse vulcanization method exhibited moderate functional group tolerance85,98,117,119,120,126–130 but for some functional groups,102,117,131–133 cosolvents such as dichlorobenzene, tetrachloroethane, pyridine or diglyme are necessary to create a homogeneous reaction system due to the limited miscibility and solubility of elemental sulphur. One of the drawbacks of the solution-based polymerization is the low conversion of sulphur which leads to low sulphur content products.133 Thus, bulk copolymerization methods have been more extensively investigated to afford useful polymeric materials. Furthermore, CHIP materials, in general, suffer from poor thermomechanical properties, poor electrical conductivity and poor handling characteristics (e.g., solution and melt processability). Hence, there is a clear technological need for exploring new monomers and polymerization methods to improve the functionality and properties of CHIPs. This section will focus on recent advances for the development of new monomers for inverse vulcanization to prepare functional CHIPs to impart improved properties and function.
2.1.1.1 Comonomers for tuning the thermal/mechanical properties of CHIPs. It was previously observed that poly(S-r-DIB) copolymers could either be thermoplastic or thermosetting materials depending on the processing conditions established to prepare or mold these materials.12,53 Hence, there are opportunities to enhance the thermomechanical properties of CHIPs by modifying the organic comonomer structure by increasing the functionality of reactive groups per molecule (f), or via modulation of the rigidity within the organic comonomer.
To increase the crosslinking density and improve the thermomechanical properties of CHIP materials, the synthesis of comonomers with a higher functionality than DIB was explored by Pyun et al. using 1,3,5-triisopropenylbenzene (TIB) for inverse vulcanization. This reaction afforded highly crosslinked copolymers (Fig. 3a)86 with improved thermomechanical properties and a dramatic enhancement of the glass transition temperature (Tg = 60–130 °C for 30–50 wt% of S) compared to the first generation poly(S-r-DIB) CHIPs which possessed a Tg of 43–49 °C determined from Dynamic Mechanical Analysis (DMA) (Fig. 3b).86
 | ||
Fig. 3 (a) Schematic structure of poly(S-r-TIB). (b) Plot of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ as a function of temperature. Adapted with permissions from ref. 86. Copyright 2016 American Chemical Society (c) Schematic structure of poly(S-r-DA). (d) Free standing films made with poly(S-r-DA). Reproduced with permission from ref. 95. Copyright 2017 Elsevier B.V. δ as a function of temperature. Adapted with permissions from ref. 86. Copyright 2016 American Chemical Society (c) Schematic structure of poly(S-r-DA). (d) Free standing films made with poly(S-r-DA). Reproduced with permission from ref. 95. Copyright 2017 Elsevier B.V. | ||
The inverse vulcanization of sulfur and a naturally occurring diene, diallyl disulfide (DA), was used to prepare flexible, freestanding CHIP films with high sulfur content (50–90 wt% of sulfur) (Fig. 3c and d).95 The film showed a very low Young modulus (1.3 to 6.1 MPa) compared to films prepared from poly(S-r-DIB) which contain the rigid aromatic moieties (185 to 460 MPa) suggesting the increased flexibility of the materials.134 These thermomechanical properties could be attributed to the presence of freely rotating low aliphatic carbon chains, which leads to the low glass transition temperatures (Tg = −13 °C to −4 °C) of these sulfur containing copolymers.
To improve the thermomechanical properties of CHIPs, copolymerization strategies with rigid aromatic comonomers have also been explored. O'Brien et al. demonstrated that the utilization of bisphenol A dimethacrylate (BPAD) for inverse vulcanization raised the melting and decomposition temperatures of the resulting CHIPs, along with the hardness of the sulfur copolymer which optimized the formation of robust polymer–nanocrystal thin films.129 The DSC analysis of S-BPAD copolymers did not exhibit an observable glass transition and only weak melting transitions at Tm = 180–245 °C were observed, which coincided with copolymer decomposition. Alternatively, Yagci et al. reported on the incorporation of polybenzoxazine moieties where the final sulfur polymers exhibited high tensile strength (100–125 MPa), high modulus (3.8–4.5 GPa), and high glass transition temperatures (Tg = 170–340 °C) which will be discussed in detail in Section 2.1.3.135
Healable polymeric materials (SHPM) have received extensive attention due to their structural design and material applications.136–140 Disulfide and polysulfide linkages have been utilized in the preparation of SHPMs due to their relatively low bond dissociation energy, indicating the self-healing properties.49,53,141–143 Polyhedral oligomeric silsesquioxane (POSS) has been demonstrated to be an effective building block to prepare materials with improved thermomechanical properties due to the size and rigidity of the POSS moiety.144–146 Liu et al. demonstrated the preparation of new hybrid (S-MMA-POSS) CHIPs prepared by the direct reaction between a multifunctional methacrylated POSS monomer (MMA-POSS) and elemental sulfur (S8) through the inverse vulcanization process (Fig. 4a).133 Due to the limited miscibility and solubility of elemental sulfur, a cosolvent (diglyme) was necessary to create a homogeneous reaction system. The tensile tests provide sufficient evidence of the healability of the S-MMA-POSS that the recovery efficiency based on the tensile strength reaches about 91% with a thermal treatment at 120 °C for 3 h (Fig. 4b).133 Moreover, S-MMA-POSS CHIPs were also useful for co-curing with other conventional thermosetting resins and preparing materials with high transparency in the mid-infrared region.
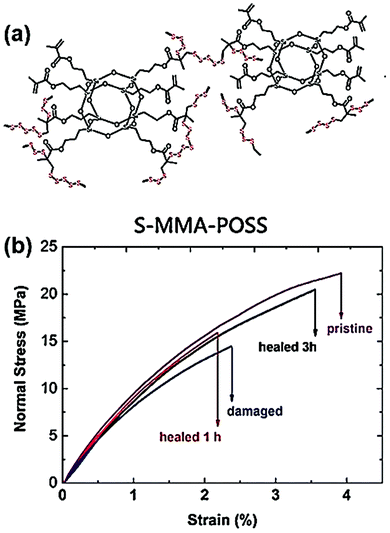 | ||
| Fig. 4 (a) Schematic structure of the S-MMA-POSS copolymer. (b) The healable properties of the thermally cured S-MMA-POSS sample evaluated with tensile tests. Reproduced with permission from ref. 133. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
2.1.1.2 Inorganic or organometallic monomers to increase the refractive index. CHIPs made from inverse vulcanization are a unique class of optical polymers that exhibit a high refractive index (n) and good IR transparency in comparison to conventional synthetic polymers.79,147,148 The refractive index could be further increased via the incorporation of other chalcogens or more polarizable elements.
Boyd et al. reported on the incorporation of Se by preparation of crystalline sulfur–selenium compounds reacted at 400 °C followed by polymerization with DIB to generate poly(S-r-Se-r-DIB) terpolymers.19 Pyun et al. reported for the first time the use of direct inverse vulcanization processes to incorporate Se units with gray selenium precursors by simple “cracking” and solubilization with liquid sulfur radicals at dramatically reduced temperatures (T = 160 °C) (Fig. 5a).124 This one-pot, streamlined synthesis enabled the controllable compositional control of Se feeds into poly(S-r-Se-r-DIB) CHIPs and resulted in a profound increase in a refractive index (n) of above n = 2.0 from 633 to 1554 nm (Fig. 5b). This unique feature of CHIP materials was also utilized to fabricate one-dimensional photonic crystals (1D-PCs)/Bragg reflectors as their ability to select the desired n, and create a large refractive index contrast(Δn), allowing for control over the optical bandgap of the assembled Bragg reflector in addition to the wavelength of reflection. Pyun then demonstrated the utilization of these ultra-high RI CHIPs for the fabrication of highly reflective all-polymer Bragg reflectors possessing a high refractive index contrast attained by the solution fabrication of alternating CHIPs and cellulose acetate films (Δn = 0.5). This allowed for very high reflectance (90–97%) at just 22 layers of alternating CHIP–cellulose acetate films and tunability over the near (NIR) and the short wave (SWIR) infrared spectrum (1–2 μm) (Fig. 5c).149 Mecerreyes et al. also demonstrated an enhanced C-rate capability and high cycling stability when poly(S-r-Se-r-DIB) was used as the cathode material in Li–S batteries.125 The incorporation of the high atomic number (Z) element Sn via the inverse vulcanization process with elemental sulfur and organometallic comonomer tetravinyltin (TVSn) was reported by Boyd et al. (Fig. 5d)100 and the obtained poly(S-r-TVSn) copolymer exhibited enhanced n and transmission in the IR region (Fig. 5e).
 | ||
| Fig. 5 (a) Synthesis of poly(S-r-Se-r-DIB) terpolymer CHIPs. (b) Refractive indices of poly(S-r-Se-r-DIB) terpolymers of varying compositions vs. wavelengths. Reproduced with permission from ref. 124. Copyright 2017 American Chemical Society. (c) Bragg reflectors from CHIP materials. Reproduced with permission from ref. 149. Copyright 2018 American Chemical Society. (d) Synthesis and digital camera image of poly(S-r-TVSn). (e) Refractive index plots for poly(S-r-TVSn) at various wavelengths. Reproduced with permission from ref. 100. Copyright 2019 American Chemical Society. | ||
2.1.1.3 New monomers to enhance electrochemical properties. Poly(S-r-DIB) was the first example of using high sulfur content (>50 wt% S) CHIPs as the active materials in cathode electrodes for Li–S batteries to improve device performance.76,78 This advance has since inspired extensive study for exploiting different polymeric sulfur materials for Li–S battery applications.11,12,150–152 The inverse vulcanization process enabled various sulfur-enriched polymeric materials to be synthesized and afforded new advanced sulfur cathodes in Li–S batteries. However, most reported sulfur-rich copolymers prepared via inverse vulcanization suffer from capacity loss during long-term cycling because of polysulfide dissolution and irreversible deposition of lower rank lithium sulfide discharge products.11,13,78,150,151,153 Extensive studies have been made on the preparation of nanostructured composite materials from sulfur copolymers with conductive carbon inclusions such as carbon nanotubes, reduced graphene oxide or carbon spheres. Li–S batteries fabricated with the obtained nanocomposites exhibited a long cycle life with a fast cycling rate and have been elegantly reviewed elsewhere as a separate class of materials.91,128,154–158 However, most reported batteries using sulfur-rich polymers (i.e., without nanocomposites) as active materials result in low cycling rates and rapid capacity degradation with the increase of the cycling rate to higher than 2C.114,151
One exception is the polymeric sulfur material made from the inverse vulcanization of sulfur and tetra(allyloxy)-1,4-benzoquinone (TABQ). Park et al. demonstrated the preparation of sulfur copolymers from elemental sulfur and different allyl ether comonomers and tested their battery performance fabricated with these sulfur materials (Fig. 6a).114 Among all these, poly(S-tetra(allyloxy)-1,4-benzoquinone), (poly(S-TABQ)), exhibited excellent cycling capability while retaining a specific capacity of 833 mA h g−1 at a cycling rate of 10C (Fig. 6b). This remarkable performance is striking for a wholly polymeric based active material in Li–S batteries.
 | ||
| Fig. 6 (a) Structures of allyl ether monomers TABQ, ANQ, BAAQ, and TATA. (b) Rate performance of lithium cells based on poly(S-TABQ) (red), poly(S-TATA) (blue), and elemental sulfur (green). (c) Long cycle life of the lithium cell based on poly(S-TABQ) at 1C. Reproduced with permission from ref. 114. Copyright 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
2.1.1.4 Inverse vulcanization with natural olefinic comonomers. Chalker and Hasell et al. investigated the inverse vulcanization of sulfur with various renewable olefinic comonomers (Fig. 7a) and the application of the obtained sulfur polymers for environmental protection and remediation has been elegantly reviewed elsewhere.90,159 These high sulfur content CHIPs have demonstrated efficient metal-ion uptake due to the affinity between S–S bonds and Lewis acids. Mechanistic studies by Chalker et al. reported on the ability of S–S bonds from CHIPs to oxidize Hg(0) to Hg(II), which exploits the intrinsic redox reactivity of S–S bonds in these materials.160 Furthermore, the processability of these high sulfur content copolymers enabled fabrication into high surface area constructs ideally suited for applications in water purificaion.159 Chalker et al. reported on the sulfur–limonene copolymerization and the application of the obtained copolymers for palladium and mercury metal capture.88 The same group later explored the sulfur copolymers made from sulfur and vegetable oils (such as canola oil) and demonstrated the use of these materials for metal scavenging, crude oil remediation and slow-release fertilizers (Fig. 7b).106,107,110,111 Hasell et al. demonstrated the preparation of porous CHIP materials from sulfur copolymerization with low-cost monomer dicyclopentadiene and renewable terpenes such as myrcene, farnesol, farnesene and perillyl alcohol followed by supercritical CO2 foaming.89,90,97,109,161,162 These polymeric sulfur materials with a high surface area exhibited efficient adsorption of mercury from aqueous solution (Fig. 7c). More recently, the preparation of electroactive CHIP materials from algae oil-based comonomers has also been reported for use in Li–S batteries.163
 | ||
| Fig. 7 (a) Renewable olefinic comonomers for inverse vulcanization. (b) Fe3+ concentration over time for porous polysulfide (green), non-porous polysulfide (blue), and no polymer (red) added to the solution. Reproduced with permission from ref. 110. Copyright 2018 The Royal Society of Chemistry. (c) The percentage Hg remaining in solution after 3 hours of exposure to each of the materials listed. Reproduced with permission from ref. 109. Copyright 2017 The Royal Society of Chemistry. | ||
2.1.1.5 Accelerated inverse vulcanization reactions. In general, the homolytic ring opening reaction of S8 requires heating to over 159 °C to generate a sufficiently high concentration of reactive sulfur radicals for appreciable rates of homopolymerization. For certain comonomers, such as styrene, reaction temperatures around 130 °C were observed to form oligomeric materials due to the presence of more reactive cyclic sulfur species.50 However, there remain opportunities and challenges to develop improved methods to enable polymerizations of S8 at lower temperatures, in organic/aqueous solution and with other functional comonomers with low reactivity and/or low boiling point. In addition, avoiding higher temperatures is crucial in minimizing the formation of hydrogen sulfide and thiols, and dehydrogenation of olefins.164 Thus, a catalytic pathway that lowers the reaction temperature for inverse vulcanization is highly desirable to avoid H2S generation and/or auto-acceleration of the reactions by the Trommsdorff–Norrish effect.77
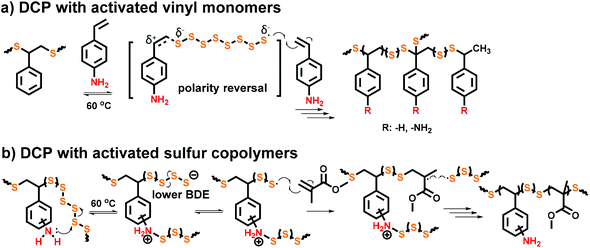
Pyun et al. were the first to report two types of organic accelerators that significantly increased the rate of inverse vulcanization.165 Activated vinyl monomers, such as 4-vinylaniline (4VA), proceeded with a profound rate enhancement during inverse vulcanization which was attributed to polarity reversal effects (Fig. 8a)130,166 and nucleophilic activators such as N-methylimidazole (NMI) are known to ring-open S8 under various conditions to enhance the reactivity of these reactions (Fig. 8b).167–169 We observed that with 1 mol% of 4VA or NMI added to the sulfur-styrene copolymerization, the rate (measured by styrene consumption) was increased by a factor of 2 and the molecular weight of the final products was comparable to the copolymerization without activators. 4-Vinylaniline also provides the pathway for copolymerizations at low temperatures (≥60 °C) which will be discussed in detail in Section 3.1. These systems were referred to as “nucleophilic activators” since the regeneration of the initial molecular structure of these small molecular accelerators could not be structurally proven to warrant proper assignment of catalysis.
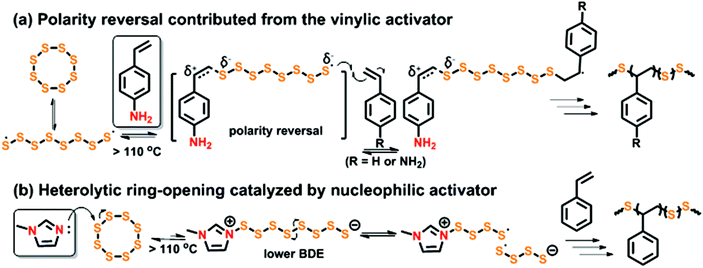 | ||
| Fig. 8 (a) Polarity reversal effects of vinylic activators (e.g., 4-VA) to S8, (b) nucleophilic ring-opening of S8 using an externally added activator (e.g., NMI). | ||
Hasell et al. demonstrated for the first time that a series of metal diethyldithiocarbamate complexes (Fig. 9c) could be used to enhance the reaction rate of the copolymerization of sulfur and organic crosslinkers at moderate temperature (135 °C).20 The metal–sulfur bond allowed for the opening of the S8 ring at temperatures lower than 159 °C, and insertion of sulfur between the metal and diethyldithiocarbamate (DTC) ligand to generate the accelerator. The accelerator then brings the sulfur into proximity to the crosslinker, and presumably lowers the energy barrier for bond formation. The copolymerization with the addition of activators was observed to proceed without H2S gas generation and promoted the reaction of sulfur and some crosslinkers such as glyoxal bis(diallylacetate) (GBDA) and 1,3,5,7-tetravinyltetramethylcyclotetrasiloxane (TVTCSi) that were unreactive without accelerators (Fig. 9b).
 | ||
| Fig. 9 (a) Monomers that can be polymerized without a “catalyst”. (b) Monomers unreactive without a “catalyst”. (c) “Catalysts” for inverse vulcanization. Reproduced with permission from ref. 20. Copyright 2019 Nature publication. | ||
Sulfur-rich copolymers have drawn much attention due to their excellent processability, flexibility and electrochemical properties. The inverse vulcanization methodology, which provides a feasible synthetic approach for preparing various sulfur-rich copolymers, is undoubtedly a great breakthrough in directly utilizing S8 for the preparation of advanced materials. However, due to the complexity of the sulfur species in the reaction system and the limitations of characterization techniques, the mechanism for the copolymerization process and the molecular structure of the product is still not fully understood. Thus, developing new model systems to investigate the reactivity and mechanism aspects of this polymerization is needed to enable the rational-design of novel CHIPs with improved properties.
| S8 + HS-R-SH → [(S)n − R]m + H2S | (1) |
These studies indicated that the reaction proceeded via a free radical process and yielded a variety of hydrogen terminated polysulfides or sulfanes.170 The reaction of thiols with sulfur was conducted in the temperature range from 130–160 °C to form polysulfide polymers via a base catalyzed process.73 In the presence of an amine catalyst, the reaction has been reported to occur even at ambient temperatures. Bordoloi et al. demonstrated the amine catalyzed reactions between sulfur and dithiols to generate polymers through a thiol–ene type reaction which accounted for the deviation observed in molecular weight versus the theoretical values. It was observed that as the molecular weight of the polymer increased, the sulfur rank plateaued to a value of 2.5.73,171
Vitrimers are novel polymeric network materials connected by dynamic covalent bonds, that combine the network structure of thermosets and the remoldability of thermoplastics, which considerably expands under the processing conditions of these polymers.172–174 Liu et al. demonstrated the synthesis of dynamic polysulfide networks through the solution polycondensation of pentaerythritol tetra(3-mercaptopropionate) (PTMP) with elemental sulfur in toluene at 80 °C for 12 h, forming dynamic polysulfide networks with 51 wt% of sulfur (Fig. 10).175 Due to the presence of dynamic S–S bonds,176–178 reversible scission of these units in the material was postulated to impart high malleability to the poly(S-PTMP) network. The polysulfide thermoset could also be rapidly plasticized by the photo-cleavage of the photo-initiator in the polymer matrix through ultraviolet (UV) irradiation.179,180 The photoplasticity of poly(S-PTMP) enabled it to be manipulated into complicated 3D shapes with a radiation of 360 nm UV light under mild conditions (Fig. 10).
 | ||
| Fig. 10 Dynamic polysulfide shape memory networks derived from elemental sulfur. Reproduced with permission from ref. 175. Copyright 2017 Elsevier B.V. | ||
As alluded to earlier in Section 2.1.1.3, elemental sulfur and high sulfur content CHIPs are attractive cathode active materials in lithium batteries because of their high theoretical specific capacity. Cui and Fu et al. demonstrated the condensation reaction of 1,4-benzenedithiol with elemental sulfur in CS2/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solvents to synthesize a highly flexible polymer polyphenylene tetrasulfide (PPTS) as a cathode material (Fig. 11a).181 The high reactivity of the thiol groups, facilitated by the conjugated nature of the benzyl ring, favors the elimination of the protons on a condensation reaction with elemental sulfur. These elastic polysulfide materials were observed to accommodate strains of up to 334% (Fig. 11b), while the carbon nanotube-based cathodes were found to be slightly more brittle (fracture observed at 107% strain (Fig. 11c)). This flexibility of these materials was associated with favorable cycling performance in a lithium battery with a high capacity (633 mA h g−1 at 1C), good rate performance, high coulombic efficiency (∼99.4%), and a low capacity decay (under 0.07% per cycle).
1 v/v) solvents to synthesize a highly flexible polymer polyphenylene tetrasulfide (PPTS) as a cathode material (Fig. 11a).181 The high reactivity of the thiol groups, facilitated by the conjugated nature of the benzyl ring, favors the elimination of the protons on a condensation reaction with elemental sulfur. These elastic polysulfide materials were observed to accommodate strains of up to 334% (Fig. 11b), while the carbon nanotube-based cathodes were found to be slightly more brittle (fracture observed at 107% strain (Fig. 11c)). This flexibility of these materials was associated with favorable cycling performance in a lithium battery with a high capacity (633 mA h g−1 at 1C), good rate performance, high coulombic efficiency (∼99.4%), and a low capacity decay (under 0.07% per cycle).
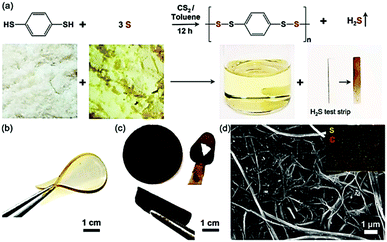 | ||
| Fig. 11 (a) Synthesis of polyphenylene tetrasulfide (PPTS) with visual representation. (b) Image of the cast PPTS membrane showing its flexibility and transparency. (c) Images of the PPTS-CNT cathode membrane showing its flexible nature. (d) SEM image of the PPTS-CNT cathode (EDS image in the inset). Reproduced with permission from ref. 181. Copyright 2018 American Chemical Society. | ||
Sulfur based cathode materials classically possess poor electrical conductivity and are subjected to polysulfide dissolution during cycling, both of which impact device performance and lifetimes.11,150,151,153 Hence, efforts have been made to improve the battery performance by chemical modification of the sulfur-based active materials.151,152 Yan et al. reported the covalent attachment of sulfur to sulfhydryl functionalized graphene nanosheets for high coulombic efficiency sulfur cathodes.182 Park et al. reported the utilization of porous trithiocyanuric acid crystals (TTCA) as a soft template, where the ring opening polymerization of elemental sulfur takes place along the thiol surfaces to create the three-dimensionally interconnected sulfur-rich polymeric materials S-TTCA (75 wt% of sulfur).183 Recently, Chen et al. synthesized the conducting polymer poly(m-aminothiophenol) (PMAT) by homopolymerization of m-aminothiophenol and the abundant thiol groups in PMAT were used for post-synthesis copolymerization with elemental sulfur at 170 °C to form the highly crosslinked cp(S-PMAT) copolymer containing approximately 80 wt% of sulfur (Fig. 12a).184 When sulfur was covalently bonded to the conductive polymer chains, the polysulfide anions were insoluble in organic electrolytes during the cell operations (Fig. 12b), resulting in the improved cycling capability of Li–S batteries. The utilization of cp(S-PMAT) as a cathode retained a high capacity of 495 mA h g−1 after 1000 cycles at a high charge–discharge current of 2C (Fig. 12c). Other approaches to prepare sulfur materials with conjugate polymer fragments such as polyaniline or polypyrrole have also been reported.185–190 However, the structural characterization of the materials remains ambiguous due to the intractability of the uncharacterized final products. Furthermore, Li–S batteries fabricated from these materials did not exhibit profound improvement in the device performance upon cycling.
 | ||
| Fig. 12 (a) Synthesis of the cp(S-PMAT) copolymer. (b) The structural evolution in the synthesis of cp(S-PMAT) and discharge–charge process. (c) Cycling performance and coulombic efficiency of cp(S-PMAT)/C cathodes at 2C. Reproduced with permission from ref. 184. Copyright 2017 Wiley-VCH Verlag GmbH&Co. KGaA, Weinheim. | ||
The first example of combining elemental sulfur with polybenzoxazines via inverse vulcanization was elegantly demonstrated by Yagci and co-workers (Fig. 14a).121 Allyl functional benzoxazine comonomers (BA-ala) were synthesized using allylamine, bisphenol A, formaldehyde and molten sulfur by heating the mixture of BA-ala and elemental sulfur at various feed ratios. The allyl groups of BA-ala were able to undergo thiol–ene reactions with generated sulfur radicals to form C–S covalent bonds between benzoxazine and sulfur units. Concurrently, ring-opening reactions of the oxazine groups also take place producing polybenzoxazine fragments. In this way, the polysulfide chains were stabilized with polybenzoxazine bridges to give a copolymer (abbreviated as poly(BA-ala-co-sulfur)). These copolymers (50–90 wt% of sulfur) were found to be soluble in THF which suggested that the homopolymerization of the benzoxazine moiety to form a crosslinked network did not occur to appreciable conversion. With low sulfur feed ratios (such as 20 wt% of sulfur), the crosslinking density was much higher and, insoluble copolymer materials were obtained. The DSC analysis of curing kinetics revealed a more than 40 °C decrease of the on-set curing temperature and 90 °C decrease of the end-set curing temperature of sulfur-benzoxazine copolymers in comparison to the homopolymerization of benzoxazines without the addition of sulfur. The copolymer poly(BA-ala-co-sulfur) also exhibited higher glass transition temperatures (Tg) of 52–66 °C compared with poly(S-r-DIB). The acidity of the reaction system was checked by a litmus paper and the acidic medium was associated with H2S gas formation which could be detected with AgNO3 solution. The gas release during the copolymerization resulted in a microporous structure with an excess benzoxazine monomer (such as 80 wt% BA-ala) feed ratio. This sponge-like morphology is advantageous for applications that demand high mass transport, such as catalysis, separations, and extraction science. Kiskan et al. demonstrated the utilization of this microporous-structured poly(BA-ala-co-sulfur) as the sorbent for mercury removal from aqueous solution which exhibited comparable adsorption capacity with functionalized poly(glycidyl methacrylate) and metal–organic framework (MOF)-74-Zn.206 The S–S bonds in polysulfide chains were observed to be dynamic upon heating and regenerated at lower temperatures. The self-healing properties of the sulfur-benzoxazine polymer were also studied.135
 | ||
| Fig. 14 (a) Synthesis of poly(BA-Ala-co-sulfur), reproduced with permission from ref. 121. Copyright 2015 American Chemical Society. (b) Sulfur-embedded polybenzoxazine (S-BOP) for Li–S batteries. Reproduced with permission from ref. 207. Copyright 2016 American Chemical Society. (c) Preparation of polybenzoxazine/sulfur hybrid material (S-PBz). (d) SEM micrographs showing repairing behavior of S-PBz. Reproduced with permission from ref. 209. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Other types of benzoxazines have been used to prepare advanced sulfur-based polymeric materials. Choi et al. demonstrated the copolymerization of sulfur and thiol-functionalized benzoxazine (Fig. 14b).207 Sulfur reacted with the thiol group through the condensation reaction and the oxazine moiety proceeded through the ring-opening polymerization to form a sulfur-embedded polybenzoxazine (S-BOP) with a high sulfur content of 72 wt%. The resulting S-BOP bears a homogeneous distribution of sulfur due to in situ formation of the polymer backbone. This unique internal structure endows S-BOP with high initial coulombic efficiency (96.6%) and robust cyclability (92.7% retention after 1000 cycles at 1C cycling rate) when tested as a sulfur cathode. Lochab et al. reported using an agro-waste origin cardanol benzoxazine (Ca) monomer as a suitable sustainable linker to chemically bind elemental sulfur via bulk polymerization to form random copolymers poly(S-r-Ca) for Li–S battery applications.208
The reaction between the sulfur radical and oxazine rings was studied in depth by Liu and co-workers.203,209 They found that sulfur was able to copolymerize with 3-phenyl-3,4-dihydro-2H-1,3-benzoxazine (Pa) through a process termed the sulfur radical transfer and coupling (SRTC) process (Fig. 13c).209 In the SRTC reaction, sulfur radicals were first generated at elevated temperatures (>159 °C) and then the radical transferred from polysulfide to the benzoxazine ring and generated a thiol group at the polysulfide chain end. Benzoxazine radicals then coupled with sulfur radicals to form C–S bonds. The generated thiols from this process were then able to react with oxazine moieties resulting in the ring-opening of benzoxazine and coupling with in situ generated polysulfide radicals. This SRTC reaction process could explain the low on-set temperature in the copolymerization of sulfur and allyl functional benzoxazines (BA-ala) and the high solubility of the copolymer with 50–90 wt% of sulfur content. The hyperbranched segments were formed through the SRTC of sulfur radicals and oxazine rings with high sulfur loading and the cross-linked polymer was generated through the homopolymerization of benzoxazine only at a higher benzoxazine feed ratio. This further afforded CHIPs from elemental sulfur and various benzoxazine monomers with improved processability (Fig. 14c and d).209
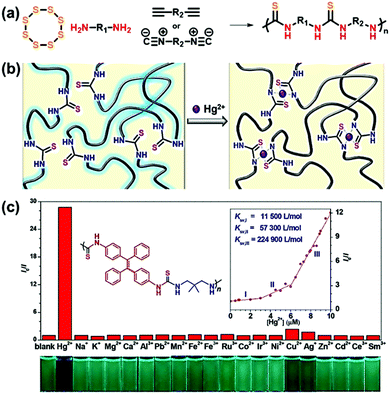 | ||
| Fig. 15 (a) Synthetic scheme of multicomponent polymerizations with elemental sulfur. (b) Proposed mechanism for mercury removal with polythioureas. (c) Fluorescence detection of mercury ions with polythiourea. Reproduced with permission from ref. 211. Copyright 2018 American Chemical Society. | ||
A solvent-free and catalyst-free synthetic method of benzazoles from methyl aromatics and arylamines in the presence of sulfur has been developed.212,213 Coskun et al. reported on a related bulk polymerization strategy that enabled direct utilization of elemental sulfur in the synthesis of highly microporous benzothiazole polymers (BTAPs) (Fig. 16a)214 with Brunauer–Emmett–Teller (BET) surface areas up to 750 m2 g−1. For the preparation of BTAPs, 2D organic linkers, 1,3,5-tris(4-tolyl)benzene (M1) and 1,3,5-tris(4-aminophenyl)benzene (A1), and 3D organic linkers, tetrakis(4-methylphenyl)methane (M2) and tetrakis(4-aminophenyl) methane (A2) were synthesized and reacted with elemental sulfur at 275 °C for 5 h under an Ar atmosphere. During the polymer synthesis, benzothiazole rings formed and sulfur radicals simultaneously attached onto the aromatic rings via C–H insertion reactions.164,215 Up to 41 wt% of sulfur content was observed in the obtained product. With additional thermal treatment at 400 °C, BTAPs with a high surface area were prepared and showed excellent CO2 separation performance under flue gas, natural gas, and landfill gas conditions (Fig. 16b).
 | ||
| Fig. 16 Synthesis of microporous polymers with elemental sulfur for natural gas sweetening. Reproduced with permission from ref. 214. Copyright 2016 Elsevier Inc. | ||
2.2 Condensation copolymerization with elemental sulfur
There are several methods of preparing solutions of inorganic polysulfides from elemental sulfur (Fig. 17a): (i) reaction of sodium hydroxide with sulfur, (ii) reaction of sodium sulfide with sulfur, (iii) reaction of sodium hydroxide, sodium hydrosulfide and sulfur, and (iv) reaction of sodium with sulfur.221,222 A large number of organic halides can be used in the preparation of polysulfide polymers. The relative reactivities of the halides with alkali polysulfides follow the pattern expected for nucleophilic substitution reactions. Alkyl bromides are more reactive than alkyl chlorides, and alkyl fluorides are generally unreactive.24 Patrick and Jorczak presented several industrially available alkyl halides which are suitable for this condensation reaction: methylene dichloride, ethylene dichloride, propylene dichloride, glycerol dichlorohydrin, dichloroethyl ether, bis(chloroethyl)ether and triglycol.217,222 Kishore et al. demonstrated the condensation reaction of styrene dibromide with Na2Sn or Na2Sen (n = 2–4) generating copolymers with weak linkages (poly(styrene disulfide), poly(styrene tetrasulfide), and poly(styrene diselenide)).223
 | ||
| Fig. 17 (a) Synthetic scheme of alkali polysulfides. (b) Polycondensation of alkyl halides and inorganic polysulfides. | ||
This condensation polymerization method has also been used recently with functional alkyl halides for building up diverse polymer materials.224–229 Chujo et al. reported the synthesis of new hybrid materials with sulfur as an inorganic element. Na2Sn (n = av. 5) was synthesized with elemental sulfur and Na in THF at 70 °C for 3 h and reacted with octakis(3-iodopropyl)POSS at room temperature to prepare network polymers with polysulfide-bridged polyhedral oligomeric silsesquioxane (Fig. 18a).227 Polyoctahedral oligomeric silsesquioxanes (POSS) are well known as molecular building blocks to construct thermally-stable materials as well as to improve the mechanical properties of materials,230–232 while the introduction of sulfur-bridging moieties results in increased refractive indices of the polymer matrices.233–236 Polymer blends of polysulfide polymers with conventional polymers, such as PMMA and PS, were prepared as a route to afford blends with improved thermal and optical properties. With 10 wt% addition of the polysulfide-bridging POSS network, the decomposition temperatures with 20 wt% mass loss (T20d) of PMMA increased from 263 °C to 360 °C, indicating a higher thermal stability of the resulting material, along with the enhancement of the refractive indices (nD) which increased from 1.4931 to 1.4994 (Fig. 18b).
 | ||
| Fig. 18 (a) Synthesis of the polysulfide-bridging POSS network and the estimated chemical structure of the single POSS unit in the network. (b) Refractive indices (nD) of the polymer blends with various amounts of the polysulfide bridging POSS network. Reproduced with permission from ref. 227. Copyright 2014 Wiley Periodicals, Inc. | ||
The preparation of CHIPs from poly(anthraquinonyl sulfides) (PAQS) has also been investigated as a route to introduce redox active moieties for use as electroactive cathode materials for various battery technologies such as sodium, magnesium and potassium batteries.237–240 The electrochemical activity of the anthraquinone moiety coupled to a S–S backbone displayed intriguing orthogonal redox behavior which was anticipated to improve Li–S battery performance. Mecerreyes et al. elegantly demonstrated the synthesis of new redox active sulfur polymers poly(anthraquinonyl sulfides) (PAQxS) with 1,5-dicholoroanthraquinone for use as cathode materials in Li–S batteries (Fig. 19).224 A series of PAQxS polymers with different polysulfide segment lengths (sulfur rank x = 2–9) have been synthesized in high yields by reacting the in situ formed sodium polysulfides with 1,5-dicholoroanthraquinone in N-methyl-2-pyrrolidone (NMP) at 120 °C overnight. The electrochemical characterization of these materials exhibited a dual reversible redox mechanism associated with both the anthraquinone and polysulfide electrochemistry. Li–S batteries fabricated using the PAQxS redox polymers as cathodes showed high experimental initial capacity values and less capacity loss compared to elemental sulfur based cathodes.
 | ||
| Fig. 19 Synthesis of poly(anthraquinonyl sulfides) (top); proposed structures for the synthesized polymers with different sulfur equivalents and digital photographs showing the obtained powdery materials (bottom). Reproduced with permission from ref. 224. Copyright 2018 American Chemical Society. | ||
Polymeric nanoparticles (NPs) consisting of S–S bond units have been the focus of intense research. They exhibit a variety of interesting properties, such as high specific surface area, good solubility and processability, and characteristic sulfur-based properties.241–244 Mori et al. employed sodium polysulfide, originated from elemental sulfur and Na2S, a cross-linking agent, and an amphiphilic block copolymer, composed of 4-bromophenyl vinyl sulfide (BPVS) and N-isopropyl acrylamide (NIPAM), to synthesize sulfur-rich nanoparticles (S-NPs) with the sulfur content up to 80 wt% (Fig. 20).226 Four different halogen compounds were selected as the crosslinking agents, including 1,2,3-trichloropropane, 1,2-dibromoethane, 2,5-dibromothiophene, and 1,4-dibromobenzene, which afford core crosslinked S-NP(C3), S-NP(C2), S-NP(Th), and S-NP(Bz), respectively. The core–shell NPs with sulfur-based crosslinked cores and a thermoresponsive poly(NIPAM) shell were prepared by the reaction of the halogen compound and sodium polysulfide, which could also react with Br groups in the assembled block copolymer in water. Amphiphilic block copolymers with reactive Br groups in the hydrophobic segment were chosen as polymeric surfactants to introduce good solubility in organic solvents and water while also imparting thermoresponsiveness to the material.245,246 This system demonstrated the feasibility of incorporating various functionalities and the formation of sulfur-rich nanoparticles with stable core–shell structures, which are attractive for developing novel sulfur-based advanced materials.
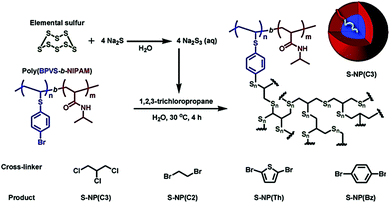 | ||
| Fig. 20 Synthesis of sulfur-rich nanoparticles (S-NPs), S-NP(C3), S-NP(C2), S-NP(Th), and S-NP(Bz), from 1,2,3-trichloropropane, 1,2-dibromoethane, 2,5-dibromothiophene, and 1,4-dibromobenzene, respectively. Reproduced with permission from ref. 226. Copyright 2017 Elsevier Ltd. | ||
Char et al. demonstrated the preparation of monodisperse, spherical, and water-dispersible sulfur-rich polymer nanoparticles for heavy metal ion remediation (Fig. 22).250 The nanoparticles (PSnDVS) were synthesized by precipitation polymerization of sodium polysulfides (Na2Sn), prepared directly from elemental sulfur and Na2S in the aqueous solution, with divinyl sulfone in the presence of an anionic surfactant (sodium dodecyl sulfate, SDS) and a neutral steric stabilizer (polyvinylpyrrolidone, PVP). Spherical nanoparticles were synthesized with SDS and the introduction of PVP led to a narrow size distribution (Fig. 22c). Under the optimized conditions, spherical particles with diameters in the range of 220–310 nm were obtained. The elemental analyses of the PSnDVS indicated sulfur contents of 55.58%, 59.15%, and 64.89% for n = 2, 3, and 4, respectively, demonstrating that the sulfur composition could be systematically controlled. Followed by subsequent reduction, thiol functionalized polymer nanoparticles (PSnDVS-SH) were obtained. PSnDVS-SH nanoparticles exhibited negligible capturing capability for nontoxic, hard ions such as Mg2+, Zn2+, Na+, K+, and Ca2+, and notable capturing capacity for toxic soft metals such as Pb2+, Cd2+, and Hg2+ (Fig. 22d), which provided a promising platform for water purification applications.
 | ||
| Fig. 22 (a) Synthesis of PSnDVS and PSnDVS-SH. (b) Illustration of PSnDVS and PSnDVS-SH nanoparticle synthesis. (c) SEM images of PS4DVS (scale bar indicates 1 μm). (d) Adsorption selectivity tests of PSnDVS-SH in the presence of metal ions: Mg2+, Zn2+, Na+, K+, Ca2+, Pb2+, Cd2+, and Hg2+. Reproduced with permission from ref. 250. Copyright 2018 The Royal Society of Chemistry. | ||
3. Post-polymerization functionalization of sulfur-containing prepolymers
3.1 Post-polymerization functionalization through dynamic S–S bonds in CHIPs
The concept of accessing the dynamic S–S bonds in CHIPs has been investigated as a route to post-polymerization processes to prepare functional sulfur-containing polymers. Poly(S-r-DIB) copolymers were demonstrated to exhibit dynamic covalent bond character due to the presence of long S–S bonds in the copolymer backbone,49 which were further exploited for healable “scratch and heal” IR windows.53 Based on the earlier findings of Tobolsky et al., the homolytic scission of di, tri- and tetrasulfide bonds in various polysulfide materials was observed at elevated temperatures (T > 100 °C).251 Pyun & Mackay et al. conducted time-dependent rheological studies on CHIPs which confirmed the dynamic nature of S–S bonds in poly(S-r-DIB) copolymers (Fig. 23b).49 The dynamic nature of S–S bonds was chemically exploited as a new approach to incorporate functional monomers into CHIP materials which was later termed, dynamic covalent polymerizations (DCP) (Fig. 24). Due to the limited miscibility and high melt viscosity of poly(S-r-DIB), liquid-like CHIP resins that can be used in bulk as the reaction medium were desirable for the DCP process. | ||
| Fig. 23 (a) Schematic representation of dynamic behavior under stimulus (T = 100 °C) in poly(S-r-DIB) as a function of dynamic S–S bond content. (b) In situ rheological characterization of dynamic behavior in poly(S-r-DIB) (80 wt% sulfur) at T = 130 °C and 100 rad s−1. Reproduced with permission from ref. 49. Copyright 2014 American Chemical Society. | ||
The copolymerization of elemental sulfur and styrene was reported as early as the 1960s and 1970s.252–256 However, the formation of low molar mass oligomeric materials was primarily observed from these systems and in-depth studies on the free radical copolymerization of S8 with styrene have not been conducted. Zhang and Pyun et al. reported the use of styrene as a comonomer to prepare poly(sulfur-random-styrene) (poly(S-r-Sty)) copolymers through the inverse vulcanization process.50 Detailed NMR spectroscopic studies were employed to gain mechanistic and structural insights into these sulfur-rich copolymers prepared from inverse vulcanization processes. These copolymers exhibit relatively low Tg (−11.2 °C–0.9 °C for 90–50 wt% S) and much better miscibility with organic solvents. Furthermore, the dynamic S–S bonds in the polysulfide backbones were accessible for thermally induced thiol–ene additions to vinylic comonomers at elevated temperatures to generate sulfur radicals. Hence, this new Tg CHIP material becomes an excellent new sulfur reagent and low Tg prepolymer resin for further functionalization reactions. Taking advantage of the improved miscibility of poly(S-r-Sty) copolymers, these low Tg oligomers were used as reactive resins for thermally induced reactions with acrylates and other functional vinyl monomers using dynamic covalent polymerizations (DCP).52,255 This approach allowed for the first time the chemical entry of organic comonomers to prepare CHIPs that were not initially miscible (or too volatile) with liquid sulfur.
The first demonstration of utilizing pre-made sulfur based resins to incorporate other vinyl monomers was reported by Todorov et al.255 The Thiokol poly(styrene polysulfide) (PStPS) liquid resins were synthesized from elemental sulfur and styrene and used as the starting material for modification with methacrylic acid (MAA). The MAA modified polysulfide terpolymer poly(methacrylic acid polysulfide) (PMAAPS) was characterized with NMR and IR spectroscopy. FTIR suggested the chemical modification of the liquid resin via vinyl addition processes, where methacrylic acid units were bound chemically to the sulfur units in the copolymer backbone. The liquid polysulfides were introduced as additives into a basic elastomer composition utilized for the packing of high-voltage cable sleeves. An improvement of the adhesion by, approximately, 43% was achieved after the modification with 10.3 mol% MAA. This seminal study confirmed the viability of the DCP process to overcome the poor solubility and miscibility of elemental sulfur to synthesize functional polysulfide polymers.
The dynamic covalent polymerization (DCP) process was further expanded to a number of acrylates, styrenics and allylic monomers by Zhang and Pyun (Fig. 25).52 Kinetic studies suggested that the DCP could be conducted at T ≥ 110 °C with a series of poly(S-r-Sty) resins of varying compositions (90–50 wt% sulfur, 10–50 wt% styrene). Size exclusion chromatography (SEC) of these terpolymers indicated the formation of low molar mass terpolymers (Mn = 1554 g mol−1; Mw/Mn = 1.3) and a slight increase in molar mass relative to the initial poly(S-r-Sty) precursors. Since dynamic S–S bonds were present in the copolymer backbone, it was anticipated that marginal differences in the terpolymer molar mass were observed. Since sulfur radical generation must be accompanied by fragmentation, decreases in the molar mass would be expected from the DCP process. However, since thiol–ene reactions in the DCP process form new S–C bonds in the terpolymer backbones, molar mass increases to nearly the original molecular weight of the poly(S-r-Sty) precursor when a monofunctional acrylate comonomer (f = 1) is used. The improved miscibility of poly(S-r-Sty) enabled the incorporation of some vinyl monomers with relatively polar functional groups via the DCP process. 4-(Poly(ethylene glycol)) styrene (PEGSty), for example, was not miscible with elemental sulfur due to the relatively polar PEG functional group and heating the S8 and 4-(poly(ethylene glycol)) styrene mixture generated heterogeneous products. The NMR spectrum of the product from heating elemental sulfur and PEGSty indicated the homopolymerization of 4-(poly(ethylene glycol)) styrene. When using poly(S-r-Sty) (70 wt% sulfur) for polymerization with 4-(poly(ethylene glycol)) styrene, the reaction mixture became homogeneous and the incorporation of the 4-(poly(ethylene glycol)) styrene unit was confirmed by NMR analysis. The polar functional group on the styrenic monomers significantly increased the solubility of the terpolymer in polar organic solvents such as methanol. The DCP process of poly(S-r-Sty) also enabled the tunability of molecular weight and thermomechanical properties of CHIPs by using multifunctional comonomers. Using this sequential process, a wide range of functional comonomers that were otherwise immiscible with liquid sulfur could be incorporated into sulfur materials.
 | ||
| Fig. 25 Dynamic covalent polymerization of poly(S-r-Sty) with acrylates, functional styrenes and an acrylic crosslinker. Reproduced with permission from ref. 52. Copyright 2017 The Royal Society of Chemistry. | ||
The DCP process could also be conducted with other sulfur polymers. Sung et al. demonstrated the thermal generation of sulfur radicals from the copolymer poly(S-r-DIB) materials for covalent attachment of polysulfide chains to oleylamine (OLA)-functionalized reduced graphene oxide (O-rGO).257 Because of the relatively high glass transition temperatures (Tg), poly(S-r-DIB) needed to be dissolved in a high boiling point solvent 1,3-dichlorobenzene (DCB) in order to mix with O-rGO. The reaction mixture was heated at 165 °C and yielded a homogeneous nanocomposite (poly S-O-rGO) where the end of the aliphatic chain of OLA is covalently linked to the polysulfide chains. Jenkins demonstrated the incorporation of vinyl and allyl ethers using DCP with poly(sulfur-r-divinylbenzene) (poly(S-r-DVB)).258 This method reduces the reaction temperature from above 160 °C to 90 °C and the incorporation of different monomers along with varying feed ratios enabled the physical properties such as Tg to be tailored.
Conventional inverse vulcanization of S8 required elevated temperatures (T ≥ 130 °C) and high S8 concentrations (bulk polymerization conditions) to promote homolytic ring-opening of S8 in order to generate a sufficiently high concentration of reactive sulfur radicals to achieve appreciable reaction rates in the polymerization. For nonactivated monomers such as acrylates, the floor temperature of this DCP process was found to be 110 °C due to the lower bond dissociation energy of polysulfide polymers expanding the scope of comonomers available that could be incorporated into polymeric sulfur materials.52 However, there remain opportunities and challenges to develop improved methods and catalytic systems to enable polymerizations of S8 at lower temperatures and with other functional comonomers. Pyun et al. observed a profound enhancement of polymerization rate when performing the inverse vulcanization of elemental sulfur and 4-vinylaniline, which was attributed to a polarity reversal effect.130,166 Motivated by this rate enhancement effect, 4-vinylaniline was introduced into the sulfur polymer precursor for low-temperature DCP (Fig. 26).165 A terpolymer containing pendant aniline group poly(S-r-Sty-r-VA) was synthesized to investigate the low-temperature DCP with volatile monomers such as methyl methacrylate (MMA). The styrene segment lowered the Tg of the copolymer and improved the miscibility with MMA, while the pendant amine group served as the activator to promote homolytic cleavage of S–S at temperatures lower than 110 °C (Fig. 26b). Kinetic studies showed that the DCP process of poly(S-r-Sty-r-VA) could be conducted at as low as 60 °C which could be applied to incorporate low boiling point comonomers that are incompatible with classical high-temperature inverse vulcanization or DCP. This low-temperature DCP process provides a new synthetic and accelerated method to activate S8 for copolymerization processes with functional comonomers at a broader temperature range enabling the preparation of new sulfur materials.
 | ||
| Fig. 26 (a) DCP of the sulfur polymer with an activated vinyl monomer. (b) DCP with sulfur copolymers containing amine moieties at lower temperatures. Reproduced with permission from ref. 165. Copyright 2018 Wiley Periodicals, Inc. | ||
3.2 Post polymerization functionalization through reactive side-chain functional groups
Wei and coworkers utilized a modified inverse vulcanization methodology to prepare soluble inverse-vulcanized hyperbranched polymers (SIVHPs) (Fig. 27).18 These SIVHPs were synthesized via thiol–ene addition of sulfur radicals to DIB in solution. This solution polymerization took place in an autoclave under an N2 atmosphere followed by heating to 150 °C–180 °C for 0.5–7 hours. The solution environment provides high molecular weights and a high degree of branching. The branching molecular architecture endows hyperbranched polymers (HPs) with better solubility259,260 than copolymers by bulk polymerization. The described SIVHPs exhibited good solubility in various organic solvents, such as toluene, CHCl3, DMF, 1,4-dioxane, THF and anisole. SIVHPs contained unreacted vinyl groups from DIB and could be further modified with sulfhydryl through thiol–ene click chemistry of 3-(dimethylamino)-1-propanethiol (DPT) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds under UV initiation, followed by the Menschutkin click reaction with propargyl bromide.261 Different from SIVHPs, the quaternary ammonium SIVHPs showed superior solubility in water, which directly demonstrated the chemical conversion from SIVHPs to quaternary ammonium SIVHPs. The hyperbranched polymers proved to be solution processible and could be processed into conductive frameworks of graphene-based ultralight aerogels (GUAs)262 by facile fluid infiltration for cathode-active materials of Li–S batteries. The SIVHP-based cells showed high initial specific capacities of 1247.6 mA h g−1 with 400 charge–discharge cycles. The cells also demonstrated an excellent rate capability, being able to cycle at 5C.
C bonds under UV initiation, followed by the Menschutkin click reaction with propargyl bromide.261 Different from SIVHPs, the quaternary ammonium SIVHPs showed superior solubility in water, which directly demonstrated the chemical conversion from SIVHPs to quaternary ammonium SIVHPs. The hyperbranched polymers proved to be solution processible and could be processed into conductive frameworks of graphene-based ultralight aerogels (GUAs)262 by facile fluid infiltration for cathode-active materials of Li–S batteries. The SIVHP-based cells showed high initial specific capacities of 1247.6 mA h g−1 with 400 charge–discharge cycles. The cells also demonstrated an excellent rate capability, being able to cycle at 5C.
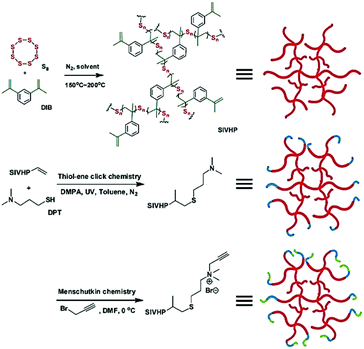 | ||
| Fig. 27 Functionalization of poly(S-r-DIB) via thiol–ene and Menschutkin click chemistry. Reproduced with permission from ref. 18. Copyright 2015 The Royal Society of Chemistry. | ||
The preparation of side-chain functional polymers was developed by Dirlam and Pyun et al., where functional groups with orthogonal reactivity to the inverse vulcanization process, followed by post-polymerization reactions of these latent moieties. This strategy was employed as a method to improve the electrical conductance of sulfur and CHIP materials. The high resistivity of sulfur (ca. 1015 Ω m) is a principal limitation in fully exploiting its exceptional capacity versus lithium in electrochemical devices. The typical approach for the electrical conductance improvement is the preparation of the wrapped sulfur particles with conductive materials, conductive polymers or various carbon materials.263 The wrapped small sulfur particles or micrometer-sized sulfur wires with conductive polymers or carbon materials have been studied for use in Li–S batteries.152,153,264 Dirlam and Pyun et al. demonstrated the post-functionalization of side chain functionalized sulfur polymers via electro-polymerization of ProDOT groups (Fig. 28a).85 The ProDOT side chains were incorporated into sulfur polymers by tandem inverse vulcanization of elemental sulfur, styrenic functional 3,4-propylenedioxythiophene (ProDOT-Sty) and DIB. With this approach, ProDOT-Sty was initially reacted with liquid sulfur diradicals to afford linear copolymeric species and a modest amount of DIB was added after the initial poly(ProDOT-Sty-r-sulfur) copolymerization, which afforded a soluble and chemically stable sulfur polymer precursor poly(ProDOT-Sty-random-DIB-random-sulfur) (ProDIBS) with ProDOT side chain groups. ProDIBS was spun cast from a copolymer solution in CHCl3/toluene onto a supporting electrode substrate indium tin oxide (ITO) for performing electrochemical experiments. Post-electropolymerization of the pendant ProDOT moieties then enabled the formation of interconnected poly(ProDOT) segments in the sulfur copolymer matrix. Electrochemical impedance spectroscopy (EIS) was conducted at varying stages of electro-polymerization to investigate the effect of introducing conjugated polymer moieties on the electrical properties of ProDIBS thin films.265 The impedance response of pProDIBS showed an over 95% decrease in charge transfer resistance (RCT) in comparison to the pristine ProDIBS after 20 or more polymerization scans. These results clearly confirmed the presence of conjugated polythiophene segments to improve the conductivity of CHIP materials and demonstrate the viability of this synthetic approach to new functional polymers (Fig. 28b).
 | ||
| Fig. 28 (a) Tandem inverse vulcanization and electropolymerization of S8 and ProDOT-Sty. (b) Nyquist plots of impedance spectra of (p)ProDIBS at an increasing number of potentiodynamic oxidative polymerization scans with expansions of lower impedance regions (top to bottom). Frequency ranging from 150 kHz to 0.1 Hz. Reproduced with permission from ref. 85. Copyright 2015 American Chemical Society. | ||
Tsutsumi et al. reported the preparation of soluble sulfur-based copolymers from elemental sulfur and alkenyl alcohol and post-functionalized with a diisocyanate crosslinker for improving the battery performance of lithium–sulfur batteries.127 The copolymerization of elemental sulfur and 10-undecen-1-ol (UDOL) was conducted in bulk at 175 °C for 3 h yielding the sulfur-rich copolymer poly(S-r-UDOL-1.0) (Fig. 29). The obtained poly(S-r-UDOL-1.0) copolymers have functional hydroxyl groups in their side chain and were soluble in organic solvents, such as tetrahydrofuran (THF), N-methyl-2-pyrrolidone (NMP) and chloroform, where the hydroxyl group was exploited for post-polymerization modification. Ketjen black (KB) was used as the porous carbon material to support the electroactive polymers for the fabrication of the electrode.266,267 The Ketjen Black (KB) powder was blended with poly(S-r-UDOL) by immersing the KB powder into a THF-poly(S-r-UDOL) solution and then processed into electrode sheets for electrochemical measurements. The hydroxyl side chain groups on poly(S-r-UDOL) were then reacted with hexamethylene diisocyanate (HDI) to crosslink the polymer phase within the cathode coating (Fig. 29). Electrochemical measurements and charge–discharge studies of Li–S batteries fabricated from these cathodes showed that the crosslinking of poly(S-r-UDOL-1.0) by HDI effectively improved the capacity retention of the cell, which was proposed to occur via the suppression of discharge product dissolution from the positive electrode at early discharge cycles.
 | ||
| Fig. 29 Post-functionalization of the sulfur-alkenyl alcohol copolymer with hexamethylene diisocyanate (HDI). | ||
Pyun et al. reported on an amine functionalized CHIP poly(sulfur-random-vinylaniline) (poly(S-r-VA)) synthesized via the inverse vulcanization process with elemental sulfur and 4-vinylaniline and post-functionalization with a variety of acid chlorides and isocyanates (Fig. 30).130 The poly(sulfur-random-4-vinylaniline) (poly(S-r-VA)) copolymer was synthesized by the inverse vulcanization method wherein sulfur was heated above its melting points (Tm = 108–120 °C) to 130 °C and 4-vinylaniline was added to the liquid sulfur dropwise. The reaction mixture vitrified rapidly to form a red, transparent glass. The retention of the amine groups in the sulfur copolymer was confirmed by IR and NMR spectroscopy. Earlier studies have reported the reaction of sulfur compounds activated by amine compounds,268,269 hence it was initially surprising to find that stable copolymers were formed via the reaction of sulfur and 4-vinylaniline. The stability of the copolymer was tested by heating poly(S-r-VA) with elemental sulfur and in dichlorobenzene solution at 120 °C for 24 h and the SEC analysis of the product after heat treatment revealed that the copolymer did not decompose through the reaction of sulfur and amine moieties. DSC analysis of these CHIPs revealed higher glass transition temperatures (Tg) for poly(S-r-VA) (Tg(70wt% S) = 31 °C and Tg(50wt% S) = 50 °C) relative to poly(S-r-Sty) (Tg = −11.2 °C–0.9 °C), which further resulted in increasing Tg for copolymers with a higher feed ratio of the organic comonomer, 4-vinylaniline. Post-polymerization modification of the sulfur copolymer via the amidation of the amine group with acid chloride under basic conditions was demonstrated for the first time. The model reaction of amination of poly(S-r-VA) was initially studied with benzoyl chloride. Poly(sulfur-random-(4-vinylphenyl)benzamide) (poly(S-r-VPBA)) (2) was synthesized with poly(S-r-VA) and benzoyl chloride in THF at 0 °C with K2CO3 as the proton sponge. The 1H NMR spectrum confirmed the formation of poly(S-r-VPBA) and SEC analysis confirmed the comparable molecular weight of sulfur copolymers before and after amination, which suggested that the sulfur backbone was intact during the amination reaction. Under the same reaction conditions, acryloyl chloride was used to synthesize the sulfur copolymer poly(sulfur-random-(4-vinylphenyl)acrylamide) (poly(S-r-VPAA) (3) with crosslinkable acrylic groups. Poly(S-r-VPAA) could be thermally crosslinked (4) by heating the material to 100 °C. Besides acid chloride, the amine group could also be functionalized with isocyanates. Poly(sulfur-random-1-phenyl-3-(4-vinylphenyl)-urea) (poly(S-r-PVPU)) (5) was synthesized with poly(S-r-VA) and isocyanatobenzene in THF solution at room temperature. This reaction provides another approach for post-modification of poly(S-r-VA) to tune the mechanical properties.
Sulfur-rich organic materials (organic polysulfanes) are promising organosulfur electrode candidates for Li–S batteries due to their unique structure and natural attractive polysulfide adsorption ability.76,91 Organic polysulfanes can achieve high sulfur loading and hence high capacity. However, the use of this type of organic polysulfane as an electrode material encounters several drawbacks: (i) the polymeric sulfur chains are unstable and they always break up during the discharge process, resulting in poor mechanical properties and bad stability; (ii) the large volume change (≈80%) of these organic polysulfanes during charge and discharge cycles is always accompanied by an irreversible conversion of the electrode materials from homogeneous sulfur-rich organic polysulfanes to heterogeneous sulfur-lean organic polysulfanes and elemental sulfur. This irreversible conversion causes an inevitable loss of sulfur and structure variation, leading to low coulombic efficiency and poor cycle life. Thus, designing more stable and length controllable polysulfane bonded in the polymer so that low-order polysulfides can directly form during discharging is an important direction to improve the cycle life time.151
Liu et al. reported the synthesis of a new kind of organic polysulfane nanosheet (OPNS) with high sulfur content (up to 93 wt% sulfur) using poly(acrylic acid) (PAA) and sulfur nanospheres as raw materials, where sulfur directly grafts on the carbon frame of PAA through direct coupling reactions initiated with decarboxylation of PAA (Fig. 31a).270 The coupling reaction proceeded with high selectivity and can be performed in the solid phase, which does not require harsh reaction conditions, expensive materials, or special solvents. The final products retained desirable electrochemical activity in devices as a consequence of this new material fabrication. Compared with the structure of conventional organic polysulfane synthesized by inverse vulcanization, the structure of our organic polysulfane with polycyclic sulfur grafted on the carbon chain of PAA possesses the advantages of stability during lithiation/delithiation cycling (Fig. 31b). Specifically, the OPNS (with 72 wt% sulfur content) cathodes for Li–S batteries demonstrated excellent rate performance (562 mA h g−1 at 10C) (Fig. 31c) and high initial capacity 891 mA h g−1 at 1C rate (based on the mass of organic polysulfane), together with only 0.014% capacity fading per cycle over 620 cycles. Additionally, this facile and solvent-free method can be easily scaled up while maintaining the high purity of the final products.
 | ||
| Fig. 31 (a) Decarboxylation-initiated coupling reaction with elemental sulfur. (b) Schematic of the structure variation for conventional organic polysulfane polymers synthesized by inverse vulcanization (red) and the organic polysulfane nanosheets (OPNS) (blue) before and after lithiation/delithiation cycles. (c) Rate performance of OPNS-50 (50 wt% S), OPNS-72 (72 wt% S) and OPNS-80 (80 wt% S) within a voltage window of 1.7–2.7 V. Reproduced with permission from ref. 270. Copyright 2019 Elsevier Ltd. | ||
Mecerreyes et al. reported a new synthetic method for sulfur-containing poly(ionic liquid)s with a unique combination of properties related to the sulfur and poly(ionic liquid) constituents (Fig. 32).101 In the first step, elemental sulfur was copolymerized with 4-vinylbenzyl chloride yielding poly(sulfur-co-4-vinylbenzyl chloride) (P(S-VBC)). These poly(ionic liquid)s were further modified via alkylation with N-methyl imidazole to prepare poly(sulfur-co-vinylbenzyl imidazolium)chloride (P(S-VBMImC)). These polyelectrolyte CHIPs were found to exhibit enhanced solubility in polar solvents such as methanol and even water. Ion exchange functionalization of the chloride anion was then carried out using anions, such as, bis(trifluoromethanesulfonyl)imide (TFSI). The obtained poly(sulfur-co-4-vinylbenzyl imidazolium)TFSI (P(S-VBMImTFSI)) having TFSI counter-anions exhibited an appreciable ionic conductivity of 10−6 to 10−9 S cm−1 in comparison to the state of the art poly(ionic liquid) materials (Fig. 32b) as determined by electrochemical impedance spectroscopy (EIS). The sulfur-containing poly(ionic liquid)s also demonstrated unique redox properties arising from the redox activity of S–S bonds in the CHIP copolymer backbone.
 | ||
| Fig. 32 (a) Synthetic scheme of the post-polymerization modification reactions toward sulfur-containing poly(ionic liquid)s. (b) Ionic conductivity versus temperature of P(S-VBMImTFSI). Reproduced with permission from ref. 101. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
4. Perspective and future outlook
The future is bright for this new area of polymer science as evidenced by the extensive reports in recent years on sulfur utilization for the creation of advanced polymeric materials. However, it is clear that this field remains in its infancy as noted by the limited number of synthetic methodologies that have been developed by sulfur polymerizations. While numerous efforts have largely focused on free radical or anionic polymerization methods, there remain numerous opportunities to apply the concepts of classical and modern polymer chemistry to prepare CHIPs with useful properties. The development of new step-growth, addition and condensation polymerization methods that directly utilize elemental sulfur remains an important opportunity to prepare polymeric materials with worthwhile thermomechanical properties. The development of new accelerated or catalysed processes will be important to afford new materials. MCP processes described in this review are an example of new synthetic methods that use sulphur for polymer synthesis that should be further explored. Alternatively, revisiting the elegant anionic ring-opening chain polymerization reported by Penczek et al. is an intriguing research opportunity to create living polymerization methods and afford well-defined CHIP homopolymers and copolymers.271–273 A major challenge for many of these polymerization methods that contain a high sulphur rank will be the suppression of chain transfer reactions of propagating, or reactive end-groups to dynamic S–S bonds in the backbone of CHIP materials. Hence, it is evident that novel and clever synthetic solutions remain to enhance the scope of methods and materials, which is the key to unlocking the true technological potential of this field.5. Conclusions
In this review, we have discussed different synthetic approaches to prepare functional CHIPs from elemental sulfur. The two primary methods to prepare polymers, inverse vulcanization and condensation copolymerization, were reviewed, followed by the discussion of post-polymerization modification methods. Condensation copolymerization was traditionally utilized to produce Thiokol liquid polysulfide and has recently been applied to introduce new functional groups from various alkyl halides or unsaturated hydrocarbons. The inverse vulcanization methodology is a feasible and atom economical approach to prepare advanced sulfur materials. However, this process is fundamentally challenged to understand the mechanistic aspects of the polymerization, along the structural characterization of the final materials. Thus, there remain opportunities to develop new model systems to further gain deeper chemical insights into this new polymerization process. Accelerated or “catalytic” inverse vulcanization approaches also provide a promising way to make functional CHIPs to incorporate unreactive monomers and allow reactions at lower temperatures. Some other methods to prepare polymeric sulfur materials such as multicomponent polymerization, sulfur-mediated cyclization and sulfur-benzoxazine reaction were also discussed. Post-polymerization modification is another important approach to introduce new functionalities. Post-polymerization modification via reactive side chain groups for sulfur polymers was limited by the functional groups that could be incorporated with sulfur polymers. The recently developed dynamic covalent polymerization (DCP) process utilizing pre-made sulfur-rich resins as the reaction medium provides the opportunity to incorporate new functionalities at a broader temperature range to prepare novel CHIP materials.Conflicts of interest
The authors declare the following competing financial interest(s): JP declares an actual or potential financial conflict of interest and is a co-founder/equity holder in Innovative Energetics, a licensee of University of Arizona (UA) intellectual property. This relationship has been disclosed to the UA Institutional Review Committee and is managed by a Financial Conflict of Interest Management Plan.Acknowledgements
We acknowledge the NSF (CHE- 1807395, DMR- 1607971) for support of this work. KC acknowledges the support from the NRF for the National Creative Research Initiative Center for Intelligent Hybrids (2010-0018290).References
- F. Seel, Sulfur in History: The Role of Sulfur in “Black Powder”, in Sulfur - Its Significance for Chemistry, for the Geo-, Bio- and Cosmosphere and Technology, ed. A. Müller and B. Krebs, Elsevier, 1984, vol. 5, pp. 55–66 Search PubMed.
- K. H. Wedepohl, Sulfur in the Earth's Crust, its Origin and Natural Cycle, in Sulfur - Its Significance for Chemistry, for the Geo-, Bio- and Cosmosphere and Technology, ed. A. Müller and B. Krebs, Elsevier, 1984, vol. 5, pp. 39–54 Search PubMed.
- R. J. Angelici, Acc. Chem. Res., 1988, 21(11), 387–394 CrossRef CAS.
- L. A. Apodoca, Sulfur: US Geological Survey, mineral commodity summaries, 2014, pp. 156–157 Search PubMed.
- E.P.A. Reregistration, eligibility document facts: Sulfur, Office of Pesticide and Toxic Substances, Washington D.C., 1991 Search PubMed.
- N. T. Binh, Adv. Synth. Catal., 2017, 359(7), 1066–1130 CrossRef.
- J. A. Ober, Materials flow of sulfur–, 2002, 2002–298 Search PubMed.
- S. R. Rafikov, Polym. Sci. U.S.S.R., 1979, 21(11), 2780–2792 CrossRef.
- A. Duda and S. Penczek, Sulfur-containing polymers, in Encyclopedia of Polymer Science and Engineering, ed. J. I. Kroschwitz, John Wiley & Sons, Inc, New York, 1989, vol. 16, pp. 246–368 Search PubMed.
- D. Vietti, Polysulfides, in Encyclopedia of Polymer Science and Technology, 2001 Search PubMed.
- P. T. Dirlam, R. S. Glass, K. Char and J. Pyun, J. Polym. Sci., Part A: Polym. Chem., 2017, 55(10), 1635–1668 CrossRef CAS.
- J. J. Griebel, R. S. Glass, K. Char and J. Pyun, Prog. Polym. Sci., 2016, 58, 90–125 CrossRef CAS.
- J. Lee, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 CrossRef PubMed.
- A. Kultys, in Sulfur-containing polymers, John Wiley & Sons, Inc., 2014, pp. 286–352 Search PubMed.
- H. Mutlu, E. B. Ceper, X. Li, J. Yang, W. Dong, M. M. Ozmen and P. Theato, Macromol. Rapid Commun., 2019, 40(1), 1800650 CrossRef PubMed.
- A. Kausar, S. Zulfiqar and M. I. Sarwar, Polym. Rev., 2014, 54(2), 185–267 CrossRef CAS.
- N. Spassky, Phosphorus, Sulfur Silicon Relat. Elem., 1993, 74(1–4), 71–92 CrossRef CAS.
- Y. Y. Wei, X. Li, Z. Xu, H. Y. Sun, Y. C. Zheng, L. Peng, Z. Liu, C. Gao and M. X. Gao, Polym. Chem., 2015, 6(6), 973–982 RSC.
- D. A. Boyd, C. C. Baker, J. D. Myers, V. Q. Nguyen, G. A. Drake, C. C. McClain, F. H. Kung, S. R. Bowman, W. Kim and J. S. Sanghera, Chem. Commun., 2016, 53(1), 259–262 RSC.
- X. Wu, J. A. Smith, S. Petcher, B. Zhang, D. J. Parker, J. M. Griffin and T. Hasell, Nat. Commun., 2019, 10(1), 647 CrossRef PubMed.
- R. Steudel, Chem. Rev., 2002, 102(11), 3905–3946 CrossRef CAS PubMed.
- C. P. Tsonis, Polysulfides, in Advances in Engineering Fluid Mechanics: Multiphase Reactor and Polymerization System Hydrodynamics, ed. N. P. Cheremisinoff, Gulf Professional Publishing, Burlington, 1996, pp. 737–746 Search PubMed.
- R. Steudel, Inorganic Polysulfides Sn2− and Radical Anions Sn·−, in Topic in Current Chemistry, ed. R. Steudel, Springer Berlin Heidelberg, Berlin, Heidelberg, 2003, pp. 127–152 Search PubMed.
- K. Kishore and K. Ganesh, Polymers containing disulfide, tetrasulfide, diselenide and ditelluride linkages in the main chain, in Polymer Synthesis/Polymer Engineering, Springer Berlin Heidelberg, Berlin, Heidelberg, 1995, pp. 81–121 Search PubMed.
- A. M. Usmani, Polym.-Plast. Technol. Eng., 1982, 19(2), 165–199 CrossRef CAS.
- N. D. Ghatge, S. P. Vernekar and S. V. Lonikar, Rubber Chem. Technol., 1981, 54(2), 197–210 CrossRef CAS.
- S. S. Maharajan and N. D. Ghatge, Pop. Plast., 1979, 24(6), 13–15 CAS.
- E. J. Goethals, J. Macromol. Sci., Rev. Macromol. Chem., 1968, 2(1), 73–144 CrossRef CAS.
- L. G. Isaacs and R. B. Fox, J. Appl. Polym. Sci., 1965, 9(10), 3489–3497 CrossRef CAS.
- R. Steudel, Sulfur: Organic Polysulfanes, in Encyclopedia of Inorganic Chemistry, 2007 Search PubMed.
- E.-K. Bang, M. Lista, G. Sforazzini, N. Sakai and S. Matile, Chem. Sci., 2012, 3(6), 1752–1763 RSC.
- Z.-R. Lu and X. Wu, Isr. J. Chem., 2010, 50(2), 220–232 CrossRef CAS PubMed.
- B. Meyer, Chem. Rev., 1976, 76(3), 367–388 CrossRef CAS.
- B. R. Currell and A. J. Williams, Thermochim. Acta, 1974, 9(3), 255–259 CrossRef CAS.
- R. E. Harris, J. Phys. Chem., 1970, 74(16), 3102–3111 CrossRef CAS.
- B. Meyer, Chem. Rev., 1964, 64(4), 429–448 CrossRef CAS.
- G. W. Miller, J. Appl. Polym. Sci., 1971, 15(8), 1985–1994 CrossRef CAS.
- J. Chiu, Anal. Chem., 1963, 35(7), 933–934 CrossRef.
- G. Gee, Trans. Faraday Soc., 1952, 48(6), 515–526 RSC.
- F. Fairbrother, G. Gee and G. T. Merrall, J. Polym. Sci., 1955, 16(82), 459–469 CrossRef CAS.
- A. V. Tobolsky and A. Eisenberg, J. Am. Chem. Soc., 1959, 81(4), 780–782 CrossRef CAS.
- R. Steudel, S. Passlackstephan and G. Holdt, Z. Anorg. Allg. Chem., 1984, 517(10), 7–42 CrossRef CAS.
- A. V. Tobolsky, J. Polym. Sci., Part C: Polym. Symp., 1966, 12, 71–78 CrossRef.
- R. Steudel, Liquid sulfur, in Elemental Sulfur and Sulfur-Rich Compounds 1, ed. R. Steudel, Springer-Verlag, Berlin, Germany, 2003, vol. 230, pp. 81–116 Search PubMed.
- R. Steudel, R. Strauss and L. Koch, Angew. Chem., Int. Ed. Engl., 1985, 24(1), 59–60 CrossRef.
- W. Klement and J. C. Koh, J. Phys. Chem., 1970, 74(24), 4280–4284 CrossRef CAS.
- R. Steudel, Phosphorus Sulfur Relat. Elem., 2007, 16(3), 251–255 CrossRef.
- P. D. Bartlett, G. Lohaus and C. D. Weis, J. Am. Chem. Soc., 1958, 80(19), 5064–5069 CrossRef CAS.
- J. J. Griebel, N. A. Nguyen, A. V. Astashkin, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, ACS Macro Lett., 2014, 3(12), 1258–1261 CrossRef CAS.
- Y. Zhang, J. J. Griebel, P. T. Dirlam, N. A. Nguyen, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, J. Polym. Sci., Part A: Polym. Chem., 2017, 55(1), 107–116 CrossRef CAS.
- R. Steudel, Angew. Chem., Int. Ed. Engl., 1975, 14(10), 655–664 CrossRef.
- Y. Zhang, K. M. Konopka, R. S. Glass, K. Char and J. Pyun, Polym. Chem., 2017, 8(34), 5167–5173 RSC.
- J. J. Griebel, N. A. Nguyen, S. Namnabat, L. E. Anderson, R. S. Glass, R. A. Norwood, M. E. Mackay, K. Char and J. Pyun, ACS Macro Lett., 2015, 4(9), 862–866 CrossRef CAS.
- J. L. Kice, J. Polym. Sci., 1956, 19(91), 123–140 CrossRef CAS.
- P. D. Bartlett and H. Kwart, J. Am. Chem. Soc., 1952, 74(16), 3969–3973 CrossRef CAS.
- C. G. Krespan and W. R. Brasen, J. Org. Chem., 1962, 27(11), 3995–4000 CrossRef CAS.
- C. G. Krespan, Fluorinated polysulfides, in The Chemistry of Sulfides, ed. A. V. Tobolsky, Wiley-Intersciences, New York, 1968, pp. 211–219 Search PubMed.
- C. G. Krespan, W. R. Brasen and H. N. Cripps, Adv. Chem. Ser., 1972, 110(110), 179 CrossRef CAS.
- W. H. Carothers, A. M. Collins and J. E. Kirby, Polymerizing 2-halo-1,3-butadiene, US1950438, 1934 Search PubMed.
- S. W. Schmitt and C. Anolick, Rubber Chem. Technol., 1978, 51(5), 888–896 CrossRef CAS.
- W. E. Mochel, J. Polym. Sci., 1952, 8(6), 583–592 CrossRef CAS.
- A. L. Klebanskii, N. Ja. Zukerman and L. P. Fomina, J. Polym. Sci., 1958, 30(121), 363–374 CrossRef CAS.
- Y. Miyata and M. Sawada, Polymer, 1988, 29(9), 1683–1688 CrossRef CAS.
- Y. Miyata and M. Sawada, Polymer, 1988, 29(8), 1495–1500 CrossRef CAS.
- P. A. Kirpichnikov, Vysokomol. Soedin., Ser. A, 1979, 21, 2457 CAS.
- L. Blight, B. R. Currell, B. J. Nash, R. A. M. Scott and C. Stillo, Adv. Chem. Ser., 1978, 165, 13–30 CrossRef CAS.
- H. Fritz and C. D. Weis, Tetrahedron Lett., 1974,(18), 1659–1660 CrossRef CAS.
- E. H. Farmer and F. W. Shipley, J. Chem. Soc., 1947, 1519–1532 RSC.
- T. C. Shields and A. N. Kurtz, J. Am. Chem. Soc., 1969, 91(19), 5415–5416 CrossRef CAS.
- J. Emsley and D. W. Griffiths, Phosphorus Sulfur Relat. Elem., 1980, 9(2), 227–230 CrossRef CAS.
- J. Emsley, D. W. Griffiths and G. J. J. Jayne, J. Chem. Soc., Perkin Trans. 1, 1979,(1), 228–232 RSC.
- B. K. Bordoloi, E. M. Pearce, L. Blight, B. R. Currell, R. Merrall, R. A. M. Scott and C. Stillo, J. Polym. Sci., Part A: Polym. Chem., 1980, 18(2), 383–406 CAS.
- B. K. Bordoloi and E. M. Pearce, J. Polym. Sci., Part A: Polym. Chem., 1978, 16(12), 3293–3300 CAS.
- A. R. Kemp and F. S. Malm, Ind. Eng. Chem., 1935, 27(2), 141–146 CrossRef CAS.
- W. J. Chung, A. G. Simmonds, J. J. Griebel, E. T. Kim, H. S. Suh, I. B. Shim, R. S. Glass, D. A. Loy, P. Theato, Y. E. Sung, K. Char and J. Pyun, Angew. Chem., Int. Ed., 2011, 50(48), 11409–11412 CrossRef CAS PubMed.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y. E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5(6), 518–524 CrossRef CAS PubMed.
- J. J. Griebel, G. X. Li, R. S. Glass, K. Char and J. Pyun, J. Polym. Sci., Part A: Polym. Chem., 2015, 53(2), 173–177 CrossRef CAS.
- A. G. Simmonds, J. J. Griebel, J. Park, K. R. Kim, W. J. Chung, V. P. Oleshko, J. Kim, E. T. Kim, R. S. Glass, C. L. Soles, Y. E. Sung, K. Char and J. Pyun, ACS Macro Lett., 2014, 3(3), 229–232 CrossRef CAS.
- J. J. Griebel, S. Namnabat, E. T. Kim, R. Himmelhuber, D. H. Moronta, W. J. Chung, A. G. Simmonds, K. J. Kim, J. van der Laan, N. A. Nguyen, E. L. Dereniak, M. E. Mackay, K. Char, R. S. Glass, R. A. Norwood and J. Pyun, Adv. Mater., 2014, 26(19), 3014–3018 CrossRef CAS PubMed.
- T. R. Martin, K. A. Mazzio, H. W. Hillhouse and C. K. Luscombe, J. Visualized Exp., 2016,(111), e54047 Search PubMed.
- T. R. Martin, K. A. Mazzio, H. W. Hillhouse and C. K. Luscombe, Chem. Commun., 2015, 51(56), 11244–11247 RSC.
- I. Gomez, D. Mecerreyes, J. A. Blazquez, O. Leonet, H. Ben Youcef, C. M. Li, J. L. Gomez-Camer, O. Bundarchuk and L. Rodriguez-Martinez, J. Power Sources, 2016, 329, 72–78 CrossRef CAS.
- I. Gracia, H. Ben Youcef, X. Judez, U. Oteo, H. Zhang, C. M. Li, L. M. Rodriguez-Martinez and M. Armand, J. Power Sources, 2018, 390, 148–152 CrossRef CAS.
- M. K. Salman, B. Karabay, L. C. Karabay and A. Cihaner, J. Appl. Polym. Sci., 2016, 133(28), 43655 CrossRef.
- P. T. Dirlam, A. G. Simmonds, R. C. Shallcross, K. J. Arrington, W. J. Chung, J. J. Griebel, L. J. Hill, R. S. Glass, K. Char and J. Pyun, ACS Macro Lett., 2015, 4(1), 111–114 CrossRef CAS.
- T. S. Kleine, N. A. Nguyen, L. E. Anderson, S. Namnabat, E. A. LaVilla, S. A. Showghi, P. T. Dirlam, C. B. Arrington, M. S. Manchester, J. Schwiegerling, R. S. Glass, K. Char, R. A. Norwood, M. E. Mackay and J. Pyun, ACS Macro Lett., 2016, 5(10), 1152–1156 CrossRef CAS.
- C. Y. Fu, G. H. Li, J. Zhang, B. Cornejo, S. S. Piao, K. N. Bozhilov, R. C. Haddon and J. C. Guo, ACS Energy Lett., 2016, 1(1), 115–120 CrossRef CAS.
- M. P. Crockett, A. M. Evans, M. J. Worthington, I. S. Albuquerque, A. D. Slattery, C. T. Gibson, J. A. Campbell, D. A. Lewis, G. J. Bernardes and J. M. Chalker, Angew. Chem., Int. Ed., 2016, 55(5), 1714–1718 CrossRef CAS PubMed.
- J. C. Bear, J. D. McGettrick, I. P. Parkin, C. W. Dunnill and T. Hasell, Microporous Mesoporous Mater., 2016, 232, 189–195 CrossRef CAS.
- D. J. Parker, H. A. Jones, S. Petcher, L. Cervini, J. M. Griffin, R. Akhtar and T. Hasell, J. Mater. Chem. A, 2017, 5(23), 11682–11692 RSC.
- F. Wu, S. Chen, V. Srot, Y. Huang, S. K. Sinha, P. A. van Aken, J. Maier and Y. Yu, Adv. Mater., 2018, 30(13), 1706643 CrossRef PubMed.
- S. Yu, H. Kwon, H. R. Noh, B. I. Park, N. K. Park, H. J. Choi, S. C. Choi and G. D. Kim, RSC Adv., 2015, 5(45), 36030–36035 RSC.
- I. Gomez, O. Leonet, J. A. Blazquez and D. Mecerreyes, ChemSusChem, 2016, 9(24), 3419–3425 CrossRef CAS PubMed.
- K. Wang, M. Groom, R. Sheridan, S. Z. Zhang and E. Block, J. Sulfur Chem., 2013, 34(1–2), 55–66 CrossRef CAS.
- S. Z. Khawaja, S. V. Kumar, K. K. Jena and S. M. Alhassan, Mater. Lett., 2017, 203, 58–61 CrossRef CAS.
- C. Zhang, C. Yan, Z. Xue, W. Yu, Y. Xie and T. Wang, Small, 2016, 12(38), 5320–5328 CrossRef CAS PubMed.
- J. S. M. Lee, D. J. Parker, A. I. Cooper and T. Hasell, J. Mater. Chem. A, 2017, 5(35), 18603–18609 RSC.
- K. Yamabuki, K. Itaoka, I. T. Kim, N. Yoshimoto and H. Tsutsumi, Polymer, 2016, 91, 1–6 CrossRef CAS.
- H. K. Lin, Y. S. Lai and Y. L. Liu, ACS Sustainable Chem. Eng., 2019, 7(4), 4515–4522 CrossRef CAS.
- D. A. Boyd, V. Q. Nguyen, C. C. McClain, F. H. Kung, C. C. Baker, J. D. Myers, M. P. Hunt, W. Kim and J. S. Sanghera, ACS Macro Lett., 2019, 8(2), 113–116 CrossRef CAS.
- I. Gomez, A. F. De Anastro, O. Leonet, J. A. Blazquez, H. J. Grande, J. Pyun and D. Mecerreyes, Macromol. Rapid Commun., 2018, 39(21), e1800529 CrossRef PubMed.
- L. L. Yan, D. M. Han, M. Xiao, S. Ren, Y. N. Li, S. J. Wang and Y. Z. Meng, J. Mater. Chem. A, 2017, 5(15), 7015–7025 RSC.
- J. Liu, B. Campbell, R. Ye, J. Bell, Z. Mutlu, C. Li, Y. Yan, M. Ozkan and C. Ozkan, Facile and Scalable Synthesis of Copolymer-Sulfur Composites as Cathodes for High Performance Lithium-Sulfur Batteries, MRS Advances, 2017, pp. 3271–3276.
- P. T. Dirlam, A. G. Simmonds, T. S. Kleine, N. A. Nguyen, L. E. Anderson, A. O. Klever, A. Florian, P. J. Costanzo, P. Theato, M. E. Mackay, R. S. Glass, K. Char and J. Pyun, RSC Adv., 2015, 5(31), 24718–24722 RSC.
- Z. J. Sun, M. Xiao, S. J. Wang, D. M. Han, S. Q. Song, G. H. Chen and Y. Z. Meng, J. Mater. Chem. A, 2014, 2(24), 9280–9286 RSC.
- N. A. Lundquist, M. J. H. Worthington, N. Adamson, C. T. Gibson, M. R. Johnston, A. V. Ellis and J. M. Chalker, RSC Adv., 2018, 8(3), 1232–1236 RSC.
- M. J. H. Worthington, R. L. Kucera, I. S. Albuquerque, C. T. Gibson, A. Sibley, A. D. Slattery, J. A. Campbell, S. F. K. Alboaiji, K. A. Muller, J. Young, N. Adamson, J. R. Gascooke, D. Jampaiah, Y. M. Sabri, S. K. Bhargava, S. J. Ippolito, D. A. Lewis, J. S. Quinton, A. V. Ellis, A. Johs, G. J. L. Bernardes and J. M. Chalker, Chemistry, 2017, 23(64), 16219–16230 CrossRef CAS PubMed.
- A. Hoefling, Y. J. Lee and P. Theato, Macromol. Chem. Phys., 2017, 218(1), 1600303 CrossRef.
- D. J. Parker, S. T. Chong and T. Hasell, RSC Adv., 2018, 8(49), 27892–27899 RSC.
- M. J. H. Worthington, C. J. Shearer, L. J. Esdaile, J. A. Campbell, C. T. Gibson, S. K. Legg, Y. Yin, N. A. Lundquist, J. R. Gascooke, I. S. Albuquerque, J. G. Shapter, G. G. Andersson, D. A. Lewis, G. J. L. Bernardes and J. M. Chalker, Adv. Sustainable Syst., 2018, 2(6), 1800024 CrossRef.
- M. Mann, J. E. Kruger, F. Andari, J. McErlean, J. R. Gascooke, J. A. Smith, M. J. H. Worthington, C. C. C. McKinley, J. A. Campbell, D. A. Lewis, T. Hasell, M. V. Perkins and J. M. Chalker, Org. Biomol. Chem., 2019, 17(7), 1929–1936 RSC.
- S. F. Valle, A. S. Giroto, R. Klaic, G. G. F. Guimarães and C. Ribeiro, Polym. Degrad. Stab., 2019, 162, 102–105 CrossRef CAS.
- X. Qin, Y. He, S. Khan, B. H. Zhang, F. X. Chen, D. Dong, Z. Wang and L. Q. Zhang, ACS Sustainable Chem. Eng., 2018, 6(10), 12865–12871 CrossRef CAS.
- H. Kang, H. Kim and M. J. Park, Adv. Energy Mater., 2018, 8(32), 1802423 CrossRef.
- A. Hoefling, D. T. Nguyen, Y. J. Lee, S. W. Song and P. Theato, Mater. Chem. Front., 2017, 1(9), 1818–1822 RSC.
- T. Thiounn, M. K. Lauer, M. S. Bedford, R. C. Smith and A. G. Tennyson, RSC Adv., 2018, 8(68), 39074–39082 RSC.
- E. T. Kim, W. J. Chung, J. Lim, P. Johe, R. S. Glass, J. Pyun and K. Char, Polym. Chem., 2014, 5(11), 3617–3623 RSC.
- V. Tzitzios, K. Dimos, I. Lelidis, N. K. Boukos, V. S. Wadi, G. Basina, G. Nounesis and S. M. Alhassan, RSC Adv., 2018, 8(72), 41480–41483 RSC.
- M. Arslan, B. Kiskan, E. C. Cengiz, R. Demir-Cakan and Y. Yagci, Eur. Polym. J., 2016, 80, 70–77 CrossRef CAS.
- S. Shukla, A. Ghosh, U. K. Sen, P. K. Roy, S. Mitra and B. Lochab, ChemistrySelect, 2016, 1(3), 594–600 CrossRef CAS.
- M. Arslan, B. Kiskan and Y. Yagci, Macromolecules, 2016, 49(3), 767–773 CrossRef CAS.
- S. B. Zeng, L. G. Li, J. P. Yu, N. Wang and S. W. Chen, Electrochim. Acta, 2018, 263, 53–59 CrossRef CAS.
- P. Dong, K. S. Han, J. I. Lee, X. Zhang, Y. Cha and M. K. Song, ACS Appl. Mater. Interfaces, 2018, 10(35), 29565–29573 CrossRef CAS PubMed.
- L. E. Anderson, T. S. Kleine, Y. Zhang, D. D. Phan, S. Namnabat, E. A. LaVilla, K. M. Konopka, L. R. Diaz, M. S. Manchester, J. Schwiegerling, R. S. Glass, M. E. Mackay, K. Char, R. A. Norwood and J. Pyun, ACS Macro Lett., 2017, 6(5), 500–504 CrossRef CAS.
- I. Gomez, D. Mantione, O. Leonet, J. A. Blazquez and D. Mecerreyes, ChemElectroChem, 2018, 5(2), 260–265 CrossRef CAS.
- K. Itaoka, I. T. Kim, K. Yamabuki, N. Yoshimoto and H. Tsutsumi, J. Power Sources, 2015, 297, 323–328 CrossRef CAS.
- K. Yamabuki, K. Itaoka, T. Shinchi, N. Yoshimoto, K. Ueno and H. Tsutsumi, Polymer, 2017, 117, 225–230 CrossRef CAS.
- A. Ghosh, S. Shukla, G. S. Khosla, B. Lochab and S. Mitra, Sci. Rep., 2016, 6, 25207 CrossRef CAS PubMed.
- P. D. McNaughter, J. C. Bear, A. G. Mayes, I. P. Parkin and P. O'Brien, R. Soc. Open Sci., 2017, 4(8), 170383 CrossRef CAS PubMed.
- Y. Zhang, T. S. Kleine, K. J. Carothers, D. D. Phan, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, Polym. Chem., 2018, 9(17), 2290–2294 RSC.
- A. Chang, Q. Wu, X. Du, S. Chen, J. Shen, Q. Song, J. Xie and W. Wu, Chem. Commun., 2016, 52(24), 4525–4528 RSC.
- B. Oschmann, J. Park, C. Kim, K. Char, Y. E. Sung and R. Zentel, Chem. Mater., 2015, 27(20), 7011–7017 CrossRef CAS.
- H. K. Lin and Y. L. Liu, Macromol. Rapid Commun., 2017, 38(10), 1700051 CrossRef PubMed.
- W. E. Mahmoud, S. A. Mansour, M. Hafez and M. A. Salam, Polym. Degrad. Stab., 2007, 92(11), 2011–2015 CrossRef CAS.
- M. Arslan, B. Kiskan and Y. Yagci, Sci. Rep., 2017, 7(1), 5207 CrossRef PubMed.
- M. Scheiner, T. J. Dickens and O. Okoli, Polymer, 2016, 83, 260–282 CrossRef CAS.
- M. D. Hager, P. Greil, C. Leyens, S. van der Zwaag and U. S. Schubert, Adv. Mater., 2010, 22(47), 5424–5430 CrossRef CAS PubMed.
- D. G. Bekas, K. Tsirka, D. Baltzis and A. S. Paipetis, Composites, Part B, 2016, 87, 92–119 CrossRef CAS.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295(5560), 1698–1702 CrossRef CAS.
- E. B. Murphy and F. Wudl, Prog. Polym. Sci., 2010, 35(1–2), 223–251 CrossRef CAS.
- M. Pepels, I. Filot, B. Klumperman and H. Goossens, Polym. Chem., 2013, 4(18), 4955–4965 RSC.
- J. A. Yoon, J. Kamada, K. Koynov, J. Mohin, R. Nicolay, Y. Z. Zhang, A. C. Balazs, T. Kowalewski and K. Matyjaszewski, Macromolecules, 2012, 45(1), 142–149 CrossRef CAS.
- J. Canadell, H. Goossens and B. Klumperman, Macromolecules, 2011, 44(8), 2536–2541 CrossRef CAS.
- T. S. Haddad and J. D. Lichtenhan, Macromolecules, 1996, 29(22), 7302–7304 CrossRef CAS.
- J. D. Lichtenhan, Y. A. Otonari and M. J. Carr, Macromolecules, 1995, 28(24), 8435–8437 CrossRef CAS.
- J. D. Lichtenhan, Comments Inorg. Chem., 1995, 17(2), 115–130 CrossRef CAS.
- J. Lim, Y. Cho, E. H. Kang, S. Yang, J. Pyun, T. L. Choi and K. Char, Chem. Commun., 2016, 52(12), 2485–2488 RSC.
- S. Namnabat, J. J. Gabriel, J. Pyun, R. A. Norwood, E. L. Dereniak and J. van der Laan, Pt. 2, Infrared Technology and Applications XL, Proc. SPIE, 2014, 9070, 90702H Search PubMed.
- T. S. Kleine, L. R. Diaz, K. M. Konopka, L. E. Anderson, N. G. Pavlopolous, N. P. Lyons, E. T. Kim, Y. Kim, R. S. Glass, K. Char, R. A. Norwood and J. Pyun, ACS Macro Lett., 2018, 7(7), 875–880 CrossRef CAS.
- F. L. Zhao, Y. Li and W. Feng, Small Methods, 2018, 2(11), 1800156 CrossRef.
- J. Liu, M. F. Wang, N. Xu, T. Qian and C. L. Yan, Energy Storage Mater., 2018, 15, 53–64 CrossRef.
- L. Chen and L. L. Shaw, J. Power Sources, 2014, 267, 770–783 CrossRef CAS.
- Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Angew. Chem., Int. Ed., 2013, 52(50), 13186–13200 CrossRef CAS PubMed.
- G. Hu, Z. Sun, C. Shi, R. Fang, J. Chen, P. Hou, C. Liu, H.-M. Cheng and F. Li, Adv. Mater., 2017, 29(11), 1603835 CrossRef PubMed.
- S. Choudhury, P. Srimuk, K. Raju, A. Tolosa, S. Fleischmann, M. Zeiger, K. I. Ozoemena, L. Borchardt and V. Presser, Sustainable Energy Fuels, 2018, 2(1), 133–146 RSC.
- C. H. Chang and A. Manthiram, ACS Energy Lett., 2018, 3(1), 72–77 CrossRef CAS.
- J. Sun, J. Ma, F. Jingbiao, J. Pyun and J. Geng, APL Mater., 2019, 7, 020904 CrossRef.
- K. Shen, H. L. Mei, B. Li, J. W. Ding and S. B. Yang, Adv. Energy Mater., 2018, 8(4), 1701527 CrossRef.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19(12), 2748–2761 RSC.
- M. Worthington, R. Kucera, I. Albuquerque, C. Gibson, A. Sibley, A. Slattery, J. Campbell, S. Alboaiji, K. A. Muller, J. Young, N. Adamson, J. Gascooke, D. Jampaiah, Y. Sabri, S. Bhargava, S. J. Ippolito, D. Lewis, J. Quinton, A. Ellis, A. Johs, G. Bernardes and J. M. Chalker, Chem. – Eur. J., 2017, 64, 16219–16230 CrossRef PubMed.
- J. A. Smith, X. Wu, N. G. Berry and T. Hasell, J. Polym. Sci., Part A: Polym. Chem., 2018, 56(16), 1777–1781 CrossRef CAS PubMed.
- T. Hasell, D. J. Parker, H. A. Jones, T. McAllister and S. M. Howdle, Chem. Commun., 2016, 52(31), 5383–5386 RSC.
- S. Oishi, K. Oi, J. Kuwabara, R. Omoda, Y. Aihara, T. Fukuda, T. Takahashi, J.-C. Choi, M. Watanabe and T. Kanbara, ACS Appl. Polym. Mater., 2019, 1(5), 1195–1202 CrossRef CAS.
- B. A. Trofimov, Sulfur Rep., 2003, 24(3), 283–305 CrossRef CAS.
- Y. Zhang, N. G. Pavlopoulos, T. S. Kleine, M. Karayilan, R. S. Glass, K. Char and J. Pyun, J. Polym. Sci., Part A: Polym. Chem., 2019, 57(1), 7–12 CrossRef CAS.
- O. Ito and M. Matsuda, J. Am. Chem. Soc., 1982, 104(6), 1701–1703 CrossRef CAS.
- E. Ramli, T. B. Rauchfuss and C. L. Stern, J. Am. Chem. Soc., 1990, 112(10), 4043–4044 CrossRef CAS.
- S. Dev, E. Ramli, T. B. Rauchfuss and C. L. Stern, J. Am. Chem. Soc., 1990, 112(17), 6385–6386 CrossRef CAS.
- B. L. Chenard and T. J. Miller, J. Org. Chem., 1984, 49(7), 1221–1224 CrossRef CAS.
- H. J. Langer and J. B. Hyne, The Reaction of Mercaptans with Liquid Sulfur, in Sulfur Research Trends, American Chemical Society, 1972, vol. 110, pp. 113–129 Search PubMed.
- B. K. Bordoloi and E. M. Pearce, J. Appl. Polym. Sci., 1979, 23(9), 2757–2761 CrossRef CAS.
- W. Denissen, G. Rivero, R. Nicolay, L. Leibler, J. M. Winne and F. E. Du Prez, Adv. Energy Mater., 2015, 25(16), 2451–2457 CAS.
- M. Rottger, T. Domenech, R. van der Weegen, A. Breuillac, R. Nicolay and L. Leibler, Science, 2017, 356(6333), 62–65 CrossRef PubMed.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7(1), 30–38 RSC.
- S. H. Zhang, L. L. Pan, L. Y. Xia, Y. B. Sun and X. K. Liu, React. Funct. Polym., 2017, 121, 8–14 CrossRef CAS.
- P. J. Hanhela, R. J. Hook and D. B. Paul, J. Appl. Polym. Sci., 1994, 51(8), 1441–1452 CrossRef CAS.
- A. Takahashi, T. Ohishi, R. Goseki and H. Otsuka, Polymer, 2016, 82, 319–326 CrossRef CAS.
- A. Rekondo, R. Martin, A. R. de Luzuriaga, G. Cabanero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1(2), 237–240 RSC.
- S. Chatani, C. J. Kloxin and C. N. Bowman, Polym. Chem., 2014, 5(7), 2187–2201 RSC.
- T. F. Scott, A. D. Schneider, W. D. Cook and C. N. Bowman, Science, 2005, 308(5728), 1615–1617 CrossRef CAS PubMed.
- A. Bhargav, M. E. Bell, Y. Cui and Y. Z. Fu, ACS Appl. Energy Mater., 2018, 1(11), 5859–5864 CrossRef.
- N. Xu, T. Qian, X. Liu, J. Liu, Y. Chen and C. Yan, Nano Lett., 2017, 17(1), 538–543 CrossRef CAS PubMed.
- J. H. Maxwell and A. M. Granda, Nat. Commun., 1975, 15(1), 131–132 CAS.
- S. Zeng, L. Li, L. Xie, D. Zhao, N. Wang and S. Chen, ChemSusChem, 2017, 10(17), 3378–3386 CrossRef CAS PubMed.
- J. Yan, B. Li and X. Liu, Nano Energy, 2015, 18, 245–252 CrossRef CAS.
- Z. Shichao, Z. Lan and Y. Jinhua, J. Power Sources, 2011, 196(23), 10263–10266 CrossRef.
- L. Xiao, Y. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24(9), 1176–1181 CrossRef CAS PubMed.
- S. C. Zhang, L. Zhang, W. K. Wang and W. J. Xue, Synth. Met., 2010, 160(17–18), 2041–2044 CrossRef CAS.
- S. B. Zeng, L. G. Li, D. K. Zhao, J. Liu, W. H. Niu, N. Wang and S. W. Chen, J. Phys. Chem. C, 2017, 121(5), 2495–2503 CrossRef CAS.
- J. E. Hyun, P. C. Lee and I. Tatsumi, Electrochim. Acta, 2015, 176, 887–892 CrossRef CAS.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32(11), 1344–1391 CrossRef CAS.
- H. Ishida, in Overview and historical background of polybenzoxazine research, Elsevier B.V., 2011, pp. 3–81 Search PubMed.
- X. Ning and H. S. Ishida, J. Polym. Sci., Part B: Polym. Phys., 1994, 32(5), 921–927 CrossRef CAS.
- H. Ishida and D. J. Allen, J. Polym. Sci., Part B: Polym. Phys., 1996, 34(6), 1019–1030 CrossRef CAS.
- A. Tuzun, B. Kiskan, N. Alemdar, A. T. Erciyes and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2010, 48(19), 4279–4284 CrossRef CAS.
- Z. Fu, K. Xu, X. Liu, J. C. Wu, C. J. Tan and M. C. Chen, Macromol. Chem. Phys., 2013, 214(10), 1122–1130 CrossRef CAS.
- Y. L. Liu and C. I. Chou, J. Polym. Sci., Part A: Polym. Chem., 2005, 43(21), 5267–5282 CrossRef CAS.
- S. B. Shen and H. Ishida, J. Polym. Sci., Part B: Polym. Phys., 1999, 37(23), 3257–3268 CrossRef CAS.
- M. Kukut, B. Kiskan and Y. Yagci, Des. Monomers Polym., 2009, 12(2), 167–176 CrossRef CAS.
- M. W. Wang, R. J. Jeng and C. H. Lin, Macromolecules, 2015, 48(3), 530–535 CrossRef CAS.
- M. Baqar, T. Agag, R. Z. Huang, J. Maia, S. Qutubuddin and H. Ishida, Macromolecules, 2012, 45(20), 8119–8125 CrossRef CAS.
- Y. X. Wang and H. Ishida, Polymer, 1999, 40(16), 4563–4570 CrossRef CAS.
- Y. J. Han, C. Y. Lin, M. Liang and Y. L. Liu, Macromol. Rapid Commun., 2016, 37(10), 845–850 CrossRef CAS PubMed.
- M. A. Tasdelen, B. Kiskan and Y. Yagci, Macromol. Rapid Commun., 2006, 27(18), 1539–1544 CrossRef CAS.
- Z. Beyazkilic, M. U. Kahveci, B. Aydogan, B. Kiskan and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2012, 50(19), 4029–4036 CrossRef CAS.
- S. Akay, B. Kayan, D. Kalderis, M. Arslan, Y. Yagci and B. Kiskan, J. Appl. Polym. Sci., 2017, 134(38), 45306 CrossRef.
- S. H. Je, T. H. Hwang, S. N. Talapaneni, O. Buyukcakir, H. J. Kim, J. S. Yu, S. G. Woo, M. C. Jang, B. K. Son, A. Coskun and J. W. Choi, ACS Energy Lett., 2016, 1(3), 566–572 CrossRef CAS.
- S. Shukla, A. Ghosh, P. K. Roy, S. Mitra and B. Lochab, Polymer, 2016, 99, 349–357 CrossRef CAS.
- H. K. Lin and Y. L. Liu, Macromol. Rapid Commun., 2018, 39(8), 1700832 CrossRef PubMed.
- W. Z. Li, X. Y. Wu, Z. J. Zhao, A. J. Qin, R. R. Hu and B. Z. Tang, Macromolecules, 2015, 48(21), 7747–7754 CrossRef CAS.
- T. Tian, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2018, 140(19), 6156–6163 CrossRef CAS PubMed.
- L. F. Zhang, Z. H. Ni, D. Y. Li, Z. H. Qin and X. Y. Wei, Chin. Chem. Lett., 2012, 23(3), 281–284 CrossRef CAS.
- T. B. Nguyen, L. Ermolenko, W. A. Dean and A. Al-Mourabit, Org. Lett., 2012, 14(23), 5948–5951 CrossRef CAS PubMed.
- S. H. Je, O. Buyukcakir, D. Kim and A. Coskun, Chem, 2016, 1(3), 482–493 CAS.
- M. Akiba and A. S. Hashim, Prog. Polym. Sci., 1997, 22(3), 475–521 CrossRef CAS.
- J. C. Patrick and H. R. Ferguson, Polysulfide polymer, US2466963, 1949 Search PubMed.
- J. C. Patrick, Trans. Faraday Soc., 1936, 32(1), 0347–0357 RSC.
- S. M. Matin and J. C. Patrick, Ind. Eng. Chem., 1936, 28(10), 1144–1149 CrossRef.
- P. J. Daly, W. G. Duncan, A. R. Fiorillo, J. R. Gilmore and K. B. Potts, Polym. Mater. Sci. Eng., 1992, 67, 417–418 CAS.
- J. C. Patrick and N. M. Mnookin, Plastic compositions, US1890231, 1932 Search PubMed.
- E. M. Fettes and J. S. Jorczak, Ind. Eng. Chem., 1950, 42(11), 2217–2223 CrossRef CAS.
- J. S. Jorczak and E. M. Fettes, Ind. Eng. Chem., 1951, 43(2), 324–328 CrossRef CAS.
- K. Kishore and K. Ganesh, Macromolecules, 1993, 26(17), 4700–4705 CrossRef CAS.
- I. Gomez, O. Leonet, J. A. Blazquez, H. J. Grande and D. Mecerreyes, ACS Macro Lett., 2018, 7(4), 419–424 CrossRef CAS.
- Y. Zhang, Y. Peng, Y. Wang, J. Li, H. Li, J. Zeng, J. Wang, B. J. Hwang and J. Zhao, Sci. Rep., 2017, 7(1), 11386 CrossRef PubMed.
- K. Nakabayashi, T. Takahashi, K. Watanabe, C. T. Lo and H. Mori, Polymer, 2017, 126, 188–195 CrossRef CAS.
- K. Tanaka, H. Yamane, K. Mitamura, S. Watase, K. Matsukawa and Y. Chujo, J. Polym. Sci., Part A: Polym. Chem., 2014, 52(18), 2588–2595 CrossRef CAS.
- J. Lim, U. Jung, W. T. Joe, E. T. Kim, J. Pyun and K. Char, Macromol. Rapid Commun., 2015, 36(11), 1103–1107 CrossRef CAS.
- H. Zhou, F. Yu, M. Wei, Y. Su, Y. Ma, D. Wang and Q. Shen, Chem. Commun., 2019, 55(26), 3729–3732 RSC.
- A. Dasari, Z. Z. Yu, Y. W. Mai, G. P. Cai and H. H. Song, Polymer, 2009, 50(6), 1577–1587 CrossRef CAS.
- K. Tanaka and Y. Chujo, J. Mater. Chem., 2012, 22(5), 1733–1746 RSC.
- J. Wu and P. T. Mather, Polym. Rev., 2009, 49(1), 25–63 CrossRef CAS.
- Y. Suzuki, K. Murakami, S. Ando, T. Higashihara and M. Ueda, J. Mater. Chem., 2011, 21(39), 15727–15731 RSC.
- C. L. Lu, Z. C. Cui, Y. X. Wang, B. Yang and J. C. Shen, J. Appl. Polym. Sci., 2003, 89(9), 2426–2430 CrossRef.
- Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Eur. Polym. J., 2010, 46(1), 34–41 CrossRef CAS.
- R. Okutsu, S. Ando and M. Ueda, Chem. Mater., 2008, 20(12), 4017–4023 CrossRef CAS.
- J. Bitenc, K. Pirnat, T. Bancic, M. Gaberscek, B. Genorio, A. Randon-Vitanova and R. Dominko, ChemSusChem, 2015, 8(24), 4128–4132 CrossRef CAS PubMed.
- Z. Song, H. Zhan and Y. Zhou, Chem. Commun., 2009,(4), 448–450 RSC.
- Z. L. Jian, Y. L. Liang, I. A. Rodriguez-Perez, Y. Yao and X. L. Ji, Electrochem. Commun., 2016, 71, 5–8 CrossRef CAS.
- B. Haupler, A. Wild and U. S. Schubert, Adv. Energy Mater., 2015, 5(11), 1402034 CrossRef.
- F. Jasinski, E. Lobry, B. Tarablsi, A. Chemtob, C. Croutxé-Barghorn, D. Le Nouen and A. Criqui, ACS Macro Lett., 2014, 3(9), 958–962 CrossRef CAS.
- S. Fujii, S. P. Armes, R. Jeans, R. Devonshire, S. Warren, S. L. McArthur, M. J. Burchell, F. Postberg and R. Srama, Chem. Mater., 2006, 18(11), 2758–2765 CrossRef CAS.
- B. L. Allen, J. D. Johnson and J. P. Walker, ACS Nano, 2011, 5(6), 5263–5272 CrossRef CAS PubMed.
- A. Rehor, N. Tirelli and J. A. Hubbell, J. Controlled Release, 2003, 87(1–3), 246–247 Search PubMed.
- H. Wei, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Prog. Polym. Sci., 2009, 34(9), 893–910 CrossRef CAS.
- Z. M. O. Rzaev, S. Dincer and E. Piskin, Prog. Polym. Sci., 2007, 32(5), 534–595 CrossRef CAS.
- B. A. Trofimov, A. G. Mal'kina, I. A. Dorofeev, G. F. Myachina, I. V. Rodionova, T. I. Vakul'skaya, L. M. Sinegovskaya and T. A. Skotheim, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179(1), 35–47 CrossRef CAS.
- J. M. Catala, J. M. Pujol and J. Brossas, Makromol. Chem., 1987, 188(11), 2517–2522 CrossRef CAS.
- B. A. Trofimov, A. G. Mal'kina, I. A. Dorofeev, G. F. Myachina, I. V. Rodionova, T. I. Vakul'skaya, L. M. Sinegovskaya and T. A. Skotheim, Russ. J. Gen. Chem., 2007, 77(9), 1559–1566 CrossRef CAS.
- H. Shin, J. Kim, D. Kim, V. H. Nguyen, S. Lee, S. Han, J. Lim and K. Char, J. Mater. Chem. A, 2018, 6(46), 23542–23549 RSC.
- G. D. T. Owen, W. J. Macknight and A. V. Tobolsky, J. Phys. Chem., 1964, 68(4), 784–786 CrossRef CAS.
- L. B. Blight, B. R. Currell, B. J. Nash, R. T. M. Scott and C. Stillo, Br. Polym. J., 2007, 12(1), 5–11 Search PubMed.
- A. Ahmed and L. P. Blanchard, J. Appl. Polym. Sci., 1984, 29(4), 1225–1239 CrossRef CAS.
- G. Montaudo, C. Puglisi, M. Blazso, K. Kishore and K. Ganesh, J. Anal. Appl. Pyrolysis, 1994, 29(2), 207–222 CrossRef CAS.
- D. Todorova and S. Todorov, Spectrosc. Lett., 1999, 32(2), 321–331 CrossRef CAS.
- L. J. Xue, D. G. Hristova-Bogaerds, J. G. van Berkel, G. Verbist and P. J. Lemstra, Polym. Degrad. Stab., 2010, 95(2), 126–131 CrossRef CAS.
- J. Park, E. T. Kim, C. Kim, J. Pyun, H. S. Jang, J. Shin, J. W. Choi, K. Char and Y. E. Sung, Adv. Energy Mater., 2017, 7(19), 1700074 CrossRef.
- C. R. Westerman and C. L. Jenkins, Macromolecules, 2018, 51(18), 7233–7238 CrossRef CAS.
- P. J. Flory, J. Am. Chem. Soc., 1952, 74(11), 2718–2723 CrossRef CAS.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29(3), 183–275 CrossRef CAS.
- J. Han, Y. Zheng, S. Zheng, S. Li, T. Hu, A. Tang and C. Gao, Chem. Commun., 2014, 50(63), 8712–8714 RSC.
- C. C. Lee, J. A. MacKay, J. M. Frechet and F. C. Szoka, Nat. Biotechnol., 2005, 23(12), 1517–1526 CrossRef CAS PubMed.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50(26), 5904–5908 CrossRef CAS PubMed.
- H. Wang, Y. Yang, Y. Liang, J. T. Robinson, Y. Li, A. Jackson, Y. Cui and H. Dai, Nano Lett., 2011, 11(7), 2644–2647 CrossRef CAS PubMed.
- J. F. Rubinson and Y. P. Kayinamura, Chem. Soc. Rev., 2009, 38(12), 3339–3347 RSC.
- D. Tashima, T. Kishita, S. Maeno and Y. Nagasawa, Mater. Lett., 2013, 110, 105–107 CrossRef CAS.
- D. Tashima, H. Yoshitama, M. Otsubo, S. Maeno and Y. Nagasawa, Electrochim. Acta, 2011, 56(24), 8941–8946 CrossRef CAS.
- K. Mori and Y. Nakamura, J. Org. Chem., 1971, 36(20), 3041–3042 CrossRef CAS.
- T. B. Nguyen, Adv. Synth. Catal., 2017, 359(7), 1066–1130 CrossRef CAS.
- H. Hu, B. T. Zhao, H. Y. Cheng, S. G. Dai, N. Kane, Y. Yu and M. L. Liu, Nano Energy, 2019, 57, 635–643 CrossRef CAS.
- A. Duda and S. Penczek, Macromolecules, 1982, 15(1), 36–40 CrossRef CAS.
- S. Penczek and A. Duda, Pure Appl. Chem., 1981, 53(9), 1679–1687 CAS.
- S. Penczek, R. Slazak and A. Duda, Nature, 1978, 273(5665), 738–739 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |










