 Open Access Article
Open Access ArticleEffect of Mn2+ doping on exciton recombination and carrier trapping in the Cs2CdCl4 metal halide†
Dayu
Huang
ab,
Ziyong
Cheng
ab,
Qiuyun
Ouyang
*a,
Hongzhou
Lian
*ab and
Jun
Lin
 *ab
*ab
aKey Laboratory of In-Fiber Integrated Optics, Ministry Education of China, and College of Physics and Opotoelectronic Engineering, Harbin Engineering University, Harbin 150001, China. E-mail: qyouyang@hrbeu.edu.cn; hzlian@ciac.ac.cn; jlin@ciac.ac.cn
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
First published on 17th April 2023
Abstract
The lattice defects that occur in material preparation processes are closely related to carrier migration. The carrier migration can be regulated by artificially modulating the defects to improve the luminescence performance, which is important for the development and application of luminescent materials. Here, we achieved a 3.29-fold increase in the high-temperature luminescence intensity by introducing Mn2+ to generate Cl vacancy defects in Cs2CdCl4. Due to thermally induced electron de-trapping, Cs2CdCl4:Mn2+ also displays anti-thermal quenching (ATQ) behavior and good thermal quenching resistance (329% at 175 °C). The increase in the photoionization effect and de-trapping of the captured electrons from shallow trap states were appropriately attributed to this exceptional phenomenon, based on thermoluminescence (TL) analysis. Based on comprehensive optical characterization, thermoluminescence and temperature-dependent X-ray diffraction (XRD), we reveal that the Cl vacancy trap state generated by Mn doping is the reason for the enhanced PL in air. The discovery of these materials helps to better understand the optical properties of all-inorganic perovskites.
1. Introduction
The study of metal halides has grown exponentially due to their excellent photoelectric properties.1–24 One aspect of this work involves replacing Pb in the metal halide with other metals to form materials with different chemical or physical properties or better stability than Pb halide perovskites.25–30 The characteristic 3D double perovskite structure of CsPbX3 (X = Cl, Br, and I) is retained, that is, the Pb2+ cation in the compound is replaced by monovalent and trivalent cations in pairs, as described by the Cs2M+M′+X6 (X = Cl, Br, and I) formula unit.31–38 Another research direction is to explore low-dimensional perovskites with metal halide octahedra forming 2D structures or isolated 0D units. In 2D perovskites, layers composed of metal halide octahedral and monovalent cations appear alternately. In this structure, the size limitation of monovalent cation selection in 3D perovskites is greatly relaxed, such that hybrid 2D perovskites can be formed in which the 2D metal halide layers are alternately arranged with organic monovalent cation layers of different sizes.39,40 Such organic–inorganic hybrid perovskites exhibit excellent optical properties, such as a tunable emission range, a narrow emission line width and a high photoluminescence quantum yield (PLQY). Among all the possible arrangements of metal halide octahedral layers, there is the unique Ruddlesden–Popper (RP) phase, which has attracted extensive attention due to its chemical diversity, stability and properties that are similar to 3D structures.40,41 The RP phase is in the stoichiometric ratio of the formula unit A2MX4 (A and X = monovalent or divalent ions; M = metal cations), which are composed of co-angular MX6 octahedral sheets stacked in an ABAB sequence along the c axis. Such a structure, e.g., oxide Sr2TiO4, was first discovered by Ruddlesden and Popper.42 In the past century, halide-based RP phases have attracted much attention due to their excellent photoelectric properties, and are considered to be a new class of semiconductor materials. The host Cs2CdCl4 in this paper has also been identified to have the same RP structure.43 Koshimizu's group reported that Cs2CdCl4 as an X-ray scintillator material can emit light by doping Sb3+ or Tl+.44 Among them, the prominent feature of Cs2CdCl4:Sb3+ is that there is only a single emission band. The maximum emission peak is located at 516 nm. However, compared with the corresponding bulk compounds, RP phase materials exhibit unique physical and photoelectric properties as colloidal nanocrystals. The research of Locardi's group involved the formation of the Cs2CdCl4:Sb3+ RP phase as colloidal nanocrystals.45 Under UV excitation, Cs2CdCl4:Sb3+ has a strong cyan emission. Doping transition metals into perovskites is a unique strategy to expand the family of perovskite materials. However, undoped materials cannot change their optical and electronic properties. Based on the unique properties of Cd-based halide perovskites, their photoelectric properties were studied under the activation of photoluminescence center signals. It is proved that the doping effect is crucial for photon upconversion and a higher excited state emission. So far, Mn2+ activated luminescent materials exhibit high thermal quenching due to strong electron–lattice coupling during heating. Therefore, it is a meaningful work to develop Mn2+ ion doped perovskites with high thermal stability and high efficiency for lighting applications.In this work, we studied the relationship between radiative exciton recombination and carrier trapping on the surface of the Cs2CdCl4:Mn2+ perovskite crystal via high temperature spectra. It is found that the photoluminescence (PL) intensity of the Cs2CdCl4:Mn2+ crystal can be increased 3.29 fold by increasing the temperature from room temperature to 175 °C. Based on comprehensive steady-state and dynamic optical characterizations, we attribute the enhanced PL with increasing temperature to the reduced density of states associated with Cl vacancies near the crystal surface. We found that the carriers trapped by Cl vacancies are activated at high temperature, contributing to the PL intensity. Therefore, we speculate that the trap density of the Cs2CdCl4:Mn2+ crystal is more than that of the Cs2CdCl4 crystal. It is worth noting that high temperature is detrimental to the performance of perovskite LEDs. Our results provide a new means of improving the stability of devices based on all-inorganic perovskites, and highlight the interesting photoelectric properties of Cs2CdCl4:Mn2+ crystals due to high temperature.
2. Experimental
2.1 Chemicals
All chemicals were used without further purification: CdO (cadmium oxide, Aladdin), CsCl (cesium chloride, Aladdin), HCl (hydrochloric acid, 36–38%) aqueous solution, N,N-dimethylformamide (Aladdin), and (CH3COO)2Mn (manganous acetate, Aladdin). All reactions were performed under ambient conditions.2.2 Synthesis of Cs2CdCl4 and Cs2CdCl4:xMn2+
Cs2CdCl4 and Cs2CdCl4:Mn2+ were synthesized via a hydrothermal method. For the Cs2CdCl4 host, CsCl (4 mmol) and CdO (1 mmol) were added to a 25 mL Teflon-lined autoclave. Then, 3 mL of HCl aqueous solution was added to the Teflon-lined autoclave followed by 3 mL DMF, and the autoclave was heated at 150 °C for 2 h before being cooled slowly at 2 °C h−1 to room temperature. Finally, the obtained single crystal particles were washed two times with ethanol and dried at 80 °C for 1 h in an oven. For Cs2CdCl4:Mn2+, (CH3COO)2Mn (0.1 mmol) was added to the Teflon-lined autoclave, and other steps are the same.2.3 Characterization
The single-crystal X-ray diffraction (SCXRD) data were recorded using a Bruker Apex II CCD diffractometer with X-ray Mo Kα radiation (λ = 0.71073 Å). A suitable single crystal was selected and kept at room temperature during data collection. Data reduction, experimental absorption correction, and cell refinement were obtained using the APEX3software package. Using Olex2 software, the structure was solved by the ShelXT structural solution program using the Intrinsic Phasing method and refined by the ShelXL refinement package using least-squares minimization. Phase identification was confirmed through X-ray diffraction using a D8 Focus diffractometer (Bruker) with Cu-Kα radiation (λ = 1.5405 Å). The diffuse reflectance spectra (DRS) were obtained using a UV-visible diffuse reflectance spectrophotometer (UV-2550 PC, Shimadzu Corporation, Japan). TEM was carried out using a FEI Tecnai G2S-Twin microscope with a field-emission gun operating at 200 kV. Photoluminescence (PL) spectra and temperature-dependent PL spectra (from −180 to 100 °C) were obtained using a fluorescence spectrophotometer with a 450 W xenon lamp as the excitation source equipped with a temperature controller (Edinburgh Instruments FLS-920). The photoluminescence quantum yield (PLQY) values were collected using an absolute PL quantum yield measurement system equipped with a temperature controller (Hamamatsu Photonics K.K., C9920-02 Japan). A LTTL-3DS spectrometer was used to record the two-dimensional (2D) thermoluminescence (TL) curves at a heating rate of 2 K s−1. Thermogravimetric studies were carried out using a simultaneous thermal analyzer (STA 449 F3).3. Results and discussion
High-quality Cs2CdCl4:Mn2+ perovskite crystals were prepared using a hydrothermal technique. The results of powder X-ray diffraction (PXRD) tests are consistent with those of single-crystal X-ray diffraction (SCXRD), and the Cs2CdCl4:Mn2+ crystal has a clear I4/m space group (Fig. 1a). Table 1 lists the detailed structural parameters. According to Fig. 1b, the structure units of Cs2CdCl4 are built up of [CdCl6]4− and [CsCl9]8− polyhedra, in which Mn2+ ions are known to selectively occupy the 6-coordinated Cd2+ sites because of their similar valence state and ionic radii (RCd2+ = 0.95 Å and RMn2+ = 0.67 Å). The XPS outcomes are displayed in Fig. 1c. In particular, the peak of Mn2+ binding energy at 653.04 eV corresponds to the 2p orbital of Mn2+. The XPS peak position of Mn2+ is consistent with many previous reports,19 demonstrating the successful introduction of Mn2+, and the signal peaks of Cs, Cd, C and Cl are well shown. From the EPR spectrum of Mn2+ (Fig. 1d) under the influence of an external magnetic field, it is further confirmed that Mn2+ exists in the material and that just one site in the lattice is suitable for Mn2+. A weak Mn–Mn dipole contact also exists.46 | ||
| Fig. 1 (a) X-ray diffraction pattern of the Cs2CdCl4:Mn2+ perovskite crystal, showing the I4/m space group that coincides well with CCDC 2235089 (a = b = 5.2464 Å, c = 16.854 Å, α = β = γ = 90°). (b) Crystal structure of Cs2CdCl4. (c) XPS spectra corresponding to Cs2CdCl4, Cs, Cd, Mn, C and Cl. (d) EPR spectrum of Cs2CdCl4:Mn2+. | ||
| Structural parameter | Cs2CdCl4:Mn2+ |
|---|---|
| a | 5.2464 Å |
| b | 5.2464 Å |
| c | 16.854 Å |
| Volume | 463.9 Å3 |
| Space group | I4/m |
| Z | 4 |
| h | 7 |
| k | 7 |
| l max | 23 |
| R (reflections) | 0.0663 |
| wR2 (reflections) | 0.1851 |
| CCDC | 2235089 |
Fig. 2a displays the UV/Vis diffuse reflectance (DR) spectrum of Cs2CdCl4:Mn2+. Here, Cs2CdCl4:Mn2+ exhibits several absorption sites and an indirect bandgap (Fig. 2b). Indeed, under observation with the naked eye, Cs2CdCl4:Mn2+ exhibited yellow emission under UV irradiation, which is clearly brighter than that of pristine Cs2CdCl4. The host's own luminescence is very weak or even invisible to the naked eye (Fig. 2c). After Mn2+ doping, the Mn2+ d–d emission corresponding to the spin-forbidden 4T1 → 6A1 transition can be observed as a broadband yellow emission peak at around 585 nm with excitation at 417 nm (Fig. 2d). The emission bands of these samples share many solitary excitation bands at 250–325, 325–375, 417, and 475–550 nm, which perhaps belong to a charge transfer band and electronic transitions from 6A1g(S) to the excited state. The corresponding excitation spectrum is shown in Fig. 2d. The distinct transitions show different excitation peaks. The excitation peak at 250–325 nm belongs to the charge transfer band of the host. The excitation peak at 325–375 nm is attributed to the 4Eg(4D)/4T2g(4D)–6A1 transition. The 4E2g(4G)/4T2g(4G)/4A1(4G)–6A1 transition is responsible for the excitation peak at 417 nm. Moreover, the 4T1(4G)–6A1 transition is involved in the excitation peak at 475–550 nm.
Fig. 2e and f present photographs of the luminescence of the samples under visible light and ultraviolet light, respectively. The size and the yellow emission of the Cs2CdCl4:Mn2+ are clearly shown. In addition, grinding the single crystal into powder does not affect the luminescence intensity of the sample. The PLQY of the material can reach 90%.
In order to study the temperature dependence of the photoluminescence of the Cs2CdCl4:Mn2+ crystal, we monitored the crystal luminescence at high temperature. Fig. 3a shows the PL spectra of the Cs2CdCl4:Mn2+ crystal excited at 275 nm at different temperatures. Surprisingly, as shown in Fig. 3c, the PL intensity at high temperature increases by 3.29 fold (I(175°C) = 3.29I(25°C), where I(175°C) is the integrated intensity at 175 °C, and I(25°C) is the integrated intensity at 25 °C). As shown in Fig. 3a, the PL peak of the Cs2CdCl4:Mn2+ crystal shows a clear enhancement and a blue shift as the temperature is increased. The blue shift of the emission peak at high temperature is because the crystal field strength of the material becomes weaker, which is generally accepted (Fig. 3b). It can be seen that the PL intensity decreases slowly before −100 °C, and then begins to increase slowly. As the temperature continues to rise, between 75 and 175 °C, the luminescence intensity increases rapidly and reaches the strongest state. Such a heating process can be repeated many times without any signs of change (Fig. 3d).
In order to obtain the mechanism of the temperature-dependent PL, it is necessary to understand the reasons for the PL enhancement of the Cs2CdCl4:Mn2+ perovskite crystals at high temperature, which is crucial for further analysis. In general, two possible scenarios can be assumed to explain the higher PL intensity at temperatures higher than room temperature. (1) The PL origin is related to the non-intrinsic radiation defect state, and the defect concentration increases at high temperature. This has recently been reported, and energy transfer from the trap state to Mn2+ has been observed at high temperatures.47 (2) If the PL originates from intrinsic interband or exciton recombination, it is necessary to verify the reduction of non-radiative recombination centers at high temperature. However, the luminescence enhancement is clearly not due to the decrease of the radiation centers, but the increase in the energy transfer path to Mn2+ at high temperature. Since significant self-trapped exciton (STE) emission can be observed at low temperature, the whole temperature-rise process refers to multiple photophysical behaviors.
First, we observe the normal emission intensity of Cs2CdCl4:Mn2+ in the region from −180 to −100 °C (I−180°C to I−100°C) as shown Fig. 3c. This is initially used to return to the ground state radiation transition energy due to the phonon vibration loss, that is, thermal quenching behavior. Defect levels are not involved during this low-temperature process due to the lack of effective thermal activation energy. In region II (from −80 to 250 °C), the emission intensity at 175 °C is 329% of that at 20 °C. Nonetheless, Cs2CdCl4:Mn2+ exhibits the zero-thermal quenching phenomenon close to the working temperature of the LED chip, indicating its practical application potential. Generally, electron–phonon coupling becomes stronger and leads to the loss of luminescence intensity at high temperature. Hence, the thermal quenching and thermo-stimulated radiation dynamic process may form a competitive relationship to affect the luminescence intensity. Our initial hypothesis was that the STE energy of the host was transferred to Mn2+; however, since the STE luminescence is so faint, it is hard to double the luminescence of Mn2+.
Through XPS, we discovered an intriguing phenomenon in which the signal peaks of Cl in Cs2CdCl4 and Cl in Cs2CdCl4:Mn2+ moved and expanded significantly (Fig. 4a and b). The material itself has Cl− vacancy defects, and the introduction of Mn2+ can control the Cl− vacancy within a certain range, thereby maintaining stability at high temperature. Due to the high temperature, excessive Cl vacancy defects will lead to luminescence quenching. Therefore, the introduction of Mn avoids a large number of Cl vacancies at high temperatures and maintains a relatively balanced Cl vacancy ratio, which significantly enhances the anti-thermal quenching of Mn2+. This is because a large number of Cl defects will bring about a large number of non-radiative complexes. This is the reason why we observe the anti-thermal quenching effect at high temperature.
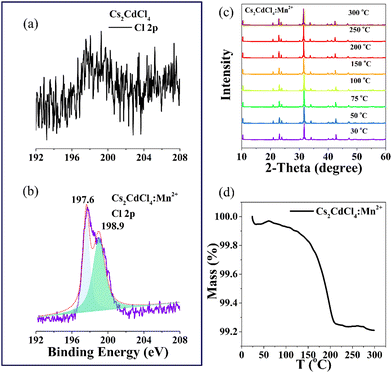 | ||
| Fig. 4 XPS spectrum of Cl in (a) Cs2CdCl4 and (b) Cs2CdCl4:Mn2+. (c) Temperature-dependent XRD patterns. (d) TG curve of Cs2CdCl4:Mn2+. | ||
To test the hypothesis proposed above, we obtained the following series of high temperature material characterization data. The material's luminous phase did not change at high temperatures, according to high temperature XRD (Fig. 4c). The TG curve proves that the quality of the sample does not change with the increase in temperature. The water adsorbed by the sample is lost (Fig. 4d). Subsequently, we examined the material's PLQY at different temperatures and discovered that, in addition to the PLQY of the material increasing at high temperatures, the absorption also increased, reaching a maximum before then declining (Table 2). The trend in the temperature-dependent quantum yield is similar to that of the temperature-dependent luminescence. The luminescence of the material is enhanced with the increase in temperature. It is further proved that the luminescence of the material is indeed enhanced at high temperature, which is very beneficial for practical applications of the material.
| Temperature (°C) | Quantum yield | Absorption |
|---|---|---|
| 25 | 0.90 | 0.36 |
| 40 | 0.91 | 0.37 |
| 55 | 0.92 | 0.38 |
| 70 | 0.92 | 0.385 |
| 85 | 0.92 | 0.389 |
| 100 | 0.93 | 0.391 |
| 115 | 0.94 | 0.393 |
| 130 | 0.94 | 0.396 |
| 145 | 0.94 | 0.398 |
| 160 | 0.95 | 0.41 |
| 175 | 0.95 | 0.412 |
| 190 | 0.94 | 0.43 |
| 205 | 0.94 | 0.40 |
| 220 | 0.94 | 0.402 |
In the information that follows, we demonstrate that the evidence points to the presence of several traps. Initially, a certain depth of lattice disorder is seen on the surface of the particles, probably as a result of the substitution of Mn2+ ions (Fig. 5a). Hence, the above finding indicated that the Cl vacancies, as electron trapping and storing sites, contributed to the enhanced emission intensity. To further support this view, the thermoluminescence (TL) glow curve, as a powerful tool to investigate the trapping state, was employed. Five trap levels were located between 300 and 600 K at various depths (Fig. 5b). E = Tm/500, where E is the average trap depth and Tm is the temperature corresponding to the peak position, is the empirical formula of Urbach.48 The computed trap depths are 0.68, 0.74, 0.86, 0.99 and 1.04 eV. Fig. 5c shows several processes of electron transfer. Some of the excited electrons transfer to the excited state of Mn2+ at room temperature, enhancing the Mn2+ emission. Deep traps catch some of them. In addition, the shallow trap's electron component contributes to the Mn2+ emission, which boosts the Mn2+ emission. Deep traps have all captured the remaining electrons. Deep trap electrons have a key role in the emission of Mn2+ at high temperatures. The abnormal luminescence phenomena are caused by the electrons of deep traps obtaining energy to jump out of the deep trap and transfer to the excited state of Mn2+. Such a host is unusual. A localized Cl vacancy trap energy level is created in the material's forbidden band through the addition of Mn2+. When the temperature increases, the electrons bound by the trap energy level are de-trapped, reaching the excited state energy level via the conduction band and undergoing electron–hole complexation when returning to the ground state. The energy is emitted in the form of yellow emission to compensate for the weakened luminescence caused by the non-radiative transitions at high temperatures. When the two processes reach dynamic equilibrium, zero-thermal quenching can be achieved. Confirmed by the Jahn–Teller effect, slight distortions lead to easier STE formation due to the enhanced electron–phonon coupling (Fig. 5a). Secondly, the doping of Mn2+ introduces a large number of defect energy states, which is a basis for the enhanced STE phenomenon compared with the host. The trap states of the Mn2+ materials were calculated via the thermoluminescence spectrum, taking into account the Cl vacancies in the lattice, to further demonstrate that defects are involved in the process of anti-thermal quenching. The energy supplement process: the light irradiation at room temperature/high temperature makes these suitable defect states capture the excited carriers. The carriers trapped by the defect state will be released to the luminescence center under the influence of thermal disturbance as the temperature increases, making up for the energy loss enforced by the thermal quenching effect and enhancing the thermal stability of the luminous material.
We further demonstrated the process of energy transfer from STE to Mn2+ by monitoring the decay time of different emission peaks at different temperatures (Fig. 5d). The conclusion that all data can be explained using a double exponential decay model, y = y0 + A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2), suggests that Cs2CdCl4:Mn2+ phosphors contain only a double light center (Table 3). By monitoring the excitation and emission spectra of Mn2+ at different temperatures, it is proved that energy transfer occurs between STE and Mn2+ at different temperatures (Fig. 5e). In addition, the decay time test further proves the energy transfer efficiency of Cs2CdCl4 and Mn2+. The lifetime of Mn2+ increases with increasing temperature, which is strongly related to the material's deep traps. Partly as a result of energy transfer, the host's lifetime keeps getting shorter.
exp(−t/τ2), suggests that Cs2CdCl4:Mn2+ phosphors contain only a double light center (Table 3). By monitoring the excitation and emission spectra of Mn2+ at different temperatures, it is proved that energy transfer occurs between STE and Mn2+ at different temperatures (Fig. 5e). In addition, the decay time test further proves the energy transfer efficiency of Cs2CdCl4 and Mn2+. The lifetime of Mn2+ increases with increasing temperature, which is strongly related to the material's deep traps. Partly as a result of energy transfer, the host's lifetime keeps getting shorter.
| T (°C) | Decay time (ms) | T (°C) | Decay time (ms) |
|---|---|---|---|
| −180 | 12.95 | 25 | 17.65 |
| −160 | 12.58 | 50 | 19.56 |
| −140 | 11.90 | 75 | 21.16 |
| −120 | 11.78 | 100 | 23.25 |
| −100 | 11.26 | 125 | 26.52 |
| −80 | 13.36 | 150 | 28.61 |
| −60 | 13.62 | 175 | 30.81 |
| −40 | 13.89 | 200 | 16.62 |
| −20 | 14.36 | 225 | 15.84 |
| 0 | 14.51 | 250 | 15.43 |
4. Conclusions
The corresponding defects that result from the substitution of Mn2+-doped equivalent cations will also cooperate with the intrinsic defects to form functional trap energy levels to achieve a dynamic balance of carrier storage and response under external excitation, which will improve the luminescence performance of the material and even bring new luminescence properties. It has been clearly shown that the PL modulation is related to the density of surface trap states. We speculate that one possible reason for the observed PL enhancement is the reversible physical adsorption of oxygen, bromine and water.49,50 Here, we aim to study the effect of Mn2+ doping on the photophysics of Cs2CdCl4, which is very beneficial to basic research. We demonstrate that Cl vacancy defects contribute to the luminescence enhancement of Cs2CdCl4:Mn2+ at high temperature. The understanding of Cl vacancy defects is still in its infancy. This work highlights the interaction between the introduction of Cl vacancies on Mn2+-doped Cs2CdCl4 crystals and luminescence enhancement. These findings are helpful to understand the optical properties of Cs2CdCl4 materials measured at high temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Key Research and Development Program of China (2022YFB3503800), the National Natural Science Foundation of China (NSFC No. 51932009, 61205113, 51902226, 52172166, U2005212), the Natural Science Fund of Heilongjiang Province (LH2019F013), the 111 project (B13015) of Ministry Education of China to Harbin Engineering University, and the Fundamental Research Funds for the Central Universities (No. 3072020CF2520). Project of High-level Scientific Research Guidance of Harbin Engineering University (No. 3072022TS2508).Notes and references
- J. S. Manser, J. A. Christians and P. V. Kamat, Chem. Rev., 2016, 116, 12956–13008 CrossRef CAS PubMed.
- L. N. Quan, B. P. Rand, R. H. Friend, S. G. Mhaisalkar, T.-W. Lee and E. H. Sargent, Chem. Rev., 2019, 119, 7444–7477 CrossRef CAS PubMed.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS PubMed.
- E. Shi, B. Yuan, S. B. Shiring, Y. Gao, Y. Guo, C. Su, M. Lai, P. Yang, J. Kong and B. M. Savoie, Nature, 2020, 580, 614–620 CrossRef CAS PubMed.
- S. C. Erwin, L. Zu, M. I. Haftel, A. L. Efros, T. A. Kennedy and D. J. Norris, Nature, 2005, 436, 91–94 CrossRef CAS PubMed.
- D. A. Berry, Nature, 2008, 454, 692–693 CrossRef CAS PubMed.
- Y. Wang, T. Wu, J. Barbaud, W. Kong, D. Cui, H. Chen, X. Yang and L. J. S. Han, Science, 2019, 365, 687–691 CrossRef CAS PubMed.
- D. J. Norris, A. L. Efros and S. C. Erwin, Science, 2008, 319, 1776–1779 CrossRef CAS PubMed.
- D. Mocatta, G. Cohen, J. Schattner, O. Millo, E. Rabani and U. Banin, Science, 2011, 332, 77–81 CrossRef CAS PubMed.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Science, 2017, 358, 745–750 CrossRef CAS PubMed.
- J. Hou, P. Chen, A. Shukla, A. Krajnc, T. Wang, X. Li, R. Doasa, L. H. Tizei, B. Chan and D. N. Johnstone, Science, 2021, 374, 621–625 CrossRef CAS PubMed.
- Y. Wei, Z. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310–350 RSC.
- E. Shi, Y. Gao, B. P. Finkenauer, A. H. Coffey and L. Dou, Chem. Soc. Rev., 2018, 47, 6046–6072 RSC.
- M. D. Smith, B. A. Connor and H. I. Karunadasa, Chem. Rev., 2019, 119, 3104–3139 CrossRef CAS PubMed.
- X. Zhou, K. Han, Y. Wang, J. Jin, S. Jiang, Q. Zhang and Z. Xia, Adv. Mater., 2023, 2212022 CrossRef CAS PubMed.
- B. Su, M. Li, E. Song and Z. Xia, Adv. Funct. Mater., 2021, 31, 2105316 CrossRef CAS.
- J. Jin, Y. Peng, Y. Xu, K. Han, A. Zhang, X.-B. Yang and Z. Xia, Chem. Mater., 2022, 34, 5717–5725 CrossRef CAS.
- Y. Jing, Y. Liu, X. Jiang, M. S. Molokeev, Z. Lin and Z. Xia, Chem. Mater., 2020, 32, 5327–5334 CrossRef CAS.
- B. Su, K. Han and Z. Xia, J. Mater. Chem. C, 2023 10.1039/D2TC04249E.
- M. Li and Z. Xia, Chem. Soc. Rev., 2021, 50, 2626–2662 RSC.
- G. Zhou, B. Su, J. Huang, Q. Zhang and Z. Xia, Mater. Sci. Eng., R, 2020, 141, 100548 CrossRef.
- K. Han, J. Jin, B. Su and Z. Xia, Trends Chem., 2022, 4, 1034–1044 CrossRef CAS.
- B. Su, G. Zhou, J. Huang, E. Song, A. Nag and Z. Xia, Laser Photonics Rev., 2021, 15, 2000334 CrossRef CAS.
- Y. Jing, Y. Liu, M. Li and Z. Xia, Adv. Opt. Mater., 2021, 9, 2002213 CrossRef CAS.
- B. Zhao, S. Bai, V. Kim, R. Lamboll, R. Shivanna, F. Auras, J. M. Richter, L. Yang, L. Dai and M. J. N. P. Alsari, Nat. Photonics, 2018, 12, 783–789 CrossRef CAS.
- T. Chiba, Y. Hayashi, H. Ebe, K. Hoshi, J. Sato, S. Sato, Y.-J. Pu, S. Ohisa and J. J. N. P. Kido, Nat. Photonics, 2018, 12, 681–687 CrossRef CAS.
- Z. Yuan, C. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. van de Burgt, K. Kountouriotis, Y. Xin, E. Holt, K. Schanze, R. Clark, T. Siegrist and B. Ma, Nat. Commun., 2017, 8, 14051 CrossRef CAS PubMed.
- X. Yang, X. Zhang, J. Deng, Z. Chu, Q. Jiang, J. Meng, P. Wang, L. Zhang, Z. Yin and J. You, Nat. Commun., 2018, 9, 1–8 CrossRef PubMed.
- R. N. Bhargava, D. Gallagher, X. Hong and A. Nurmikko, Phys. Rev. Lett., 1994, 72, 416–419 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Chem. Rev., 2019, 119, 3296–3348 CrossRef CAS PubMed.
- A. Zhang, Y. Liu, G. Liu and Z. Xia, Chem. Mater., 2022, 34, 3006–3012 CrossRef CAS.
- Y. Liu, A. Nag, L. Manna and Z. Xia, Angew. Chem., Int. Ed., 2021, 133, 11696–11707 CrossRef.
- Y. Liu, X. Rong, M. Li, M. S. Molokeev, J. Zhao and Z. Xia, Angew. Chem., Int. Ed., 2020, 59, 11634–11640 CrossRef CAS PubMed.
- Y. Jing, Y. Liu, J. Zhao and Z. Xia, J. Phys. Chem. Lett., 2019, 10, 7439–7444 CrossRef CAS PubMed.
- Y. Liu, Y. Jing, J. Zhao, Q. Liu and Z. Xia, Chem. Mater., 2019, 31, 3333–3339 CrossRef CAS.
- J. Zhou, Z. Xia, M. S. Molokeev, X. Zhang, D. Peng and Q. Liu, J. Mater. Chem. A, 2017, 5, 15031–15037 RSC.
- K. Han, J. Qiao, S. Zhang, B. Su, B. Lou, C. G. Ma and Z. Xia, Laser Photonics Rev., 2023, 17, 2200458 CrossRef CAS.
- M. D. Smith, B. A. Connor and H. I. Karunadasa, Chem. Rev., 2019, 119, 3104–3139 CrossRef CAS PubMed.
- B. Vargas, G. Rodriguez-Lopez and D. Solis-Ibarra, ACS Energy Lett., 2020, 5, 3591–3608 CrossRef CAS.
- X. Li, J. M. Hoffman and M. G. Kanatzidis, Chem. Rev., 2021, 121, 2230–2291 CrossRef CAS PubMed.
- S. N. Ruddlesden and P. Popper, Acta Crystallogr., 1957, 10, 538–540 CrossRef CAS.
- S. Siegel and E. Gebert, Acta Crystallogr., 1964, 17, 790 CrossRef CAS.
- T. Sakai, M. Koshimizu, Y. Fujimoto, D. Nakauchi, T. Yanagida and K. Asai, Sens. Mater., 2018, 30, 1565–1575 CAS.
- F. Locardi, M. Samoli, A. Martinelli, O. Erdem, D. V. Magalhaes, S. Bals and Z. Hens, ACS Nano, 2021, 15, 17729–17737 CrossRef CAS PubMed.
- Y. Liu, J. Zhang, B. Han, X. Wang, Z. Wang, C. Xue, G. Bian, D. Hu, R. Zhou, D. Li, Z. Wang, Z. Ouyang, M. Li and T. Wu, J. Am. Chem. Soc., 2020, 142(14), 6649–6660 CrossRef CAS PubMed.
- S. He, Q. Qiang, T. Lang, M. Cai, T. Han, H. You, L. Peng, S. Cao, B. Liu, X. Jing and B. Jia, Angew. Chem., Int. Ed., 2022, 134, e202208937 Search PubMed.
- F. Urbach, Sitzungsberichte Akad. Der Wiss, 1930, 139, 363–372 Search PubMed.
- Y. Wang, Y. Ren, S. Zhang, J. Wu, J. Song, X. Li, J. Xu, C. H. Sow, H. Zeng and H. Sun, Commun. Phys., 2018, 1, 96 CrossRef CAS.
- H.-H. Fang, S. Adjokatse, H. Wei, J. Yang, G. R. Blake, J. Huang, J. Even and M. A. Loi, Sci. Adv., 2016, 2, e1600534 CrossRef PubMed.
Footnote |
| † CCDC 2235089. For crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc00982c |
| This journal is © The Royal Society of Chemistry 2023 |



