Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Inhibitors of thiol-mediated uptake†
Yangyang
Cheng
 ,
Anh-Tuan
Pham
,
Anh-Tuan
Pham
 ,
Takehiro
Kato
,
Bumhee
Lim
,
Dimitri
Moreau
,
Takehiro
Kato
,
Bumhee
Lim
,
Dimitri
Moreau
 ,
Javier
López-Andarias
,
Javier
López-Andarias
 ,
Lili
Zong‡
,
Lili
Zong‡
 ,
Naomi
Sakai
,
Naomi
Sakai
 and
Stefan
Matile
and
Stefan
Matile
 *
*
Department of Organic Chemistry, University of Geneva, Geneva, Switzerland. E-mail: stefan.matile@unige.ch; Web: http://www.unige.ch/sciences/chiorg/matile/ Tel: +41 22 379 6523
First published on 18th November 2020
Abstract
Ellman's reagent has caused substantial confusion and concern as a probe for thiol-mediated uptake because it is the only established inhibitor available but works neither efficiently nor reliably. Here we use fluorescent cyclic oligochalcogenides that enter cells by thiol-mediated uptake to systematically screen for more potent inhibitors, including epidithiodiketopiperazines, benzopolysulfanes, disulfide-bridged γ-turned peptides, heteroaromatic sulfones and cyclic thiosulfonates, thiosulfinates and disulfides. With nanomolar activity, the best inhibitors identified are more than 5000 times better than Ellman's reagent. Different activities found with different reporters reveal thiol-mediated uptake as a complex multitarget process. Preliminary results on the inhibition of the cellular uptake of pseudo-lentivectors expressing SARS-CoV-2 spike protein do not exclude potential of efficient inhibitors of thiol-mediated uptake for the development of new antivirals.
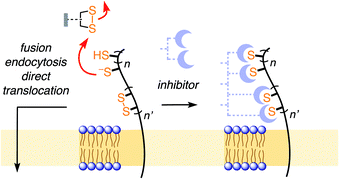
Thiol-mediated uptake1–10 has been developed to explain surprisingly efficient cellular uptake of substrates attached to thiol-reactive groups, most notably disulfides. The key step of this mechanism is the dynamic covalent thiol-disulfide exchange between disulfides of the substrates and exofacial thiols on cell surfaces (Fig. 1). The covalently bound substrate then enters the cell either by fusion, endocytosis, or direct translocation across the plasma membrane into the cytosol. Thiol-disulfide exchange has been confirmed to play an essential role in the cellular entry of some viruses1,11–14 and toxins.2 Indeed, diphtheria toxin and HIV were among the first to be recognized to enter cells via thiol-mediated uptake.1,2 The involvement of cell-surface thiols in cellular uptake is most often probed by inhibition with Ellman's reagent (DTNB). However, this test is not always reliable, in part due to the comparably poor reactivity of DTNB, and the comparably high reactivity of the disulfide obtained as a product. Thus, the importance of thiol-mediated uptake for viral entry and beyond remains, at least in part, unclear.
We became interested in thiol-mediated uptake3–5 while studying the cytosolic delivery of substrates such as drugs, probes and also larger objects like proteins or quantum dots with cell-penetrating poly(disulfide)s.6 Our recent focus shifted to cyclic oligochalcogenides (COCs) to increase speed and selectivity of dynamic covalent thiol-oligochalcogenide exchange, and, most importantly, to assure reversibility, i.e., mobility during uptake, with a covalently tethered, intramolecular leaving group.7 With increasingly unorthodox COC chemistry, from strained disulfides7,8 and diselenides9 to adaptive dynamic covalent networks produced by polysulfanes,10 uptake activities steadily increased. Their high activities suggested that the same, or complementary, COCs could also function as powerful inhibitors of thiol-mediated uptake that ultimately might perhaps lead to antivirals. In the following, this hypothesis is developed further.
Fluorescently labeled COCs 18 and 210 were selected as reporters for the screening of thiol-mediated uptake inhibitors because of their high activity, their destination in the cytosol, and their different characteristics (Fig. 2). The COC in 1 is an epidithiodiketopiperazine (ETP). With a CSSC dihedral angle ∼0°, ETPs drive ring tension to the extreme.15,16 Ring-opening thiol-disulfide exchange is ultrafast, and the released thiols are acidic enough to continue exchanging in neutral water, including ring closure.8 This unique exchange chemistry coincides with efficient cellular uptake and poor retention on thiol affinity columns.8
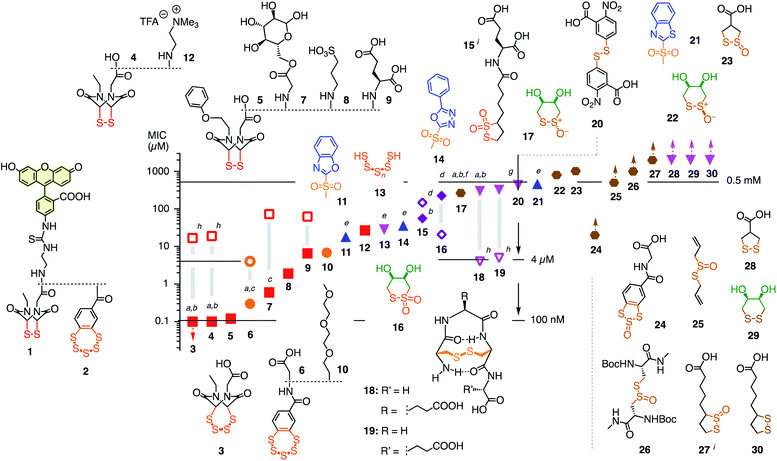 | ||
| Fig. 2 Structure of reporters 1 and 2 and inhibitor candidates 3–30 with their concentrations needed to inhibit by ∼15% (MIC) the uptake of 1 (1 h pre-incubation with inhibitors, 30 min incubation with reporter, filled symbols) and 2 (4 h pre-incubation, empty symbols). Red squares: ETPs; orange circles: BPSs; blue upward triangles: heteroaromatic sulfones; purple diamonds: thiosulfonates; magenta downward triangles: di- and polysulfides; brown hexagons: thiosulfinates. Symbols with upward arrows: MIC not reached at the highest concentration tested. Symbols with downward arrows indicate the lowest concentration tested already exceeds the MIC. (a) Similarly active upon co-incubation of reporters and inhibitor; (b–d) similarly (b), less (c), or more (d) active upon co-incubation in the presence of serum (mostly 6 h); (e) pre-incubation for 15 min; (f) isomerizes into cis22; (g) V-shaped DRC (see Fig. 3f); (h) pre-incubation for 30 min, co-incubation with 2; (i) mixture of regioisomers. | ||
The COC in 2 is a benzopolysulfane (BPS). Like ETPs, BPSs occur in natural products and have inspired total synthesis.17 Unlike ETPs, BPSs are not strained but evolve into adaptive networks of extreme sulfur species for cells to select from. Uptake efficiencies and retention on thiol affinity columns exceed other COCs clearly.10,18
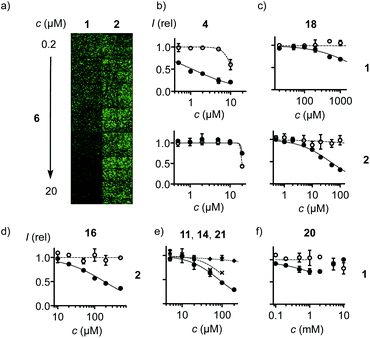
With COCs 1 and 2 as cell-penetrating reporters, a fully automated, fluorescent microscopy image-based high-content high-throughput (HCHT)19 inhibitor screening assay was developed. HeLa cells in multiwell plates are incubated with a reporter at constant and inhibitors at varying concentrations and incubation times. Hindered reporter uptake then causes decrease of fluorescence inside of cells (Fig. 3a). Automated data analysis19 was established to extract average fluorescence intensity per cell and, at the same time, cell viability from propidium iodide negative nuclei count (Fig. 3 and S3–S6†). Standard assay conditions consisted of pre-incubation of HeLa cells with inhibitors for different periods of time, followed by the removal of inhibitors and the addition of reporters, thus excluding possible interactions between the two in the extracellular environment. In alternative co-incubation conditions, inhibitors were not removed before the addition of reporters to allow for eventual interactions between the two.
Among the very high number of thiol-reactive probes, compounds 3–30 were selected based on promise, experience, availability and accessibility. Main focus was on COCs offering increasingly extreme sulfur chemistry because dynamic covalent thiol-oligochalcogenide exchange with different intramolecular leaving groups promises access to different exchange cascades for the intramolecular and, perhaps, also intermolecular crosslinking of the target proteins. More hydrophilic, often anionic COCs were preferred to prevent diffusion into cells and thus minimize toxicity. The expectation was that from such a sketchy outline of an immense chemical space, leads could be identified for future, more systematic exploration. Reporters 1 and 2 and candidates 3–30 were prepared by substantial multistep synthesis (Schemes S1–S11 and Fig. S47–S93,† commercially available: 20, 25, 30). Inhibitors were numbered in the order of efficiency against reporter 1, evaluated by their minimum inhibitory concentrations (MICs), i.e., concentrations that cause a ∼15% reduction of reporter uptake in cells (Fig. 2 and Tables S1–S37†). We chose to use MICs because half-maximal inhibitions could not always be reached due to the onset of toxicity, formally anticooperative, or even V-shaped dose–response curves (DRCs, e.g., Fig. 3b–f, all DRCs can be found in the ESI, Fig. S7–S43†). MICs are usually below the half-maximal cell growth inhibition concentration (GI50, Tables S1–S37†).
Among the most potent inhibitors of ETP reporter 1 were ETPs 4 and 5 (Fig. 2, 3b). This intriguing self-inhibition was even surpassed by the expanded cyclic tetrasulfide ETP43 (MIC < 0.1 μM), which was of interest because they are much poorer transporters.10 Further formal ring expansion leads to cyclic pentasulfides BPS56 as equally outstanding inhibitors (MIC ≈ 0.3 μM). This trend toward the adaptive networks, reminiscent of elemental sulfur chemistry, did not extend toward inorganic polysulfides 13 (MIC ≈ 20 μM). ETPs 4 and 5 were sensitive to modification of the carboxylate, with the cationic 12 being the worst (MIC ≈ 30 μM) and the neutral glucose hemiacetal 7 the most promising (MIC ≈ 0.5 μM).
Although this study focuses on increasingly extreme dynamic covalent COC chemistry, the inclusion of one example for covalent C–S bond formation was of interest for comparison. The classical iodoacetamides7 and maleimides4 were more toxic than active (not shown). However, nucleophilic aromatic substitution of heteroaromatic sulfones,20 just developed for the efficient bioorthogonal conversion of thiols into sulfides, was more promising. Weaker than dynamic covalent COCs, this irreversible inhibition was best with benzoxazole 11 (MIC ≈ 15 μM) and decreased in accordance with reactivity toward free thiols to oxadiazole 14 and benzothiazole 21 (MIC ≈ 300 μM, Fig. 3e).
At constant pH, Ellman's reagent 20 was confirmed to be erratic also in this assay. The DRC showed minor inhibition up to around 2 mM, which disappeared again at higher concentrations (Fig. 3f). Other cyclic disulfides were inactive as well (28–30). Also disappointing were oxidized disulfides, that is thiosulfinates, including allicin 25, the main odorant component of garlic,21,22 oxidized cystine 26 and oxidized lipoic acid 27. Thiosulfinates were of interest because they should selectively target the vicinal thiols of reduced disulfides bridges, producing two disulfides.23 The most active trans dithioerythrol (DTE) thiosulfinate 17 isomerized with time into the less active, hydrogen-bonded cis isomer 22 (Fig. S46†).
Reporter 2 was more difficult to inhibit than 1, as expected from high activity with extreme retention on thiol affinity columns.10,18 For instance, BPS 6 was very efficient against ETP 1 but much less active against BPS 2 (Fig. 3a), although longer pre-incubation could lower the MIC down to 4 μM (Fig. 2, S41†). The complementary ETP 4 “self-inhibited” ETP 1 but was also unable to inhibit BPS 2 as efficiently (Fig. 3b). Among the best inhibitors of BPS 2 upon co-incubation were disulfide bridged γ-turn24 peptides 18 and 19 (MIC ≈ 5 μM), both less active against 1 (MIC ≈ 300 μM, Fig. 3c). Disulfide-bridged γ-turn CXC peptides consist of an 11-membered ring with significant Prelog strain. They were introduced by Wu and coworkers as transporters for efficient cytosolic delivery.5 The cyclic thiosulfonates 15 and 16 showed promising activities against both 1 and 2, and were tolerant toward the presence of serum (Fig. 2d, S33 and S42†). Contrary to thiosulfinate 27, the oxidation of lipoic acid to pure thiosulfonates was not successful so far. However, weakly detectable activity of the lipoyl-glutamate conjugate oxidized to the thiosulfinate (MIC ≈ 350 μM, not shown) compared to the inactive thiosulfinate 27 implied that lipoic acid oxidized to the thiosulfonate would also be less active than the glutamate conjugate 15.
The oxidized DTE 1625–28 was particularly intriguing because it was more potent against 2 and could achieve nearly complete inhibition (MIC ∼ 20 μM, Fig. 3d). Highly selective for thiols, the cyclic thiosulfonate 16 was stable for weeks at room temperature, without precaution, in all solvents tested. The disulfides and sulfinates obtained from exchange with thiols were stable as well, and the latter can further react with disulfides27 for intramolecular or eventually intermolecular crosslinking of the target proteins.
The overall mismatched inhibition profiles found for reporters 1 and 2 supported that thiol-mediated uptake proceeds through a series of at least partially uncoupled parallel multitarget systems instead of a specific single protein or membrane target. From proteomics studies with cysteine-reactive irreversible probes, it is known that different probes generally target different proteins.29b Proteomics analysis29a for asparagusic acid derived transporters supports the involvement of many targets beyond the commonly considered protein disulfide isomerases and the confirmed transferrin receptor.12–14,26–30 The unusual, formally anti-cooperative (Hill coefficients < 1) DRCs further supported thiol-mediated uptake as complex multitarget systems.
Despite the complexity of these systems, results did not much depend on assay conditions. Compared to the standard protocol of pre-incubation with inhibitors followed by inhibitor removal and incubation with reporters 1 or 2 for detection, the co-incubation protocol, in which pre-incubation with inhibitors is followed by co-incubation with reporters 1 or 2 without inhibitor removal, gave reasonably similar results (Fig. 2). Inhibition characteristics naturally depended on pre-incubation time, with weaker activities at shorter and longer times, reflecting incomplete exchange and cellular response or other ways of inhibitor destruction, respectively. The presence of serum also did not affect the activities much (Fig. 2b–d).
Preliminary studies on antiviral activity were performed with pseudo-lentivectors31 that express the D614G mutant11 of the SARS-CoV-2 spike protein and code for a luciferase reporter gene, which is expressed by the infected cells.12 A549 human lung alveolar basal epithelium cell line constitutively overexpressing ACE2 and TMPRSS2 was selected to facilitate the entry of the SARS-CoV-2 spike pseudo-lentivirus. The most significant activities were found for DTE thiosulfonate 16 with an IC50 around 50 μM, while toxicity was detected only at 500 μM (Fig. S44†). The onset of inhibition could be observed for tetrasulfide ETP 3 at 50 μM, but it coincided with the appearance of cytotoxicity. Protease inhibition is less likely to be the mode of action, as similar activity was found with wild type A549 cells transduced with a standard lentivirus expressing vesicular-stomatitis virus G surface protein VSVG (Fig. S45†).13 Short incubation times of cells and inhibitors before the addition of viruses disfavored contributions from changes in gene expression. More detailed studies are ongoing.
The lessons learned from this study are that, firstly, thiol-mediated uptake can be inhibited efficiently by thiol-reactive reagents, confirming that thiol-mediated uptake exists and transporters like ETP 1 and BPS 2 do not simply diffuse into cells; the best inhibitors are more than 5000 times better than Ellman's reagent. Secondly, inhibitor efficiencies vary with the transporters, supporting that thiol-mediated uptake operates as a complex multitarget system. The best inhibitors are COCs that operate with fast dynamic covalent exchange, suggesting that the reversibility provided by COCs is important. The inhibition of thiol-mediated uptake might contribute to activities of thiol-reactive antivirals such as 16, ETPs or ebselen, although they have been shown to bind to zinc fingers or inhibit proteases.16,25,32–34 Finally, the inhibitors reported here could also be of interest for delivery applications and might be worth investigation with regard to antiviral activity. We currently plan to focus more systematically on the most promising leads within COCs, particularly cyclic thiosulfonates, and to expand the screening campaign toward new attractive motifs.33–35
Experimental section
See ESI.†Conflicts of interest
A U.S. Patent application has been filed (No. 63/073863).Acknowledgements
We thank Neurix and the NMR, MS and Bioimaging platforms for services, and the University of Geneva, the National Centre for Competence in Research (NCCR) Chemical Biology, the NCCR Molecular Systems Engineering and the Swiss NSF for financial support. pCG1_SCoV-2 plasmid encoding SARS-CoV-2 S-protein was provided by Stefan Pöhlmann (Deutsches Primatenzentrum, Leibniz-Institute for Primate Research, Göttingen).Notes and references
- (a) E. P. Feener, W. C. Shen and H. J. Ryser, J. Biol. Chem., 1990, 265, 18780–18785 CAS; (b) H. J.-P. Ryser and R. Flückiger, Drug Discovery Today, 2005, 10, 1085–1094 CrossRef CAS.
- (a) H. J. Ryser, R. Mandel and F. Ghani, J. Biol. Chem., 1991, 266, 18439–18442 CAS; (b) P. Ø. Falnes and S. Olsnes, J. Biol. Chem., 1995, 270, 20787–20793 CrossRef CAS.
- (a) S. Ulrich, Acc. Chem. Res., 2019, 52, 510–519 CrossRef CAS; (b) Y. Zhang, Y. Qi, S. Ulrich, M. Barboiu and O. Ramström, Mater. Chem. Front., 2020, 4, 489–506 RSC; (c) J. Zhou, Z. Shao, J. Liu, Q. Duan, X. Wang, J. Li and H. Yang, ACS Appl. Bio Mater., 2020, 3, 2686–2701 CrossRef CAS; (d) A. G. Torres and M. J. Gait, Trends Biotechnol., 2012, 30, 185–190 CrossRef CAS; (e) S. Aubry, F. Burlina, E. Dupont, D. Delaroche, A. Joliot, S. Lavielle, G. Chassaing and S. Sagan, FASEB J., 2009, 23, 2956–2967 CrossRef CAS; (f) J. Lu, H. Wang, Z. Tian, Y. Hou and H. Lu, J. Am. Chem. Soc., 2020, 142, 1217–1221 CrossRef CAS; (g) C. Wu, C. Belenda, J.-C. Leroux and M. A. Gauthier, Chem.–Eur. J., 2011, 17, 10064–10070 CrossRef CAS; (h) R. Bej, P. Dey and S. Ghosh, Soft Matter, 2020, 16, 11–26 RSC; (i) S. Du, S. S. Liew, L. Li and S. Q. Yao, J. Am. Chem. Soc., 2018, 140, 15986–15996 CrossRef CAS; (j) D. Oupický and J. Li, Macromol. Biosci., 2014, 14, 908–922 CrossRef; (k) A. Kohata, P. K. Hashim, K. Okuro and T. Aida, J. Am. Chem. Soc., 2019, 141, 2862–2866 CrossRef CAS; (l) C. R. Drake, A. Aissaoui, O. Argyros, M. Thanou, J. H. G. Steinke and A. D. Miller, J. Controlled Release, 2013, 171, 81–90 CrossRef CAS; (m) Z. Shu, I. Tanaka, A. Ota, D. Fushihara, N. Abe, S. Kawaguchi, K. Nakamoto, F. Tomoike, S. Tada, Y. Ito, Y. Kimura and H. Abe, Angew. Chem., Int. Ed., 2019, 58, 6611–6615 CrossRef CAS; (n) J. Zhou, L. Sun, L. Wang, Y. Liu, J. Li, J. Li, J. Li and H. Yang, Angew. Chem., Int. Ed., 2019, 58, 5236–5240 CrossRef CAS; (o) X. Li, Y. Hou, X. Meng, C. Ge, H. Ma, J. Li and J. Fang, Angew. Chem., Int. Ed., 2018, 57, 6141–6145 CrossRef CAS; (p) A. Tirla and P. Rivera-Fuentes, Biochemistry, 2019, 58, 1184–1187 CrossRef CAS; (q) P. Yuan, X. Mao, X. Wu, S. S. Liew, L. Li and S. Q. Yao, Angew. Chem., Int. Ed., 2019, 58, 7657–7661 CrossRef CAS; (r) M. Balakirev, G. Schoehn and J. Chroboczek, Chem. Biol., 2000, 7, 813–819 CrossRef CAS.
- (a) A. Kichler, J. S. Remy, O. Boussif, B. Frisch, C. Boeckler, J. P. Behr and F. Schuber, Biochem. Biophys. Res. Commun., 1995, 209, 444–450 CrossRef CAS; (b) T. Li and S. Takeoka, Int. J. Nanomed., 2014, 9, 2849–2861 Search PubMed.
- (a) X. Meng, T. Li, Y. Zhao and C. Wu, ACS Chem. Biol., 2018, 13, 3078–3086 CrossRef CAS; (b) T. Li, W. Gao, J. Liang, M. Zha, Y. Chen, Y. Zhao and C. Wu, Anal. Chem., 2017, 89, 8501–8508 CrossRef CAS.
- (a) E.-K. Bang, G. Gasparini, G. Molinard, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2013, 135, 2088–2091 CrossRef CAS; (b) G. Gasparini, E.-K. Bang, G. Molinard, D. V. Tulumello, S. Ward, S. O. Kelley, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2014, 136, 6069–6074 CrossRef CAS; (c) E. Derivery, E. Bartolami, S. Matile and M. Gonzalez-Gaitan, J. Am. Chem. Soc., 2017, 139, 10172–10175 CrossRef CAS.
- G. Gasparini, G. Sargsyan, E.-K. Bang, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2015, 54, 7328–7331 CrossRef CAS.
- L. Zong, E. Bartolami, D. Abegg, A. Adibekian, N. Sakai and S. Matile, ACS Cent. Sci., 2017, 3, 449–453 CrossRef CAS.
- N. Chuard, A. I. Poblador-Bahamonde, L. Zong, E. Bartolami, J. Hildebrandt, W. Weigand, N. Sakai and S. Matile, Chem. Sci., 2018, 9, 1860–1866 RSC.
- Y. Cheng, L. Zong, J. López-Andarias, E. Bartolami, Y. Okamoto, T. R. Ward, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2019, 58, 9522–9526 CrossRef CAS.
- L. Zhang, C. B. Jackson, H. Mou, A. Ojha, E. S. Rangarajan, T. Izard, M. Farzan and H. Choe, 2020, bioRxiv:2020.06.12.148726.
- M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T. S. Schiergens, G. Herrler, N.-H. Wu, A. Nitsche, M. A. Müller, C. Drosten and S. Pöhlmann, Cell, 2020, 181, 271–280 CrossRef CAS.
- X. Sun, S. L. Roth, M. A. Bialecki and G. R. Whittaker, Future Virol., 2010, 5, 85–96 CrossRef CAS.
- (a) S. Hati and S. Bhattacharyya, ACS Omega, 2020, 5, 16292–16298 CrossRef CAS; (b) E. Fenouillet, R. Barbouche and I. M. Jones, Antioxid. Redox Signaling, 2007, 9, 1009–1034 CrossRef CAS; (c) J. Shang, Y. Wan, C. Luo, G. Ye, Q. Geng, A. Auerbach and F. Li, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 11727–11734 CrossRef CAS; (d) L. Mathys and J. Balzarini, Expert Opin. Ther. Targets, 2016, 20, 123–143 CrossRef CAS.
- (a) H. R. Kilgore, C. Olsson, K. A. D'Angelo, M. Movassaghi and R. T. Raines, J. Am. Chem. Soc., 2020, 142, 15107–15115 CrossRef CAS; (b) J. Kim and M. Movassaghi, Acc. Chem. Res., 2015, 48, 1159–1171 CrossRef CAS; (c) T. R. Welch and R. M. Williams, Nat. Prod. Rep., 2014, 31, 1376–1404 RSC.
- C. R. M. Asquith, B. C. Sil, T. Laitinen, G. J. Tizzard, S. J. Coles, A. Poso, R. Hofmann-Lehmann and S. T. Hilton, Bioorg. Med. Chem., 2019, 27, 4174–4184 CrossRef CAS.
- (a) R. Steudel, Chem. Rev., 2002, 102, 3905–3946 CrossRef CAS; (b) L. S. Konstantinova, O. A. Rakitin and C. W. Rees, Chem. Rev., 2004, 104, 2617–2630 CrossRef CAS; (c) V. Behar and S. J. Danishefsky, J. Am. Chem. Soc., 1993, 115, 7017–7018 CrossRef CAS; (d) F. D. Toste and I. W. J. Still, J. Am. Chem. Soc., 1995, 117, 7261–7262 CrossRef CAS; (e) J. Xu, M. Chatterjee, T. D. Baguley, J. Brouillette, P. Kurup, D. Ghosh, J. Kanyo, Y. Zhang, K. Seyb, C. Ononenyi, E. Foscue, G. M. Anderson, J. Gresack, G. D. Cuny, M. A. Glicksman, P. Greengard, T. T. Lam, L. Tautz, A. C. Nairn, J. A. Ellman and P. J. Lombroso, PLoS Biol., 2014, 12, e1001923 CrossRef; (f) A. Mahendran, A. Vuong, D. Aebisher, Y. Gong, R. Bittman, G. Arthur, A. Kawamura and A. Greer, J. Org. Chem., 2010, 75, 5549–5557 CrossRef CAS.
- J. López-Andarias, J. Saarbach, D. Moreau, Y. Cheng, E. Derivery, Q. Laurent, M. González-Gaitán, N. Winssinger, N. Sakai and S. Matile, J. Am. Chem. Soc., 2020, 142, 4784–4792 CrossRef.
- R. Martinent, J. López-Andarias, D. Moreau, Y. Cheng, N. Sakai and S. Matile, Beilstein J. Org. Chem., 2020, 16, 2007–2016 CrossRef CAS.
- H. F. Motiwala, Y.-H. Kuo, B. L. Stinger, B. A. Palfey and B. R. Martin, J. Am. Chem. Soc., 2020, 142, 1801–1810 CrossRef CAS.
- U. Münchberg, A. Anwar, S. Mecklenburg and C. Jacob, Org. Biomol. Chem., 2007, 5, 1505–1518 RSC.
- T. M. Shojai, A. G. Langeroudi, V. Karimi, A. Barin and N. Sadri, Avicenna J. Phytomed., 2016, 6, 458–467 CAS.
- D. P. Donnelly, M. G. Dowgiallo, J. P. Salisbury, K. C. Aluri, S. Iyengar, M. Chaudhari, M. Mathew, I. Miele, J. R. Auclair, S. A. Lopez, R. Manetsch and J. N. Agar, J. Am. Chem. Soc., 2018, 140, 7377–7380 CrossRef CAS.
- (a) R. Kishore and P. Balaram, Biopolymers, 1985, 24, 2041–2043 CrossRef CAS; (b) B. Schmidt, S. Lindman, W. Tong, G. Lindeberg, A. Gogoll, Z. Lai, M. Thörnwall, B. Synnergren, A. Nilsson, C. J. Welch, M. Sohtell, C. Westerlund, F. Nyberg, A. Karlén and A. Hallberg, J. Med. Chem., 1997, 40, 903–919 CrossRef CAS; (c) E. Vass, M. Hollósi, F. Besson and R. Buchet, Chem. Rev., 2003, 103, 1917–1954 CrossRef CAS; (d) I. Cacciatore, A. D. Stefano, S. Duprè, E. Morera, F. Pinnen and A. Spirito, Bioorg. Chem., 2003, 31, 109–121 CrossRef CAS; (e) K. J. Woycechowsky and R. T. Raines, Biochemistry, 2003, 42, 5387–5394 CrossRef CAS; (f) U. Derewenda, T. Boczek, K. L. Gorres, M. Yu, L. Hung, D. Cooper, A. Joachimiak, R. T. Raines and Z. S. Derewenda, Biochemistry, 2009, 48, 8664–8671 CrossRef CAS.
- D. C. Baker, L. E. Henderson, W. G. Rice and R. R. Schultz, WO1998001440, PCT/US1997/010870, 1998.
- B. K. Law and R. K. Castellano, WO2019241644, PCT/US2019/037209, 2019.
- R. B. Ferreira, M. E. Law, S. C. Jahn, B. J. Davis, C. D. Heldermon, M. Reinhard, R. K. Castellano and B. K. Law, Oncotarget, 2015, 6, 10445–10459 CrossRef.
- K. Klann, D. Bojkova, G. Tascher, S. Ciesek, C. Münch and J. Cinatl, Mol. Cell, 2020, 80, 164–174 CrossRef CAS.
- (a) D. Abegg, G. Gasparini, D. G. Hoch, A. Shuster, E. Bartolami, S. Matile and A. Adibekian, J. Am. Chem. Soc., 2017, 139, 231–238 CrossRef CAS; (b) D. Abegg, R. Frei, L. Cerato, D. P. Hari, C. Wang, J. Waser and A. Adibekian, Angew. Chem., Int. Ed., 2015, 54, 10852–10857 CrossRef CAS.
- (a) H.-S. Cho, K. Mason, K. X. Ramyar, A. M. Stanley, S. B. Gabelli, D. W. Denney and D. J. Leahy, Nature, 2003, 421, 756–760 CrossRef CAS; (b) M. A. Lemmon and J. Schlessinger, Cell, 2010, 141, 1117–1134 CrossRef CAS; (c) M. C. Yi and C. Khosla, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 197–222 CrossRef CAS; (d) H. A. Khan and B. Mutus, Front. Chem., 2014, 2, 70 Search PubMed.
- M. Giry-Laterrière, E. Verhoeyen and P. Salmon, Methods Mol. Biol., 2011, 737, 183–209 CrossRef.
- (a) W. G. Rice, C. A. Schaeffer, B. Harten, F. Villinger, T. L. South, M. F. Summers, L. E. Henderson, J. W. Bess, L. O. Arthur, J. S. McDougal, S. L. Orloff, J. Mendeleyev and E. Kun, Nature, 1993, 361, 473–475 CrossRef CAS; (b) W. G. Rice, J. G. Supko, L. Malspeis, R. W. Buckheit, D. Clanton, M. Bu, L. Graham, C. A. Schaeffer, J. A. Turpin, J. Domagala, R. Gogliotti, J. P. Bader, S. M. Halliday, L. Coren, R. C. Sowder, L. O. Arthur and L. E. Henderson, Science, 1995, 270, 1194–1197 CrossRef CAS.
- (a) Z. Jin, X. Du, Y. Xu, Y. Deng, M. Liu, Y. Zhao, B. Zhang, X. Li, L. Zhang, C. Peng, Y. Duan, J. Yu, L. Wang, K. Yang, F. Liu, R. Jiang, X. Yang, T. You, X. Liu, X. Yang, F. Bai, H. Liu, X. Liu, L. W. Guddat, W. Xu, G. Xiao, C. Qin, Z. Shi, H. Jiang, Z. Rao and H. Yang, Nature, 2020, 582, 289–293 CrossRef CAS; (b) C. A. Menéndez, F. Byléhn, G. R. Perez-Lemus, W. Alvarado and J. J. de Pablo, Sci. Adv., 2020, eabd3045 Search PubMed; (c) K. Sargsyan, C.-C. Lin, T. Chen, C. Grauffel, Y.-P. Chen, W.-Z. Yang, H. Yuan and C. Lim, Chem. Sci., 2020, 11, 9904–9909 RSC; (d) H. Sies and M. J. Parnham, Free Radical Biol. Med., 2020, 156, 107–112 CrossRef CAS.
- Preliminary results confirm that also ebselen inhibits thiol-mediated uptake.
- (a) R. Gabizon, A. Shraga, P. Gehrtz, E. Livnah, Y. Shorer, N. Gurwicz, L. Avram, T. Unger, H. Aharoni, S. Albeck, A. Brandis, Z. Shulman, B.-Z. Katz, Y. Herishanu and N. London, J. Am. Chem. Soc., 2020, 142, 11734–11742 CrossRef CAS; (b) L. J. Macpherson, A. E. Dubin, M. J. Evans, F. Marr, P. G. Schultz, B. F. Cravatt and A. Patapoutian, Nature, 2007, 445, 541–545 CrossRef CAS; (c) E. D. Evans and B. L. Pentelute, Org. Biomol. Chem., 2019, 17, 1862–1868 RSC; (d) R. Tessier, R. K. Nandi, B. G. Dwyer, D. Abegg, C. Sornay, J. Ceballos, S. Erb, S. Cianférani, A. Wagner, G. Chaubet, A. Adibekian and J. Waser, Angew. Chem., Int. Ed., 2020, 59, 10961–10970 CrossRef CAS; (e) C. Zambaldo, E. V. Vinogradova, X. Qi, J. Iaconelli, R. M. Suciu, M. Koh, K. Senkane, S. R. Chadwick, B. B. Sanchez, J. S. Chen, A. K. Chatterjee, P. Liu, P. G. Schultz, B. F. Cravatt and M. J. Bollong, J. Am. Chem. Soc., 2020, 142, 8972–8979 CrossRef CAS; (f) J. C. Lukesh, B. Vanveller and R. T. Raines, Angew. Chem., Int. Ed., 2013, 52, 12901–12904 CrossRef CAS; (g) C. Bravin and C. A. Hunter, Chem. Sci., 2020, 11, 9122–9125 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed procedures and results for all reported experiments. See DOI: 10.1039/d0sc05447j |
| ‡ Current address: School of Pharmaceutical Sciences, Xiamen University, Xiamen 361102, China |
| This journal is © The Royal Society of Chemistry 2021 |