Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in electrospun nanofibrous membrane sensors for ion detection
Liangqiang Wu
a,
Yan Songb,
Shuo Xinga,
Yapeng Li a,
Hai Xu
*a,
Qingbiao Yang
a,
Hai Xu
*a,
Qingbiao Yang
 *a and
Yaoxian Lia
*a and
Yaoxian Lia
aCollege of Chemistry, Jilin University, Changchun 130021, P. R China. E-mail: yangqb@jlu.edu.cn; haixu@jlu.edu.cn
bCollege of Materials Science and Engineering, Jilin Institute of Chemical Technology, Jilin 132022, P. R. China
First published on 6th December 2022
Abstract
Harmful metal ions and toxic anions produced in industrial processes cause serious damage to the environment and human health. Chemical sensors are used as an efficient and convenient detection method for harmful ions. Electrospun fiber membranes are widely used in the field of solid-state chemical sensors due to high specific surface area, high porosity, and strong adsorption. This paper reviews the solid-state chemical sensors based on electrospinning technology for the detection of harmful heavy metal ions and toxic anions in water over the past decade. These electrospun fiber sensors have different preparation methods, sensing mechanisms, and sensing properties. The preparation method can be completed by physical doping, chemical modification, copolymerization, surface adsorption and self-assembly combined with electrospinning, and the material can also be combined with organic fluorescent molecules, biological matrix materials and precious metal materials. Sensing performance aspects can also be manifested as changes in color and fluorescence. By comparing the literature, we summarize the advantages and disadvantages of electrospinning technology in the field of ion sensing, and discuss the opportunities and challenges of electrospun fiber sensor research. We hope that this review can provide inspiration for the development of electrospun fiber sensors.
 Hai Xu | Hai Xu is currently an associate professor at Jilin University. The main research direction is molecular simulation and quantitative calculation of organic optoelectronic functional materials. |
Introduction
With the development of industry, while bringing progress to human society, it also causes damage to the environment. For example, the harmful metal ions (Cu2+, Zn2+, Fe3+, Al3+, Pb2+, Hg2+) and anions (CN−, F−, HSO3−, ClO−) in wastewater produced in industrial processes will enter the environment and cause serious health problems to animals and plants in water bodies, including humans.1–5Among them, iron, copper and zinc are essential trace elements for human beings and play vital roles in human physiological activities.6–9 However, high levels of them can also affect the normal physiological activities of the body, causing a series of diseases. Excessive intake of Cu2+ may cause Alzheimer's disease, familial amyotrophic lateral sclerosis, and Mikan Wilson diseases.10–12 Excessive intake of Zn2+ can cause poisoning with gastrointestinal symptoms such as vomiting and diarrhea. In addition, it can cause damage to liver, kidney function and immunity.13–17 Excessive Fe3+ accumulate in body can lead to tissue damage, organ failure and eventually death.18–20 Excessive aluminum ions in the body can also lead to physiological dysfunction, which may cause mental retardation, Alzheimer's syndrome, etc.21–23 At the same time, some harmful heavy metal ions (Pb2+, Hg2+) in the water body will directly cause poisoning to our body. Ingesting trace amounts of harmful heavy metal ions can wreak havoc on our bodies and cause serious diseases such as cancer, cardiovascular disease, brain damage, failure of kidney and nervous system.24–29
CN−, F− are widely used in metallurgy and production of daily chemicals. However, cyanide will rapidly combine with iron in cytochrome oxidase, inhibiting the activity of oxidase, resulting in the inability of cells to use oxygen, causing symptoms such as vomiting, convulsions, loss of consciousness, and even death.30–32 Fluoride plays an important role in preventing dental caries and treating osteoporosis, but excessive fluoride can lead to dental fluorosis and bone fluorosis.33–36 Hypochlorite is widely used as a common disinfectant and bleach. However, as a kind of reactive oxygen species, it has a significant impact on the physiological activities of the human body. Studies have shown that high levels of hypochlorite in the human body will react with proteins, DNA and RNA to cause various diseases. Such as osteoarthritis, cardiovascular disease, atherosclerosis, pneumonia, pulmonary fibrosis, and even cancer.37–40 Sulfur dioxide, sulfite and hydrogen sulfite are common food additives that can be effectively preserved and they also have important physiological roles. For example, SO2 can act synergistically with NO to relieve vascular smooth muscle and dilate blood vessels. However, high levels of sulfur dioxide and SO32−/HSO3− are also toxic. Lung cancer, migraine, stroke and other respiratory, nervous system, cardiovascular and cerebrovascular diseases are all associated with high levels of sulfur dioxide and SO32−/HSO3−.41,42
These harmful ions pose a threat to human health. Therefore, it is an inevitable trend to develop a method that can quickly detect the type and content of harmful anions and metal ions in water. At present, plasma emission spectroscopy,43 mass spectrometry,44 chromatography45 and electrochemical analysis methods46 have been widely used in the detection of harmful ions in the environment. Although these detection methods can well complete the qualitative and quantitative detection of harmful ions in the environment, they have the disadvantages of relying on large-scale equipment, complex sample pretreatment, low detection efficiency, and high cost. Despite its advantages of simple operation, fast response time, low cost, naked eye identification and colorimetric detection, fluorescent probes have the disadvantage that they cannot be recovered and separated from the detection system.47,48 Solid-phase sensors have the advantages of convenient operation, environmental friendliness, easy separation, and reusability, and are widely used in environmental monitoring. Therefore, the development of solid-phase composite nano-fluorescence sensors has become an important direction for fluorescence sensing.49,50
Electrospinning technology is an efficient method for the preparation of nanofibers. Compared with traditional nanofibers, electrospun nanofibers have the advantages of high porosity, ultra-high specific surface area and uniform fiber diameter, which are ideal sensing substrates. In addition, electrospinning technology is simple to operate, low cost, widely applicable (almost all linear polymers and sols) and capable of continuous production in large batches.51,52 Reneker once pointed out that electrospinning technology can prepare nanofibers up to one kilometer long.53 Yuris Dzenis define it as an effective method that can realize the preparation of “Continuous Fibers for Nanotechnology".54 It has been widely used in the fields of environment,55,56 energy,57,58 biomedicine,59,60 sensing,61,62 EMI shielding,63 fiber reinforced composites,64,65 smart textiles,66 food packaging,67 electrocatalysts,68 actuator,69,70 water treatment.71–73 Usually, some sensing units (fluorescent dyes, quantum dots, metal–organic framework luminescent metal nanoclusters, etc.) and nanofiber materials are combined to form a composite sensing material and prepared into test strips for sensing (Scheme 1). The current electrospun fiber test strips for colorimetric or fluorescent detection have made great progress in sensing pH,74 temperature,75 harmful ions,76 toxic gases77 and biomolecules.78 After the test, the test strip can be directly removed from the water, and can even be prepared as a reusable test strip. There are many ways to combine the materials of electrospun nanofibers with chemical sensor, such as physical doping, chemical modification, copolymerization, surface adsorption and self-assembly.
Physical doping method is through the sensing unit (fluorescent dye, quantum dots, metal–organic framework, etc.) directly dispersed in a certain concentration of polymer solution, and then using electrospinning technology to prepare nanofiber membrane. This method has the advantages of simple preparation and wide application. However, the binding mode of sensing units and polymers only relies on weak intermolecular interactions, so some hydrophilic sensing units fall off the nanofibers in aqueous environment, which affects the sensing effect. Moreover, many luminescent units will undergo aggregation-induced quenching in the aggregated state, which is not suitable for direct physical doping. The utilization rate of the large number of sensing units wrapped inside the fiber is not high.
In the copolymerization method, the sensing unit is prepared into monomer and copolymerized with some high performance polymer monomer to form block polymer, which is then prepared into nanofiber film. This method effectively avoids the shortcomings of the sensor unit falling off the fiber, but the problems of aggregation-induced quenching and the inability to utilize the sensor unit inside the fiber are not solved.
Chemical modification is made by grafting sensing units on the polymer and reusing electrospinning technology to prepare nanofibers or grafting sensing units on the surface of nanofibers. The idea is to form strong chemical bonds between the sensing unit and the polymer chain. Among them, the method of chemical modification of sensing unit on fiber surface can reduce the influence of aggregation induced quenching and make the sensing unit directly contact with the measured object, so that the sensing efficiency is higher.
The method of surface adsorption is similar to surface chemical modification, except that one uses strong chemical bond binding while surface adsorption uses electrostatic interaction binding between fiber surface and sensing unit. This method can also effectively reduce the effect of aggregation-induced quenching and enhance the utilization of sensing units.
The host-guest self-assembly method makes use of the ion–dipole, hydrogen bond, van der Waals force and hydrophobic interactions of the host and guest to form substances with specific structures. In the preparation of electrospun nanofibers, we synthesize polymers and sensing units with subject or guest structures, respectively. The modified polymer is then prepared into nanofibers, which are combined by host–guest interaction. This method is similar to surface chemical modification and surface adsorption, except that we can change the structure of host and guest by changing the conditions to regulate the binding and separation between them.
This article will review the related nanofiber sensors in the past ten years (2012–2022) from the aspects of fiber material, preparation method, sensing mechanism, and sensor performance based on the different types of ions to be detected.
Electrospun nanofiber fluorescent sensor for detection of harmful metal ions
Generally, transition metal ions have empty orbitals and usually have strong coordination. Based on this characteristic, many compound of ligands with N, S and O atoms can be designed to detect metal ions such as organic molecular probes, surface-modified noble metal clusters and quantum dots. In order to enhance the convenience of use and sensing sensitivity of the sensor and reduce the pollution to the environment, metal-ion composite sensors based on electrospun nanofibers have made remarkable progress in the past decade. Some electrospun fiber membrane metal ion sensor base on organic molecular probes, metal nanoclusters, nanoparticles and quantum dots have been prepared by physical doping, chemical modification, copolymerization and self-assembly.Electrospun nanofiber fluorescent sensor for detection of Cu2+
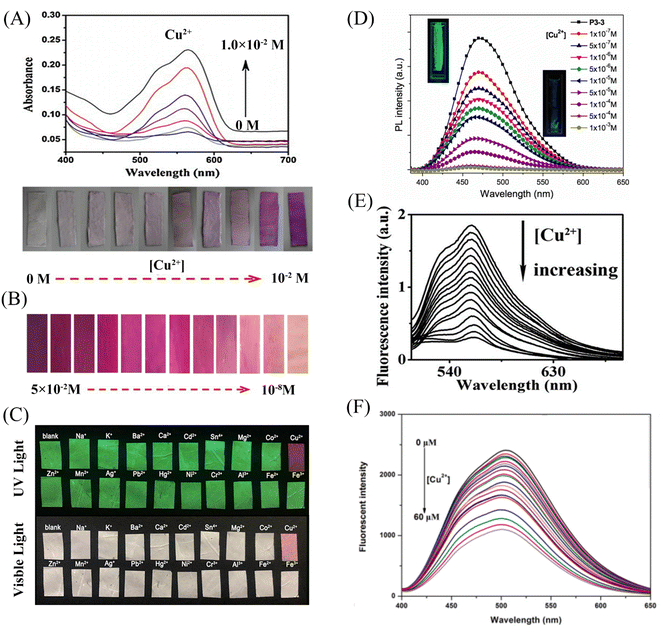
Min et al.79 doped salicylaldehyde-modified rhodamine dye into poly(ether-sulfone) (PES) to prepare a sensor for copper ion response in aqueous medium by electrospinning technology. The nanofiber membrane sensor can achieve high sensitivity and selective response to copper ions. The detection limit is as low as 1.1 nM and it exhibits remarkable fluorescence enhancement and colorimetric effect. The fiber membrane shows good reusability after treatment with EDTA (Fig. 1(A) and 2(A)). Zhang et al.80 fabricated an electrospun fiber membrane from chitosan/polyacrylonitrile (CS/PAN) doped with rhodamine hydrazide salicylaldehyde Schiff base as a Cu2+ nanofiber membrane (NFM) sensor. The response mechanism of the NFM to copper ions is the coordination of copper ions and oxygen leads to the opening of the cyclic lactam, which causes the color change. The naked-eye detection limit of NFM is as low as 10−8 M, which is about three orders of magnitude lower than that of pure probe solution. The NFM shows good reusability after treatment with EDTA (Fig. 1(B) and 2(B)). In 2020, Jin et al.81 doped rhodamine-phenothiazine-based Schiff base derivative RB into polymethyl methacrylate (PMMA) to prepare a reusable ratio fluorescence-colorimetric nanofibrous membrane sensor by electrospinning technology. The excited-state intramolecular proton transfer (ESIPT) process of the probe molecule was inhibited by the coordination of copper ion with the oxygen atom on the carbonyl group on RB and the nitrogen atom on the pyridine, which manifested as a red shift of the emission peak and enhanced UV absorption and the limit of detection (LOD) is 0.11 μM/0.28 μM. After adding adenosine triphosphate (ATP), the copper ions will fall off from the RB molecule, and the ESIPT effect of the molecule will restore the blue-shift of the emission peak to the original fluorescence and weakened ultraviolet absorption (Fig. 1(C) and 2(C)). It can be seen that the Schiff base derivative based on rhodamine is a good copper ion recognition unit and the sensing mechanism of copper ion is the same. Moreover, the fiber membrane sensor prepared by physical doping method still shows good fluorescence and colorimetric detection effects, which indicates that rhodamine derivatives are not easily affected by aggregation-induced quenching. It showed good reusability after strong ligand treatment. It provides a good model for fabricating fiber membrane copper ion sensor easily.
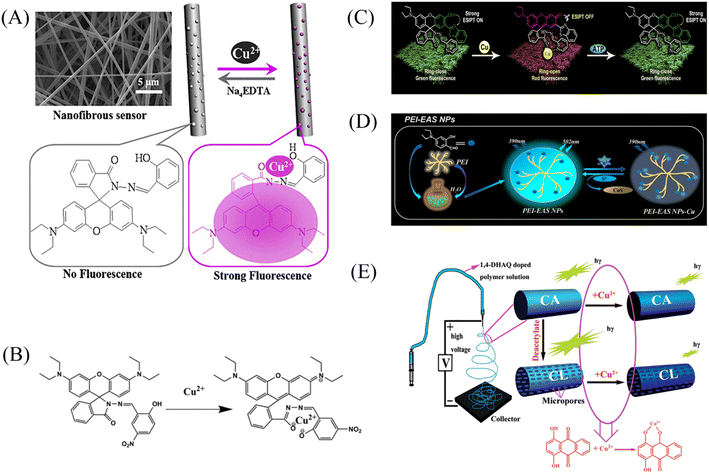 | ||
| Fig. 1 Preparation of copper ion sensor by physical doping method and their sensing mechanism. (A) Reprinted with permission from ref. 79. Copyright 2013, Elsevier. (B) Reprinted with permission from ref. 80. Copyright 2021, The Korean Fiber Society. (C) Reprinted with permission from ref. 81. Copyright 2020, Elsevier. (D) Reprinted with permission from ref. 84. Copyright 2021, Elsevier. (E) Reprinted with permission from ref. 82. Copyright 2012, American Chemical Society. | ||
 | ||
| Fig. 2 Sensing properties of copper ion sensors preparted by physical doping. (A) Reprinted with permission from ref. 79. Copyright 2013, Elsevier. (B) Reprinted with permission from ref. 80. Copyright 2021, the Korean Fiber Society. (C) Reprinted with permission from ref. 81. Copyright 2020, Elsevier. (D) Reprinted with permission from ref. 83. Copyright 2015, Springer Science Business Media New York. (E) Reprinted with permission from ref. 82. Copyright 2012, American Chemical Society. (F) Reprinted with permission from ref. 84. Copyright 2021, Elsevier. | ||
Some phenols, pyridines and salicylaldehyde-based Schiff base derivatives can also be used as recognition probes for copper ions. Wang et al.82 prepared 1,4-dihydroxyanthraquinone (1,4-DHAQ) and cellulose acetate (CA) co-doped nanofibrous membranes (1,4-DHAQ@CA) by electrospinning technology. 1,4-DHAQ@CA was then deacetylated to obtain a microporous fibrous membrane (1,4-DHAQ@CL). Since 1,4-DHAQ can coordinate with Cu2+ in aqueous solution to form phenolate, resulting in obvious fluorescence quenching. The experimental results show that the fluorescence intensity of the fiber membrane has a good linear relationship in the range of copper ion concentration of 2.5 to 37.5 × 10−9 M, the LOD is 3 × 10−9 M. The nanofiber membrane can complete the specific response to copper ions under a variety of interfering metal ions. Therefore, the 1,4-DHAQ@CL fiber membrane prepared by simple doping method and deacetylation can achieve high sensitivity and selectivity for Cu2+ fluorescence detection. Under the treatment of Cr3+, the sensor shows good reusability (Fig. 1(E) and 2(E)). Lin et al.83 doped 1,10-phenanthroline fluorescence sensor (F-phen) into poly (N-isopropylacrylamide-co-N-methylol acrylamide) (P(NIPAAm-co-NMA)). A nanofiber membrane copper ion sensor with a thermally responsive switching mechanism was fabricated by electrospinning technology. The sensor exhibits a dynamic linear relationship in the range of copper ion concentration from 1.0 × 10−5 to 1.0 × 10−4 M and exhibits significant fluorescence quenching. When the temperature exceeds lower critical solution temperature (LCST), the conformational transition of poly (N-isopropylacrylamide (PNIPAAm) exhibits shrinkage and hydrophobic state, which leads to the inhibition of fluorescence emission by F-phen aggregation and the fibrous membrane in the hydrophobic state is also unfavorable for binding to copper ions, so the sensor exhibits characteristics of temperature-controlled copper ion detection. This approach opens up the possibility for more temperature-controlled sensor designs (Fig. 2(D)). Jin et al.84 synthesized nanoparticles (PEI-EAS NPs) using 4(N,N-diethyl) salicylaldehyde and poly(ethylene imine) (PEI). The PEI-EAS NPs were doped into PMMA and the nanofibrous membrane sensor PEI-EAS@NF was fabricated by electrospinning. PEI-EAS@NF can respond specifically to copper ions, showing the phenomenon of green fluorescence quenching. The fluorescence can be recovered by the treatment of sulfur ions, and the repeated detection of copper ions can be realized (Fig. 1(D) and 2(F)). Rhodamine derivatives have poor photostability and the spiral rings are easy to be opened under light conditions. Rhodamine-based sensors require strict light avoidance during synthesis and storage. The Schiff base derivative sensor mentioned above not only has the same good sensing performance as rhodamine derivative, but also has better photostability, which is more suitable for the preparation of solid-phase nanofiber membrane sensor.
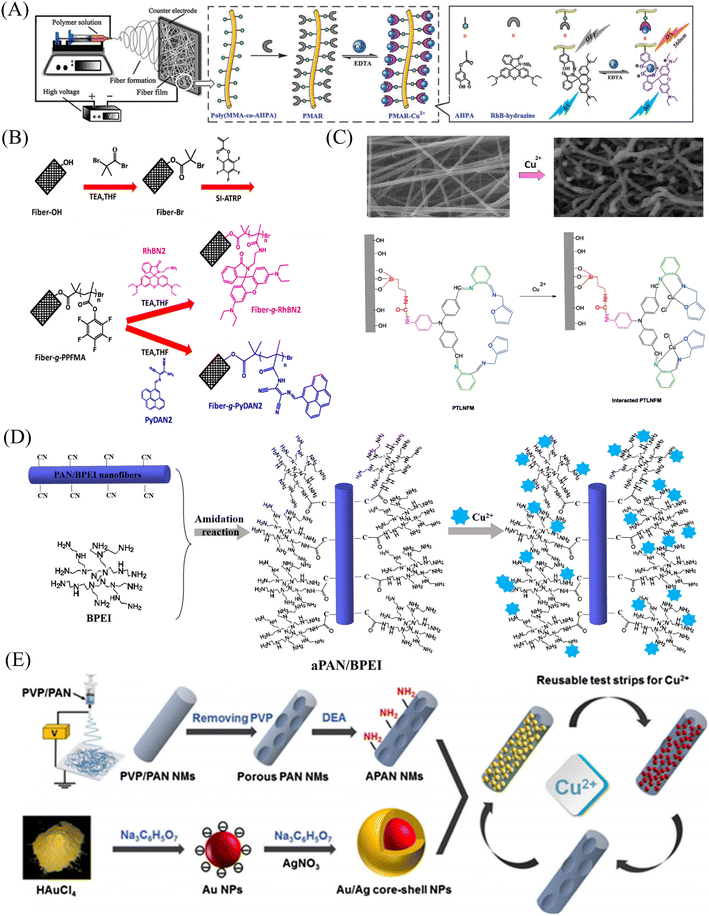
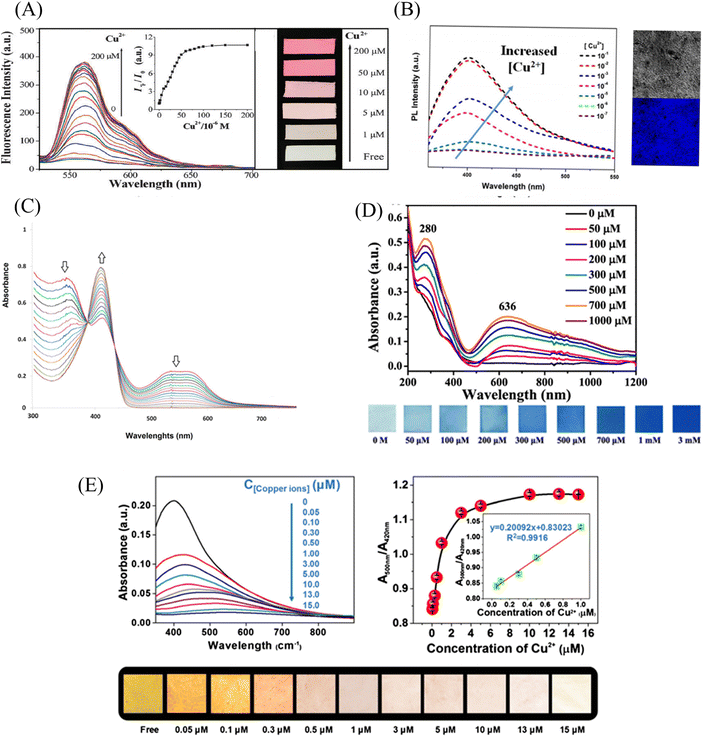
In 2012, Wang et al.85 reported a rhodamine surface-modified poly (methyl methacrylate co 4-aldehyde-3-hydroxy phenyl acrylate)nanofiber membrane (PMAR), which enables real-time colorimetric-fluorescence sensing of copper ions. PMAR can sense copper ions with high efficiency and selectivity, show obvious fluorescence enhancement and colorimetric effect. Experiments show that the response time of PMAR to copper ions is very short (<10 s), with a good linear relationship in the copper ion concentration range of 1.0 × 10−6–2.0 × 10−4 M, the detection limit is 1.5 × 10−6 M. Copper ions can be detected repeatedly by EDTA-treated PMAR (Fig. 3(A) and 4(A)). Cho et al.86 grafted pyrene derivatives (PyDAN2) on the surface of electrospun nanofibers using poly (2-hydroxyethylmethacrylic acid-co-N-methacrylic acid acrylamide) (P(HEMA-co-NMA)) as raw material prepared a fluorescent fiber membrane sensor (Fiber-g-PyDAN2) with high sensitivity to copper ions. Fiber-g-PyDAN2 nanofibers can chelate with copper ions in aqueous solution, resulting in a blue fluorescence enhancement. The lowest and highest LOD of 10−7 to 10−6 M and 10−2 to 10−1 M, respectively. Treat it with EDTA and it can be reused at least four times (Fig. 3(B) and 4(B)). Gao et al.87 synthesized a tri-ethoxy silane-modified triphenylamine-based symmetric Schiff base derivative (L) using ultrasound-assisted technology, and grafted L onto the surface of polyvinyl alcohol (PVA) electrospun nanofiber membranes. A highly sensitive copper ion colorimetric sensor (PTLNFM) was prepared. PTLNFM can chelate Cu2+ in aqueous solution, resulting in changes in UV absorption. It exhibits a good linear relationship in the copper ion concentration range of 9.34 × 10−8 to 1.15 × 10−5 M and the detection limit is 1.27 × 10−8 M to achieve an ultrasensitive response to copper ions (Fig. 3(C) and 4(C)).
 | ||
| Fig. 3 Preparation of copper ion sensor by chemical modification method and their sensing mechanism. (A) Reprinted with permission from ref. 85. Copyright 2013, The Royal Society of Chemistry. (B) Reprinted with permission from ref. 86. Copyright 2016, Elsevier. (C) Reprinted with permission from ref. 87. Copyright 2018, Elsevier. (D) Reprinted with permission from ref. 88. Copyright 2021, American Chemical Society. (E) Reprinted with permission from ref. 89. Copyright 2018, Springer-Verlag GmbH Austria, part of Springer Nature. | ||
 | ||
| Fig. 4 Sensing properties of copper ion sensors prepared by chemical modification. (A) Reprinted with permission from ref. 85. Copyright 2013, The Royal Society of Chemistry. (B) Reprinted with permission from ref. 86. Copyright 2016, Elsevier. (C) Reprinted with permission from ref. 87. Copyright 2018, Elsevier. (D) Reprinted with permission from ref. 88. Copyright 2021, American Chemical Society. (E) Reprinted with permission from ref. 89. Copyright 2018, Springer-Verlag GmbH Austria, part of Springer Nature. | ||
PEI is rich in amino groups and can form tetra-amino-Cu2+ complexes with copper ions, showing a special dark blue color, which can realize copper ion adsorption and colorimetric detection. In 2021, Shao et al.88 grafted branched polyethyleneimine (BPEI) onto the surface of polyacrylonitrile (PAN)-based electrospun nanofibrous membranes to prepare copper ion colorimetrically responsive nanofibrous membrane sensor (aPAN/BPEI NMs). The aPAN/BPEI NMs were able to adsorb copper ions in water, and the color of the fiber membrane changed from yellow to blue. With the addition of copper ions, the UV absorption peaks at 280 nm and 636 nm gradually increased, and showed a good linear relationship in the range of copper ion concentration from 0 to 700 μM. The detection limits of Cu2+ is λ280 nm = 11.5 μM, λ636 nm = 4.8 μM. And it also showed a remarkable adsorption effect on copper ions, and the adsorption capacity reached 209.53 mg g−1 (Fig. 3(D) and 4(D)).
Noble metal nanoparticles can be used for sensor design due to their easy surface modification and good stability. Abedalwafa et al.89 modified Au/Ag NPs with shell–core structure onto porous ammoniated PAN electrospun fibers, and successfully prepared a fiber membrane sensor Au/Ag NPs@aPAN for colorimetric detection of copper ions. With the increase of copper ions, the yellow color of the fiber membrane gradually faded. The mechanism of discoloration is the leaching of Au/Ag NPs from NFM in the presence of ammonium chloride, thiosulfate and Cu2+, forming a soluble complex of Ag+/Au3+/Cu2+-thiosulfate on their surface. The discolored fiber membrane can be restored to its original yellow color by placing it in the Au/Ag NP solution, that is, it can be regenerated. The detection limit of Au/Ag NPs@aPAN for copper ions is 50 nM (Fig. 3(E) and 4(E)).
Grafting on the fiber surface with rhodamine derivatives, Schiff base compounds, gold nanoparticles is a good way to prepare nanofiber membrane sensors that respond to copper ions. This method allows the sensing unit to be distributed on the surface of the fiber, and the detected object can fully contact the identification site. In addition, for some fluorescent dyes that are induced by aggregation, a small amount of grafting on the surface can also slow down the quenching phenomenon.
 | ||
| Fig. 5 Preparation of copper ion sensor by copolymerization and surface adsorption and their sensing mechanism. (A) Reprinted with permission from ref. 90. Copyright 2016, Springer Science Business Media Dordrecht. (B) Reprinted with permission from ref. 91. Copyright 2021 Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Published by Elsevier. | ||
 | ||
| Fig. 6 Sensing properties of copper ion sensors preparted by copolymerization and surface adsorption. (A) Reprinted with permission from ref. 90. Copyright 2016, Springer Science Business Media Dordrecht. (B) Reprinted with permission from ref. 92. Copyright 2015, Senthamizhan, A. et al. | ||
It is also an effective method to prepare the electrospun nanofiber membrane sensor. Moreover, this method makes the copper ion probe less susceptible to detachment from the fiber due to solubility.
Using electrostatic interactions, van der Waals forces, and coordination to adsorb sensors onto nanofiber membrane surfaces is also a method to fabricate probe/nanofiber membrane composite sensors. This method is easier to operate, and the surface-adsorbed probe can quickly contact with copper ions in water, enhancing the sensing efficiency.
Electrospun nanofiber fluorescent sensor for detection of Fe3+
As a transition metal, iron has abundant empty orbitals and is easy to form complexes with many ligands. Therefore, the related iron ion sensor is prepared according to this characteristic.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coordination with the N atom of the naphthalene ring and the carbonyl oxygen of the amide moiety (Fig. 7(A) and 8(C)). Rijin et al.95 doped 4,4′-fluoresceinoxybisphthalonitrile (FPN) into polycaprolactone (PCL) solution. A nanofibrous membrane sensor (PCL/FPN) capable of selectively detecting iron ions in aqueous solution was fabricated by electrospinning technology. The nitrogen atoms on the four cyano groups in the FPN molecule can chelate with two iron ions through weak van der Waals forces, resulting in fluorescence quenching. The response of PCL/FPN to Fe ions was tested in the concentration range of Fe ions from 10 to 70 nM, and the results showed that the detection limit of Fe ions was 2.9413 nM (Fig. 7(B)).
1 coordination with the N atom of the naphthalene ring and the carbonyl oxygen of the amide moiety (Fig. 7(A) and 8(C)). Rijin et al.95 doped 4,4′-fluoresceinoxybisphthalonitrile (FPN) into polycaprolactone (PCL) solution. A nanofibrous membrane sensor (PCL/FPN) capable of selectively detecting iron ions in aqueous solution was fabricated by electrospinning technology. The nitrogen atoms on the four cyano groups in the FPN molecule can chelate with two iron ions through weak van der Waals forces, resulting in fluorescence quenching. The response of PCL/FPN to Fe ions was tested in the concentration range of Fe ions from 10 to 70 nM, and the results showed that the detection limit of Fe ions was 2.9413 nM (Fig. 7(B)).
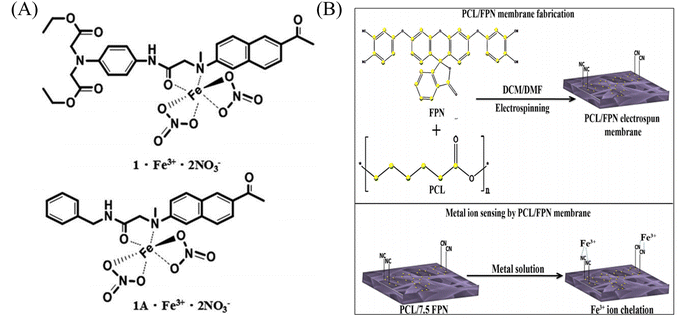 | ||
| Fig. 7 Preparation of copper ion sensor by physical doping method and their sensing mechanism. (A) Reprinted with permission from ref. 94. Copyright 2016, The Royal Society of Chemistry. (B) Reprinted with permission from ref. 95. Copyright 2019, Springer Science Business Media, LLC, part of Springer Nature. | ||
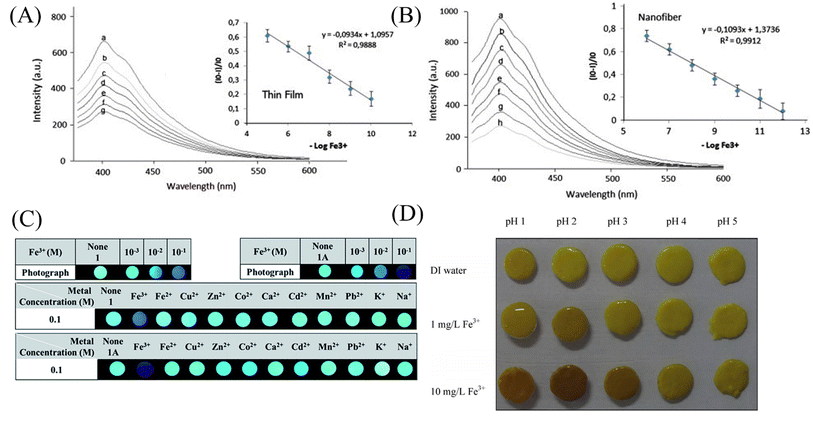 | ||
| Fig. 8 Sensing properties of iron ion sensors preparted by physical doping method. (A) and (B) Reprinted with permission from ref. 93. Copyright 2013, Elsevier. (C) Reprinted with permission from ref. 94. Copyright 2016, The Royal Society of Chemistry. (D) Reprinted with permission from ref. 101. Copyright 2014, Elsevier. | ||
The complexes of rare earth elements usually have good luminescent properties. Lanthanide rare earth MOF materials have the characteristics of large Stokes shift and narrow emission wavelength, which can be used for ion detection.96 Zhou et al.97 prepared an Eu-MOF material using Eu3+ of the lanthanide series. The Eu-MOF@PAN nanofiber membrane sensor responsive to iron ions was prepared by electrospinning Eu-MOF-doped PAN solution. After adsorption of iron ions in Eu-MOF@PAN, effective collisions between Fe3+ and Eu3+ occur. This process will cause the energy of the excited state to be damaged and lead to the quenching of the fluorescence of Eu3+-MOF. The sensor exhibits excellent selectivity and sensitivity for iron ions with a detection limit of 63 nM. Bai et al.98 doped a chain complex (1-Eu3+) formed by Eu3+ and organic molecular ligands into PAN solution, and fabricated an iron ion-responsive nanofibrous membrane sensor (1-Eu3+@PAN). The sensor showed obvious red fluorescence quenching under iron ion treatment, and the detection limit was 6.685 × 10−4 M.
As an environmentally friendly material, biomaterials have good application prospects and can be used in the fields of environment, energy and sensing.99,100 Saithongdee et al.101 prepared a nanofibrous membrane sensor from a curcumin-doped zein solution by electrospinning and amidation cross-linking under the conditions of citric acid and heating. The sensor is able to achieve a colorimetric response to iron ions with a color change from yellow to brown with an optical detection limit of 0.4 mg L−1 (Fig. 8(D)).
Many probe molecules, MOFs, and biological matrices etc. Which can selectively detect iron ions can be mixed with some polymers by means of physical doping to prepare nanofiber membrane sensors that respond to iron ions by electrospinning. This simple mixing method is suitable for a wide range of linear polymers with a wide range of applications and is easy to operate. However, due to simple doping, there is no strong connection between the sensing unit and the polymer, and the polymer and the sensing unit are easily separated by solvents. For some sensing units that are easily soluble in water, they are easy to dissolve in water under the water system, which affects the sensing effect.
Wang et al.102 used chemical modification to graft pyrene derivatives to triblock copolymers to form a pyrene-containing copolymer (PPy-b-PNIPAAm-b-PNMA). The pyrene-containing copolymers were then prepared into nanofibrous membranes by electrospinning. The fibers are aggregated from nanospheres formed by self-assembly of PPy-b-PNIPAAm-b-PNMA copolymer, and the pyrene-containing molecules are on the outermost side of the nanospheres. The fiber membrane can quench the fluorescence in response to iron ions and temperature. Even at the concentration of 10−5 M iron ion, the fluorescence of the fiber membrane can be quenched (Fig. 9(A) and 10(A)). Zhou et al.103 modified coumarin derivatives on copolymers of acrylic acid and acrylonitrile (PANA) to prepare iron ion-responsive nanofibrous membranes (PANADC) by electrospinning. The fiber membrane can be used for the adsorption and detection of iron ions, and the fluorescence is quenched with the addition of iron ions to the fiber membrane, and the detection limit and adsorption amount are 10.63 μM and 13.93 mg g−1, respectively (Fig. 9(B) and 10(B)).
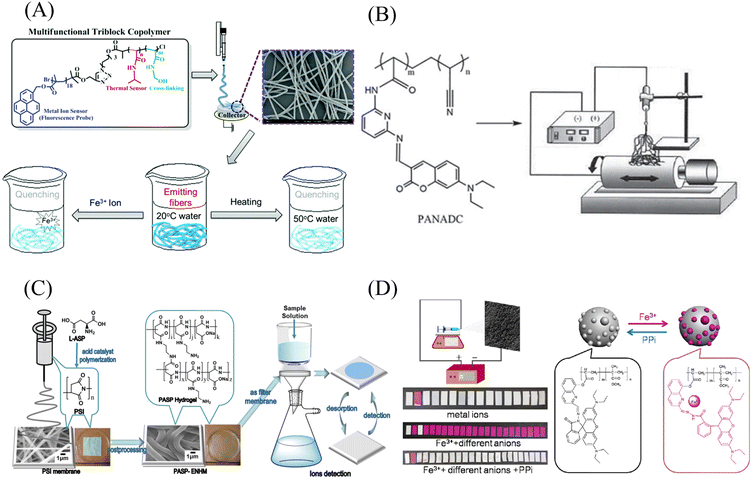 | ||
| Fig. 9 Preparation and sensing mechanism of iron ion sensor by chemical modification and copolymerization. (A) Reprinted with permission from ref. 102. Copyright 2015, The Royal Society of Chemistry. (B) Reprinted with permission from ref. 103. Copyright 2018, Journal of The Brazilian Chemical Society. (C) Reprinted with permission from ref. 104. Copyright 2019, American Chemical Society. (D) Reprinted with permission from ref. 105. Copyright 2015, Elsevier. | ||
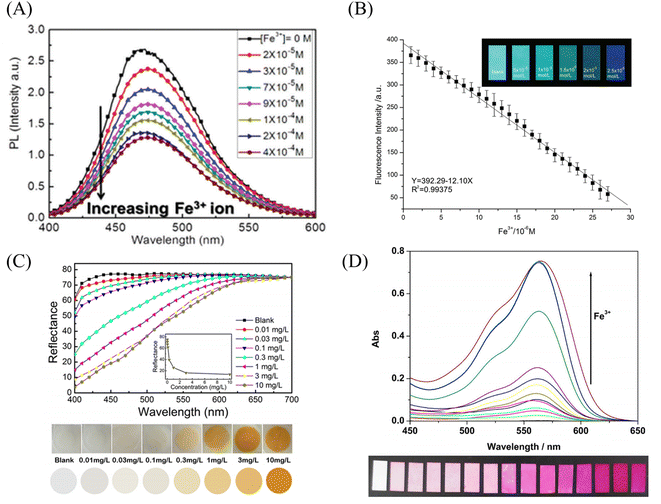 | ||
| Fig. 10 Sensing properties of iron ion sensors preparted by chemical modification and copolymerization. (A) Reprinted with permission from ref. 102. Copyright 2015, The Royal Society of Chemistry. (B) Reprinted with permission from ref. 103. Copyright 2018, Journal of The Brazilian Chemical Society. (C) Reprinted with permission from ref. 104. Copyright 2019, American Chemical Society. (D) Reprinted with permission from ref. 105. Copyright 2015, Elsevier. | ||
Utilizing the adsorption of some amino acids to iron ions can also be used to detect iron ions by chemical cross-linking after some amino acids are prepared into polymers. Zhang et al.104 prepared poly(aspartic acid) (PASP) into nanofibrous membranes by electrospinning and then cross-linked with ethylenediamine to obtain an electrospun fibrous hydrogel film (PASP-ENHM). PASP-ENHM showed a colorimetric change from white to yellow under iron treatment with a detection limit of 0.1 mg L−1. And the fiber membrane can continue to be used for the detection of iron ions after being treated with EDTA (Fig. 9(C) and 10(C)).
Copolymerization method
Directly polymerizing molecular probes with other monomers is also a method for preparing sensing materials. Li et al.105 synthesized a rhodamine Schiff base derivative monomer RQ, RQ and methyl acrylate were prepared by emulsion polymerization to obtain a block copolymer poly(MMA-co-RQ). Poly(MMA-co-RQ) was obtained by electrospinning prepared nano-films stacked with nano-microspheres. The film can be used for rapid colorimetric detection of iron ions from white to pink with a detection limit of 1.19 μM, and can be reused after treatment with ppi solution (Fig. 9(D) and 10(D)).The iron ion sensor prepared by the above chemical modification method has good sensing performance. And it solves the problem that the sensing unit is easy to fall off in the physical co-doping method.
Electrospun nanofiber fluorescent sensor for detection of Hg2+
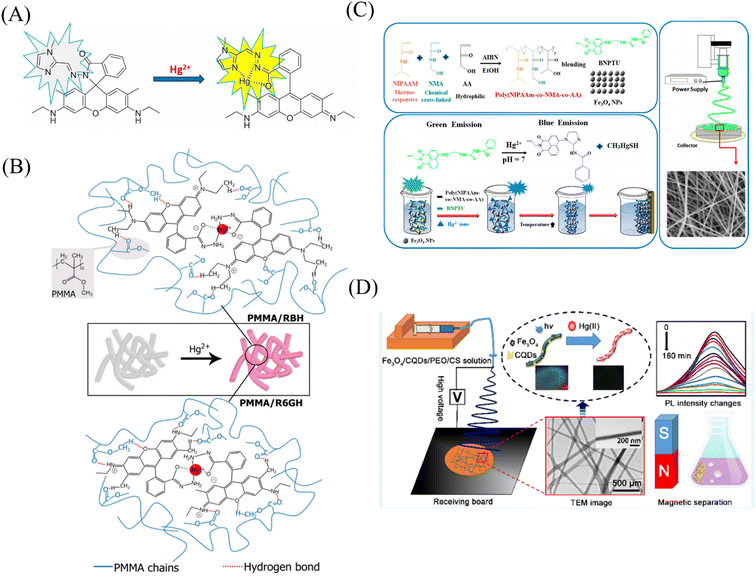
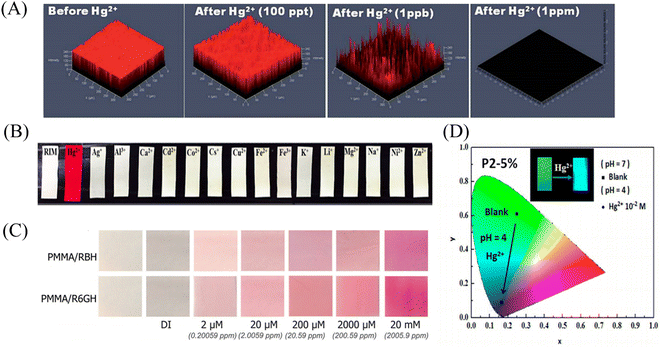
Gold nanoparticles, rhodamine derivatives and quantum dots can form composite fiber membrane sensors that respond to mercury ions through physical doping, chemical modification, surface adsorption, self-assembly, and copolymerization.Rao et al.107 synthesized a mercury ion chemical sensor RIM using rhodamine 6G as a raw material. The RIM and polyurethane were mixed uniformly, and a nanofiber membrane was prepared by electrospinning. The fibrous membrane is able to respond to mercury ion, the mechanism of sensing of mercury ion by RIM is the ring opening of spirolactum resulting in enhanced fluorescence (Fig. 11(A) and 12(B)). Girdthep et al.108 doped rhodamine B hydrazide (RBH) and rhodamine 6G hydrazide (R6GH) into PMMA, respectively. And prepared PMMA/RBH and PMMA/R6G mercury ion colorimetric and fluorescent nanofiber membrane sensor by electrospinning. Both sensors can chelate with mercury ions in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, resulting in a white-to-pink color change and fluorescence enhancement due to the opening of the spiro ring. The detection limits of the PMMA/RBH and PMMA/R6G are 0.8–1.099 ppb and 3.6–4.3 ppb, respectively. The two sensors can be recycled under the treatment of EDA solution (Fig. 11(B) and 12(C)).
1 ratio, resulting in a white-to-pink color change and fluorescence enhancement due to the opening of the spiro ring. The detection limits of the PMMA/RBH and PMMA/R6G are 0.8–1.099 ppb and 3.6–4.3 ppb, respectively. The two sensors can be recycled under the treatment of EDA solution (Fig. 11(B) and 12(C)).
 | ||
| Fig. 11 Preparation and sensing mechanism of mercury ions sensor by physical doping (A) reprinted with permission from ref. 107. Copyright 2017, Elsevier. (B) Reprinted with permission from ref. 108. Copyright 2021, Elsevier. (C) Reprinted with permission from ref. 109. Copyright 2017, MDPI. (D) Reprinted with permission from ref. 110. Copyright 2018, American Chemical Society. | ||
 | ||
| Fig. 12 Sensing properties of mercury ion sensors prepared by physical doping (A) reprinted with permission from ref. 106. Copyright 2015, The Royal Society of Chemistry. (B) Reprinted with permission from ref. 107. Copyright 2017, Elsevier. (C) Reprinted with permission from ref. 108. Copyright 2021, Elsevier. (D) Reprinted with permission from ref. 109. Copyright 2017, MDPI. | ||
Liang et al.109 fabricated a temperature-on–off magnetic nanofiber membrane sensor (P2-5%ESNFs) for mercury ion response. The sensor was prepared by electrospinning by mixing poly (NIPAAm-co-NMA-co-AA) (P2), naphthalimide derivative (BNPTU) and Fe3O4 nanoparticles. P2-5% ESNFs showed the performance of temperature control in response to mercury ions. The temperature change changed the hydrophilic–hydrophobic state of the fiber membrane, and the chelation of mercury ions and BNPTU was more likely to occur in the hydrophilic state of the fiber membrane. Therefore, the on and off effects of temperature are exhibited. The response of P2-5% ESNFs to mercury ions changed from green to blue. The addition of Fe3O4 makes the fiber membrane magnetic for easy removal from water (Fig. 11(C) and 12(D)). Li et al.110 prepared a one-dimensional electrospun fiber membrane material using a mixed solution of polyethylene oxide (PEO), chitosan (CS), Fe3O4 and CQDs. The fiber membrane material can monitor the adsorption of mercury ions by real-time fluorescence monitoring. The adsorption of mercury ions reaches equilibrium within 100 min, and the maximum single-layer adsorption capacity is 148.148 mg g−1. The fluorescence of the fiber membrane was gradually quenched with the adsorption of mercury ions. The fiber membrane is magnetic and can be easily removed from the aqueous solution, due to the addition of Fe3O4 (Fig. 11(D)). Mercury ion probes based on rhodamine derivatives, naphthalimide derivatives, quantum dots and gold nanoparticles can be simply doped into polymers to quickly prepare electrospun fiber membrane sensors with good sensing effects. However, the sensing unit inside the fiber in this preparation method is not used, and the sensing unit on the surface of the fiber is easy to fall off due to the combination of the polymer only by weak molecular force, which affects the sensing effect.
 | ||
| Fig. 13 Preparation and sensing mechanism of mercury ions sensor by chemical modification, self-assembly and surface adsorption (A) reprinted with permission from ref. 111. Copyright 2012, Elsevier. (B) Reprinted with permission from ref. 112. Copyright 2019, American Chemical Society. (C) Reprinted with permission from ref. 113. Copyright 2018, MDPI. (D) Reprinted with permission from ref. 114. Copyright 2016, Elsevier. (E) Reprinted with permission from ref. 115. Copyright 2012, The Royal Society of Chemistry. | ||
 | ||
| Fig. 14 Sensing properties of mercury ion sensors prepared by chemical modification, self-assembly and surface adsorption (A) reprinted with permission from ref. 111. Copyright 2012, Elsevier. (B) Reprinted with permission from ref. 112. Copyright 2019, American Chemical Society. (C) Reprinted with permission from ref. 114. Copyright 2016, Elsevier. (D) Reprinted with permission from ref. 115. Copyright 2014, American Chemical Society. (E) Reprinted with permission from ref. 116. Copyright 2015, Anitha Senthamizhan et al. (F) Reprinted with permission from ref. 117. Copyright 2012, The Royal Society of Chemistry. | ||
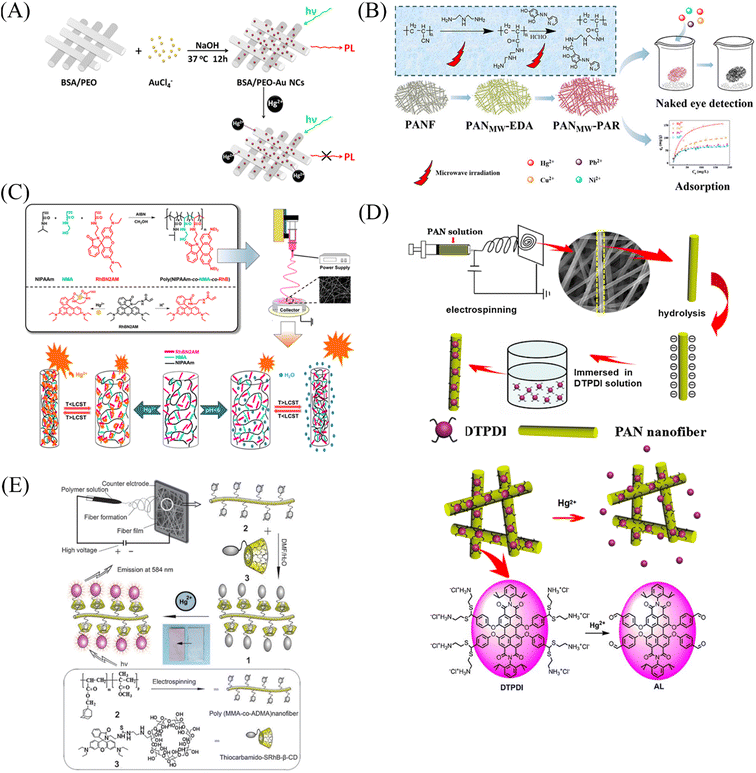
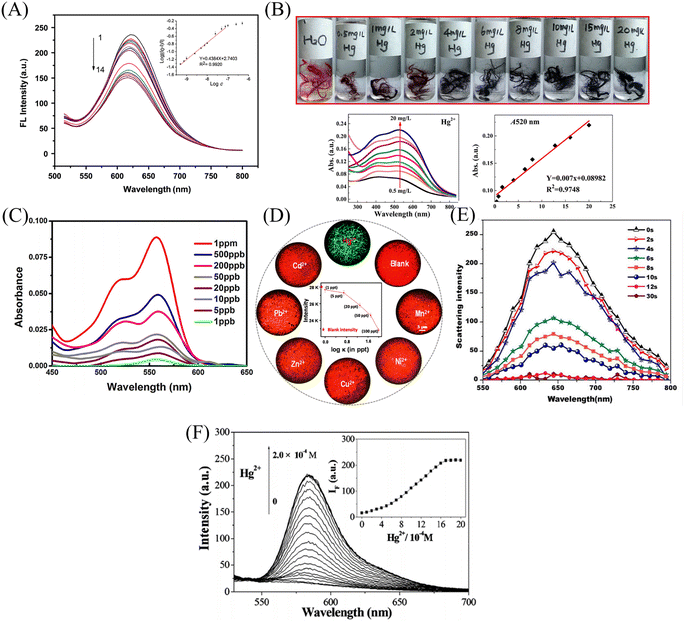
Nanofiber membrane sensors were prepared by copolymerization to form block polymers between mercury ion probes and polymers. It can effectively prevent the probe from wandering into the detection environment, but the probe utilization rate and physical doping method are not much different. Chemical modification, surface adsorption and self-assembly methods can modify the mercury ion sensing unit on the surface of the fiber membrane to increase the utilization rate of the mercury ion sensing unit and reduce the aggregation and quenching phenomenon of fluorescent molecules.
Electrospun nanofiber fluorescent sensor for detection of Pb2+
Some biological matrix materials and noble metal nanoclusters are very useful for preparing lead ion nanofiber membrane sensors by co-doping, chemical modification and other methods.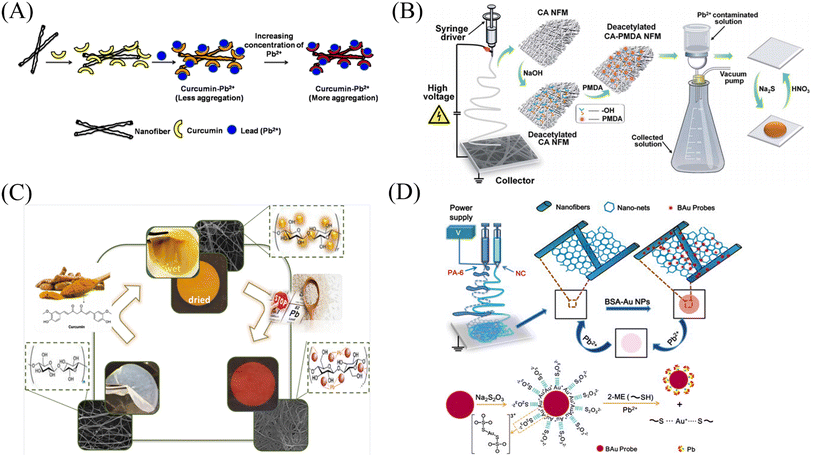 | ||
| Fig. 15 Preparation and sensing mechanism of Pb2+ sensor. (A) Reprinted with permission from ref. 118. Copyright 2015, Elsevier. (B) Reprinted with permission from ref. 119. Copyright 2015, The Royal Society of Chemistry. (C) Reprinted with permission from ref. 120. Copyright 2021, Iranian Chemical Society. (D) Reprinted with permission from ref. 121. Copyright 2013, Elsevier. | ||
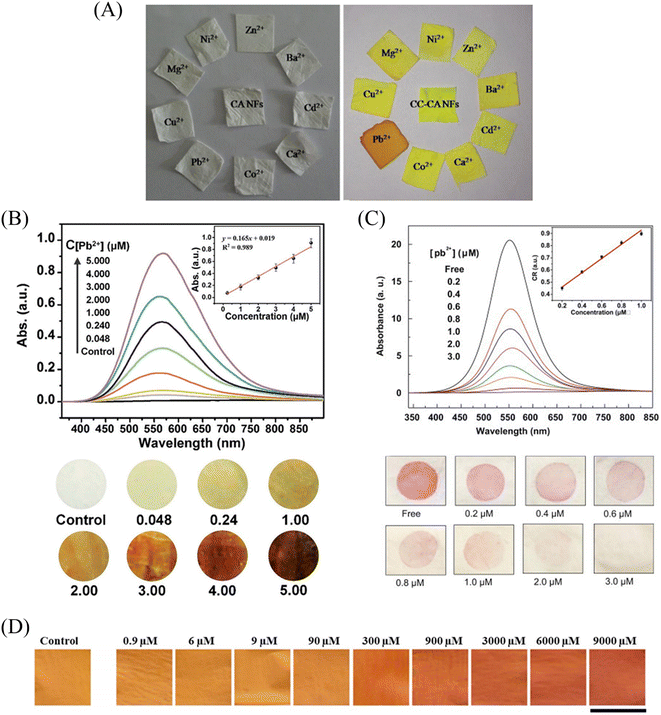 | ||
| Fig. 16 Sensing properties of Pb2+ sensors. (A) Reprinted with permission from ref. 118. Copyright 2015, Elsevier. (B) Reprinted with permission from ref. 119. Copyright 2015, The Royal Society of Chemistry. (C) Reprinted with permission from ref. 121. Copyright 2021, Iranian Chemical Society. (D) Reprinted with permission from ref. 120. Copyright 2013, Elsevier. | ||
Electrospun nanofiber membranes for Al3+ detection
Kim et al.122 blended a rhodamine-based colorimetric-fluorescent chemical sensor (R2PP) with polyurethane to prepare an aluminum ion-responsive fiber membrane sensor by electrospinning. Under the treatment of aluminum ions, the fibrous membrane changed from white to pink and showed a significant enhancement of yellow fluorescence, and the detection limit was 8.5 × 10−9 M. The sensing mechanism is due to the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between R2PP and aluminum ions, which results in the opening of the spiro ring structure of R2PP, which changes the conjugation structure and changes the UV and fluorescence. In addition, it can be reused under the treatment of EDTA (Fig. 17(A), (B) and 18(A), (B)).
1 complex between R2PP and aluminum ions, which results in the opening of the spiro ring structure of R2PP, which changes the conjugation structure and changes the UV and fluorescence. In addition, it can be reused under the treatment of EDTA (Fig. 17(A), (B) and 18(A), (B)).
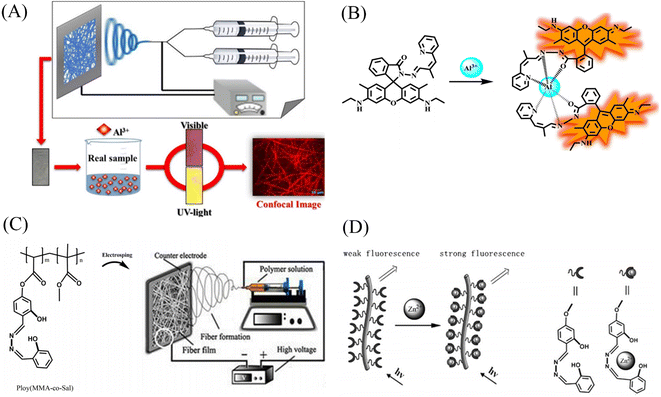 | ||
| Fig. 17 Preparation and sensing mechanism of Zn2+, Al3+ sensor. (A) and (B) Reprinted with permission from ref. 122. Copyright 2016, Elsevier. (C) and (D) Reprinted with permission from ref. 124. Copyright 2017, Chen Zhou et al. | ||
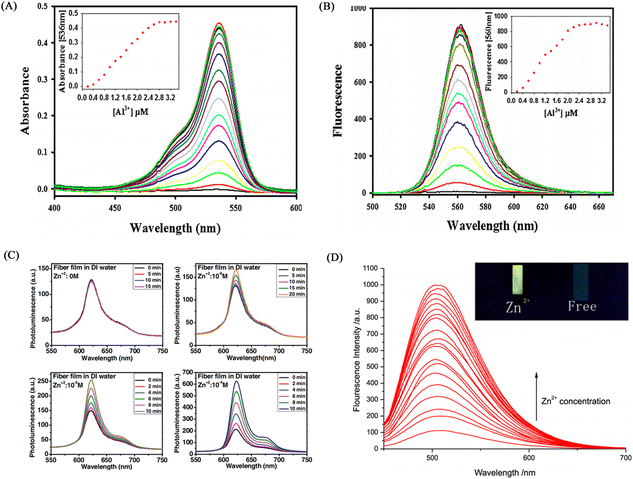 | ||
| Fig. 18 Sensing properties of Zn2+, Al3+ sensor. (A) and (B) reprinted with permission from ref. 122. Copyright 2016, Elsevier. (C) Reprinted with permission from ref. 123. Copyright 2013, Wiley-VCH (D) reprinted with permission from ref. 124. Copyright 2017, Chen Zhou et al. | ||
Electrospun nanofiber membranes for Zn2+ detection
Syu et al.123 doped the zinc ion probe meso-2,6-dichlorophenyltripyrrinone (TPN-Cl2) into poly(2-hydroxyethyl methacrylate) (PHEM) solution and fabricated a fiber membrane sensor by electrospinning. The sensor can respond to zinc ions in the normal physiological range, and the time resolution can reach 5 min at the zinc ion concentration of 10−6 M (Fig. 18(C)). Zhou et al.124 obtained poly (MMA-co-Sal) by polymerizing and modifying 2-hydroxy-4-acryloyloxy-benzaldehyde (HAB), methyl methacrylate (MMA) and salicylaldehyde-hydrazine as raw materials. The polymer poly (MMA-co-Sal) can be prepared into nanofibrous membranes by electrospinning technology. The fiber membrane can be used for fluorescence detection and adsorption of zinc ions. The fluorescence at 504 nm gradually increased with the addition of zinc ions, the detection limit was 1.95 × 10−5 mol L−1, and the adsorption capacity was 11.45 mg g−1 (Fig. 17(C), (D) and 18(D)).Regardless of the form of binding, the sensing of metal ions is always inseparable from the coordination and oxidation of metal ions. Therefore, the design of metal ion sensing mainly considers the influence of the way the sensing unit is introduced on the sensor performance.
Electrospun nanofiber fluorescent sensor for detection of harmful anions
The preparation of electrospun fiber membrane sensor for harmful anion detection can utilize physical doping, chemical grafting, and host–guest self-assembly to combine CDs, organic molecular probes, and noble metal nanoparticles with polymers.Electrospun nanofiber fluorescent sensor for detection of CN−
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone = 2
acetone = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
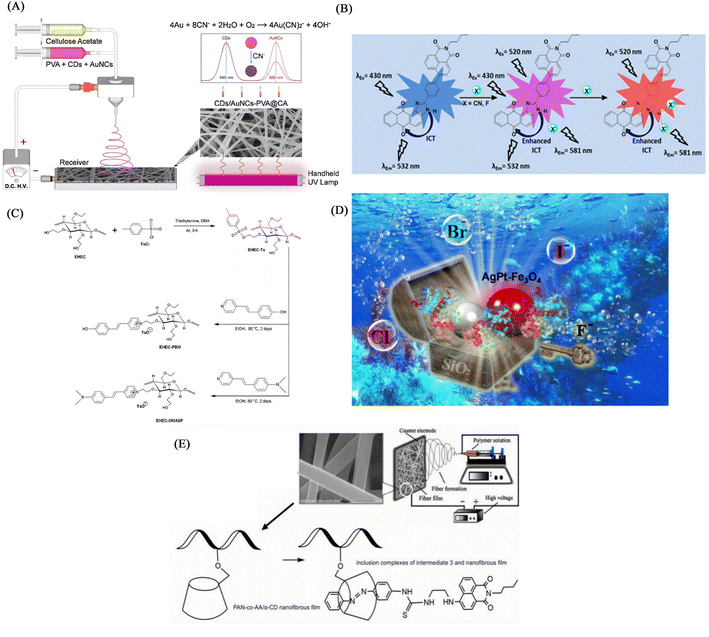
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of cellulose acetate for 30 minutes, then used the PVA aqueous solution in which CDs and AuNC were uniformly dispersed for 30 minutes, and finally used the organic cellulose acetate solution. Solution electrospinning for 30 min. A fluorescent nanofiber membrane sensor (CDs/AuNCs-PVA@CA NFM) for ratiometric detection of cyanide was successfully fabricated. The CDs/AuNCs-PVA@CA NFM fluorescence intensity of 445 nm/650 nm gradually increased with the addition of cyanide, showing a ratiometric detection of cyanide. Since the relative content of Au (0) decreased due to the etching effect of cyanide on AuNCs, the fluorescence intensity at 650 nm of AuCNs decreased, while the fluorescence intensity at 445 nm of CDs was not affected, so NFM showed the effect of ratiometric detection. Experiments show that the detection limit of NFM is 0.15 μM, which is much lower than the WHO standard for cyanide content in drinking water (Fig. 19(A) and 20(A)).
1 solution of cellulose acetate for 30 minutes, then used the PVA aqueous solution in which CDs and AuNC were uniformly dispersed for 30 minutes, and finally used the organic cellulose acetate solution. Solution electrospinning for 30 min. A fluorescent nanofiber membrane sensor (CDs/AuNCs-PVA@CA NFM) for ratiometric detection of cyanide was successfully fabricated. The CDs/AuNCs-PVA@CA NFM fluorescence intensity of 445 nm/650 nm gradually increased with the addition of cyanide, showing a ratiometric detection of cyanide. Since the relative content of Au (0) decreased due to the etching effect of cyanide on AuNCs, the fluorescence intensity at 650 nm of AuCNs decreased, while the fluorescence intensity at 445 nm of CDs was not affected, so NFM showed the effect of ratiometric detection. Experiments show that the detection limit of NFM is 0.15 μM, which is much lower than the WHO standard for cyanide content in drinking water (Fig. 19(A) and 20(A)).
 | ||
| Fig. 19 Preparation and sensing mechanism of CN−, F-sensor (A) reprinted with permission from ref. 125. Copyright 2019, Elsevier. (B) Reprinted with permission from ref. 126. Copyright 2018, Elsevier. (C) Reprinted with permission from ref. 127. Copyright 2020, Elsevier. (D) Reprinted with permission from ref. 128. Copyright 2022, American Chemical Society. (D) Reprinted with permission from ref. 129. Copyright 2021, Huan Zhang et al. | ||
 | ||
| Fig. 20 Sensing properties of CN−, F-sensor (A) reprinted with permission from ref. 125. Copyright 2019, Elsevier. (B) Reprinted with permission from ref. 126. Copyright 2018, Elsevier. (c) Reprinted with permission from ref. 127. Copyright 2020, Elsevier. (D) Reprinted with permission from ref. 128. Copyright 2022, American Chemical Society. (E) Reprinted with permission from ref. 129. Copyright 2021, Huan Zhang et al. | ||
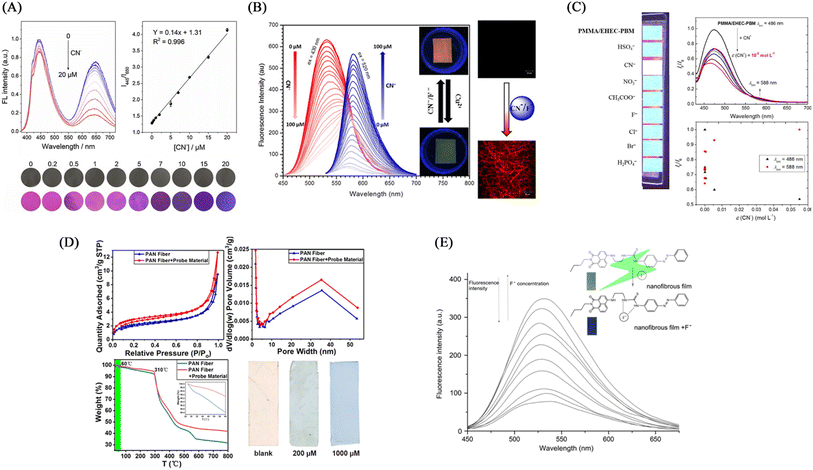
Kim et al.126 synthesized an imidazole derivative (M) based on naphthalimide and anthraquinone for cyanide detection. A cyanide detection test strip was prepared by blending M and polyurethane in dimethylacetamide and electrospinning. The test strip showed the effect of colorimetric and fluorescence detection under the treatment of cyanide. It changes from green to orange under visible light, and exhibits an orange–red fluorescence enhancement under UV light irradiation. The reason for this change is that the cyanide can strongly interact with the H on the N–H unit of imidazole and dissociate the proton, resulting in an enhanced ICT effect but a sharp change in UV and fluorescence (Fig. 19(B) and 20(B)).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (wt/wt) to obtain a cyanide fiber membrane sensor PMMA/EHEC-PBM by electrospinning. PMMA/EHEC-PBM has good hydrophilicity and can be used for the detection of enhancers in total aqueous solution. With the addition of cyanide, the fluorescence at 486 nm is gradually quenched, and the fluorescence at 588 nm is gradually enhanced. The sensing mechanism is due to the change of fluorescence caused by the proton detachment of the phenolic hydroxyl group in the presence of cyanide.31 The detection limit and quantification limit of this sensor were 2.15 × 10−5 and 7.17 × 10−5 mol L−1, respectively (Fig. 19(C) and 20(C)).
2 (wt/wt) to obtain a cyanide fiber membrane sensor PMMA/EHEC-PBM by electrospinning. PMMA/EHEC-PBM has good hydrophilicity and can be used for the detection of enhancers in total aqueous solution. With the addition of cyanide, the fluorescence at 486 nm is gradually quenched, and the fluorescence at 588 nm is gradually enhanced. The sensing mechanism is due to the change of fluorescence caused by the proton detachment of the phenolic hydroxyl group in the presence of cyanide.31 The detection limit and quantification limit of this sensor were 2.15 × 10−5 and 7.17 × 10−5 mol L−1, respectively (Fig. 19(C) and 20(C)).Electrospun nanofiber fluorescent sensor for detection of F−
As a strong electronegative element, F− easily forms a hydrogen bond with N–H or O–H to deprotonate the molecule, thereby causing the change of the conjugated structure and the change of fluorescence and color. In addition, fluoride, as a lewis acid, can react with silicon-containing compounds, and fluoride sensors can be designed according to this characteristic.Electrospun nanofiber sensor for detection of ClO−
Virginia et al.130 used electrospinning technology to encapsulate active chlorine-responsive graphene quantum dots (GQDs) in PAN to prepare a nanofibrous membrane chemical sensor with superior stability. The GQDs-PAN nanofibrous membrane exhibited irreversible fluorescence quenching effect and excellent selectivity under free active chlorine treatment. The GQDs-PAN nanofibrous membrane exhibited better photostability and lower detection limit than the single GQDs solution, also had good luminescence performance under light irradiation for 2 months and the detection limit for hypochlorite was as low as 2 μM. Based on its excellent stability and low detection limit, it has a good application prospect in practical environmental monitoring (Fig. 21(A)).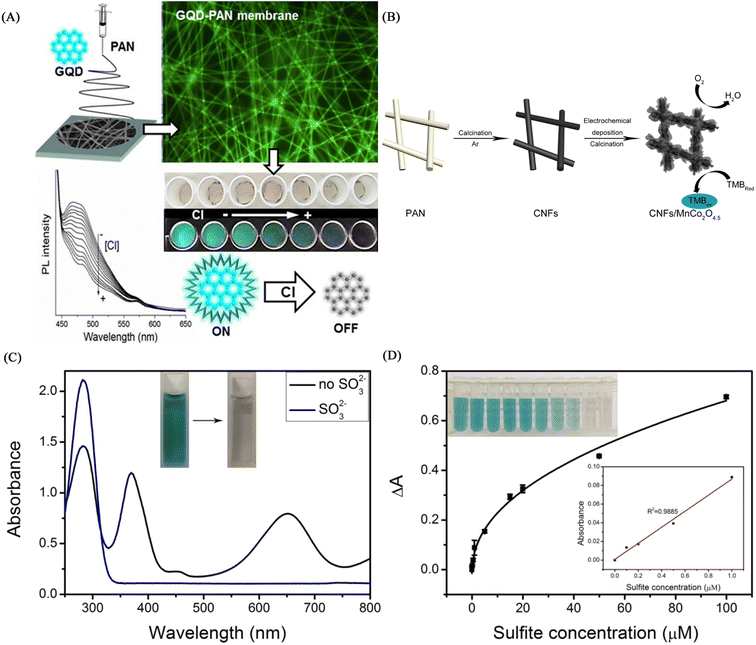 | ||
| Fig. 21 Fabrication and properties of ClO−, So32−nanofiber membrane sensors (A) reprinted with permission from ref. 130. Copyright 2018, Elsevier. (B), (C) and (D) reprinted with permission from ref. 131. Copyright 2017, IOP Publishing Ltd. | ||
Electrospun nanofiber sensor for detection of SO32−
Gao et al.131 prepared a CNFs/MnCo2O4.5 composite fiber membrane material by electrospinning technology and electrochemical deposition technology. The fiber membrane can be used for the colorimetric detection of sulfite, showing a good linear relationship in the sulfite concentration range of 0–1 μM, and the detection limit was calculated to be 15.9 nM. Its excellent colorimetric effect and high sensitivity enable the application of manganese cobalt oxide/carbon nanofiber composites in chemical sensing (Fig. 21(B), (C) and (D)).Cyanide, as a strong electron-withdrawing ion, can achieve sensing by forming strong interactions with active N–H and phenolic hydroxyl groups to deprotonate them, resulting in changes in fluorescence and color. It is also possible to use the etching effect of cyanide on the metal to cause the change of fluorescence to realize the sensing of the reinforcement. Hypochlorite and sulfite except according to the inorganic sensing unit described above. It is also possible to use the oxidation of hypochlorite to design some organic dyes containing sulfur and selenium as sensing units, or to use the nucleophilicity of sulfites to design organic dyes containing carbon positive structure as sensing units.
Conclusion and future prospects
In this paper, we summarize and compare the recent advances in electrospun fiber membrane chemical sensors in the field of ion detection in the past decade (Table 1). The preparation of electrospun fiber membrane sensors is a unit that will be able to act as sensing through physical doping, chemical modification, copolymerization, surface adsorption and self-assembly, such as organic molecular probes, MOFs, carbon quantum dots, noble metal nanoparticles and natural biomaterials etc. are immobilized on the electrospun fibers.| Analyte | Fiber material | Sensing unit | Preparation | Sensor types | LOD | Repeatability | Ref. |
|---|---|---|---|---|---|---|---|
| Cu2+ | PES | Rhodamine derivatives | Physical doping | Fluorescence/colorimetric | 1.1 nM | Yes | 79 |
| Cu2+ | CS/PAN | Rhodamine derivatives | Physical doping | Colorimetric | 10−8 M | Yes | 80 |
| Cu2+ | PMMA | RB | Physical doping | Fluorescence/colorimetric | 0.11 μM/0.28 μM | Yes | 81 |
| Cu2+/Cr3+ | CA | 1,4-DHAQ | Physical doping | Fluorescence | 3 × 10−9 M | Yes | 82 |
| Cu2+ | Poly (NIPAAm-co-NMA) | F-phen | Physical doping | Fluorescence | — | No | 83 |
| Cu2+ | PMMA | PEI-EAS NPs | Physical doping | Fluorescence | — | Yes | 84 |
| Cu2+ | PMAR | Rhodamine B-hydrazine | Chemical modification | Fluorescence/colorimetric | 1.5 × 10−6 M | Yes | 85 |
| Cu2+/Hg2+ | P(HEMA-co-NMA) | PyDAN2/RhBN2 | Chemical modification | Fluorescence | 10−7 M/10−6 M | Yes | 86 |
| Cu2+ | PVA | L | Chemical modification | Colorimetric | 1.27 × 10−8 M | No | 87 |
| Cu2+ | aPAN | BPEI | Chemical modification | Colorimetric | 11.5 μM/4.8 μM | No | 88 |
| Cu2+ | aPAN | Au/Ag NPs | Chemical modification | Colorimetric | 50 nM | Yes | 89 |
| Cu2+ | PNNR | RHPMA | Copolymerization | Fluorescence | — | Yes | 90 |
| Cu2+ | PEI/PVA | CdTe-QDs | Surface adsorption | Fluorescence | 11.1 nM | No | 91 |
| Cu2+ | CA | DTT.AuNC | Surface adsorption | Fluorescence | 16 μM | No | 92 |
| Fe3+ | EC | CBINH | Physical doping | Fluorescence | 7 × 10−14 M | Yes | 93 |
| Fe3+ | PMMA | 1/1A | Physical doping | Fluorescence | 174 ppb/59 ppb | No | 94 |
| Fe3+ | PCL | FPN | Physical doping | Fluorescence | 2.9413 nM | No | 95 |
| Fe3+ | PAN | Eu-MOF | Physical doping | Fluorescence | 63 nM | No | 97 |
| Fe3+ | PAN | 1-Eu3+ | Physical doping | Fluorescence | 6.685 × 10−4 M | No | 98 |
| Fe3+ | Zein | Curcumin | Physical doping | Colorimetric | 0.4 mg L−1 | No | 99 |
| Fe3+ | PPy-b-PNIPAAm-b-PNMA | PPy | Chemical modification | Fluorescence | — | No | 102 |
| Fe3+ | PANA | Coumarin derivatives | Chemical modification | Fluorescence | 10.63 μM | No | 103 |
| Fe3+ | PASP | PASP | Chemical modification | Colorimetric | 0.1 mg L−1 | No | 104 |
| Fe3+ | Poly (MMA-co-RQ) | RQ | Copolymerization | Colorimetric | 1.19 μM | Yes | 105 |
| Hg2+ | PVA | AuCN | Physical doping | Fluorescence | 1 ppb | No | 106 |
| Hg2+ | Polyurethane | RIM | Physical doping | Fluorescence | — | No | 107 |
| Hg2+ | PMMA | RBH/R6GH | Physical doping | Fluorescence/colorimetric | 0.8 ppb/3.6 ppb | Yes | 108 |
| Hg2+ | Poly(NIPAAm-co-NMA-co-AA) | BNPTU | Physical doping | Fluorescence | — | No | 109 |
| Hg2+ | PEO/CS | CQDs | Physical doping | Fluorescence | — | Yes | 110 |
| Hg2+ | BSA/PEO | Au–NCs | Chemical modification | Fluorescence | 57 pM | No | 111 |
| Hg2+ | PAN | PAR | Chemical modification | Colorimetric | 35 μg L−1 | No | 112 |
| Hg2+ | P(NIPAAm-co-NMA-co-RhBN2AM) | RhBN2AM | Copolymerization | Fluorescence | — | Yes | 113 |
| Hg2+ | PAN | DTPDI | Surface adsorption | Colorimetric | 1 ppb | No | 114 |
| Hg2+ | N6/FITC | Au@BSA | Surface adsorption | Fluorescence | 1 ppt | No | 115 |
| Hg2+ | PCL-NF | AuNC | Surface adsorption | Fluorescence | ppt level | No | 116 |
| Hg2+ | Poly(MMA-co-ADMA) | Thiocarbamido-SRhB-β-CD | Self-assembly | Fluorescence/colorimetric | 6.0 × 10−5 mol L−1 | No | 117 |
| Pb2+ | CA | Curcumin | Physical doping | Colorimetric | 20 μM | No | 118 |
| Pb2+ | DCA | PMAD | Chemical modification | Colorimetric | 0.048 mM | Yes | 119 |
| Pb2+ | Bacterial cellulose | Curcumin | Surface adsorption | Colorimetric | 9 μM/0.9 μM | No | 120 |
| Pb2+ | PA-6/NC | BAu probe | Surface adsorption | Colorimetric | 0.2 μM | Yes | 121 |
| Al3+ | Polyurethane | R2PP | Physical doping | Fluorescence | 8.5 × 10−9 M | Yes | 122 |
| Zn2+ | PHEM | TPN-Cl2 | Physical doping | Fluorescence | — | No | 123 |
| Zn2+ | Poly (MMA-co-Sal) | HAB | Copolymerization | Fluorescence | 1.95 × 10−5 mol L−1 | No | 124 |
| CN− | PVA@CA | CDs/AuNC | Physical doping | Fluorescence | 0.15 μM | No | 125 |
| CN−/F− | Polyurethane | M | Physical doping | Fluorescence/colorimetric | — | No | 126 |
| CN− | PMMA/EHCE | PBM | Chemical modification | Fluorescence | 2.15 × 10−5 M | No | 127 |
| F− | PAN | AgPt–Fe3O4@SiO2 | Physical doping | Colorimetric | 3.73 μM | No | 128 |
| F− | PAN-co-AA/β-CD | Intermediate 3 | Self-assembly | Fluorescence | — | No | 129 |
| ClO− | PAN | GQDs | Physical doping | Fluorescence | 2 μM | No | 130 |
| SO32− | CNFs | MnCo2O4.5 | Chemical deposition | Colorimetric | 15.9 nM | No | 131 |
In conclusion, electrospun nanofiber membranes for ion detection can be prepared using physical doping, copolymerization, chemical modification, self-assembly and surface adsorption. Among them, the physical doping method is the simplest and the widest range of use, but there are also shortcomings such as low utilization rate of the sensing unit and easy separation of the sensing unit from the polymer. The copolymerization method enables the sensing unit and the polymer to be tightly bonded, but the low utilization of the sensing unit is not improved. Chemical modification, surface adsorption and self-assembly can solve the above problems and improve the utilization rate of the sensing unit while making the combination more stable. This provides some ideas for the development of ion electrospun fiber sensors. The electrospun fiber membrane sensor can rapidly and sensitively detect metal ions and anions in water by colorimetric-fluorescence, due to the large specific surface area and high permeability of the fiber membrane and most of the fiber membrane sensors also have good regeneration ability. It is not easy to cause pollution in the process of detecting harmful substances and it has the characteristics of environmental friendliness, because solid-state sensors can be easily separated from water.
However, there are some challenges in the development of electrospun membrane sensors. At present, most of the electrospun fibers use polymers that are not easy to degrade, which are easy to form microplastics in the environment. The pollution of microplastics has become a major environmental problem in today's world. Although electrospun membrane sensors have a very high specific surface area and porosity, the heterogeneous system formed with water limits the contact between the detected ions and the sensor itself. Therefore, the detection limit of the sensor is still relatively high, and the detection of trace ions cannot be completed. In addition, the current electrospun fiber membranes for ion detection mainly focus on the detection of metal ions, but for the case of water pollution, some electrospun fiber membranes for common highly toxic anion detection have not been reported. The reusability of the sensor is an important indicator. However, only a very small number of electrospun fiber membrane sensors have been reported to study their reusability (Scheme 2).
At the same time as the challenges, there are many opportunities for electrospun membrane sensors. There are many polymeric materials with good degradability. Examples include chitosan, cellulose, protein and amino acid polymers.132 These polymeric materials can be decomposed by microorganisms in the environment into environmentally friendly inorganic substances such as carbon dioxide, nitrogen and water. Moreover, in order to improve the detection sensitivity of nanofiber membrane sensors to various ions, electrochemical methods can be introduced to improve the sensing sensitivity. Alternatively, a fiber membrane material that can enrich ions in water can be designed based on the electrostatic interaction of anions and cations so that low concentrations of ions can also respond to the fiber membrane sensor.133–135 Expanding the detection of ion types of sensors is also the future development direction of electrospun fiber membrane sensors. Some anions containing arsenic and chromium in the environment pose a serious threat to human health and the environment. It is necessary to design fiber membranes that can efficiently detect and remove these ions. Finally, for the sustainable development of electrospun membrane sensors, exploring their reusability is also a highlight.
Author contributions
Liangqiang Wu: conceptualization; writing–original draft; investigation, Yan Song: writing – review & editing; supervision, Shuo Xing: resources; investigation, Yapeng Li: resources; writing – review & editing, Hai Xu: writing – review & editing, Qingbiao Yang: writing – review & editing; funding acquisition, supervision, Yaoxian Li: supervision; writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks to the generous funding of the National Natural Science Foundation of China (No. 21174052) for this research.Notes and references
- J. J. Kim, Y. S. Kim and V. Kumar, J. Trace Elem. Med. Biol., 2019, 54, 226–231 CrossRef CAS PubMed.
- Y. Y. Si, J. N. Li, B. Cui, D. J. Tang, L. Yang and V. Murugadoss, et al., Adv. Compos. Hybrid Mater., 2022, 5(2), 1180–1195 Search PubMed.
- A. T. Ubando, A. D. M. Africa, M. C. Maniquiz-Redillas, A. B. Culaba, W. H. Chen and J. S. Chang, J. Hazard. Mater., 2021, 402, 12341 CrossRef.
- C. Wang, H. M. Hu, M. Chen, Q. Wang, C. Liu and M. F. Chen, et al., J. Clean. Prod., 2022, 340, 130747 CrossRef CAS.
- W. W. Zhou, G. Liu, B. Yang, Q. Y. Ji, W. M. Xiang and H. He, et al., Sci. Total Environ., 2021, 780, 146483 CrossRef CAS.
- N. E. Hellman and J. D. Gitlin, Annu. Rev. Nutr., 2002, 22, 439 Search PubMed.
- S. Lutsenko, N. L. Barnes, M. Y. Bartee and O. Y. Dmitriev, Physiol. Rev., 2007, 87, 1011 CrossRef CAS.
- R. Sladek, G. Rocheleau, J. Rung, C. Dina, L. Shen, D. Serre, P. Boutin, D. Vincent, A. Belisle, S. Hadjadj, B. Balkau, B. Heude, G. Charpentier, T. J. Hudson, A. Montpetit, A. V. Pshezhetsky, M. Prentki, B. I. Posner, D. J. Balding, D. Meyre, C. Polychronakos and P. Froguel, Nature, 2007, 445, 881–885 CrossRef CAS.
- A. Waseem, J. Arshad, F. Iqbal, A. Sajjad, Z. Mehmood and G. Murtaza, BioMed Res. Int., 2014, 2014, 813206 Search PubMed.
- J. S. Valentine and P. J. Hart, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3617–3622 CrossRef CAS PubMed.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 Search PubMed.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed.
- Z. Guo, G. H. Kim, I. Shin and J. Yoon, Biomaterials, 2012, 33, 7818–7827 CrossRef CAS PubMed.
- X. Fang, H. Li, G. Zhao, X. Fang, J. Xu and W. Yang, Biosens. Bioelectron., 2013, 42, 308–313 Search PubMed.
- M. Hosseini, A. Ghafarloo, M. R. Ganjali, F. Faridbod, P. Norouzi and M. S. Niasari, Sens. Actuators, B, 2014, 198, 411–415 Search PubMed.
- D. Noy, I. Solomonov, O. Sinkevich, T. Arad, K. Kjaer and I. Sagi, J. Am. Chem. Soc., 2008, 130, 1376–1383 Search PubMed.
- P. O. Tsvetkov, I. A. Popov, E. N. Nikolaev, A. I. Archakov, A. A. Makarov and S. A. Kozin, ChemBioChem, 2008, 9, 1564–1567 CrossRef CAS PubMed.
- S. Chakraborty, M. Mandal and S. Rayalu, Chem. Commun., 2020, 121, 108189 CAS.
- X. Nan, Y. Huyan, H. Li, S. Sun and Y. Xu, Coord. Chem. Rev., 2021, 426, 213580 CrossRef CAS.
- S. K. Sahoo and G. Crisponi, Molecules, 2019, 24, 3267 CrossRef CAS PubMed.
- J. Zhu, L. Lu, M. Wang, T. Sun, Y. Huang, C. Wang, W. Bao, M. Wang, F. Zou and Y. Tang, Tetrahedron Lett., 2020, 61, 151893 Search PubMed.
- J. Fu, K. Yao, Y. Chang, B. Li, L. Yang and K. Xu, Spectrochim. Acta, Part A, 2019, 222, 117234 Search PubMed.
- X. He, W. Xiong, L. Zhang, C. Xu, J. Fan, Y. Qian, J. Wen, F. Ding and J. Shen, Dyes Pigments, 2020, 174, 108059 Search PubMed.
- H. Ali, E. Khan and I. Ilahi, J. Chem., 2019, 2019, 1–14 Search PubMed.
- X. Li, X. Lan, W. Liu, X. Cui and Z. Cui, J. Hazard. Mater., 2020, 395, 122676 CrossRef CAS.
- M. Lu, R. Xiao, X. Zhang, J. Niu, X. Zhang and Y. Wang, Biosens. Bioelectron., 2016, 85, 267–271 Search PubMed.
- K. M. Rice, E. M. Walker, Jr., M. Wu, C. Gillette and E. R. Blough, J. Prev. Med. Public. Health., 2014, 47, 74–83 Search PubMed.
- R. Verma and B. D. Gupta, Food Chem., 2015, 166, 568–575 CrossRef CAS PubMed.
- Y. Zhou, X. He, H. Chen, Y. Wang, S. Xiao, N. Zhang, D. Li and K. Zheng, Sens. Actuators, B, 2017, 247, 626–631 CrossRef CAS.
- K. H. Jung and K. H. Lee, Anal. Chem., 2015, 87, 9308–9314 Search PubMed.
- L. G. Nandi, C. R. Nicoleti, V. G. Marini, I. C. Bellettini, S. R. Valandro, C. C. S. Cavalheiro and V. G. Machado, Carbohydr. Polym., 2017, 157, 1548–1556 CrossRef CAS PubMed.
- W.-J. Qu, W.-T. Li, H.-L. Zhang, T.-B. Wei, Q. Lin, H. Yao and Y.-M. Zhang, Sens. Actuators, B, 2017, 241, 430–437 Search PubMed.
- S. L. Ashok Kumar, M. Saravana Kumar, P. B. Sreeja and A. Sreekanth, Spectrochim. Acta, Part A, 2013, 113, 123–129 Search PubMed.
- H. Chen, M. Yan, X. Yang, Z. Chen, G. Wang, D. Schmidt-Vogt, Y. Xu and J. Xu, J. Hazard. Mater., 2012, 235–236, 201–209 CrossRef CAS PubMed.
- W.-H. Ding, D. Wang, X.-J. Zheng, W.-J. Ding, J.-Q. Zheng, W.-H. Mu, W. Cao and L.-P. Jin, Sens. Actuators, B, 2015, 209, 359–367 Search PubMed.
- X. Wang, T. Li and C. Ma, J. Hazard. Mater., 2021, 413, 125384 CrossRef CAS.
- Y. L. Pak, S. J. Park, G. Song, Y. Yim, H. Kang and H. M. Kim, et al., Anal. Chem., 2018, 90(21), 12937–12943 CrossRef CAS PubMed.
- G. P. Kotchey, J. A. Gaugler, A. A. Kapralov, V. E. Kagan and A. Star, J. Mater. Chem., 2013, 1(3), 302–309 Search PubMed.
- C. Gorrini, I. S. Harris and T. W. Mak, Nat. Rev. Drug Discov., 2013, 12(12), 931–947 Search PubMed.
- J. Yang, X. Zhang, P. Yuan, J. Yang, Y. Xu and J. Grutzendler, et al., Proc. Natl. Acad. Sci. U. S. A., 2017, 114(47), 12384–12389 CrossRef CAS.
- V. S. Lin, W. Chen, M. Xian and C. J. Chang, Chem. Soc. Rev., 2015, 44(14), 4596–4618 RSC.
- K. Shimamoto and K. Hanaoka, Nitric Oxide, 2015, 46, 72–79 Search PubMed.
- E. C. Ferreira, D. b. M. B. P. Milori, E. J. Ferreira, L. M. dos Santos, L. Martin-Neto and A. R. d. A. j. Nogueira, Talanta, 2011, 85, 435–440 CrossRef CAS PubMed.
- B. Li, X. Zou, H. Wang, Y. Lu, C. Shen and Y. Chu, Anal. Biochem., 2019, 581, 113344 Search PubMed.
- F. Diaz, Y. Wang, R. Weyhe and B. Friedrich, Waste Manag., 2019, 84, 102–111 CrossRef CAS.
- J. Shang, M. Zhao, H. Qu, H. Li, R. Gao and S. Chen, J. Electroanal. Chem., 2019, 855, 113624 CrossRef CAS.
- S. Dong, L. Zhang, Y. Lin, C. Ding and C. Lu, Analyst, 2020, 145, 5068–5089 RSC.
- L. Zhu, J. Nie, Q. Li, J. Du, X. Fan, F. Bai, Q. Yang, Y. Shan and Y. Li, J. Lumin., 2019, 215, 116620 CrossRef CAS.
- H. J. Yoon, D. H. Jun, J. H. Yang, Z. Zhou, S. S. Yang and M. M.-C. Cheng, Sens. Actuators, B, 2011, 157, 310–313 Search PubMed.
- V. Patel, P. Kruse and P. R. Selvaganapathy, Biosensors, 2021, 11, 9 CrossRef CAS PubMed.
- J. Valtera, T. Kalous, P. Pokorny, O. Batka, M. Bilek, J. Chvojka, P. Mikes, E. K. Kostakova, P. Zabka, J. Ornstova, J. Beran, A. Stanishevsky and D. Lukas, Sci. Rep., 2019, 9, 1801 CrossRef.
- Z. Jiang, H. Zhang, M. Zhu, D. Lv, J. Yao, R. Xiong and C. Huang, J. Appl. Polym. Sci., 2018, 135, 45766 Search PubMed.
- D. H. Reneker and I. Chun, Nanotechnology, 1996, 7, 216–223 CrossRef CAS.
- Y. Dzenis, Science, 2004, 304, 1917–1919 Search PubMed.
- X. Ding, Y. Li, Y. Si, X. Yin, J. Yu and B. Ding, Compos. Commun., 2019, 13, 57–62 CrossRef.
- D. Lv, M. Zhu, Z. Jiang, S. Jiang, Q. Zhang, R. Xiong and C. Huang, Macromol. Mater. Eng., 2018, 303, 1800336 CrossRef.
- W. Xu, X. Hu, S. Zhuang, Y. Wang, X. Li, L. Zhou, S. Zhu and J. Zhu, Adv. Energy Mater., 2018, 8, 1702884 CrossRef.
- T. Cai, M. Huang, Y. Huang and W. Zheng, Int. J. Hydrogen Energy, 2019, 44, 3088–3098 Search PubMed.
- G. Birhanu, S. Tanha, H. Akbari Javar, E. Seyedjafari, A. Zandi-Karimi and B. Kiani Dehkordi, Pharm. Dev. Technol., 2019, 24, 338–347 CrossRef CAS PubMed.
- H. Li, X. Chen, W. Lu, J. Wang, Y. Xu and Y. Guo, Nanomaterials, 2021, 11, 1822 Search PubMed.
- A. Camposeo, M. Moffa and L. Persano, Cham, 2015, 129–155 Search PubMed.
- A. Petropoulou, S. Kralj, X. Karagiorgis, I. Savva, E. Loizides, M. Panagi, T. Krasia-Christoforou and C. Riziotis, Sci. Rep., 2020, 10, 367 CrossRef CAS PubMed.
- H. Guo, Y. Chen, Y. Li, W. Zhou, W. Xu and L. Pang, et al., Composites, Part A, 2021, 143, 106309 CrossRef.
- S. Jiang, Y. Chen, G. Duan, C. Mei, A. Greiner and S. Agarwal, Polym. Chem., 2018, 9(20), 2685–2720 Search PubMed.
- Y. Chen, L. Sui, H. Fang, C. Ding, Z. Li and S. Jiang, et al., Compos. Sci. Technol., 2019, 174, 20–26 Search PubMed.
- L. Liu, W. Xu, Y. Ding, S. Agarwal, A. Greiner and G. Duan, Compos. Commun., 2020, 22, 100506 Search PubMed.
- L. Zhao, G. Duan, G. Zhang, H. Yang, S. He and S. Jiang, Nanomaterials, 2020, 10(1), 150 CrossRef CAS PubMed.
- S. Li, H. Guo, S. He, H. Yang, K. Liu and G. Duan, et al., Mater. Des., 2022, 214, 110406 CrossRef CAS.
- X. Wei, L. Chen, Y. Wang, Y. Sun, C. Ma and X. Yang, et al., Chem. Eng. J., 2022, 433, 134258 CrossRef CAS.
- S. Agarwal, S. Jiang and Y. Chen, Macromol. Mater. Eng., 2019, 304(2), 1800548 Search PubMed.
- S. Jian, Z. Tian, K. Zhang, G. Duan, W. Yang and S. Jiang, Chem. Res. Chin. Univ., 2021, 37(3), 565–570 Search PubMed.
- S. Jian, Y. Cheng, X. Ma, H. Guo, J. Hu and K. Zhang, et al., New J. Chem., 2022, 46, 490–497 RSC.
- S. Jian, F. Shi, R. Hu, Y. Liu, Y. Chen and W. Jiang, et al., Compos. Commun., 2022, 33, 101191 CrossRef.
- C. K. da Silva, D. J. D. Mastrantonio, J. A. V. Costa and M. G. de Morais, Food Chem., 2019, 294, 397–404 CrossRef PubMed.
- J. Z. Li, X. Liu, H. Sun, L. M. Wang, J. Q. Zhang, L. Deng and T. H. Ma, Sensors, 2020, 20, 6996 CrossRef CAS PubMed.
- Y. Wang, M. Q. Wang, L. L. Lei, Z. Y. Chen, Y. S. Liu and S. J. Bao, Microchim. Acta, 2018, 185, 140 CrossRef.
- S. W. Choi, J. Y. Park and S. S. Kim, Nanotechnology, 2009, 20, 465603 CrossRef PubMed.
- J. P. Lee, F. Jannah, K. Bae and J. M. Kim, Sens. Actuators, B, 2020, 309, 127771 CrossRef CAS.
- M. Min, X. Wang, Y. Chen, L. Wang, H. Huang and J. Shi, Sens. Actuators, B, 2013, 188, 360–366 Search PubMed.
- H. Zhang, C. Yao and X. Qin, Fibers Polym., 2021, 22, 1844–1852 Search PubMed.
- X. Jin, X. Wu, F. Zhang, H. Zhao, W. Zhong, Y. Cao, X. Ma, X. Leng, H. Zhou and M. She, Dyes Pigments, 2020, 182, 108677 Search PubMed.
- M. Wang, G. Meng, Q. Huang and Y. Qian, Environ. Sci. Technol., 2012, 46, 367–373 CrossRef CAS PubMed.
- H.-J. Lin and C.-Y. Chen, J. Mater. Sci., 2015, 51, 1620–1631 Search PubMed.
- X. Jin, M. She, J. Yang, J. Chen, X. Ma, H. Zhou, W. Chen, X. Leng and J. Li, Dyes Pigments, 2021, 192, 109417 Search PubMed.
- W. Wang, X. Wang, Q. Yang, X. Fei, M. Sun and Y. Song, Chem. Commun., 2013, 49, 4833–4835 RSC.
- C.-J. Cho, S.-T. Lu, C.-C. Kuo, F.-C. Liang, B.-Y. Chen and C.-C. Chu, React. Funct. Polym., 2016, 108, 137–147 CrossRef CAS.
- W. Gao, P. Haratipour, M. R. R. Kahkha and A. Tahvili, Ultrason. Sonochem., 2018, 44, 152–161 CrossRef CAS PubMed.
- H. Shao, D. Yin, D. Li, Q. Ma, W. Yu and X. Dong, ACS Appl. Mater. Interfaces, 2021, 13, 49288–49300 CrossRef CAS PubMed.
- M. A. Abedalwafa, Y. Li, D. Li, N. Sanbhal, J. Yang and L. Wang, Microchim. Acta, 2018, 185, 402 CrossRef PubMed.
- W. C. Wu and H. J. Lai, J. Polym. Res., 2016, 23, 223 Search PubMed.
- X.-C.-Q. Li, T. Yang, J. Wang and C.-Z. Huang, Chin. J. Anal. Chem., 2021, 49, 207–215 CAS.
- A. Senthamizhan, A. Celebioglu, B. Balusamy and T. Uyar, Sci. Rep., 2015, 5, 15608 Search PubMed.
- S. Kacmaz, K. Ertekin, M. Gocmenturk, A. Suslu, Y. Ergun and E. Celik, React. Funct. Polym., 2013, 73, 674–682 Search PubMed.
- G. Mun, S. H. Jung, A. Ahn, S. S. Lee, M. Y. Choi, D. H. Kim, J.-Y. Kim and J. H. Jung, RSC Adv., 2016, 6, 53912–53918 Search PubMed.
- K. K. Rijin, P. Sagitha, G. S. Amitha, S. Vasudevan and A. Sujith, J. Mater. Sci., 2019, 54, 13433–13444 Search PubMed.
- J. Wang, D. Q. Li, Y. X. Ye, Y. Qiu, J. W. Liu, L. Huang, B. Liang and B. L. Chen, Adv. Mater., 2021, 33, 2008020 CrossRef CAS PubMed.
- Z. Zhou, M. Shang, Z. Yao and J. Zhang, Dyes Pigments, 2022, 198, 110016 CrossRef CAS.
- C. Bai, L. Yan, H.-M. Hu, F. Zou, T.-H. Zhang and G. Xue, ACS Appl. Polym. Mater., 2021, 3, 4504–4511 CrossRef CAS.
- C. Wu, P. Zhu, Y. Liu, L. Du and P. Wang, Sensors, 2021, 21, 7874 CrossRef CAS PubMed.
- X. W. Fu and W. H. Zhong, Adv. Energy Mater., 2019, 9, 1901774 Search PubMed.
- A. Saithongdee, N. Praphairaksit and A. Imyim, Sens. Actuators, B, 2014, 202, 935–940 CrossRef CAS.
- J.-T. Wang, Y.-C. Chiu, H.-S. Sun, K. Yoshida, Y. Chen, T. Satoh, T. Kakuchi and W.-C. Chen, Polym. Chem., 2015, 6, 2327–2336 RSC.
- C. Zhou, Y. Zhang and H. Liu, J. Braz. Chem. Soc., 2018, 29, 457–462 CAS.
- C. Zhang, H. Li, Q. Yu, L. Jia and L. Y. Wan, ACS Omega, 2019, 4, 14633–14639 CrossRef CAS PubMed.
- Z. Li, H. Li, C. Shi, W. Zhang, W. Zhou, L. Wei and M. Yu, Sens. Actuators, B, 2016, 226, 127–134 CrossRef CAS.
- A. Senthamizhan, A. Celebioglu and T. Uyar, J. Mater. Chem. A, 2014, 2, 12717–12723 RSC.
- P. G. Rao, B. Saritha and T. Siva Rao, J. Fluoresc., 2019, 29, 353–360 CrossRef CAS PubMed.
- S. Girdthep, O. Hanmeng, N. Triamnak, N. Chailek and N. Wanichacheva, Polym. Test., 2021, 97, 107151 Search PubMed.
- F. C. Liang, Y. L. Luo, C. C. Kuo, B. Y. Chen, C. J. Cho, F. J. Lin, Y. Y. Yu and R. Borsali, Polymers, 2017, 9, 136 Search PubMed.
- L. Li, F. Wang, Y. Lv, J. Liu, H. Bian, W. Wang, Y. Li and Z. Shao, ACS Omega, 2018, 3, 4220–4230 Search PubMed.
- Y. Cai, L. Yan, G. Liu, H. Yuan and D. Xiao, Biosens. Bioelectron., 2013, 41, 875–879 Search PubMed.
- S. Deng, G. Zhang and P. Wang, ACS Sustain. Chem. Eng., 2018, 7, 1159–1168 CrossRef.
- B. Y. Chen, Y. C. Lung, C. C. Kuo, F. C. Liang, T. L. Tsai, D. H. Jiang, T. Satoh and R. J. Jeng, Polymers, 2018, 10, 1259 CrossRef PubMed.
- L. Ma, K. Liu, M. Yin, J. Chang, Y. Geng and K. Pan, Sens. Actuators, B, 2017, 238, 120–127 CrossRef CAS.
- A. Ghosh, V. Jeseentharani, M. A. Ganayee, R. G. Hemalatha, K. Chaudhari, C. Vijayan and T. Pradeep, Anal. Chem., 2014, 86, 10996–11001 CrossRef CAS PubMed.
- A. Senthamizhan, A. Celebioglu and T. Uyar, Sci. Rep., 2015, 5, 10403 CrossRef PubMed.
- W. Wang, Y. Li, M. Sun, C. Zhou, Y. Zhang, Y. Li and Q. Yang, Chem. Commun., 2012, 48, 6040–6042 RSC.
- S. Raj and D. R. Shankaran, Sens. Actuators, B, 2016, 226, 318–325 CrossRef CAS.
- Y. Li, Y. Wen, L. Wang, J. He, S. S. Al-Deyab, M. El-Newehy, J. Yu and B. Ding, J. Mater. Chem. A, 2015, 3, 18180–18189 Search PubMed.
- E. Sheikhzadeh, S. Naji-Tabasi, A. Verdian and S. Kolahi-Ahari, J. Iran. Chem. Soc., 2021, 19, 283–290 Search PubMed.
- Y. Li, Y. Si, X. Wang, B. Ding, G. Sun, G. Zheng, W. Luo and J. Yu, Biosens. Bioelectron., 2013, 48, 244–250 Search PubMed.
- C. Kim, J.-Y. Hwang, K.-S. Ku, S. Angupillai and Y.-A. Son, Sens. Actuators, B, 2016, 228, 259–269 Search PubMed.
- J.-H. Syu, Y.-K. Cheng, W.-Y. Hong, H.-P. Wang, Y.-C. Lin, H.-F. Meng, H.-W. Zan, S.-F. Horng, G.-F. Chang, C.-H. Hung, Y.-C. Chiu, W.-C. Chen, M.-J. Tsai and H. Cheng, Adv. Funct. Mater., 2013, 23, 1566–1574 CrossRef CAS.
- C. Zhou and H. Liu, J. Braz. Chem. Soc., 2017, 28, 1947–1952 CAS.
- Y. Hu, X. Lu, X. Jiang and P. Wu, J. Hazard. Mater., 2020, 384, 121368 CrossRef CAS PubMed.
- M. Kim, N. Mergu and Y.-A. Son, J. Lumin., 2018, 204, 244–252 Search PubMed.
- J. P. Dreyer, R. I. Stock, L. G. Nandi, I. C. Bellettini and V. G. Machado, Carbohydr. Polym., 2020, 236, 115991 CrossRef CAS PubMed.
- Z. Qiu, W. Duan, S. Cao, T. Zeng, T. Zhao, J. Huang, X. Lu and J. Zeng, Environ. Sci. Technol., 2022, 56, 1713–1723 CrossRef PubMed.
- H. Zhang, C. Zhou, J. Sun, X. Li and J. Tang, Materia, 2021, 26, 12958 Search PubMed.
- V. Ruiz, A. Pérez-Marquez, J. Maudes, H. J. Grande and N. Murillo, Sens. Actuators, B, 2018, 262, 902–912 CrossRef CAS.
- Mu Gao, et al., Nanotechnology, 2017, 28, 485708 Search PubMed.
- M. Vetrik, M. Pradny, L. Kobera, M. Slouf, M. Rabyk, A. Pospisilova, P. Stepanek and M. Hruby, RSC Adv., 2013, 3, 15282–15289 RSC.
- Z. Wang, S. Bakshi, C. Li, S. J. Parikh, H. S. Hsieh and J. J. Pignatello, J. Colloid Interface Sci., 2020, 579, 258–268 Search PubMed.
- P. Tang, N. Kaspersky and G. Sun, Talanta, 2020, 219, 121149 CrossRef CAS PubMed.
- Q. Chen, R. Sheng, P. Wang, Q. Ouyang, A. Wang, S. Ali, M. Zareef and M. M. Hassan, Spectrochim. Acta, Part A, 2020, 241, 118654 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |