 Open Access Article
Open Access ArticleEmerging investigator series: the dynamics of particle size distributions need to be accounted for in bioavailability modelling of nanoparticles
Martina G.
Vijver†
 *a,
Yujia
Zhai†
a,
Zhuang
Wang
c and
Willie J. G. M.
Peijnenburg
*a,
Yujia
Zhai†
a,
Zhuang
Wang
c and
Willie J. G. M.
Peijnenburg
 ab
ab
aInstitute of Environmental Sciences (CML), Leiden University, P.O. Box 9518, 2300 RA, Leiden, The Netherlands. E-mail: vijver@cml.leidenunv.nl
bNational Institute of Public Health and the Environment (RIVM), Center for Safety of Substances and Products, P.O. Box 1, 3720 BA, Bilthoven, The Netherlands
cSchool of Environmental Science and Engineering, Nanjing University of Information Science and Technology, Nanjing 210044, China
First published on 28th September 2018
Abstract
We propose to include the time-dependent size distribution of dispersed and internalized nanoparticles (NPs) in the ecotoxicological evaluation of exposure of biota to NPs and to develop tools to add the particle dynamics in the bioavailability modelling of NPs. The challenges that we face are that: 1) NPs are hardly ever present in dispersions within a narrow size range but rather as size distributions. This affects the overall particle behavior as size does matter in many processes. 2) In exposure media or environmental matrices, the size distribution of NPs changes over time due to transformation and aggregation processes and subsequent sedimentation. 3) The physico-chemical properties and solubility of internalized NPs are modified during biodistribution, while the interactions between NPs and the components of biological fluids have not been well explored. This makes bioavailability modelling and hence quantifying the dose–response relationship on the basis of the actual number of bioavailable particles in the exposure medium questionable. The myriad of processes indicate that exposure concentrations of NPs are not a straightforward expression of the dose–response relationship. The classical dose–response relationship is suggested to include the fate assessment of external and internal NPs when attempting to predict the response of organisms. Various conventional ideas for modelling bioavailability and effects are discussed, and they were found to be not fully tailored to NPs. We think that currently size-dependent features still require a little more experimental data and should be verified for a broader range of specific test species and a variety of testing conditions. Understanding of the underlying processes is achievable and the first steps in developing mechanistic-based modelling can be performed. Before such mechanistic evidence becomes available, we advocate to keep the modelling as simple as it can be.
Environmental significanceNano-specific features as well as the fact that they come in different size distributions typically determines the availability in the exposure medium as well as in the organisms. We state that assessing the time-dependent size and concentration of NPs during exposure, uptake and biodistribution allows for a more realistic effect quantification. Thus, the conventional dose–response relationship is suggested to include proper fate assessment of NPs in biological fluids when attempting to predict the response of tested organisms. |
1. Introduction
In recent years increasing knowledge has been gained about the toxicity of nanoparticles (NPs) to various model species. The particle properties (e.g. shape, size, and surface properties) as well as the external conditions (e.g. pH, natural organic matter, and electrolytes) influence the fate and behavior (e.g. dissolution, aggregation, and sedimentation) of NPs in the environment.1–3 Also, the uptake and accumulation of NPs in biota have been observed.4In vitro toxicity assays encompassing endpoints like cell growth, membrane integrity, or microbial activity have been reported to induce the negative effects of NP exposure.5 Moreover, adverse responses due to exposure to NPs were found in various invertebrates with fitness damage and bioaccumulation as endpoints.6,7 Bioavailability generally is approached from a process-oriented point of view within a toxicological framework, which is applicable to all types of chemicals. Hamelink et al.8 were the first to present bioavailability as a sequestration of three principal processes. The first process is chemical availability which can be defined as the fraction of the total dosage of toxicants present in an environmental compartment that contributes to the actual exposure of an organism. The second process is the “actual or potential uptake”, described as the toxicokinetics of a substance and reflecting the development over time of the concentration of a toxicant on (adsorbed) and in (internalized) the organism. The third process describes the internal distribution of the substance leading to its interaction(s) at the site of action. This so-called “toxico-availability” includes the biochemical and physiological processes resulting from the effects of the toxicant at the site of action. Kinetics is involved in all the three basic processes. The time frame can vary from very brief (less than seconds) to extremely long (up to hundreds of years). Current state-of-the art ideas about all processes underlying the bioavailability of NPs along the chain from exposure, uptake to physiological responses and adverse effects, are depicted in Fig. 1.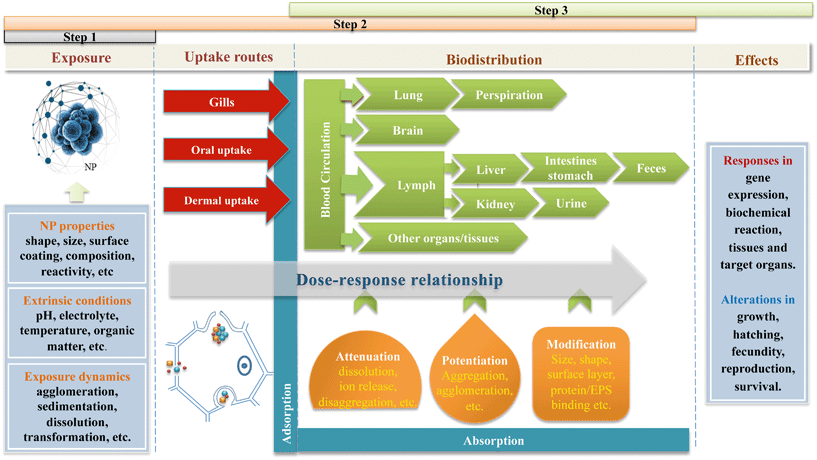 | ||
| Fig. 1 Schematic illustration of the bioavailability processes inducing the toxic effect of NPs. The steps reflect the processes according to Hamelink et al.8 The physico-chemical properties of NPs in the exposure medium are influenced by the environmental conditions. Thus, actual exposure is changed due to agglomeration, aggregation, sedimentation and dissolution of NPs (step 1). At the environment–organism interface, the bioavailability of NPs changes upon changing the uptake route, where gill uptake and dermal uptake relate to the composition of the environmental matrix while oral uptake relates to the conditions in the gastro-intestinal tract. After uptake, the biodistribution of internalized NPs may cause potentiation in size (e.g. aggregation and agglomeration), attenuation in size (e.g. dissolution and/or loss of surface coating), or modification of the surface properties (e.g. protein binding) (step 2). The toxico-availability of NPs may differ from the original state when the target site is reached. The dynamic fate of external and internal NPs may induce altered effects at the site of action compared with the response predicted by the exposure dose (step 3). | ||
A specific characteristic of NPs is that their size distribution is changing over time, which may alter their impacts on the organisms tested.9 Dose–response relationships have been established to quantify and predict the responses of organisms to exposure to NPs.10 Given the fact that the toxicity of NPs is significantly driven by the particle size, there is a strong need to elucidate the dynamic (time-dependent) aggregation and dissolution profiles of NPs in both the exposure medium and the biological fluid when evaluating and predicting biological responses. The aim of this perspective is to discuss the existing trends in the assessment of adverse effects of NPs on organisms, explicitly accounting for the time-dependent size distribution of dispersed and internalized NPs, and giving recommendations to develop bioavailability modelling and dose–response relationships accounting for NP-specific process kinetics. The starting hypothesis is that similar to metals and organic chemicals, the individual free NPs have the highest uptake potential and are actually causing toxic responses; the agglomerated NPs are not directly a bioavailable fraction but a source releasing individual particles. The same principles of binding and agglomeration yield the internalized NPs.
Several types of dose–response models have been developed for conventional chemicals (see Table 1). These are based on the three principal processes of the bioavailability concept, and we evaluate how these processes fit the NP-specific properties.
| Dose–response relationship | Based on | Example | Ref. |
|---|---|---|---|
| Free ion activity model | External concentrations | Metal ions | 11 |
| Critical body concentration | Internal concentrations | Neurotoxic compounds | 12 |
| Biotic ligand models | Adsorption onto the uptake site | Metals in solutions | 13 |
| Physiologically based pharmacokinetics | Toxico-kinetics and toxico-dynamics | Drugs and medicines | 14 |
| Empirical fits | Initial chemical properties | Quantitative structure–activity relationship (QSAR) models | 15 |
2. NP-specific exposure characteristics
The population of particles in a powder is described by its particle size distribution (PSD) and affects the properties of a powder and its dispersions in various ways (Fig. 2). The PSD can be quantified and a single value (e.g. average ± standard deviation) can be obtained. When the particles are suspended in an exposure medium, the size distribution of the NP entities changes over time due to collisions between particles of various sizes. Primary particles can be in the medium. These are inorganic or organic structures held together by atomic or molecular bonding. Also, two or more primary particles tightly bound together by rigid chemical bonding can be found. These so-called aggregates have a large interfacial area of contact between each particle and the force necessary to rupture these bonds is considerable. Also, collections of aggregates can form agglomerates, loosely held together at a point-to-point contact by weak electromagnetic forces, van der Waals forces, mechanical friction, and interlocking. They can be broken apart with dispersion techniques. Although the terms are used interchangeably, in nano(eco)toxicology, most publications follow only one.16 The authors propose to exclusively use the term “agglomerate” when particle assemblages are described.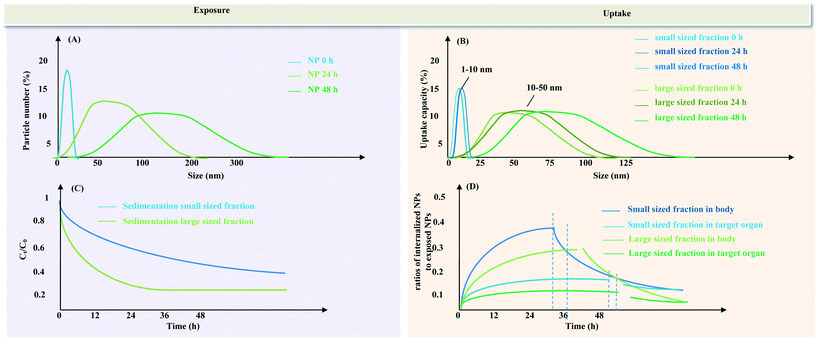 | ||
| Fig. 2 Schematic illustration of the time-dependent size distribution at the different stages of bioavailability and its relationship with the exposure availability (A) and uptake bioavailability (B) according to the principal processes of Hamelink et al.8 The exposure availability refers to NPs still suspended in the water column (and assuming that NPs that have sedimented are not bioavailable and will not resuspend). The uptake availability refers to the amount of different fractions of NPs (small sized fraction inducing increased uptake and large sized fraction inducing lower uptake) that can be taken up by organisms (which can be measured as the sum of adsorbed and absorbed NPs); the sedimentation dynamics (C) and the time-dependent ratios of internalized NPs to dispersed NPs (D). The vertical dotted lines in Fig. 2(D) delineates the partition between the uptake period in NP exposure media and the depuration period in clean water. | ||
Currently, a functional assay-rooted approach is proposed to provide parameter estimates for environmental fate and effect models.17 Quantitative information on the process of exposure e.g. transformation rates, surface affinity, and dissolution rates using functional assays allows protocols to determine the intrinsic/extrinsic properties of NPs and system properties.18,19 The surface affinity (collision between particles) and dissolution rate are proposed as two critical contents of functional assays for the characterization of NPs in various important systems. The dynamics of aggregation/agglomeration of NPs in liquid suspensions are dependent on the physico-chemical properties of the NPs20 and the composition of the medium.21 Newly formed aggregates subsequently influence the structure and reactivity of NPs, which should be taken into consideration when evaluating the effective exposure concentration of NPs. An approach to tackle this is to monitor the size distribution and particle number concentration of the NPs over time in order to assess the effective exposure concentration.22 In general, differently sized particles will have different agglomeration kinetics. The rate of agglomeration for the different fractions should be experimentally assessed (Fig. 2) but in most cases this agglomeration kinetics will be a matter of minutes to hours and depends on the particle concentration and the attachment efficiency of the particles. In addition to aggregation/agglomeration, some types of NPs, e.g. metal-based NPs and biodegradable polymeric NPs used as drug delivery systems, also undergo dissolution or ion release in the exposure media. The dissolution rate depends on the type of NP and the properties of the exposure medium, and these variables can be spatially and temporally highly heterogeneous. Thus, continuous evaluation of the particle size over time is required since the dynamics of aggregation/agglomeration of NPs during exposure inevitably affect the effective exposure.
There is a quest for proper expression of the estimated exposure. In other words, there is a quest for an unambiguous description of the dose of NPs, i.e. a dose metric. An adequate dose metric includes all particle characteristics that are necessary to explain differences between responses in experiments. Mass is traditionally a unique measure of administered dose in toxicity studies with conventional chemical substances. Because of the variety of specific physical properties of NPs, other dose metrics are likely to be more appropriate.23 The total number of particles, surface area or volume has been suggested as potential simplified dose metrics.24–26 For those nanomaterials that shed off ions, it sometimes is assumed that these drive the toxicity, and this is the case for AgNPs that often fully dissolve. Here the free-ion-activity model (FIAM) would be an example, the external dosage having a relationship with the adverse responses. The FIAM model is developed explicitly for metal ions, being reactive species. These models have been applied in modeling the toxicity of metallic nanoparticles (e.g. AgNPs, ZnNPs, CuNPs etc.) that could release metal ions.27
Although each of these metrics has been shown to be useful in isolated cases, a systematic evaluation of their applicability is lacking.28,29
At small scales, the NP exposure is dynamic. Abiotic factors such as rain and flooding events, weather conditions, and redox status may alter the fate of NPs. To make it more complicated, when organisms enter the water, they too modify the exposure conditions. Amongst others, excretion products of the tested organisms (e.g. feces, mucus; extracellular polymeric substances) can also influence the aggregation/agglomeration of NPs.30 This alters the environmental conditions and hence affects the fate of particles administered in the system. Also, the production of root exudates by plants may have similar impacts.31 On top of that, biotic activities like bioturbation by organisms modify the exposure conditions by re-suspending particles into water, or earthworms aerate the soil via their typical digging behavior and excrete enzymes via their gut and skin mucus that may stimulate microbial activity.32
The lack of process-based models respecting the particle size distribution in the exposure medium, the dynamics of dissolution and aggregation/agglomeration, as well as the issue of proper dosimetry to express the effective dose that is available for organisms, make the dose–response modelling challenging and certainly not straightforward.
3. Adsorption and uptake of NPs
A theoretical framework33 revealed that the uptake of NPs is highly dependent on particle size (Fig. 2). Focusing on uptake at the cellular interface, NPs with sizes ranging from 4 to 10 nm can pass the membrane bilayer via direct penetration. Compared with larger sized NPs, the larger surface area to volume ratio of smaller sized NPs enables the particles to more efficiently interact with cells.34 The key uptake pathway of NPs with sizes between 10 and 50 and maybe even up to 100 nm is pinocytosis. Agglomerated or functionally modified NPs with sizes larger than 100 nm can enter the cell via phagocytosis.33 By performing modelling in analogy to the BLM (Biotic Ligand Model) in which the idea is that the adsorbed dosage is proportionally related to the initial effects, an important threshold for induction of biological activity by NPs was a size distribution fraction of 20–30 nm.35Most studies show effective adsorption of NPs by various organisms.36,37 NPs larger than 50 nm were found to be efficiently adsorbed.38 It is likely that the adsorbed NP fraction can be seen as the effective exposure source from which the NPs penetrate through epidermal membranes when they shed off from the agglomerated particle cluster. The uptake of NPs into cells is driven by the surface facets of the particle, with high-atom-density surface facets enabling NPs to most effectively interact with cell surfaces.39In vivo studies revealed that transformation of NPs changes the cellular uptake. Transformations of internalized NPs due to the aggregation/agglomeration, dissolution or nanoparticle-biochemical substance binding are dictated by the composition of the gut media and the properties of the primary particles.40 This implies that the bioavailability of NPs changes with the exposure route.41 For instance, oral uptake generally decreases bioavailability due to gastrointestinal barriers compared to instillation which in principle is accompanied by 100% bioavailability. Moreover, the uptake of NPs by the tested organism occurs not only at a steady and constant pace but can also be variable along with time (Fig. 2), as it is for instance dependent on selective feeding that is related to the dynamic aggregation/agglomeration of the bioavailable NPs.42
Additionally, the NPs adsorbed onto the body surface can cause direct limitations of movements. This is described for particles adsorbed onto the antennae and filtering screens of Daphnia magna inhibiting movement and thus increasing mortality.43 Adsorbed NPs may act as point sources for metal dissolution or for other pollutants adsorbed onto the particle. Bruinink et al.44 stated that agglomeration of NPs inside organisms reduces translocation across primary barriers such as the gastrointestinal tract, lungs or gills, and skin, effectively preventing exposure of “secondary” organs. Despite the importance of the dynamics of absorption/adsorption for NP bioavailability, adsorption itself can cause the environment–organism barrier to be exposed, causing toxic effects.45 Support comes from studies on the exposure of adult medaka to fluorescent latex particles, in which it was found that particle uptake via environment–organism barriers induced subsequent effects in gills, intestine, liver, gallbladder and kidney.46 NPs may not be able to cross the barriers to translocate into the organism, while effects initiated at the barriers could be indirectly propagated to other tissues or organs. Overall, the uptake dynamics of NPs during adsorption and absorption and the indirect exposure via chain reactions initiated at the environment–organism barriers need to be taken into consideration in the process of “actual or potential uptake”.
4. Internal concentrations and internal biodistribution
Over the last decade, scientific results show that tissue residues can be a predictor of bioavailability. This especially is the case for simplified biological systems such as unicellular systems e.g. cell lines,47 algae,48 and Escherichia coli.49 The uptake and accumulation kinetics can in these cases be described by the so-called 'single compartment models'. These models consider the individual as a single, well-mixed vessel. Thus, it is either assumed that the chemical is evenly distributed over the organism, or only part of the organism is considered to be relevant with respect to toxicity. A generally accepted approach for assessing possible adverse effects to biota, no matter what kind of organism, is the Critical Body Concentration (CBC) concept.12 The key assumption is that independent of exposure time or exposure dosage, effects occur at a more or less fixed internal dosage. The CBC is defined as the highest internal dosage of a toxicant in an organism that does not yet cause an adverse effect. By comparing the internal dosage measured in exposed organisms to CBC values derived in the laboratory, a measure of risk is obtained. In this way, the actual exposure concentration in the environment does not need to be known for performing a hazard assessment. The CBC applies both to lethal and to sub-lethal effects.It is unlikely that the CBC is applicable to NPs. In cases of multiple internal compartments, the actual toxicant concentration in organisms is not suited to explain toxicity properly.50–52 The biodistribution of internalized NPs is highly dependent on their physico-chemical properties, their fate and transport in biological fluids, and the NP–protein interaction (Fig. 1). The translocation of NPs from tissue to tissue may cause potentiation in size (e.g. aggregation and agglomeration) that decreases the efficiency of NPs in breaching cell membrane barriers.53 Meanwhile, NPs may also undergo attenuation in size (e.g. dissolution and/or loss of surface coating) during biodistribution.54 In addition to potentiation and attenuation, the shape, size and surface properties of NPs can also be modified due to binding of NPs to target tissues. When NPs migrate in biological fluids, the proteins present in plasma and tissues can cover the surface of the NPs, forming a biomolecular corona.55 Corona formation depends on the ratio between the surface areas of NPs, the nature of the proteins and the protein concentration.56 The interactions between protein corona and NPs can alter the size distribution of NPs, impact the capability of particles to cross biological barriers, and induce conformational changes in adsorbed proteins, the sum of which may cause significant changes in NP biodistribution.57 The type of ligand to which an NP is bound and how this ligand is transported or stored in the body determine to a great extent where the NP will accumulate. Sensitive targets or critical biochemical processes differ between species and this may also lead to modified toxicity profiles. This means that internal concentrations of NPs are not a straightforward expression of the dose–response relationship.
5. Physiologically based pharmacokinetic modelling
Physiologically based pharmacokinetic (PBPK) modeling is a mathematical modeling technique for predicting the absorption, distribution, metabolism and excretion (ADME) of synthetic or natural chemical substances in biota following the principles of mass transport, fluid dynamics, and biochemistry of the substance.14 The rate of uptake in a critical target organ is a more superior toxicity predictor. After absorption, the distribution of chemical substances from organ to organ may induce potential accumulation in secondary organs such as the liver and kidneys which are mainly responsible for metabolism. The accumulation and metabolism of chemical substances in these organs therefore need to be considered to facilitate the excretion kinetics, which is important in the context of understanding their elimination and biopersistence.58,59 Well-validated PBPK modelling may be applied in the toxicity evaluation of NPs. In this case, the fate and dose of NPs in plasma and target tissues over time need to be systematically identified and quantified enabling the integration of available experimental and theoretical studies.60 Since the currently available data are not comprehensive to systemically assess both the pharmacokinetics and cellular toxicity of NPs across species, as well as to allow for in vitro to in vivo extrapolations, PBPK modelling is not the most suited option for bioavailability assessment of NPs at the moment.6. Uptake rate as a superior and most suited predictor of particle bioavailability
It has been shown that the rate of uptake is a superior predictor of bioavailability instead of the external or the internal dose.58,59 According to the parsimony principle,61 we should look for the simplest possible explanation of observed phenomena rather than postulating complex processes without empirical evidence. This prevents over-parameterization of the experimental observations as performed by means of ecotoxicological assays. The initial slope of the uptake curve is to be considered as the best indicator of bioavailability of chemicals. In the simplest case, the exposure concentration is constant, the organism starts with an internal concentration of zero, and accumulation is linear. Under these assumptions, the data can be described by an equation of the form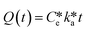 | (1) |
In case the exposure dose is not constant over time and consists of different fractions (Fig. 2), the description becomes more complicated. Considering a dynamic particle size distribution due to dissolution, aggregation/agglomeration and subsequent sedimentation processes, the amount of internalized NPs can be described by the exponential equation
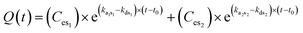 | (2) |
Sometimes it is difficult to quantify the uptake of NPs (especially for carbon materials and cells) in complex biological matrices based on the mass concentration due to limitations of analytical techniques.45 Thus, indirect qualitative and quantitative methods can be used to determine the uptake of NPs based on a biological response and damage signal (e.g. oxidative stress) detected in a certain tissue or organ:
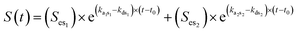 | (3) |
To further visualize the promoted relationships between the particle size distribution and uptake of particles, a pilot case study on the relative NP uptake ability, normalized to the total NP-derived oxidative damage signals in algal cells and freshwater fish larva, was conducted (Fig. 3). It is found that the intracellular reactive oxygen species levels in two aquatic organisms of different trophic levels increase upon decreasing the agglomerated size of the studied NPs. This also implicates a potential increase in the cellular uptake of the NPs when the particles are in the lower size range. It can be concluded that the particle size distribution is important for explaining the toxicity of particles that are present as agglomerates. Although data at this moment are only obtained for microalga species and fish larva, the concept of inclusion of the dynamics of particle size distributions in bioavailability is also applicable for other model species that could uptake and bioaccumulate NPs. Moreover, in the two case studies we selected organic matter as a factor that influenced the particle size distribution and bioavailability. It cannot be denied that in the process of biodistribution the interactions between protein corona and internalized NPs can also alter the size distribution of NPs, which needs to be tackled in future studies.
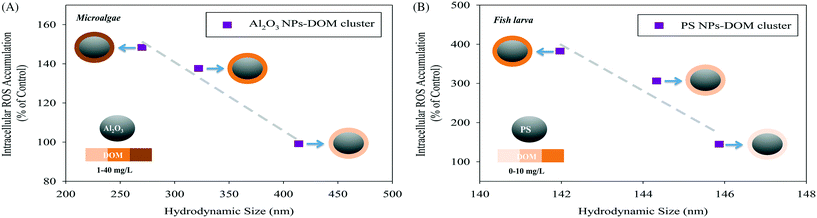 | ||
| Fig. 3 Variation of intracellular reactive oxygen species (ROS) accumulation (indicating the uptake of particles) in (A) a freshwater microalga species (Scenedesmus obliquus) as a function of the hydrodynamic size of Al2O3 NPs-DOM clusters (data from Ye et al.62) and (B) a freshwater fish larva (Danio rerio) as a function of the hydrodynamic size of polystyrene (PS) NPs-DOM clusters (the data were newly generated using the procedures described in Ye et al.62). DOM stands for dissolved organic matter. | ||
7. The way forward
The main quest currently debated in the scientific community is what models to use to quantify NP bioavailability, for which conventional mass-based models cannot be applied? We highlighted that fate processes such as aggregation and dissolution in the exposure medium need to be measured continuously to incorporate exposure dynamics into uptake models. The time-weighted average size of NPs based on the assessment of the dynamic size distribution was suggested to be included as a first indicator that can be used to resolve some of the issues identified above as the dynamic size distribution is directly linked to (cellular) particle uptake. The time-weighted average concentration of NPs was proposed to offer a more realistic display for exposure concentrations compared to initially measured concentrations. We highlighted that the size distribution and concentration of internalized NPs in the body of tested organisms are required to be systematically analyzed to provide information for toxico-kinetic modelling. This dependence of particle size distribution is typical for availability in the exposure medium as well as in the organisms. We did not reject our starting hypothesis that the individual free NPs have the highest uptake potential and cause toxic responses; the agglomerated NPs are not directly a bioavailable fraction but a source releasing individual particles. The same principles of binding and agglomeration yield the internalized NPs. Therefore we state that assessing the time-dependent size and concentration of NPs during exposure, uptake and biodistribution allows for a more realistic effect quantification. Thus, the conventional dose–response relationship is suggested to include proper fate assessment of NPs in biological fluids when attempting to predict the response of tested organisms.Future research should consider filling the current gaps regarding the integration of the exposure dynamics, uptake and internal toxicokinetics of NPs into risk assessment in order to offer integral understanding and realistic prediction of the ecotoxicity of NPs. As soon as more experimentally underpinned information is available and verified for specific test species, extension towards mechanisms can be made within modelling. It should be realized that nano-research is proceeding at a fast pace and that even though many analytical detection techniques are still in their infancy, they are rapidly developing. We therefore believe that within a short time span of 2–4 years, validated methods for determining the size distribution in external media as well as in biological matrices will be developed and established. It should be noted that many nano-specific OECD protocols are currently in progress and reviewed by experts. Before such evidence becomes available, we advocate to keep the modelling as simple as it can be. This means we now highlight the gaps and use “easy” simplified modelling but we need to report already all data measured. This means that within the next 4 years data will be available to fit more process-based models.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research described in this work was supported by NWO-VIDI project number 864.13.010 granted to MV. The CSC is gratefully acknowledged for its financial support to Y. Z., project number 201506510003.References
- Y. Xiao, M. G. Vijver, G. Chen and W. J. Peijnenburg, Toxicity and accumulation of Cu and ZnO nanoparticles in Daphnia magna, Environ. Sci. Technol., 2015, 49, 4657–4664 CrossRef CAS PubMed.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles, Environ. Health Perspect., 2005, 113, 823 CrossRef PubMed.
- J. R. Conway, A. S. Adeleye, J. Gardea-Torresdey and A. A. Keller, Aggregation, dissolution, and transformation of copper nanoparticles in natural waters, Environ. Sci. Technol., 2015, 49, 2749–2756 CrossRef CAS PubMed.
- Z. Wang, Y.-H. Lee, B. Wu, A. Horst, Y. Kang, Y. J. Tang and D.-R. Chen, Anti-microbial activities of aerosolized transition metal oxide nanoparticles, Chemosphere, 2010, 80, 525–529 CrossRef CAS PubMed.
- E. Lefevre, N. Bossa, M. R. Wiesner and C. K. Gunsch, A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): behavior, transport and impacts on microbial communities, Sci. Total Environ., 2016, 565, 889–901 CrossRef CAS PubMed.
- K. Schlich, T. Klawonn, K. Terytze and K. Hund-Rinke, Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test, Environ. Toxicol. Chem., 2013, 32, 181–188 CrossRef CAS PubMed.
- W.-C. Hou, P. Westerhoff and J. D. Posner, Biological accumulation of engineered nanomaterials: a review of current knowledge, Environ. Sci.: Processes Impacts, 2013, 15, 103–122 RSC.
- J. Hamelink, P. F. Landrum, H. Bergman and W. H. Benson, in Bioavailability: physical, chemical, and biological interactions, Lewis Publishers, Boca Raton, Florida, 1992 Search PubMed.
- U. M. Graham, G. Jacobs, R. A. Yokel, B. H. Davis, A. K. Dozier, M. E. Birch, M. T. Tseng, G. Oberdörster, A. Elder and L. DeLouise, in Modelling the Toxicity of Nanoparticles, ed. L. Tran, M. A. Bañares and R. Rallo, Springer, New York, 2017, pp. 71–100 Search PubMed.
- H. F. Krug, Nanosafety research—are we on the right track?, Angew. Chem., Int. Ed., 2014, 53, 12304–12319 CAS.
- P. G. C. Campbell, in Metal Speciation and Bioavailability in Aquatic Systems, ed. A. Tessier and D. R. Turner, Wiley, New York, 1995, pp. 45–102 Search PubMed.
- L. S. McCarty and D. Mackay, Enhancing ecotoxicological modeling and assessment. Body residues and modes of toxic action, Environ. Sci. Technol., 1993, 27, 1718–1728 CrossRef CAS.
- P. R. Paquin, R. C. Santore, K. B. Wu, C. D. Kavvadas and D. M. Di Toro, The biotic ligand model: a model of the acute toxicity of metals to aquatic life, Environ. Sci. Policy, 2000, 3, 175–182 CrossRef.
- K. Bischoff, in Pharmacokinetics in Risk Assessment. Drinking Water and Health, National Academy Press, Washington, DC, 1987, vol. 8, pp. 36–64 Search PubMed.
- T. Puzyn, B. Rasulev, A. Gajewicz, X. Hu, T. P. Dasari, A. Michalkova, H.-M. Hwang, A. Toropov, D. Leszczynska and J. Leszczynski, Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles, Nat. Nanotechnol., 2011, 6, 175 CrossRef CAS PubMed.
- G. Nichols, S. Byard, M. J. Bloxham, J. Botterill, N. J. Dawson, A. Dennis, V. Diart, N. C. North and J. D. Sherwood, A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization, J. Pharm. Sci., 2002, 91, 2103–2109 CrossRef CAS PubMed.
- C. O. Hendren, G. V. Lowry, J. M. Unrine and M. R. Wiesner, A functional assay-based strategy for nanomaterial risk forecasting, Sci. Total Environ., 2015, 536, 1029–1037 CrossRef CAS PubMed.
- N. K. Geitner, N. J. O'Brien, A. A. Turner, E. J. Cummins and M. R. Wiesner, Measuring nanoparticle attachment efficiency in complex systems, Environ. Sci. Technol., 2017, 51, 13288–13294 CrossRef CAS PubMed.
- N. K. Geitner, S. M. Marinakos, C. Guo, N. O'Brien and M. R. Wiesner, Nanoparticle surface affinity as a predictor of trophic transfer, Environ. Sci. Technol., 2016, 50, 6663–6669 CrossRef CAS PubMed.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- D. Zhou, Z. Ji, X. Jiang, D. R. Dunphy, J. Brinker and A. A. Keller, Influence of material properties on TiO2 nanoparticle agglomeration, PLoS One, 2013, 8, e81239 CrossRef PubMed.
- M. Baalousha, Y. Nur, I. Römer, M. Tejamaya and J. Lead, Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles, Sci. Total Environ., 2013, 454, 119–131 CrossRef PubMed.
- M. Hull, A. Kennedy, C. Detzel, P. Vikesland and M. Chappell, Moving beyond mass: the unmet need to consider dose metrics in environmental nanotoxicology studies, Environ. Sci. Technol., 2012, 46, 10881–10882 CrossRef CAS PubMed.
- J. Hua, M. G. Vijver, G. Chen, M. K. Richardson and W. J. Peijnenburg, Dose metrics assessment for differently shaped and sized metal-based nanoparticles, Environ. Toxicol. Chem., 2016, 35, 2466–2473 CrossRef CAS PubMed.
- C. J. Delmaar, W. J. Peijnenburg, A. G. Oomen, J. Chen, W. H. de Jong, A. J. Sips, Z. Wang and M. V. Park, A practical approach to determine dose metrics for nanomaterials, Environ. Toxicol. Chem., 2015, 34, 1015–1022 CrossRef CAS PubMed.
- J. Jiang, G. Oberdörster, A. Elder, R. Gelein, P. Mercer and P. Biswas, Does nanoparticle activity depend upon size and crystal phase?, Nanotoxicology, 2008, 2, 33–42 CrossRef CAS PubMed.
- Y. Liu, J. Baas, W. J. Peijnenburg and M. G. Vijver, Evaluating the combined toxicity of Cu and ZnO nanoparticles: utility of the concept of additivity and a nested experimental design, Environ. Sci. Technol., 2016, 50, 5328–5337 CrossRef CAS PubMed.
- S. J. Klaine, P. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS PubMed.
- R. J. Griffitt, J. Luo, J. Gao, J. C. Bonzongo and D. S. Barber, Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms, Environ. Toxicol. Chem., 2008, 27, 1972–1978 CrossRef CAS PubMed.
- N. Joshi, B. T. Ngwenya and C. E. French, Enhanced resistance to nanoparticle toxicity is conferred by overproduction of extracellular polymeric substances, J. Hazard. Mater., 2012, 241, 363–370 CrossRef PubMed.
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Interaction of nanoparticles with edible plants and their possible implications in the food chain, J. Agric. Food Chem., 2011, 59, 3485–3498 CrossRef CAS PubMed.
- G. Cornelis, K. Hund-Rinke, T. Kuhlbusch, N. Van den Brink and C. Nickel, Fate and bioavailability of engineered nanoparticles in soils: a review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2720–2764 CrossRef CAS.
- M. Zhu, G. Nie, H. Meng, T. Xia, A. Nel and Y. Zhao, Physicochemical properties determine nanomaterial cellular uptake, transport, and fate, Acc. Chem. Res., 2012, 46, 622–631 CrossRef PubMed.
- M.-S. Wong, D.-S. Sun and H.-H. Chang, Bactericidal performance of visible-light responsive titania photocatalyst with silver nanostructures, PLoS One, 2010, 5, e10394 CrossRef PubMed.
- M. Auffan, J. Rose, J.-Y. Bottero, G. V. Lowry, J.-P. Jolivet and M. R. Wiesner, Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective, Nat. Nanotechnol., 2009, 4, 634 CrossRef CAS PubMed.
- P. Lodeiro, T. J. Browning, E. P. Achterberg, A. Guillou and M. S. El-Shahawi, Mechanisms of silver nanoparticle toxicity to the coastal marine diatom Chaetoceros curvisetus, Sci. Rep., 2017, 7, 10777 CrossRef PubMed.
- X. Zhu, L. Zhu, Y. Chen and S. Tian, Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna, J. Nanopart. Res., 2009, 11, 67–75 CrossRef CAS.
- M. Van Pomeren, N. Brun, W. Peijnenburg and M. Vijver, Exploring uptake and biodistribution of polystyrene (nano) particles in zebrafish embryos at different developmental stages, Aquat. Toxicol., 2017, 190, 40–45 CrossRef CAS PubMed.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346 CrossRef CAS PubMed.
- A. A. Taylor, I. M. Marcus, R. L. Guysi and S. L. Walker, Metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota, Environ. Eng. Sci., 2015, 32, 602–612 CrossRef CAS.
- J. E. Riviere, Pharmacokinetics of nanomaterials: an overview of carbon nanotubes, fullerenes and quantum dots, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 26–34 CAS.
- R. D. Holbrook, K. E. Murphy, J. B. Morrow and K. D. Cole, Trophic transfer of nanoparticles in a simplified invertebrate food web, Nat. Nanotechnol., 2008, 3, 352–355 CrossRef CAS PubMed.
- N. A. Lewinski, H. Zhu, H.-J. Jo, D. Pham, R. R. Kamath, C. R. Ouyang, C. D. Vulpe, V. L. Colvin and R. A. Drezek, Quantification of water solubilized CdSe/ZnS quantum dots in Daphnia magna, Environ. Sci. Technol., 2010, 44, 1841–1846 CrossRef CAS PubMed.
- A. Bruinink, J. Wang and P. Wick, Effect of particle agglomeration in nanotoxicology, Arch. Toxicol., 2015, 89, 659–675 CrossRef CAS PubMed.
- K. Schirmer, R. Behra and L. Sigg, Ecotoxicological aspects of nanomaterials in the aquatic environment, in Safety Aspects of Engineered Nanomaterials, ed. W. Luther and A. Zweck, Pan Stanford Publishing Pte. Ltd., Singapore, 2013, ch. 5, pp. 141–161 Search PubMed.
- S. Kashiwada, Distribution of nanoparticles in the see-through medaka (Oryzias latipes), Environ. Health Perspect., 2006, 114, 1697 CrossRef CAS PubMed.
- J. M. Hillegass, A. Shukla, S. A. Lathrop, M. B. MacPherson, N. K. Fukagawa and B. T. Mossman, Assessing nanotoxicity in cells in vitro, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 219–231 CAS.
- A. Ivask, I. Kurvet, K. Kasemets, I. Blinova, V. Aruoja, S. Suppi, H. Vija, A. Käkinen, T. Titma and M. Heinlaan, Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro, PLoS One, 2014, 9, e102108 CrossRef PubMed.
- S. Pal, Y. K. Tak and J. M. Song, Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS PubMed.
- M. G. Vijver, C. A. Van Gestel, R. P. Lanno, N. M. Van Straalen and W. J. Peijnenburg, Internal metal sequestration and its ecotoxicological relevance: a review, Environ. Sci. Technol., 2004, 38, 4705–4712 CrossRef CAS PubMed.
- S. N. Luoma and P. S. Rainbow, Why is metal bioaccumulation so variable? Biodynamics as a unifying concept, Environ. Sci. Technol., 2005, 39, 1921–1931 CrossRef CAS PubMed.
- P. S. Rainbow, Trace metal concentrations in aquatic invertebrates: why and so what?, Environ. Pollut., 2002, 120, 497–507 CrossRef CAS PubMed.
- A. Verma and F. Stellacci, Effect of surface properties on nanoparticle–cell interactions, Small, 2010, 6, 12–21 CrossRef CAS PubMed.
- W.-M. Li and W.-X. Wang, Distinct biokinetic behavior of ZnO nanoparticles in Daphnia magna quantified by synthesizing 65 Zn tracer, Water Res., 2013, 47, 895–902 CrossRef CAS PubMed.
- M. P. Monopoli, C. Åberg, A. Salvati and K. A. Dawson, Biomolecular coronas provide the biological identity of nanosized materials, Nat. Nanotechnol., 2012, 7, 779–786 CrossRef CAS PubMed.
- M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. B. Bombelli and K. A. Dawson, Physical− chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles, J. Am. Chem. Soc., 2011, 133, 2525–2534 CrossRef CAS PubMed.
- K. Choi, J. E. Riviere and N. A. Monteiro-Riviere, Protein corona modulation of hepatocyte uptake and molecular mechanisms of gold nanoparticle toxicity, Nanotoxicology, 2017, 11, 64–75 CrossRef CAS PubMed.
- N. Van Straalen, M. Donker, M. Vijver and C. Van Gestel, Bioavailability of contaminants estimated from uptake rates into soil invertebrates, Environ. Pollut., 2005, 136, 409–417 CrossRef CAS PubMed.
- F. R. Khan, K. Syberg, Y. Shashoua and N. R. Bury, Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio), Environ. Pollut., 2015, 206, 73–79 CrossRef CAS PubMed.
- J. E. Riviere, Of mice, men and nanoparticle biocoronas: are in vitro to in vivo correlations and interspecies extrapolations realistic?, Nanomedicine, 2013, 8, 1357–1359 CrossRef CAS PubMed.
- A. Blumer, A. Ehrenfeucht, D. Haussler and M. K. Warmuth, Occam's razor, Inf. Process. Lett., 1987, 24, 377–380 CrossRef.
- N. Ye, Z. Wang, S. Wang, H. Fang and D. Wang, Dissolved organic matter and aluminum oxide nanoparticles synergistically cause cellular responses in freshwater microalgae, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2018, 1–8 Search PubMed.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2018 |
