Hydrogen-evolving photocathodes consisting of Cu2SnxGe1−xS3 particles synthesized by polymerized complex method and sulphurization†
Yosuke
Kageshima
 *ab,
Yusuke
Ooka
a,
Hiromu
Kumagai
*ab,
Yusuke
Ooka
a,
Hiromu
Kumagai
 *c,
Fumiaki
Takagi
a,
Katsuya
Teshima
*c,
Fumiaki
Takagi
a,
Katsuya
Teshima
 ab,
Kazunari
Domen
ab,
Kazunari
Domen
 bd and
Hiromasa
Nishikiori
bd and
Hiromasa
Nishikiori
 *ab
*ab
aDepartment of Materials Chemistry, Faculty of Engineering, Shinshu University, 4-17-1 Wakasato, Nagano 380-8553, Japan. E-mail: kage_ysk@shinshu-u.ac.jp; nishiki@shinshu-u.ac.jp
bResearch Initiative for Supra-Materials (RISM), Shinshu University, 4-17-1 Wakasato, Nagano 380-8553, Japan
cResearch Center for Advanced Science and Technology, The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8904, Japan. E-mail: kumagai@enesys.rcast.u-tokyo.ac.jp
dOffice of University Professors, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 21st September 2023
Abstract
A facile, scalable approach to synthesizing Cu2SnxGe1−xS3 (CTGS) photocatalytic particles was developed, based on the sulphurization of particulate oxide precursors prepared by a polymerized complex (PC) method. The combination of this PC technique and sulphurization enabled the synthesis of relatively small CTGS particles having more uniform size distributions compared with materials produced using a conventional solid-state reaction (SSR). Consequently, a photocathode consisting of CTGS particles synthesized by the PC method followed by sulphurization exhibited superior photoelectrochemical (PEC) performance during hydrogen evolution relative to that of specimens synthesized via the SSR. The effects of the Sn/Ge ratio and of Cu deficiency on the crystalline structure, optical properties and PEC performance of CTGS particles synthesized by the PC technique and sulphurization were elucidated. A photocathode consisting of Cu1.94Sn0.5Ge0.5S3 (Sn/(Sn + Ge) = 0.5, 3% Cu deficient) particles showed the highest hydrogen evolution performance among the present specimens, providing a photocurrent of −8.1 mA cm−2 at an applied potential of 0 V vs. a reversible hydrogen electrode (RHE) under simulated sunlight and a 0.59% half-cell solar-to-hydrogen conversion efficiency at 0.15 VRHE.
Introduction
Photoelectrochemical (PEC) water splitting has been intensively studied as a promising means of harvesting solar energy in the form of storable, conveyable hydrogen.1,2 Since the majority of solar radiation consists of visible and infrared (IR) light, the development of narrow-gap semiconductor photocatalysts responsive to long wavelengths is one of the most important steps to achieving the high solar-to-hydrogen (STH) energy conversion efficiencies required for practical artificial photosynthetic systems.3Cu2SnxGe1−xS3 (CTGS) polycrystalline thin films have been researched as light-absorbing materials with applications in efficient solid-state photovoltaic devices and hydrogen-evolving photocathodes.4–6 These compounds exhibit narrow bandgaps and so are able to absorb light up to the near-IR region. The bandgap energy of CTGS can also be tuned by adjusting the Sn/Ge ratio. Finally, the chemical stability and the low toxicity of the constituent elements of CTGS are advantageous compared with conventional photovoltaic materials such as Cu(In,Ga)Se2 and CdTe. Recently, our group reported that photocathodes consisting of CTGS particles synthesized by solid-state reaction (SSR) or flux methods in sealed quartz ampules were capable of promoting PEC hydrogen evolution with relatively high efficiencies.7,8 It has been proposed that particulate photocatalysts, rather than polycrystalline thin films, might be better suited to mass-production in an economically feasible manner and could be applied to the construction of integrated solar energy conversion devices.9,10 However, CTGS particles prepared by an SSR method in a previous study exhibited a wide range of sizes ranging from the submicron to micron scale as well as ragged and undefined shapes.7 In contrast, crystalline particles synthesized via flux methods have been grown to sizes larger than 10 μm.8 Therefore, it should be possible to improve the hydrogen evolution performance of these materials through controlling the size and shape of the CTGS particles. Furthermore, synthesis using a sealed ampule under vacuum is not suitable for the future mass-production of these catalysts and it would be helpful to assess flow-type reactor processes.
On this basis, the present study developed a new, facile route to the fabrication of photocatalytic CTGS particles that could be scaled up to permit mass production. In this process, an amorphous mixed metal oxide is prepared by a polymerized complex (PC) method and subsequently heated under a continuous H2S flow such that it is converted to the corresponding sulphide. This PC method involves the synthesis of a metal-polyamide resin from an aqueous solution containing a metal–citrate complex and ethylene diamine uniformly mixed at the atomic level followed by pyrolysis. Fine oxide precursors intimately mixed with metal cations are obtained in this manner.11–13 Sulphurization using a tubular furnace as demonstrated herein might also be applicable to the future mass production of these materials. The effects of the Sn/Ge ratio and of Cu deficiency on the crystalline structure, optical properties and PEC performance of CTGS particles synthesized using the PC method combined with sulphurization were also elucidated. The sulphurization of oxide precursors containing the desired constituent elements in a homogeneously dispersed state is especially beneficial as a means of fine tuning the compositions of multielement compound semiconductors such as CTGS.
Experimental
Synthesis of CTGS particles
Fabrication of photocathodes
Characterizations
The morphologies of the specimens were examined using field-emission scanning electron microscopy (FE-SEM; Hitachi, SU8000). The particle size distributions and average particle sizes were estimated from the resulting SEM images, employing a commercial image analysis software package (MIPAR). The crystalline structures of the specimens were characterized based on X-ray diffraction (XRD; Rigaku, MiniFlex600) using the Cu Kα line, while the optical properties of the materials were evaluated by ultraviolet-visible-near-IR diffuse reflectance (DR) spectroscopy (Jasco, V-770). X-ray absorption fine structure (XAFS) analyses were conducted using Beamline 9C at the Photon Factory operated by the High Energy Accelerator Research Organization in Tsukuba, Japan. XAFS spectra were collected in transmission mode under ambient conditions and were processed using the Athena software package. TG-DTA data were acquired using a Rigaku Thermoplus EVO2 apparatus.PEC measurements
A typical three-electrode setup was employed to assess the PEC performance of each photocathode. A Ag/AgCl electrode in a saturated aqueous KCl solution and a Pt coil were used as the reference and counter electrode, respectively. A 1.0 M potassium phosphate buffer solution (KPi, 0.5 M KH2PO4/0.5 M K2HPO4, adjusted to pH 7 with KOH) was employed as the aqueous electrolyte. This electrolyte was vigorously stirred and purged with Ar during the measurements and a simulated AM 1.5G sunlight source (100 mW cm−2) was used.Results and discussion
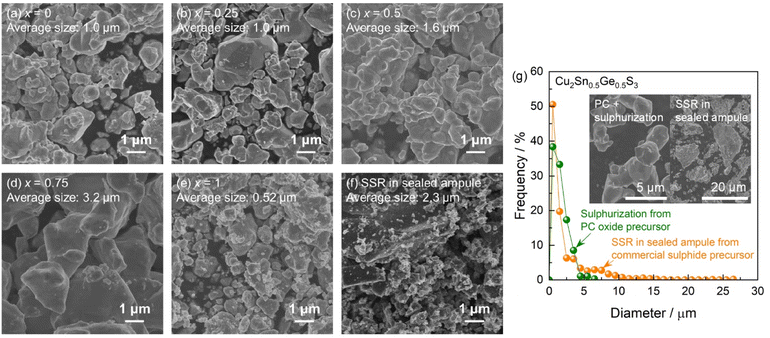
The Cu–Sn–Ge mixed oxides prepared by the PC method comprised small particles less than 1 μm in size with undefined shapes and relatively narrow size distributions (Fig. S6†). The specimens calcined at 550 °C had slightly smaller particle sizes than those carbonized at 400 °C (Fig. S7†), possibly due to the evaporation of residual organic carbon. Typically, it is desirable for mixed metal oxides prepared by the PC method to be amorphous because this indicates that the constituent metals are homogeneously mixed.11 In the present case, the Cu–Sn–Ge oxides generated weak XRD peaks possibly assignable to each oxide (Fig. S8†), unfortunately implying partial separation of the crystallized oxides. Nevertheless, the XRD peaks obtained for the present PC oxides were significantly weaker than those for a commercial sulphide reagent that has been used as a precursor for the conventional SSR process in a sealed ampule.7 These findings imply that a significant portion of the Cu–Sn–Ge PC oxides produced in this study possessed an amorphous-like structure with minimal crystallinity when compared with more highly-crystalline commercially-available reagents. It appears that the metal cations in the present oxides generated by the PC method were more homogeneously dispersed relative to the state of dispersion in the simple mixtures of commercial sulphide reagents used in prior work.SEM images of the CTGS particles obtained from sulphurization of the PC oxides are presented in Fig. 1. It was confirmed that the bulk of CTGS particles represented pure sulphide, while the immediate vicinity of the photocatalyst surface contained oxidized species (Fig. S9†). These specimens had ragged, nonspecific shapes with an average size of approximately 1 μm. The average particle size gradually increased as the proportion of Sn in the material was increased while the size of the Cu2SnS3 particles (that is, particles not containing Ge, x = 1) decreased. Although the details of the associated mechanism remain unclear, the Sn/Ge ratio might affect the degree of crystal growth. It should also be noted that the CTGS synthesized from commercial sulphide precursors through the conventional SSR process in sealed ampules exhibited various particles sizes ranging from the submicron scale to greater than several microns (Fig. 1f).7 Particle size distributions were estimated from SEM images and the results for Cu2Sn0.5Ge0.5S3 specimens prepared by the PC method followed by sulphurization and by conventional SSR are provided in Fig. 1g. The distributions for CTGS samples having different Sn/Ge ratios are also summarized in Fig. S10† for comparison purposes. The specimens synthesized from commercial sulphide precursors using the SSR method in sealed ampules (that is, the conventional method7) each provided bimodal size distributions. These distributions consisted of a prominent peak derived from relatively small particles less than 5 μm in size together with a second small peak associated with particles larger than 5 μm. Notably, the materials made using the SSR method in sealed ampules with commercial sulphide precursors comprised extremely large particles (greater than several tens of microns) in some cases. Conversely, the CTGS specimens fabricated via the PC method followed by sulphurization contained particles with sizes less than 5 μm and no extremely large particles over 10 μm. Thus, relatively small CTGS particles with narrow size distributions were synthesized through the present method combining a PC process and sulphurization.
The effects of the precursor type and the synthesis method on the morphologies of the CTGS particles were also examined. In these trials, Cu2Sn0.5Ge0.5S3 particles were synthesized through four different routes; (1) commercial sulphide precursors were heated in sealed quartz ampules (the conventional SSR method7), (2) commercial sulphide precursors were heated in a tubular furnace under a flow of H2S, (3) commercial oxide precursors were heated in a tubular furnace under a flow of H2S or (4) oxide precursors produced by the PC method were heated in a tubular furnace under a flow of H2S (the present method). Interestingly, heating the mixture of commercial sulphide precursors under the continuous N2 flow was incapable of synthesizing pure phase of CTGS, possibly due to the volatilization of sulphur and germanium-related species (Fig. S11 and S12†). It should be noted that the commercial sulphide reagents (Cu2S, SnS2 and GeS2) used in these experiments contained extremely large particles over 10 μm in size (Fig. S13†). The commercial Cu2O and GeO2 were made of particles having sizes on the micron scale with relatively narrow distributions while the commercial SnO2 comprised relatively large aggregates of fine particles (Fig. S14†). The CTGS particles synthesized from the commercial sulphide precursors contained both relatively small submicron-sized particles and extremely large particles on the micron scale, regardless of whether the heat treatment was conducted in sealed ampules (route 1) or under a H2S flow (route 2) (Fig. S15a and S15b†). Relatively large CTGS particles on the order of several microns with moderately wide size distributions were obtained following sulphurization of the commercial oxide precursors (route 3) (Fig. S15c†). Among the present trials, only the combination of the PC technique and sulphurization (that is, route 4) provided reasonably small CTGS particles (approximately 1 μm) with relatively uniform size distributions (Fig. S15d†). These differences in the sizes of the synthesized CTGS particles can be attributed to variations in the particle size distributions of the precursors. The Cu–Sn–Ge mixed oxides synthesized via the PC method were significantly less crystalline than the commercial precursors (Fig. S8†). Even so, the resulting CTGS particles exhibited a consistent crystalline structure (based on XRD peak positions) and high crystallinity (based on full-width at half-maximum (FWHM) values) regardless of the crystallinity of the precursors (Fig. S16†). Therefore, the fine, amorphous oxide particles prepared using the PC method in this work would be expected to serve as a suitable precursor for the synthesis of relatively small, uniform CTGS particles.
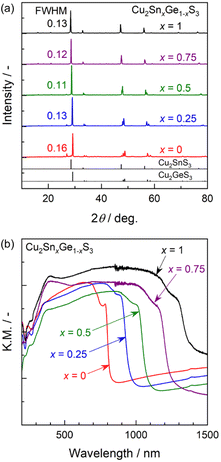
Sulphide specimens having various Sn/Ge ratios synthesized through the PC method followed by sulphurization all generated similar XRD patterns assignable to a monoclinic crystalline CTGS phase17–20 (Fig. 2a). The diffraction peaks also gradually shifted to lower angles as the proportion of Sn in the material was increased. This effect reflected the larger ionic radius of Sn4+ (69 pm) relative to that of Ge4+ (53 pm) in the four-coordinated state. This result suggests that the particles comprised a solid solution of Cu2SnS3 and Cu2GeS3. The XRD peaks generated by the present specimens showed similar FWHM values other than a slight decrease as the Sn proportion was increased. There was also a slight increase in the FWHM at x values larger than 0.5, such that the sharpest peak was obtained from the Cu2Sn0.5Ge0.5S3. This observation also implies that the coexistence of Sn and Ge species might promote the growth of the CTGS crystals to a greater extent than would occur if one metal was present in excess. The DR spectra of the CTGS particles containing various Sn/Ge ratios are presented in Fig. 2b. These data demonstrate that the absorption edges underwent a gradual red-shift with increases in this ratio, moving from approximately 800 nm (x = 0) to 1400 nm (x = 1). This variation in the bandgap energy according to the Sn/Ge ratio is consistent with previous results obtained from studies of polycrystalline thin films or particles synthesized by SSR.7,17–20 The CTGS particles produced in this work evidently absorbed at very long wavelengths up to the near-IR region of the solar spectrum. The weak absorption at wavelengths longer than the absorption edge is attributed to defects and/or impurities. Hence, light absorbed at these longer wavelengths would not be expected to be involved in the PEC reaction.21,22
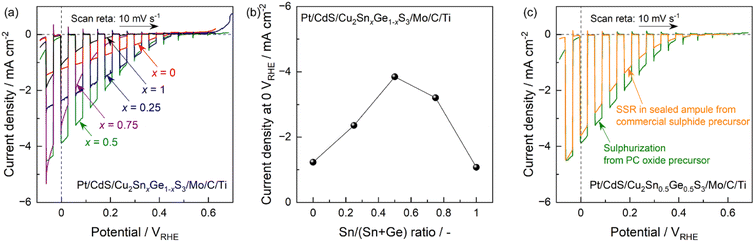
Current–potential curves obtained for photocathodes made from CTGS particles having various Sn/Ge ratios and synthesized through the PC method and sulphurization are shown in Fig. 3a. These CTGS photocathodes generated cathodic photocurrents in response to simulated sunlight regardless of the Sn/Ge ratio. The cathodic photocurrents at negative potentials (such as 0 V vs. a reversible hydrogen electrode (RHE)) gradually increased as the proportion of Sn was increased. The highest photocurrent value of −3.8 mA cm−2 at 0 VRHE was obtained for x = 0.5 (Fig. 3b). Interestingly, further increases in the Sn ratio (to x = 0.75 and 1) decreased the photocurrent, especially at positive potentials. Consequently, the specimens with relatively low Sn contents (x = 0 to 0.5) were capable of generating cathodic photocurrents beginning at approximately 0.5 VRHE, whereas the materials for which x was 0.75 or 1 exhibited a negative shift in the onset potential to approximately 0.3 VRHE. Intriguingly, incident-photon-to-current conversion efficiencies (IPCEs) under an illumination of monochromatic light with a fixed wavelength of which the materials show the similar degree of absorption coefficients irrespective to the composition also showed the similar volcano-type tendency (Fig. S17†). Prior work by our group established that a greater proportion of Sn induced a positive shift in the conduction band minimum while the valence band maximum was almost unchanged regardless of the Sn/Ge ratio.7 Thus, the volcano-type photocurrent trend shown by the data according to the Sn/Ge ratio can be attributed to the trade-off between the increased number of photons absorbed by the semiconductor and the decreased driving force of the photoexcited electrons in the conduction band. The greater quantity of photons that the CTGS was able to absorb due to the red-shift of the absorption edge should certainly be one possible parameter contributing to the enhanced photocurrent up to an x value of 0.5. Here, it should be noted that increasing of IPCEs as the Sn/(Sn + Ge) ratio increases even under the monochromatic light (Fig. S17†) implies the existence of another parameter (such as accelerated crystal grows associated with the increased Sn/Ge ratio as described in Fig. 1) contributing to the enhanced photocurrent. Meanwhile, the positive shift in the conduction band minimum originating from further increases in the Sn ratio could have decreased the reductive driving force of the photoexcited electrons for hydrogen evolution. The noticeable drop of the IPCE observed when Sn/(Sn + Ge) > 0.5 (Fig. S17†) should support this adverse effect of positive shift of conduction band level. The optimal Sn/(Sn + Ge) composition for the present synthesis method was different from the optimal value for the case of SSR,7 although the origin of the difference is still unclear. Moreover, CTGS particles synthesized by the present method (that is, the combination of the PC process and sulphurization) generated higher photocurrents compared with the specimen prepared through the conventional route (SSR in the sealed ampule7) especially at positive potentials (Fig. 3c). We have reported that an appropriate particle size (that is neither too large nor too small) should be required to obtain efficient PT photoelectrodes.23 Additionally, the typical polycrystalline thin films intended for photovoltaic applications comprise a light-absorbing layer with a thickness of several micrometres. Because the extremely large particles contained in the specimens synthesized through the conventional SSR should be inadequate for the photocathode applications, this improved performance might be attributable to the relatively small, uniform particle sizes of the CTGS synthesized via the new method. However, CTGS particles synthesized by the combination of PC process and sulphurization unfortunately showed lower photocatalytic activity than the conventional SSR specimen in the powder suspension system (Fig. S18†). To enhance the photocatalytic activity rather than the PEC performance, another designing of photocatalyst surface (e.g., surface etching and/or loading more appropriate cocatalysts) might be necessary.
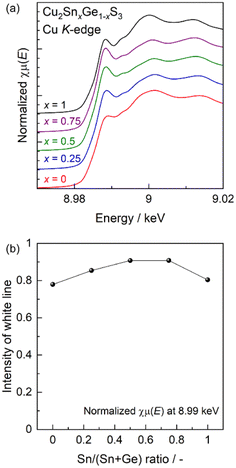
To further investigate the origin of the volcano-type tendency shown by the photocurrent values with changes in the Sn/Ge ratio, XAFS analyses were also conducted with the results provided in Fig. 4. The present CTGS particles produced similar Cu K-edge X-ray absorption near edge structure (XANES) spectra at all Sn/Ge ratios (Fig. 4a). These data also confirmed that the onset of X-ray absorption gradually shifted to higher energies in the order of Cu metal, Cu2O and CuO, reflecting the increased valence state of the Cu in these materials (Fig. S19†).24 It should be noted that the Cu2S used as a reference showed a similar X-ray absorption onset to that of Cu metal, possibly due to the electrical conductivity of Cu2S.25 Nevertheless, because the CTGS particles fabricated in this work exhibited similar X-ray absorption onsets to that for Cu2O regardless of the Sn/Ge ratio, the Cu incorporated in the CTGS appears to have been in the monovalent state. The extended X-ray absorption fine structure (EXAFS) oscillations for these specimens were also barely affected by the Sn/Ge ratio. In addition, the corresponding Fourier transformed radial distribution functions for all specimens were consistent with the first nearest-neighbour Cu–S distances reported in the literature (Fig. S20†).26–29 These results provide evidence that the local structure around the Cu atoms was only minimally affected by the Sn/Ge ratio. Here, it should be noted that the XANES white line intensity at approximately 8.99 keV, ascribed to the 1s to 4p transition associated with tetrahedrally-coordinated Cu+ ions in sulphides (such as in a chalcopyrite-type structure),28–30 (Fig. 4a) varied according to the Sn/Ge ratio. The intensity of this white line is plotted as a function of the ratio in Fig. 4b. From these data, it is apparent that the intensity gradually increased along with the proportion of Sn but then dropped at an x value of 1. As such, the most prominent peaks were obtained with x = 0.5 to 0.75. It has been reported that a more intense white line is associated with an increase in positive charge (meaning a decrease in electronic density) around the absorbing atom.30,31 Thus, the present result implies that the carrier density (meaning the hole concentration) was affected by the Sn/Ge ratio and that the highest density was achieved in the x range of 0.5 to 0.75. This increased carrier density obtained by alloying Sn and Ge might be another possible reason for the highest photocurrent observed at x = 0.5.
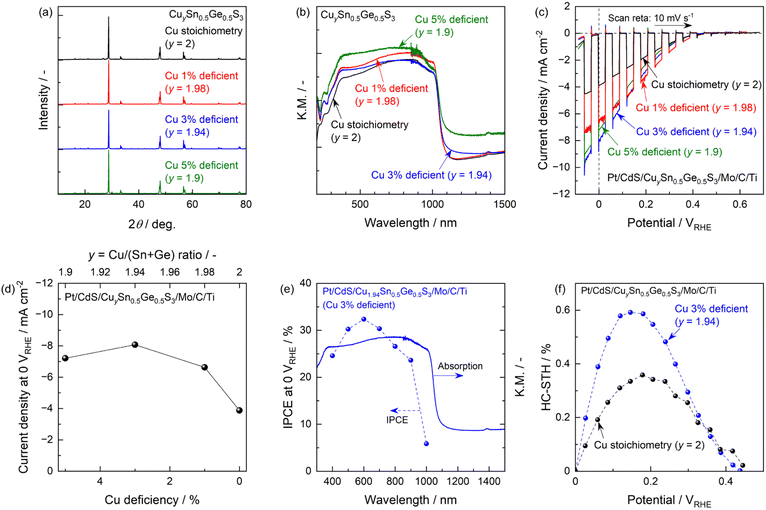
The synthesis of Cu-chalcogenide materials under Cu-deficient conditions is known to improve the photovoltaic and PEC performance of these materials.32–34 Our own group previously reported that Cu deficiency improved the PEC performance of CTGS particles synthesized via the conventional SSR technique in sealed ampules.7 Thus, the present work examined the effect of Cu deficiency on the PEC performance of CTGS particles synthesized through the PC method and sulphurization. Specimens having a Sn/(Sn + Ge) ratio of 0.5 and various Cu proportions were found to generate identical XRD patterns. These findings indicate that the Cu content only minimally affected the crystal structure and the degree of crystallinity (Fig. 5a). All specimens also showed the same absorption edge position of approximately 1000 nm, providing evidence that the optical bandgap energy was unaffected by the Cu proportion (Fig. 5b). However, the absorption baseline at wavelengths longer than the absorption edge was slightly increased as the Cu content was decreased. This effect might indicate a slight increase in the concentration of electronic defects.21,22 SEM images of oxide precursors prepared by the PC method and the corresponding sulphides having various Cu contents are provided in Fig. S21 and S22.† The morphologies of the oxides generated by the PC method were evidently unaffected by the Cu proportion while the size of the Cu-deficient sulphide particles was slightly increased. The current–potential profiles for the photocathodes consisting of CTGS particles synthesized via the PC process and sulphurization and having various Cu deficits are summarized in Fig. 5c. All specimens generated cathodic photocurrents beginning at an electrode potential of approximately 0.5 VRHE. In addition, the photocurrent gradually increased in conjunction with a negative shift of the electrode potential. The cathodic photocurrents were −3.8, −6.6, −8.1 and −7.2 mA cm−2 at 0 VRHE for specimens with a stoichiometric amount of Cu or 1%, 3% and 5% Cu deficient compositions, respectively. Notably, the CTGS specimens synthesized with Cu deficiencies all generated higher photocurrents than the stoichiometric specimen at almost all potentials. The highest performance was obtained for the 3% Cu deficient composition (Cu/(Sn + Ge) = 1.94) (Fig. 5d). The photocurrent obtained from this specimen was approximately 2.1 times that generated by the specimen having a stoichiometric composition. Faradaic efficiency during the PEC hydrogen evolution using the optimal specimen was confirmed to be almost unity (Fig. S23†). The IPCE spectrum obtained by the 3% Cu deficient specimen is presented in Fig. 5e. The onset of IPCE spectrum was located in the vicinity of 1000 nm, in agreement with the absorption edge of CTGS particles. This indicates that the observed photocurrent originated from the bandgap photoexcitation of CTGS. The IPCE values gradually became greater in response to the illumination of shorter wavelength light, and reached 32% under illumination of 600 nm monochromatic light at an applied potential of 0 VRHE. The effects of electrode potential on the half-cell STH (HC-STH) for the optimal CTGS and the stoichiometric specimen are shown in Fig. 5f. The HC-STH value for the former material was as high as 0.59% at an applied potential of 0.15 VRHE while that for the latter specimen (Cu2Sn0.5Ge0.5S3) was a maximum of 0.36% at 0.18 VRHE. This evident improvement in cathodic photocurrent upon reducing the Cu content can possibly be attributed to an increase in the carrier density and/or the generation of Cu-deficient secondary phases, as has been reported to occur in similar Cu-chalcogenide materials.32–34 The slight decrease in photocurrent induced by an excessive reduction in the Cu content (5% deficient) might have been related to an increase in electronic defects. This possibility is supported by the increased absorption baseline at wavelengths beyond the absorption edge in the DR spectra.21,22 Although there is still room for improvement, these PEC characteristics certainly rank relatively high for this type of particulate photocathode.35–41 Thus, the facile, readily scaled-up synthesis route introduced herein, combining the PC method and sulphurization as well as composition tuning, could be a viable means of designing an efficient hydrogen-evolving CTGS particulate photocathode. Such cathodes would be responsive to wavelengths up to the IR region of the solar spectrum.
Conclusions
A new, simple fabrication process that could be applied to mass-production was demonstrated in this work. This process allows the synthesis of photocatalytic CTGS particles based on the PC method and subsequent sulphurization in a tubular furnace. The PC technique was found to generate fine, amorphous mixed metal oxide particles less than 1 μm in size and having relatively narrow size distributions. Despite the low crystallinity of these Cu–Sn–Ge mixed oxides synthesized via the PC method, CTGS particles exhibiting a consistent crystalline structure, and a high degree of crystallinity were obtained after sulphurization under a H2S flow. The present method enabled the synthesis of relatively small, uniform CTGS particles and so was superior to other routes using commercial oxide or sulphide precursors. Variations in the morphologies of the synthesized CTGS particles reflected differences in the particle size distributions of the precursors. The relatively small and uniform particle sizes of the CTGS synthesized via the PC process with sulphurization may have resulted in PEC performance during hydrogen evolution that was superior to that for materials made using the conventional SSR technique. The cathodic photocurrents generated by CTGS photocathodes gradually increased with increasing Sn content over the range of x = 0 to 0.5, but then decreased at higher Sn proportions (x = 0.75 and 1). This volcano-type trend associated with changes in the Sn/Ge ratio can possibly be explained by the trade-off between increases in the number of photons that the CTGS can absorb and decreases in the driving force for the photoexcited electrons, originating from variations in the bandgap energy. This work also demonstrated an additional enhancement of PEC performance based on adjusting the Cu content of the catalyst. A photocathode consisting of Cu1.94Sn0.5Ge0.5S3 (x = 0.5, 3% Cu deficient) particles showed the highest hydrogen evolution performance, with a −8.1 mA cm−2 photocurrent at 0 VRHE under simulated sunlight and a 0.59% HC-STH value at 0.15 VRHE.Author contributions
Yosuke Kageshima: conceptualization, formal analysis, funding acquisition, investigation, methodology, and writing – original draft. Yusuke Ooka: formal analysis and investigation. Hiromu Kumagai: conceptualization, formal analysis, investigation, and methodology. Fumiaki Takagi: investigation. Katsuya Teshima: supervision. Kazunari Domen: supervision. Hiromasa Nishikiori: conceptualization, funding acquisition, and supervision. All authors: writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by a Grant-in-aid for Scientific Research (B) (no. 21H01715) from the Japan Society for the Promotion of Science (JSPS). This study was also funded in part by a Grant-in-Aid for Scientific Research (C) (no. 22K05297). The XAFS analyses reported herein were performed under the approval of the Photon Factory Program Advisory Committee (proposal no. 2021G633).References
- J. W. Ager, M. R. Shaner, K. A. Walczak, I. D. Sharp and S. Ardo, Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting, Energy Environ. Sci., 2015, 8, 2811–2824 RSC.
- W. Yang, R. R. Prabhakar, J. Tan, S. D. Tilley and J. Moon, Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting, Chem. Soc. Rev., 2019, 48, 4979–5015 RSC.
- K. Maeda, Photocatalytic water splitting using semiconductor particles: history and recent developments, J. Photochem. Photobiol., C, 2011, 12, 237–268 CrossRef CAS.
- M. Umehara, Y. Takeda, T. Motohiro, T. Sakai, H. Awano and R. Maekawa, Cu2Sn1-xGexS3 (x = 0.17) Thin-Film Solar Cells with High Conversion Efficiency of 6.0, Appl. Phys. Express, 2013, 6, 045501 CrossRef.
- H. Hayashi, J. Chantana, Y. Kawano, T. Nishimura, A. Mavlonov and T. Minemoto, Zn1−xMgxO second buffer layer of Cu2Sn1−xGexS3 thin-film solar cell for minimizing carrier recombination and open-circuit voltage deficit, Sol. Energy, 2020, 204, 769–779 CrossRef CAS.
- S. Kamemoto, Y. Matsuda, M. Takahashi and S. Higashimoto, Photocatalytic water splitting on Cu2SnS3 photoelectrode: effects of Cu/Sn composite ratio on the photoelectrochemical performance, Catal. Today, 2023, 411–412, 113820 CrossRef CAS.
- Y. Kageshima, S. Shiga, T. Ode, F. Takagi, H. Shiiba, M. T. Htay, Y. Hashimoto, K. Teshima, K. Domen and H. Nishikiori, Photocatalytic and Photoelectrochemical Hydrogen Evolution from Water over Cu2SnxGe1–xS3 Particles, J. Am. Chem. Soc., 2021, 143, 5698–5708 CrossRef CAS PubMed.
- Y. Kageshima, S. Kato, S. Shiga, F. Takagi, H. Minamisawa, M. Horita, T. Yamakami, K. Teshima, K. Domen and H. Nishikiori, Impact of Ball Milling on the Hydrogen Evolution Performance of Cu2Sn0.38Ge0.62S3 Photocatalytic Particles Synthesized via a Flux Method, ACS Appl. Mater. Interfaces, 2023, 15, 13108–13120 CrossRef CAS PubMed.
- B.-J. Ng, L. K. Putri, X. Y. Kong, P. Pasbakhsh and S.-P. Chai, Z-scheme photocatalyst sheets with P-doped twinned Zn0.5Cd0.5S1-x and Bi4NbO8Cl connected by carbon electron mediator for overall water splitting under ambient condition, Chem. Eng. J., 2021, 404, 127030 CrossRef CAS.
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata, Y. Li, I. D. Sharp, A. Kudo, T. Yamada and K. Domen, Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%, Nat. Mater., 2016, 15, 611–615 CrossRef CAS PubMed.
- M. Kakihana, Synthesis of high-performance ceramics based on polymerizable complex method, J. Ceram. Soc. Jpn., 2009, 117, 857–862 CrossRef CAS.
- X. Sun and X. Xu, Efficient photocatalytic hydrogen production over La/Rh co-doped Ruddlesden-Popper compound Sr2TiO4, Appl. Catal., B, 2017, 210, 149–159 CrossRef CAS.
- H. Nakamura, K. Shinozaki, T. Okumura, K. Nomura and T. Akai, Massive red shift of Ce3+ in Y3Al5O12 incorporating super-high content of Ce, RSC Adv., 2020, 10, 12535–12546 RSC.
- H. G. Langer, Solid complexes with tetravalent metal ions and ethylenediamime tetra-acetic acid (EDTA), J. Inorg. Nucl. Chem., 1964, 26, 59–72 CrossRef CAS.
- C. S. Quintans, H. Kato, M. Kobayashi, H. Kaga, A. Iwase, A. Kudo and M. Kakihana, Improvement of hydrogen evolution under visible light over Zn1−2x(CuGa)xGa2S4 photocatalysts by synthesis utilizing a polymerizable complex method, J. Mater. Chem. A, 2015, 3, 14239–14244 RSC.
- T. Minegishi, N. Nishimura, J. Kubota and K. Domen, Photoelectrochemical properties of LaTiO2N electrodes prepared by particle transfer for sunlight-driven water splitting, Chem. Sci., 2013, 4, 1120–1124 RSC.
- D. M. Berg, R. Djemour, L. Gütay, S. Siebentritt, P. J. Dale, X. Fontane, V. Izquierdo-Roca and A. Pérez-Rodriguez, Raman analysis of monoclinic Cu2SnS3 thin films, Appl. Phys. Lett., 2012, 100, 192103 CrossRef.
- D. Tiwari, T. K. Chaudhuri, T. Shripathi, U. Deshpande and R. Rawat, Non-toxic, earth-abundant 2% efficient Cu2SnS3 solar cell based on tetragonal films direct-coated from single metal-organic precursor solution, Sol. Energy Mater. Sol. Cells, 2013, 113, 165–170 CrossRef CAS.
- M. T. Htay, T. Mandokoro, H. Seki, T. Sakaizawa, N. Momose, T. Taishi, Y. Hashimoto and K. Ito, Influence of Ge composition in the Cu2Sn1-xGexS3 thin-film photovoltaic absorber prepared by sulfurization of laminated metallic precursor, Sol. Energy Mater. Sol. Cells, 2015, 140, 312–319 CrossRef.
- Y. Matsumoto, N. Aihara, N. Saito and K. Tanaka, Growth of Cu2GeS3 bulk single crystals by chemical vapor transport with iodine, Mater. Lett., 2017, 194, 16–19 CrossRef CAS.
- M. Miyauchi, M. Takashio and H. Tobimatsu, Photocatalytic Activity of SrTiO3 Codoped with Nitrogen and Lanthanum under Visible Light Illumination, Langmuir, 2004, 20, 232–236 CrossRef CAS PubMed.
- K. Maeda, M. Higashi, B. Siritanaratkul, R. Abe and K. Domen, SrNbO2N as a Water-Splitting Photoanode with a Wide Visible-Light Absorption Band, J. Am. Chem. Soc., 2011, 133, 12334–12337 CrossRef CAS PubMed.
- F. Takagi, Y. Kageshima, K. Teshima, K. Domen and H. Nishikiori, Enhanced photoelectrochemical performance from particulate ZnSe:Cu(In,Ga)Se2 photocathodes during solar hydrogen production via particle size control, Sustain. Energy Fuels, 2021, 5, 412–423 RSC.
- K. Iwashina, A. Iwase, S. Nozawa, S. Adachi and A. Kudo, Visible-Light-Responsive CuLi1/3Ti2/3O2 Powders Prepared by a Molten CuCl Treatment of Li2TiO3 for Photocatalytic H2 Evolution and Z-Schematic Water Splitting, Chem. Mater., 2016, 28, 4677–4685 CrossRef CAS.
- P. Kumar, R. Nagarajan and R. Sarangi, Quantitative X-ray absorption and emission spectroscopies: electronic structure elucidation of Cu2S and CuS, J. Mater. Chem. C, 2013, 1, 2448–2454 RSC.
- R. Bacewicz, J. Antonowicz, S. Podsiadło and S. Schor, Local structure in Cu2ZnSnS4 studied by the XAFS method, Solid State Commun., 2014, 177, 54–56 CrossRef CAS.
- P. T. Erslev, M. R. Young, J. V. Li, S. C. Siah, R. Chakraborty, H. Du, R. J. Lad, T. Buonassisi and G. Teeter, Tetrahedrally coordinated disordered Cu2SnS3–Cu2ZnSnS4–ZnS alloys with tunable optical and electronic properties, Sol. Energy Mater. Sol. Cells, 2014, 129, 124–131 CrossRef CAS.
- W. Hu, J. Ludwig, B. Pattengale, S. Yang, C. Liu, X. Zuo, X. Zhang and J. Huang, Unravelling the Correlation of Electronic Structure and Carrier Dynamics in CuInS2 Nanoparticles, J. Phys. Chem. C, 2018, 122, 974–980 CrossRef CAS.
- M. J. Turnbull, Y. M. Yiu, M. Goldman, T.-K. Sham and Z. Ding, Favorable Bonding and Band Structures of Cu2ZnSnS4 and CdS Films and Their Photovoltaic Interfaces, ACS Appl. Mater. Interfaces, 2022, 14, 32683–32695 CrossRef CAS PubMed.
- Y. L. Mikhlin, M. N. Likhatski, O. A. Bayukov, Y. Knyazev, D. A. Velikanov, Y. V. Tomashevich, A. S. Romanchenko, S. A. Vorobyev, M. V. Volochaev, S. M. Zharkov and D. M. Meira, Valleriite, a Natural Two-Dimensional Composite: X-ray Absorption, Photoelectron, and Mössbauer Spectroscopy, and Magnetic Characterization, ACS Omega, 2021, 6, 7533–7543 CrossRef CAS PubMed.
- W. van der Stam, M. de Graaf, S. Gudjonsdottir, J. J. Geuchies, J. J. Dijkema, N. Kirkwood, W. H. Evers, A. Longo and A. J. Houtepen, Tuning and Probing the Distribution of Cu+ and Cu2+ Trap States Responsible for Broad-Band Photoluminescence in CuInS2 Nanocrystals, ACS Nano, 2018, 12, 11244–11253 CrossRef CAS PubMed.
- M. Kemell, M. Ritala and M. Leskelä, Thin Film Deposition Methods for CuInSe2 Solar Cells, Crit. Rev. Solid State Mater. Sci., 2005, 30, 1–31 CrossRef CAS.
- A. Walsh, S. Chen, S. H. Wei and X. G. Gong, Kesterite Thin-Film Solar Cells: Advances in Materials Modelling of Cu2ZnSnS4, Adv. Energy Mater., 2012, 2, 400–409 CrossRef CAS.
- T. Nishimura, S. Toki, H. Sugiura, K. Nakada and A. Yamada, Effect of Cu-deficient layer formation in Cu(In,Ga)Se2 solar-cell performance, Prog. Photovoltaics, 2018, 26, 291–302 CAS.
- X. Guo, P. Diao, D. Xu, S. Huang, Y. Yang, T. Jin, Q. Wu, M. Xiang and M. Zhang, CuO/Pd composite photocathodes for photoelectrochemical hydrogen evolution reaction, Int. J. Hydrog. Energy, 2014, 39, 7686–7696 CrossRef CAS.
- J. E. Park, Y. Hu, J. W. Krizan, Q. D. Gibson, U. T. Tayvah, A. Selloni, R. J. Cava and A. B. Bocarsly, Stable Hydrogen Evolution from an AgRhO2 Photocathode under Visible Light, Chem. Mater., 2018, 30, 2574–2582 CrossRef CAS.
- T. Hayashi, R. Niishiro, H. Ishihara, M. Yamaguchi, Q. Jia, Y. Kuang, T. Higashi, A. Iwase, T. Minegishi, T. Yamada, K. Domen and A. Kudo, Powder-based (CuGa1−yIny)1−xZn2xS2 solid solution photocathodes with a largely positive onset potential for solar water splitting, Sustain. Energy Fuels, 2018, 2, 2016–2024 RSC.
- G. S. Pawar, A. Elikkottil, S. Seetha, K. S. Reddy, B. Pesala, A. A. Tahir and T. K. Mallick, Enhanced Photoactivity and Hydrogen Generation of LaFeO3 Photocathode by Plasmonic Silver Nanoparticle Incorporation, ACS Appl. Energy Mater., 2018, 1, 3449–3456 CrossRef CAS.
- D. Huang, L. Li, K. Wang, Y. Li, K. Feng and F. Jiang, Wittichenite semiconductor of Cu3BiS3 films for efficient hydrogen evolution from solar driven photoelectrochemical water splitting, Nat. Commun., 2021, 12, 3795 CrossRef CAS PubMed.
- S. Wojtyła and T. Baran, Copper-Nickel-Oxide Nanomaterial for Photoelectrochemical Hydrogen Evolution and Photocatalytic Degradation of Volatile Organic Compounds, Mater. Res. Bull., 2021, 142, 111418 CrossRef.
- H. Fukai, K. Nagatsuka, Y. Yamaguchi, A. Iwase and A. Kudo, Powder-Based Cu3VS4 Photocathode Prepared by Particle-Transfer Method for Water Splitting Using the Whole Range of Visible Light, ECS J. Solid State Sci. Technol., 2022, 11, 063002 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: TG-DTA results, optimization of the sulphurization temperature, SEM images and XRD patterns for oxide precursors prepared by the PC method, XPS depth profile, particle size distributions for various Sn/(Sn + Ge) ratios, characterizations for a specimen prepared by heating the mixture of commercial sulphide precursors under N2 flow, SEM images of commercial oxide and sulphide precursors, SEM images and XRD patterns for CTGS particles prepared by various synthesis routes, effects of Sn/Ge ratio on IPCE, results of photocatalysis in powder suspension system, XAFS data, SEM images of PC oxides and corresponding sulphides with various Cu proportions and results of product gas analysis. See DOI: https://doi.org/10.1039/d3se00871a |
| This journal is © The Royal Society of Chemistry 2023 |