Synthesis of semi-rigid-biobased polyesters from renewable furanic cyclobutane diacid
Abstract
The pursuit of novel sustainable materials is driving advancements in polymer science, with the consolidation of furfural and hydroxymethylfurfural derivatives as key renewable building blocks. 3,4-Di(furan-2-yl)cyclobutane-1,2-dicarboxylic acid (CBDA) is a highly promising rigid-structure biobased monomer that is readily synthesized from furfural. In this study, we expanded the scope of CBDA-based polymers by synthesizing this platform molecule and investigating its polymerization with a series of aliphatic diols of varying chain lengths. CBDA was successfully synthesized from furfural-derived 3-(2-furyl)acrylic acid through a green and efficient UV-mediated solid-state dimerization reaction. Subsequent polymerization was carried out in bulk via a two-step method. The success of the polymerization was confirmed through ATR-FTIR, 1H NMR, and 13C CP/MAS NMR spectroscopy. The resulting polymers presented average molecular weights (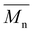 ) of up to 11 200 g mol−1. Thermogravimetric analysis (TGA) revealed good thermal stability, with a Td10% ranging from 263 to 284 °C and 50% weight retention observed up to 388 °C. Furthermore, DSC analysis indicated that the glass transition temperature of the polymers could be tailored, varying from 6 to 52 °C depending on the chain length of the utilized diol. These results underscore the potential of CBDA as a renewable rigid monomer for the development of sustainable biobased materials.
) of up to 11 200 g mol−1. Thermogravimetric analysis (TGA) revealed good thermal stability, with a Td10% ranging from 263 to 284 °C and 50% weight retention observed up to 388 °C. Furthermore, DSC analysis indicated that the glass transition temperature of the polymers could be tailored, varying from 6 to 52 °C depending on the chain length of the utilized diol. These results underscore the potential of CBDA as a renewable rigid monomer for the development of sustainable biobased materials.

- This article is part of the themed collection: Make polymers sustainable, why and how?


 Please wait while we load your content...
Please wait while we load your content...