An anti-poisoning nanosensor for in situ monitoring of intracellular endogenous hydrogen sulfide†
Xi
Chen
,
Wen-Tao
Wu
,
Yu-Ting
Jiao
,
Yi-Ran
Kang
,
Xin-Wei
Zhang
 * and
Wei-Hua
Huang
* and
Wei-Hua
Huang
 *
*
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, China. E-mail: xinweizhang@whu.edu.cn; whhuang@whu.edu.cn
First published on 17th January 2023
Abstract
Intracellular H2S plays an important regulatory role in cell metabolism. The limited sensing materials and severe sensor passivation hinder its quantification. We functionalized conductive nanowires with MoS2 and quercetin in a large-scale manner, developed single nanowire sensors with excellent electrocatalytic and anti-poisoning performance, and achieved the accurate quantification of H2S within single cells.
H2S is an important endogenous signaling molecule in organisms.1 As a strong reductive and nucleophilic molecule (H2S has lone pair electrons), H2S participates in regulating various physiological and pathological processes; for instance, the regulation of redox balance by directly reacting with reactive oxygen/nitrogen species (ROS/RNS) such as H2O2 and NO, promoting vasodilation by persulfidating KATP channels to cause K+ influx, and so on.2 Notably, these processes are not only related to the intrinsic chemical properties of H2S, but also concentration-dependent.3 At micromolar concentration, H2S generally has a cytoprotective effect. Higher (millimolar) H2S tends to be cytotoxic to cells and might lead to inhibition of cell proliferation and pro-inflammatory effects; this is due to the insufficient ATP synthesis and over-expression of pro-inflammatory cytokines, like tumor necrosis factor TNF-α, etc.4
Therefore, the accurate quantification of the concentration of H2S facilitates a deeper understanding of its biological significance. Up to now, a variety of analytical methods have been developed for H2S detection, including fluorescence,5 chemiluminescence and electrochemical methods.6,7 Among them, the electrochemical method stands out because of its quantification ability, high sensitivity and extremely fast response speed, which is very suitable for the detection of H2S with considerable reactivity and fast catabolism.8 Some micrometer-sized electrodes developed for detecting H2S in biological matrices have been reported,9,10 but most focus on the intercellular H2S-mediated physiological processes (neuromodulation and regulation of vascular tone),1b,2c while many important biological effects occurring within cells, such as the effect on mitochondrial ATP and inflammation regulation,2a,b,4 are less studied. In addition, the synthesis site of H2S mainly locates within the cells, including CBS (cystathionine-β synthase) and CSE (cystathionine-γ lyase) in the cytosol and 3-MST (mercaptopyruvate transferase) in the mitochondrial matrix.1 These microsensors placed outside the cells cannot easily reflect the accurate concentration of intracellular H2S and its biological effects within living cells.11 Therefore, an in situ measurement strategy is indispensable. However, to our best knowledge, the real-time electrochemical detection of intracellular H2S has not been reported. Nanoelectrodes provide the possibility for intracellular detection, with their extremely small size and high temporal–spatial resolution, which are suitable for in situ analysis within single living cells.12 Nevertheless, there is an outstanding problem that the electrochemical sensors for detecting H2S are easily poisoned owing to the adsorption of the electrooxidation product of H2S – elemental sulfur on the electrode surface. This passivation is particularly obvious on the nano-sized electrodes. So, there is an urgent need to develop a robust and anti-sulfur poisoning nanoelectrode to monitor H2S within single living cells.
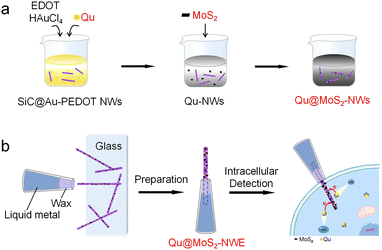
Herein, based on the SiC@Au–PEDOT nanowires with proper aspect ratio and excellent electrical conductivity previously synthesized,13 we loaded MoS2 and quercetin (Qu) on these nanowires by a large-scale synthesis method. Here, MoS2 shows excellent anti-sulfur poisoning and good electrocatalytic performance.14 Qu, as a kind of catechol derivative, has several hydroxyl groups and a conjugated π system, which might perform as an excellent electron transfer mediator,15 further enhancing the electrocatalytic performance of MoS2. We further fabricated nanoelectrodes with Qu–MoS2 composite functionalized nanowires. These functionalized nanowire sensors exhibit excellent electrocatalytic and anti-sulfur poisoning performance toward H2S detection. With such nanosensors, we realized the sensitive and accurate detection of endogenous H2S in single living cells.
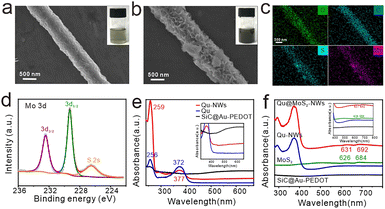
The synthetic process of the MoS2–Qu composite functionalized nanowires is illustrated in Scheme 1. SiC@Au–PEDOT NWs were first synthesized as substrates according to our previous work.13 3,4-Ethylenedioxythiophene (EDOT), Qu and HAuCl4 were then added to the SiC@Au–PEDOT NW dispersion. During the polymerization of EDOT initiated by HAuCl4, Qu was simultaneously bound to the generated polyEDOT (PEDOT), thus being stably and uniformly modified on SiC@Au–PEDOT NWs to form Qu-NWs (SiC@Au–PEDOT@Qu nanowires). The negatively charged MoS2 (zeta potential = −21.3 mV) was added and adsorbed on the positively charged Qu-NWs (zeta potential = +28.4 mV) (Fig. S1, ESI†),16 thus forming the Qu@MoS2-NWs (SiC@Au–PEDOT@Qu@MoS2 nanowires). The morphology of the Qu-NWs and Qu@MoS2-NWs was characterized by scanning electron microscopy. Compared with the smooth surface of the Qu-NWs (Fig. 1a), the Qu@MoS2-NWs showed a porous structure that was hierarchically assembled by the MoS2 nanosheets (Fig. 1b). The energy-dispersive X-ray (EDX) mapping showed that O, C, S and Mo were spread over the prepared nanowires (Fig. 1c). In addition, the X-ray photoelectron spectroscopy results showed the presence of Mo (IV) indicated by the Mo 3d peak at 229.3 and 232.4 eV (Fig. 1d),17 the Qu was indicated by the O 1s peaks at 533.2 eV (–OH and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 531.3 eV (C–O–C in the pyran functional group) and the PEDOT by the C–O–C bond peak at 532.6 eV (Fig. S2, ESI†).18,19 Moreover, the UV-Vis spectra showed the typical absorption peaks from the benzoyl system (256 nm) and cinnamoyl system (372 nm) in Qu,20 and the transition peaks of excitons from MoS2 at 626 nm and 684 nm (Fig. 1e and f).21 These results confirm the successful preparation of Qu@MoS2-NWs.
O) and 531.3 eV (C–O–C in the pyran functional group) and the PEDOT by the C–O–C bond peak at 532.6 eV (Fig. S2, ESI†).18,19 Moreover, the UV-Vis spectra showed the typical absorption peaks from the benzoyl system (256 nm) and cinnamoyl system (372 nm) in Qu,20 and the transition peaks of excitons from MoS2 at 626 nm and 684 nm (Fig. 1e and f).21 These results confirm the successful preparation of Qu@MoS2-NWs.
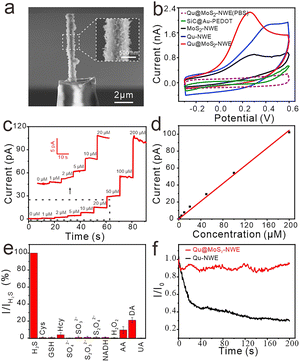
According to the previously reported protocol for fabricating single nanowire electrodes,13 we assembled the Qu@MoS2-NW to form a nanowire electrode (Qu@MoS2-NWE) with a diameter of ∼800 nm (Fig. 2a). Its good conductivity was demonstrated by cyclic voltammetry, which was applied on the Qu@MoS2-NWE in 1 mM Ru(NH3)63+ solution (Fig. S3, ESI†). The cyclic voltammogram of Qu@MoS2-NWEs in 0.01 M PBS showed a pair of characteristic redox peaks of Qu, which result from the conversion between the phenol and benzoquinone groups (Fig. S4, ESI†).20,22 Moreover, cyclic voltammetry of Qu@MoS2-NWE in 1 mM Ru(NH3)63+ and 0.01 M PBS was performed for 20 cycles to demonstrate its good stability (Fig. S5 and S6, ESI†). This advantage can be attributed to the stable modification of Qu and the strong electrostatic interaction between the Qu-NWs and MoS2.
The electrocatalytic ability of the Qu@MoS2-NWEs for H2S was further assessed. 1 mM Na2S solution was used to prepare the standard solution, which can instantaneously generate HS− and S2− at the physiological pH. Its electrochemical oxidation behavior at the SiC@Au–PEDOT NWE, Qu-NWE, MoS2-NWE and Qu@MoS2-NWE was examined by cyclic voltammetry (Fig. 2b). It could be seen that the SiC@Au–PEDOT NWE only exhibited a weak upturned current response, while the Qu@MoS2-NWE exhibited a more significant catalytic performance, which started oxidizing Na2S around −0.1 V and reached a maximum oxidation current around 0.25 V. Compared with the Qu-NWE and MoS2-NWE, the Qu@MoS2-NWE possesses obviously higher peak current and lower peak potential for oxidation of Na2S. These results indicated that the MoS2 and Qu composites had a better electrocatalytic ability than individual MoS2 or Qu. This excellent electrochemical performance might be attributed to a larger specific area of the porous structure as well as the synergistic effect of MoS2 and Qu. Specifically, the MoS2 efficiently catalyzed the oxidation of Na2S, while the Qu acted as a good electron transfer mediator which increased the charge transfer rate. The detection ability of the Qu@MoS2-NWE towards H2S was further examined by the amperometric method, and a good linear relationship was obtained between the oxidation peak currents and the Na2S concentration from 1 μM to 200 μM (Fig. 2c and d). The detection limit was calculated to be about 300 nM (S/N = 3, R2 = 0.997). Furthermore, Qu@MoS2-NWE exhibited an excellent selectivity toward Na2S compared to some interferents, including sulfur-containing reagents and other common biomolecules (Fig. 2e). As mentioned above, during the electrochemical detection of Na2S, the deposited sulfur usually passivates the electrode active surface and greatly decreases the sensitivity. But the MoS2 with good sulfur tolerance might present a high anti-poisoning performance. To verify this ability of MoS2, we recorded amperometric responses of 50 μM Na2S (this level is higher than the endogenous H2S concentration of ca. 15 μM in MCF-7 cell lines23) at the Qu@MoS2-NWE and Qu-NWE (E = 300 mV). The Qu@MoS2-NWE could output a stable current response to 50 μM Na2S, whereas the Qu-NWE showed a decreasing current response (Fig. 2f and Fig. S7 and S8, ESI†). Furthermore, the average normalized current (I/I0) recorded at Qu@MoS2-NWEs at 200 s was 93%, which was much higher than 30% compared with that at Qu-NWEs (Fig. S9, ESI†). These results confirmed that MoS2 presents a good anti-sulfur poisoning performance, which may be attributed to the repulsive force between the sulfur atom layers of MoS2 and the elemental sulfur produced by H2S oxidation. When elemental sulfur was produced on the electrode surface, it may be repelled by the sulfur layers of MoS2 and preferably escape to the bulk solution instead of depositing on the electrode surface.14
H2S is an important signaling molecule within cells and plays concentration-dependent regulation roles in intracellular metabolism. Herein, we applied Qu@MoS2-NWEs for the quantitative monitoring of H2S in living cells. The capability of Qu@MoS2-NWEs for intracellular detection was first evaluated. Due to the nano-size and good mechanical properties of the Qu@MoS2-NWEs, they could easily penetrate through the cell membrane and be tightly sealed by the membrane. This process was confirmed by monitoring the limiting reduction currents of Ru(NH3)63+ (1 mM in the extracellular bath solution), which can be reduced at the electrode but cannot cross the cellular membranes. As the depth of NWE insertion into cells increased, the limiting reduction current of Ru(NH3)63+ decreased correspondingly, and also almost recovered (∼95%) when the electrode was completely withdrawn from the cells (Fig. S10a, ESI†).24 Meanwhile, the high viability of the penetrated cells was proved by the fluorescence staining with calcein-AM and propidium iodide (PI), indicating that the insertion of Qu@MoS2-NWEs caused no significant damage to the cells (Fig. S10b, ESI†). These results demonstrated that Qu@MoS2-NWEs were suitable for intracellular detection.
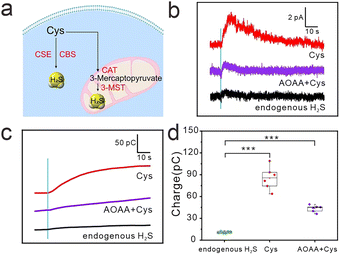
For intracellular H2S detection, we first applied a constant potential of 300 mV to the Qu@MoS2-NWEs and then positioned the Qu@MoS2-NWEs in contact with the MCF-7 cell membrane. It could be seen that there were no amperometric responses when the NWEs were outside the cells (amperometric trace on the left of the blue line in Fig. 3b). When the NWEs were completely inserted, weaker amperometric response signals were detected in the cells without any treatment. When the cells were treated with 10 mM Cys (the main substrate of the H2S synthesis, Fig. 3a) for 1 h to upregulate the intracellular H2S levels, an obviously elevated amperometric response was recorded. Whereas when the cells were pre-treated with 1 mM aminooxy acetic acid (AOAA, an inhibitor of PLP (pyridoxal-5-phosphate monohydrate)-dependent enzymes such as CBS and CSE) for 1 h,25 and subsequently treated with 10 mM Cys for 1 h, significantly smaller amperometric responses were detected (Fig. 3b). By integrating the current over the time, the corresponding charges are shown in Fig. 3c. The statistical charge data are illustrated in Fig. 3d, and it can be seen that the total charge generated by intracellular endogenous H2S oxidation was 11.8 ± 1.8 pC, which was lower than the 74.0 pC in Cys-treated cells and 31.6 pC in AOAA + Cys-treated cells.
Moreover, according to Faraday's law and the volume of a cell (the average diameter of spherical MCF-7 cells measured as ∼9 μm), the endogenous H2S concentration in a single MCF-7 cell was calculated to be 20.4 ± 3.1 μM. At this concentration, H2S is considered physiologically beneficial, and acts as an electron donor to stimulate mitochondrial electron transport and promote ATP synthesis, thus providing energy for the proliferation and migration of MCF-7 cells.26 In addition, since the electrode could output a stable current response to 50 μM Na2S for 200 s, which was longer than the acquisition time (∼100 s) of intracellular detection, the anti-sulfur poisoning performance of the Qu@MoS2-NWEs ensured the accurate quantification of intracellular endogenous H2S.
In conclusion, we prepared a novel single nanowire sensor by versatile and facile co-modification of MoS2 and Qu on nanowires. The excellent catalytic and good anti-sulfur poisoning properties of the MoS2–Qu composite endow the nanowire electrodes with outstanding H2S sensing performance, thus ensuring the accurate quantification of endogenous H2S in MCF-7 cells. The measured concentration of endogenous H2S in MCF-7 cells is approximately 20 μM, which was considered to be physiologically beneficial. The nanowire sensor would be an effective tool for the accurate quantification of H2S levels in various pathological conditions such as cancer, neurodegenerative diseases and cardiovascular diseases, which will be of great significance for better understanding the biphasic biological roles of H2S in these processes.
We gratefully acknowledge financial support from the National Natural Science Foundation of China (Grants 21725504, 22090050, 22090051, and 21721005).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. Aroca, C. Gotor, D. C. Bassham and L. C. J. A. Romero, Antioxidants, 2020, 9, 621 CrossRef CAS PubMed; (b) H. Kimura, N. Shibuya and Y. Kimura, Antioxid. Redox Signaling, 2012, 17, 45–57 CrossRef CAS PubMed.
- (a) M. R. Filipovic, J. Zivanovic, B. Alvarez and R. Banerjee, Chem. Rev., 2018, 118, 1253–1337 CrossRef CAS PubMed; (b) L. Li, P. Rose and P. K. Moore, Annu. Rev. Pharmacol. Toxicol., 2011, 51, 169–187 CrossRef CAS PubMed; (c) N. Skovgaard, A. Gouliaev, M. Aalling and U. Simonsen, Curr. Pharm. Biotechnol., 2011, 12, 1385–1393 CrossRef CAS PubMed.
- B. D. Paul, S. H. Snyder and K. Kashfi, Redox Biol., 2021, 38, 101772 CrossRef CAS PubMed.
- (a) C. Szabo, Cells, 2021, 10, 220 CrossRef CAS PubMed; (b) C. Szabo, Nat. Rev. Drug Discovery, 2007, 6, 917–935 CrossRef CAS PubMed; (c) C. Szabo, C. Ransy, K. Módis, M. Andriamihaja, B. Murghes, C. Coletta, G. Olah, K. Yanagi and F. Bouillaud, Br. J. Pharmacol., 2014, 171, 2099–2122 CrossRef CAS PubMed.
- (a) Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688–1691 CrossRef CAS PubMed; (b) Y. Qian, J. Karpus, O. Kabil, S.-Y. Zhang, H.-L. Zhu, R. Banerjee, J. Zhao and C. He, Nat. Commun., 2011, 2, 495 CrossRef PubMed.
- (a) B. Ke, W. Wu, W. Liu, H. Liang, D. Gong, X. Hu and M. Li, Anal. Chem., 2016, 88, 592–595 CrossRef CAS PubMed; (b) T. S. Bailey and M. D. Pluth, J. Am. Chem. Soc., 2013, 135, 16697–16704 CrossRef CAS PubMed.
- (a) S. Wang, X. Liu and M. Zhang, Anal. Chem., 2017, 89, 5382–5388 CrossRef CAS PubMed; (b) K. S. Shalini Devi and A. Senthil Kumar, Analyst, 2018, 143, 3114–3123 RSC; (c) Y. Qian, L. Zhang and Y. Tian, Anal. Chem., 2022, 94, 1447–1455 CrossRef CAS PubMed.
- J. Myszkowska, I. Derevenkov, S. V. Makarov, U. Spiekerkoetter and L. Hannibal, Antioxidants, 2021, 10, 1065 CrossRef CAS PubMed.
- X.-B. Hu, Y.-L. Liu, H.-W. Zhang, C. Xiao, Y. Qin, H.-H. Duo, J.-Q. Xu, S. Guo, D.-W. Pang and W.-H. Huang, ChemElectroChem, 2016, 3, 1998–2002 CrossRef CAS.
- H. Dong, Q. Zhou, L. Zhang and Y. Tian, Angew. Chem., Int. Ed., 2019, 58, 13948–13953 CrossRef CAS PubMed.
- F.-S. Quan and G.-J. Lee, BioMed Res. Int., 2021, 5473965 CAS.
- (a) N. T. N. Phan, X. Li and A. G. Ewing, Nat. Rev. Chem., 2017, 1, 0048 CrossRef CAS; (b) W. Wang, S.-H. Zhang, L.-M. Li, Z.-L. Wang, J.-K. Cheng and W.-H. Huang, Anal. Bioanal. Chem., 2009, 394, 17–32 CrossRef CAS PubMed; (c) X.-W. Zhang, A. Hatamie and A. G. Ewing, Curr. Opin. Electrochem., 2020, 22, 94–101 CrossRef CAS; (d) K. Ino, Y. Nashimoto, N. Taira, J. R. Azcon and H. Shiku, Electroanalysis, 2018, 30, 2195–2209 CrossRef CAS.
- W.-T. Wu, H. Jiang, Y.-T. Qi, W.-T. Fan, J. Yan, Y.-L. Liu and W.-H. Huang, Angew. Chem., Int. Ed., 2021, 60, 19337–19343 CrossRef CAS PubMed.
- (a) N. Jeromiyas, V. Mani, P.-C. Chang, C.-H. Huang, K. N. Salama and S.-T. Huang, Sens. Actuators, B, 2021, 326, 128844 CrossRef CAS; (b) V. Mani, S. Selvaraj, N. Jeromiyas, S.-T. Huang, H. Ikeda, Y. Hayakawa, S. Ponnusamy, C. Muthamizhchelvan and K. N. Salama, J. Mater. Chem. B, 2020, 8, 7453–7465 RSC.
- L. V. da Silva, A. K. A. de Almeida, J. A. Xavier, C. B. Lopes, F. d A. d S. Silva, P. R. Lima, N. D. dos Santos, L. T. Kubota and M. O. F. Goulart, J. Electroanal. Chem., 2018, 827, 230–252 CrossRef CAS.
- S. Khaledian, D. Kahrizi, S. Tofik Jalal Balaky, E. Arkan, M. Abdoli and F. Martinez, J. Mol. Liq., 2021, 334, 115989 CrossRef CAS.
- H. Nan, Z. Wang, W. Wang, Z. Liang, Y. Lu, Q. Chen, D. He, P. Tan, F. Miao, X. Wang, J. Wang and Z. Ni, ACS Nano, 2014, 8, 5738–5745 CrossRef CAS PubMed.
- K. Bourhis, S. Blanc, C. Mathe, J.-C. Dupin and C. Vieillescazes, Appl. Clay Sci., 2011, 53, 598–607 CrossRef CAS.
- Z. Liu, J. Xu, R. Yue, T. Yang and L. Gao, Electrochim. Acta, 2016, 196, 1–12 CrossRef CAS.
- W. Liu and R. Guo, J. Colloid Interface Sci., 2006, 302, 625–632 CrossRef CAS PubMed.
- T. Chen, H. Zou, X. Wu, C. Liu, B. Situ, L. Zheng and G. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 12453–12462 CrossRef CAS PubMed.
- Y. Dilgin, B. Kızılkaya, B. Ertek, N. Eren and D. G. Dilgin, Talanta, 2012, 89, 490–495 CrossRef CAS PubMed.
- M. Zuo, Q. Duan, C. Li, J. Ge, Q. Wang, Z. Li and Z. Liu, Anal. Chem., 2021, 93, 5635–5643 CrossRef CAS PubMed.
- Y. Wang, J.-M. Noël, J. Velmurugan, W. Nogala, M. V. Mirkin, C. Lu, M. Guille Collignon, F. Lemaître and C. Amatore, Proc. Nat. Acad. Sci., 2012, 109, 11534–11539 CrossRef CAS PubMed; X.-W. Zhang, Q.-F. Qiu, H. Jiang, F.-L. Zhang, Y.-L. Liu, C. Amatore and W.-H. Huang, Angew. Chem., Int. Ed., 2017, 56, 12997 CrossRef PubMed.
- A. Asimakopoulou, P. Panopoulos, C. T. Chasapis, C. Coletta, Z. Zhou, G. Cirino, A. Giannis, C. Szabo, G. A. Spyroulias and A. Papapetropoulos, Br. J. Pharmacol., 2013, 169, 922–932 CrossRef CAS PubMed.
- C. Szabo, C. Coletta, C. Chao, K. Módis, B. Szczesny, A. Papapetropoulos and M. R. Hellmich, Proc. Nat. Acad. Sci., 2013, 110, 12474–12479 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06729c |
| This journal is © The Royal Society of Chemistry 2023 |