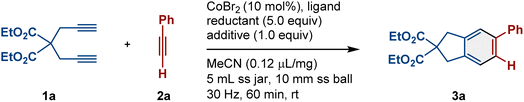Open Access Article
Open Access ArticleA mechanochemical [2+2+2] cycloaddition facilitated by a cobalt(II) catalyst and piezoelectric materials†
Kalipada Janaa,
Koji Kubota *ab and
Hajime Ito
*ab and
Hajime Ito *ab
*ab
aInstitute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Sapporo, Hokkaido, Japan. E-mail: kbt@eng.hokudai.ac.jp; hajito@eng.hokudai.ac.jp
bDivision of Applied Chemistry, Faculty of Engineering, Hokkaido University, Sapporo, Hokkaido, Japan
First published on 23rd May 2025
Abstract
In this study, we present the first general mechanochemical protocol for the catalytic [2+2+2] cycloaddition of alkynes via a simple and inexpensive cobalt(II)-based catalytic system. The key to achieving the high efficiency of this mechanochemical transformation is the use of piezoelectric BaTiO3 as a reductant in combination with an amine additive to facilitate the generation of a catalytically active low-valent cobalt species. This method is characterized by a broad substrate scope, short reaction times, solvent-less conditions, and the feasibility of reacting poorly soluble substrates. Thus, we provide a more efficient, broadly applicable, and sustainable alternative to existing solution-based protocols.
Introduction
Mechanochemistry, particularly when ball milling is used, has gained recognition as a sustainable and environmentally friendly alternative to traditional organic synthesis in solution.1–20 Ball milling eliminates the need for large amounts of harmful solvents in the reaction step and accelerates various organic transformations, making it more versatile, atom-efficient, energy-efficient, and environmentally beneficial than traditional solution-based methods. These advantages have been demonstrated in a variety of organic transformations that include synthetically important transition-metal-catalyzed processes such as cross-coupling reactions.1–20 Building on our recent success in leveraging ball-milling techniques to address challenges in traditional solution-based organic synthesis,21–33 we envisioned the development of a mechanochemical protocol for transition-metal-catalyzed [2+2+2] cycloadditions (Scheme 1A).34–38 Given the widespread use of the [2+2+2] cycloaddition in modern organic synthesis and materials science, we anticipate that such a mechanochemical approach would offer a more efficient and sustainable method that is complementary to existing solution-based methods. In 2016, Mack and co-workers have conducted a pioneering study describing the first attempt at a mechanochemical [2+2+2] cycloaddition of terminal alkynes using a nickel catalyst.39 Interestingly, the ball-milling reaction gave [2+2+2+2] cycloadducts as the major products, while only a trace amount of the corresponding [2+2+2] products were obtained (Scheme 1B).39 Thus, the synthetic potential of mechanochemical [2+2+2] cycloadditions remains to be explored further.The transition-metal-catalyzed [2+2+2] cycloaddition can be catalyzed by a variety of different transition metals.34–38 Of these metals, cobalt has proven to be an inexpensive and robust catalyst.40–48 In particular, catalytic systems using a cobalt(II) salt40–43,47,48 have been widely used as simpler alternatives to conventional more expensive and air-sensitive cobalt(I)-based catalysts, such as CpCo(CO)2 and CoCl(PPh3)3.44–46 In cobalt(II) catalytic methods, the efficient in situ reduction of cobalt(II) to low-valent cobalt species [Co(I) or Co(0)] using a metal-based reductant such as zinc or manganese is important for achieving effective catalysis under mild conditions.40–43 Rovis and co-workers have demonstrated that an iridium-based photocatalyst can be used for reducing cobalt(II) to generate a catalytically active cobalt(0) species in situ, enabling efficient [2+2+2] cycloaddition reactions.47 It has also been reported that at high temperature (>130 °C), cobalt(II) can directly catalyze the cycloaddition in the absence of a reductant.48 The reductant-free reaction presumably proceeds through a Co(II)/Co(IV) catalytic cycle.48 Despite recent advances in this area, these solution-based methods commonly require the use of substantial amounts of toxic solvents, high temperatures, and extended reaction times, all of which represent important disadvantages to solution-based approaches.40–48
Herein, we report the first mechanochemical [2+2+2] cycloaddition of alkynes achieved via a simple, inexpensive, and highly robust catalytic system based on cobalt(II) bromide, tricyclohexylphosphine (PCy3), and piezoelectric BaTiO3 (Scheme 1c). Notably, the use of piezoelectric BaTiO329–31,49–59 as a reductant in combination with N,N-diisopropylethylamine (DIPEA) proved to be crucial for facilitating a highly efficient mechanochemical [2+2+2] cycloaddition, as the efficiency was much lower under conditions where traditional metal reductants were used. The mechanochemical system presented here allows synthesizing a diverse range of substituted benzenes in short reaction times (∼60 min), under mild conditions without the need for heating or reaction solvents, thereby complementing existing solution-based [2+2+2] cycloaddition strategies. This work also demonstrates the effectiveness of using a unique reduction system that uses piezoelectricity to generate catalytically active low-valent metal species under mechanochemical conditions.31,49
Results and discussion
Our study began with the optimization of the reaction conditions using diyne 1a and ethynylbenzene 2a as model substrates (Table 1). All mechanochemical reactions were conducted using a Retsch MM400 mill [stainless-steel (ss) milling jar (5 mL), 30 Hz; ss ball (10 mm diameter); (for details, see the ESI†)]. First, we conducted the reaction using cobalt(II) bromide (CoBr2) in the presence of MeCN as a liquid-assisted-grinding (LAG) additive, and no product was formed (entry 1, Table 1). The desired product (2a) was obtained in 55% yield when tricyclohexylphosphine (PCy3) was added as a ligand (entry 2, Table 1).47 This was an unexpected result because the reaction had proceeded without a reductant under very mild conditions.48 Although the mechanism of the reductant-free cycloaddition is still unclear at present, we think that the reaction might proceed through a phosphine-assisted Co(II)/Co(IV) catalytic cycle due to the generation of hot spots during ball milling.60 Moreover, a mechanism where thermal disproportionation generates low-valent cobalt species cannot be ruled out unequivocally.34,47 In order to promote the [2+2+2] cycloaddition, the addition of metal-based reductants was investigated (entries 3 and 4, Table 1). When zinc was used, the yield of 3a slightly improved to 60% (entry 3, Table 1). Performing the reaction with manganese did not significantly improve the yield of 3a (66%, entry 4, Table 1). These results indicate that traditional metal-based reductants are unable to effectively generate catalytically active low-valent cobalt species under mechanochemical conditions. To overcome this challenge, we turned our attention to piezoelectric materials.29–31,49–58 Our group first reported that piezoelectric BaTiO3 can act as a charge-transfer catalyst under ball-milling conditions to enable force-induced redox reactions.29 Thus, we anticipated that piezoelectric BaTiO3 could be used as a unique and highly efficient reductant to promote the [2+2+2] cycloaddition. However, when BaTiO3 was used as a reductant in our reaction the desired product (3a) was obtained in a yield comparable to those obtained with the traditional metal-based reductants (61%, entry 5, Table 1). Nevertheless, we discovered that the addition of DIPEA as an additive in the presence of BaTiO3 dramatically increased the yield (96%, entry 6, Table 1). On the other hand, the same reaction in the absence of BaTiO3 gave 3a in a much lower yield (54%, entry 7, Table 1). Importantly, the combination of the traditional metal reductants (zinc and manganese) and the amine additive did not improve the yield of 3a (for details, see the ESI†). These results suggest that the combination of BaTiO3 and the amine additive is essential to achieve a high yield with this mechanochemical protocol. We explored other piezoelectric materials, such as lithium titanate (Li2TiO3), zinc oxide (ZnO), lithium tantalate (Li2TaO3), and lithium niobate (LiNbO3), in the presence of DIPEA. These materials produced lower yields of 3a (21–52%, entries 8–11, Table 1). Non-piezoelectric materials such as aluminum oxide (Al2O3) and barium oxide (BaO) did not yield 3a (entries 12 and 13, Table 1). Reducing the amount of 2a resulted in a lower yield of 3a (68%, entry 14, Table 1). Finally, we screened other phosphine ligands, such as triphenylphosphine (PPh3), 1,3-bis(diphenylphosphino)propane (dppp), and 1,4-bis(diphenylphosphino)butane (dppb), and found that PCy3 was still the most effective (entries 15–17, Table 1).| Entry | 2a (equiv.) | Ligand (20 mol%) | Reductant (5.0 equiv.) | Additive (1.0 equiv.) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol), ethynylbenzene 2a, CoBr2 (10 mol%), and DIPEA (1.0 equiv.) in a stainless-steel (ss) ball-milling jar (5 mL) with one ss ball (diameter: 10 mm), ball milling for 1 h.b Determined from the NMR spectrum of the crude reaction mixture using 1,3,5-trimethoxybenzene as an internal standard.c Isolated yield.d 10 mol%. | |||||
| 1 | 3 | None | None | None | <1 |
| 2 | 3 | PCy3 | None | None | 55 |
| 3 | 3 | PCy3 | Zn | None | 60 |
| 4 | 3 | PCy3 | Mn | None | 66 |
| 5 | 3 | PCy3 | BaTiO3 | None | 61 |
| 6 | 3 | PCy3 | BaTiO3 | DIPEA | 96 (86c) |
| 7 | 3 | PCy3 | None | DIPEA | 54 |
| 8 | 3 | PCy3 | Li2TiO3 | DIPEA | 32 |
| 9 | 3 | PCy3 | ZnO | DIPEA | 52 |
| 10 | 3 | PCy3 | Li2TaO3 | DIPEA | 36 |
| 11 | 3 | PCy3 | LiNbO3 | DIPEA | 21 |
| 12 | 3 | PCy3 | Al2O3 | DIPEA | 0 |
| 13 | 3 | PCy3 | BaO | DIPEA | 0 |
| 14 | 2 | PCy3 | BaTiO3 | DIPEA | 68 |
| 15 | 3 | PPh3 | BaTiO3 | DIPEA | 31 |
| 16 | 3 | dpppd | BaTiO3 | DIPEA | 6 |
| 17 | 3 | dppbd | BaTiO3 | DIPEA | 44 |
To highlight the importance of the mechanical impact caused by ball milling for the effectiveness of the catalysis in the presence of piezoelectric BaTiO3, we conducted the reaction using different ball-milling frequencies. Interestingly, when the reaction was performed at 15 Hz, only a trace amount of the product was obtained (entry 1, Table 2A). However, increasing the milling frequency to 20, 25, and 30 Hz resulted in dramatically improved yields of 51%, 71%, and 96%, respectively (entries 2–4, Table 2A). This result clearly demonstrates that the reaction efficiency is proportional to the amount of force applied through ball milling, which is consistent with a piezoelectrically induced acceleration mechanism.29–31,49–58 Furthermore, we conducted a solution-based version of the reaction using MeCN as the solvent in a sealed tube under argon with BaTiO3 (Table 2B). After 1 hour, we only recovered the starting materials, and 3a was not obtained (Table 2B). This result further supports the notion that the reaction with BaTiO3 specifically requires ball-milling conditions.
| a Reaction conditions: 1a (0.20 mmol), ethynylbenzene 2a (3.0 equiv.), CoBr2 (10 mol%), and DIPEA (1.0 equiv.) in a stainless-steel (ss) ball milling jar (5 mL) with one ss ball (diameter: 10 mm). |
|---|
 |
Based on the optimization results and control experiments, we would like to propose a plausible role for BaTiO3 in this catalytic system (Scheme 2). Under ball-milling conditions, piezoelectric materials generate highly polarized particles (Scheme 2A).2–31,49–58 These polarized particles could then oxidize DIPEA to generate highly reducing, negatively charged particles (Scheme 2A). Subsequently, the activated particles can be expected to reduce cobalt(II) to cobalt(I), which is a catalytically active species in the [2+2+2] cycloaddition reaction (Scheme 2B). A mechanism where the generation of cobalt(0) is followed by the [2+2+2] cycloaddition is also possible (Scheme 2B).47 We speculate that the generated low-valent cobalt species are transient, as they can be readily oxidized by the tertiary amine radical cation or the polarized BaTiO3 particles (Scheme 2B). Although further mechanistic investigations are necessary, we propose that the low-valent cobalt species undergoes oxidative cyclization, followed by alkyne insertion and reductive elimination to furnish the desired product (see ESI† for details).
With the optimized reaction conditions in hand, we explored the reaction scope by varying the alkyne substrate while keeping the diyne (1a) constant (Table 3). Unsubstituted phenylacetylene gave the desired cycloaddition product (3a) in excellent isolated yield (86%). We examined the electronic effects of different phenylacetylene substrates by introducing different electron-donating and -withdrawing groups at the para position of the benzene ring. A methoxy group at the 4-position provided the corresponding arene (3b) in 81% yield. Halides such as bromide, chloride, and fluoride were well tolerated under the applied reaction conditions, producing satisfactory yields of 3c (75%), 3d (75%), and 3e (86%). Butyl and hexyl substituents at the para position were also well tolerated and furnished the corresponding products in good yield (3f: 72%; 3g: 67%). An alkyne with an electron-withdrawing trifluoromethyl group also provided the corresponding product in good yield (3h: 70%). The reaction proceeded smoothly with a thiophene-substituted alkyne, resulting giving the heterocyclic biaryl compound 3j in 70% yield. Alkyl-substituted alkynes were also investigated and gave the desired products in excellent yields (3l: 81%; 3m: 80%). The use of an alkyne with a polyaromatic ring, phenanthrene, resulted in the efficient formation of arene 3k in 82% yield. A trimethylsilyl (TMS)-substituted alkyne provided the corresponding product (3n) in 50% yield. Furthermore, this method proved to be efficient for internal alkynes, producing highly substituted arenes in moderate to good yields (3o: 44%; 3p: 52%)
| a Reaction conditions: diyne 1a (0.20 mmol), alkyne 2 (3.0 equiv.), CoBr2 (10 mol%), PCy3 (20 mol%), DIPEA (1.0 equiv.), and MeCN (0.2 mL mg−1) in a stainless-steel (ss) ball-milling jar (5 mL) with one ss ball (diameter: 10 mm) for 60 min, 30 Hz. |
|---|
 |
Next, we investigated the scope of different diynes (1) while ethenyl benzene (2a) was kept constant as a reaction counterpart (Table 4). Symmetrical diynes with diketone (1b), sulfonyl (1c and 1d), ester (1e), N-tosylate (1f), and ether (1g) moieties furnished the expected products in good to moderate yields (3q-3v; 64–92%). This comprehensive study demonstrates the robustness and versatility of this mechanochemical [2+2+2] cycloaddition protocol. In our substrate scope studies, side products such as homocoupled products of the diynes were scarcely detected.
| a Reaction conditions: diyne 1 (0.20 mmol), alkyne 2a (3.0 equiv.), CoBr2 (10 mol%), PCy3 (20 mol%), DIPEA (1.0 equiv.), and MeCN (0.2 mL mg−1) in a stainless-steel (ss) ball milling jar (5 mL) with one ss ball (diameter: 10 mm) for 60 min, 30 Hz. |
|---|
 |
To demonstrate the scalability of our reaction, we conducted a gram-scale experiment (Scheme 3). Starting from 1.00 g of 1a, we successfully obtained 1.15 g of 3a in 81% yield without significant loss of yield compared to the small-scale reaction. This result showcases the practical utility of this mechanochemical protocol.
To further illustrate the advantages of our mechanochemical method over traditional solution-based techniques, we tested the reaction with alkyne 2q, which is poorly soluble in common organic solvents (Scheme 4). For example, the solubility of 2q in THF at room temperature is 1.7 × 10−1 M, and in MeCN at room temperature 1.2 × 10−2 M. Therefore, the previously reported solution-based reaction of 2q using a cobalt(II)/zinc catalytic system in THF61 is slow, yielding only 18% of the desired product (3w). We also tested a photoredox/cobalt(II) dual metallophotoredox system in MeCN,47 albeit that the reaction did not afford 3w (<1%). We confirmed that these reactions were carried out in slurry states, suggesting that the unsuccessful results were most likely due to the low solubility of 2q. Another possibility is that the pyrene moiety of 2q is capable of accepting excitation energy from the excited photocatalyst, thus suppressing the [2+2+2] cycloaddition.62–64 In contrast, under our mechanochemical conditions, the desired product (3w) was obtained in much higher yield (51%). These results demonstrate that the unique advantages of our mechanochemical approach will be able to expand the scope of [2+2+2] cycloadditions for poorly soluble alkynes and molecules capable of accepting excitation energy.
 | ||
| Scheme 4 Reactions of poorly soluble alkyne 2q; for the details of the reaction conditions, see the ESI.† | ||
Conclusion
In summary, we have successfully developed the first general mechanochemical protocol for the [2+2+2] cycloaddition reaction of alkynes that employs a simple cobalt(II)-based catalyst. In this mechanochemical system, the modulation of the piezoelectrically induced oxidation state of the cobalt center is used to facilitate a highly efficient [2+2+2] cycloaddition. Importantly, the use of traditional metal reductants such as zinc and manganese resulted in low reactivity, highlighting the effectiveness of the piezoelectrically induced approach. This method is characterized by a broad substrate scope, short reaction times, and the use of an almost negligible quantity of solvent in the reaction step. Moreover, the mechanochemical approach can be readily scaled up to the gram scale and has proved advantageous for poorly soluble substrates, achieving yields unattainable by conventional solution-based methods. Beyond the practical utility of the mechanochemical [2+2+2] cycloaddition, this work showcases the great potential of our unique piezoelectric reduction system to generate catalytically active low-valent metal species, thus enabling highly efficient mechanochemical transformations.31,49Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
KK and HI conceived and designed the study. KJ, KK and HI co-wrote the paper. KJ performed the chemical experiments and analysed the data. All authors discussed the results and the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from JSPS KAKENHI grants 24H00453 (to KK), 24H01050 (to KK), 24H01832 (to KK), 22H00318 (to HI) and 22K18333 (to HI), as well as from the JST via CREST grant JPMJCR19R1 (to HI) and FOREST grant JPMJFR201I (to KK). The authors are furthermore thankful to the Institute for Chemical Reaction Design and Discovery (ICReDD), established by the World Premier International Research Initiative (WPI), MEXT, Japan. The authors thank Mr Keisuke Kondo for his help in cross-checking the experiments.Notes and references
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2011, 41, 413–447 RSC.
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC.
- T. K. Achar, A. Bose and P. Mal, Beilstein J. Org. Chem., 2017, 13, 1907–1931 CrossRef CAS PubMed.
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC.
- T.-X. Métro, J. Martinez and F. Lamaty, ACS Sustainable Chem. Eng., 2017, 5, 9599–9602 CrossRef.
- C. Bolm and J. G. Hernández, Angew. Chem., Int. Ed., 2019, 58, 3285–3299 CrossRef CAS PubMed.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steedk and D. C. Waddelli, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- A. Porcheddu, E. Colacino, L. De Luca and F. Delogu, ACS Catal., 2020, 10, 8344–8394 CrossRef CAS.
- K. Kubota and H. Ito, Trends Chem., 2020, 2, 1066–1081 CrossRef CAS.
- G. A. Bowmaker, Chem. Commun., 2013, 49, 334–348 RSC.
- J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC.
- D. Braga, L. Maini and F. Grepioni, Chem. Soc. Rev., 2013, 42, 7638–7648 RSC.
- O. Eguaogie, J. S. Vyle, P. F. Conlon, M. A. Gîlea and Y. Liang, Beilstein J. Org. Chem., 2018, 14, 955–970 CrossRef CAS PubMed.
- A. C. Jones, J. A. Leitch, S. E. Raby-Buck and D. L. Browne, Nat. Synth., 2022, 1, 763–775 CrossRef CAS.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Nat. Rev. Chem., 2023, 7, 51–65 CrossRef CAS PubMed.
- K. Kondo, K. Kubota and H. Ito, Chem. Sci., 2024, 15, 4452–4457 RSC.
- K. Kubota, T. Endo and H. Ito, Chem. Sci., 2024, 15, 3365–3371 RSC.
- Y. Gao, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2023, 62, e202217723 CrossRef CAS PubMed.
- T. Seo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2023, 145, 6823–6837 CrossRef CAS PubMed.
- R. Takahashi, P. Gao, K. Kubota and H. Ito, Chem. Sci., 2023, 14, 499–505 RSC.
- P. Gao, J. Jiang, S. Maeda, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2022, 61, e202207118 CrossRef CAS PubMed.
- R. Takahashi, A. Hu, P. Gao, Y. Gao, Y. Pang, T. Seo, J. Jiang, S. Maeda, H. Takaya, K. Kubota and H. Ito, Nat. Commun., 2021, 12, 6691–6700 CrossRef CAS PubMed.
- R. Takahashi, T. Seo, K. Kubota and H. Ito, ACS Catal., 2021, 11, 14803–14810 CrossRef CAS.
- K. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500–1504 CrossRef CAS PubMed.
- Y. Pang, J. W. Lee, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2020, 59, 22570–22576 CrossRef CAS PubMed.
- T. Seo, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2023, 62, e202311531 CrossRef CAS PubMed.
- K. Kubota, R. Hisazumi, T. Seo and H. Ito, RSC Mechanochem., 2024, 1, 250–254 RSC.
- K. Kubota, N. Shizukuishi, S. Kubo and H. Ito, Chem. Lett., 2024, 53, upae056 CrossRef CAS.
- G. Domínguez and J. Pérez-Castells, Chem. Soc. Rev., 2011, 40, 3430–3444 RSC.
- P. Matton, S. Huvelle, M. Haddad, P. Phansavath and V. Ratovelomanana-Vidal, Synthesis, 2022, 54, 4–32 CrossRef CAS.
- A. Roglans, A. Pla-Quintana and M. Solà, Chem. Rev., 2021, 121, 1894–1979 CrossRef CAS PubMed.
- S. Saito and Y. Yamamoto, Chem. Rev., 2000, 100, 2901–2916 CrossRef CAS PubMed.
- N. Weding and M. Hapke, Chem. Soc. Rev., 2011, 40, 4525–4538 RSC.
- R. A. Haley, A. R. Zellner, J. A. Krause, H. Guan and J. Mack, ACS Sustainable Chem. Eng., 2016, 4, 2464–2469 CrossRef CAS.
- G. P. Chiusoli, M. Costa, S. Reverberi and M. G. Terenghi, Transition Met. Chem., 1989, 14, 238–240 CrossRef CAS.
- F. Slowinski, C. Aubert and M. Malacria, Adv. Synth. Catal., 2001, 343, 64–67 CrossRef CAS.
- N. Saino, D. Kogure and S. Okamoto, Org. Lett., 2005, 7, 3065–3067 CrossRef CAS PubMed.
- G. Hilt, T. Vogler, W. Hess and F. Galbiati, Chem. Commun., 2005, 1474–1475 RSC.
- K. P. C. Vollhardt, Angew. Chem., Int. Ed. Engl., 1984, 23, 539–556 CrossRef.
- V. Gandon, C. Aubert and M. Malacria, Chem. Commun., 2006, 2209–2217 RSC.
- T. Gläsel, B. N. Baumann and M. Hapke, Chem. Rec., 2021, 21, 3727–3745 CrossRef PubMed.
- K. E. Ruhl and T. Rovis, J. Am. Chem. Soc., 2016, 138, 15527–15530 CrossRef CAS PubMed.
- T. Gläsel, H. Jiao and M. Hapke, ACS Catal., 2021, 11, 13434–13444 CrossRef.
- C. Schumacher, J. G. Hernández and C. Bolm, Angew. Chem., Int. Ed., 2020, 59, 16357–16360 CrossRef CAS PubMed.
- M. M. Amer, R. Hommelsheim, C. Schumacher, D. Kong and C. Bolm, Faraday Discuss., 2023, 241, 79–90 Search PubMed.
- X. Wang, X. Zhang, L. Xue, Q. Wang, F. You, L. Dai, J. Wu, S. Kramer and Z. Lian, Angew. Chem., Int. Ed., 2023, 62, e202307054 CrossRef CAS PubMed.
- R. Qu, S. Wan, X. Zhang, X. Wang, L. Xue, Q. Wang, G.-J. Cheng, L. Dai and Z. Lian, Angew. Chem., Int. Ed., 2024, 63, e202400645 Search PubMed.
- S.-L. Niu, W. Yuan, X. Gong, B. Bao, Z.-W. Wu, B. Xu, R. Zeng, Q.-W. Yang and Q. Ouyang, ACS Sustainable Chem. Eng., 2023, 11, 17816–17825 Search PubMed.
- H. Wang, W. Ding and G. Zou, J. Org. Chem., 2023, 88, 12891–12901 Search PubMed.
- C. B. Lennox, T. H. Borchers, L. Gonnet, C. J. Barret, S. G. Koenig, K. Nagapudi and T. Friščić, Chem. Sci., 2023, 14, 7475–7481 RSC.
- J. A. Leitch and D. L. Browne, Chem.–Eur. J., 2021, 27, 9721–9726 CrossRef CAS PubMed.
- S. M. Zeitler and M. R. Golder, Chem. Commun., 2024, 60, 26–35 RSC.
- H. Sudrajat, H.-Y. Hsu, F. Jérôme and J. C. Colmenares, ChemCatChem, 2025, e202401814 CrossRef CAS.
- S. Mkrtchyan, O. Shalimov, M. G. Garcia, J. Zapletal and V. O. Iaroshenko, Chem. Sci., 2024, 15, 9155–9163 RSC.
- A. W. Tricker, G. Samaras, K. L. Hebisch, M. J. Realff and C. Sievers, Chem. Eng. J., 2020, 382, 122954–122967 CrossRef CAS.
- A. Goswami, T. Ito and S. Okamoto, Adv. Synth. Catal., 2007, 349, 2368–2374 CrossRef CAS.
- F. Glaser, C. Kerzig and O. S. Wenger, Angew. Chem., Int. Ed., 2020, 59, 10266–10284 CrossRef CAS PubMed.
- I. Ghosh, J. I. Bardagi and B. König, Angew. Chem., Int. Ed., 2017, 56, 12822–12824 CrossRef CAS PubMed.
- A. Chatterjee and B. König, Angew. Chem., Int. Ed., 2019, 58, 14289–14294 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02074c |
| This journal is © The Royal Society of Chemistry 2025 |