Catalytic oxidation of carbohydrates into organic acids and furan chemicals
Abstract
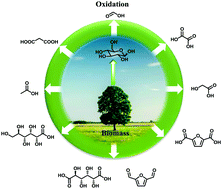
A wide variety of commodity chemicals can be produced from the catalytic oxidation of carbohydrates or carbohydrate derived molecules including formic acid, acetic acid, glycolic acid, gluconic acid, glucaric acid, malonic acid, oxalic acid, 2,5-diformylfuran (DFF), 5-hydroxymethyl-2-furancarboxylic acid (HFCA), 5-formyl-2-furancarboxylic acid (FFCA), and 2,5-furandicarboxylic acid (FDCA). This review will highlight the recent research progress in the development of new routes for the production of organic acids and furan compounds via catalytic oxidation reactions. Particular attention will be paid to these one-pot reactions with the requirements of an acidic site and a metal site. For the one-pot transformation of cellobiose or lignocellulose into gluconic acid, these reactions were performed via a one-step strategy using a single catalyst containing an acidic site and a metal site. However, a two-step strategy was adopted for the oxidative transformation of carbohydrates into DFF or FDCA in order to avoid the oxidation of the carbohydrates. The first step was performed for the dehydration of carbohydrates into 5-hydroxylmethylfuran (HMF) in the presence of an acid catalyst, and the second step was performed for the oxidation of HMF into DFF or FDCA with a metal catalyst.


 Please wait while we load your content...
Please wait while we load your content...