Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Are cosmetics a significant source of PFAS in Europe? product inventories, chemical characterization and emission estimates†
Kerstin Winkens
Pütz‡
*a,
Shahla
Namazkar‡
 *b,
Merle
Plassmann
b and
Jonathan P.
Benskin
*b,
Merle
Plassmann
b and
Jonathan P.
Benskin
 *b
*b
aIVL Swedish Environmental Research Institute, Stockholm, Sweden. E-mail: Kerstin.Putz@ivl.se
bDepartment of Environmental Science, Stockholm University, Stockholm, Sweden. E-mail: Shahla.Namazkar@aces.su.se; Jon.Benskin@aces.su.se
First published on 21st July 2022
Abstract
In this study, emission of per- and polyfluoroalkyl substances (PFAS) from the use of cosmetics in the European Economic Area (EEA; not including Lichtenstein and Iceland) was estimated for the first time. Using the European Commission database for information on cosmetic substances and ingredients (CosIng) ∼170 structures containing at least –CF2– or –CF3 were identified as ingredients in cosmetics on the European market. These structures were then cross referenced against the Cosmetic Database “CosmEthics” to identify PFAS-containing products. Among these products, polytetrafluoroethylene (PTFE) and C9-15 fluoroalcohol phosphate were the most frequently listed PFAS ingredients. Thereafter, a sample of 45 cosmetics spanning 5 product categories was purchased in Sweden and characterized for total fluorine (TF), extractable organofluorine (EOF), and target PFAS. Using measured concentrations, the share of PFAS-containing products in each product category, sales data from Cosmetics Europe, as well as other parameters and assumptions, the annual emission of PFAS from cosmetics after use was estimated. Annual EEA-wide TF and EOF-based emissions ranged from ∼17–38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg F per year and 37–5100 kg F per year, respectively, representing combined emission to wastewater and solid waste (low to high emission scenario). Sum perfluoroalkyl carboxylic acid (PFCA) emissions were considerably lower (21 kg ∑PFCAs per year; high scenario). While TF- and EOF-based emissions are significant, they are considerably lower than estimates of TF emission from washing of PFAS-coated textiles in the EU. This work provides the first estimate of PFAS emissions from cosmetics and highlights the importance of using a multi-platform analytical approach for PFAS emission estimates.
000 kg F per year and 37–5100 kg F per year, respectively, representing combined emission to wastewater and solid waste (low to high emission scenario). Sum perfluoroalkyl carboxylic acid (PFCA) emissions were considerably lower (21 kg ∑PFCAs per year; high scenario). While TF- and EOF-based emissions are significant, they are considerably lower than estimates of TF emission from washing of PFAS-coated textiles in the EU. This work provides the first estimate of PFAS emissions from cosmetics and highlights the importance of using a multi-platform analytical approach for PFAS emission estimates.
Environmental significancePer- and polyfluoroalkyl substances (PFAS) are listed ingredients in European cosmetic products, but their contribution to PFAS contamination in liquid and solid waste remains unclear. In this work the prevalence of PFAS in cosmetic products from the European market was investigated using a combination of database mining and comprehensive analytical characterization. These data, together with information on consumer habits related to cosmetic removal, were applied to estimate PFAS emissions to wastewater and solid waste for the first time. Since waste treatment does not remove PFAS completely, the disposal of PFAS-containing cosmetics may lead to environmental contamination. While PFAS emissions from cosmetics are significant, they are lower than emissions from other sources, such as washing of PFAS-coated textiles. |
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a diverse group of over 4700 chemicals, which have been widely used since the 1950s in industrial processes and consumer products.1 The OECD/UNEP Global PFC Group defines PFAS as substances that contain 1 or more fluorinated methyl or methylene carbon atoms (i.e. –CF3 or –CnF2n–; n ≥ 1), with some noted exceptions.2 The extraordinary strength of the C–F bond together with the combined lipophobic and hydrophobic properties of perfluoroalkyl chains make PFAS useful for a wide variety of applications. Examples include non-stick cookware, textiles, food contact paper, medical equipment, motor oil fluids, firefighting foams, cosmetics and personal care products.1,3–5Since the first detection of PFAAs globally in humans and wildlife,6 concerns surrounding the risks of PFAS have steadily increased. PFAS have been associated with a wide range of health effects, including cancer, thyroid and hormonal disturbance, premature birth, neuro-developmental deficiencies and immunotoxicity.7,8 Phase-out and regulation of certain legacy PFAS such as perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) has led to declining concentrations in humans from some parts of the world.9–11 However, some exemptions exist, and numerous PFAS remain unregulated and continue to be manufactured and used in consumer products globally.
Ingredients in cosmetics and personal care products are regulated by the European Parliament and Council of the European Union,12 which stipulates that cosmetic manufacturers must ensure the safety of their products for human health. However, this regulation does not contain requirements on the use and release of substances that may impact the environment, such as PFAS. Instead, these substances are addressed by REACH, which stipulates that polymers and low molecular weight substances imported or manufactured in quantities of <1 tonne per year do not require hazard and risk assessments.13 PFOA and PFOA-related substances (i.e. substances that can degrade to PFOA) have been banned since the 4th of July 2020 in all products sold in the EU with limits of 25 and 1000 ng g−1, respectively (REACH Annex XVII list of restricted substances (entry 68)). In addition, Sweden requires manufacturers and importers to register the occurrence of PFAS in products in the Swedish Chemicals Agency Products Register, regardless of the concentration used in the product. However, in other EU countries, such regulations are not in place and some PFAS may be completely overlooked in consumer products.
In addition to a variety of PFAS that are intentionally added to consumer products (mostly as emulsifiers, antistatics, stabilizers, surfactants, film formers, viscosity regulators and solvents), PFAS may occur unintentionally as impurities and/or degradation products.14 Fujii et al. (2013) were the first to measure PFAS in 24 cosmetics and personal care products, and found that 88% contained PFCAs, with sum PFCA concentrations up to 19 μg g−1 in sunscreens and up to 5.9 μg g−1 in other cosmetic products.15 Thereafter, Schultes et al. (2018) reported target PFAS in a wide range of cosmetic products from the Swedish market, showing high concentrations of PFCAs and PFCA-precursors in some samples, and even higher concentrations of total fluorine (TF) and extractable organofluorine (EOF).16 Most recently, a study of 231 cosmetics from Canada and the US reported that over 50% contained high levels of TF.17 While the possible occurrence of inorganic fluorine hampered determination of the exact proportion of PFAS-containing products, re-analysis of a subset of 29 products (i.e. ∼13%) using targeted methods revealed detectable PFAS in all samples, with concentrations ranging from 22–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ng g−1 product weight.17 This is concerning, in particular since North American cosmetic producers are not necessarily required to list PFAS ingredients,17 thereby hampering consumer efforts to avoid PFAS-containing products.
500 ng g−1 product weight.17 This is concerning, in particular since North American cosmetic producers are not necessarily required to list PFAS ingredients,17 thereby hampering consumer efforts to avoid PFAS-containing products.
Given the large surface area of the skin (∼22 m2)18 and the liberal application of some cosmetic products, dermal uptake may represent an important route of exposure to PFAS in cosmetics.16 However, many PFAS in cosmetics are ionic or polymeric, both of which are expected to be poorly absorbed by the skin.19 Dermal absorption of non-ionizable, low molecular weight PFAS is largely unknown. In addition to the potential for exposure from dermal uptake and ingestion of PFAS in cosmetics, PFAS used in these products may pose a risk to the environment. Environmental releases may occur during manufacture and use of cosmetics, or after use, when the products are intentionally removed from the skin or hair. These residues ultimately enter landfills or wastewater treatment plants (WWTPs) where they are often poorly retained and may subsequently enter the environment via landfill leachate,20 WWTP effluent,21 or the use of sludge for agricultural fertilizer.22 However, to date, the prevalence of PFAS in cosmetics on large scale has not been determined; nor have emissions to waste streams.
The overarching aim of this work was to assess the importance of cosmetics from the European market as a source of PFAS to waste streams. To achieve this goal, an inventory of PFAS in cosmetics was carried out using European cosmetic databases. Thereafter, a selection of decorative cosmetics, hair care, and skin care products were characterized using a combination of targeted PFAS analysis and fluorine-specific measurements. Finally, using measured concentrations, the share of products containing PFAS, sales data, as well as other parameters and assumptions, the annual emission of PFAS into European wastewater and solid waste from cosmetics after use was estimated for the first time.
Materials and methods
Sample collection
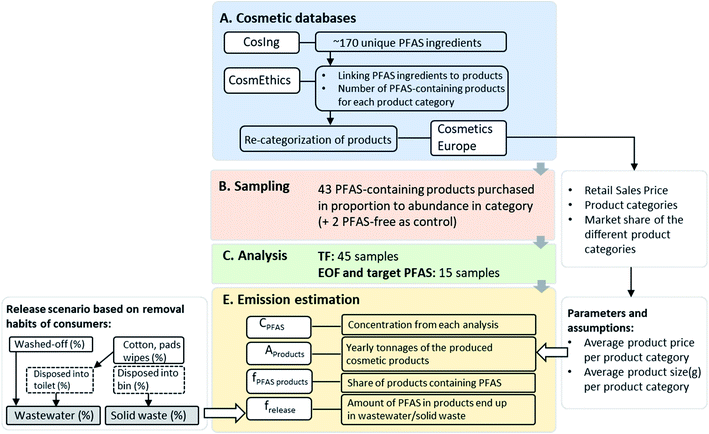
An inventory of PFAS and their frequency of occurrence in cosmetic products was determined through database searches (Fig. 1). The European Commission database for information on cosmetic substances and ingredients (CosIng) was searched (for “fluoro”) to extract unique PFAS ingredients potentially occurring in cosmetic products using International Nomenclature of Cosmetic Ingredients (INCI) identifiers. All substances containing at least a –CF2– or –CF3 (including polymers), were included in the search. Note that substances considered “exceptions” (i.e. a carbon atom with a H/Cl/Br/I atom attached to it2) were also included. It is pertinent to note that one INCI name may comprise several PFAS (e.g. “C9–C15 fluoroalcohol phosphate”, seven PFAS) but for practical reasons, each INCI name was considered as a single PFAS for the database analysis.The ∼170 unique PFAS ingredients identified in CosIng were then cross referenced against the CosmEthics European database, in order to connect PFAS INCI names to specific cosmetic products within 11 product categories (Table S1†). Each of the product categories contained a range of sub-categories. For example, anti-aging cream, masks, and eye gel were among the sub-categories within the category “Facial Care” (Table S1†). In order to maximize sampling of sub-categories containing a high share of PFAS-containing products, the following approach was used: First, summary statistics on PFAS-containing products received from CosmEthics (EU/EEA barcode product database extract only) were reviewed and the share (%) of PFAS-containing products was calculated for each product sub-category. The most relevant sub-categories were identified as those with both the largest share of PFAS-containing products and the largest total number of products. Thereafter, a total of 50 anticipated samples was distributed among the most relevant product categories and sub-categories according to the number of PFAS-containing products in a given sub-category (i.e. percent distribution). Sampling aimed to collect the number of products identified from each of the targeted sub-categories (Table S1†).
In September 2020, a total of 45 different cosmetics were purchased (43 listing PFAS as ingredients, 2 which did not) either online (1 product) or from local stores in Stockholm. For further consideration and emissions calculations, products were re-categorized into the following 5 product categories: “decorative cosmetics”, “hair care”, “skin care”, “toiletries”, and “perfumes and fragrances”. This was necessary in order to align with the categories of the retail sales data provided in a report by Cosmetics Europe23 which was later used for emissions calculations (see below). Among purchased products, a total of 24 were “decorative cosmetics”, 6 were “hair care” and 15 were “skin care” (out of which 2 products were for males). An overview of the investigated samples, brand names and listed fluorinated ingredients (i.e. INCI names) is available in Table S2.†
Chemical analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/6
2/6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diPAP), bis(1H,1H,2H,2H-perfluorodecyl) phosphate (8
2 diPAP), bis(1H,1H,2H,2H-perfluorodecyl) phosphate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/8
2/8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diPAP) and (1H,1H, 2H,2H-perfluorooctyl) (1H,1H,2H,2H-perfluorodecyl) phosphate (6
2 diPAP) and (1H,1H, 2H,2H-perfluorooctyl) (1H,1H,2H,2H-perfluorodecyl) phosphate (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/8
2/8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diPAP) were purchased from Wellington Laboratories (Guelph, Canada). A list of isotopically-labelled internal standards is provided in Table S3.† Additional reagents used for sample preparation are provided in the ESI.†
2 diPAP) were purchased from Wellington Laboratories (Guelph, Canada). A list of isotopically-labelled internal standards is provided in Table S3.† Additional reagents used for sample preparation are provided in the ESI.†
For comparison to TF and EOF data, targeted PFAS concentrations were converted to fluorine equivalents (CF_PFAS; ng F g−1) using eqn (1):
| CF_PFAS = nF × AF/MWPFAS × CPFAS | (1) |
Interlaboratory comparison of EOF measurements in water and sludge performed at Stockholm University have shown reasonable agreement with other labs.25 For cosmetics, accuracy and precision were assessed by replicate matrix spike/recovery experiments, consisting of a PFAS-free cosmetic product which was extracted and analysed with and without fortification of a suite of PFAS (277 ng F fortification level; n = 3). The average percent recovery from these experiments was 61% (14% RSD) and data were not corrected for losses. Furthermore, an additional spiking experiment was performed using 250 ng sodium fluoride (NaF; n = 3), which was not recovered, confirming that inorganic fluorine was removed during the extraction procedure. In addition to analysing 10% of samples in triplicate (i.e. 2 individual samples analyzed in triplicate for EOF), three procedural blanks were handled in the same way as the samples to check for potential contamination introduced during the extraction procedure. A standard of PFOS (1 μg mL−1) analyzed routinely with samples revealed good comparability to theoretical concentrations (92–113%), confirming that the response measured on the CIC was F-specific. Finally, potential carry-over and instrumental stability were monitored using the same procedure as explained for TF analysis. All EOF concentrations were blank-corrected and the limits of detection were calculated based three times the standard deviation of the procedural blanks.
For target PFAS measurements, accuracy and precision were assessed using the same replicate spike/recovery experiments as used for EOF, i.e. a PFAS-free cosmetic product which was extracted and analyzed with and without fortification of 10 ng of individual PFAS (Table S3†). In addition to analyzing 10% of samples in triplicate (i.e. 2 individual samples analyzed in triplicate), three procedural blanks (i.e. no matrix) were handled in the same way as the samples to monitor for potential contamination introduced during the extraction procedure. Finally, during instrumental analysis, instrumental blanks and a mid-level calibration standard were run intermittently to monitor potential carry-over and instrumental stability, respectively. Procedural blanks did not show detectable contamination for any target PFAS. Therefore, limits of detection (LODs) were determined using the concentration obtained from the lowest calibration point with a well-shaped peak displaying an intensity >1000 and a signal-to-noise (S/N) ratio of >3 and converted to a weight-based concentration (e.g. ng g−1) using the average sample weight (∼0.1 g). Replicate spike/recovery experiments with target PFAS revealed an average percent recovery for all targets of 83% (RSD 13%; n = 3). Most substances fell within the range of 85–95%, with the exception of PFHxDA and PFOcDA, which had lower recoveries (61 and 36%, respectively). Considering that internal standards were added after extraction (necessary because a portion of the same extract was used for EOF analysis), these recoveries are reasonable, and reflect some losses incurred during the extraction procedure and/or matrix effects arising from the absence of exactly matching, isotopically labelled internal standards. Consequently, no correction for losses was made. Detection limits ranged from 1 to 18 ng PFAS g−1 cosmetic product sample, which are higher than for environmental sample analysis due to the low quantity of sample used.
As a final QC measure, a sample previously analyzed in Schultes et al. (2018) was re-analysed in the present study for TF, EOF and target PFAS concentrations. TF and EOF concentrations measured here were in good agreement with previous measurements (3100 vs. 2900 ng F g−1 and 1260 vs. 1380 ng F g−1, respectively). For target analysis, sum PFCA (∑PFCA) concentrations were also in good agreement (6.27 ng F g−1vs. 8.11 ng F g−1, respectively), but higher diPAP concentrations were observed in the present work compared to Schultes et al. (2018) (487 ng F g−1 here vs. 72 ng F g−1 in Schultes et al. respectively). This is perhaps not surprising considering that a different method was used for diPAP determination in Schultes et al. (2018) and because challenges were noted in the previous study for determination of PAPs in these samples due to their extremely high concentrations.
Emission estimates
Annual PFAS or fluorine emission (EPFAS; kg PFAS per year or kg F per year) was estimated after cosmetic use to wastewater and/or solid waste for the European Economic Area (EEA; not including Lichtenstein and Iceland, population roughly 450 million people) according to eqn (2).| EPFAS = CPFAS × Aproducts × fPFAS products × frelease | (2) |
Each variable contains a number of calculations and assumptions which are explained in more detail in ESI (see Section S3, Tables S8–S11†). CPFAS is the concentration of PFAS (or fluorine) in a product obtained based on measurement of target PFAS (μg PFCAs per g product) or TF (μg F g−1 product), EOF (μg F g−1 product). The total amount of products sold annually (Aproducts; tons per year) was derived from the retail sales price from the European cosmetic products market (EEA, not including Lichtenstein and Iceland), the market share of the different product categories,23 and the assumed average product price and size per product category. The share of products containing PFAS (fPFAS products; unitless) was derived by dividing the total number of cosmetic products containing PFAS by the total number of products for each product category (numbers from the CosmEthics database, but product subcategories recategorized into cosmetics Europe categories to match the sales statics categories, see Section S3, Table S12†). Finally, the fraction of the chemical released (frelease; %) from the product into a certain compartment (i.e. wastewater or solid waste; see Section S3†) was obtained by considering consumer habits26 and assumptions described in detail in the ESI (Section S3, Table S14†).
Finally, three different waste stream emission scenarios (i.e. low, average and high) were calculated for each of the three measurements (i.e. TF, EOF and targeted PFAS) based on: (i) concentrations (minimum, average and maximum, Tables S5–S7 and explanation in ESI†) in each product category; (ii) three release scenarios (i.e. frelease % into wastewater and solid waste, Table S14†) based on statistics on consumer removal habits26 (Table S13†) and (iii) three product price levels (Table S10†), for the overall product categories. The difference between the total amount of PFAS in cosmetics and the amount that is emitted via wastewater was considered to end up in solid waste (i.e. disposal of cosmetic products removal pads/tissues etc. into waste, leftover product amount in the package, disposal after a product's lifetime). Further, PFAS emissions during the production of cosmetic products are not considered, neither are releases of volatile PFAS into the air. A detailed explanation of each input parameter for the different emission scenarios is provided in the ESI,† as well as an overview table on the ingoing parameters and their values applied in the emission calculation for the different scenarios (Tables S15–18†).
Result and discussion
Product characterization
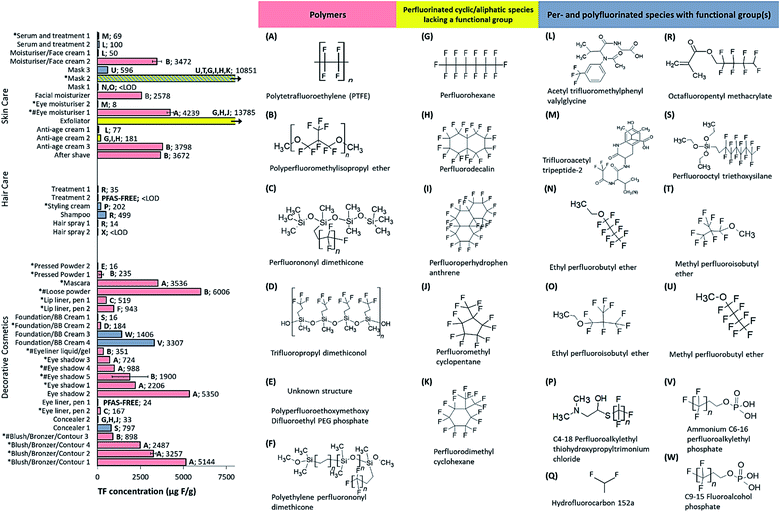 | ||
| Fig. 2 TF concentrations (μg F g−1) of different cosmetics, grouped according to product category. Bar colors represent samples containing polymers (red), perfluorinated cyclic/aliphatic species lacking a functional group (yellow) and PFAS species with functional group(s) (grey) that appeared on the ingredient list. Samples of mask 1, treatment 2 and hairspray 2 are < LOD. Structures T and Y are adopted from literature,29 but are uncertain based on the INCI name provided. Samples labelled PFAS-FREE did not list any PFAS ingredients. *Mica listed among ingredients; #synthetic fluorphlogopite listed among ingredients. | ||
As was shown in Schultes et al. (2018), there was some evidence that inorganic fluorine contributed to the TF signal in some samples.16 For instance, TF concentrations were above the LOD for eye liner pen 1, which did not list any PFAS ingredients. Assuming that the fluorine does not occur from a residual or undeclared PFAS ingredient, it is likely attributable to inorganic substances (e.g. synthetic fluorphlogopite/mica or fluoride). Indeed 24 samples listed “mica” (not specified as synthetic or natural) and 7 samples listed “synthetic fluorphlogopite” (Fig. 2 and 3) but the exact quantities remain unclear. While EOF and targeted PFAS analysis do not capture inorganic fluorine and can be used to rule out its occurrence (as was the case here), these approaches may underestimate the total PFAS concentration (depending on the PFAS present), since some organofluorine substances may not be extracted with the solvent and/or measured in either case (i.e. during EOF or targeted PFAS analyses). We conclude that all three approaches may be susceptible to bias (TF – over-reporting; EOF and targeted PFAS – under-reporting); therefore, a combined approach, presenting upper and lower bound estimates, is the most transparent and comprehensive strategy for product characterization.
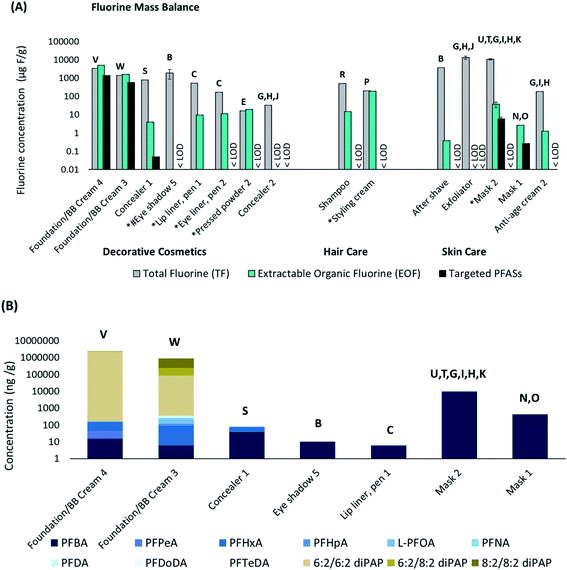 | ||
| Fig. 3 (a) Fluorine mass balance showing TF, EOF and targeted PFAS concentrations (μg F g−1 sample). The targeted PFAS (∑PFAS) concentrations were converted to fluorine concentrations for comparative purposes. (LODTF = 35.45 μg F g−1, LODEOF = 0.32 μg F g−1, LOD∑PFAS = 0.0015 μg F g−1). *Mica listed among ingredients; #synthetic fluorphlogopite listed among ingredients. (b) Concentrations (ng g−1 sample) of targeted PFAS in 5 decorative cosmetics and 2 skin care samples (masks). Samples not shown (8 out of 15) were <LOD. Samples are plotted on a logarithmic scale (base 10) to enhance readability. The letters on top of each bar correspond to structures provided in Fig. 2. | ||
EOF measurements were performed in 15 of the 45 cosmetic products. Concentrations of EOF ranged from <LOD (<162 ng F g−1 for exfoliator, <325 ng F g−1 for concealer 2 and Eyeshadow 5) up to 4.93 mg F g−1 for foundation/BB cream 4 (Fig. 3a, Table S6†). The second highest EOF concentration was detected in foundation/BB cream 3 (1.58 mg F g−1). Similar to TF, EOF concentrations varied both within product categories and sub-categories. While skin care samples appeared to have lower EOF concentrations than hair care and decorative cosmetic products, the small sample size (n = 6) made it difficult to draw firm conclusions about trends of PFAS concentrations among product types. Overall these results compared well with prior work by Schultes et al. (2018) which reported EOF concentrations of <LOD up to 1.72 mg F g−1, with the exception of one product in the current study which contained EOF concentrations up to 4.93 mg F g−1 (foundation/BB cream 4).
For most samples, EOF concentrations were ∼10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold lower than TF concentrations, indicating that the listed PFAS ingredient(s) were poorly extracted and/or that the products contained high concentrations of inorganic fluorine (Table S16†). The few instances of EOF exceeding TF concentrations (e.g. foundation/BB creams 3 and 4, pressed powder 2) are attributable to the variability in TF and EOF analyses, and have been documented previously.28 In these three cases the EOF and TF are considered equivalent, indicating that the organofluorine ingredient is efficiently extracted and that inorganic fluorine is negligible (Table S16†). However, the observation of EOF concentrations at similar levels to TF in the case of pressed powder 2, was surprising, considering that the listed ingredient (polyperfluoroethoxymethoxy difluoroethyl PEG phosphate) appears to be polymeric and was not expected to be extracted efficiently with methanol. In comparison, all other polymer-containing products (e.g. Eyeshadow 5 and lip liner, pen 1, eye liner, pen 2 and aftershave) displayed EOF levels that were much lower than TF (range 0.01–6.8%). It can be speculated that the polymer in pressed powder 2 is much smaller (i.e. shorter chain length) and more soluble in methanol than the two other polymers in the aforementioned products.
000-fold lower than TF concentrations, indicating that the listed PFAS ingredient(s) were poorly extracted and/or that the products contained high concentrations of inorganic fluorine (Table S16†). The few instances of EOF exceeding TF concentrations (e.g. foundation/BB creams 3 and 4, pressed powder 2) are attributable to the variability in TF and EOF analyses, and have been documented previously.28 In these three cases the EOF and TF are considered equivalent, indicating that the organofluorine ingredient is efficiently extracted and that inorganic fluorine is negligible (Table S16†). However, the observation of EOF concentrations at similar levels to TF in the case of pressed powder 2, was surprising, considering that the listed ingredient (polyperfluoroethoxymethoxy difluoroethyl PEG phosphate) appears to be polymeric and was not expected to be extracted efficiently with methanol. In comparison, all other polymer-containing products (e.g. Eyeshadow 5 and lip liner, pen 1, eye liner, pen 2 and aftershave) displayed EOF levels that were much lower than TF (range 0.01–6.8%). It can be speculated that the polymer in pressed powder 2 is much smaller (i.e. shorter chain length) and more soluble in methanol than the two other polymers in the aforementioned products.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ng g−1, with 6
500 ng g−1, with 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 fluorotelomer alcohols (FTOHs) and methacrylates (FTMAs) among the dominant PFAS. While FTOHs and FTMAs were not included in the present study, it was expected that both classes of PFAS (if present) would be captured by EOF measurements. Overall, the diversity of PFAS ingredients and residuals in consumer products which are not captured by targeted approaches highlights the importance of including TF- and EOF-based measurements for a comprehensive picture of PFAS contamination in consumer products.
2 fluorotelomer alcohols (FTOHs) and methacrylates (FTMAs) among the dominant PFAS. While FTOHs and FTMAs were not included in the present study, it was expected that both classes of PFAS (if present) would be captured by EOF measurements. Overall, the diversity of PFAS ingredients and residuals in consumer products which are not captured by targeted approaches highlights the importance of including TF- and EOF-based measurements for a comprehensive picture of PFAS contamination in consumer products.
Our targeted PFAS analysis also detected intentionally added PFAS ingredients in two products. Foundation/BB cream 3 and 4 contained “perfluoroalkylethyl phosphate” and “fluoroalcohol phosphate”, respectively, which assumably refers to polyfluoroalkyl phosphate esters (PAPs). Indeed, diPAPs were measured in both products, at concentrations up to 2.3 mg g−1 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diPAP; Fig. 3b). 6
2 diPAP; Fig. 3b). 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/8
2/8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 8
2 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diPAP were detected at concentrations up to 0.67 mg g−1 (Fig. 3b), which far exceeds the EU limit of 1000 ng g−1 for PFOA-related substances.30 These diPAP concentrations exceeded concentrations of all other PFAS by several orders of magnitude in some cases, demonstrating that targeting listed ingredients will greatly increase ∑PFAS concentrations, compared to if exclusively impurities are measured (e.g. PFCAs, FTOHs, etc.). This underlines the need for more analytical standards and methods in order to quantify the diversity of PFAS which are intentionally added to products.
2 diPAP were detected at concentrations up to 0.67 mg g−1 (Fig. 3b), which far exceeds the EU limit of 1000 ng g−1 for PFOA-related substances.30 These diPAP concentrations exceeded concentrations of all other PFAS by several orders of magnitude in some cases, demonstrating that targeting listed ingredients will greatly increase ∑PFAS concentrations, compared to if exclusively impurities are measured (e.g. PFCAs, FTOHs, etc.). This underlines the need for more analytical standards and methods in order to quantify the diversity of PFAS which are intentionally added to products.
PFAS emissions
Low, high, and average emission scenarios for PFAS from the use of cosmetics were estimated using TF, EOF, and ∑PFCA (C4–C18)-concentrations, resulting in a total of 9 estimates for combined emission to wastewater and solid waste (Table 1). These estimates should not be confused with environmental emissions, since the fraction of PFAS retained and/or destroyed during treatment (i.e. by wastewater treatment plants, landfills, or incineration plants) has not been included in the calculation, nor were emissions occuring during cosmetic production. For TF-based estimates, emissions ranged from 17 kg F (low emission scenario) to 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg F (high emission scenario) per year. Since TF concentrations capture all fluorinated substances (both organic and inorganic) these estimates will err on the side of overestimation. Moreover, caution is warranted when converting these fluorine concentrations to PFAA-equivalents (for example to estimate surface water concentrations), since many of the fluorinated ingredients captured by TF measurements are not PFAA-precursors and may not be water soluble. Rather, they are polymers which may contribute to particulate (i.e. nano- or microplastic) pollution, or possibly retained in the wastewater treatment plant. By comparison, EOF-based emission estimates, which ranged from 37 kg F (low emission scenario) to 5100 kg F (high emission scenario) per year do not include contributions from inorganic fluorine, but are limited to (semi-)polar PFAS, typically PFAAs and/or PFAA-precursors. Polymeric PFAS and low molecular weight non-polar PFAS are unlikely to be captured in these estimates. Finally, ∑PFCA-based estimates, which are expressed on a kg ∑C4–C18 PFCAs per year basis (instead of fluorine equivalents per year) ranged from negligible (low emission scenario) to 21 kg ∑C4–C18 PFCAs per year (high emission scenario), and were dominated by emissions to wastewater in all 3 scenarios. These estimates represent an even more severe underestimate of total PFAS emissions, since only PFCAs are included, yet they provide a good estimate of contamination introduced from unintentionally added residual PFAAs and can be more readily used to estimate contributions to PFAS concentrations in surface water.
000 kg F (high emission scenario) per year. Since TF concentrations capture all fluorinated substances (both organic and inorganic) these estimates will err on the side of overestimation. Moreover, caution is warranted when converting these fluorine concentrations to PFAA-equivalents (for example to estimate surface water concentrations), since many of the fluorinated ingredients captured by TF measurements are not PFAA-precursors and may not be water soluble. Rather, they are polymers which may contribute to particulate (i.e. nano- or microplastic) pollution, or possibly retained in the wastewater treatment plant. By comparison, EOF-based emission estimates, which ranged from 37 kg F (low emission scenario) to 5100 kg F (high emission scenario) per year do not include contributions from inorganic fluorine, but are limited to (semi-)polar PFAS, typically PFAAs and/or PFAA-precursors. Polymeric PFAS and low molecular weight non-polar PFAS are unlikely to be captured in these estimates. Finally, ∑PFCA-based estimates, which are expressed on a kg ∑C4–C18 PFCAs per year basis (instead of fluorine equivalents per year) ranged from negligible (low emission scenario) to 21 kg ∑C4–C18 PFCAs per year (high emission scenario), and were dominated by emissions to wastewater in all 3 scenarios. These estimates represent an even more severe underestimate of total PFAS emissions, since only PFCAs are included, yet they provide a good estimate of contamination introduced from unintentionally added residual PFAAs and can be more readily used to estimate contributions to PFAS concentrations in surface water.
| Product category | Emission | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low emission scenario | Average emission scenario | High emission scenario | |||||||
| Total (Wastewater + solid waste) | Wastewater | Solid waste | Total (Wastewater + solid waste) | Wastewater | Solid waste | Total (Wastewater + solid waste) | Wastewater | Solid waste | |
| TF (kg F per year) | |||||||||
| Skin care | 8.0 | 5.3 | 2.7 | 8200 | 6200 | 2000 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0 |
| Toiletries | 1.0 | 0.82 | 0.21 | 560 | 500 | 58 | 1500 | 1500 | 0 |
| Hair care | 1.9 | 1.5 | 0.35 | 1000 | 930 | 86 | 2700 | 2700 | 0 |
| Decorative cosmetics | 5.6 | 1.0 | 4.6 | 1200 | 650 | 570 | 4100 | 4100 | 0 |
| Cosmetics total | 17 | 8.6 | 7.9 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8300 | 2700 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| EOF (kg F per year) | |||||||||
| Skin care | 0.17 | 0.11 | 0.059 | 11 | 8.7 | 2.8 | 78 | 78 | 0 |
| Toiletries | 13 | 10 | 2.7 | 310 | 270 | 32 | 570 | 570 | 0 |
| Hair care | 24 | 20 | 4.4 | 560 | 510 | 47 | 1000 | 1000 | 0 |
| Decorative cosmetics | 0.11 | 0.020 | 0.092 | 380 | 200 | 180 | 3400 | 3400 | 0 |
| Cosmetics total | 37 | 30 | 7.3 | 1300 | 1000 | 260 | 5100 | 5100 | 0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| ∑PFCAs (kg ∑C 4 –C 18 PFCAs per year) | |||||||||
| Skin care | 0 | 0 | 0 | 2.7 | 2.02 | 0.64 | 20 | 20 | 0 |
| Toiletries | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hair care | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Decorative cosmetics | 0 | 0 | 0 | 0.035 | 0.019 | 0.016 | 0.24 | 0.24 | 0 |
| Cosmetics total | 0 | 0 | 0 | 2.7 | 2.0 | 0.66 | 21 | 21 | 0 |
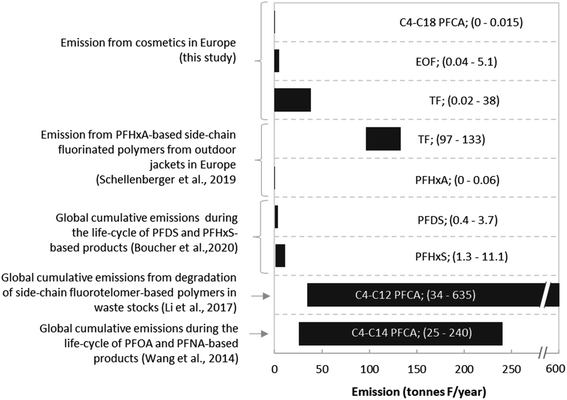
To investigate the importance of PFAS emission from cosmetics relative to other sources, emission estimates calculated here were compared to literature data, expressed on a fluorine equivalent basis (Fig. 4). While cosmetics after use release up to 0.015 tonnes F from C4–C18 PFCAs annually within the EEA, annual emission of C4–C14 PFCAs during the entire life-cycle of PFOA and PFNA-based products between 1951 and 2015 were much higher (25–240 tonnes F per year).31 In comparison, Li et al. (2017) estimated that degradation of side-chain fluorotelomer-based polymers in waste stocks would release ∼34–635 tonnes of C4–C12 PFCAs per year from 2015 onward into the environment32 (Fig. 4). Considering PFCA emissions alone, the contribution from cosmetics appears low. However, it is pertinent to note that our emission estimates for cosmetics only include release from the products themselves, whereas other studies included the emission from production as well. Considering the high emission scenario using TF data, emissions from cosmetics are only 3.5-fold lower than emissions from outdoor textile washing (i.e. 38 tonnes F per year vs. 133 tonnes F per year)33 and TF data are in the same range as estimates for the global emissions of fluorine during the life cycle of PFHxS- and PFDS-based products (0.4–11.1 tonnes F per year compared to the cosmetic range 0.02–38 tonnes F per year).34
Implications
The present work demonstrates that both regulated and unregulated PFAS exist in cosmetic products from the EEA, sometimes at concentrations exceeding EU limits for PFOA (25 ng g−1) and PFOA-related substances (1000 ng g−1) since the 4th of July 2020 for products sold in the EU.30 Considering that regulation of PFOA and PFOA-related substances under REACH has existed since 2019 and are now included under Annex A of the Stockholm Convention, new products or product version ought to exclude these compounds. While the proportion of products listing PFAS ingredients is small relative to the total number of products on the market, the emissions to wastewater and solid waste may be significant, albeit lower than other sources (e.g. outdoor textiles). Nevertheless, questions remain as to why these products are needed in cosmetics or why some regulated PFAS still appear in these products and how to exclude them in case of impurities.Study limitations, uncertainties and future improvements
This study was subject to a number of limitations and uncertainties related to inventory development, product sales estimates, analytical characterization and emission estimates, which are described in detail in the ESI.† A brief overview of the most important sources of error from each parameter is described here:Conflicts of interest
There are no conflicts to declare.Preprint
A preprint version of this work was published as a government report (https://www.kemi.se/en/publications/pms/2021/pm-9-21-pfass-in-cosmetics).Acknowledgements
Funding for this work was provided by the Swedish Research Council FORMAS (Grant 2018-00930) and The Swedish Chemicals Agency (KEMI). KEMI is thanked for an initial INCI list and Jenny Ivarsson and Mattias Carlsson Feng (KEMI) are thanked for helpful feedback and discussions.References
- OECD, Toward a new comprehensive global database of per-and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per-and polyfluoroalkyl substances, PFASs, 2018, https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en Search PubMed.
- Z. Wang, A. M. Buser, I. T. Cousins, S. Demattio, W. Drost, O. Johansson, K. Ohno, G. Patlewicz, A. M. Richard, G. W. Walker, G. S. White and E. Leinala, A New OECD Definition for Per- and Polyfluoroalkyl Substances, Environ. Sci. Technol., 2021, 55, 15575–15578 CrossRef CAS PubMed.
- J. Glüge, M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, X. Trier and Z. Wang, An overview of the uses of per- and polyfluoroalkyl substances (PFAS), Environ. Sci.: Processes Impacts, 2020, 22, 2345–2373 RSC.
- R. Lohmann, I. T. Cousins, J. C. DeWitt, J. Glüge, G. Goldenman, D. Herzke, A. B. Lindstrom, M. F. Miller, C. A. Ng, S. Patton, M. Scheringer, X. Trier and Z. Wang, Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS?, Environ. Sci. Technol., 2020, 54, 12820–12828 CrossRef CAS.
- D. Herzke, E. Olsson and S. Posner, Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway – A pilot study, Chemosphere, 2012, 88, 980–987 CrossRef CAS PubMed.
- J. P. Giesy and K. Kannan, Global Distribution of Perfluorooctane Sulfonate in Wildlife, Environ. Sci. Technol., 2001, 35, 1339–1342 CrossRef CAS PubMed.
- S. E. Fenton, A. Ducatman, A. Boobis, J. C. DeWitt, C. Lau, C. Ng, J. S. Smith and S. M. Roberts, Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research, Environ. Toxicol. Chem., 2021, 40, 606–630 CrossRef CAS PubMed.
- J. C. DeWitt, S. J. Blossom and L. A. Schaider, Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence, J. Exposure Sci. Environ. Epidemiol., 2019, 29, 148–156 CrossRef CAS.
- B. Göckener, M. Eichhorn, R. Lämmer, M. Kotthoff, J. Kowalczyk, J. Numata, H. Schafft, M. Lahrssen-Wiederholt and M. Bücking, Transfer of Per- and Polyfluoroalkyl Substances (PFAS) from Feed into the Eggs of Laying Hens. Part 1: Analytical Results Including a Modified Total Oxidizable Precursor Assay, J. Agric. Food Chem., 2020, 145, 106123 Search PubMed.
- L. T. Miaz, M. M. Plassmann, I. Gyllenhammar, A. Bignert, O. Sandblom, S. Lignell, A. Glynn and J. P. Benskin, Temporal trends of suspect- and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996–2017, Environ. Sci.: Processes Impacts, 2020, 22, 1071–1083 RSC.
- L.-M. L. Toms, J. Thompson, A. Rotander, P. Hobson, A. M. Calafat, K. Kato, X. Ye, S. Broomhall, F. Harden and J. F. Mueller, Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011, Environ. Int., 2014, 71, 74–80 CrossRef CAS.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products, 151. https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf.
- European Parliament and Council, Regulation (EC) No 1907/2006 of the European Parliament and Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC (Text with EEA relevance), 2006 Search PubMed.
- Risk assessment of fluorinated substances in cosmetic products, Danish Environmental Protection Agency, 2018, ISBN: 978-87-93710-94-8 Search PubMed.
- Y. Fujii, K. H. Harada and A. Koizumi, Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents, Chemosphere, 2013, 93, 538–544 CrossRef CAS PubMed.
- L. Schultes, R. Vestergren, K. Volkova, E. Westberg, T. Jacobson and J. P. Benskin, Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure, Environ. Sci.: Processes Impacts, 2018, 20, 1680–1690 RSC.
- H. D. Whitehead, M. Venier, Y. Wu, E. Eastman, S. Urbanik, M. L. Diamond, A. Shalin, H. Schwartz-Narbonne, T. A. Bruton, A. Blum, Z. Wang, M. Green, M. Tighe, J. T. Wilkinson, S. McGuinness and G. F. Peaslee, Fluorinated Compounds in North American Cosmetics, Environ. Sci. Technol. Lett., 2021, 8(7), 538–544 CrossRef CAS.
- B. Godin and E. Touitou, Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models, Adv. Drug Delivery Rev., 2007, 59, 1152–1161 CrossRef CAS.
- J. Franko, B. J. Meade, H. F. Frasch, A. M. Barbero and S. E. Anderson, Dermal Penetration Potential of Perfluorooctanoic Acid (PFOA) in Human and Mouse Skin, J. Toxicol. Environ. Health, Part A, 2012, 75, 50–62 CrossRef CAS.
- J. P. Benskin, B. Li, M. G. Ikonomou, J. R. Grace and L. Y. Li, Per- and Polyfluoroalkyl Substances in Landfill Leachate: Patterns, Time Trends, and Sources, Environ. Sci. Technol., 2012, 46, 11532–11540 CrossRef PubMed.
- R. Aro, U. Eriksson, A. Kärrman, F. Chen, T. Wang and L. W. Y. Yeung, Fluorine Mass Balance Analysis of Effluent and Sludge from Nordic Countries, ACS EST Water, 2021, 9, 2087–2096 CrossRef.
- K. Röhler, A. A. Haluska, B. Susset, B. Liu and P. Grathwohl, Long-term behavior of PFAS in contaminated agricultural soils in Germany, J. Contam. Hydrol., 2021, 241, 103812 CrossRef PubMed.
- Cosmetics Europe, Market Performance 2019 – European Cosmetic, Toiletry & Perfumery, 2020 Search PubMed.
- L. Schultes, G. F. Peaslee, J. D. Brockman, A. Majumdar, S. R. McGuinness, J. T. Wilkinson, O. Sandblom, R. A. Ngwenyama and J. P. Benskin, Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform?, Environ. Sci. Technol. Lett., 2019, 6, 73–78 CrossRef CAS.
- A. Kärrman, L. W. Y. Yeung, K. M. Spaan, F. T. Lange, M. A. Nguyen, M. Plassmann, C. A. de Wit, M. Scheurer, R. Awad and J. P. Benskin, Can determination of extractable organofluorine (EOF) be standardized? First interlaboratory comparisons of EOF and fluorine mass balance in sludge and water matrices, Environ. Sci.: Processes Impacts, 2021, 23, 1458–1465 RSC.
- TNS Kantar, Consumer habits on removal and disposal of leave-on cosmetics products commissioned by Cosmetics, Europe, 2018 Search PubMed.
- A. Brinch, A. A. Jensen and F. Christensen, Risk Assessment of Fluorinated Substances in Cosmetic Products, The Danish Environmental Protection Agency, 2018, pp. 1–116 Search PubMed.
- K. M. Spaan, C. van Noordenburg, M. M. Plassmann, L. Schultes, S. Shaw, M. Berger, M. P. Heide-Jørgensen, A. Rosing-Asvid, S. M. Granquist, R. Dietz, C. Sonne, F. Rigét, A. Roos and J. P. Benskin, Fluorine Mass Balance and Suspect Screening in Marine Mammals from the Northern Hemisphere, Environ. Sci. Technol., 2020, 54, 4046–4058 CrossRef CAS PubMed.
- C. Peng, J. Da, Y. Hsu and T. E. Hogen-Esch, Polymeric Fluorosurfactant-Mediated Perfluorocarbon Emulsions, J. Dispersion Sci. Technol., 2006, 27, 377–387 CrossRef CAS.
- Commission Regulation (EU) 2017/1000 – amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards perfluorooctanoic acid (PFOA), its salts and PFOA-related substances, 2017 Search PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources, Environ. Int., 2014, 70, 62–75 CrossRef CAS.
- L. Li, J. Liu, J. Hu and F. Wania, Degradation of Fluorotelomer-Based Polymers Contributes to the Global Occurrence of Fluorotelomer Alcohol and Perfluoroalkyl Carboxylates: A Combined Dynamic Substance Flow and Environmental Fate Modeling Analysis, Environ. Sci. Technol., 2017, 51, 4461–4470 CrossRef CAS PubMed.
- S. Schellenberger, C. Jönsson, P. Mellin, O. A. Levenstam, I. Liagkouridis, A. Ribbenstedt, A.-C. Hanning, L. Schultes, M. M. Plassmann, C. Persson, I. T. Cousins and J. P. Benskin, Release of Side-Chain Fluorinated Polymer-Containing Microplastic Fibers from Functional Textiles During Washing and First Estimates of Perfluoroalkyl Acid Emissions, Environ. Sci. Technol., 2019, 53, 14329–14338 CrossRef CAS PubMed.
- J. M. Boucher, I. T. Cousins, M. Scheringer, K. Hungerbühler and Z. Wang, Toward a Comprehensive Global Emission Inventory of C 4 –C 10 Perfluoroalkanesulfonic Acids (PFSAs) and Related Precursors: Focus on the Life Cycle of C 6 - and C 10 -Based Products, Environ. Sci. Technol. Lett., 2019, 6, 1–7 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2em00123c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |