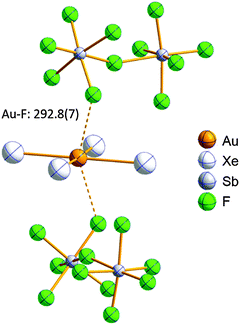
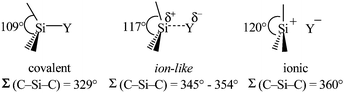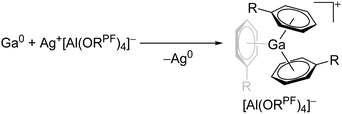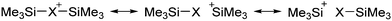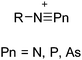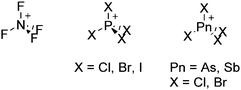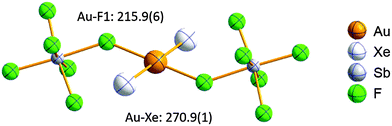Open Access Article
Open Access ArticleReactive p-block cations stabilized by weakly coordinating anions
Tobias A.
Engesser
,
Martin R.
Lichtenthaler
,
Mario
Schleep†
and
Ingo
Krossing
*
Institut für Anorganische und Analytische Chemie and Freiburger Materialforschungszentrum (FMF), Universität Freiburg, Albertstr. 21, 79104 Freiburg, Germany. E-mail: krossing@uni-freiburg.de
First published on 27th November 2015
Abstract
The chemistry of the p-block elements is a huge playground for fundamental and applied work. With their bonding from electron deficient to hypercoordinate and formally hypervalent, the p-block elements represent an area to find terra incognita. Often, the formation of cations that contain p-block elements as central ingredient is desired, for example to make a compound more Lewis acidic for an application or simply to prove an idea. This review has collected the reactive p-block cations (rPBC) with a comprehensive focus on those that have been published since the year 2000, but including the milestones and key citations of earlier work. We include an overview on the weakly coordinating anions (WCAs) used to stabilize the rPBC and give an overview to WCA selection, ionization strategies for rPBC-formation and finally list the rPBC ordered in their respective group from 13 to 18. However, typical, often more organic ion classes that constitute for example ionic liquids (imidazolium, ammonium, etc.) were omitted, as were those that do not fulfill the – naturally subjective – “reactive”-criterion of the rPBC. As a rule, we only included rPBC with crystal structure and only rarely refer to important cations published without crystal structure. This collection is intended for those who are simply interested what has been done or what is possible, as well as those who seek advice on preparative issues, up to people having a certain application in mind, where the knowledge on the existence of a rPBC that might play a role as an intermediate or active center may be useful.
Introduction
Main group chemistry continues to reside at the heart of fundamental as well as applied chemistry. As such, recent years have seen an enormous growth of concepts that shed new light on hitherto undiscovered, or more correctly, underdeveloped areas of main group chemistry. Thus, the availability of stable singlet carbenes1 as strong donors offered tremendous new perspectives as did the establishment of the frustrated Lewis pairs (FLP) concept2 or the systematic investigation of (often low valent) cationic mixed main group-transition metal salts.3–5 In the framework of those approaches, next to other fundamental6 and applied questions,7–10 also the stabilization or use of reactive p-block cations (rPBC) with weakly coordinating anions (WCAs) was one focus that led to fascinating new rPBC. This review gives a comprehensive overview on recent rPBC developments since about 2000, but also cites all-time classics in the field. It also includes the fascinating class of transition metal substituted rPBC for which the assignment of the positive charge to one specific moiety is often not clear.Scope of this review
Many of the p-block elements have relatively high ionization potentials and electronegativities. Thus, most of the stable examples base on delocalization and other electronic or steric effects. In addition, rPBC are often very electrophilic and/or oxidizing. Therefore, chemically stable and inert weakly coordinating anions (WCAs) and solvents are needed to access their salts. These ingredients allowed the syntheses of a large number of fundamentally interesting rPBC of the groups 13–18 in the condensed phase. We discuss typical synthesis routes, give a brief overview of the WCAs, and describe the rPBC ordered according to their main group as well as cation class. However, typical, often more organic ion classes that constitute for example ionic liquids (imidazolium, ammonium, etc.) were omitted, as were those that do not fulfill the – naturally subjective – “reactive”-criterion of the rPBC. As a rule, we only included rPBC with crystal structure and only rarely refer to important cations published without crystal structure.Handling of substance classes with recent reviews
Some of the substance classes, which fit into this review were just recently and sometimes very comprehensively reviewed (cf. our contribution describing the advances in the synthesis of homopolyatomic cations of the non-metals since 200011). To reduce the overlap, we decided to give an overview on general aspects such as WCAs in Table 1 and include a short table with relevant reviews for each main group at the beginning of each main group chapter and only list the compounds in these cases. Therefore, we mainly list, but do not describe the cations of this category in the chapters of their corresponding element. Nevertheless, the scope of this review is rather large, which in any case precludes extensive discussions and mainly serves as an overview on what is known.| Year | Topic | Title | Ref. |
|---|---|---|---|
| 1993 | WCAs | The search for larger and more weakly coordinating anions | 15 |
| 1998 | WCAs | Carboranes: a new class of weakly coordinating anions for strong electrophiles, oxidants, and superacids | 16 |
| 2004 | WCAs | Noncoordinating anions—fact or fiction? A survey of likely candidates | 13 and 12 |
| 2006 | WCAs | Chemistry with weakly-coordinating fluorinated alkoxyaluminate anions: gas phase cations in condensed phases? | 14, 15 and 17 |
| 2006 | WCAs | Chemistry of the carba-closo-dodecaborate(−) anion, [CB11H12]− | 18 |
| 2008 | π-Complexation of post-transition metals by neutral aromatic hydrocarbons: the road from observations in the 19th century to new aspects of supramolecular chemistry | 19 | |
| 2013 | WCAs | Weakly coordinating anions: halogenated borates and dodecaborates | 20 |
| 2013 | WCAs | Weakly coordinating anions: fluorinated alkoxyaluminates | 21 |
| 2013 | WCAs | Weakly coordinating anions: highly fluorinated borates | 22 |
| 2015 | WCAs | Taming the cationic beast: novel developments in the synthesis and application of weakly coordinating anions (Publication in progress by IK) | 23 |
Reactive p-block cations
The rPBC in this article need a WCA as counterion and, therefore, we first briefly describe typical WCAs and give some advice on their selection before turning to typical ionization and synthetic procedures for rPBC preparations. Thereafter, the ordering of the cation classes for the individual sections is described, and finally the rPBC are grouped according to the main group of the relevant cationic entry. In addition, first applications emerged for rPBC salts and will be highlighted in the respective cation sections.WCA overview
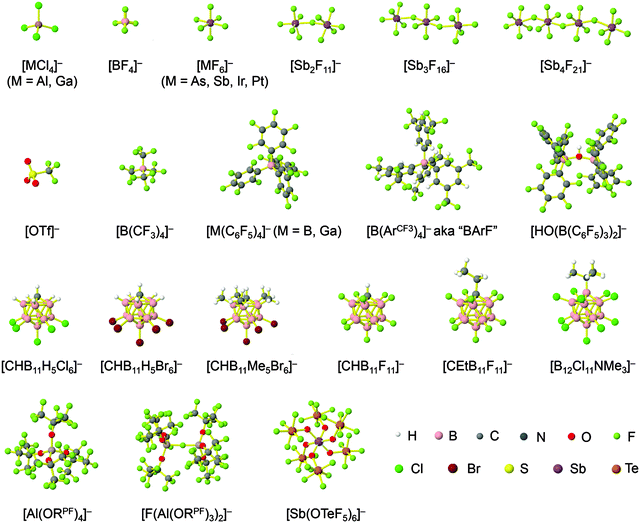
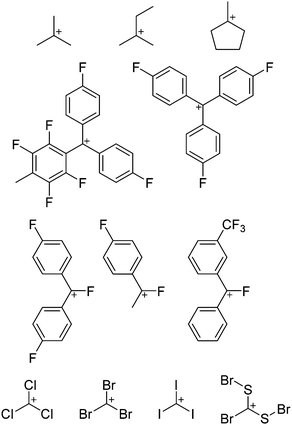
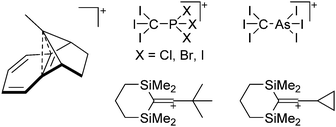
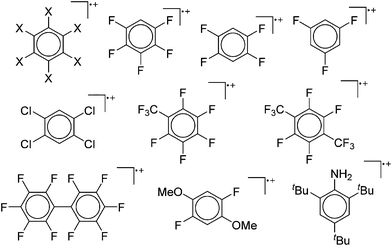
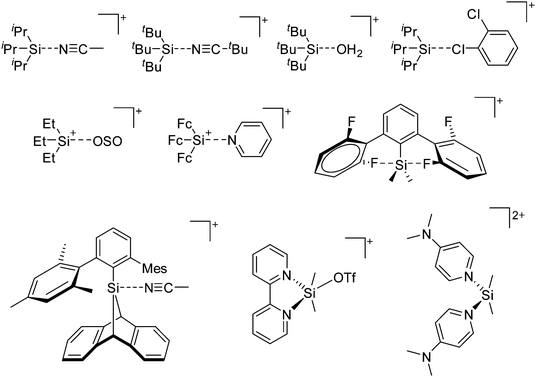
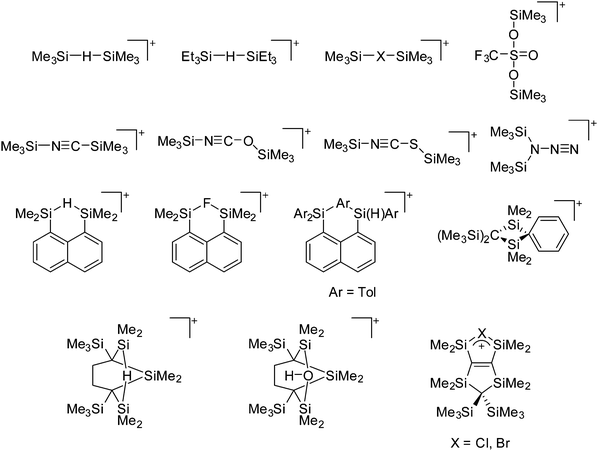
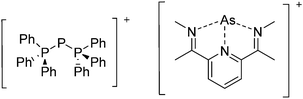
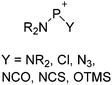
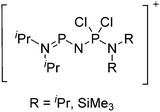
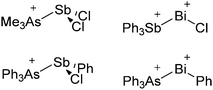
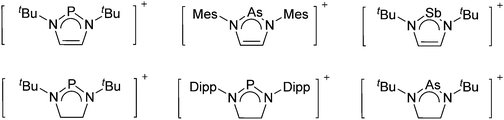
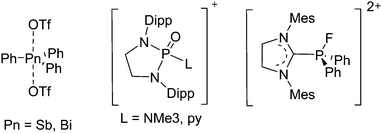
Because of their potential in fundamental and applied chemistry,12–15 a great variety of different WCA types are currently known (Fig. 2) and was frequently reviewed (Table 1).But which out of the multitude of published WCAs shown in Fig. 2 should be used for a given problem…? Is there one best WCA that fulfills all needs…?
Clearly holds: the more reactive the rPBC are, the more demanding is the task for the anions, to meet the requirements for a successful stabilization in the condensed phase. Some of this reactivity may be dampened kinetically by the use of suitable bulky ligands, e.g. for the silylium ions. However, there is not one ultimate WCA that fulfills all requirements to allow for use with all in here described rPBC. Typically, rPBC follow at least one of the following classifications:
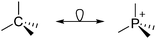
• Being a strong electrophile, thus having a strong tendency to coordinate an anion or solvent. Silylium ions SiR3+ are good examples for this. This coordination is often the entrance towards an anion degradation by heterolytic cleavage of a bond in the WCA.
• Being a strong oxidant, thus needing anions and solvents compatible with this need. Halogen and noble gas cations are typical examples.
• Being a weakly bound complex, in which the interesting main group particle can easily be displaced by anion or solvent, just as in many metal–non-metal clusters. This includes protonated, weakly basic molecules that tend to pass the proton to more basic and more coordinating anions or solvents.
Thus, the demand for very weak coordination behaviour is only medium for several very oxidizing cations, but the necessity of the WCA being stable against oxidation is a prerequisite of highest importance. For example, the typical counterions of group 16 to 18 rPBC are fluorometallates like [MF6]− or [M2F11]− (M = As, Sb) compatible with (i) the oxidizing power of the cation and (ii) the typically used super acid solvents. However, despite the fact that fluoroantimonates allow for the synthesis of tremendously oxidizing cations like [Xe2]+, they fail to stabilize the extreme electrophiles [SiR3]+ and form F-SiR3 and antimony pentafluoride. On the other hand, with some steric protection at the silylium ion, already the [B(C6F5)4]− WCA suffices to stabilize for example the [Si(Mes)3]+ cation. By contrast, and due to the aromatic system, [B(C6F5)4]− is not compatible with the only mildly oxidizing [NO]+ or [NO2]+ cation. Some thoughts that allow for the selection of a suitable WCA for a given problem may be summarized by the triangle shown in Fig. 1.
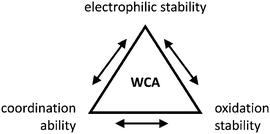 | ||
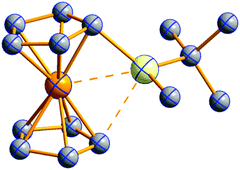
| Fig. 1 Triangle delineating the independent demands of a rPBC that lead to different mixtures of the WCA properties necessary for its successful stabilization. | ||
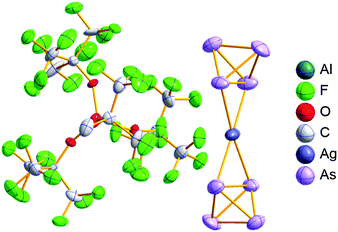
With Fig. 1 in mind, a personal selection of the “best WCAs” includes [1-H-CB11Me5Br6]−,24 [1-Et-CB11F11]−,25 [CB11(CF3)12]−,26 [Sb4F21]−,27 [Sb(OTeF5)6]−,28 [Al(ORPF)4]−,29–31 [B(C6F5)4]−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32–34 and [B(CF3)4]−.35 A recent noteworthy addition overcoming the frequent disorder of the also towards fluoride abstraction less stable [B(ArCF3)4]− anion is the [B(ArCl)4]− WCA.36
32–34 and [B(CF3)4]−.35 A recent noteworthy addition overcoming the frequent disorder of the also towards fluoride abstraction less stable [B(ArCF3)4]− anion is the [B(ArCl)4]− WCA.36
Other aspects that will influence the choice, are the synthetic availability of the entire WCA class, or the specific starting material necessary to ionize the system of interest. In this respect, most of the WCAs known so far also do have disadvantages: the carborates are hard to synthesize and have often low yields. [CB11(CF3)12]− is even explosive, as is the LiC6F5 intermediate needed for the [B(C6F5)4]− synthesis. In addition, starting materials such as solvent free Ag+ salts or [NO]+, [NO2]+ are not accessible as salts of [B(C6F5)4]−. Anions with multiple –CF3 groups often tend to disorder in the solid state, which sometimes makes it hard to solve or refine the crystal structure. The problems associated with the refinement of structures containing the [Al(ORPF)4]− WCA even led to the development of the software tool DSR.37 It allows for the simplified refinement of such disordered structures and is now implemented with standard programs like OLEX2.38
Therefore, the search for new useful anions is still in progress. With the amminated chloroborate cluster anion [1-Me3N-B12Cl11]− another promising candidate that refined earlier ideas by S. Strauss et al.,39 was just recently presented by Jenne et al. in 2014.40 The positive charge of the ammonium function leads to an overall −1 charge and makes it possible to use the in 30 g scale accessible –B12Cl11 cluster residue. Important starting materials M+[1-Me3N-B12Cl11]− (M+ = Na+, [HNMe3]+, [HNOct3]+, [NO]+, [CPh3]+, [NnBu4]+, [Et3Si]+) have been described facilitating the application.40,41 More details on typical WCA starting materials to introduce a counterion into the given system can be found in the synthesis section below as well in the numerous WCA reviews cited in Table 1.
Synthesis routes to reactive main group cation salts
At the beginning, each proposal to prepare a target-rPBC needs to consider the choice of the WCA as delineated in the preceding section, as well as the available starting materials, ionization method and reaction medium.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44) a halide (e.g. Li+,45 Na+, Ag+
44) a halide (e.g. Li+,45 Na+, Ag+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46), hydride- or alkyl-abstractor ([CPh3]+
46), hydride- or alkyl-abstractor ([CPh3]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47), a Brønsted acid ([H(OEt2)2]+,48 [H(NMe2Ph)]+) or a metal cation, if a simple metal complex is desired as product (e.g. Cu+
47), a Brønsted acid ([H(OEt2)2]+,48 [H(NMe2Ph)]+) or a metal cation, if a simple metal complex is desired as product (e.g. Cu+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49,50) (Table 2). Neutral Lewis acids for bond heterolysis are available in great variety and include the classical simple halides MIIIX3 and MVX5 (MIII = B, Al, Ga; MV = P, As, Sb, Bi; X = F, Cl, Br, I; not all combinations useful), the rather fine tunable B(aryl)3 acids (aryl = fluorinated,51 chlorinated52 or fluoroalkylated53 aromatic residue), or aluminum based systems like Al(C6F5)354 and Al(ORF)3.55 Also the ion-like R3Si(WCA) compounds have frequently been used.56,57 Recent systematic work analyzed the potency of a given Lewis acid versus fluoride, chloride, hydride and methanide as a base. It includes benchmark Lewis acidity values for a smaller set of simple MXn acids.58 Neutral Brønsted acids like HF, HNTf2 and derivatives thereof,59 or combinations of Brønsted and Lewis acids like HBr/nAlBr360,61 are suitable for protonations. Novel, and in large quantity available very strong acids like RHFOSO3H62 should also be mentioned.
49,50) (Table 2). Neutral Lewis acids for bond heterolysis are available in great variety and include the classical simple halides MIIIX3 and MVX5 (MIII = B, Al, Ga; MV = P, As, Sb, Bi; X = F, Cl, Br, I; not all combinations useful), the rather fine tunable B(aryl)3 acids (aryl = fluorinated,51 chlorinated52 or fluoroalkylated53 aromatic residue), or aluminum based systems like Al(C6F5)354 and Al(ORF)3.55 Also the ion-like R3Si(WCA) compounds have frequently been used.56,57 Recent systematic work analyzed the potency of a given Lewis acid versus fluoride, chloride, hydride and methanide as a base. It includes benchmark Lewis acidity values for a smaller set of simple MXn acids.58 Neutral Brønsted acids like HF, HNTf2 and derivatives thereof,59 or combinations of Brønsted and Lewis acids like HBr/nAlBr360,61 are suitable for protonations. Novel, and in large quantity available very strong acids like RHFOSO3H62 should also be mentioned.
| Acr. | Type | Example | Ref. |
|---|---|---|---|
| a This type of reaction is sometimes referred to as Bartlett–Condon–Schneider (BCS) type hydride transfer reaction.75 | |||
| Com | Complexation reaction |
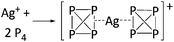
|
72 |
| Ox | Oxidation reaction; including 1e− and 2e− oxidations. |

|
30 and 31 |
| Lewis | Lewis acid induced halogen bond heterolysis with neutral Lewis acids, including ion-like compounds. |
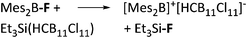
|
32, 33 and 73 |
| Salt | Salt elimination reaction |

|
74 |
| Hyd | Hydride metathesis reaction with neutral or ionic H−-acceptor |
|
76 |
| Alk | Alkyl metathesis reaction with neutral or ionic R−-acceptor |
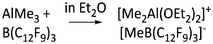
|
77 |
| Ins | Insertion reaction |

|
78 |
| Prot | Protonation reaction |
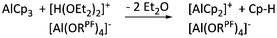
|
79 |
| Lig | Ligand exchange reaction |
|
80 |
| Ion | Ionization |
|
81 |
| Other | Other reaction not classified as one of the above | — | — |
The recently established concepts of absolute acidity,69 absolute reducity70 and their two-dimensional combination as the protoelectric potential map70 can be used to understand protonation and/or redox chemistry over medium/solvent and even phase boundaries. This also includes ILs and therefore a thermodynamically sound pH definition has been introduced for IL media.61,71
On the representations of the cation chemical structures
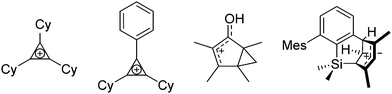
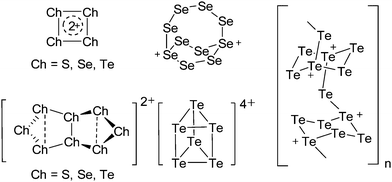
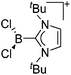
Note that the structural diagrams used throughout this review obey a distance criterion for interactions, but not necessarily a 2e2c-understanding of every interaction line. However, at least in the organic residue we attempted to follow an electron precise 2e2c picture. Necessarily, this gets difficult for structures with N-heterocyclic carbenes that formally allow for a neutral dative (imidazolyl) as well as ionic (imidazolium) description (Fig. 3).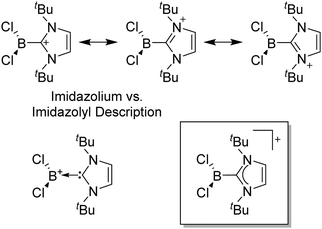 | ||
| Fig. 3 Possible descriptions of NHC-containing structures exemplified for the simple [Cl2B-ItBu]+ cation. | ||
For simplicity, we chose the representation shown in the box in Fig. 3 and adopted similar drawings for related cases throughout. Thus, we only use arrows for relatively weak interactions with the bonding situation in ammine-borane H3B←:NH3 being the prototype as suggested by A. Haaland,83 and later contributions.84 For thoughts on these ongoing discussions, see these recent publications.85 Only if the positive charge can clearly (and not just formally) be attributed to one atom, we assigned the charge to this atom. More common is the case in the box in Fig. 3, in which the charge may be delocalized to quite a series of atoms and therefore we placed the charge at the upper right corner.
Ordering of the cation classes
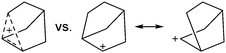
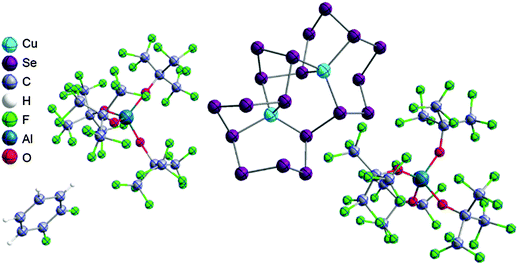
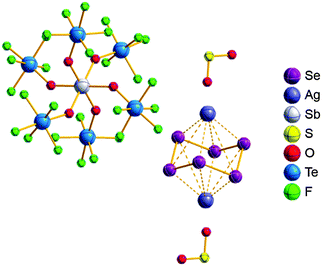
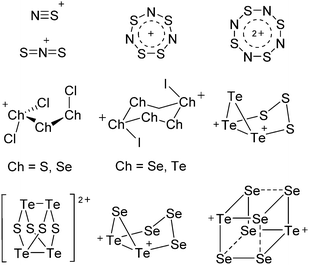
The rPBC were as far as possible ordered according to accepted cation classes that may either refer to the number of valence electrons (i.e. the onium/enium/inium-series) or to the structure. In each subchapter, we intend to go from homoatomic, to binary and then to more complex cation compositions. The not always consistently used classification according to onium- (8 VE), enium- (6 VE) and inium-cations (4 VE) presents some problems. Note, that the coordination number of a group 14 onium ion may not always be four, as the σ-donation of π-density of a donating double bond may increase the coordination number to 5 as, for example, in the 2-norbornyl cation, a carbonium ion.86 Similar considerations hold for other donor coordinated onium- and enium-ions. Thus, we typically include the coordination number in the cation classification, for example as ligand substituted, (CN = 2). By contrast to these cation assignments, the group 15 to 18 cations were in addition classified by the oxidation state of the central atoms. This is often used for such rPBC. In addition, we included ion-like compounds that were initially defined for silylium compounds with coordinated counterions that structurally have to be addressed as a tight ion-pair but from the reactivity still bear a considerable amount of reactivity related to the free cation, e.g. see the ion like silylium compounds R3Si(WCA) in Fig. 4. Related cases were published for coordinated aluminum cations, e.g. R2Al(WCA), and were used in a similar manner.87 For ion-like compounds we keep the notation with the anion in parentheses and no charges written, as in R3Si(WCA) and R2Al(WCA). Heteropolyatomic clusters were discussed in the group of their most electropositive element (e.g. [P3Se4]+ in group 15, but [S4N4]2+ in group 16).In the following chapters we describe the rPBCs of the Group 13 to 18 elements and give selected representative examples for each cation type. However, for reasons of legibility, the full tables that comprehensively cover the rPBC entries of the groups, are collected in landscape format at the end of this document.
Group 13 cations
Traditionally, group 13 chemistry is dominated by compounds in the +III oxidation state.88 Of those, the simple halides are commonly applied as Lewis acid catalysts and initiators (e.g. BF3) and usually associated with anion formation (e.g. [BF4]−). However, discrete trivalent group 13 cations have been found to be more reactive, owing to their greater electrophilicity if paired with coordinative unsaturation.10,89 Except for boron, it has become increasingly possible to stabilize group 13 cations in their +I oxidation state, e.g. by employing bulky substituents and/or WCAs31,90–92 (for thallium, this is the favored oxidation state due to the inert pair effect93). Featuring a lone pair of electrons and empty p-orbitals, the +I cations are ambiphilic and can function both as Lewis base or acid, thus offering unique reactivities and selectivities in organometallic chemistry,94–97 as well as organic98 and polymer7,99 syntheses. Overall, different aspects of the chemistry of cationic group 13 compounds were reviewed and these contributions are compiled in Table 3. In this context, this sections intends to give a comprehensive overview of reactive group 13 cations of the larger WCAs since about 2000. Due to the large scope of this chapter, we mainly omit rPBC with the simple halometallate based counterions and only include those in special cases of high relevance.| Year | Group | Title | Ref. |
|---|---|---|---|
| 1985 | 13 | Arene complexes of univalent gallium, indium and thallium | 100 and 101 |
| 1998 | 13 | Cationic group 13 complexes | 102 |
| 2004 | 13 | From group 13–group 13 donor–acceptor bonds to triple-decker cations | 94 |
| 2005 | 13 | Borinium, borenium, and boronium ions: synthesis, reactivity, and applications | 89 |
| 2007 | 13 | Development of the chemistry of indium in formal oxidation states lower than +III | 103 |
| 2008 | 13 | Borylene transfer from transition metal borylene complexes | 13 and 12 |
| 2008 | 13 | Synthesis, characterization, and applications of group 13 cationic compounds | 95 |
| 2009 | 13 | Highly electrophilic main group compounds: ether and arene thallium and zinc complexes | 90 |
| 2009 | 13 | Transition metal borylene complexes: boron analogues of classical organometallic systems | 104 |
| 2010 | 13 | Electron-precise coordination modes of boron-centered ligands | 105 |
| 2011 | 13 | Coordination chemistry of group 13 monohalides | 96 |
| 2011 | 13 | New light on the chemistry of the group 13 metals | 88 |
| 2011 | 13 | The chemistry of the group 13 metals in the +I oxidation state | 106 |
| 2011 | 13 | Mixed or intermediate valence group 13 metal compounds | 107 |
| 2011 | 13 | Coordination and solution chemistry of the metals: biological, medical and environmental relevance | 108 |
| 2012 | 13 | Cationic tricoordinate boron intermediates: borenium chemistry from the organic perspective | 109 |
| 2012 | 13 | Cyclopentadiene based low-valent group 13 metal compounds: ligands in coordination chemistry and link between metal rich molecules and intermetallic materials | 110 |
| 2012 | 13 | Low-oxidation state indium-catalyzed C–C bond formation | 98 |
| 2013 | 13 | 1.17-low-coordinate main group compounds – group 13 | 97 |
| 2013 | 13 | Transition metal borylene complexes | 5 |
| 2013 | 13 | Boron, aluminum, gallium, indium and thallium | 111 |
| 2015 | 13 | Discrete cationic complexes for ring-opening polymerization catalysis of cyclic esters and epoxides | 10 |
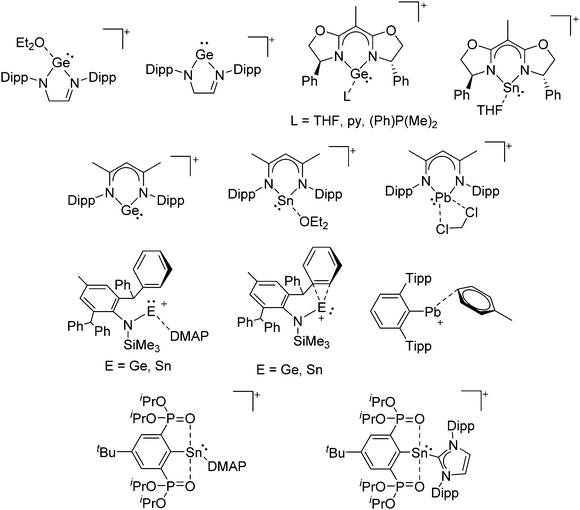
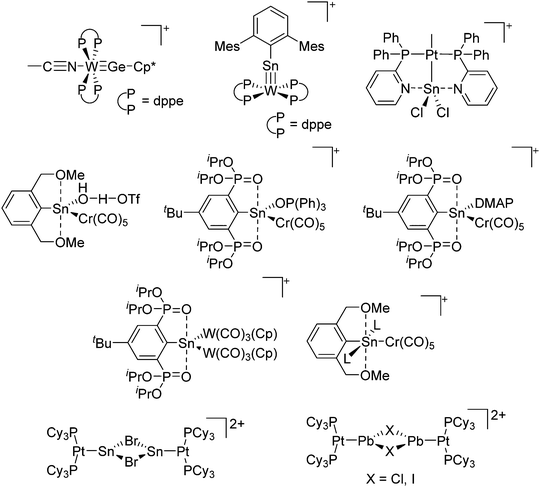
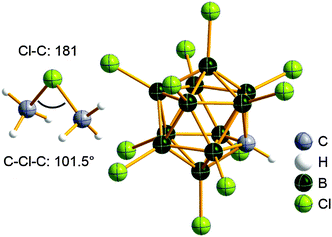
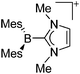
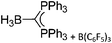
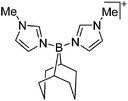
Boron cations
For a long time, boron cations have remained a chemical curiosity due to their redox lability. However and partly owing to the developments in the field of WCAs, more and more boron-based cations are being reported. Overall, the cations can be classified according to the coordination number at boron: i.e., di-, tri-, and tetra-coordinated boron cations are referred to as borinium, borenium and boronium cations. To this day, the boron cations have been most notably reviewed by Nöth (1985; a milestone in cationic boron chemistry),112 Piers (2005; structural and bonding aspects)89 and Vedejs (2012; reactivties and applications).109For the isolation of the discrete [BH2(PR2H)2]+ cation, the nature of the WCA is again essential: compared to [OTf]−, [B(ArCF3)4]− features no hydrogen bond with the cation, thus allowing for increased reactivities.120
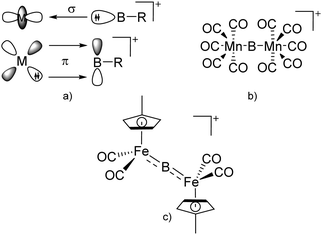 | ||
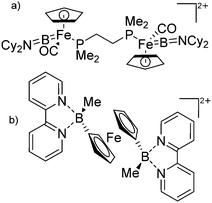
| Fig. 6 (a) Orbital interaction between borylenes and transition-metal fragments; (b) and (c) exemplarily selected transition-metal substituted borinium cations. | ||
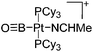
Another notable substance class are the cationic T-shaped platinum boryl complexes that are usually accessible via salt metathesis reactions: e.g., [(Cy3P)2(MeCN)Pt(B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O)]+ can be synthesized by reacting (Cy3P)2Pt(B
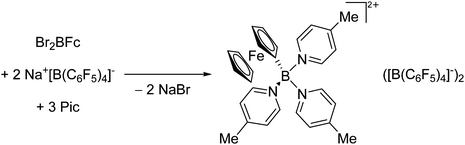
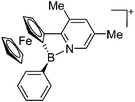
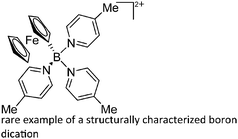
O)]+ can be synthesized by reacting (Cy3P)2Pt(B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O)(Br) with the halide abstracting reagent Ag+[B(ArCF3)4]−.121 Employing a ferrocenyl ligand on the other hand, Braunschweig et al. were able to isolate a rare example of a structurally characterized boron dication: [FcB(Pic)3]2+ (Fig. 7).122
O)(Br) with the halide abstracting reagent Ag+[B(ArCF3)4]−.121 Employing a ferrocenyl ligand on the other hand, Braunschweig et al. were able to isolate a rare example of a structurally characterized boron dication: [FcB(Pic)3]2+ (Fig. 7).122
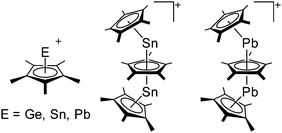
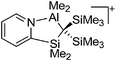
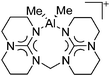
Aluminum cations
Among the group 13 cations, the lower- and higher-coordinated derivatives of aluminum have been of significant interest as they feature increased Lewis acidities and ligand labilities, thus allowing for higher catalytic activities compared to their neutral analogs.95 While Atwood (1998)102 and Dagorne (2008)95 have given a good overview on cationic aluminum species from a fundamental perspective, Sarazin and Carpentier (2015)10 recently reviewed various discrete cationic aluminum complexes that are able to catalyze ring-opening polymerizations.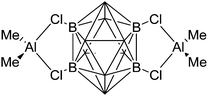 | ||
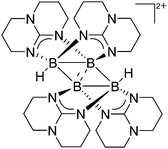
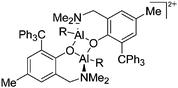
| Fig. 10 The ion-like (Me2Al)2B12Cl12 salt.87 For clarity, all BCl moieties of the perchlorinated closo-dodecaborate that feature no contact to the “[Me2Al]+” cation have been omitted. | ||
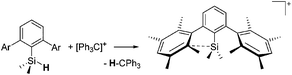
The [(2,6-Mes2C6H3)2Al]+ cation on the other hand, is a discrete and therefore almost linear di-coordinate aluminum cation that features no contact to the WCA [B(C6F5)4]−.129 The occurrence of the highly Lewis acidic aluminum cation is attributable to the intrinsic stabilization effect of the 2,6-Mes2C6H3 ligand: i.e., bending of the flanking Mes-moieties towards the aluminum center.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131 and [(η5-Cp*)2Al]+ [MeB(C6F5)3]−.132,133 Moreover, the salts offer insights into the relationship between the nucleophilicity of Cp, Cp′ and Cp*, the corresponding WCAs and the resultant Lewis acidities and reactivities of the aluminum cations: i.e., with increasing nucleophilicity of the Cp ligands (Cp < Cp′ < Cp*) the WCAs can be less coordinating ([MeB(C6F5)3]− > [B(C6F5)4]− > [Al(ORPF)4]−). The more interacting anions induce decreased Lewis acidities and lower reactivities of the aluminum cations for the initiation of olefin polymerizations: [(η5-Cp)2Al]+ > [(η5-Cp′)2Al]+ > [(η5-Cp*)2Al]+.79
131 and [(η5-Cp*)2Al]+ [MeB(C6F5)3]−.132,133 Moreover, the salts offer insights into the relationship between the nucleophilicity of Cp, Cp′ and Cp*, the corresponding WCAs and the resultant Lewis acidities and reactivities of the aluminum cations: i.e., with increasing nucleophilicity of the Cp ligands (Cp < Cp′ < Cp*) the WCAs can be less coordinating ([MeB(C6F5)3]− > [B(C6F5)4]− > [Al(ORPF)4]−). The more interacting anions induce decreased Lewis acidities and lower reactivities of the aluminum cations for the initiation of olefin polymerizations: [(η5-Cp)2Al]+ > [(η5-Cp′)2Al]+ > [(η5-Cp*)2Al]+.79
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 and [(β-diketiminate)Al–Me]+
135 and [(β-diketiminate)Al–Me]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136 complexes, respectively (Fig. 12).
136 complexes, respectively (Fig. 12).
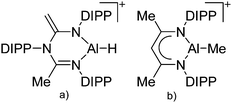 | ||
| Fig. 12 (a) The [(β-diketiminate)Al–H]+ cation derives from the reaction of a N-imidoylamidine ligand with AlH3·NMe2Et and [Ph3C]+[B(C6F5)4]−.135 (b) The [(β-diketiminate)Al–Me]+ cation is formed by reacting the neutral precursor (β-diketiminate)Al(Me)2 with the demethylating reactants [CPh3]+[B(C6F5)4]− and B(C6F5)3, respectively.136 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 and [H2Al(NMe3)2]+
144 and [H2Al(NMe3)2]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145 (cf.Fig. 13 for the complex synthesis of the [H2Al(NMe3)2]+ cation and the in situ generation of the corresponding WCA).
145 (cf.Fig. 13 for the complex synthesis of the [H2Al(NMe3)2]+ cation and the in situ generation of the corresponding WCA).
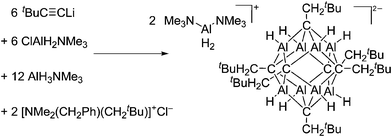 | ||
| Fig. 13 Salt metathesis and hydroalumination reactions lead to the formation of the weakly coordinating carbaalanate cluster that allows for the synthesis of two equivalents of the [H2Al(NMe3)2]+ cation.145 | ||
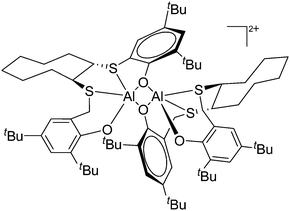 | ||
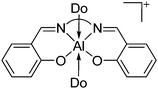
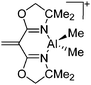
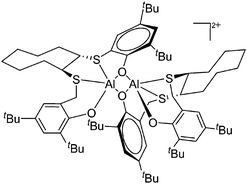
| Fig. 15 In the [{(OSSO)Al}2]2+ cation one aluminum atom is coordinated in a trigonal-bipyramidal and the other in a distorted-square-pyramidal fashion. The cationic species is a potential catalyst for the ring opening polymerization of propylene oxide.155 | ||
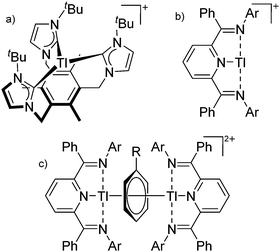
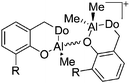
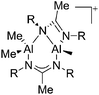
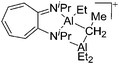
On the other hand, there are various dinuclear, yet singly charged aluminum cations in which the latter usually feature different coordination modes. Notable contributions to this field of research have been made by Jordan et al., such as the cationic aluminum aminotroponiminate141 and amidinate156 complexes in Table 8.
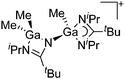
Gallium cations
As mentioned above, gallium in its +I oxidation state is thermodynamically unstable and usually disproportionates into the metal and the +III ions. Notable contributions to the field of reactive gallium cations therefore allow for the stabilization of the +I oxidation state of gallium.31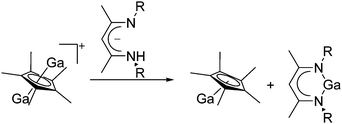 | ||
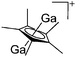
| Fig. 16 The [Ga2(η5-Cp*)]+[B(ArCF3)4]− salt cleanly reacts as a gallium(I) source with ligands such as DDP. | ||
The coordination mode of the Cp* ligands in the [(η1-Cp*)(η3-Cp*)Ga]+ cation on the other hand differs.160 Hence, the originally expected η5,η5-ferrocene-like structure that was also observed for the aluminum analogue is likely perturbed by the more interacting [BF4]− counterion.
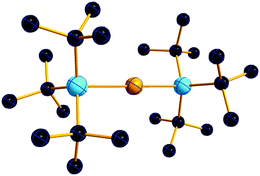 | ||
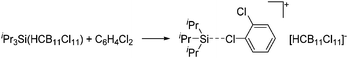
| Fig. 18 Molecular structure of the [tBu3Si–Ga–SitBu3]+ cation. A. Budanow, T. Sinke, J. Tilmann, M. Bolte and M. Wagner, Two-coordinate gallium ion [tBu3Si–Ga–SitBu3]+ and the halonium ions [tBu3Si–X–SitBu3]+ (X = Br, I): sources of the supersilyl cation [tBu3Si]+, Organometallics, 2012, 31, 7298–7301. Data from this reference were used to draw this figure and the hydrogen atoms were omitted for clarity.163 | ||
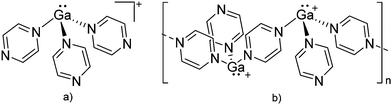 | ||
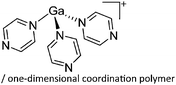
| Fig. 19 (a) Monomeric [Ga(pyrazine)3]+ complex and (b) one-dimensional coordination polymer [{Ga(μ-pyrazine)2(η1-pyrazine)}+]∞. The propagation of the polymer into the second dimension was not possible as each cationic strand is surrounded by strands of the corresponding [Al(ORPF)4]− anions.164 | ||
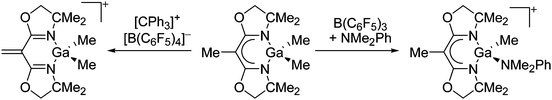 | ||
| Fig. 20 Hydride vs. methyl abstraction of neutral BOX ligated gallium complexes.165 | ||
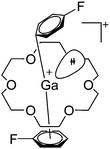 | ||
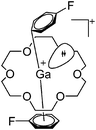
| Fig. 21 The [Ga([18]crown-6)(η6-/η1-C6H5F)2]+ cation. The η6- and η1-coordination modes could be an indication for a stereoactive lone pair on the side of the weaker and only η1-coordinated C6H5F molecule.168 | ||
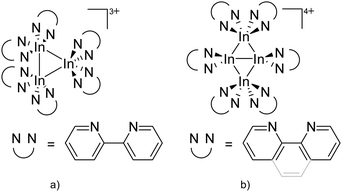
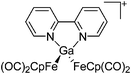
As the N-heterocyclic arenes are potential ligands for univalent gallium (see above), Krossing et al. additionally reacted the chelating bipy with the [Ga(η6-C6H5F)2]+ complex. Instead of witnessing a simple ligand exchange reaction, they isolated the paramagnetic and distorted octahedral [GaIII{(bipy)3}˙]2+ complex due to the non-innocence of the bipy ligand.17 This is reminiscent to transition metal chemistry where for example the [RuIII{(bipy)3}˙]2+ complex features similar bonding.
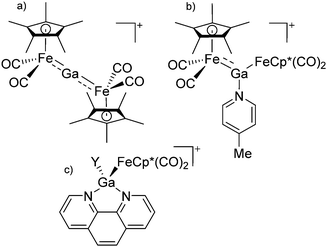 | ||
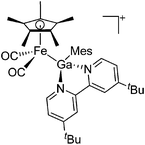
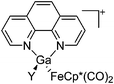
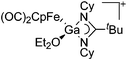
| Fig. 22 (a) The linear di-coordinated cation [(FP*)2(μ-Ga)]+ derives from a salt metathesis of (FP*)2GaCl and Na+[B(ArCF3)4]−. The Fe–Ga–Fe moiety features a significant π bonding component (population analysis).169 (b) The [(FP*)2(μ-Ga)(4-Pic)]+ cation is an addition product of [(FP*)2Ga]+ and 4-Pic and the second structurally characterized complex containing a cationic tricoordinate gallium centre.170 (c) Applying the chelating phen ligand, Ueno et al. isolated the tetra-coordinated [(FP*)Ga(phen)(Y)]+ (Y = Cl, SpTol) cations, i.e. the first transition-metal complex with a thiolate group on the gallium atom.171 | ||
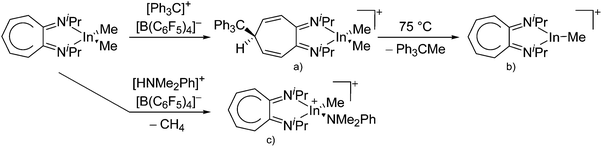
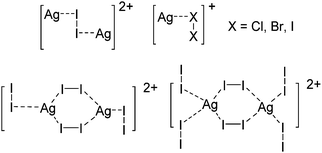
Indium cations
Compared to the lighter homologue gallium, well-defined indium(I) halides exist, though they are practically insoluble in organic solvents. The synthesis of In+[OTf]− by Macdonald et al. as a soluble alternative is therefore an important development concerning the indium(I) chemistry.182![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192,193 and [In(arene)n]+
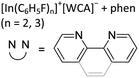
192,193 and [In(arene)n]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 168 (n = 2, 3) can be reacted with the crown ether [18]crown-6, yielding cationic indium complexes with similar structures to the gallium congener (cf.Fig. 21) and strong anion–cation interactions in the case of the [OTf]− anion. Reacting In+[OTf]− with [15]crown-5 on the other hand, the sandwich complex [In([15]crown-5)2]+ was isolated.194
168 (n = 2, 3) can be reacted with the crown ether [18]crown-6, yielding cationic indium complexes with similar structures to the gallium congener (cf.Fig. 21) and strong anion–cation interactions in the case of the [OTf]− anion. Reacting In+[OTf]− with [15]crown-5 on the other hand, the sandwich complex [In([15]crown-5)2]+ was isolated.194
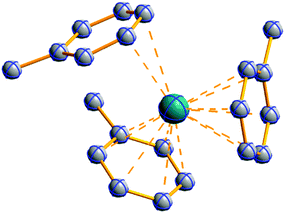
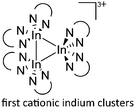
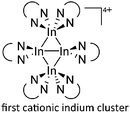
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 168 complexes with n = 2, 3 are a powerful starting material. Hence a dicationic [{(PPh3)3In}2(μ-PPh3)]2+ complex in which one PPh3 ligand bridges both indium(I) cations was isolated.162 Applying the non-innocent and chelating bipy and phen ligands on the other hand, Krossing et al. surprisingly isolated the first cationic tri- and tetra-nuclear indium clusters: [In3(bipy)5–6]3+ and [In4(Do)6]4+ (Do = phen, bipy) (Fig. 26).17 This result very much differs from the above mentioned synthesis of the paramagnetic [GaIII{(bipy)3}˙]2+ complex and can be attributed to the higher redox-stability of indium compared to gallium. In addition and to our knowledge, these are the first higher charged clusters that have been reported to this day: i.e., for cluster formations usually reductive syntheses are applied, yielding neutral and anionic clusters.
168 complexes with n = 2, 3 are a powerful starting material. Hence a dicationic [{(PPh3)3In}2(μ-PPh3)]2+ complex in which one PPh3 ligand bridges both indium(I) cations was isolated.162 Applying the non-innocent and chelating bipy and phen ligands on the other hand, Krossing et al. surprisingly isolated the first cationic tri- and tetra-nuclear indium clusters: [In3(bipy)5–6]3+ and [In4(Do)6]4+ (Do = phen, bipy) (Fig. 26).17 This result very much differs from the above mentioned synthesis of the paramagnetic [GaIII{(bipy)3}˙]2+ complex and can be attributed to the higher redox-stability of indium compared to gallium. In addition and to our knowledge, these are the first higher charged clusters that have been reported to this day: i.e., for cluster formations usually reductive syntheses are applied, yielding neutral and anionic clusters.
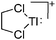
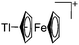
Thallium cations
In contrast to the lighter homologues, thallium's thermodynamic most stable oxidation state is +I. Hence, various syntheses of unsubstituted thallium(I) cations of different WCAs have been reported: i.e., the protonation of TlOEt using [H(OEt2)2]+[B(ArCF3)4]−/[B(C6F5)4]−,196,197 the Lewis acid base reaction of Tl+[OTeF5]− and B(OTeF5)3198 and the salt metathesis of TlF and Li+[Al(ORHF/PF)4]−.199,200 The thallium(I) salts are relatively stable (cf. the silver congeners decompose upon exposure to light) and mainly used as reactants to introduce WCAs (e.g. salt metathesis reactions). Tl+[Al(ORHF)4]− could only be isolated, if the precursors were applied in an exact 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. An excess of TlF however, led to the formation of the cationic multinuclear [Tl3F2Al(ORHF)3]+ complex.200
1 stoichiometry. An excess of TlF however, led to the formation of the cationic multinuclear [Tl3F2Al(ORHF)3]+ complex.200
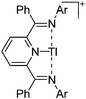
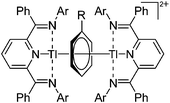
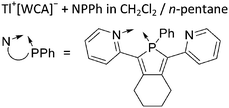
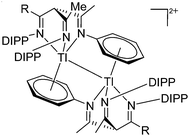
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 and bis(imino)pyridines206 can be applied to isolate tricoordinate thallium(I) cations (Fig. 27).
205 and bis(imino)pyridines206 can be applied to isolate tricoordinate thallium(I) cations (Fig. 27).
An even higher coordinated thallium cation is the [Tl([18]crown-6)]+ complex, which features a similar structure as the [18]crown-6 complexes of the lighter homologues gallium and indium.203
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
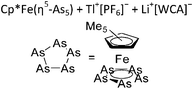
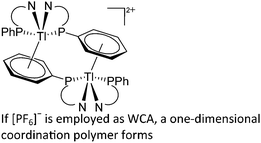
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.203 Increasing the amount of FeCp2 from 1 to 2.2 equivalents, only the [Tl(FeCp2)]+ complex was isolated.204 In contrast to the lighter homologues, the reaction of Cp*Fe(η5-As5) with Tl+[PF6]− and Li+[FAl{OC6F10(C6F5)}3]− did not result in aggregation and formation of a cationic one-dimensional coordination polymer, but rather yielded the pseudo-trigonal-planar [Tl{(η5-As5)FeCp*}3]+ complex.176 Performing a similar chemistry in the presence of the very good WCA [Al(ORPF)4]− however, one-dimensional polymers were isolated (cf.Fig. 23), proving the importance of the WCA.207 Reacting the neutral Pt(CH2Ph)Cl(PCH2-ox) complex with Tl+[PF6]−, Braunstein et al. did not isolate any chloride abstraction product but a “trapped” thallium(I) cation: the cationic [{P(Ph2)CH2ox}(Cl)(Tl)Pt-CH2Ph}]+ complex.208 Herein, the ligand functions as a chelate and interacts with thallium via a Pt–Tl bond and a η6-benzyl coordination (Fig. 29).
1 ratio.203 Increasing the amount of FeCp2 from 1 to 2.2 equivalents, only the [Tl(FeCp2)]+ complex was isolated.204 In contrast to the lighter homologues, the reaction of Cp*Fe(η5-As5) with Tl+[PF6]− and Li+[FAl{OC6F10(C6F5)}3]− did not result in aggregation and formation of a cationic one-dimensional coordination polymer, but rather yielded the pseudo-trigonal-planar [Tl{(η5-As5)FeCp*}3]+ complex.176 Performing a similar chemistry in the presence of the very good WCA [Al(ORPF)4]− however, one-dimensional polymers were isolated (cf.Fig. 23), proving the importance of the WCA.207 Reacting the neutral Pt(CH2Ph)Cl(PCH2-ox) complex with Tl+[PF6]−, Braunstein et al. did not isolate any chloride abstraction product but a “trapped” thallium(I) cation: the cationic [{P(Ph2)CH2ox}(Cl)(Tl)Pt-CH2Ph}]+ complex.208 Herein, the ligand functions as a chelate and interacts with thallium via a Pt–Tl bond and a η6-benzyl coordination (Fig. 29).
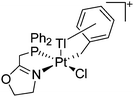 | ||
| Fig. 29 The first fully characterized metal–metal bonded Tl–Pt–Cl complex. If Ag+[OTf]− and Ag+[BF4]− is applied, the expected chloride abstraction takes place.208 | ||
Reacting RuCl2(DMeOPrPE)2 with Tl+[PF6]− an “arrested” chloride abstraction occurs.209 In the resultant one-dimensional coordination polymer, the thallium(I) cations are coordinated in an unusual octahedral fashion with a stereoactive 6s2 lone pair at thallium.
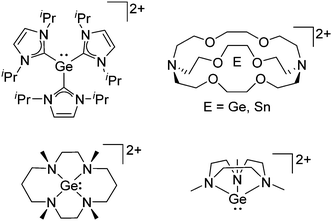
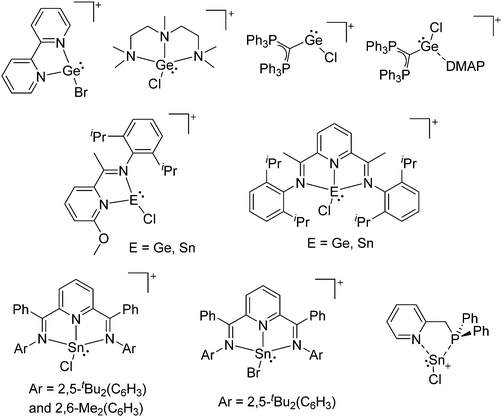
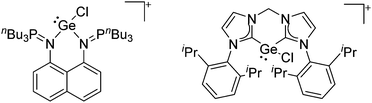
Group 14 cations
Already in 1887, Henderson described the synthesis of trityl malonate starting from triphenylmethyl bromide and ethylic sodiomalonate246 and 15 years later, Bayer and Villiger realized that the yellow color of a solution of triphenylmethane in concentrated sulfuric acid is the result of the formation of a carbocation.247 Despite these early discoveries, it took another 63 years until the structure of this cation could be determined.248 While the first structure determination succeeded with [ClO4]−, the structure of the trityl cation is nowadays known with several different anions (e.g.ref. 249) and it has become a common reagent for the generation of various other cations. Only one earlier structure determination of a carbocation was published: the structure of triphenylcyclopropenium perchlorate in 1963.250 Since then, many rPBCs of group 14 were synthesized and characterized. Carbocations have drawn a lot of interest, due to their role as intermediates in organic chemistry. Silylium ions are more electrophilic and more reactive than their carbon analogues, so that the structural characterization of a truly free silylium ion was only achieved in 200224 and is still in the focus of interest. But also the heavier elements of group 14 were subject to extensive research and today a multitude of interesting rPBCs are known, part of which have been reviewed in the articles included with Table 4.| Year | Title | Ref. |
|---|---|---|
| 1995 | Modern approaches to silylium cations in condensed phase | 251 |
| 2005 | Cations of group 14 organometallics | 252 |
| 2005 | Carbon, silicon, germanium, tin and lead | 253 |
| 2010 | Silylium ions in catalysis | 254 |
| 2010 | H+, CH3+, and R3Si+ carborane reagents: when triflates fail | 255 |
| 2011 | N-heterocyclic carbene analogues with low-valent group 13 and group 14 elements: syntheses, structures, and reactivities of a new generation of multitalented ligands | 256 |
| 2013 | Catenated compounds – group 14 (Ge, Sn, Pb) | 257 |
| 2015 | Cations and dications of heavier group 14 elements in low oxidation states | 258 |
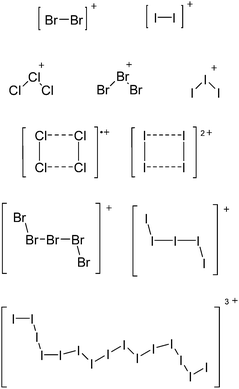
Carbon
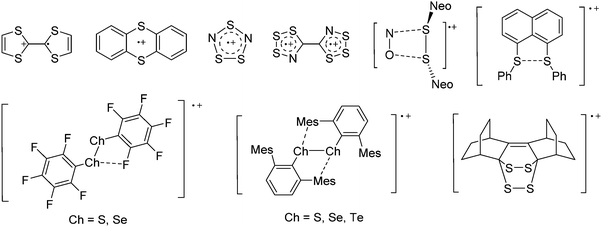
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 and an additional structure of the tert-butyl cation with the [HCB11Cl11]− anion.267 In 2000, ion-like (CH3)2CF(AsF6) was the first structural characterized example of a fluorinated carbocation and was published together with a higher substituted variant.268 In both compounds, each cation is stabilized by two stronger contacts to the anion. The higher substituted [(m-CF3C6H4)(C6H5)CF]+ derivative, contains the less coordinating [As2F11]− anion in the structure with only weak interaction between the ions.268 With [HCB11I11]−, two more fluoro-substituted carbocations and one with fluorine substituted aryl residues could be isolated (see Fig. 32).56 Apart from [CF3]+, all [CX3]+ cations are now synthesized and structurally characterized (see Fig. 32). First, [CI3]+[Al(ORPF)4]− was published in 2003269 and shortly after [CCl3]+ and [CBr3]+ with [Sb(OTeF5)6]− as the counterion.270 In addition, the latter was used to stabilize related [C(OTeF5)3]+.270 Later, also [CCl3]+ and [CBr3]+ were synthesized with the [Al(ORPF)4]− and the [(RPFO)3Al–F–Al(ORPF)3]− counterions.271 In all of those compounds containing [CX3]+ cations, still some weak interactions between cation (mainly halogen atoms) and anion exist. These interactions are weaker between [Br-C(SBr)2]+ and the mentioned alkoxyaluminate, due to delocalization of the charge.117 Although comparable, far stronger interaction between cation and anion was found in [(MeO)(MeS)CSH]+[SbF6]−.272 However, there is no close contact between the carbon atom and the fluorine atoms of the anion. Instead, the anion forms hydrogen bonds to the thiol group of the cation.
266 and an additional structure of the tert-butyl cation with the [HCB11Cl11]− anion.267 In 2000, ion-like (CH3)2CF(AsF6) was the first structural characterized example of a fluorinated carbocation and was published together with a higher substituted variant.268 In both compounds, each cation is stabilized by two stronger contacts to the anion. The higher substituted [(m-CF3C6H4)(C6H5)CF]+ derivative, contains the less coordinating [As2F11]− anion in the structure with only weak interaction between the ions.268 With [HCB11I11]−, two more fluoro-substituted carbocations and one with fluorine substituted aryl residues could be isolated (see Fig. 32).56 Apart from [CF3]+, all [CX3]+ cations are now synthesized and structurally characterized (see Fig. 32). First, [CI3]+[Al(ORPF)4]− was published in 2003269 and shortly after [CCl3]+ and [CBr3]+ with [Sb(OTeF5)6]− as the counterion.270 In addition, the latter was used to stabilize related [C(OTeF5)3]+.270 Later, also [CCl3]+ and [CBr3]+ were synthesized with the [Al(ORPF)4]− and the [(RPFO)3Al–F–Al(ORPF)3]− counterions.271 In all of those compounds containing [CX3]+ cations, still some weak interactions between cation (mainly halogen atoms) and anion exist. These interactions are weaker between [Br-C(SBr)2]+ and the mentioned alkoxyaluminate, due to delocalization of the charge.117 Although comparable, far stronger interaction between cation and anion was found in [(MeO)(MeS)CSH]+[SbF6]−.272 However, there is no close contact between the carbon atom and the fluorine atoms of the anion. Instead, the anion forms hydrogen bonds to the thiol group of the cation.
In 2004, the structure of the benzonorbornenyl cation was published, with an intramolecular stabilization of the cationic center by the aromatic ring.273 Intermolecular stabilized carbenium cations are known of the [CI3]+ with the weak bases PX3 (X = Cl, Br, I) and AsI3 (Fig. 33).274 Only two related vinyl cations are known (see Fig. 33).275,276 Both are β-substituted by two silyl groups, which help to stabilize the positive charge.
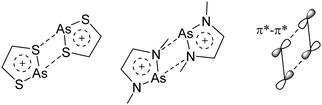
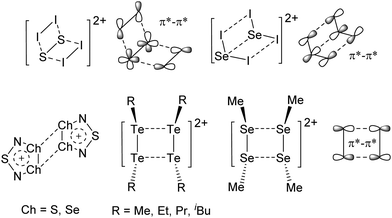
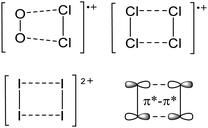
An exception is the radical cation [C6F6]˙+ in the solid state structures with [Sb2F11]− and [Os2F11]−: it yields two different forms.278 One cation can be described as a quinoidal cation and the other as a bisallyl cation (see Fig. 35) and both are separated by a barrier of around 13 kJ mol−1 according to calculations.
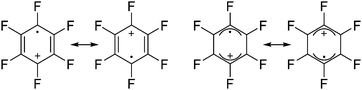 | ||
| Fig. 35 Lewis structures of the canonical forms of the quinoid and the bisallyl cationic form of [C6F6]˙+. | ||
Shortly after the publication of the radical cation of the hexafluorobenzene, some more related structures were presented. Among them, the other perhalogenated benzene radical cations279,280 and some partially and mixed substituted analogs, including the [C6F5–C6F5]˙−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280,281 (Fig. 36). The only other example displaying both a quinoidal and a bisallyl cationic form is [2,4,6-tBu3C6H2NH2]˙+.282 At 123 K, this cation adopts the bisallylic structure but upon heating, a transition to the quinoidal form occurs.
280,281 (Fig. 36). The only other example displaying both a quinoidal and a bisallyl cationic form is [2,4,6-tBu3C6H2NH2]˙+.282 At 123 K, this cation adopts the bisallylic structure but upon heating, a transition to the quinoidal form occurs.
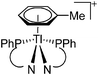
A different type of delocalized cations are the allyl cations amongst which the cyclopropenyl cations take a special position. Already since 1986, two examples, [(Cy)3C3]+ and [(Cy)2(Ph)C3]+, are known283 and in the same year, an allyl cation stabilized by an hydroxyl group has been published (Fig. 37).284 In 2002, the structure of [C5Me5H2]+ was determined although it was by mistake addressed as an [C5Me5]+ cation, probably due to its unexpected formation during the reaction of C5Me5H with [Ph3C]+.285 Finally a silyl stabilized allyl cation was characterized, which formed via an interesting mechanism that starts with the formation of a silylium cation (Fig. 37).286
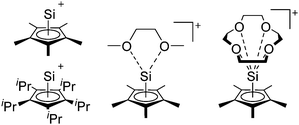
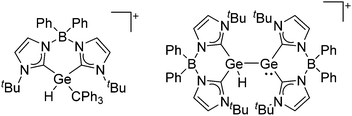
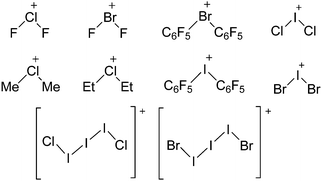
Silicon
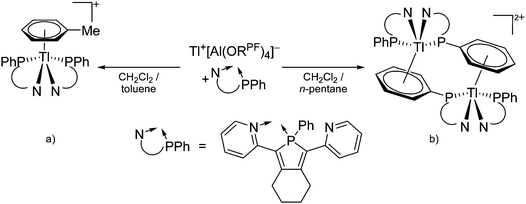
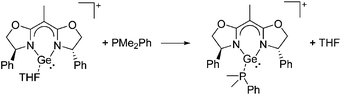
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and in 2013, ([Pemp3Si]+)2[B12Cl12]2− was published (Fig. 38).290 The latter was afterwards also synthesized and characterized with [Al(ORPF)4]−.291 In all three structures, the cation has no closer contacts to the anion.
24 and in 2013, ([Pemp3Si]+)2[B12Cl12]2− was published (Fig. 38).290 The latter was afterwards also synthesized and characterized with [Al(ORPF)4]−.291 In all three structures, the cation has no closer contacts to the anion.
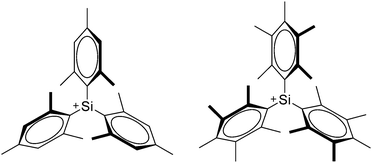
[R3Si-L]+ ions with π-donor ligands L = arenes like the before mentioned [Et3Si(C7H8)] are less stabilized than those with σ-donors (Fig. 42).289 Several different arene adducts of [Me3Si]+ were reported by Schulz and Villinger et al. (Fig. 42).304 As can be seen in Fig. 41, some of these compounds are coordinated by a second arene molecule binding in an η6-fashion to the proton ipso to the silylium center. This shows that these arena adducts are also very strong cationic Brønsted acids.
Comparable to the before mentioned 2,6-bis(2,6-difluorophenyl)phenyldimethylsilylium ion without any additional ligand, a 2,6-diarylphenyldimethylsilyl cation is existing, which is stabilized by intramolecular π-donation (Fig. 42).76
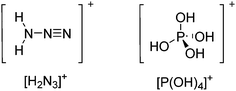
Compounds of the type [R3Si–X–SiR3]+ have to be treated as a special case of ligand stabilization. The first example of this type is the initially as [Et3Si]+ misinterpreted [Et3Si–H–SiEt3]+,305 whose structure determination has been published about two years ago.306 [Me3Si–H–SiMe3]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 is also known as well as the analogous [Me3Si–X–SiMe3]+ compounds with X = F, Cl, Br, I307 and trifluoromethanesulfonate.308 The X-bridged species are typically addressed as halonium ions, but it appears more reasonable to address them as ligand-stabilized silylium ions (see Fig. 43).
81 is also known as well as the analogous [Me3Si–X–SiMe3]+ compounds with X = F, Cl, Br, I307 and trifluoromethanesulfonate.308 The X-bridged species are typically addressed as halonium ions, but it appears more reasonable to address them as ligand-stabilized silylium ions (see Fig. 43).
Calculations state that the positive charge is still located at the silicon atoms and F, Cl and Br are negatively charged.307 Only in the case of iodine, a small positive charge is located at the bridging atom.307 Additionally, bissilylated pseudohalonium cations [Me3Si–X–SiMe3]+ with X = CN, OCN, SCN, and NNN are known.309 Of these, only in [(Me3Si)2NNN]+ both silyl groups are attached to the same atom,309 so that the structure of the cation is analog to the protonated hydrogen azide310 (see Fig. 44 and Table 5 for [H2N3]+[SbF6]−). Some more examples with bridged SiR3-groups, in which both groups are connected with each other, are known (Fig. 44).311–315
| Year | Title | Ref. |
|---|---|---|
| 2004 | Homoatomic cages and clusters of the heavier group 15 elements. Neutral species and cations | 394 |
| 2008 | Catena–phosphorus cations | 392 |
| 2011 | Homo- and heteroatomic polycations of groups 15 and 16. recent advances in synthesis and isolation using room temperature ionic liquids | 66 |
| 2012 | Multiple-charged P1-centered cations: perspectives in synthesis | 395 |
| 2013 | Catenated phosphorus compounds | 391 |
| 2013 | Recent advances in the syntheses of homopolyatomic cations of the non-metallic elements C, N, P, S, Cl, Br, I and Xe | 11 |
| 2013 | Catenated compounds – group 15 (As, Sb, Bi) | 396 |
| 2014 | Interpnictogen cations: exploring new vistas in coordination chemistry | 393 |
| 2014 | The chemistry of cationic polyphosphorus cages – syntheses, structure and reactivity | 397 |
| 2015 | Coordination chemistry of homoatomic ligands of bismuth, selenium and tellurium | 398 and 399 |
| Year | Title | Ref. |
|---|---|---|
| 2000 | Recent advances in the understanding of the syntheses, structures, bonding and energetics of the homopolyatomic cations of groups 16 and 17 | 503 |
| 2003 | Homoatomic sulfur cations | 504 |
| 2004 | Cages and clusters of the chalcogens | 505 |
| 2006 | Synthesis, reactions and structures of telluronium salts | 506 |
| 2011 | Homo- and heteroatomic polycations of groups 15 and 16. Recent advances in synthesis and isolation using room temperature ionic liquids | 66 |
| 2013 | Catenated sulfur compounds | 507 |
| 2013 | Catenated compounds – group 16 (Se, Te) | 508 |
| 2013 | Recent advances in the syntheses of homopolyatomic cations of the non-metallic elements C, N, P, S, Cl, Br, I and Xe | 11 |
| 2013 | RCNSSS+: a novel class of stable sulfur rich radical cations | 509 |
| 2015 | Coordination chemistry of homoatomic ligands of bismuth, selenium and tellurium | 398 and 399 |
| Year | Title | Ref. |
|---|---|---|
| 2000 | Recent advances in the understanding of the syntheses, structures, bonding and energetics of the homopolyatomic cations of groups 16 and 17 | 503 |
| 2008 | Polyvalent perfluoroorgano- and selected polyfluoroorgano-halogen(III and V) compounds | 640 |
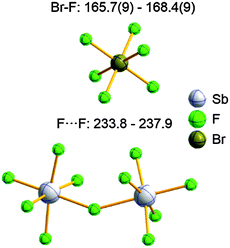
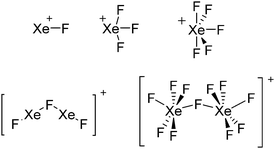
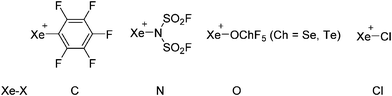
| 2013 | Recent advances in the syntheses of homopolyatomic cations of the non-metallic elements C, N, P, S, Cl, Br, I and Xe | 11 |
| Cation | WCA | Classa | Synthesis | Comment/structure | Ref. |
|---|---|---|---|---|---|
| a Classification according to the introduction (Table 2). | |||||
| Unsubstituted | |||||
| In+ | [OTf]− | Prot | InCp* + H+[WCA]− | Soluble in organic solvents in contrast to the In(I) halides | 182 |
| Tl+ | [B(ArCF3)4]−/[B(C6F5)4]− | Prot | TlOEt + [H(OEt2)2]+[WCA]− | — | 196 and 197 |
| Tl+ | [B(OTeF5)4]− | Lewis | Tl+[OTeF5]− + B(OTeF5)3 in CH2Cl2/1,2-C2Cl3F3 | — | 198 |
| Tl+ | [Al(ORPF)4]−/[Al(ORHF)4]−/[Al(ORMeF)4]− | Salt | TlF + Li+[WCA]− | — | 199 and 200 |
| Alkyl/aryl substituted | |||||
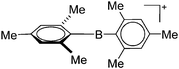
| [Mes2B]+ | [HCB11Cl11]−/[B(C6F5)4]− | Salt | Mes2BF + Et3Si(HCB11Cl11)/[Et3Si(Mes)]+[WCA]− |
|
32 and 113 |
| (R2Al) (R = Me, Et) | [B12Cl12]2− | Alk | R3Al + {[CPh3]+}2[B12Cl12]2− | Ion-like compound | 87 |
| (Et2Al) | [CB11H6X6]− (X = Cl, Br) | Alk | Et3Al + [CPh3]+[CB11H6X6]− | Ion-like compound | 128 |
| [(2,6-Mes2C6H3)2Al]+ | [B(C6F5)4]− | Hyd | (2,6-Mes2C6H3)2AlH + [CPh3]+[WCA]− | Related structure to the [Mes2B]+ cation, though the Mes moieties of the 2,6-Mes2C6H3 substituent additionally shield the aluminum cation | 129 |
| [(2,6-Mes2C6H3)2Ga]+ | [Li{Al(ORHF)4}2]− | Salt | (2,6-Mes2C6H3)2GaCl + 2Li+[WCA]− | Similar structure as the [(2,6-Mes2C6H3)2Al]+ cation | 158 |
| Cyclopentadienyl complexed | |||||
| [(η5-Cp)2Al]+ | [Al(ORPF)4]− | Prot | AlCp3 + [H(OEt2)2]+[WCA]− | — | 79 |
| [(η5-Cp′)2Al]+ | [B(C6F5)4]− | Alk | Cp′3Al + [CPh3]+[WCA]− | — | 131 |
| [(η5-Cp*)2Al]+ | [{Ph(Me)B(η5-C5H4)2}ZrCl2]− | Alk | Cp*2AlMe + {Ph(SMe2)B-(η5-C5H4)2}ZrCl2 + [Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3]+Cl− PPh3]+Cl− |
— | 132 |
| [(η5-Cp*)2Al]+ | [MeB(C6F5)3]− | Alk | Cp*2AlMe + B(C6F5)3 | — | 133 |
| [(η5-Cp)2(Et2O)2Al]+ | [Al(ORPF)4]− | Prot | AlCp3 + [H(OEt2)2]+[WCA]− | Et2O can coordinate the [(η5-Cp)2Al]+ cation | 79 |
| [Ga2(η5-Cp*)]+ | [B(ArCF3)4]− | Prot | [H(OEt2)2]+[WCA]− + GaCp* |
|
159 |
| [(η1-Cp*)(η3-Cp*)Ga]+ | [BF4]− | Prot | Cp*3Ga + HBF4 | cf. [B(η5/η1-Cp*)2]+ and [Al(η5/η5-Cp*)2]+ | 160 |
| [In2(η5-Cp)]+ | [Cp3In–Cp–InCp3]− | Com | In+[OTf]− + Cp2Mn in C6H5Me | Inverted sandwich structure (cf. the related [Ga2(η5-Cp*)]+ cation) | 183 |
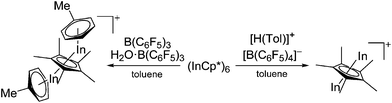
| [In2(η5-Cp*)]+ | [B(C6F5)4]− | Prot | [(C6H5Me)H]+[WCA]− + InCp* | Similar structure to the [Ga2(η5-Cp*)]+ cation | 185 |
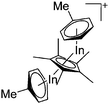
| [(μ-η5-C5Me5)In2(η6-Tol)2]+ | [(C6F5)3BO(H)B(C6F5)3]− | Prot, Com | (Cp*In)6 + B(C6F5)3 + H2O·B(C6F5)3 |
|
184 and 185 |
| Arene complexed | |||||
| [Ga(η6-C6H5R)n]+ (R = F, Me; n = 2, 3) | [Al(ORPF)4]− | Ox | Ga0 + Ag+[WCA]− in arene |
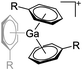
|
31, 91 and 92 |
| [Ga(η6-arene)n]+ (n = 2, 3) | [Al(ORPF)4]− | Com | [Ga(C6H5F)2–3]+[WCA]− + arene (arene = Mes, p-Xyl, C6Me6) | Bent-sandwich (2 ligands) or tubby coordinated complex (3 ligands) | 7 |
| [Ga(η6-DPE)]+ | [Al(ORPF)4]− | Com | [Ga(C6H5F)2–3]+[WCA]− + DPE | First structurally characterized bent-sandwich ansa-arene complex | 8 and 99 |
| [In(η6-C6H5F)n]+ (n = 2, 3) | [Al(ORPF)4]− | Ox | In0 + Ag+[WCA]− in C6H5F | Bent-sandwich complex (cf. gallium analogue) | 162 |
| [In(η6-o-C6H4F2)2]+ | [Al(ORPF)4]− | Salt | InCl + Li+[WCA]− in o-C6H4F2 | Bent-sandwich complex (cf. gallium analogue) | 186 |
| [Tl(η6-C6Me6)]+ | [H2N{B(C6F5)3}2]− | Other | [Tl(C6Me6)2]+ in Et2O + C6H5Me, vacuum | First example of a mono-η6-coordinated thallium complex | 204 |
| [Tl(η6-C6H5Me)2]+ | [HCB11H5Br6]− | Salt | Cs+[HCB11H5Br6]− + TlF | Bent-sandwich complex (cf. gallium analogue) | 201 |
| [Tl(η6-C6H5Me)3]+ | [H2N{B(C6F5)3}2]− | Com | [Tl(OEt2)2]+[WCA]− + C6H5Me | Tubby coordinated complex (cf. gallium analogue) | 203 |
| [Tl(η6-Mes)2]+ | [B(OTeF5)4]− | Lewis, Com | Tl+[OTeF5]− + B(OTeF5)3 in Mes | Tubby coordinated complex (cf. gallium analogue) | 202 |
| [Tl(η6-C6Me6)2]+ | [H2N{B(C6F5)3}2]− | Com | [Tl(OEt2)3]+[WCA]− + C6Me6 | Tubby coordinated complex (cf. gallium analogue) | 203 |
| Ligand substituted (CN = 2) | |||||
| [Cp*B(IMes)]2+ | [AlCl4]− | Lewis | Cp*BCl2(IMes) + 2AlCl3 |
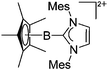
|
210 |
| [(tBu3PN)2B]+ | [B(C6F5)4]− | Hyd | (tBu3PN)2BH + [Ph3C]+[WCA]− |
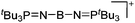
|
114 |
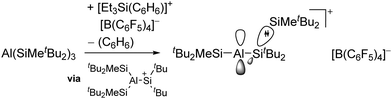
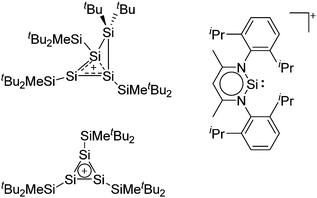
| [tBu2MeSi–Al–SitBu2–SitBu2Me]+ | [B(C6F5)4]− | Alk | Al(SiMetBu2)3 + [Et3Si]+[WCA]− | Hyperconjugation with a neighboring Si–Si bond | 134 |
| [Ga(IR)2]+ (R = Pr, Mes) | [Al(ORPF)4]− | Com | [Ga(C6H5F)2]+[WCA]− + IR |
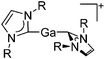
|
161 |
| [tBu2MeSi–Ga–SitBu2–Si–MetBu2]+ | [B(C6F5)4]− | Alk | Ga(SiMetBu2)3 + [Et3Si(C6H6)]+[WCA]− | Stabilized by hyperconjugation with a neighboring Si–Si bond | 134 |
| [tBu3Si–Ga–SitBu3]+ | [Al(ORPF)4]− | Salt | (tBu3Si)2GaCl + Ag+[WCA]− | Linear arrangement | 163 |
| [Ga(PtBu3)2]+ | [Al(ORPF)4]− | Com | [Ga(C6H5F)2]+[WCA]− + PtBu3 |
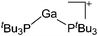
|
162 |
| [In(Mes2py)(η6-C6H5F)]+ | [B(ArCF3)4]− | Salt, Com | InBr + Na+[WCA]− + Mes2py |
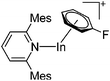
|
187 |
| [In(IPr)2]+ | [Al(ORPF)4]− | Com | [In(C6H5F)2]+ + IPr |
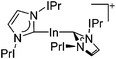
|
161 |
| [In(Mes2py)2]+ | [B(ArCF3)4]− | Salt | In+Br− + Na+[WCA]− + 2Mes2py |
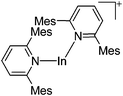
|
187 |
| [Tl(1,2-Cl2C2H4)]+ | [B(OTeF5)4]− | Lewis, Com | Tl+[OTeF5]− + B(OTeF5)3 in 1,2-C2H4Cl2 |
|
198 |
| Ligand substituted (CN = 3) | |||||
| [BMes2(IMe)]+ | [OTf]− | Salt | Mes2BF + [Me3Si]+[OTf]− + [Ag(IMe)2]+[Ag2I3]− |
|
211 |
| [BCl2(ItBu)]+ | [B(ArCl)4]− | Salt | BCl3(ItBu) + Na+[WCA]− |
|
74 |
| [{(PPh3)2C}BH2]+ | [HB(C6F5)3]− | Hyd |
|

|
118 and 119 |
| [BMes2(DMAP)]+/[B(ArN)2(DMAP)]+ ArN = 4-(Me2N)-2,6-Me2-C6H2) | [OTf]− | Salt | Mes2BF + Me3Si-OTf + ArN2BF + DMAP |
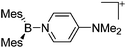
|
57 |
| [B(SubPc)]+(Sub = C24H12N6) | [HCB11Me5Br6]− | Salt | B(SubPc)Cl + Et3Si(HCB11Me5Br6) |
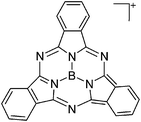
|
115 |
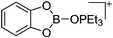
[CatB(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PEt3)]+ PEt3)]+ |
[HCB11H5Br6]− | Salt, Com | Ag+[WCA]− + CatBBr + OPEt3 |
|
116 |
| [(CatB)(PNP)PdH]+ | [B(ArCF3)4]−/[CB11H12]− | Other | [(PNP)Pd(THF)]+[WCA]− + CatBH |
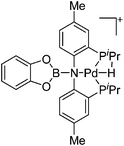
|
117 |
[ArN(C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)NAr)(C(Me)NAr)AlH]+ (Ar = DIPP) CH2)NAr)(C(Me)NAr)AlH]+ (Ar = DIPP) |
[B(C6F5)4]− | Hyd | ArN(CMeNAr)2 + AlH3·NMe2Et + [Ph3C]+[WCA]− |
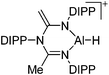
|
135 |
| [{HC(CMeNAr)2}AlMe]+ (Ar = DIPP) | [B(C6F5)4]−/[MeB(C6F5)3]− | Alk | {HC(CMeNAr)2}AlMe2 + [CPh3]+[WCA]−/B(C6F5)3 |
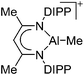
|
136 |
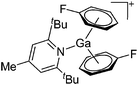
| [Ga(η6-C6H5F)2(DTBMP)]+ | [Al(ORPF)4]− | Com | [Ga(η6-μC6H5F)n]+[WCA]− + DTBMP (n = 2, 3) |
|
164 |
| [Ga(pyrazine)3]+/[{Ga(μ-pyrazine)2-(η1-pyrazine)}+]∞ | [Al(ORPF)4]− | Com | [Ga(C6H5F)]+[WCA]− + pyrazine (n = 2, 3) |
|
164 |
| [Ga(PPh3)3]+ | [Al(ORPF)4]− | Com | [Ga(C6H5Me)2]+[WCA]− + PPh3 |
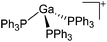
|
31, 91 and 92 |
| [(iPr2-ATI)InMe]+ | [B(C6F5)4]− | Other | Thermolysis of [{iPr2-ATI(CPh3)}InMe2]+[WCA]− |
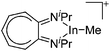
|
190 |
[{ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3)In]+ (Ar = 2,4-tBu2C6H3, 2,5-tBu2C6H3, 2,6-Et2C6H3, 2,6-iPr2C6H3,) CPh}2(NC5H3)In]+ (Ar = 2,4-tBu2C6H3, 2,5-tBu2C6H3, 2,6-Et2C6H3, 2,6-iPr2C6H3,) |
[OTf]− | Com | In+[WCA]− + bis(imino)pyridine ligand |
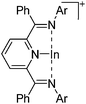
|
188 and 189 |
| [In(PPh3)3]+ | [Al(ORPF)4]− | Com | [In(C6H5F)n]+[WCA]− + 3 PPh3 (n = 2, 3) | Trigonal pyramidal (cf. gallium analogue) | 162 |
| [Tl(Mes2py)(η6-C6H5R)2]+ (R = F, Me) | [B(ArCF3)4]− | Salt, Com | TlCl + Na+[WCA]− + Mes2py in C6H5R |
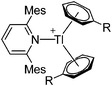
|
187 |
| [Tl(timtmbtBu)]+ | [OTf]− | Com | Tl+[WCA]− + timtmbtBu |
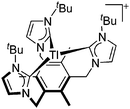
|
205 |
[{ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3)Tl]+ (Ar = 2,6-Et2C6H3, 2,5-tBu2C6H3) CPh}2(NC5H3)Tl]+ (Ar = 2,6-Et2C6H3, 2,5-tBu2C6H3) |
[OTf]− | Com | Tl+[WCA]− + bis(imino)pyridine ligand |
|
206 |
| Ligand substituted (CN = 4) | |||||
| [{(PPh3)2C}BH2(DMAP)]+ | [HB(C6F5)3]− | Com | [{(PPh3)2C}BH2]+[WCA]− + DMAP |
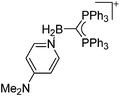
|
118 and 119 |
| [BH2(PR2H)2]+ (R = tBu, Cy, Ph) | [B(ArCF3)4]− | Salt | [BH2(PR2H)]+Br− + Na+[WCA]− |
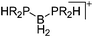
|
120 |
| [(1-MIM)2(9BBN)]+ | [B(ArCF3)4]− | Com | [PMAF–9BBN)]+[WCA]− + 1-MIM |
|
212 |
| [Me2Al(OEt2)2]+ | [MeB(C12F9)3]− | Alk | AlMe3 + B(12F9)3 in Et2O | — | 77 |
| [Me2Al(THF)2]+ | [{Me2Si(NDIPP)2}2Zr2Cl5]− | Alk | Al2Me6 + {Me2Si(NDIPP)2}ZrCl2(THF)2 | — | 143 |
| [Me2Al(NPhMe2)2]+ | [B(C6F5)4]− | Prot, Com | Al2Me6 + [HNMe2Ph]+[WCA]− | — | 144 |
| [H2Al(NMe3)2]+ | [(AlH)8(CCH2tBu)6]2− | Other |
t
Bu![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CLi + AlH3·NMe3 + ClAlH2·NMe3 + [tBuCH2(Bzl)NMe2]+Cl− CLi + AlH3·NMe3 + ClAlH2·NMe3 + [tBuCH2(Bzl)NMe2]+Cl− |
— | 145 |
| [(Pytsi)AlMe]+ | [MeB(C6F5)3]− | Alk | (Pytsi)AlMe2 + B(C6F5)3 |
|
137 |
| [H2C{hpp}2AlMe2]+ | [BPh4]− | Prot | [{hpp}H2C{hpp}H]+[WCA]− + AlMe3 |
|
138 |
[{H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(BOX-Me2)2}Al-(Me)2]+ C(BOX-Me2)2}Al-(Me)2]+ |
[B(C6F5)4]− | Hyd | {BOX-Me2}Al(Me)2 + [CPh3]+[WCA]− |
|
139 |
| [{BOX-Me2}Al(Me)(NMe2Ph)]+ | [MeB(C6F5)3]− | Alk | {BOX-Me2}Al(Me)2 + B(C6F5)3 in NMe2Ph |
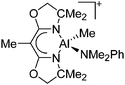
|
139 |
| [{6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al(iBu)(NMe2Ph)]+ | [HB(C6F5)3]− | Hyd | {6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al-(iBu)2 + B(C6F5)3 + NMe2Ph |
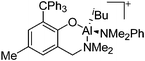
|
213 and 214 |
| [{HC(CPhNSiMe3)2}-Al(Do)Me]+ (Do = Et2O, THF) | [B(C6F5)4]−/[MeB(C6F5)3]− | Prot/Alk, Com | {HC(CPhNSiMe3)2}AlMe2 + [HNMe2Ph]+[WCA]− + Et2O/B(C6F5)3 + THF |
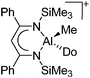
|
215 |
| [(ArN)C(Me)CHPPh2(NAr)AlMe(OEt2)]+ (Ar = DIPP) | [B(C6F5)4]− | Alk, Com | (ArN)C(Me)CHPPh2(NAr)MMe2 + [Ph3C]+[WCA]− in Et2O |
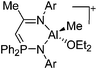
|
216 |
| [(iPr2-ATI)Al(Et)(Do)]+ (Do = ClPh, NCMe) | [B(C6F5)4]− | Alk, Com | (iPr2-ATI)AlEt2 + [CPh3]+[WCA]− in PhCl/ + MeCN |
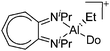
|
140 and 141 |
| [(SchNMe2)AlMe]+ | [BPh4]− | Salt | (SchNMe2)AlMeCl + Na+[WCA]− |
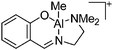
|
142 |
| [{η2-O,P-(2-PPh2-4-Me-6-tBu-C6H2O)}2Al]+ | [MeB(C6F5)3]− | Alk | {η2-O,P-(2-PPh2-4-Me-6-tBu-C6H2O)}2AlMe + B(C6F5)3 |
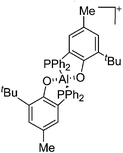
|
217 |
[{H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(BOX-Me2)2}Ga-(Me)2]+ C(BOX-Me2)2}Ga-(Me)2]+ |
[B(C6F5)4]− | Hyd | {BOX-Me2}Ga(Me)2 + [CPh3]+[WCA]− |
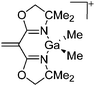
|
165 |
| [{BOX-Me2}Ga(Me)]+ | [MeB(C6F5)3]− | Alk | {BOX-Me2}Ga(Me)2 + B(C6F5)3 in NMe2Ph |
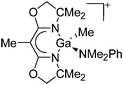
|
165 |
| [(iPr2-ATI)Ga(Me)(ClPh)]+ | [B(C6F5)4]− | Alk, Com | (iPr2-ATI)GaMe2 + [CPh3]+[WCA]− in PhCl |
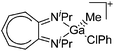
|
140 |
| [{1,2-(NiPr)2-5-CPh3-cyclohepta-3,6-diene}InMe2]+ | [B(C6F5)4]− | Other | (iPr2-ATI)InMe2 + [Ph3C]+[WCA]− |
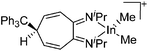
|
190 |
| [(iPr2-ATI)In(Me)(NMe2Ph)]+ | [B(C6F5)4]− | Prot | (iPr2-ATI)InMe2 + [HNMe2Ph]+[WCA]− |
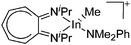
|
190 |
| [Tl(OEt2)4]+ | [H2N{B(C6F5)3}2]− | Prot | TlOEt + [H(OEt2)2]+[WCA]− in Et2O |
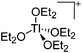
|
203 |
| Ligand substituted (CN = 5) | |||||
| [{SalenCF3}Al(OEt2)]+ | [MeB(C6F5)3]− | Alk | {SalenCF3}AlMe + B(C6F5)3 in Et2O |
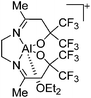
|
147 |
| [Ga(η1-C3H5)2(THF)n]+ (n = 2, 3) | [B(C6F5)4]−/[B(ArCl)4]− | Prot | Ga(η1-C3H5)3(THF) + [HNMe2Ph]+[WCA]− |
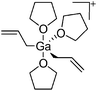
|
166 |
| [In(CH2SiMe3)2(THF)3]+ | [B(C6F5)4]− | Prot | In(CH2SiMe3)3 + [HNMe2Ph]+[WCA]− in THF |
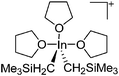
|
191 |
| [Tl(NPPh)2(η6-C6H5Me)]+ NPPh = 2,5-bis(2-pyridyl)-1-phenylphosphole | [Al(ORPF)4]− | Com |
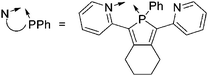
|
|
218 |
| Ligand substituted (CN ≥ 6) | |||||
| [DoAl(MeOH)2]+ (Do = Salen, Acen) | [BPh4]− | Salt, Com | DoAlCl + Na+[WCA]− + MeOH |
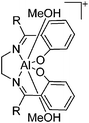
|
148 and 149 |
| [Salpen(tBu)Al(THF)2]+ | [BPh4]− | Salt, Com | Salpen(tBu)AlCl + Na+[WCA]− + THF |
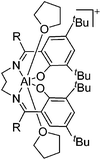
|
150 and 151 |
| [(SchNMe2)Al(OPh)-(THF)2]+ | [BPh4]− | Com | [(SchNMe2)AlPh]+[WCA]− + O2 in THF |
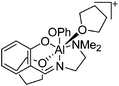
|
142 |
| [GaH(THF)4(OTf)]+ | [Ga(THF)4(OTf)2]− | Prot | GaCp* + HOSO2CF3 in THF |
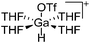
|
167 |
| [GaIII{(bipy)3}˙]2+ | [Al(ORPF)4]− | Com |
|

|
17 |
| [Ga([18]crown-6)(η6-/η1-C6H5F)2]+ | [Al(ORPF)4]− | Com | [Ga(η6-C6H5F)]+[WCA]− + [18]crown-6 (n = 2, 3) |
|
168 |
| [In([18]crown-6)]+ | [OTf]− | Com | In+[WCA]− + [18]crown-6 | No coordinated solvent, but a strong anion–cation interaction: cf. In–O = 227.2 pm and 278.5 pm (sum of the van der Waals radii 345 pm) | 192 and 193 |
| [In([18]crown-6)(η6-/η1-C6H5F)2]+ | [Al(ORPF)4]− | Com | [In(η6-C6H5F)n]+[WCA]− + [18]crown-6 (n = 2, 3) | Similar structure to the gallium analogue (see above) | 168 |
| [In([15]crown-5)2]+ | [OTf]− | Com | In+[WCA]− + [15]crown-5 | Sandwich complex | 194 |
| [{HC(3,5-Me2pz)3}nTl]+ (n = 1, 2) | [PF6]− | Com | Tl+[WCA]− + HC(3,5-Me2pz)3 |
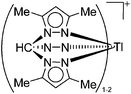
|
219 |
| [Tl([18]crown-6)]+ | [H2N{B(C6F5)3}2]− | Com | [Tl(C6H5Me)2]+[WCA]− + [18]crown-6 | Similar to gallium analogue, yet featuring significant Tl–F interactions to two counteranions | 203 |
| Transition-metal substituted | |||||
| [(FP*)(BMes)]+ | [B(ArCF3)4]− | Salt | (FP*)(BMes)Br + Na+[WCA]− |
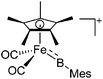
|
220 |
| [CpFe(CO)(PCy3)-(BNCMes2)]+ | [B(ArCl)4]− | Salt | CpFe(CO)(PCy3)(B(Cl)-NCMes2) + Na+[WCA]− |
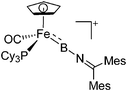
|
221 |
| [CpM(CO)(R){B(NCy2)}]+ (M = Fe, Ru; Do = CO, PMe3, PPh3) | [B(ArCF3)4]− | Salt | CpM(CO)(R){B(NCy2)Cl} + Na+[WCA]− |
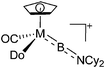
|
12 and 124 |
[(Cy3P)2(MeCN)Pt(B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O)]+ O)]+ |
[B(ArCF3)4]− | Salt, Com | (Cy3P)2Pt(B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O)(Br) + Ag+[WCA]− + MeCN O)(Br) + Ag+[WCA]− + MeCN |
|
121 |
| [{(OC)5Mn}2(μ-B)]+ | [B(ArCF3)4]− | Salt | {(OC)5Mn}2(μ-BBr) + Na+[WCA]− |
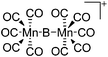
|
222 |
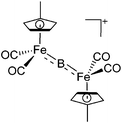
| [(FP’)2(μ-B)]+ | [B(ArCF3)4]− | Salt | (FP′)2B(Cl) + Na+[WCA]− |
|
222 |
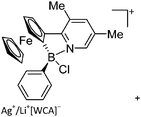
| [Fc(NC5H2Me2)BPh]+ | [Al(ORPF)4]− | Salt |
|
|
223 |
| [(FP){B(NiPr2)(OPPh3)}]+ | [B(ArCF3)4]− | Com | [(FP)B(NiPr2)]+[WCA]− + Ph3PO |
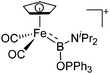
|
224 |
[(FP)B{N(iPr)(CMe2)}(Do)]+ (Do = Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Me2C O, Me2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr) NiPr) |
[B(ArCF3)4]− | Com, other | [(FP)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr2]+[WCA]− + Do NiPr2]+[WCA]− + Do |
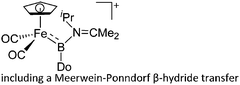
|
225 |
| [(FP)B(NCy2)(Do)]+ (Do = C5H4PPh3, 4-Pic) | [B(ArCF3)4]− | Com | [(FP)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy2]+[WCA]− + Do NCy2]+[WCA]− + Do |
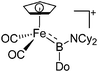
|
12 |
| [CpRu(CO)2{B(NCy2)-(4-Pic)}]+ | [B(ArCF3)4]− | Salt, Com | CpRu(CO)2{B(NCy2)Cl} + Na+[WCA]− + 4-Pic |
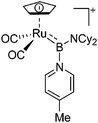
|
124 |
| [(FP*)B(Cl)(LB)]+ (Do = 3,5-lutidine, PMe3, IMe) | [B(ArCl)4]− | Salt | (FP*)B(Cl2)(Do) + Na+[WCA]− |
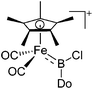
|
226 |
| [(FP*)B(nacnac)]+ | [B(ArCF3)4]− | Salt, other |
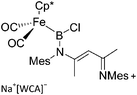
|
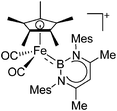
|
227 |
| [(FP)C(NCy)2BNR2]+ (R = iPr, Cy) | [B(ArCF3)4]− | Ins | [(FP)(BNR)2]+[WCA]− + RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR (substoichiometric) NR (substoichiometric) |
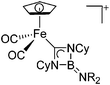
|
228 |
| [(H)(PNP)Pd(BCat)]+ | [B(ArCF3)4]− | Other | [(BCat)(PNP)Pd(BCat)]+[WCA]− + H2O |
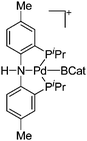
|
117 |
| [(R3P)2Pt{B(Fc)Br}]+ (R = iPr, Cy) | [B(ArCF3)4]− | Salt | (R3P)2Pt(Br){B(Fc)Br} + Na+[WCA]− |
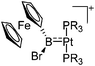
|
229 and 230 |
| [(Cy3P)2Pt{B(X)X′}]+ (X = Br; X′ = ortho-tolyl, tBu, NMe2, Pip, Br; XX′ = (NMe2)2, CatB) | [B(ArCF3)4]−/[B(C6F5)4]− | Salt | (Cy3P)2Pt(Br){B(X)X′} + Na+/K+[WCA]− |
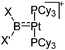
|
231 |
| [(Cy3P)2Pt(Br){B(NC5H4-4-R)X}]+ (R = Me, X = NMe2, Pip, Br; R = tBu, X = Pip) | [B(ArCF3)4]− | Salt | (Cy3P)2Pt(Br){B(Br)-(NC5H4-4-R)X} + Na+[WCA]− |
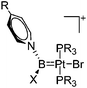
|
125 |
| [(Cy3P)2Pt{B(Br)(NMe2)}-(NCMe)]+ | [B(ArCF3)4]−/[B12Cl12]2− | Com/salt, Com | (Cy3P)2Pt{B(Br)(NMe2)} + NCMe/(Cy3P)2Pt{B(Br)(NMe2)}Br + {Na+}2[WCA]2− + MeCN |
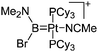
|
230 |
| [(Cy3P)2Pt(BCl2)]+ | [B(ArCF3)4]− | Salt | (Cy3P)2Pt(BCl2)Cl + Na+[WCA]− |
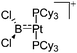
|
230 |
| [Cp*Ru(PiPr3)(BH2Mes)]+ | [B(C6F5)4]− | Salt | Cp*Ru(PiPr3)-(BH2MesCl) + Li+[WCA]−·2.5OEt2 |
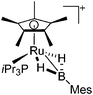
|
232 |
| [(PMAF)2BH2]+ | [B(C6F5)4]− | Hyd, Com | PMAF–BH3 + [CPh3]+[WCA]− + PMAF |
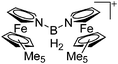
|
212 |
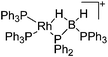
| [Rh(PPh3)2(κ1,η-PPh2BH2·PPh3)]+ | [B(ArCF3)4]− | Salt, Com | ClRh(PPh3)3 + Na+[WCA]− + H3B·PPh2H |
|
120 |
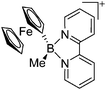
| [FcBMe(bipy)]+ | [PF6]− | Salt, Com | FcBBrMe + bipy + [NH4]+[WCA]− |
|
126 |
| [FcB(Pic)3]2+ | [B(ArCF3)4]− | Salt, Com | Br2BFc + 2Na+[WCA]− + 3Pic |
|
122 |
| [(FP){C(NCy)2B-(NCy)2CNR2}]+ (R = iPr, Cy) | [B(ArCF3)4]− | Ins | [(FP){B(NR2)}]+ + CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy NCy |
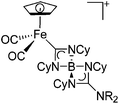
|
124 and 228 |
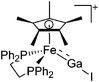
| [(dppe)Cp*FeGaI]+ | [B(ArCF3)4]− | Salt | (dppe)Cp*FeGaI2 + Na+[WCA]− |
|
233 |
| [(FP*)2Ga]+ | [B(ArCF3)4]− | Salt | (FP*)2GaCl + Na+[WCA]− |
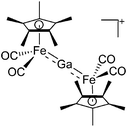
|
169 |
| [(FP*)2Ga(4-Pic)]+ | [B(ArCF3)4]− | Salt, Com | (FP*)2GaCl + Na+[WCA]− + 4-Pic |
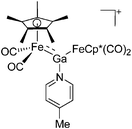
|
170 |
| [(FP*)Ga(Mes)(dtbpy)]+ | [B(ArCF3)4]− | Salt, Com | (FP*)Ga(Mes)I + Na+[WCA]− + dtbpy |
|
234 |
| [(FP*)Ga(phen)(Y)]+ (Y = Cl, SpTol) | [BPh4]− | Salt, Com/Lewis | 2(FP*)GaCl2 + Na+[WCA]− + phen/[(FP*)Ga(phen)(Cl)]+ + Me3SiSpTol |
|
171 |
| [(FP)Ga(OEt2){(NCy)2-CtBu}]+ | [B(ArCF3)4]− | Salt, Com | (FP)Ga(Cl){(NCy)2CtBu} + Na+[WCA]− in Et2O |
|
235 |
| [(FP)2Ga(bipy)]+ | [Cl2Ga(FP)2]− | Lewis, Com | 2ClGa(FP)2 + bipy |
|
236 |
| [InPt(PPh3)3]+ | [B(ArCF3)4]− | Com | In+[WCA]− + Pt(PPh3)4 |
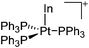
|
179 and 180 |
| [(phen)2In-Ag(η3-C6H5F)]2+ | [Al(ORPF)4]− | Com |
|
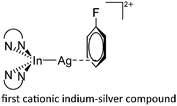
|
17 |
| [(FP*)2In]+ | [B(ArCF3)4]− | Salt | (FP*)2InCl + Na+[WCA]− |
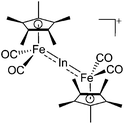
|
195 |
| [(FP*)2In(THF)]+ | [B(ArCF3)4]− | Com | [(FP*)2In]+[WCA]− + THF |
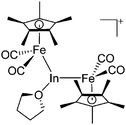
|
195 |
| [Tl(η5-FeCp2)]+ | [H2N{B(C6F5)3}2]− | Others | [Tl(η6-C6H5Me)3]+[WCA]− + 2.2FeCp2 |
|
204 |
| [Tl2(η5-FeCp2)3]2+ | [H2N{B(C6F5)3}2]− | Com | [Tl(η6-C6H5Me)2]+[WCA]− + FeCp2 |
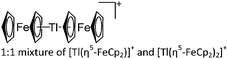
|
203 |
| [Tl{(η5-As5)FeCp*}3]+ | [FAl{OC6F10(C6F5)}3]− | Salt, Com |
|

|
176 |
| [{P(Ph2)CH2ox}(Cl)(Tl)Pt-CH2Ph}]+ | [PF6]− | Other | Tl+[WCA]− + {P(Ph2)CH2ox}Pt(Cl)-CH2Ph} |
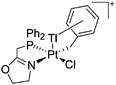
|
208 |
| Multinuclear | |||||
| [{IPr(H2B)}2(μ-H)]+ | [HB(C6F5)3]− | Hyd | IPr + B(C6F5)3 |
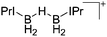
|
118 and 119 |
| [{Me3N(H2B)}2(μ-H)]+ | [B(C6F5)4]− | Hyd | Me3N–BH3 + [CPh3]+[WCA]− |
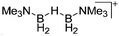
|
237 |
| [B4H2(μ-hpp)4]2+ | [HB(C6F5)3]− | Hyd, Com | [HB(μ-hpp)]2 + B(C6F5)3 |
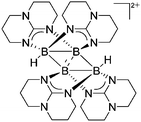
|
123 |
| [[{6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al(R)]2]2+ (R = C6H13) | [B(C6F5)4]− | Com | [{6-(CH2NMe2)-2-CPh3-4-Me-C6H2O}Al(iBu)(BrPh)]+[WCA]− + 1-hexene |
|
152 |
| [{2-(CH2Do)-6-R-C6H3O}AlMe({2-(CH2Do)-6-R-C6H3O}AlMe2)]+ (R = Ph, tBu; Do = NMe2, NC4H8, NC5H10) | [MeB(C6F5)3]− | Alk | {2-(CH2Do)-6-R-C6H3O}AlMe2 + B(C6F5)3 |
|
238 and 214 |
| [{MeC(NR)2}2Al2Me3]+ (R = iPr, Cy) | [B(C6F5)4]−/[MeB(C6F5)3]− | Alk, Com | {MeC(NR)2}AlMe2 + [CPh3]+[WCA]−/B(C6F5)3 |
|
156 |
| [AlEt(μ-η2,η1-iPr2-ATI)-(μ-Et)AlEt2]+ | [B(C6F5]− | Com | [(iPr2-ATI)Al(Et)]+[WCA]−+AlEt3 |
|
141 |
| [{(iPr2-ATI)AlMe}2(μ-Me)]+ | [B(C6F5)4]− | Alk, Com | (iPr2-ATI)AlMe2 + [CPh3]+[WCA]− |
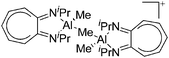
|
141 and 239 |
| [{(iPr2-ATI)Al(μ-OiPr)}2]2+ | [B(C6F5)4]− | Com | [(iPr2-ATI)Al(Et)]+[WCA]− + acetone |
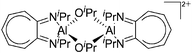
|
141 and 153 |
| [Me2Al(μ-OSi(R123)3)2Al-Me(NMe2Ph)]+ (R1, R2 = Me; R3 = Me, tBu) | [B(C6F5)4]− | Prot | Me2Al(μ-OSiR3)2AlMe2 + [HNMe2Ph]+[WCA]− |
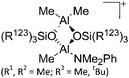
|
154 |
[{(iPr2-ATI)Al-(μ-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)}2]2+ CtBu)}2]2+ |
[B(C6F5)4]− | Com | [(iPr2-ATI)Al(Et)]+[WCA]− + tert-butyl acetylene |
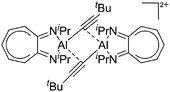
|
141 and 153 |
| [{(tacn)AlMe}2]2+ | [MeB(C6F5)3]− | Alk | [(tacn)AlMe2]2 + B(C6F5)3 |
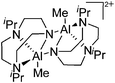
|
240 |
| [{(OSSO)Al}2]2+ | [MeB(C6F5)3]− | Alk | (OSSO)AlMe + B(C6F5)3 |
|
155 |
| [(η6-C6H5F)Ga-(μ-η6-m-TP)2-Ga(η6-C6H5F)]2+ | [Al(ORPF)4]− | Com | [Ga(η6-C6H5F)n]+[WCA]− + m-TP (n = 2, 3) |
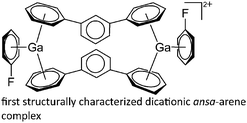
|
8 and 99 |
| [{tBuC(NiPr)2}GaMe-{tBuC(NiPr)2}GaMe2]+ | [B(C6F5)4]− | Alk, Com | {tBuC(NiPr)2}GaMe2 + [Ph3C]+[WCA]− |
|
156 |
| [{(iPr2-ATI)GaMe}2(μ-OH)]+ | [B(C6F5)4]− | Other | [(iPr2-ATI)Ga(Me)(NMe2Ph)]+[WCA]− + H2O |
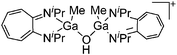
|
172 |
| [{(Salomphen)Ga}(μ-Cl)]+ | [BPh4]− | Salt | (Salomphen)GaCl + Na+[BPh4]− |
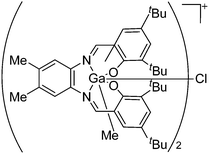
|
241 |
| [(BuGa)4(μ-OH)6]2+ | [HCB11Br6Me5]− | Other | [(2,6-Mes2C6H3)GaBu]+ [WCA]− + H2O |
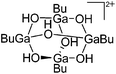
|
173 |
| [{(PPh3)3In}2(μ-PPh3)]2+ | [Al(ORPF)4]− | Com | [In(C6H5F)n]+[WCA]− + PPh3 (n = 2, 3) | One PPh3 moiety functions as a bridge between both InI cations | 162 |
| [In3(bipy)5–6]3+ | [Al(ORPF)4]− | Com |
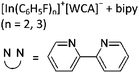
|
|
17 |
| [In4(Do)6]4+ (Do = bipy, phen) | [Al(ORPF)4]− | Com |
|
|
17 |
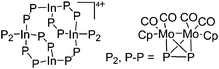
| [In4{(CpMo(CO)2)2P2}8]4+ | [Al(ORPF)4]− | Com | [In(o-C6H4F2)2]+[WCA]− + {CpMo(CO)2}2(P2) |
|
186 |
[{{ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3)Tl}2(μ-η6-C6H5R)]2+ (Ar = 2,6-Et2C6H3, 2,5-tBu2C6H3; R = H, Me) CPh}2(NC5H3)Tl}2(μ-η6-C6H5R)]2+ (Ar = 2,6-Et2C6H3, 2,5-tBu2C6H3; R = H, Me) |
[OTf]− | Com | 2[(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh)2(C5H3N)Tl]+[WCA]− + C6H5R CPh)2(C5H3N)Tl]+[WCA]− + C6H5R |
|
206 |
| [Tl2(NPPh)4]2+ NPPh = 2,5-bis(2-pyridyl)-1-phenylphosphole | [Al(ORPF)4]− | Com |
|
|
218 |
| [Tl(β-triketimine)2]2+(R = Me, tBu) | [B(ArCF3)4]− | Com | Tl+[WCA]− + β-triketimine |
|
14 |
| [Tl2({CpMo(CO)2}2)6]2+ | [Al(ORPF)4]− | Com | {CpMo(CO)2}2(P2) + Tl+[WCA]− |
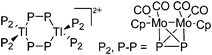
|
207 |
| [Tl3F2Al(ORHF)3]+ | [Al(ORHF)4]− | Salt, other | TlF + 2Li+[WCA]− |
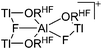
|
200 |
| [Tl4(μ-OH)2]2+ | [H2N{B(C6F5)3}2]− | Other | [Tl(OEt2)2]+[WCA]− + H2O |
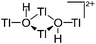
|
203 |
| [{Tl(OR)4(μ-Cl)2}+]n | [PF6]− | Other | RuCl2(DMeOPrPE)2 + Tl+[WCA]− | “Arrested” chloride abstraction yielding a one-dimensional coordination polymer | 209 |
| Multinuclear transition-metal substituted | |||||
| [(CpFe(CO){B(NCy2)})2-(μ-dmpe)]2+ | [B(ArCF3)4]− | Com, salt | (FP){B(NCy2)}Cl + dmpe + Na+[WCA]− |
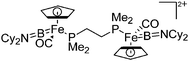
|
124 |
| [(BCat)(PNP)Pd(BCat)]+ | [B(ArCF3)4]−/[CB11H12]− | Other | [(PNP)Pd(THF)]+[WCA]−/(PNP)Pd(CB11H12) + CatB–BCat |
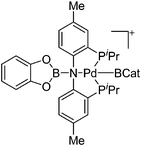
|
117 |
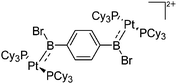
| [(Cy3P)2{Pt(BBr)}2-(μ-C6H4)]2+ | [B(C6F5)4]− | Salt | {(Cy3P)2Pt(Br)(BBr2)}2-(μ-C6H4) + K+[WCA]− |
|
125 |
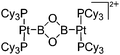
| [{(Cy3P)2PtB}2(μ-O)2]2+ | [Al(ORPF)4]− | Salt | (Cy3P)2BrPt(B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) + Ag+[WCA]− O) + Ag+[WCA]− |
|
242 |
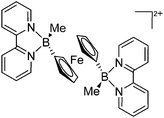
| [{(bipy)(Me)B}2(μ-Fc)]2+ | [PF6]− | Salt, Com | Fc(BBrMe)2 + 2bipy + [NH4]+[WCA]− |
|
126 and 127 |
| [{(FP)Ga(Mes)}2(μ-Cl)]+ | [B(ArCF3)4]− | Salt, Com | (FP)Ga(Mes)(Cl) + Na+[WCA]− |
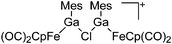
|
195 |
| [{{FeCp(CO)2}Ga{(NCy)2-CtBu}}2(μ-OH)]+ | [B(ArCF3)4]− | Other | [FpGa(OEt2)-{(NCy)2CtBu}]+[WCA]− + H2O |
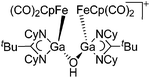
|
235 |
| [{Ga(P5FeCp*)3}+]n | [Al(ORPF)4]− | Com |
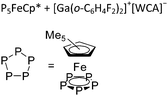
|
|
176 |
| [{(DDP)(THF)Ga}2Au]+ | [B(ArCF3)4]− | Salt, Com | {(DDP)Ga}2AuCl + Na+[WCA]− in THF |
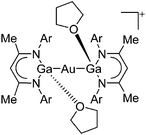
|
174 |
| [{(THF)(DDP)GaZn(THF)}2-(μ-Cl)2]2+ | [B(ArCF3)4]− | Salt, Com | (DDP)(Cl)GaZn(Cl)(THF)2 + Na+[WCA]− in THF |
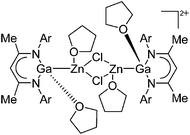
|
175 |
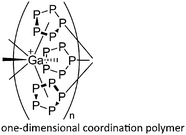
| [{In{η5-E5)FeCp*}3}+]n (E = P, As) | [Al(ORPF)4]− | Com |
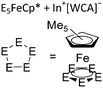
|
One-dimensional coordination polymer/similar structure to [{Ga(P5FeCp*)3}+]n | 176 |
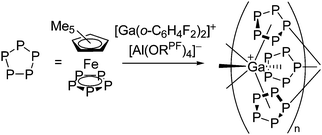
| [{Tl{(η5-E5)FeCp*}3}+]n (E = P, As) | [Al(ORPF)4]− | Com |
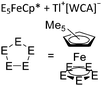
|
One-dimensional coordination polymer/similar structure to [{Ga(P5FeCp*)3}+]n | 207 and 176 |
| ECp* substituted (E = Al, Ga) | |||||
| [Rh(COD)(AlCp*)3]+ | [B(ArCF3)4]− | Com | [Rh(COD)2]+[WCA]− + 3AlCp* |
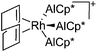
|
157 |
| [Cp*Fe(GaCp*)3]+ | [B(ArCF3)4]− | Com | [Fe(MeCN)6]2+{[WCA]−}2 + 4GaCp* |
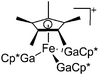
|
243 |
| [Cp*Co(GaCp*)3]2+ | [B(ArCF3)4]− | Ox, Com | [Co(MeCN)6]2+{[WCA]−}2 + 4GaCp* |
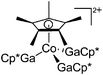
|
243 |
| [Cu(GaCp*)4]+ | [B(ArCF3)4]− | Com | [Cu(MeCN)4]+[WCA]− + 4GaCp* |
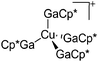
|
243 |
| [Zn(GaCp*)4]2+ | [B(ArCF3)4]− | Prot, Com | ZnMe2 + [H(OEt2)2]+[WCA]− + 4GaCp* |
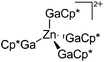
|
175 |
| [Zn2(GaCp*)6]2+ | [B(ArCF3)4]− | Other | Zn2Cp*2 + [Ga2Cp*]+[WCA]− mechanism unclear |
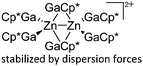
|
244 |
| [Rh(COD)(GaCp*)3]+ | [B(ArCF3)4]− | Com | [Rh(COD)2]+[WCA]− + 3GaCp* |
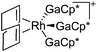
|
157 |
| [{Rh(NBD)(PCy3)-(GaCp*)2}]+ | [B(ArCF3)4]− | Com | [Rh(NBD)(PCy3)2]+[WCA]− + 2GaCp* |
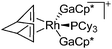
|
157 |
| [Pt(H)(GaCp*)4]+ | [B(ArCF3)4]− | Prot | Pt(GaCp*)4 + [H(OEt2)2*]+[WCA]− |
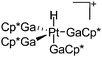
|
180 |
| [(Ga)Ru(PCy3)2(GaCp*)2]+ | [B(ArCF3)4]− | Other | Ru(PCy3)2(GaCp*)2(H)2 + [Ga2Cp*]+[WCA]− |
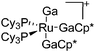
|
177 |
| [(Ga)Ni(GaCp*)4]+ | [B(ArCF3)4]− | Other | Ni(GaCp*)4 + [FeCp2]+[WCA]− |
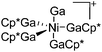
|
178 |
| [(Ga)Pt(GaCp*)4]+ | [B(ArCF3)4]− | Com | Pt(GaCp*)4 + [Ga2Cp*]+[WCA]− |
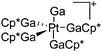
|
179 and 180 |
| [(Cp*Ga)4Rh{Ga(Me)}]+ | [B(ArCF3)4]− | Prot | (Cp*Ga)4Rh-(η1-Cp*GaMe) + [H(OEt2)2]+[WCA]− |
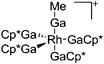
|
181 |
| [(Cp*Ga)4Rh{Ga(Me)-(py)}]+ | [B(ArCF3)4]− | Com | [(Cp*Ga)4Rh(GaMe)]+[WCA]− + py |
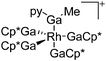
|
181 |
| [Ru(COD)(H)(GaCp*)3]+ | [B(ArCF3)4]− | Com | [Ru(COD)(H)(DMH)3]+[WCA]− + 3GaCp* |
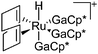
|
245 |
| [Ru(GaCp*)4-{η3-(CH2)2C(Me)}]2+ | [B(ArCF3)4]− | Prot | Ru(GaCp*)3(TMM) + [H(OEt2)2]+[WCA]− TMM = η4-C(CH2)3 |
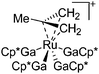
|
177 |
| [{Ru(GaCp*)3-[(CH2)2C{CH2(μ-Ga)}]}2]+ | [B(ArCF3)4]− | Com | Ru(GaCp*)3(TMM) + [Ga2Cp*]+[WCA]− |
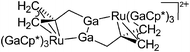
|
177 |
| [{(GaCp*)4Pt}{Pt(H)-(GaCp*)3}(μ-Ga)]2+ | B(ArCF3)4]− | Prot, Com | Pt(GaCp*)4 + [H(OEt2)2*]+[WCA]− |
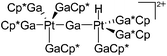
|
180 |
| [Pt3(GaCp*)6(μ-Ga)]+ | [B(ArCF3)4]− | Other | Pt(GaCp*)4 + [FeCp2]+[WCA]− (substoichiometric) |
|
178 |
| Cation | Anion | Class.a | Synthesis | Comment | Ref. |
|---|---|---|---|---|---|
| Homopolyatomic and cage cations | |||||
| [C76]+ | [HCB11H5Br6]− | Ox | C76 + [Ar3N]+[WCA]− | 44 | |
| [C60]+ | [HCB11H5Cl6]− | Ox | C60 + [Ar3N]+[WCA]− | 259 | |
| [C60]2+ | [AsF6]− | Ox | C60 + 3 AsF5 | Polymeric | 260 |
| [HC60]+ | [HCB11H5Cl6]− | Prot | C60 + H(WCA) | 259 | |
| [C59N]+ | [Ag(HCB11H5Cl6)2]− | Ox | (C59N)2 + 2[HBPC]˙+[WCA]− | 261 | |
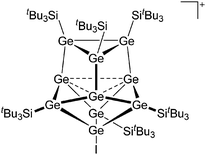
|
[B(C6F4H)4]− | Other |
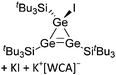
|
332 | |
| Onium ions | |||||
|
[Al2Br7]− | Lewis |
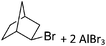
|
86 | |
| Enium ions | |||||
| [(CH3)3C]+ | [HCB11Me5Cl6]− | Hyd | n BuH + Me+[WCA]− or tBuH + Me(WCA) | 265 | |
| [(CH3)3C]+ | [Al2Br7]− | Lewis | t BuBr + 2 AlBr3 | 266 | |
| [(CH3)3C]+ | [HCB11Cl11]− | Other | Thermal decomposition of [Et2Cl][CHB11Cl11] | 267 | |
|
[HCB11Me5Br6]− | Hyd |

|
265 | |
|
[HCB11Me5Br6]− | Hyd |

|
265 | |
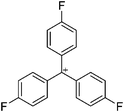
|
[HCB11I11]− | Lewis | p-CH3-C6F4-CF3 + Et3Si(WCA) + PhF | 56 | |
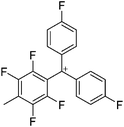
|
[HCB11Cl11]− | Lewis | p-CH3-C6F4-CF3 + Et3Si(WCA) + PhF | 56 | |
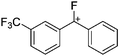
|
[As2F11]− | Lewis | C6H5CF3 + AsF5 | Excess AsF5 | 268 |
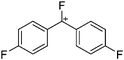
|
[HCB11I11]− | Lewis | p-F-C6H4CF3 + Et3Si(WCA) + PhF | 56 | |
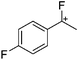
|
[HCB11I11]− | Lewis | CH3CF3 + Et3Si(WCA) + PhF | 56 | |
| [CI3]+ | [Al(ORPF)4]− | Salt | CI4 + Ag+[WCA]− | 269 | |
| [CCl3]+ | [Sb(OTeF5)6]− | Ox | CCl4 + [XeOTeF5]+[WCA]− | 270 | |
| [CCl3]+ | [Al(ORPF)4]−, [(RPFO)3Al-F-Al(ORPF)3]− | Salt | CCl4 + Ag+[WCA]− | 271 | |
| [CBr3]+ | [Sb(OTeF5)6]− | Ox | CBr4 + [XeOTeF5]+[WCA]− | 270 | |
| [CBr3]+ | [Al(ORPF)4]−, [(RPFO)3Al-F-Al(ORPF)3]− | CBr4 + Ag+[WCA]− | 271 | ||
| [C(OTeF5)3]+ | [Sb(OTeF5)6]− | Ox | CBr4 + [XeOTeF5]+[WCA]− | 270 | |
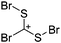
|
[Al(ORPF)4]− | Ox | CS2 + [AsBr4]+[WCA]− and CS2 + Br2 + Ag+[WCA]− | 117 | |
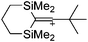
|
[B(C6F5)4]− | Hyd |
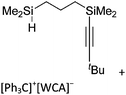
|
275 | |
|
[B(C6F5)4]− | Hyd |
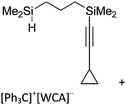
|
276 | |
| [Mes3Si]+ | [HCB11Me5Br6]− | Other | Mes3Si(CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) + Et3Si(WCA) CH2) + Et3Si(WCA) |
24 | |
| [Pemp3Si]+ | [B12Cl12]2− | Hyd | 2Pemp2MeSiH + [Ph3C]2+[WCA]− | 290 | |
| [Pemp3Si]+ | [Al(ORPF)4]− | Hyd | 1.5Pemp2MeSiH + [Ph3C]+[WCA]− | 291 | |
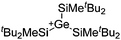
|
[B(C6F5)4]− | Ox |
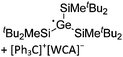
|
334 | |
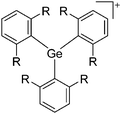
|
[Al(ORPF)4]− | Salt | (Ar)3GeBr + Ag+[WCA]− | R = OtBu | 336 |
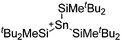
|
[B(C6F5)4]− | Ox |
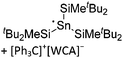
|
335 | |
| [(Tipp)3Sn]+ | [B(C6F5)4]− | Other | (allyl)(Tipp)3Sn + [E]+[WCA]− | [E]+ not exactly defined, likely [Et3Si(C6H6)]+ or comparable | 34 |
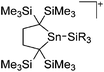
|
[B(C6F5)4]− | Com, Lig |
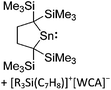
|
R = Et, iPr | 337 |
| Delocalized (cyclic) cations | |||||
| [C6H7]+ | [HCB11Me5Br6]− | Prot | C6H6 + H(WCA) | 386 | |
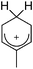
|
[HCB11H5Br6]− | Prot | C6H6 + H(WCA) | 387 | |
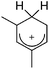
|
[HCB11H5Br6]− | Prot | C6Me2H4 + H(WCA) | 387 | |
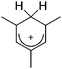
|
[HCB11H5Br6]− | Prot | C6Me3H3 + H(WCA) | 387 | |
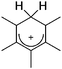
|
[B(C6F5)4]− | Prot | Et3Si(WCA) + HCl + C6(Me)5H | C–H⋯F–C interactions | 388 |
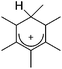
|
[HCB11H5Br6] | Prot | C6Me6 + H(WCA) | 387 | |
| [C6Me7]+ | [AlCl4]− | Other | C6Me6 + CH3Cl + AlCl3 | 277 | |
| [C6F6]˙+ | [Sb2F11]− | Ox | C6F6 + [O2]+[WCA]− | Crystallized out of HF | 278 |
| [C6F6]˙+ | [Os2F11]− | Ox | C6F6 + OsF6 + SbF5 | In HF | 278 |
| [C6Cl6]˙+ | [Sb2F11]− | Ox | C6F6 + SbF5 | 279 | |
| [C6Br6]˙+ | [As2F11]− | Ox | C6Br6 + [O2]+[AsF6]− + HSO3F | 280 | |
| [C6I6]˙+ | [AsF6]− | Ox | C6I6 + AsF5 | In HF | 279 |
| [C6I6]˙+ | [SbF6]− | Ox | C6I6 + SbF5 | In HF | 279 |
| [C6I6]˙+ | [OTf]− | Other | [C6I6][AsF6] + HOTf | 279 | |
| [C6HF5]˙+ | [AsF6]− | Ox | C6HF5 + [O2]+[WCA]− | 279 | |
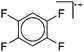
|
[SbF6]− | Ox | C6H2F4 + [O2]+[WCA]− | 279 | |
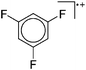
|
[AsF6]− | Ox | C6H3F3 + [O2]+[WCA]− + AsF5 | 279 | |
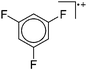
|
[SbF6]− | Ox | C6H3F3 + [O2]+[Sb2F11]− | In HF | 279 |
|
[Sb2F11]− | Ox | C6F5(CF3) + [O2]+[WCA]− | In HF | 280 |
|
[Sb2F11]− | Ox | C6F4(CF3)2 + SbF5 | In HF | 280 |
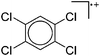
|
[Sb2F11]− | Ox | C6H2Cl4 + [O2]+[WCA]− | 280 | |
|
[Sb3F16]− | Ox | [C6F5–C6F5] + [O2]+[WCA]− + SbF5 | 280 | |
|
[Nb2F11]− | Ox |
|
281 | |
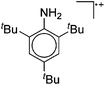
|
[SbF6]− | Ox |
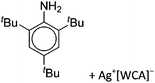
|
282 | |
| [Cy3C3]+ | [SbF6]− | Salt | [Cy3C3]Cl + Ag+[WCA]− | 283 | |
| [Cy2PhC3]+ | [BF4]− | Lewis | [Cy2(Ph)C3]F + BF3 | 283 | |
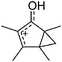
|
[SbCl6]− | Prot |
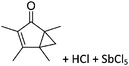
|
284 | |
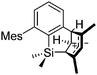
|
[HCB11Me5Br6]− | Hyd | ArMe2SiH + [Ph3C]+[WCA]− | 286 | |
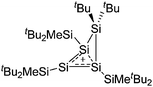
|
[B(C6F5)4]− | Other |
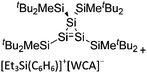
|
292 | |
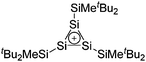
|
[B(C6F4R)4]− | Other |
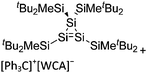
|
R = 4-SiMe2tBu | 293 |
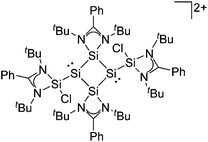
|
[Zr2Cl7Cp*2]− | Lewis |
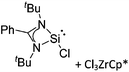
|
294 | |
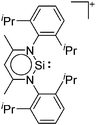
|
[B(C6F5)4]− | Prot |
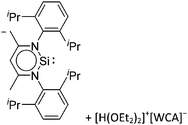
|
295 | |
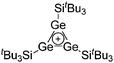
|
[B(C6H5)]−, [B(ArCF3)4]−, [B(C6F4R)4]− | Other |
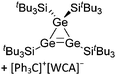
|
R = 4-Si(Me)2(tBu) | 339–341 |
| Ligand-stabilized cations | |||||
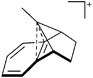
|
[Sb2F11]− | Lewis |
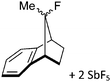
|
273 | |
| [I3C-PX3]+ | [Al(ORPF)4]− | Comp | [CI3]+[WCA]− + PX3 | X = Cl, Br, I | 274 |
| [I3C-PI3]+ | [(RPFO)3Al-F-Al(ORPF)3] | Comp | [CI3]+[WCA]− + PI3 | 274 | |
| [I3C-AsI3]+ | [Al(ORPF)4]− | Comp | [CI3]+[WCA]− + AsI3 | 274 | |
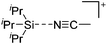
|
[HCB9H4Br5]− | Hyd | iPr3SiH + [Ph3C]+[WCA]− + MeCN | 297 | |
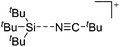
|
[B(C6F5)4]− | Other | t Bu3Si–SitBu3 + 2[Ph3C]+[WCA]− + tBuCN | 298 | |
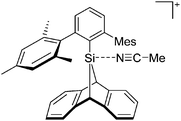
|
[B(C6F5)4]− | Com |
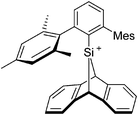
|
+ MeCN | 299 |
| [Fc3Sipy]+ | [B(ArCF3)4]− | Lig | [Fc3Si(THF)]+ + py | 300 | |
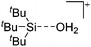
|
[HCB11H5Br6]− | Ion | t Bu3Si(WCA) + H2O | 301 | |
|
[HCB11Cl11]− | Ion | iPr3Si(WCA) + C6H4Cl2 | 81 | |
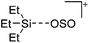
|
[HCB11Me5Br6]− | Ion | Et3Si(WCA) + SO2 | 81 | |
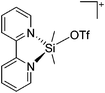
|
[OTf]− | Ion | Me2Si(OTf)2 + bipy | 302 | |
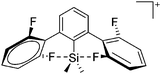
|
[B(C6F5)4]− | Hyd | Me2ArSiH + [Ph3C]+[WCA]− | 303 | |
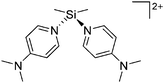
|
[OTf]− | Ion | Me2Si(OTf)2 + 2DMAP | 302 | |

|
[B(C6F5)4]− | Hyd | Et3SiH + [Ph3C]+[WCA]− | 289 | |
| [Me3Si(Ar)]+ | [B(C6F5)4]− | Lig | [Me3SiHSiMe3]+[WCA]− + arene | Ar = benzene, toluene, ethylbenzene, n-propylbenzene, and iso-propylbenzene, o-xylene, m-xylene, p-xylene, 1,2,3-trimethylbenzene, 1,2,4-trimethylbenzene, mesitylene | 304 |
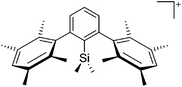
|
[B(C6F5)4]− | Hyd |
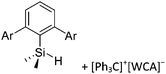
|
76 | |
| [Me3SiHSiMe3]+ | [HCB11HCl11]− | Hyd | 2Me3SiH + [Ph3C]+[WCA]− | 81 | |
| [Et3SiHSiEt3]+ | [B(C6F5)4]− | Hyd | 2Et3SiH + [Ph3C]+[WCA]− | 306 | |
| [Me3SiXSiMe3]+ | [B(C6F5)4]− | Ion | Me3SiX + Me3Si(WCA) | X = F, Cl, Br, I | 307 |
| [Me3Si-CN-SiMe3]+ | [B(C6F5)4]− | Ion | Me3SiCN + Me3Si(WCA) | 309 | |
| [Me3Si-OCN-SiMe3]+ | [B(C6F5)4]− | Ion | Me3SiOCN + Me3Si(WCA) | 309 | |
| [Me3Si-SCN-SiMe3]+ | [B(C6F5)4]− | Ion | Me3SiSCN + Me3Si(WCA) | 309 | |
| [(Me3Si)2NNN]+ | [B(C6F5)4]− | Ion | Me3SiNNN + Me3Si(WCA) | 309 | |
|
[B(C6F5)4]− | Ion | Me3Si(OTf) + Me3Si(WCA) | 308 | |
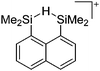
|
[B(C6F5)4]− | Hyd |
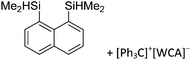
|
311 | |
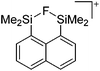
|
[B(C6F5)4]− | Other |
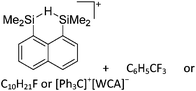
|
311 | |
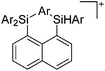
|
[B(C6F5)4]− | Hyd |
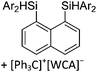
|
Ar = Tol | 312 |
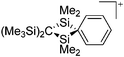
|
[B(C6F5)4]− | Other | (Me3Si)3CSiMePhH + [Ph3C]+[WCA]− | 313 | |
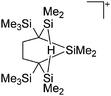
|
[B(C6F5)4]− | Other |
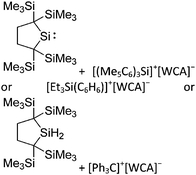
|
314 | |
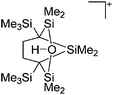
|
[B(C6F5)4]− | Other |
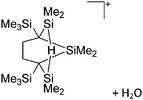
|
314 | |
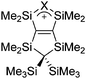
|
[B(C6F5)4]− | Other |
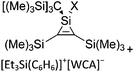
|
X = Cl, Br | 315 |
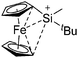
|
[B12Cl12]2− | Hyd | 2FcMetBuSiH + [Ph3C]2+[WCA]− | 316 | |
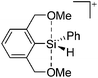
|
[OTf]− | Lewis |
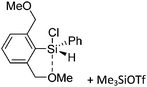
|
317 | |
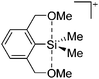
|
[OTf]− | Lewis |
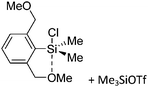
|
317 | |
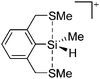
|
[OTf]− | Lewis |
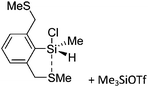
|
317 | |
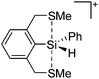
|
[OTf]− | Lewis |
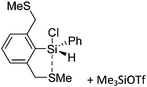
|
317 | |
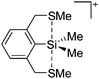
|
[OTf]− | Lewis |
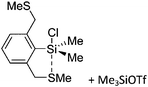
|
317 | |
|
Cl− | Ion |
|
318 | |
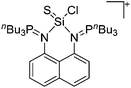
|
Cl− | Ox |
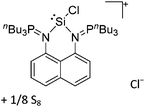
|
318 | |
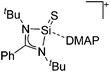
|
[OTf]− | Ox |
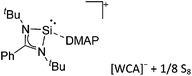
|
319 | |
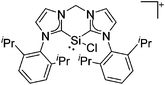
|
Cl− | Ion |
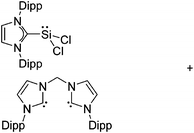
|
320 | |
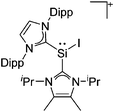
|
I− | Ion |
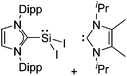
|
321 | |
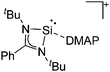
|
[OTf]− | Ox |
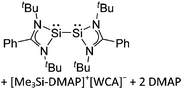
|
319 | |
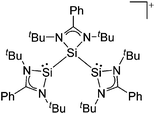
|
Other |
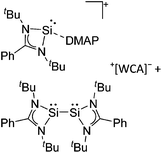
|
319 | ||
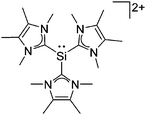
|
I− | Ion |
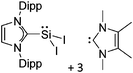
|
321 | |
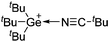
|
[B(C6F5)4]− | Ox | t Bu3Ge–GetBu3 + 2[Ph3C]+[WCA]− + tBuCN | 298 | |
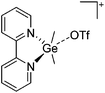
|
[OTf]− | Ion | Me2Ge(OTf)2 + bipy | 302 | |
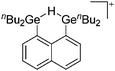
|
[B(C6F5)4]− | Hyd |
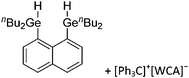
|
343 | |
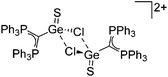
|
[AlCl4]− | Ox |
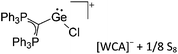
|
344 | |
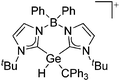
|
[B(C6F5)4]− | Ox or Com |
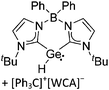
|
349 | |
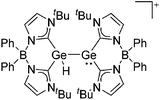
|
[B(C6F5)4]− | Hyd |
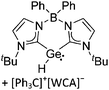
|
349 | |
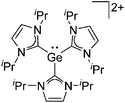
|
I− | Ion |
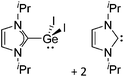
|
354 | |
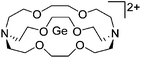
|
[OTf]− | Ion, Lig |
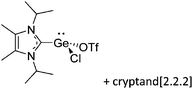
|
355 | |
| [Ge([12]-crown-4)2]2+ | [GeCl3]− | Ion | GeCl2·dioxane + [12]crown-4 | 357 | |
| [Ge([12]-crown-4)2]2+ | [OTf]− | Ion | GeCl2·dioxane + [12]crown-4 + 2Me3Si(OTf) | 357 | |
| [GeCl([15]-crown-5)]+ | [GeCl3]− | Ion | 2GeCl2·dioxane + [15]crown-5 | 357 | |
| [Ge(OTf)([15]-crown-5)]+ | [OTf]− | Ion | GeCl2·dioxane + [15]crown-5 + 2Me3Si(OTf) | 357 | |
| [GeCl([18]-crown-6)]+ | [GeCl3]− | Ion | 2GeCl2·dioxane + 1.5[18]crown-6 | 357 | |
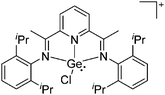
|
[GeCl3]− | Ion |
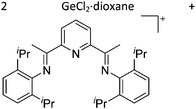
|
360 | |
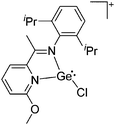
|
[GeCl3]− | Ion |
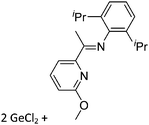
|
361 | |
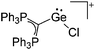
|
[AlCl4]− | Lewis |
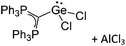
|
344 | |
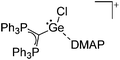
|
[B12Cl12]2− | Com |
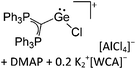
|
344 | |
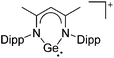
|
[HO{B(C6F5)3}2]− | Lewis |
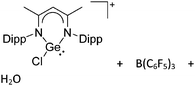
|
369 | |
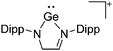
|
[B(C6F5)4]− | Prot |
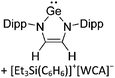
|
Protonated by acidified benzene | 365 |
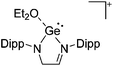
|
[B(C6F5)4]− | Prot |
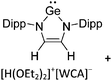
|
365 | |
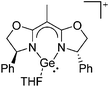
|
[SbF6]− | Salt |
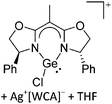
|
80 | |
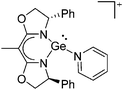
|
[SbF6]− | Lig |
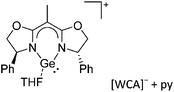
|
80 | |
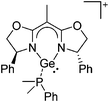
|
[SbF6]− | Lig |
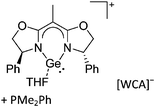
|
80 | |
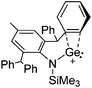
|
[Al(ORPF)4]− | Salt |
|
366 | |
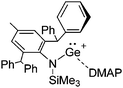
|
[Al(ORPF)4]− | Lig |
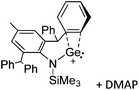
|
366 | |
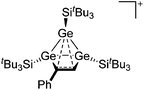
|
[B(C6F5)4]− | Lewis |
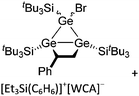
|
347 | |
| [Me3Sn(OPPh3)2]+ | [(MeSO2)2N]− | Ion | Me3SnN(SO2Me)2 + 2OPPh3 | 342 | |
|
[B(Ph)4]− | Ox |
|
L = DMAP | 345 |
| [Sn([15]crown-5)2]2+ | [SnCl3]− | Ion | 2SnCl2 + 2[15]crown-5 | 350 | |
| [Sn([12]crown-4)2]2+ | [OTf]− | Ion | Sn(OTf)2 + 2[12]crown-4 | 352 | |
| [Sn([15]crown-5)2]2+ | [OTf]− | Ion | Sn(OTf)2 + 2[15]crown-5 | 352 | |
| [Sn([18]crown-6)(OTf)]+ | [OTf]− | Ion | Sn(OTf)2 + [18]crown-6 | 352 | |
| [Sn([18]crown-6)(F)]+ | [PF6]− | Other | [Sn([18]crown-6)]2+[WCA]2− + KF | 353 | |
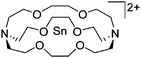
|
[OTf]− | Ion | Sn(OTf)2 + cryptand[2.2.2] or 2SnCl2 + cryptand[2.2.2] + 4Me3Si(OTf) | 356 | |
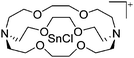
|
[SnCl3]− | Ion | 2SnCl2 + cryptand[2.2.2] | 356 | |
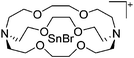
|
[SnBr3]− | Ion | 2SnCl2 + cryptand[2.2.2] + 4Me3SiBr | 356 | |
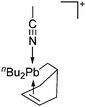
|
[B(C6F5)4]− | Com |
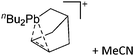
|
346 | |
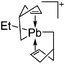
|
[B(C6F5)4]− | Other |
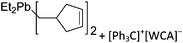
|
348 | |
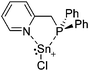
|
[SnCl3]− | Salt |
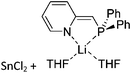
|
389 | |
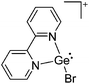
|
[GeBr3]− | Ion | GeBr2 + bipy | Bulk product is GeBr2(bipy) | 359 |
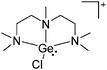
|
[GeCl3]− | Ion | GeCl2·dioxane + pmdta | 359 | |
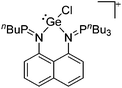
|
Cl− | Ion |
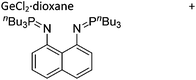
|
363 | |
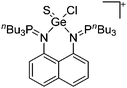
|
Cl− | Ox |
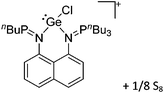
|
363 | |
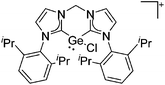
|
Cl− | Ion |
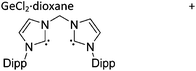
|
364 | |
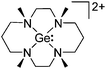
|
[GeCl3]− | Ion | GeCl2·dioxane + Me4-cyclam | 358 | |
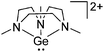
|
[GeBr3]− + Br− | Ion | GeBr2 + Me3-tacn | 358 | |
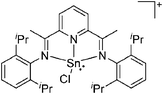
|
[SnCl3]− | Ion |
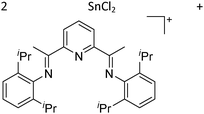
|
360 | |
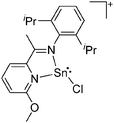
|
[SnCl3]− | Ion |
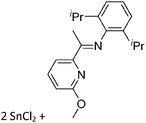
|
361 | |
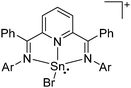
|
[SnBr3]− | Ion |
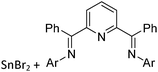
|
Ar = 2,5-tBu2(C6H3) | 362 |
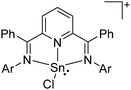
|
[SnCl3]− | Ion |
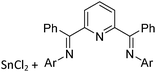
|
Ar = 2,5-tBu2(C6H3) and 2,6-Me2(C6H3) | 362 |
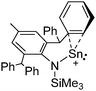
|
[Al(ORPF)4]− | Salt |
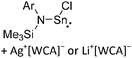
|
366 | |
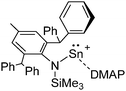
|
[Al(ORPF)4]− | Lig |
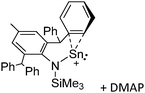
|
366 | |
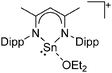
|
[MeB(C6F5)3]− | Lewis |
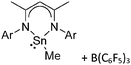
|
367 | |
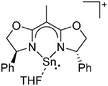
|
[SbF6]− | Salt |
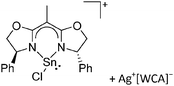
|
368 | |
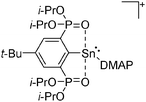
|
[B(Ph)4]− | Salt |
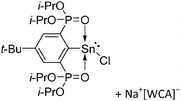
|
345 | |
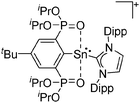
|
[B(ArCF3)4]− | Salt |
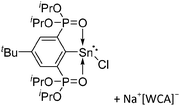
|
345 | |
|
[B(C6F5)4]− | Other | (Tipp)2Sn + [Et3Si(C7H8)]+[WCA]− | 371 | |
| [Pb(NO3)([12]-crown-4)2]+ | [Pb(NO3)3([12]crown-4)]− | Ion | Pb(NO3)2 + [12]crown-4 | 351 | |
| [Pb([15]-crown-5)2]2+ | [Pb(NO3)3([15]crown-5)]− | Ion | Pb(NO3)2 + 2[15]crown-5 | 351 | |
| [Pb(benzo-[15]-crown-5)2]2+ | [Pb(NO3)3(benzo-[15]crown-5)]− | Ion | Pb(NO3)2 + benzo-[15]crown-5 | 351 | |
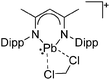
|
[B(C6F5)4]− | Salt |
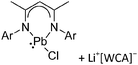
|
367 | |
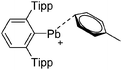
|
[B(C6F5)3(CH3)]− | Lewis |
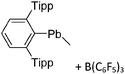
|
370 | |
| Cyclopentadienyl substituted cations | |||||
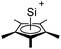
|
[B(C6F5)4]− | Prot | (Me5C5)2Si + [Me5C5H2]+[WCA]− | 322 | |
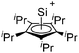
|
[Al(ORPF)4]− | Prot | (Me5C5)(iPr5C5)Si + [H(OEt2)2]+[WCA]− | 323 | |
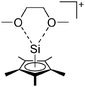
|
[B(C6F5)4]− | Com | [(Me5C5)Si]+[WCA]− + dme | 324 | |
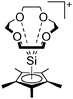
|
[B(C6F5)4]− | Com | [(Me5C5)Si]+[WCA]− + [12]crown-4 | 324 | |
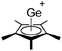
|
[BF4]− | Prot | (C5Me5)2Ge + H(WCA) | 376 | |
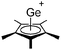
|
[SnCl3]− | Lewis | (C5Me5)GeCl + SnCl2 | 377 | |
|
[BF4]− | Prot | (C5Me5)2Ge + H(WCA) | 373 | |
|
[B(C6F5)4]− | Salt | (C5Me5)SnCl + Li+[WCA]− | 375 | |
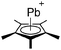
|
[B(C6F5)4]− | Salt | (C5Me5)PbCl + Li+[WCA]− | 375 | |
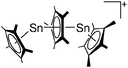
|
[Ga(C6F5)4]− | Other | (C5Me5)2Sn + Ga(C6F5)3 | 378 | |
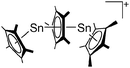
|
[B(C6F5)4]− | Com | [(C5Me5)Sn]+[WCA]− + (C5Me5)2Sn | 375 | |
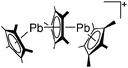
|
[B(C6F5)4]− | Com | [(C5Me5)Pb]+[WCA]− + (C5Me5)2Pb | 375 | |
| Ion-like compounds | |||||
| Me2(B12Cl12) | Salt | [Li]2+[WCA]− + 2.2MeF + 2.6AsF5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture with [Li]2+[WCA]− 1 mixture with [Li]2+[WCA]− |
288 | |
| (Me2CH)(HCB11Me5Br6) | Lewis | (H3C)2CHCl + (H3C)(HCB11H5Br6) | 287 | ||
| Me2CF(AsF6) | Lewis | (H3C)2CF2 + AsF5 | 268 | ||
| (m-CF3-C6H4)(Ph)CF(AsF6) | Lewis | C6H5CF3 + AsF5 | 268 | ||
| Me3Si(HCB11F11) | Hyd | Me3SiH + [Ph3C]+[WCA]− | 326 | ||
| Me3Si(C2H5CB11F11) | Hyd | Me3SiH + [Ph3C]+[WCA]− | 326 | ||
| Me3Si(FAl(ORPF)3) | Other | Ag+[Al(ORPF)4]− + Me3SiCl or AlEt3 + 3HORPF + Me3SiF | 327 | ||
| Et3Si(HCB11H5Br6) | Hyd | Et3SiH + [Ph3C]+[WCA]− | 328 | ||
| Et3Si(HCB11Cl11) | Hyd | Et3SiH + [Ph3C]+[WCA]− | 81 | ||
| iPr3Si(HCB11H5Br6) | Hyd | iPr3SiH + [Ph3C]+[WCA]− | 325 | ||
| iPr3Si(HCB11H5Cl6) | Hyd | iPr3SiH + [Ph3C]+[WCA]− | 329 | ||
| iPr3Si(HCB11H5I6) | Hyd | iPr3SiH + [Ph3C]+[WCA]− | 329 | ||
| iPr3Si(HCB9H4Br5) | Hyd | iPr3SiH + [Ph3C]+[WCA]− | 297 | ||
| t Bu3Si(HCB11H5Br6) | Hyd | t Bu3SiH + [Ph3C]+[WCA]− | 328 | ||
| t Bu3Si(FAl(ORPF)3) | Other | t Bu3SiI + 15 (tBu)3SiF + Ag+[Al(ORPF)4]− | 330 | ||
| t Bu2MeSi(HCB11H5Br6) | Hyd | t Bu2MeSiH + [Ph3C]+[WCA]− | 328 | ||
| Fc3Si(OTf) | Prot |
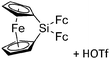
|
331 | ||
| Et3Ge(HCB11H5Br6) | Hyd | Et3GeH + [Ph3C]+[WCA]− | 338 | ||
| Et3Sn(HCB11H5Br6) | Lewis | Et3SnCl + (Et)3Si(WCA) | 338 | ||
| n Bu3Sn(CB11Me12) | Ox | n Bu6Sn2 + 2 CB11Me12 | 333 | ||
| Et3Pb(HCB11H5Br6) | Lewis | Et3PbCl + (Et)3Si(WCA) | 338 | ||
| Transition-metal substituted cations | |||||
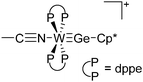
|
[B(C6F5)4]− | Salt |
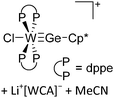
|
380 | |
|
[PF6]− | Salt |
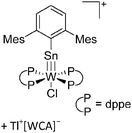
|
379 | |
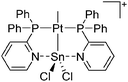
|
[BF4]− | Com |
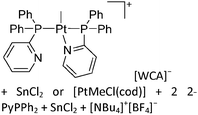
|
381 | |
|
Salt |
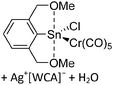
|
382 | ||
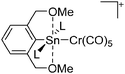
|
[CB11H12]− | Salt |
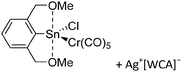
|
L = THF | 382 |
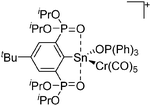
|
[ClO4]− | Ion |
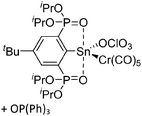
|
383 | |
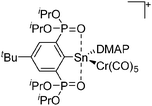
|
[ClO4]− | Ion |
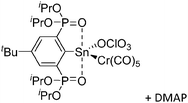
|
383 | |
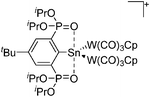
|
[W(CO)3Cp]− | Prot |
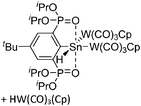
|
384 | |
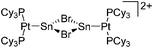
|
[AlBr4]− | Lewis |
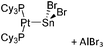
|
385 | |
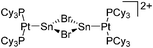
|
[B(ArCl)4]− | Salt |
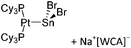
|
385 | |
| {(Cy3P)2Pt}2Sn(AlBr4)2 | Lewis |
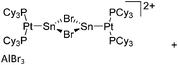
|
385 | ||
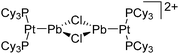
|
[AlCl4]− | Lewis |
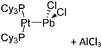
|
385 | |
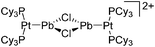
|
[B(ArCl)4]− | Salt |
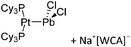
|
385 | |
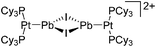
|
[AlCl4]− | Salt |
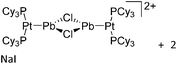
|
385 | |
| {(Cy3P)2Pt}2Pb(AlCl4)2 | Lewis |
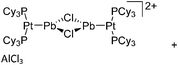
|
385 | ||
A special case of intramolecular ligand stabilization can be observed in [FcSiMetBu]+.316 Here the silicon is dipped towards the iron atom due to two 3c2e bonds between Cipso, Si and Fe and C′ipso, Si and Fe, respectively (Fig. 45).316
More intramolecular σ-donor stabilized silylium ions are known: [RSi(R′)2]+ or [RSi(R′)(R′′)]+ with R being a pincer ligand can be seen as an extra class of ligand-stabilized silicon cations. In 2009, several silylium ions with OCO and SCS pincer ligands were published by Jutzi et al. (Fig. 46).317
 | ||
| Fig. 46 Structurally characterized silylium ion salts with intramolecular stabilization by pincer ligands. | ||
All before mentioned ligand-stabilized silicon cations contain an inter- or intramolecularly by additional donor atoms stabilized [R3Si]+ cation. Two more different types of ligand-stabilized silicon cations were published with silicon in oxidation state +IV. Both were synthesized by oxidation of silicon(II) cations through elemental sulfur (Fig. 47).318,319 These cations containing subvalent silicon are very rare and most of the known examples are bearing a cyclopentadienyl substituent (see Cyclopentadienyl substituted cations). However, with well stabilizing ligands, two [LSiCl]+ cations were synthesized (Fig. 47).318,320 Both are prepared just by adding the chelating ligand to NHC·SiCl2. The NHC ligand is being replaced by L and yields the [LSiCl]+ cation with chloride as the anion. This shows that the silicon cationic center is largely stabilized by coordination. By using well stabilizing NHCs, it was possible to generate an [(L)(L′)SiI]+[I]− and even the dication [L3Si]2+([I]−)2.321 In addition, two related silicon(II) monocations [RSi(L)n]+ were structurally characterized in which the residue R is not a halogen atom (Fig. 48).319
Germanium, tin and lead
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 334 and [(tBu2MeSi)3Sn]+,335 all with [B(C6F5)4]− as their counterpart (Fig. 51). Although enium ions with aryl substituents have always been under the first examples for carbon, silicon and tin, it kept lacking an example for germanium until in 2009 [Ge({2,6-OtBu}2C6H3)3]+[Al(ORPF)4]− was synthesized and characterized.336 However, the cationic center is stabilized by contacts to the oxygen atoms of the tert-butoxy residues at 286 and 288 pm.336 More recently, a mixed substituted enium ion of tin has been published (Fig. 51).337 Examples for lead are still missing and the only formally [R3Pb]+ containing ion-like substance Et3Pb(HCB11H5Br6) has like its Si, Ge and Sn analogs stronger interactions between the ions.338
334 and [(tBu2MeSi)3Sn]+,335 all with [B(C6F5)4]− as their counterpart (Fig. 51). Although enium ions with aryl substituents have always been under the first examples for carbon, silicon and tin, it kept lacking an example for germanium until in 2009 [Ge({2,6-OtBu}2C6H3)3]+[Al(ORPF)4]− was synthesized and characterized.336 However, the cationic center is stabilized by contacts to the oxygen atoms of the tert-butoxy residues at 286 and 288 pm.336 More recently, a mixed substituted enium ion of tin has been published (Fig. 51).337 Examples for lead are still missing and the only formally [R3Pb]+ containing ion-like substance Et3Pb(HCB11H5Br6) has like its Si, Ge and Sn analogs stronger interactions between the ions.338
Norbornyl cations with the heavier group 14 elements were classified in here as ligand-stabilized cations, although one may address them as onium ions. Although the heavier norbornyl cation analogues were all published – also with silicon – no crystal structure could be determined.346 However, by addition of acetonitrile to the norbornyl cations, the stronger σ-donor replaces the weaker π-donating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. An exception is the plumbanorbornyl cation, which gets coordinated by acetonitrile additionally and remains coordinated by the alkene (scXRD).346 A comparable π-stabilization as in the norbornyl cations can be found in the 1,4,5-trigermabicyclo[2.1.0]pent-2-en-5-ylium ion, in which the cationic center is coordinated intramolecularly by a C
C double bond. An exception is the plumbanorbornyl cation, which gets coordinated by acetonitrile additionally and remains coordinated by the alkene (scXRD).346 A comparable π-stabilization as in the norbornyl cations can be found in the 1,4,5-trigermabicyclo[2.1.0]pent-2-en-5-ylium ion, in which the cationic center is coordinated intramolecularly by a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.347 Another unique π-stabilization can be observed in bis(cyclopentenemethyl)plumbylium.348 This cation is intramolecularly stabilized by the C–C double bonds of the two cyclopentene substituents (Fig. 52).
C double bond.347 Another unique π-stabilization can be observed in bis(cyclopentenemethyl)plumbylium.348 This cation is intramolecularly stabilized by the C–C double bonds of the two cyclopentene substituents (Fig. 52).
Hard to classify are two germanium cations stabilized by a monoanionic bidentate bis(NHC)borate ligand (Fig. 53).349 Both originate from the attempt to synthesize a germanium dication stabilized by the before mentioned ligand through the reaction of LGeH with [Ph3C]+[B(C6F5)4]−. Instead of delivering the desired germanium dication, two different products were obtained. In one, instead of abstracting the hydride, the trityl cation attacks the lone pair of the Ge(II) cation, forming the adduct. In the other, the hydride is indeed abstracted by the trityl cation, but the resulting germanium dication is coordinated by unreacted starting material.
Apart from those examples, the ligand-stabilized cations of the heavier group 14 elements are in oxidation state +II. Already as early as 1989, [Sn([15]crown-5)2]2+ has been published along with its crystal structure.350 This cation is accessible directly through the reaction of SnCl2 with two equivalents of the crown ether, which is why [SnCl3]− serves as the counterion. In this or a similar fashion it has been possible to synthesize a portfolio of different crown ether complexes of tin(II) and lead(II).351–353
To isolate the first related Ge(II) compound, better stabilizing ligands were needed. By employing NHC ligands, a germanium dication was isolated (Fig. 54).354 The germanium center is highly stabilized by its ligands, and – although iodides are the counterions – only weak interactions between the ions are present. Another germanium containing dication was synthesized with the encapsulating cryptand[2.2.2],355 and a few years later the analogous tin complex.356 Today, quite a few different crown ether complexes of germanium are known as well (Table 9).357 By using other well stabilizing chelating N-donor ligands, it was also possible to isolate [(L)Ge]2+ cations.358
The autoionization reaction used for the preparation of many of the crown ether complexes has also been applied to synthesize most of the structurally characterized [(L)EX]+[WCA]− compounds of germanium and tin (Fig. 55).344,359–362,389 With even stronger donating ligands, comparable salts [(L)GeCl]+Cl− were prepared.363,364 These compounds are strongly stabilized so that even halides are sufficient as anions (Fig. 56). Related [RE(L)]+ cations with the residue R not being an halogen atom are also known. In these cations, the residue is capable to stabilize the cationic center by an additional σ- or π-donation (Fig. 57).80,345,365–368 In case of bulky residues it was possible to work without an additional ligand and to obtain the free [RE]+ cations (Fig. 57).365,366,369 For lead, one additional [RE(L)]+ cation is known with R being a bulky aryl ligand and with a toluene molecule coordinating to the lead atom.370
A rather special case is [Sn(C7H8)3]2+, in which a tin(II) cation is coordinated by three toluene molecules.371 Although lots of arene complexes of tin(II) are known, almost all of them do still have strong interactions to the anions, mostly halides and/or [AlCl4]− (see for some examples ref. 19). An exception is the Sn(II) complex with [2.2.2]paracyclophane.372 Only one of the two [AlCl4]− ions is coordinated to the tin atom, the other one does not have interactions with the cation. However, [Sn(C7H8)3]2+([B(C6F5)4]−)2 is the first example of a tin(II) complex with independent arenes and without additional stabilization by the anion (Fig. 58).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376 and [SnCl3]−
376 and [SnCl3]−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377 as its counterion and not with any larger WCA. In addition, interesting triple-decker cations are known for tin and lead: [({Me5C5}Sn)2(μ-Me5C5)]+ was first synthesized and structurally characterized with the [Ga(C6F5)4]− anion,378 its structure was subsequently published with [B(C6F5)4]− together with the analogous lead compound (Fig. 59).375
377 as its counterion and not with any larger WCA. In addition, interesting triple-decker cations are known for tin and lead: [({Me5C5}Sn)2(μ-Me5C5)]+ was first synthesized and structurally characterized with the [Ga(C6F5)4]− anion,378 its structure was subsequently published with [B(C6F5)4]− together with the analogous lead compound (Fig. 59).375
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Sn-C6H3-2,6-Mes2]+ was synthesized with [PF6]− as its counterion in which the W–Sn–C angle is close to 180°.379 A similar germanium compound was published one year after, in 2004. In [(MeCN)(dppe)2W
Sn-C6H3-2,6-Mes2]+ was synthesized with [PF6]− as its counterion in which the W–Sn–C angle is close to 180°.379 A similar germanium compound was published one year after, in 2004. In [(MeCN)(dppe)2W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ge-(η1-Cp*)]+, the germanium is substituted by a Cp* and the tungsten atom is coordinated additionally by an acetonitrile molecule.380 As WCA serves [B(C6F5)4]− in this case. A new complex cation featuring a Sn–Pt bond was published in 2010. In trans-[Pt(Me)(SnCl2)(2-PyPPh2)2][BF4], the tin atom is pentacoordinated and adopts a trigonal-bipyramidal geometry.381 Along with that, comparable compounds were synthesized with the remaining group 10 metals, but no crystal structure determination was performed. By using an OCO-pincer ligand, a chromiumpentacorbonyl coordinated tin(II) cation was synthesized. Two variants were published, {2,6-(MeOCH2)2C6H3}(H2O)SnCr(CO)5(OTf), in which the tin is coordinated additionally by a water molecule and [{2,6-(MeOCH2)2C6H3}(THF2)SnCr(CO)5]+[CB11H12]−, in which the tin is hexacoordinated with two THF molecules complementing the coordination sphere.382 The former has indeed no contact between the triflate and the tin atom, but a strong hydrogen bond between the coordinated water molecule and the anion is existing, with an O–O distance of about 261 pm. Two more chromiumpentacorbonyl coordinated tin(II) cations were published in 2013, both also with a pincer-type ligand (Fig. 60).383 The same ligand was used to prepare the [RSn{W(CO)3Cp}2]+, with R = R = 4-tBu-2,6-{P(O)(OiPr)2}2C6H2) and [W(CO)3Cp]− as its counterion.384 Recently, new platinum-coordinated cations of tin and lead were published. Starting from (Cy3P)2Pt(SnBr2), [{(Cy3P)2Pt-SnBr}2]+ was synthesized with two different anions.385 The analogous dimeric lead cation [{(Cy3P)2Pt-PbCl}2]+ was accessible by using (Cy3P)2Pt(PbCl2) as a starting material.385 Through further reaction with AlX3, {(Cy3P)2Pt}2Sn(AlBr4)2 respectively {(Cy3P)2Pt}2Pb(AlCl4)2 were synthesized.385 However, in both dications some interactions between the ions are present. Additionally, the dimeric lead cation was also synthesized with iodine instead of chlorine.385
Ge-(η1-Cp*)]+, the germanium is substituted by a Cp* and the tungsten atom is coordinated additionally by an acetonitrile molecule.380 As WCA serves [B(C6F5)4]− in this case. A new complex cation featuring a Sn–Pt bond was published in 2010. In trans-[Pt(Me)(SnCl2)(2-PyPPh2)2][BF4], the tin atom is pentacoordinated and adopts a trigonal-bipyramidal geometry.381 Along with that, comparable compounds were synthesized with the remaining group 10 metals, but no crystal structure determination was performed. By using an OCO-pincer ligand, a chromiumpentacorbonyl coordinated tin(II) cation was synthesized. Two variants were published, {2,6-(MeOCH2)2C6H3}(H2O)SnCr(CO)5(OTf), in which the tin is coordinated additionally by a water molecule and [{2,6-(MeOCH2)2C6H3}(THF2)SnCr(CO)5]+[CB11H12]−, in which the tin is hexacoordinated with two THF molecules complementing the coordination sphere.382 The former has indeed no contact between the triflate and the tin atom, but a strong hydrogen bond between the coordinated water molecule and the anion is existing, with an O–O distance of about 261 pm. Two more chromiumpentacorbonyl coordinated tin(II) cations were published in 2013, both also with a pincer-type ligand (Fig. 60).383 The same ligand was used to prepare the [RSn{W(CO)3Cp}2]+, with R = R = 4-tBu-2,6-{P(O)(OiPr)2}2C6H2) and [W(CO)3Cp]− as its counterion.384 Recently, new platinum-coordinated cations of tin and lead were published. Starting from (Cy3P)2Pt(SnBr2), [{(Cy3P)2Pt-SnBr}2]+ was synthesized with two different anions.385 The analogous dimeric lead cation [{(Cy3P)2Pt-PbCl}2]+ was accessible by using (Cy3P)2Pt(PbCl2) as a starting material.385 Through further reaction with AlX3, {(Cy3P)2Pt}2Sn(AlBr4)2 respectively {(Cy3P)2Pt}2Pb(AlCl4)2 were synthesized.385 However, in both dications some interactions between the ions are present. Additionally, the dimeric lead cation was also synthesized with iodine instead of chlorine.385
Group 15 cations
Of all the pnictogen elements, especially phosphorus has a rich cation chemistry. The analogy between CR4 and [PR4]+ displays the possibility of creating a large variety of cationic phosphorus frameworks (Fig. 61).Over the last decades a multitude of catenated phosphorus cations were synthesized. The classical phosphino-phosphonium cation (Fig. 62, left), which can be synthesized through halide abstraction from PR2Cl and formal insertion/coordination of the resulting [PR2]+ (see section “Oxidation state +III” below) into a R2P–R bond/to PR3 stands for an entire substance class of compounds typically containing organic residues R.390 However, we refer the interested reader to the multitude of recent reviews especially on these cations,391,392 the analogous interpnictogen cations (Fig. 63)393 and other types of cationic pnictogen compounds (Table 5).
 | ||
| Fig. 62 Examples for catenated phosphorus cations in the formal phosphino-phosphonium or diphosphonium form. Also cyclic versions are available. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 and [P7S6I2]+.234Ref. 134 contains investigations on the nature of this formal insertion reaction, which is not as simple as thought and rather follows a concerted, orbital controlled mechanism (Fig. 67).
134 and [P7S6I2]+.234Ref. 134 contains investigations on the nature of this formal insertion reaction, which is not as simple as thought and rather follows a concerted, orbital controlled mechanism (Fig. 67).
The binary group 15 and 16 cations have also a strong tendency to form clusters. The newer examples like the antimony-chalcogen cations [Sb10Se10]2+ and [Sb7Te8]5+ were synthesized in ionic liquids or GaCl3 melts.409,410 Very recently [P3Se4]+, the first binary P–Se-cation was characterized by six different groups with three different approaches.411 It is accessible from solution, but also through solid state syntheses.412 In 2004 the synthesis of the sulphur- and selenium-bismuth cations from a chloroaluminate melt completed the series of the heterocubane cluster cations [Bi4Ch4]4+ (Ch = S, Se, Te) (Fig. 68).413,414
Bulky arylphosphines and -diphosphines (Fig. 72) can also be oxidized to their radical cations, if the cation is stabilized by a WCA.418,419
The delocalization of the single electron over a ring system also leads to stabilization. The four-membered radical cation ring systems with different pnictogen atoms (Fig. 73) were obtained through direct oxidation with silver and nitrosyl salts of WCAs.420
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421 the oxidation state of the central pnictogen “P+” can be described as +I, which is for example supported by the unusual high field shift in 31P-NMR of the central phosphorus atom in these cations (−210 to −270 ppm).422 In case of the ligand-stabilized arsenic cation [AsDppDIMPY]+ this is also supported by the synthesis: (DppDIMPY = [α,α′-{2,6-iPr2PhNC(Me)}2(C5H3N)]). The reduction of AsCl3 with SnCl2 led to a cation with a planar carbenoid-like structure.423 Under the same conditions with a different ligand an arsa-carbenoid of type [As(NR)2C2H2]+ was obtained (Fig. 78). This displays the difficulty of a clear assignment of oxidation states in such systems (Fig. 74).
421 the oxidation state of the central pnictogen “P+” can be described as +I, which is for example supported by the unusual high field shift in 31P-NMR of the central phosphorus atom in these cations (−210 to −270 ppm).422 In case of the ligand-stabilized arsenic cation [AsDppDIMPY]+ this is also supported by the synthesis: (DppDIMPY = [α,α′-{2,6-iPr2PhNC(Me)}2(C5H3N)]). The reduction of AsCl3 with SnCl2 led to a cation with a planar carbenoid-like structure.423 Under the same conditions with a different ligand an arsa-carbenoid of type [As(NR)2C2H2]+ was obtained (Fig. 78). This displays the difficulty of a clear assignment of oxidation states in such systems (Fig. 74).
For most of the reactive phosphorus cations, the decomposition is normally accompanied by the formation of strong P–X bonds (X = F, Cl…). This makes it necessary to use weakly coordinating anions stable against electrophilic cations. For the homoleptic halogen substituted cations, extremely weak anions are needed. The first examples of the less, but still highly reactive mixed amino-halogen substituted phosphenium cations were published already in 1976. Through the use of a halide-abstractor (MCl3, M = Al, Ga, Fe) is was possible to prepare [P(NR2)Cl]+ (R = Me, Et, iPr) (X = [AlCl4]−) but no crystallographic data was obtained. It was not until 2012 that the first crystal structure of a halogen and a pseudohalogen mono-substituted phosphenium cation was determined. The structures of [P(NR2)X]+ (R = TMS; X = Cl, N3, NCO, NCS) and (R = iPr; X = Cl, N3) were determined by scXRD. All cations were stabilized with the [GaCl4]− anion. Especially the azidophosphenium compound turned out to be a versatile starting material for further chemistry, and made it possible to derive more complex phosphor-centered cations like iminophosphorane-substituted-phosphonium salts [iPr2NPNP(Cl)2NR2]+[GaCl4]− [R = iPr, SiMe3] (Fig. 76) – for instance through the reaction with the corresponding chlorophosphane R2NPCl2.
Another example of ligand-stabilized pnictogen cations are the N-heterocyclic carbenoid rings [Pn(NR)2C2H2]2+ (Pn = P, As, Sb), which are formally 1,4-diaza-1,3-butadiene complexes of a pnictogen cation in oxidation state +III, but the delocalization of the positive charge supports also a description as a neutral pnictogen atom. In case of the 1,3,2-diazaphospholidinium rings [Pn(NR)2C2H4]+ (Pn = P, As) the double bond between C4 and C5 is missing.
But there are also some newer, highly oxidized cations in the literature: the formal [PnPh3]2+ cations (Fig. 80), which have a strong contact to the anion, serve as useful starting materials for further coordination chemistry of PnV compounds.425 The ligand stabilized formal “PO+” cations were prepared through the oxidation of a phosphorus carbenoid (Fig. 80, see also the phosphorus carbenoids above) with the amine-N-oxides Me3NO and pyO.426 The charge of the carbene-stabilized formal “[PFPh2]2+”, which was prepared from the carbene-stabilized “[PF2Ph2]+” through fluoride abstraction is likely partially localised on the strongly bound ligand (Fig. 80).427
| Cation | Anion | Class.a | Synthesis | Commentb | Ref. |
|---|---|---|---|---|---|
| a Classification according to the introduction: Lewis = Lewis acid halogen bond heterolysis, Ox = oxidation, Com = complexation reaction, Prot = protonation, other = all other reactions not classified. b HTS = high temperature synthesis. | |||||
| Homopolyatomic cations | |||||
| [N5]+ | [Sb2F11]− | Other | [N2F]+[SbF6]− + HN3 in aHF | 401 | |
| [P9]+ | [Al(ORPF)4]− | Ox | P4 + [NO]+[Al(OC(CF3)3)4]− | 2.5 equiv. of P4, no X-ray | 29 |
| [Sb8]2+ | [GaCl4]− | Lewis | SbCl3 + Ga+[GaCl4]− in GaCl3/C6H6 | 402 | |
| [Bi2]4+·[Bi9]5+ | [Ag3Bi3Br15]3−·6[Br]− | Lewis, Ox | Bi + BiBr3 + Ag | HTS (350 °C) | 429 |
| 2[Bi5]+·[Bi6]2+ | 2[IrBi6Br12]−·[IrBi6Br13]2− | Lewis, Ox | Bi + Ir + BiBr3 | HTS (1000 °C) | 430 |
| [Bi5]3+ | [AlCl4]− | Lewis, Ox | Bi + BiCl3 + AlCl3 | HTS | 431 |
| [Bi5]3+ | [AlCl4]− | Lewis, Ox | Bi + BiCl3 + [BMIM]Cl/AlCl3 | Ionic liquid based synthesis | 403 |
| [Bi5]3+ | [AlX4]− (X = Br, I) | Lewis, Ox | Bi + BiX3 + AlX3 | HTS (490 °C (I), 520 °C (Br)) | 432 |
| [Bi5]3+·2SO2 | [AsF6]− | Lewis, Ox | Bi + AsF5 in SO2 | No X-ray | 196 |
| [Bi8]2+ | [AlCl4]− | Lewis, Ox | Bi + BiCl3 + AlCl3 | HTS | 433 |
| [Bi8]2+ | [Ta2O2Br7]− | Lewis, Ox | Bi + BiBr3 + TaBr5 | HTS (570 °C), traces of H2O | 434 |
| [Bi9]5+ | 4[BiCl5]2−·[Bi2Cl8]2− | Lewis, Ox | Bi + BiCl3 | HTS (325 °C), Bi6Cl7 | 435 and 236 |
| [Bi9]5+ | [Bi]+·3[HfCl6]2− | Lewis, Ox | Bi + BiCl3 + HfCl4 | HTS | 436 |
| [Bi9]5+ | [Bi]+·3[NbCl6]2− | Lewis, Ox | Bi + BiCl3 + NbCl5 | HTS (550 °C), Nb(V) to Nb(IV) reduction | 434 |
| [Bi9]5+ | [Sn7Br24]10− | Lewis, Ox | Bi + BiBr3 + Sn | HTS (250 °C), C4v symmetric | 437 |
| Metal–nonmetal-cluster complexes | |||||
| [Cu(P4)2]+ | [Al(ORPF)4]− | Com | CuI + [Ag]+[Al(ORPF)4]− + P4 | 438 | |
| [Cu(P4)]+ | [GaCl4]− | Com | CuCl + GaCl3 + P4 | 439 | |
| [Ag(P4)2]+ | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + P4 | 72 | |
| [Ag(P4)]+ | [GaCl4]− | Com | AgCl + GaCl3 + P4 | 439 | |
| [Au(P4)2]+ | [GaCl4]− | Com | AuCl + GaCl3 + P4 | 439 | |
| [Cp*M(dppe)(P4)]+ (M = Fe, Ru) | [BPh4]− | Salt | [Cp*M(dppe)Cl] + P4 + Na+[BPh4]− | 440 | |
| [CpOs(PPh3)2(P4)]+ | [OTf]− | Salt | [CpOs(PPh3)2Cl] + Ag+[OTf]− | 441 | |
| [{CpRu(PPh3)2}2(P4)]2+ | [OTf]− | Com, salt | [CpRu(PPh3)2Cl] + P4 + Ag+[OTf]− | 442 | |
| [{CpRu(PPh3)2}-{CpOs(PPh3)2}(P4)]2+ | [OTf]− | Com | [CpOs(PPh3)2(P4)]+ + [{CpRu(PPh3)2}]+ | Bridging end-on/end-on | 441 |
| [Ag(As4)2]+ | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + As4 | 405 | |
| [AuPPh3(As4)]+ | [Al(ORPF)4]− | Com, salt | [Ag(As4)2]+[Al(ORPF)4]− + AuPPh3Cl | 405 | |
| [Cp*Ru-(dppe)(As4)]+ | [Al(ORPF)4]− | Com, salt | [Ag(As4)2]+[Al(ORPF)4]− + Cp*Ru-(dppe)Cl | 443 | |
| [Ag(P4S3)n]+ (n = 1, 2) | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + P4S3 | 444 | |
| [Ag2(P4S3)6]+ | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + P4S3 | 170 | |
| [{CpRu(PPh3)2}2(P4S3)]2+ | [OTf]− | Com, salt | [CpRu(PPh3)2Cl] + P4S3 + Ag+[OTf]− | 442 | |
| Clusters, cluster-like and catenated cations | |||||
| [N2Ph]+ | [BF4]− | Other | PhNH2 + NaNO2 in HCl(aq) and Na+[BF4]− | N–N triple bond | 445 |
|
[BF4]− | Other | H2NC6(CF3)5 + [NO]+[BF4]− | 446 | |
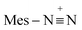
|
[OsO2(NO3)2(Mes)]− | Other | [N2Mes]+[NO3]− + [OsO2(NO3)(Mes)]+ | 447 | |
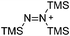
|
[GaCl4]− | Other | Hg(N2(TMS)3)2 + Ag+[GaCl4]− or Bi(N2(TMS)3)2 + GaCl3 + Cl2 | LTS (−80 °C) | 448 |
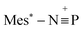
|
[AlCl4]− | Lewis | Mes*-NPCl + AlCl3 | N–P triple bond | 407 |
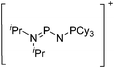
|
[GaCl4]− | Other | [P(NiPr2)N3]+[GaCl4]− + PCy3 | 449 | |
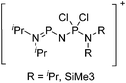
|
[GaCl4]− | Other | [P(NiPr2)N3]+[GaCl4]− + P(NR2)Cl2 | N2 as leaving group | 449 |
|
[SbF6]− | Salt | [(tBuIM)NPCl(C(PPh3)2)]+[Cl]− + Ag+[SbF6]− | Dicationic iminophosphorane | 450 |
| [P2Ph5]+ | [OTf]− | Lewis | Ph2PCl + TMSOTf + PPh3 | First homoleptic phosphine-phosphonium | 451 |
| [P2Me6]2+ | [OTf]− | Lewis | P2Me4 + MeOTf | 452 | |
| [P2Br5]+ | [Al(ORPF)4]− | Salt | PBr3 + Ag+[Al(ORPF)4]− | 453 | |
| [P2I5]+ | AlI4− | Lewis | PI3 + AlI3 | 454 | |
| [P2I5]+ | [Al(ORPF)4]− | Salt | PI3 + Ag+[Al(ORPF)4]− | 453 | |
|
[AlCl4]− | Lewis | PPh3 + PCl3 + AlCl3 | [P(PPh3)2]+ | 421 |
|
[OTf]− | Lewis | P2Me4 + PMe2Cl + TMSOTf | [(PMe2)3]+ | 455 |
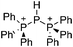
|
[AlCl4]− | Prot | [P(PPh3)2]+[AlCl4]− + AlCl3 + HCl | 456 | |
| [P3I6]+ | [F(Al(ORPF)3)2]− | Salt | P4 + I2 + Ag+[Al(ORPF)4]− | [(PI2)3]+ | 457 |
| [PMe(AsMe3)2]+ | [OTf]− | Other | MePCl2 + AsMe3 + TMSOTf | [PRL2]+ | 458 |
| [P4Ph8]2+ | [OTf]− | Lewis | PPhCl2 + PPh3 + TMSOTf | 459 | |
| [P4NO]+ | [Al(ORPF)4]− | Other | P4 + [NO]+[Al(ORPF)4]− | Insertion in P4, no X-ray | 78 |
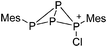
|
[GaCl4]− | Lewis | ClP(PMes*)2PCl + GaCl3 | Bicyclic phosphine-phosphonium | 460 |
| [(PPh)2(PnPh3)2]2+ (Pn = As, Sb) | [AlCl4]− | Lewis | PPhCl2 + PnPh3 + AlCl3 | 461 | |
| [P5Br2]+ | [Al(ORPF)4]− | Lewis, other | P4 + PBr3 + Ag+[Al(ORPF)4]− | Insertion in P4 | 453 and 462 |
| [P5Ph2]+, [P7Ph6]3+ | [GaCl4]− | Lewis, other | P4 + Ph2PCl + GaCl3 | Different stoichiometries, insertion in P4, HTS (60–70 °C) | 68 |
| [P5RCl]+ (R = Me, Et, iPr, Cy, Ph, C6F5) | [GaCl4]− | Lewis, other | P4 + RPCl2 + GaCl3 | Insertion in P4 | 463 |
| [P5R2]+ (R = Me, Et, iPr, Cy, Mes, Dipp) | [GaCl4]− | Lewis, other | P4 + R2PCl + GaCl3 | Insertion in P4 | 464 |
| [P5(NCy2)Cl]+ | [GaCl4]− | Lewis, other | P4 + P(NCy2)Cl2 + GaCl3 | Insertion in P4 | 465 |
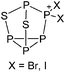
|
[Al(ORPF)4]− | Salt, other | P4S3 + PX3 + Ag+[Al(ORPF)4]− | Initial insertion in P4S3 | 134 |
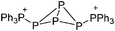
|
[AlCl4]− | Other | [P4(AsPh3)2]2+([AlCl4]−)2 + PPh3 | Ligand exchange | 466 |
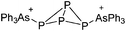
|
[AlCl4]− | Other | AsPh3 + PCl3 + AlCl3 | 466 | |
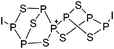
|
[Al(ORPF)4]− | Salt | P4S3I2 + Ag+[Al(ORPF)4]− | 234 | |
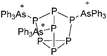
|
[OTf]− | Other | (AsPh3)(OTf)2 + PCl3 + Ph3As | 467 | |
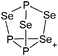
|
[AlCl4]− | Lewis, other | P(red) + Se + SeCl4 + BMImCl/AlCl3 | Ionic liquid based synthesis, rhombohedral and orthorhombic modification | 412 |
| [P3Se4]+ | [AlCl4]− | Lewis, other | P3Se4 + Me5C6Br + AlCl3 | In solution, orthorhombic modification | 412 |
| [P3Se4]+ | [Ga2Cl7]− | Lewis, other | PCl3 + SeTMS2 + GaCl3 | In solution | 412 |
| [P5Se2Ph2]+ | [GaCl4]− | Ox | [P5Ph2]+[GaCl4]− + Se | HTS (160 °C) | 464 |
| [P5Se2Cy2]+ | [GaCl4]− | Ox | [P5Cy2]+ + GaCl3 + Se | HTS (140 °C) | 464 |
Mes*–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) As As |
[GaCl4]− | Lewis | Mes*NAsCl + GaCl3 | N–As triple bond | 408 |
| [As3N3Ph3Cl2]+ | [GaCl4]− | Lewis | N2As2Ph2Cl2 + GaCl3 | As3N3 ring | 468 |
| [As2Me6]2+ | [OTf]− | Other | Me3As + PCl3 + TMSOTf | 458 | |
| [As2Ph6]2+ | [AlCl4]− | Ox | AsPh3 + PCl3 + AlCl3 | 466 | |
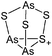
|
[AsF6]− | Ox | As4S4 + AsF5 in SO2 | 469 | |
| [As3S4]+ | [SbF6]− | Ox | As4S4 + SbF5 in SO2 | 469 | |
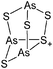
|
[AlCl4]− | Ox, Lewis | As + AsCl3 + S + AlCl3 | HTS (80 °C) | 470 |
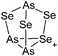
|
[SbF6]− | Ox | As + Se + SbF5 in SO2 | 469 | |
| [As3Se4]+ | [AlCl4]− | Ox, Lewis | As + AsCl3 + Se + AlCl3 | HTS (80 °C) | 470 |
| ([Sb2Se2]+)n | [AlCl4]− | Ox | Sb + Se + BMImCl/AlCl3 | Ionothermal, HTS (160 °C) | 67 |
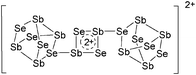
|
[AlCl4]− | Ox, others | Sb + Se + SeCl4 + BMImCl/AlCl3 | Ionic liquid based synthesis | 409 |
| ([Sb2Te2]+)n | [AlCl4]− | Ox, Lewis | Sb + Te + SbCl3 + NaCl + AlCl3 | HTS (130 °C), polymeric | 15 |
| [Sb7Te8]5+ | 3[Ga2Cl7]−·2[GaCl4]−/2[Ga2Cl7]−·3[GaCl4]− | Ox, Lewis | Sb + Te + SbCl3 + GaCl3 | AlCl3/GaCl3 melt | 410 |
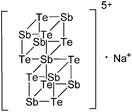
|
[MCl4]− (M = Al, Ga) | Ox, Lewis | Sb + Te + SbCl3 + MCl3 + NaCl | AlCl3/GaCl3 melt | 410 |
| [Bi2X4]2+ (X = Cl, Br) | [AlX4]− | Lewis | BiX3 + AlX3 | 471 | |
| [Bi4OF2Cl6(C6Me6)4]2+ | [Al(ORPF)4]− | Other | [Bi5]+[AsF6]− + Li+[Al(ORPF)4]− + C6Me6 | Evidence for soluble [Bin]x+ salts, partial decomposition of anion and solvent | 404 |
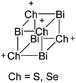
|
[AlCl4]− | Ox, Lewis | Bi + BiCl3 + Ch in AlCl3/NaCl | HTS (130 °C), heterocubane, series Bi4Ch44+ (Ch = S, Se, Te) complete | 413 |
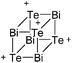
|
[AlCl4]− | Ox, Lewis | Bi + BiCl3 + Te in AlCl3/NaCl | HTS (130 °C), heterocubane | 414 |
| [PdBi10]4+ | ([BiBr4]−)∞ | Ox | Bi2Pd + Bi + Br2 | HTS (1000 °C) Pd@[Bi10]4+ | 472 |
| (4n + 2)π-cations | |||||
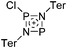
|
[GaCl4]− | Lewis | ClP(μ-NTer)2PCl + GaCl3 | P2N2 ring | 473 |
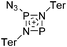
|
[N3(GaCl3)2]− | Other | [ClP(μ-NTer)2P]+[GaCl4]− + TMSN3 + GaCl3 | P2N2 ring | 473 |

|
[GaCl4]− | Other | [Mes*-NAs]+[GaCl4]− + Ph3CN3 | Cycloaddition | 408 |
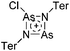
|
[GaCl4]− | Lewis | ClAs(μ-NTer)2AsCl + GaCl3 | As2N2 ring | 474 |
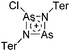
|
[OTf]− | Salt | ClAs(μ-NTer)2AsCl + Ag+[OTf]− | As2N2 ring | 474 |
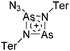
|
[N3(GaCl3)2]− | Lewis | [ClAs(μ-NTer)2As]+[GaCl4]− + TMSN3 + GaCl3 | As2N2 ring | 474 |
| [As(μ-NTer)2As]2+ | [OTf]− | Salt | ClAs(μ-NTer)2AsCl + Ag+[OTf]− | As2N2 ring, 4π system, 2 equiv. of Ag+[OTf]− | 474 |
| [ClSb(μ-NTer)2Sb]+ | [GaCl4]− | Lewis | ClSb(μ-NTer)2SbCl + GaCl3 | Sb2N2 ring | 475 |
| [Sb(μ-NTer)2Sb]2+ | [OTf]− | Salt | ClSb(μ-NTer)2SbCl + Ag+[OTf]− | Sb2N2 ring, 4π system, 2 equiv. of Ag+[OTf]− | 475 |
| [IBi(μ-NTer)2Bi]+ | [B(C6F5)4]− | Salt | IBi(μ-NTer)2BiI + [Ag(Tol)3]+[B(C6F5)4]− | Bi2N2 ring | 475 |
| [Bi(μ-NTer)2Bi]2+ | [OTf]− | Salt | ClBi(μ-NTer)2BiCl + Ag+[OTf]− | Bi2N2 ring, 4π system, 2 equiv. of Ag+[OTf]− | 475 |
| π*–π*-complexes | |||||
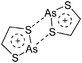
|
[GaCl4]− | Lewis | AsS2(CH)2Cl + GaCl3 | 415 | |
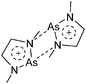
|
[MCl4]− (M = Al, Ga) | Lewis | As(NMe)2(CH)2Cl + MCl3 | 415 | |
| Radicals | |||||
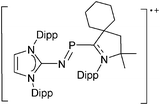
|
[B(C6F5)4]− | Ox | [P(CN(Dipp)C10H18)N(C(N(Dipp))2C2H2)] + [CPh3]+[B(C6F5)4]− | CN(Dipp)C10H18![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAAC, cyclic alkylaminocarbene CAAC, cyclic alkylaminocarbene |
416 |
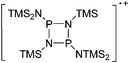
|
[SbF6]− | Ox | [(NTMS)2(PNTMS2)2] + [NO]+[SbF6]− | 420 | |
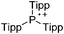
|
[X]− = [SbF6]−, [Al(ORPF)4]− | Ox | PTipp3 + Ag+[X]− | 418 | |
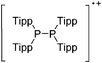
|
[Al(ORHT)4]− | Ox | P2Tipp4 + Ag+[Al(ORHT)4]− | 419 | |
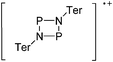
|
[B(C6F5)4]− | Ox | [P2(NTer)2] + [Ag(Tol)3]+[B(C6F5)4]− | 476 | |
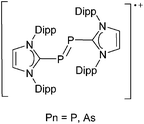
|
[B(C6F5)4]− | Ox | [P2(C(N(Dipp))2C2H2)2] + [CPh3]+[B(C6F5)4]− | C(N(Dipp))2C2H2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NHC, N-heterocyclic carbene NHC, N-heterocyclic carbene |
208 |
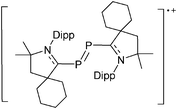
|
[B(C6F5)4]− | Ox | [P2(CN(Dipp)C10H18)2] + [CPh3]+[B(C6F5)4]− | NC10H18 = tetramethylamide | 477 |
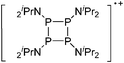
|
[Al(ORPF)4]− | Ox | [P4(NiPr2)4] + [NO]+[BF4]− + Li+[Al(ORPF)4]− | 420 | |
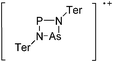
|
[B(C6F5)4]− | Ox | [AsP(NTer)2] + [Ag(Tol)3]+[B(C6F5)4]− | 476 | |
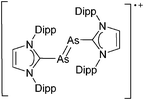
|
[GaCl4]− | Ox | [As2(C(N(Dipp))2C2H2)2] + GaCl3 | 417 | |
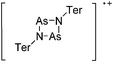
|
[B(C6F5)4]− | Ox | [As2(NTer)2] + [Ag(Tol)3]+[B(C6F5)4]− | 476 | |
| Oxidation state +I | |||||
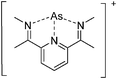
|
[SbCl5·THF]− | Lewis | AsCl3 + SnCl2 + DppDIMPY | [AsL3]+, DppDIMPY = [α,α′-{2,6-iPr2PhN-C(Me)}2(C5H3N)] | 423 |
| Oxidation state +III | |||||
| [P(NiPr2)2]+ | [GaCl4]− | Lewis | P(NiPr2)2Cl + GaCl3 | [PX2]+ | 478 and 479 |
| [P(NCy2)Cl]+ | [GaCl4]− | Lewis | P(NCy2)Cl2 + GaCl3 | [PX2]+ | 465 |
| [P(NiPr2)Cl]+ | [GaCl4]− | Lewis | P(NiPr2)Cl2 + GaCl3 | [PX2]+ | 449 |
| [P(NTMS 2)Cl]+ | [GaCl4]− | Lewis | P(NTMS2)Cl2 + GaCl3 | [PX2]+ | 480 |
| [P(NiPr2)N3]+ | [GaCl4]− | Other | [P(NiPr2)Cl]+[GaCl4]− + TMSN3 | [PX2]+ | 449 |
| [P(NTMS2)X]+ (X = N3, NCO, NCS) | [GaCl4]− | Other | [P(NTMS2)Cl]+[GaCl4]− + TMSX | [PX2]+ | 480 and 481 |
| [P(NTMS2)OTMS]+ | [GaCl4]− | Other | [P(NTMS2)Cl]+[GaCl4]− + TMSCNO | [PX2]+ | 481 |
| [PCp*Cl]+ | [Cl(Al(ORPF)3)2]− | Lewis | PCp*Cl2 + PhF·Al(ORPF)3 | [PR2]+ | 482 |
| [PCp*2]+ | [Cl(Al(ORPF)3)2]− | Lewis | PCp*2Cl + PhF·Al(ORPF)3 | [PR2]+, phosphocenium ion | 482 |
| [AsCp*Cl]+ | [Cl(Al(ORPF)3]− | Lewis | AsCp*Cl2 + PhF·Al(ORPF)3 | [AsRX]+ | 482 |
| [SbCl2(AsMe3)]+ | [OTf]− | Lewis | SbCl3 + AsMe3 + TMSOTf | [SbX2L]+ | 483 |
| [SbPhCl(AsPh3)]+ | [AlCl4]− | Lewis | SbPhCl2 + AsPh3 + AlCl3 | [SbRXL]+ | 483 |
| [BiPh(AsPh3)]2+ | [OTf]− | Lewis | BiCl2Ph + AsPh3 + TMSOTf | [BiRL]2+ | 484 |
| [BiCl(SbPh3)]2+·C6H6 | [AlCl4]− | Lewis | BiCl3 + AsPh3 + AlCl3 | [BiXL]2+ | 484 |
| [BiCl2(AsPh3)2]+ | [OTf]− | Lewis | BiCl3 + AsPh3 + TMSOTf | [BiX2L2]+ | 483 |
| [BiCl2(SbPh3)2]+·C7H8 | [AlCl4]− | Lewis | BiCl3 + SbPh3 + AlCl3 | [BiX2L]+ | 484 |
| [Bi(N2TMS3)2]+ | [GaCl4]− | Lewis | Bi(N2TMS3)2Cl + GaCl3 | [BiX2]+ | 485 |
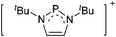
|
[BF4]− | Salt | PCl(NtBu)2C2H2 + Ag+[BF4]− | P carbenoid | 486 |
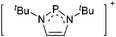
|
[PF6]− | Salt | PCl(NtBu)2C2H2 + Ag+[PF6]− | P carbenoid | 487 |
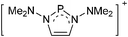
|
[GaCl4]− | Lewis | PCl(NMe)2C2H4 + GaCl3 | P carbenoid | 479 |
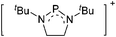
|
[GeCl5]−·[Cl]− | Other | Ge(NtBu)2C2H4 + PCl3 | P carbenoid | 488 |
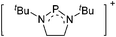
|
[PF6]− | Salt | PCl(NtBu)2C2H4 + Ag+[PF6]− | P carbenoid | 487 |
|
[GaCl4]− | Lewis | PCl(NDipp)2C2H4 + GaCl3 | P carbenoid | 426 |
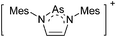
|
[SbCl5·THF]− | Lewis | AsCl3 + SnCl2 + (MesN)2C2H2 | As carbenoid | 423 |
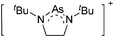
|
[GeCl5]−·[Cl]− | Other | Ge(NtBu)2C2H4 + AsCl3 | As carbenoid | 488 |
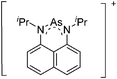
|
[GaCl4]− | Lewis | As(iPrN)2C10H6Cl + GaCl3 | As carbenoid | 489 |
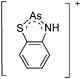
|
[AlCl4]− | Lewis | AsCl(HN)SC6H4 + AlCl3 | As carbenoid | 490 |
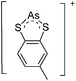
|
[AlCl4]− | Lewis | AsClS2C6H3CH3 + AlCl3 | As carbenoid | 490 |
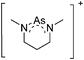
|
[GaCl4]− | Lewis | AsCl(NMe)2C3H6 + GaCl3 | As carbenoid | 491 |
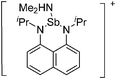
|
[OTf]− | Other | Sb(iPrN)2C10H6(NMe2) + HOTf | Not planar through the ligand NHMe2 | 489 |
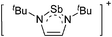
|
[Sb2Cl8]2− | Lewis | [Sb(tBuN)2C2H2]+ + SbCl3 | Sb carbenoid | 492 |
| Oxidation state +V | |||||
| [NF4]+ | [BF4]−, [SbF6]−, [Sb2F11]− | Lewis | NF3 + F2 + BF3 or SbF5 | 493 | |
| [PF4]+ | [Sb3F16]− | Lewis | PF5 + SbF5 | No X-ray | 494 |
| [PCl4]+ | [SnCl6]2− | Lewis | PCl5 + SnCl4 | For more structures see ref. 495 | 496 |
| [PBr4]+ | [Al(ORPF)4]− | Salt | PBr3 + Br2 + Ag+[Al(ORPF)4]− | 453 | |
| [PI4]+ | [AlCl4]− | Lewis | PI3 + ICl + AlCl3 in CS2 | 495 | |
| [PI4]+ | [AlBr4]− | Lewis | PI3 + IBr + AlBr3 in CS2 | 495 | |
| [PI4]+ | [AlI4]− | Lewis | PI3 + I2 + AlI3 in CS2 | 497 | |
| [PI4]+ | [GaI4]− | Lewis | PI3 + I2 + GaI3 in CS2 | 495 | |
| [PI4]+ | [Al(ORPF)4]− | Salt | PI3 + I2 + Ag+[Al(ORPF)4]− | 453 | |
| [AsCl4]+ | [AsF6]− | Lewis, Ox | AsCl3 + Cl2 + AsF5 | 498 | |
| [AsCl4]+ | [As(OTeF5)6]− | Lewis, Ox | AsCl3 + ClOTeF5 + As(OTeF5)5 | 499 | |
| [AsBr4]+ | [Al(ORPF)4]− | Salt | AsBr3 + Br2 + Ag+[Al(ORPF)4]− | 500 | |
| [AsBr4]+ | [FAs(OTeF5)5]− | Lewis, Ox | AsBr3 + BrOTeF5 + AsF(OTeF5)4 | 499 | |
| [SbCl4]+ | [Sb2F11]− | Lewis | SbCl5 + SbF5 | 501 | |
| [SbCl4]+ | [Sb(OTeF5)6]− | Ox | Sb(OTeF5)3 + Cl2 | 502 | |
| [SbBr4]+ | [Sb(OTeF5)6]− | Ox | Sb(OTeF5)3 + Br2 | 502 | |
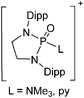
|
[GaCl4]− | Ox | [(CH2)2(NDipp)2P]+ + OL | “PO+” cation | 426 |
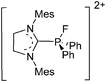
|
[B(C6F5)4]− | Lewis | [(SIMes)PF2Ph2]+ + [Et3Si(Tol)]+[B(C6F5)4]− | “PFPh22+” cation | 427 |
| [Ph3Pn]2+ (Pn = Sb, Bi) | [OTf]− | Salt | Ph3PnCl2 + Ag+[OTf]− | Strong contact to the anion | 425 |
| Protonated cations | |||||
| [H2N3]+ | [SbF6]− | Prot | HN3 + HF/SbF5 | 310 | |
| [P(OH)4]+ | [SbF6]− | Prot | H3PO4 + HF/SbF5 | 428 | |
Group 16 cations
Hundreds of chalcogen cations are known to the literature (see Table 6 for reviews). The relatively strong Ch–Ch– and Ch–X-single bonds (Ch = S, Se, Te; X = F, Cl, Br, I) led to a great diversity of reactive compounds, which include homo- and heteropolyatomic clusters, radical cations and a large number of different [ChX3]+ structures for instance. To avoid the formation of the more stable neutral compounds, weakly coordinating anions are needed to stabilize the reactive chalcogen cations. | ||
| Fig. 86 Heteroatomic cationic aromatic (6π) systems containing chalcogen atoms. (S4N3+ is a 10π system). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 or in case of the selenium containing rings with Se, [Se8]2+ or EtSeCl and [NS]+ as starting materials.516–518 [S4N3]+, a 10π-system, was synthesized through the reaction of S4N4 with Se2Cl2 and is stabilized by the polymeric ([SeCl5]−)∞ anion.519
515 or in case of the selenium containing rings with Se, [Se8]2+ or EtSeCl and [NS]+ as starting materials.516–518 [S4N3]+, a 10π-system, was synthesized through the reaction of S4N4 with Se2Cl2 and is stabilized by the polymeric ([SeCl5]−)∞ anion.519
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 or already oxidized chalcogens like [PhTe]+[SbF6]−
526 or already oxidized chalcogens like [PhTe]+[SbF6]−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 and alkynes.
526 and alkynes.
Another example of ligand-stabilized chalcogen cations are the N-heterocyclic carbenoidic rings [R2C2N2Ch]2+ (Ch = S, Se, Te), which are formally 1,4-diaza-1,3-butadiene complexes of a chalcogen cation in oxidation state +II, but the delocalization of the positive charge supports a description as a chalcogen in oxidation state +IV (Fig. 90).527 The carbene-analogues were prepared through complexation of an in situ generated Ch2+ dication, which can be obtained through halide abstraction from SCl2,528 SeCl4529 or (Dipp2BIAN)TeI2525 (Dipp2BIAN = 1,4-(2,6-diisopropyl)phenyl-bis(arylimino)-acenaphthene).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 and [Te(C6F5)3]+
532 and [Te(C6F5)3]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 and to refer to a recently published review about the chemistry and structures of these cations.506 Some newer examples of compounds with chalcogen atoms in oxidation state +IV are the triazidetelluronium cation [Te(N3)3]+
533 and to refer to a recently published review about the chemistry and structures of these cations.506 Some newer examples of compounds with chalcogen atoms in oxidation state +IV are the triazidetelluronium cation [Te(N3)3]+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 534 and a fluoride bridged version of [TeCl3]+.28
534 and a fluoride bridged version of [TeCl3]+.28
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 and [B(ArCF3)4]− (Fig. 92).535
532 and [B(ArCF3)4]− (Fig. 92).535
| Cation | Anion | Classa | Synthesis | Commentb | Ref. |
|---|---|---|---|---|---|
| a Classification according to the introduction (Table 2): Com = complexation reaction, Lewis = Lewis acid halogen bond heterolysis, Prot = protonation, Ox = oxidation, other = all other reactions not classified. b HTS = high temperature synthesis. | |||||
| Homopolyatomic cations | |||||
| [O2]+ | [PtF6]− | Ox | O2 + PtF6 | 510 | |
| [O2]+ | [PtF6]− | Ox | O2 + PtF6 | Neutron diffraction | 539 |
| [S4]2+ | [AsF6]− | Ox | S + AsF5 in SO2 | 540 | |
| [S4]2+·4[S7I]+ | [AsF6]− | Ox, Lewis | S + I2 + AsF5 in SO2 | 540 and 541 | |
| [S4]2+ | [Sb9F39]2−(![[= with combining circumflex]](https://www.rsc.org/images/entities/char_003d_0302.gif) [(SbF6)5(Sb2F4)(Sb2F5)]2−) [(SbF6)5(Sb2F4)(Sb2F5)]2−) |
Ox | S + SbF5 in SO2 | Traces of Br2 were added | 542 |
| [S4]2+·AsF3 | [AsF6]− | Ox | S + AsF5 + AsF3 in HF | Traces of Br2 were added | 543 |
| [S8]2+ | [AsF6]− | Ox | S + HF/AsF5 | 544 | |
| [S8]2+ | [SbF6]−·[Sb3F14]−(![[= with combining circumflex]](https://www.rsc.org/images/entities/char_003d_0302.gif) [(SbF6)2(SbF2)]−) [(SbF6)2(SbF2)]−) |
Ox | S + SbF5 in SO2 | 542 | |
| [S8]2+ | [AsF6]− | Ox | [S8]2+([AsF6]−)2 in SO2/SO2ClF | 73 | |
| [S19]2+ | [AsF6]− | Ox | S + AsF5 in SO2/SO2ClF | 545 | |
| [S19]2+ | [SbF6]− | Ox | S + SbF5 in SO2 | 542 | |
| [Se4]2+ | [AlCl4]− | Lewis, Ox | Se + SeCl4 + AlCl3 | 546 | |
| [Se4]2+ | [MCl6]2− (M = Zr, Hf) | Lewis, Ox | Se + SeCl4 + MCl4 | HTS (130 °C) | 235 |
| [Se4]2+ | [SbF6]−·[Sb4F11]−(![[= with combining circumflex]](https://www.rsc.org/images/entities/char_003d_0302.gif) [(SbF6)2(Sb2F5)2]−) [(SbF6)2(Sb2F5)2]−) |
Ox | Se + SbF5 in SO2 | 547 | |
| [Se4]2+ | [Sb9F39]2−(![[= with combining circumflex]](https://www.rsc.org/images/entities/char_003d_0302.gif) [(SbF6)5(Sb2F4)(Sb2F5)]2−) [(SbF6)5(Sb2F4)(Sb2F5)]2−) |
Ox | S + Se + SbF5 in SO2 | 546 | |
| [Se4]2+ | [MoOCl4]− | Ox | Se + MoOCl4 | HTS (190 °C) | 548 |
| [Se4]2+ | [Mo2O2Cl8]−·[MCl6]− (M = Zr, Hf) | Lewis, Ox | [Se4]2+[Mo2O2Cl8]2− + [Se4]2+[MCl6]2− or Se, SeCl4 + MoOCl4 + MCl4 | HTS (120 °C) | 549 |
| [Se8]2+ | [AlCl4]− | Lewis, Ox | Se + SeCl4 + AlCl3 | Crystals from vapour-phase transport | 550 |
| [Se8]2+·[Te6]4+·SO2 | [AsF6]− | Ox | Se + Te + AsF5 in SO2 | 551 | |
| [Se10]2+ | [SbF6]− | Ox | Se + SbF5 in SO2 | 552 | |
| [Se10]2+ | [SO3F]− | Ox | Se + AsF5 in SO2 | 553 | |
| [Se10]2+ | [Bi4Cl14]− | Lewis, Ox | Se + SeCl4 + BiCl3 | HTS (90 °C) | 554 |
| [Se17]2+ | [WCl6]− | Ox | Se + WCl6 | 555 | |
| [Se17]2+ | [NbCl6]− | Lewis, Ox | Se + SeCl4 + NbCl5 in SnCl4 | Solvothermal (150 °C) | 556 |
| [Se17]2+ | [TaBr6]− | Lewis, Ox | Se + SeBr4 + TaBr5 in SiBr4 | Solvothermal (150 °C) | 556 |
| [Te4]2+ | [AlCl4]−[Al2Cl7]− | Lewis, Ox | Te + TeCl4 + AlCl3 | HTS (250 °C) | 557 |
| [Te4]2+ | [SbF6]− | Ox | Te + SbF5 in SO2 | Germanium was added to obtain mixed cations | 546 |
| [Te4]2+ | [WCl6]− | Ox | Te + WCl6 | HTS (190 °C) | 558 |
| [Te4]2+ | [WCl6]− | Ox | Te + WCl6 | Traces of Br2 were added, β-mod. | 559 |
| [Te4]2+ | [Zr2Br10]2− | Lewis, Ox | Te2Br + ZrBr4 | HTS (210 °C) | 560 |
| [Te4]2+ | [HfCl6]− | Lewis, Ox | Te + TeCl4 + HfCl4 | HTS (200 °C) | 235 |
| [Te4]2+ | [MCl6]− (M = Nb, Ta), [TaBr6]−, [Ta2Cl10O]2− | Lewis, Ox | Te + TeCl4 + MCl5 | HTS (170 °C) | 561 |
| [Te4]2+ | [Bi6Cl10]2−[Bi2Br8]2− | Lewis, Ox | Te + TeX4 + BiX3 (X = Cl, Br) | HTS (170 °C) | 562 |
| [Te4]2+ | [Nb2Cl10O]2− | Lewis, Ox | Te + TeCl4 + NbCl5 + NbOCl3 | HTS (200 °C) | 563 |
| [Te4]2+ | [MoCl4O]− | Ox | Te + MoOCl4 | HTS (250 °C) | 564 |
| (Te102+)n·Te42+ | [Bi4Cl16]2− | Lewis, Ox | Te + TeCl4 + BiCl3 | HTS (150 °C) | 565 |
| [Te6]2+ | [MCl6]2− (M = Zr, Hf) | Lewis, Ox | Te + TeCl4 + MCl4 | HTS (220 °C) | 103 |
| [Te6]2+ | [WCl4O]− | Ox | Te + WOCl4 | HTS (150 °C) | 566 |
| [Te6]2+ | [NbCl4O]− | Lewis, Ox | Te + TeCl4 + NbOCl3 | HTS (200 °C) | 567 |
| [Te6]4+·2AsF3 | [AsF6]− | Ox | Te + AsF5 in SO2 | 568 | |
| (Te72+)n | [AsF6]− | Other | [Te4]2+([AsF6]−)2 + Fe(CO)5 in SO2 | Reduction of Te42+ | 569 |
| (Te72+)n | [Be2Cl6]2− | Lewis, Ox | Te + TeCl4 + BeCl2 | HTS (250 °C) | 565 |
| (Te72+)n | [WBr4O]−·[Br]− | Ox | Te + WOBr4/WBr5 | HTS (230 °C) | 570 |
| (Te72+)n | [WCl4O]−·[Cl]− | Ox | Te + WOCl4/WCl5 | HTS (150 °C) | 571 |
| (Te72+)n | [NbCl4O]−·[Cl]− | Ox | Te + TeCl4 + NbOCl3 | HTS (225 °C) | 101 |
| (Te72+)n | [NbBr4O]−·[Br]− | Ox | Te2Br + NbOBr3 | HTS (220 °C) | 101 |
| [Te8]2+ | [WCl6]− | Ox | Te + WCl6 | HTS (200 °C) | 572 |
| [Te8]2+ | [ReCl6]2− | Lewis, Ox | Te + TeCl4 + ReCl4 | HTS (230 °C) | 573 |
| [Te8]2+ | [Bi4Cl14]2− | Lewis, Ox | Te + TeCl4 + BiCl3 | HTS (160 °C) | 574 |
| [Te8]2+ | [U2Br10]2− | Lewis, Ox | Te + TeBr4 + UBr5 in SiBr4 | Solvothermal 200 °C | 575 |
| [Te8]2+ | [Ta4O4Cl16]4− | Lewis, Ox | Te + TeCl4 + TaCl5 + TaOCl3 + [BMIM]+Cl− | Ionic liquid based synthesis | 576 |
| [Te8]4+ | ([VCl4O]2−)n | Ox | Te + VOCl3 | HTS (270 °C), cubic | 577 |
| Metal–nonmetal-cluster complexes | |||||
| [Cu(S12)(S8)]+ | [Al(ORPF)4]− | Com | Cu+ + S | 512 and 578 | |
| [Cu(S12)(CH2Cl2)]+ | [Al(ORPF)4]− | Com | Cu+ + S | 512 and 578 | |
| [Ag(S8)2]+ | [Al(ORPF)4]− | Com | Ag+ + S | 579 | |
| [Cu2Se19]2+ | [Al(ORPF)4]− | Com | Cu+ + Se(red) | 50 | |
| ([Ag2(Se6)]2+)∞ | [AsF6]− | Com | Ag+ + Se | 513 | |
| [Ag(Se6)]+ | [Ag2(SbF6)3]− | Com | Ag+ + Se | 513 | |
| [Ag2(Se6)(SO2)2]2+ | [Sb(OTeF5)6]− | Com | Ag+ + Se(grey) | 513 | |
| [Ag2(Se6)(SO2)4]2+ | [Al(ORPF)4]− | Com | Ag+ + Se(grey) | 580 | |
| [Ag2Se12]2+ | [FAl(OC(C5F10)(C6F5))3]−, [Al(ORPF)4]−) | Com | Ag+ + Se(red) | 176 and 581 | |
| Clusters/cluster-like | |||||
| NS+ | [AlCl4]+ | Lewis | (NSCl)3 + AlCl3 | 517 | |
| [NS2]+ | [AlCl4]+ | Other | S4N4 + AlCl3 | 582 | |
| [S4N4]2+ | [SbCl6]− | Ox | S4N4/S3N3Cl3 + SbCl5 in SO2 | 583 | |
| [S4N4]2+ | [Sb3F14]−·[SbF6]− | Ox | S4N4 + SbF5 in SO2 | 583 | |
| [S5N5]+ | [SbCl6]− | Lewis | S3N3Cl3 + SbCl5 in SOCl2 | 584 | |
| [S3Cl3]+ | [AsF6]− | Other | [SCl3]+[AsF6]− + S8 in SO2 | 585 | |
| [(S2N2C)2]2+ | [SbF6]−, [Sb2F11]− | Other | [NS2]+[AsF6]− + (CN)2 in SO2 | 586 | |
| [S4Te4]2+·SO2 | [AsF6]− | Ox | Te + Sn + AsF5 in SO2 | 587 | |
| [S3Te3]2+ | [AsF6]− | Ox | S + Te + AsF5 in SO2 | 588 | |
| [Se3Cl3]+ | [AsF6]− | Other | [SeCl3]+[AsF6]− + Se in SO2 | 585 | |
| [Se6Ph2]2+·2SO2 | [AsF6]− | Other | [Se4]2+([AsF6]−)2 + Ph2Se2 | 589 | |
| [Se6I2]2+·2SO2 | [AsF6]− | Ox | Te + I2 + AsF5 in SO2 or [SeI3]+[AsF6]−2 + [Se8]2+([AsF6]−)2 | 590 | |
| [Te6I2]2+ | [WCl6]− | Ox | Te + I2 + WCl6 | HTS (150 °C) | 566 |
| ([Te15X4]2+)n | ([MOX4]−)n (M = Mo, X = Cl, Br; M = W, X = Br) | Other | Te2Br + MoOBr3, TeCl4 + MoNCl2/MoOCl3, Te + WBr5/WOBr3 | 591 | |
| [Se4Te2]2+ | [MF6]− (M = As, Sb) | Ox | Se + Te + MF5 in SO2 | 588 | |
| [Se4Te3]2+ | [MOCl4]− | Ox | Se + [Te6]2+([MOCl4]−)2 | HTS (190 °C) | 592 |
| [Se6Te2]2+·[Se8Te2]2+·2SO2 | [AsF6]− | Ox | S + Se + Te + AsF5 in SO2 | Heterocubane | 593 |
| [Se8Te2]2+ | [MF6]− (M = As, Sb) | Ox | Se + Te + MF5 in SO2 | Isostructural to Se102+ | 593 |
| [Se8Te2]2+·SO2 | [AsF6]− | Ox | Te + [Se8]2+([AsF6]−)2 in SO2 | 594 | |
| (4n + 2)π-cations | |||||
| [S2N3]+ | [Hg2Cl6]2− | Lewis | NSCl + HgCl2 | 595 | |
| [S3N2]2+ | [MF6]− (M = As, Sb) | Other | [SN]+[MF6]− + [S2N]+[MF6]− in SO2 | Cycloaddition | 515 |
| [S4N3]+ | ([SeCl5]−)∞ | Ox | S4N4 + Se2Cl2 in SOCl2 | 10π-aromatic | 519 |
| [S3Se]2+ | [Sb3F16]−·3[SbF6]− | Ox | S + Se + SbF5 in SO2 | Traces of Br2 were added, contains disordered mixture of [SxSex−4]2+ | 596 |
| [SSe2N2]2+ | [AsF6]− | Ox | [(SSe2N2)2]2+([AsF6]−)2 + AsF5 in SO2 | 597 | |
| [S2SeN2]2+ | [AsF6]− | Other | [NS]+[AsF6]− + [Se8]2+[AsF6]− | 516 | |
| [S2SeN2Cl]+ | [AlCl4]− | Other | [NS]+[AlCl4]− + Se/EtSeCl | 517 and 518 | |
| [Se2Te2]2+ | [Sb3F14]3−·[SbF6]− | Ox | Se + Te + SbF5 in SO2 | 598 | |
| [SeTe3]2+ | [Sb3F14]3−·[SbF6]− | Ox | Se + Te + SbF5 in SO2 | Contains disordered mixture of [SeTe3]2+, [Te4]2+ and [Se2Te2]2+ | 598 |
| π*–π*-complexes | |||||
|
[AsF6]− | Ox | S4N4 + AsF5 in SO2 | 599 | |
| [S6N4]2+ | [S2O2F]−, [SO3F]− | Ox | S4N4 + HSO3F in SO2 | 600 | |
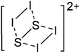
|
[AsF6]− | Ox | S + I2 + AsF5 in SO2 | 520 | |
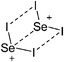
|
[Sb2F11]− | Ox | Se + [I2]+[Sb2F11]− in SO2 | 521 | |
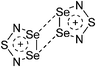
|
[MF6]− (M = As, Sb) | Other | S4N4 + [Se4]2+([MF6]−)2 in SO2 | 601 | |
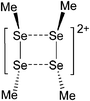
|
[BF4]− | Ox | Me2Se2 + XeF2 + BF3·OEt2 | 82 | |
| [(MeSe)4]2+ | [OTf]− | Ox | Et2Te2 + [NO]+[OTf]− | 602 | |
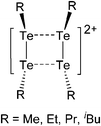
|
[OTf]− | Ox | Et2Te2 + [NO]+[OTf]− | 82 and 602 | |
| Radicals | |||||
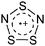
|
[AsF6]− | Ox | S4N4 + [Te4]2+([AsF6]−)2 in SO2 | 600 | |
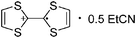
|
[NTf2]− | Ox | TTF + 0.5XeF2 + TMSNTf2 | TTF = Tetrathiafulvalene | 82 |
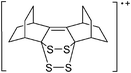
|
[SbCl6]− | Ox | (C8H10)2S2 + SbCl5 | Strong transannular interactions | 603 |
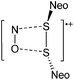
|
[OTf]− | Ox | (NeoS)2 + [NO]+ | Diorgano disulfide–nitrosonium adduct | 604 |
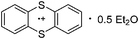
|
[FAl(ORPF)3]− | Ox | C12H8S2 + XeF2 + Al(OC(CF3)3)3 | C12H8S2 = thianthrene | 82 |
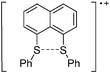
|
[Al(ORPF)4]− | Ox | 1,8-(SPh)2Nap + [NO]+ | S–S-3e–σ-bond Nap = naphthalene | 605 |
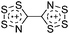
|
[AsF6]− | Ox | S + AsF5 + (CN)2 in SO2 | Traces of Br2 were added | 522 |
| (CNS3˙+)2 | [SbF6]−, [Sb2F11]− | Ox | S + SbF5 + (CN)2 in SO2 | Traces of Br2 were added | 523 |
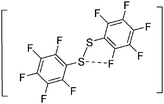
|
[Sb2F11]− | Ox | (C6F5S)2 + SbF5 | 606 | |
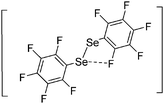
|
[As2F11]− | Ox | (C6F5Se)2 + AsF5 | 606 | |
|
[SbF6]− | Ox | (2,6-Mes2C6H3Ch)2 + [NO]+ | 606 | |
| Oxidation state +II | |||||
| [Me2S-SMe]+ | [SbCl6]− | — | — | 607 | |
| [MeS-S(Me)-SMe]+ | [SbCl6]− | Ox, Lewis | S2Me2 + SbCl5 | =[S3Me3]+ | 607 |
|
[OTf]− | Com | S(OTf)2 + (NR)2C2H2 | Sulfur carbenoid, oxidation state unclear | 528 |
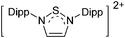
|
[B(C6F5)4]− | Other | [S(NDipp)2C2H2]2+ ([OTf]−)2 + K+[B(C6F5)4]− | Sulfur carbenoid, salt metathesis, oxidation state unclear | 528 |
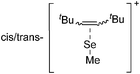
|
[X]− = [SbCl6]−, [BF4]− | Other | [S3Me3]+[X]− + C2H2tBu2 | Thiiranium ion | 608 |
|
[X]− = [BF4]− [PF6]− | Other | [S3Me3]+[X]− + C2tBu2 | Thiirenium ion | 608 |
| [MeSC2tBu2]+·CH2Cl2 | [CHB11Cl11]− | Ox | Me2S2 + C2tBu2 + XeF2 + Me3Si(CHB11Cl11) | 82 | |
|
[B(C6F5)4]− | Ox | Ph2S2 + C2tBu2 + XeF2 + [Me3Si(Tol)]+[B(C6F5)4]− | Thiirenium ion | 82 |
| [MeSe-Se(Me)-SeMe]+ | [SbCl6]− | Ox, Lewis | Se2Me2 + SbCl5 | =[Se3Me3]+ | 607 |
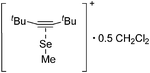
|
[SbCl6]− | Other | [Se3Me3]+[SbCl6]− + C2tBu2 | Selenirenium ion | 526 |
| [PhSeC2Ad2]+·CH2Cl2 | [SbCl6]− | Other | [PhSe]+[SbCl6]− + C2Ad2 | 526 | |
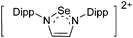
|
[SnCl6]2− | Ox, Lewis | (NDipp)2C2H2 + SnCl2 + SeCl4 | Selenium carbenoid | 529 |
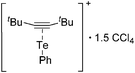
|
[SbF6]− | Other | [PhTe]+[SbF6]− + C2tBu2 | Tellurirenium ion | 526 |
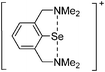
|
[PF6]− | Other | C6H3(CH2NMe2)2SeMe + tBuOCl + K+[PF6]− | 524 | |
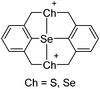
|
[PF6]− | Ox | (C6H3(CH2)2Ch)2Se + [NO]+[PF6]− | 609 | |
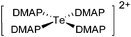
|
[OTf]− | Other | [(Dipp2BIAN)Te]2+ ([OTf]−)2 + DMAP | Ligand exchange | 525 |
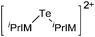
|
[OTf]− | Other | [(Dipp2BIAN)Te]2+ ([OTf]−)2 + 2 iPrIM | Ligand exchange, anions coordinating | 525 |
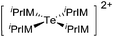
|
[OTf]− | Other | [(Dipp2BIAN)Te]2+ ([OTf]−)2 + iPrIM | Ligand exchange, 4 equivalents of iPrIM | 525 |
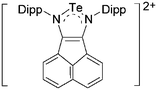
|
[OTf]− | Hal | (Dipp2BIAN)TeI2 + Ag+[OTf]− | Tellurium carbenoid, Dipp2BIAN = 1,4-(2,6-diisopropyl)phenyl-bis(arylimino)acenaphthene | 525 |
| Oxidation state +IV | |||||
| [SF3]+ | [BF4]− | Lewis | SF4 + BF3 | Crystals through sublimation | 610 |
| [SF3]+ | [GeF6]2− | Lewis | SF4 + GeF4 | 611 | |
| [SCl3]+ | [ICl4]− | Ox, Lewis | S + Cl2 + I2 | 612 | |
| [SCl3]+ | [ICl4]− | Ox, Lewis | S + Cl2 + I2 | Second modification | 613 |
| [SCl3]+ | [UCl6]− | Lewis | SOCl2 + UCl5 | 614 | |
| [SCl3]+ | [AlCl4]− | Lewis | SCl4 + AlCl3 | 615 | |
| [SCl3]+ | [SbCl6]− | Ox, Lewis | As4S4 + SbCl5 in SO2 | 531 | |
| [SCl3]+ | [MoOCl4] | Ox | S + MOCl4 | HTS (100 °C), large excess of ICl3 was added | 616 |
| [SBr1.2Cl1.8]+ | [SbCl6]− | Ox, Lewis | S + Br2 + SbCl5 in SO2 | Attempt to prepare [SBr3]+[SbCl6]− | 531 |
| [SBr3]+ | [AsF6]− | Lewis | S + Br2 + AsF5 in SO2 | 617 and 618 | |
| [(SX2)2N]+ (X = F, Cl) | [AsF6]− | Ox | [S2N]+[AsF6]− + X2 in SO2 | 619 | |
| [SeF3]+ | [NbF6]−, [Nb2F11]−, [TaF6]− | Lewis | SeF4 + NbF5 | First structure with a [ChX3]+ cation (Ch = S, Se, Te; X = F, Cl, Br, I) | 620 |
| [SeCl3]+ | [AlCl4]− | Lewis | SeCl4 + AlCl3 in SO2Cl2 | 621 | |
| [SeCl3]+ | [SbCl6]− | Ox, Lewis | As + Se + SbCl5, or SeCl4 + SbCl5 in SO2 | Melt | 531 |
| [SeCl3]+ | [MoOCl4]− | Others | [Se4]2+[MoOCl4]2− in SOCl2 | Decomposition at 150 °C, β-modification | 622 |
| [SeCl3]+ | [AuCl4]− | Lewis | SeCl4 + AuCl3 | 623 | |
| [SeBr3]+ | [AsF6]− | Ox, Lewis | Se + Br2 + AsF5 in SO2 | Small amount of AsF3 was added | 624 |
| [SeBr3]+ | [SbF6]− | Ox, Lewis | [Se4]2+([SbF6]−)2 + Br2 + AsF5 in SO2 | 624 | |
| [SeBr3]+ | [AlBr4]− | Lewis | SeBr4 + AlBr3 | HTS (150 °C) | 625 |
| [SeI3]+ | [AsF6]− | Ox, Lewis | [Se4]2+([SbF6]−)2 + I2 in AsF3 | 617 and 618 | |
| [SeI3]+ | [SbF6]− | Ox, Lewis | Se + I2 + SbF5 in SO2 | ||
| [TeF3]+ | [Sb2F11]− | Lewis | TeF4 + SbF5 | 626 | |
| [TeF3]+ | [SO4]2− | Ox, Lewis | Te + Br2 + AsF5 in SO2 | 531 | |
| [TeCl3]+ | [AlCl4]− | Lewis | TeCl4 + AlCl3 | Monoclinic | 627 |
| [TeCl3]+ | [AlCl4]− | Lewis | S7TeCl2 + AlCl3 | TeCl3[AlCl4] (triclinic) | 531 |
| [TeCl3]+ | [AsF6]− | Lewis | TeCl4 + AsF5 | 531 | |
| [TeCl3]+ | [SbF6]− | Lewis | TeF4 + SbF5 in CH2Cl2 | Solvent decomposition | 531 |
| [TeCl3]+ | [AuCl4]− | Lewis | TeCl4 + AuCl3 | 628 | |
| [TeCl3]+ | [MoCl4O]− | Lewis | TeCl4 + MoOCl3 | HTS (180 °C) | 629 |
| [TeCl3]+ | [MoCl6]2− | Lewis | TeCl4 + MoCl4 | HTS (195 °C) | 630 |
| [TeCl3]+ | [Re2Cl6]− | Lewis, Ox | Te + TeCl4 + ReCl5 | HTS (150 °C), β-modification | 630 |
| [TeCl3]+ | [MoCl6]2−·[Cl]− | Lewis | TeCl4 + MoCl4 | HTS (300 °C) | 631 |
| [TeCl3]+ | [MCl6]− (M = Nb, Ta) | Lewis | TeCl4 + MCl5 | α- and β-modification | 632 |
| [TeCl3]+ | [WCl6]− | Lewis | TeCl4 + WCl6 | 632 | |
| [TeBr3]+ | [AsF6]− | Ox, Lewis | Te + Br2 + AsF5 in SO2 | 624 | |
| [TeBr3]+·1/2Br2 | [AuBr4]− | Ox, Lewis | Te + Au + Br2 | HTS (160 °C) | 633 |
| [TeBr3]+ | [Zr2Br9]− | Other | [Te4]2+[Zr2Br10]2− | Decomposition above 250 °C | 560 |
| [TeBr3]+ | [MBr6]− (M = Ta, W) | Lewis | TeBr4 + MBr5 | 632 | |
| [TeI3]+ | [AsF6]− | Ox, Lewis | Te + I2 + AsF5 in SO2 | 634 | |
| [TeI3]+ | [SbF6]− | Ox, Lewis | Te + I2 + SbF5 in SO2 | 617 and 618 | |
| [TeI3]+ | [AlI4]− | Lewis, Ox | Te + I2 + AlI3 | HTS (150 °C) | 625 |
| [TeI3]+ | [MI4]−, (M = Ga, In) | Lewis, Ox | Te + M + I2 | HTS (350 °C) | 635 |
| [TeI3]+·1/2SO2 | [AsF6]− | Ox, Lewis | Te + I2 + AsF5 in SO2 | Hemisolvate of [TeI3]+[AsF6]− | 636 |
| [TeI3]+ | [Al(ORPF)4]− | Salt | TeX4 + Ag+[Al(ORPF)4]− | Good solubility | 637 |
| [TePh3]+ | [B(C6F5)4]− | Other | [TePh5]+[B(C6F5)4]− | Thermal decomposition at 150 °C | 532 |
| [Te(C6F5)3]+ | [OTf]− | Other | Te(C6F5)4 + TMSOTf | 533 | |
| [Te(N3)3]+ | [SbF6]− | Ox | [Te4]2+([SbF6]−)2 + KN3 in SO2 | Side products may be explosive! | 534 |
| [F(TeCl3)2]+ | [Sb(OTeF5)6]− | Ox, Lewis | TeBr4 + Ag+[Sb(OTeF5)6]− in SO2ClF | 28 | |
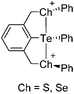
|
[BF4]− | Ox | (C6H3(CH2ChPh)2)TePh + [NO]+[BF4]− | 638 | |
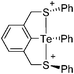
|
[OTf]− | Ox | (C6H3(CH2SPh)2)TePh + tBuOCl + O(Tf)2 | 638 | |
| Oxidation state +VI | |||||
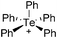
|
[B(C6F5)4]− | Hal | TePh5Cl + Ag+[OTf]− + Li+[B(C6F5)4]− | 532 | |
| [TePh5]+ | [ClO4]− | Hal | TePh5Cl + Ag+[ClO4]− | 535 | |
| [TePh5]+⋯MeCN | [B(ArCF3)4]− | Hal | TePh5Cl + Ag+[OTf]− + [B(ArCF3)4]− | 535 | |
| Protonated cations | |||||
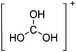
|
[AsF6]− | Prot | OC(OTMS)2 + HF/AsF5 | 537 | |
|
[Ge3F16]4− | Prot | Me2SO + HF/GeF4 | 639 | |
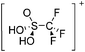
|
[SbF6]− | Prot | OTf2 + HF/SbF5 | 536 | |

|
[SbF6]− | Prot | SO2(OTMS)2 + HF/SbF5 | 538 | |
Group 17 cations
The electronegative halogen atoms are strongly oxidizing and have high ionization energies as well as electron affinities. This makes halogen cations good electrophiles, which need very oxidation-resistant WCAs to be stabilized in condensed phases. Concerning the synthesis of these highly reactive compounds, there are many similarities to the noble gas cations. Almost every halogen cation, for which a crystal structure is known, was synthesized through a halide abstraction by a strong Lewis acid like MF5 (M = As, Sb). The starting materials are normally neutral interhalogen compounds like ClF3, BrF5, IF7, I2Cl6, or IBr, and the majority of the obtained cations contains halogen atoms in oxidation state +III, +V and +VII (Table 7).Fluorine cations
Because of the high electronegativity and ionization potential of fluorine, it would be very difficult to oxidize it and obtain actual fluorine cations in the condensed phase. By contrast, in the gas phase this is possible.641 Some bulk cationic compounds contain fluorine, but it is very unlikely that the positive charge is actually localized on the fluorine atom. Such compounds like for example the in Fig. 94 shown compound that was published as a formal disilylfluoronium ion are discussed in the chapters of the element, which has a larger positive charge density (here silicon).642It should be mentioned that also electrophilic “F+-” or “N–F-” reagents like “Selectfluor” belong to this class of compounds that are very useful for organic transformations and compatible with solvents like CH2Cl2.643
Chlorine, bromine and iodine cations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 211 and the only measurable crystals of a [Br3]+ salt were obtained from a 20 year old [BrF2]+[AsF6]− solution.645 Iodine has five known cations. [I2]+, [I3]+ and [I5]+ are isostructural to the lighter homologues and can all be obtained by oxidation from I2 with the strong Lewis acids MF5 (M = As, Sb). [I4]2+ was synthesized through the entropically unfavorable dimerization of the paramagnetic [I2]+˙ radical cation at low temperature and can be described as an rectangular planar diamagnetic π*–π*-complex (see below: π*–π*-complexes of group 17) (Fig. 95).
211 and the only measurable crystals of a [Br3]+ salt were obtained from a 20 year old [BrF2]+[AsF6]− solution.645 Iodine has five known cations. [I2]+, [I3]+ and [I5]+ are isostructural to the lighter homologues and can all be obtained by oxidation from I2 with the strong Lewis acids MF5 (M = As, Sb). [I4]2+ was synthesized through the entropically unfavorable dimerization of the paramagnetic [I2]+˙ radical cation at low temperature and can be described as an rectangular planar diamagnetic π*–π*-complex (see below: π*–π*-complexes of group 17) (Fig. 95).
 | ||
| Fig. 98 [I(NCMe)2]+ cation in [I(NCMe)2][AsF6] with iodine in the formal oxidation state +I.649 | ||
In case of bromine and iodine, also some new examples were synthesized during the last 20 years. The stable cyclic bromonium and iodonium ions of sterically hindered olefins (Fig. 101) were stabilized by the [OTf]− anion and can be seen as stable intermediates of the halogenation of olefins.656
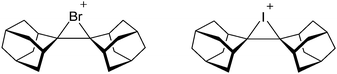 | ||
| Fig. 101 Stable [OTf]− salts of cyclic bromonium and iodonium ions.656 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 and [OBrF2]+ (Fig. 102).659
658 and [OBrF2]+ (Fig. 102).659
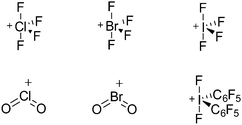 | ||
| Fig. 102 Structurally characterized halogen cations with halogen atoms in oxidation state +V.657,660–662 | ||
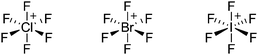 | ||
| Fig. 103 Structurally characterized halogen cations with halogen atoms in oxidation state +VII.660 | ||
| Cation | Anion | Classa | Synthesis | Comment | Ref. |
|---|---|---|---|---|---|
| a Classification according to the introduction (Table 2): Lewis = Lewis acid halogen bond heterolysis, Ox = oxidation, Com = complexation reaction, Other = all other reactions not classified. | |||||
| Homopolyatomic cations | |||||
| [Cl3]+ | [AsF6]− | Lewis | [Cl2F]+[AsF6]− + Cl2 + AsF5 | 42 | |
| [Cl3]+ | [X]− = [SbF6]−, [Sb2F11]−, [Sb3F16]− | Other | [Cl2O2]+[X]− + Cl2 in HF at RT | 42 | |
| [Br2]+ | [Sb3F16]− | Ox | Br2 + HSO3F/SbF5/3SO3 | 663 | |
| [Br3]+ | [AsF6]− | Other | [BrF2]+[AsF6]− decomposition | Store [BrF2]+[AsF6]− for 20 years (!) | 645 |
| [Br5]+ | [MF6]− (M = As, Sb) | Ox | [XeF]+[MF6]− + Br2 | 211 | |
| [I2]+ | [Sb2F11]− | Ox | I2 + SbF5 | 15 | |
| [I3]+ | [AsF6]− | Ox | I2 + AsF5 | 15 | |
| [I5]+ | [AsF6]− | Ox | I2 + AsF5 | 664 | |
| [I15]3+ | [SbF6]− | Ox | I2 + SbF5 | 15 | |
| Metal–nonmetal-cluster complexes | |||||
| [Ag(Cl2)]+ | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + Cl2 | Only stable at low temperature | |
| [Ag(Br2)]+ | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + Br2 | ||
| [Ag2(I2)x]2+ (x = 1, 4, 6) | [Al(ORPF)4]− | Com | Ag+[Al(ORPF)4]− + I2 | ||
| [Ag(I2)]nn+ | [MF6]− (M = Sb, As) | Com | Ag+[MF6]− + I2 | First complex with halogen as donor | 646 |
| π*–π*-complexes | |||||
| [Cl4]+ | [IrF6]− | Ox | Cl2 + IrF6 | Radical, homo-polyatomic cation | 644 |
| [Cl2O2]+ | [X]− = [SbF6]−, [Sb2F11]− | Com | [O2]+ + Cl2 | 42 | |
| [I4]2+ | [MF6]− (M = As, Sb), and [Sb3F14]−/[SbF6]− | Com | 2[I2]+ | Also homopoly-atomic cation | 665 |
| Oxidation state +I | |||||
| [I(NCMe)2]+ | [AsF6]− | Other | [I3]+ + MeCN | [I3]+ as I+ donor | 649 |
| Oxidation state +III | |||||
| [ClF2]+ | [AsF6]− | Lewis | ClF3 + AsF5 | 666 | |
| [ClF2]+ | [SbF6]− | Lewis | ClF3 + SbF5 | 667 | |
| [ClF2]+ | [RuF6]− | Lewis | ClF3 + RuF5 | 668 | |
| [ClMe2]+[ClEt2]+ | [CHB11Cl11]− | Prot | H(CHB11Cl11) + RCl (R = Me, Et) | Protonation of RCl | 655 |
| [BrF2]+ | [SbF6]− | Lewis | BrF3 + SbF5 | 669 | |
| [Br(C6F5)2]+ | [BF4]− | Lewis | BrF3 + (C6F5)2BF | 670 | |
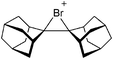
|
[OTf]− | Lewis | C20H28 + Br2 + MeOTf |
Stable bromonium ion
C20H28 = adamantyli-deneadamantane |
656 |
| [ICl2]+ | [SbF6]− | Lewis | I2Cl6 + SbF5 | 671 | |
| [IBr2]+ | [Sb2F11]− | Lewis | IBr + SbF5 | 672 | |
| [IBr0.75Cl1.25]+ | [SbCl6]− | Lewis | IBr + Cl2 + SbF5 | 672 | |
| [I3Cl2]+ | [X]− = [SbCl6]−, [AlCl4]− | Lewis, other | I2 + SbCl5 | 652–654 | |
| [I3Br2]+ | [SbCl6]− | Lewis, other | I2 + SbCl5 | 653 | |
| [I(C6F5)R]+ | [BF4]− | Lewis | I(C6F5)F2 + RBF2 R = C6H5, o-C6H4F, m-C6H4F, p-C6H4F, 2,4,6-C6H2F3, C6F5 | Strong interactions with the anions | 673 |
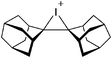
|
[OTf]− | Lewis | C20H28 + I2 + Ag+[OTf]− |
Stable iodonium ion
C20H28 = adamantyli-deneadamantane |
656 |
| Oxidation state +V | |||||
| [ClF4]+ | [SbF6]− | Lewis | ClF5 + SbF5 | 661 | |
| [ClO2]+ | [SbF6]− | Lewis | ClO2F + HF/SbF5 | 674 | |
| [ClO2]+ | [Sb2F11]− | Lewis | ClO2F + 2SbF5 | Also an oxidation product of [ClF2]+ | 675 |
| [ClO2]+ | [X]− = [BF4]−, [GeF5]− | Lewis | ClO2F + BF3, GeF4 | 611 and 676 | |
| [ClO2]+ | [ClO4]− | Ox | ClO2 + O3 | Cl2O6 | 677 |
| [ClO2]+ | [RuF6]− | Lewis Ox | ClO2F + 2RuF5 ClF3 + HF/RuO4 | 668 | |
| [BrF4]+ | [Sb2F11]− | Lewis | BrF5 + 2SbF5 | 662 and 678 | |
| [BrO2]+ | [SbF6]− | Lewis, other | BrO3F + SbF5 | Reduction of BrVII | 674 |
| [IF4]+ | [SbF6]− | Lewis | IF5 in HF/SbF5 | 662 and 679 | |
| [IF4]+ | [Sb2F11]− | Lewis | IF5 + 2HF/SbF5 | 662 and 680 | |
| [I(C6F5)2F2]+ | [BF4]− | Lewis | C6F5IF4 + C6F5BF2 | 657 | |
| Oxidation state +VII | |||||
| [ClF6]+ | [Sb2F11]− | Ox, Lewis | ClF5 + F2 + SbF5 | 660 | |
| [BrF6]+ | [Sb2F11]− | Ox, Lewis | BrF5 + F2 + SbF5 | 660 | |
| [IF6]+ | [Sb2F11]− | Lewis | IF7 + SbF5 | 660 | |
Group 18 cations
Due to the high ionization potential of the noble gases, their cations all need weakly coordinating and very oxidation resistant anions to be stabilized. The history of noble gas compounds started in the early 1960s, when Bartlett obtained “[Xe]+[PtF6]−” from a reaction of [O2]+[PtF6]− and xenon – the noble gas with the lowest ionization potential. Over the next decades the composition of this product stayed unclear. In 2000 a very comprehensive review about the nature of the product was published with the result that it is very likely a [XeF]+ salt of a polymeric (weakly coordinating) [PtF5]n anion.681 All of the hitherto known and structurally characterized noble gas cations are included with Table 13.| Cation | Anion | Class.a | Synthesis | Comment | Ref. |
|---|---|---|---|---|---|
| a Classification according to the introduction (Table 2): Lewis = Lewis acid halogen bond heterolysis, Com = complexation reaction, other = all other reactions not classified. | |||||
| Homopolyatomic cations | |||||
| [Xe2]+ | [Sb4F21]− | Lewis | [XeF]+/Xe + SbF5 | First homopoly-atomic cation | 27 |
| [Xe4]+ | [SbxF5x+1]− | Lewis | [XeF]+[Sb2F11]−, SbF5 + Xe | No X-ray | 696 |
| Metal–nonmetal-cluster complexes | |||||
| [AuXe4]2+ | [Sb2F11]− | Lewis, Com | AuF3, Xe in HF/SbF5 | First xenon metal complex, gold(II) 2 modifications: triclinic and tetragonal | 695 and 697 |
| [trans-AuXe2]2+ | [SbF6]− | Com | HAuCl4, XeF2, Xe in HF/SbF5 | Gold(II) | 695 |
| [cis-AuXe2]2+ | [Sb2F11]− | Com | [AuXe4]2+([Sb2F11]−)2−Xe at RT | Gold(II) | 695 |
| [trans-AuXe2F]2+ | [SbF6]− + [Sb2F11]− | Com | Au, XeF2, Xe in HF/SbF5 | Gold(III) | 695 |
| [Au2Xe2F]3+ | [SbF6]− | Com | Au, XeF2, Xe in HF/SbF5 | Gold(II) | 695 |
| [(F3As)AuXe]+ | [Sb2F11]− | Com | AuF3, Xe, AsF3 in HF/SbF5 | 698 | |
| [HgXe]2+ | [SbF6]− + [Sb2F11]− | Com | HgF2, Xe in HF/SbF5 | 698 | |
| Oxidation state +II | |||||
| [KrF]+ | [MF6]− (M = As, Sb, Bi) | Lewis | KrF2 + MF5 | First krypton cation | 699 |
| [KrF]+ | [AuF6]− | Lewis | KrF2 + Au | 682 | |
| [Kr2F3]+·KrF2, [Kr2F3]+·1/2KrF2, [Kr2F3]+·[KrF]+ | [SbF6]−[SbF6]−[AsF6]− | Lewis | KrF2 + MF5 | 699 | |
| [XeF]+ | [Sb2F11]− | Lewis | XeF2 + 2SbF5 | 687 | |
| [XeF]+ | [RuF6]− | Lewis | XeF2 + RuF5 | 700 | |
| [XeF]+ | [AsF6]− | Lewis | XeF2 + AsF5 | 701 | |
| [XeF]+ | [N(SO2F)2]− | Lewis | XeF2 + HN(SO2F)2 | First Xe–N bond, strong interaction with the anion | 702 |
| [XeF]+·HF | [Sb2F11]− | Lewis | XeF2 + 2SbF5 | 27 | |
| [XeF]+ | [X]− = [AsF6]−, [SbF6]−, [Sb2F11]−, [BiF6]−, [Bi2F11]− | Lewis | XeF2 + AsF5, SbF5, BiF5 | Better structures for [XeF]+[X]−: [X]− = [MF6]−, [SbF6]−, [Sb2F11]− | 691 |
| [Xe2F3]+ | [AsF6]− | Lewis | 2XeF2 + AsF5 | Monoclinic structure | 703 and 704 |
| [Xe2F3]+ | [AuF6]− | Lewis | XeF2 + AuF5 | 686 | |
| [Xe2F3]+ | [MF6]− (M = As, Sb) | Com | [XeF]+ as starting material | [Xe2F3]+[AsF6]− trigonal structure | 705 |
| [Xe(N(SO2F)2)]+ | [Sb3F16]− | Lewis | 3 step synthesis with AsF5 and SbF5 | 706 | |
| [XeC6F5]+ | [(F5C6)2BF2]− | Lewis | XeF2 + B(C6F5)3 | First Xe–C bond | 693 |
| [(MeCN)XeC6F5]+[XeC6F5]+ | [BX]− (X = CF3, C6F5) [BX]− (X = CF3, CN) | Lewis, Com | XeF2 + C6F5BF2 | Salt metatheses with [XeC6F5]+[BF4]− | 707 |
| [(C6F5Xe)2Cl]+ | [AsF6]− | Other | [XeC6F5]+ + TMSCl | First Xe–Cl bond | 708 |
| [XeOChF5]+ (Ch = Se, Te) | [AsF6]− | Lewis | FXeOChF5 + AsF5 | First Xe–O bond | 709 |
| [XeOTeF5]+·SO2ClF | [Sb(OTeF5)6]− | Other | Xe(OTeF5)2 + Sb(OTeF3)3 | OTeF5− abstraction | 710 |
| [XeCl]+ | [Sb2F11]− | Other | [XeF]+ + [Cl]− | Ligand exchange | 711 |
| [XeN(H)TeF5]+ | [AsF6]− | Lewis | [F5TeNH3]+[AsF6]− + XeF2 | First Xe–N(sp3) bond | 712 |
| [(F3SN)XeF]+ | [AsF6]− | Com | F3SN + [XeF]+ | First Xe–N(sp) bond | 713 |
| [XeNSF4]+ | [AsF6]− | Other | In solid state and HF/BrF5 solution | Rearrangement of [F3SNXeF]+[AsF6]− | 714 |
| [(F3SN)XeNSF4]+ | [AsF6]− | Com | [F3SNXeF]+ + F3SN | 715 | |
| [Xe3OF3]+ | [MF6]− (M = As, Sb) | Other | H2O + XeF2/[XeF]+ | Hydrolysis to XeFOH followed by a reaction with [Xe2F3]+ | 716 |
| Oxidation state +IV | |||||
| [XeF3]+ | [Sb2F11]− | Lewis | XeF4 + 2SbF5 | 717 | |
| [XeF3]+ | [BiF6]− | Lewis | XeF4 + BiF5 | 718 | |
| [XeF3]+ | [Sb2F11]− | Lewis | XeF4 + SbF5 | Better structure | 691 |
| [XeF3]+·HF [H5F4]+·2([XeF3]+·HF) [XeF3]+ | [Sb2F11]−[SbF6]·2[Sb2F11]−[SbF6]− | Lewis | XeOF2·xHF + HF/SbF5 | 719 | |
| Oxidation state +VI | |||||
| [XeF5]+ | [PtF6]− | Lewis | XeF6 + PtF5 | First xenon cation structure | 688 and 689 |
| [XeF5]+ | [AsF6]− | Lewis | XeF6 + AsF5 | 704 | |
| [XeF5]+ | [SbF6]− | Ox | [XeF]+[SbF6]− + F2 in aHF | 692 | |
| [XeF5]+ | [Sb2F11]− | Other | [XeF5]+[SbF6]− | Crystals from a O2SbF6/XeF5SbF6 mixture | 692 |
| [XeF5]+ | [RuF6]− | Lewis | XeF6 + RuF5 | 700 | |
| [XeF5]+ | [PdF6]2− | Lewis | 2XeF6 + PdF4 | 720 | |
| [XeF5]+ | [Ti4F19]3− | Lewis | XeF6 + TiF4 | XeF6 from XeF2, F2 and UV radiation | 721 |
| [XeF5]+ | [m-F(OsO3F2)2]−[OsO3F2]− | Lewis | XeF6 + (OsO3F2)∞ | 690 | |
| [XeF5]+ | [Cu(SbF6)3]− | Other | [XeF5]+[SbF6]− + Cu+[SbF6]− in aHF | Anion exchange | 43 |
| [XeF5]+ | [AgF4]− | Lewis | AgF2 + KrF2 + XeF6 in aHF | 722 | |
| [XeF5]+ | [AuF4]− | Lewis | XeF6 + BrF3 AuF3 | 722 | |
| [XeF5]+·XeF2·[XeF5]+·2XeF2 [XeF5]+·1/2XeF2 | [AsF6]−[AsF6]−[AsF6]− | Com | XeF2 + [XeF5]+[AsF6]− | 723 | |
| [XeF5]+·XeOF4 | [SbF6]− | Lewis | XeF6 + [H3O]+[SbF6]− | 694 | |
| [XeF5]+·NO2+ | [SbF6]− | Other | [XeF5]+[SbF6]− + [NO2]+[SbF6]− | 43 | |
| [Xe2F11]+ | [AuF6]− | Lewis | 2XeF6 + AuF5 | 724 | |
| [Xe2F11]+ | [OsO3F2]− | Lewis | XeF6 + (OsO3F2)∞ | 690 | |
| [XeO2F]+[F(XeO2F)2]+ | [MF6]− (M = As, Sb) [AsF6]− | Lewis | XeO2F2 + MF5 (M = As, Sb) | α- and β-modification of [XeO2F]+[SbF6]− | 694 |
Krypton cations
The only other noble gas besides xenon, for which cations are known, is krypton. The oxidation state +II is the only one known yet. The diversity of the amount of compounds and the number of hitherto realized Kr–X bonds is much smaller. Only two different fluorine-containing cations are known ([KrF]+ and [Kr2F3]+) and some nitrile complexes, which can be described as Kr–N compounds. [KrF]+ is a very strong oxidative fluorinating reagent682 and made is possible to synthesize [XeF5]+ from Xe,683 [ClF6]+ from ClF5,684 [BrF6]+ from BrF5685 and [O2]+ from O2.686 There are only seven crystal structures of krypton cations in the literature and five of them with the classical [AsF6]− and [SbF6]− anions.Xenon cations
The lower ionization potential calls for a richer chemistry of xenon. Thus, since the birth of noble gas chemistry, a multitude of different xenon cations containing the element in the oxidation state II, IV and VI were synthesized.Most of the syntheses use Lewis acids to abstract fluoride from the neutral xenon fluoride (XeF2, XeF4, XeF6). Most structures contain the conjugated weakly coordinating anions of these Lewis acids ([MF6]−, M = As, Sb, Au, Ru). Another mentionable approach is the reaction of XeF2 with the Lewis acid B(C6F5)3, which led to the [F5C6Xe]+ cation containing the first Xe–C bond and the unsymmetric anion [(F5C6)2BF2]−.693 Through the use of the dioxydifluoride XeO2F2 as starting material, it was also possible to obtain the mixed cation [XeO2F]+ or the fluoride bridged [F(XeO2F)2]+ (Fig. 107).694
Only the [AuXe4]2+ dication exists in a truly ionic structure with two [Sb2F11]− counterions in the lattice (Fig. 109).
Conclusion
Sparked by the availability of new WCAs and new WCA starting materials in combination with novel concepts like FLP and others, the number of rPBC exploded over the last one to two decades. Noteworthy additions were found for each p-block element and, despite their quite high moisture and air sensitivity, true applications of rPBC salts emerged.Where will this lead to over the next one or two decades…? To our understanding the blue sky synthesis of rPBC salts barely accessible with good/novel WCAs in combination with suitable media will continue to function as an “eye-opener” of what is possible. Many surprising discoveries will force us to sharpen our use of bonding concepts or lead to novel applications. It is often the combination of structural knowledge (“Wow, this crazy cation is stable…? I would have never thought so.”) that leads to the right moment of wonder and then inspiration (“Hm, if this cation is really straight forward accessible, one could use its electrophilic/acidic/oxidizing/activating properties in application XY”). In the 21st century, it is our duty as creative scientists to use this potential from fundamentals to the first application. Do not hesitate to really seek for application of your rPBC salt, as rarely others will pick up on these ideas, since the activation barrier for synthesizing a to this application group unknown rPBC is simply too high. So do not give up until you have demonstrated a possible application – yourself or through collaborations – to a level that others will continue. And on the other hand this compilation of rPBC should encourage application based groups to identify interesting cations that may have an application. Contact the people, the chances are very good that through an informal collaboration showing a proof-of-principle new and relevant application areas may be developed.
In this respect, we are looking forward to all the scientific creativity that is breaking loose, and to realize what potentially could be done with the rPBC. This is an integral element of innovation and the justification for preparing blue sky or simply beautiful and esoteric compounds.
Abbreviations
| CN | Coordination number |
| DFT | Density functional theory |
| ε r | Relative permittivity of a solvent (static dielectric constant) |
| FLP | Frustrated Lewis pairs |
| IL | Ionic liquid |
| n.a. | Not available |
| rPBC | Reactive p-block cations |
| WCA | Weakly coordinating anion |
| ArCF3 | 3,5-(CF3)2C6H3 |
| ArCl | 3,5-Cl2-C6H3 |
| 9BBN | 9-Bora[3.3.1]bicyclononane |
| bipy | 1,2-Bipyridine |
| BOX | Bis(oxazoline) |
| CatBH | Catecholborane/1,3,2-benzodioxaborole |
| COD | 1,5-Cyclooctadiene |
| Cp | C5H5 |
| Cp′ | C5Me4H |
| Cp* | C5Me5 |
| Cy | Cyclohexyl |
| DDP | 2-(DIPP)amino-4-(Dipp)imino-2-pentene |
| Dipp | 2,6-iPr2-C6H3 |
| DMAP | 4-Dimethylaminopyridine |
| DMeOPrPE | 1,2-(Bis(dimethoxypropyl)-phosphino)ethane |
| DMH | 1,1-Me2N2H4 |
| Dmp | 2,6-Dimethyl-phenyl |
| dmpe | 1,2-Bis(dimethylphosphino)ethane |
| Do | Donor |
| DPE | 1,2-Diphenylethane |
| dppe | 1,2-Bis(diphenylphosphino)ethane |
| DTBMP | 2,6-Di-tert-butyl-4-methylpyridine |
| dtbpy | 4,4′-Di-tert-butyl-2,2′-bipyridyl |
| Et | Ethyl |
| Fc | Ferrocenyl |
| FP | CpFe(CO)2 |
| FP′ | Cp′Fe(CO)2 |
| FP* | Cp*Fe(CO)2 |
| hppH | 1,3,4,6,7,8-Hexahydro-2H-pyrimido-[1,2-a]pyrimidine |
| IMe | 1,3-Bis(methyl)imidazol-2-ylidene |
| IMes | 1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene |
| iPr | iso-Propyl |
| iPr2-ATI | N,N′-Diisopropylaminotroponiminate |
| IPr | 1,3-Bis(2,6-diisopropylphenyl)imidazol-2-ylidene |
| ItBu | 1,3-Bis(tert-butyl)imidazol-2-ylidene 3,5-lutidine 3,5-dimethylpyridine |
| Me | Methyl |
| Me4-cyclam | N,N′,N′′,N′′′-Tetramethyl-1,4,8,11-tetraazacyclotetradecane |
| Mes | 2,4,6-Me3C6H2 |
| Me3SiSpTol | 1-SSiMe3-4-Me-C6H4 |
| Me3-tacn | N,N′,N′′-Trimethyl-1,4,7-triaza-cyclononane |
| nacnac | (NMesCMe)2CH |
| NBD | 2,5-Norbornadiene |
| NPPh | 2,5-Bis(2-pyridyl)-1-phenylphosphole |
| 1-MIM | N-Methylimidazole |
| m-TP | meta-Terphenyl |
| OSSO | trans-1,2-Cyclooctanediyl-bridged[OSSO]-type bis(phenolate) |
| ORPF | –OC(CF3)3 |
| ORHT | –OC(CH3)(CF3)2 |
| ORHF | –OC(H)(CF3)2 |
| ORMeF | –OC(CH3)(CF3)2 |
| Ph | –C6H5 |
| Phen | 1,10-Phenanthroline |
| 4-Pic | 4-Methylpyridine |
| Pip | Piperidyl |
| PMAF | Pentamethylazaferrocene |
| pmdta | N,N,N′,N′,N′′-Pentamethyldiethylenetriamine |
| PNP | Bis(2-iPr2P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) -Me-phenyl)amido -Me-phenyl)amido |
| Py | Pyridine |
| Pytsi | C(SiMe3)2SiMe2(2-C5H4N) |
| p-Xyl | para-Xylene |
| R | Typical univalent organic residue |
| Salen | N,N′-Ethylenebis(2-hydroxyphenyl)imine |
| SalenCF3 | N,N′-Ethylenebis(2-hydroxy-2-(CF3)2-ethyl)imine |
| Salomphen | N,N′-(4,5-Dimethyl)phenylene-bis(2-hydroxyphenyl)imine |
| Salpen | N,N′-Propylenebis(2-hydroxyphenyl)imine |
| Sch | Tridentate Schiff base |
| SubPc | Subphthalocyanine |
| tacn | 1,4-iPr2-1,4,7-Triaza-cyclononane |
| t Bu | tert-Butyl |
| Tf | –SO2CF3 |
| THF | Tetrahydrofuran |
| timtmbtBu | 1,3,5-{Tris(3-tert-butylimidazol-2-ylideno)methyl}-2,4,6-trimethylbenzene |
| TMM | η4-C(CH2)3 |
| Tol | Toluene |
| Tipp | 2,4,6-iPr3-C6H2 |
| X | Halogen |
Acknowledgements
This work was supported by the Albert-Ludwigs-Universität Freiburg, the ERC in the grant UniChem, and by the DFG in the Normalverfahren.References
- D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 2000, 100, 39 CrossRef CAS PubMed.
- G. C. Welch, R. R. San Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124 CrossRef CAS PubMed; D. W. Stephan, Org. Biomol. Chem., 2008, 6, 1535 Search PubMed.
- C. E. Anderson, H. Braunschweig and R. D. Dewhurst, Organometallics, 2008, 27, 6381 CrossRef CAS.
- D. Vidovic, G. A. Pierce and S. Aldridge, Chem. Commun., 2009, 1157 RSC.
- H. Braunschweig, R. D. Dewhurst and V. H. Gessner, Chem. Soc. Rev., 2013, 42, 3197 RSC.
- G. A. Olah, Onium ions, Wiley, New York, 1998 CrossRef CAS; C. A. Reed, Acc. Chem. Res., 1998, 31, 325 CrossRef CAS.
- M. R. Lichtenthaler, A. Higelin, A. Kraft, S. Hughes, A. Steffani, D. A. Plattner, J. M. Slattery and I. Krossing, Organometallics, 2013, 32, 6725 CrossRef CAS.
- M. R. Lichtenthaler, S. Maurer, R. J. Mangan, F. Stahl, F. Mönkemeyer, J. Hamann and I. Krossing, Chem. – Eur. J., 2015, 21, 471 CrossRef.
- M. R. Lichtenthaler, S. Maurer, R. J. Mangan, F. Stahl, F. Mönkemeyer, J. Hamann and I. Krossing, Chem. – Eur. J., 2015, 21, 157 CrossRef PubMed.
- Y. Sarazin and J.-F. Carpentier, Chem. Rev., 2015, 115, 3564 CrossRef CAS PubMed.
- T. A. Engesser and I. Krossing, Coord. Chem. Rev., 2013, 257, 946 CrossRef CAS.
- S. Aldridge, C. Jones, T. Gans-Eichler, A. Stasch, D. L. Kays, N. D. Coombs and D. J. Willock, Angew. Chem., Int. Ed., 2006, 45, 6118 CrossRef CAS PubMed.
- I. Krossing and I. Raabe, Angew. Chem., Int. Ed., 2004, 43, 2066 CrossRef CAS PubMed.
- J. Cullinane, A. Jolleys and F. S. Mair, Dalton Trans., 2013, 42, 11971 RSC.
- S. H. Strauss, Chem. Rev., 1993, 93, 927 CrossRef CAS.
- C. A. Reed, Acc. Chem. Res., 1998, 31, 133 CrossRef CAS.
- M. R. Lichtenthaler, F. Stahl, D. Kratzert, L. Heidinger, E. Schleicher, J. Hamann, D. Himmel, S. Weber and I. Krossing, Nat. Commun., 2015, 6, 8288 CrossRef CAS PubMed.
- S. Körbe, P. J. Schreiber and J. Michl, Chem. Rev., 2006, 106, 5208 CrossRef PubMed.
- H. Schmidbaur and A. Schier, Organometallics, 2008, 27, 2361 CrossRef CAS.
- C. Knapp, in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 651–679 Search PubMed.
- I. Krossing, in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 681–705 Search PubMed.
- E. Y.-X. Chen and S. J. Lancaster, in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 707–754 Search PubMed.
- P. Malinowski, A. Kraft, J. Schaefer and I. Krossing, 2015, invited review by Angew. Chem., to be submitted within 2015.
- K.-C. Kim, C. A. Reed, D. W. Elliott, L. J. Mueller, F. Tham, L. Lin and J. B. Lambert, Science, 2002, 297, 825 CrossRef CAS PubMed.
- S. M. Ivanova, S. V. Ivanov, S. M. Miller, O. P. Anderson, K. A. Solntsev and S. H. Strauss, Inorg. Chem., 1999, 38, 3756 CrossRef CAS; A. J. Lupinetti, M. D. Havighurst, S. M. Miller, O. P. Anderson and S. H. Strauss, J. Am. Chem. Soc., 1999, 121, 11920 CrossRef.
- B. T. King and J. Michl, J. Am. Chem. Soc., 2000, 122, 10255 CrossRef CAS.
- T. Drews and K. Seppelt, Angew. Chem., Int. Ed., 1997, 36, 273 CrossRef CAS.
- T. S. Cameron, I. Dionne, I. Krossing and J. Passmore, Solid State Sci., 2002, 4, 1435 CrossRef CAS.
- T. Köchner, T. A. Engesser, H. Scherer, D. A. Plattner, A. Steffani and I. Krossing, Angew. Chem., Int. Ed., 2012, 51, 6529 CrossRef PubMed.
- J. M. Slattery, A. Higelin, T. Bayer and I. Krossing, Angew. Chem., Int. Ed., 2010, 49, 3228 CrossRef CAS PubMed.
- R. J. Wehmschulte, Angew. Chem., Int. Ed., 2010, 49, 4708 CrossRef CAS PubMed.
- C. Reus and M. Wagner, Nat. Chem., 2014, 6, 466 CrossRef CAS PubMed.
- Y. Shoji, N. Tanaka, K. Mikami, M. Uchiyama and T. Fukushima, Nat. Chem., 2014, 6, 498 CrossRef CAS PubMed.
- J. B. Lambert, L. Lin, S. Keinan and T. Müller, J. Am. Chem. Soc., 2003, 125, 6022 CrossRef CAS PubMed.
- W. W. Wilson, A. Vij, V. Vij, E. Bernhardt and K. O. Christe, Chem. – Eur. J., 2003, 9, 2840 CrossRef CAS.
- R. Anulewicz-Ostrowska, T. Kliś, D. Krajewski, B. Lewandowski and J. Serwatowski, Tetrahedron Lett., 2003, 44, 7329 CrossRef CAS.
- D. Kratzert, J. J. Holstein and I. Krossing, J. Appl. Crystallogr., 2015, 48, 933 CAS.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- S. V. Ivanov, J. A. Davis, S. M. Miller, O. P. Anderson and S. H. Strauss, Inorg. Chem., 2003, 42, 4489 CrossRef CAS PubMed.
- C. Bolli, J. Derendorf, C. Jenne, H. Scherer, C. P. Sindlinger and B. Wegener, Chem. – Eur. J., 2014, 20, 13783 CrossRef CAS PubMed.
- M. Wegener, F. Huber, C. Bolli, C. Jenne and S. F. Kirsch, Chem. – Eur. J., 2015, 21, 1328 CrossRef CAS PubMed.
- T. Drews, W. Koch and K. Seppelt, J. Am. Chem. Soc., 1999, 121, 4379 CrossRef CAS.
- Z. Mazej and E. Goreshnik, Eur. J. Inorg. Chem., 2015, 1453 CrossRef CAS.
- R. D. Bolskar, R. S. Mathur and C. A. Reed, J. Am. Chem. Soc., 1996, 118, 13093 CrossRef CAS.
- S. M. Ivanova, B. G. Nolan, Y. Kobayashi, S. M. Miller, O. P. Anderson and S. H. Strauss, Chem. – Eur. J., 2001, 7, 503 CrossRef CAS.
- I. Krossing, Chem. – Eur. J., 2001, 7, 490 CrossRef CAS.
- Z. Xie, T. Jelinek, R. Bau and C. A. Reed, J. Am. Chem. Soc., 1994, 116, 1907 CrossRef CAS.
- P. Jutzi, C. Müller, A. Stammler and H.-G. Stammler, Organometallics, 2000, 19, 1442 CrossRef CAS.
- G. Santiso-Quiñones, A. Higelin, J. Schaefer, R. Brückner, C. Knapp and I. Krossing, Chem. – Eur. J., 2009, 15, 6663 CrossRef PubMed.
- J. Schaefer, A. Steffani, D. A. Plattner and I. Krossing, Angew. Chem., Int. Ed., 2012, 51, 6009 CrossRef CAS PubMed.
- A. G. Massey and A. J. Park, J. Organomet. Chem., 1964, 2, 245 CrossRef CAS.
- A. E. Ashley, T. J. Herrington, G. G. Wildgoose, H. Zaher, A. L. Thompson, N. H. Rees, T. Krämer and D. O’Hare, J. Am. Chem. Soc., 2011, 133, 14727 CrossRef CAS PubMed.
- E. L. Kolychev, T. Bannenberg, M. Freytag, C. G. Daniliuc, P. G. Jones and M. Tamm, Chem. – Eur. J., 2012, 18, 16938 CrossRef CAS PubMed.
- T. Belgardt, J. Storre, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, Inorg. Chem., 1995, 34, 3821 CrossRef CAS.
- L. O. Müller, D. Himmel, J. Stauffer, G. Steinfeld, J. Slattery, G. Santiso-Quiñones, V. Brecht and I. Krossing, Angew. Chem., Int. Ed., 2008, 47, 7659 CrossRef CAS PubMed; L. O. Müller, D. Himmel, J. Stauffer, G. Steinfeld, J. Slattery, G. Santiso-Quiñones, V. Brecht and I. Krossing, Angew. Chem., 2008, 120, 7772 CrossRef; A. Kraft, N. Trapp, D. Himmel, H. Böhrer, P. Schlüter, H. Scherer and I. Krossing, Chem. – Eur. J., 2012, 18, 9371 CrossRef PubMed.
- C. Douvris, E. S. Stoyanov, F. S. Tham and C. A. Reed, Chem. Commun., 2007, 1145 RSC.
- C.-W. Chiu and F. P. Gabbaï, Organometallics, 2008, 27, 1657 CrossRef CAS.
- H. Bohrer, N. Trapp, D. Himmel, M. Schleep and I. Krossing, Dalton Trans., 2015, 44, 7489 RSC.
- J. F. Kögel, T. Linder, F. G. Schröder, J. Sundermeyer, S. K. Goll, D. Himmel, I. Krossing, K. Kütt, J. Saame and I. Leito, Chem. – Eur. J., 2015, 21, 5769 CrossRef PubMed.
- F. Scholz, D. Himmel, L. Eisele, W. Unkrig and I. Krossing, Angew. Chem., Int. Ed., 2014, 53, 1689 CrossRef CAS PubMed.
- F. Scholz, D. Himmel, L. Eisele, W. Unkrig, A. Martens, P. Schlüter and I. Krossing, Chem. – Eur. J., 2015, 21, 7489 CrossRef CAS PubMed.
- W. Beichel, J. M. U. Panzer, J. Hätty, X. Ye, D. Himmel and I. Krossing, Angew. Chem., Int. Ed., 2014, 53, 6637 CrossRef CAS PubMed; W. Beichel, J. M. U. Panzer, J. Hätty, X. Ye, D. Himmel and I. Krossing, Angew. Chem., 2014, 126, 6755 CrossRef.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; T. Welton, Ionic liquids in synthesis, Wiley-VCH, Weinheim, 2nd edn, 2008 CrossRef PubMed; H. Weingärtner, Angew. Chem., Int. Ed., 2008, 47, 654 CrossRef PubMed.
- U. Müller, A. Isaeva and M. Ruck, Z. Anorg. Allg. Chem., 2014, 640, 1564 CrossRef.
- E. Ahmed, J. Breternitz, M. F. Groh, A. Isaeva and M. Ruck, Eur. J. Inorg. Chem., 2014, 3037 CrossRef CAS.
- E. Ahmed and M. Ruck, Coord. Chem. Rev., 2011, 255, 2892 CrossRef CAS.
- M. F. Groh, J. Breternitz, E. Ahmed, A. Isaeva, A. Efimova, P. Schmidt and M. Ruck, Z. Anorg. Allg. Chem., 2015, 641, 388 CrossRef CAS.
- J. J. Weigand, M. Holthausen and R. Fröhlich, Angew. Chem., Int. Ed., 2009, 48, 295 CrossRef CAS PubMed.
- D. Himmel, S. K. Goll, I. Leito and I. Krossing, Angew. Chem., Int. Ed., 2010, 49, 6885 CrossRef CAS PubMed; D. Himmel, S. K. Goll, I. Leito and I. Krossing, Angew. Chem., 2010, 122, 7037 CrossRef; D. Himmel, S. K. Goll, I. Leito and I. Krossing, Chem. – Eur. J., 2011, 17, 5808 CrossRef PubMed; D. Himmel, S. K. Goll, I. Leito and I. Krossing, Chem. – Eur. J., 2012, 18, 9333 CrossRef PubMed.
- V. Radtke, D. Himmel, K. Pütz, S. K. Goll and I. Krossing, Chem. – Eur. J., 2014, 20, 4194 CrossRef CAS PubMed.
- D. Himmel, S. K. Goll, F. Scholz, V. Radtke, I. Leito and I. Krossing, ChemPhysChem, 2015, 16, 1428 CrossRef CAS PubMed.
- I. Krossing, J. Am. Chem. Soc., 2001, 123, 4603 CrossRef CAS PubMed.
- T. S. Cameron, R. J. Deeth, I. Dionne, H. Du, H. D. B. Jenkins, I. Krossing, J. Passmore and H. K. Roobottom, Inorg. Chem., 2000, 39, 5614 CrossRef CAS PubMed.
- S. Muthaiah, D. C. H. Do, R. Ganguly and D. Vidovic, Organometallics, 2013, 32, 6718 CrossRef CAS.
- P. D. Bartlett, F. E. Condon and A. Schneider, J. Am. Chem. Soc., 1944, 66, 1531 CrossRef CAS.
- S. Duttwyler, Q.-Q. Do, A. Linden, K. K. Baldridge and J. S. Siegel, Angew. Chem., Int. Ed., 2008, 47, 1719 CrossRef CAS PubMed.
- J. Klosin, G. R. Roof, E. Y. X. Chen and K. A. Abboud, Organometallics, 2000, 19, 4684 CrossRef CAS.
- T. Köchner, S. Riedel, A. J. Lehner, H. Scherer, I. Raabe, T. A. Engesser, F. W. Scholz, U. Gellrich, P. Eiden, R. A. Paz-Schmidt, D. A. Plattner and I. Krossing, Angew. Chem., Int. Ed., 2010, 49, 8139 CrossRef PubMed.
- M. Huber, A. Kurek, I. Krossing, R. Mülhaupt and H. Schnöckel, Z. Anorg. Allg. Chem., 2009, 635, 1787 CrossRef CAS.
- H. Arii, F. Nakadate, K. Mochida and T. Kawashima, Organometallics, 2011, 30, 4471 CrossRef CAS.
- S. P. Hoffmann, T. Kato, F. S. Tham and C. A. Reed, Chem. Commun., 2006, 767 RSC.
- H. Poleschner and K. Seppelt, Angew. Chem., Int. Ed., 2013, 52, 12838 CrossRef CAS PubMed.
- A. Haaland, Angew. Chem., Int. Ed., 1989, 28, 992 CrossRef CAS; A. Haaland, Angew. Chem., 1989, 101, 1017 CrossRef.
- D. Himmel, I. Krossing and A. Schnepf, Angew. Chem., Int. Ed., 2014, 53, 370 CrossRef CAS PubMed; H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2013, 52, 176 CrossRef PubMed; H. Schmidbaur and A. Schier, Angew. Chem., 2013, 125, 187 CrossRef.
- R. Koppe and H. Schnoeckel, Chem. Sci., 2015, 6, 1199 RSC; N. Holzmann, M. Hermann and G. Frenking, Chem. Sci., 2015, 6, 4089 RSC; G. Frenking, Angew. Chem., Int. Ed., 2014, 53, 6040 CrossRef CAS PubMed; G. Frenking, Angew. Chem., 2014, 126, 6152 CrossRef; D. Himmel, I. Krossing and A. Schnepf, Angew. Chem., 2014, 126, 6159 CrossRef; D. Himmel, I. Krossing and A. Schnepf, Angew. Chem., Int. Ed., 2014, 53, 6047 CrossRef PubMed.
- F. Scholz, D. Himmel, F. W. Heinemann, P. von Ragué Schleyer, K. Meyer and I. Krossing, Science, 2013, 341, 62 CrossRef CAS PubMed.
- M. Kessler, C. Knapp and A. Zogaj, Organometallics, 2011, 30, 3786 CrossRef CAS.
- A. J. Downs and H.-J. Himmel, The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 1–74 Search PubMed.
- W. E. Piers, S. C. Bourke and K. D. Conroy, Angew. Chem., Int. Ed., 2005, 44, 5016 CrossRef CAS PubMed.
- M. Bochmann, Coord. Chem. Rev., 2009, 253, 2000 CrossRef CAS.
- J. D. Corbett, D. J. Prince and B. Garbisch, Inorg. Chem., 1970, 9, 2731 CrossRef CAS.
- R. J. Gillespie and G. P. Pez, Inorg. Chem., 1969, 8, 1233 CrossRef CAS.
- K. Akhbari and A. Morsali, Coord. Chem. Rev., 2010, 254, 1977 CrossRef CAS.
- A. H. Cowley, Chem. Commun., 2004, 2369 RSC.
- S. Dagorne and D. A. Atwood, Chem. Rev., 2008, 108, 4037 CrossRef CAS PubMed.
- D. Vidovic and S. Aldridge, Chem. Sci., 2011, 2, 601 RSC.
- C. J. Allan and C. L. B. Macdonald, in Comprehensive Inorganic Chemistry II, ed. J. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 485–566 Search PubMed.
- U. Schneider and S. Kobayashi, Acc. Chem. Res., 2012, 45, 1331 CrossRef CAS PubMed.
- J. Barr, R. J. Gillespie, R. Kapoor and K. C. Malhotra, Can. J. Chem., 1968, 46, 149 CrossRef CAS.
- H. Schmidbaur, Angew. Chem., Int. Ed. Engl., 1985, 24, 893 CrossRef.
- J. Beck and G. Bock, Z. Anorg. Allg. Chem., 1994, 620, 1971 CrossRef CAS.
- D. A. Atwood, Coord. Chem. Rev., 1998, 176, 407 CrossRef CAS.
- A. Baumann and J. Beck, Z. Anorg. Allg. Chem., 2004, 630, 2078 CrossRef CAS.
- D. Vidovic, G. A. Pierce and S. Aldridge, Chem. Commun., 2009, 1157 RSC.
- H. Braunschweig, R. D. Dewhurst and A. Schneider, Chem. Rev., 2010, 110, 3924 CrossRef CAS PubMed.
- C. Jones and A. Stasch, The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 285–341 Search PubMed.
- B. F. T. Cooper and C. L. B. Macdonald, The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 342–401 Search PubMed.
- P. J. Brothers and C. E. Ruggiero, The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, John Wiley & Sons, Ltd, 2011, pp. 519–611 Search PubMed.
- T. S. De Vries, A. Prokofjevs and E. Vedejs, Chem. Rev., 2012, 112, 4246 CrossRef CAS PubMed.
- S. González-Gallardo, T. Bollermann, R. A. Fischer and R. Murugavel, Chem. Rev., 2012, 112, 3136 CrossRef PubMed.
- M. J. Ingleson, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2013, 109, 28 RSC.
- P. Koelle and H. Noeth, Chem. Rev., 1985, 85, 399 CrossRef CAS.
- Y. Shoji, N. Tanaka, K. Mikami, M. Uchiyama and T. Fukushima, Nat. Chem., 2014, 6, 498 CrossRef CAS PubMed.
- S. Courtenay, J. Y. Mutus, R. W. Schurko and D. W. Stephan, Angew. Chem., Int. Ed., 2002, 41, 498 CrossRef CAS.
- T. Kato, F. S. Tham, P. D. W. Boyd and C. A. Reed, Heteroat. Chem., 2006, 17, 209 CrossRef CAS.
- A. Del Grosso, R. G. Pritchard, C. A. Muryn and M. J. Ingleson, Organometallics, 2010, 29, 241 CrossRef CAS.
- M. Gonsior and I. Krossing, Chem. – Eur. J., 2004, 10, 5730 CrossRef CAS PubMed.
- R. J. Gillespie and J. B. Milne, Inorg. Chem., 1966, 5, 1577 CrossRef CAS.
- B. Inés, M. Patil, J. Carreras, R. Goddard, W. Thiel and M. Alcarazo, Angew. Chem., Int. Ed., 2011, 50, 8400 CrossRef PubMed.
- T. A. Shuttleworth, M. A. Huertos, I. Pernik, R. D. Young and A. S. Weller, Dalton Trans., 2013, 42, 12917 RSC.
- H. Braunschweig, K. Radacki and A. Schneider, Chem. Commun., 2010, 46, 6473 RSC.
- H. Braunschweig, M. Kaupp, C. Lambert, D. Nowak, K. Radacki, S. Schinzel and K. Uttinger, Inorg. Chem., 2008, 47, 7456 CrossRef CAS PubMed.
- S. Litters, E. Kaifer, M. Enders and H.-J. Himmel, Nat. Chem., 2013, 5, 1029 CrossRef CAS PubMed.
- G. A. Pierce, D. Vidovic, D. L. Kays, N. D. Coombs, A. L. Thompson, E. D. Jemmis, S. De and S. Aldridge, Organometallics, 2009, 28, 2947 CrossRef CAS.
- H. Braunschweig, K. Radacki and K. Uttinger, Organometallics, 2008, 27, 6005 CrossRef CAS.
- F. Fabrizi de Biani, T. Gmeinwieser, E. Herdtweck, F. Jäkle, F. Laschi, M. Wagner and P. Zanello, Organometallics, 1997, 16, 4776 CrossRef CAS.
- L. Ding, K. Ma, G. Durner, M. Bolte, F. Fabrizi de Biani, P. Zanello and M. Wagner, J. Chem. Soc., Dalton Trans., 2002, 1566 RSC.
- K.-C. Kim, C. A. Reed, G. S. Long and A. Sen, J. Am. Chem. Soc., 2002, 124, 7662 CrossRef CAS PubMed.
- J. D. Young, M. A. Khan and R. J. Wehmschulte, Organometallics, 2004, 23, 1965 CrossRef CAS.
- M. Kessler, C. Knapp, I. Krossing, M. R. Lichtenthaler, 2011, unpublished results.
- S.-J. Lee, P. J. Shapiro and B. Twamley, Organometallics, 2006, 25, 5582 CrossRef CAS.
- C. T. Burns, D. S. Stelck, P. J. Shapiro, A. Vij, K. Kunz, G. Kehr, T. Concolino and A. L. Rheingold, Organometallics, 1999, 18, 5432 CrossRef CAS.
- C. T. Burns, P. J. Shapiro, P. H. M. Budzelaar, R. Willett and A. Vij, Organometallics, 2000, 19, 3361 CrossRef CAS.
- M. Gonsior, I. Krossing and E. Matern, Chem. – Eur. J., 2006, 12, 1703 CrossRef CAS PubMed.
- J. D. Masuda and D. W. Stephan, Dalton Trans., 2006, 2089 RSC.
- C. E. Radzewich, I. A. Guzei and R. F. Jordan, J. Am. Chem. Soc., 1999, 121, 8673 CrossRef CAS.
- O. Stanga, C. L. Lund, H. Liang, J. W. Quail and J. Müller, Organometallics, 2005, 24, 6120 CrossRef CAS.
- P. J. A. Saez, S. H. Oakley, M. P. Coles and P. B. Hitchcock, Chem. Commun., 2007, 816 RSC.
- S. Dagorne, S. Bellemin-Laponnaz and R. Welter, Organometallics, 2004, 23, 3053 CrossRef CAS.
- A. V. Korolev, F. Delpech, S. Dagorne, I. A. Guzei and R. F. Jordan, Organometallics, 2001, 20, 3367 CrossRef CAS.
- A. V. Korolev, E. Ihara, I. A. Guzei, V. G. Young and R. F. Jordan, J. Am. Chem. Soc., 2001, 123, 8291 CrossRef CAS PubMed.
- J. Lewiński, P. Horeglad, M. Dranka and I. Justyniak, Inorg. Chem., 2004, 43, 5789 CrossRef PubMed.
- M. S. Hill and P. B. Hitchcock, Organometallics, 2002, 21, 3258 CrossRef CAS.
- O. Wrobel, F. Schaper and H. H. Brintzinger, Organometallics, 2004, 23, 900 CrossRef CAS.
- A. Stasch, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, Inorg. Chem., 2005, 44, 5854 CrossRef CAS PubMed.
- D. A. Atwood and M. J. Harvey, Chem. Rev., 2001, 101, 37 CrossRef CAS PubMed.
- S. Dagorne, M. Bouyahyi, J. Vergnaud and J.-F. Carpentier, Organometallics, 2010, 29, 1865 CrossRef CAS.
- D. A. Atwood, J. A. Jegier and D. Rutherford, J. Am. Chem. Soc., 1995, 117, 6779 CrossRef CAS.
- D. A. Atwood, J. A. Jegier and D. Rutherford, Inorg. Chem., 1996, 35, 63 CrossRef CAS PubMed.
- M.-A. Muñoz-Hernandez, S. Parkin, B. Yearwood, P. Wei and D. Atwood, J. Chem. Crystallogr., 2000, 30, 215–218 CrossRef.
- M.-A. Munoz-Hernandez, M. L. McKee, T. S. Keizer, B. C. Yearwood and D. A. Atwood, J. Chem. Soc., Dalton Trans., 2002, 410 RSC.
- S. Dagorne, F. Le Bideau, R. Welter, S. Bellemin-Laponnaz and A. Maisse-Franois, Chem. – Eur. J., 2007, 13, 3202 CrossRef CAS PubMed.
- A. V. Korolev, I. A. Guzei and R. F. Jordan, J. Am. Chem. Soc., 1999, 121, 11605 CrossRef CAS.
- O. Wrobel, F. Schaper, U. Wieser, H. Gregorius and H. H. Brintzinger, Organometallics, 2003, 22, 1320 CrossRef CAS.
- N. Nakata, Y. Saito and A. Ishii, Organometallics, 2014, 33, 1840 CrossRef CAS.
- S. Dagorne, I. A. Guzei, M. P. Coles and R. F. Jordan, J. Am. Chem. Soc., 2000, 122, 274 CrossRef CAS.
- T. Cadenbach, C. Gemel, T. Bollermann and R. A. Fischer, Inorg. Chem., 2009, 48, 5021 CrossRef CAS PubMed.
- R. J. Wehmschulte, J. M. Steele, J. D. Young and M. A. Khan, J. Am. Chem. Soc., 2003, 125, 1470 CrossRef CAS PubMed.
- B. Buchin, C. Gemel, T. Cadenbach, R. Schmid and R. A. Fischer, Angew. Chem., Int. Ed., 2006, 45, 1074 CrossRef CAS PubMed.
- C. L. B. Macdonald, J. D. Gorden, A. Voigt and A. H. Cowley, J. Am. Chem. Soc., 2000, 122, 11725 CrossRef CAS.
- A. Higelin, S. Keller, C. Göhringer, C. Jones and I. Krossing, Angew. Chem., Int. Ed., 2013, 52, 4941 CrossRef CAS PubMed.
- A. Higelin, U. Sachs, S. Keller and I. Krossing, Chem. – Eur. J., 2012, 18, 10029 CrossRef CAS PubMed.
- A. Budanow, T. Sinke, J. Tillmann, M. Bolte, M. Wagner and H.-W. Lerner, Organometallics, 2012, 31, 7298 CrossRef CAS.
- M. R. Lichtenthaler, F. Stahl, D. Kratzert, B. Benkmil, H. A. Wegner and I. Krossing, Eur. J. Inorg. Chem., 2014, 4335 CrossRef CAS.
- S. Dagorne, S. Bellemin-Laponnaz, A. Maisse-Francois, M.-N. Rager, L. Juge and R. Welter, Eur. J. Inorg. Chem., 2005, 4206 CrossRef CAS.
- C. Lichtenberg, T. P. Spaniol and J. Okuda, Inorg. Chem., 2012, 51, 2254 CrossRef CAS PubMed.
- G. Linti and A. Seifert, Z. Anorg. Allg. Chem., 2008, 634, 1312 CrossRef CAS.
- A. Higelin, C. Haber, S. Meier and I. Krossing, Dalton Trans., 2012, 41, 12011 RSC.
- N. R. Bunn, S. Aldridge, D. L. Coombs, A. Rossin, D. J. Willock, C. Jones and L.-L. Ooi, Chem. Commun., 2004, 1732 RSC.
- N. D. Coombs, N. R. Bunn, D. L. Kays, J. K. Day, L.-L. Ooi and S. Aldridge, Inorg. Chim. Acta, 2006, 359, 3693 CrossRef CAS.
- K. Ueno, T. Watanabe and H. Ogino, Appl. Organomet. Chem., 2003, 17, 403 CrossRef CAS.
- N. Bartlett and D. H. Lohmann, J. Chem. Soc., 1962, 5253 RSC.
- R. J. Gillespie and J. B. Milne, Inorg. Chem., 1966, 5, 1236 CrossRef CAS.
- A. Kempter, C. Gemel, N. J. Hardman and R. A. Fischer, Inorg. Chem., 2006, 45, 3133 CrossRef CAS PubMed.
- A. Kempter, C. Gemel, T. Cadenbach and R. A. Fischer, Inorg. Chem., 2007, 46, 9481 CrossRef CAS PubMed.
- M. Fleischmann, S. Welsch, H. Krauss, M. Schmidt, M. Bodensteiner, E. V. Peresypkina, M. Sierka, C. Groeger and M. Scheer, Chem. – Eur. J., 2014, 20, 3759 CrossRef CAS PubMed.
- T. Cadenbach, C. Gemel, T. Bollermann, I. Fernandez, G. Frenking and R. A. Fischer, Chem. – Eur. J., 2008, 14, 10789 CrossRef CAS PubMed.
- M. Halbherr, T. Bollermann, C. Gemel and R. A. Fischer, Angew. Chem., Int. Ed., 2010, 49, 1878 CrossRef CAS PubMed.
- S. Aldridge, Angew. Chem., Int. Ed., 2006, 45, 8097 CrossRef CAS PubMed.
- B. Buchin, C. Gemel, T. Cadenbach, I. Fernández, G. Frenking and R. A. Fischer, Angew. Chem., Int. Ed., 2006, 45, 5207 CrossRef CAS PubMed.
- T. Cadenbach, C. Gemel, D. Zacher and R. A. Fischer, Angew. Chem., Int. Ed., 2008, 47, 3438 CrossRef CAS PubMed.
- C. L. B. Macdonald, A. M. Corrente, C. G. Andrews, A. Taylor and B. D. Ellis, Chem. Commun., 2004, 250 RSC.
- C. G. Andrews and C. L. B. Macdonald, J. Organomet. Chem., 2005, 690, 5090 CrossRef CAS.
- A. H. Cowley, C. L. B. Macdonald, J. S. Silverman, J. D. Gorden and A. Voigt, Chem. Commun., 2001, 175 RSC.
- J. N. Jones, C. L. B. Macdonald, J. D. Gorden and A. H. Cowley, J. Organomet. Chem., 2003, 666, 3 CrossRef CAS.
- S. Welsch, M. Bodensteiner, M. Dušek, M. Sierka and M. Scheer, Chem. – Eur. J., 2010, 16, 13041 CrossRef CAS PubMed.
- H. B. Mansaray, C. Y. Tang, D. Vidovic, A. L. Thompson and S. Aldridge, Inorg. Chem., 2012, 51, 13017 CrossRef CAS PubMed.
- T. Jurca, J. Lummiss, T. J. Burchell, S. I. Gorelsky and D. S. Richeson, J. Am. Chem. Soc., 2009, 131, 4608 CrossRef CAS PubMed.
- J. Beck, C. J. Brendel, L. Bengtsson-Kloo, B. Krebs, M. Mummert, A. Stankowski and S. Ulvenlund, Chem. Ber., 1996, 129, 1219 CrossRef CAS.
- F. Delpech, I. A. Guzei and R. F. Jordan, Organometallics, 2002, 21, 1167 CrossRef CAS.
- I. Peckermann, D. Robert, U. Englert, T. P. Spaniol and J. Okuda, Organometallics, 2008, 27, 4817 CrossRef CAS.
- C. G. Andrews and C. L. B. Macdonald, Angew. Chem., Int. Ed., 2005, 44, 7453 CrossRef CAS PubMed.
- B. F. T. Cooper and C. L. B. Macdonald, New J. Chem., 2010, 34, 1551 RSC.
- B. F. T. Cooper and C. L. B. Macdonald, J. Organomet. Chem., 2008, 693, 1707 CrossRef CAS.
- N. R. Bunn, S. Aldridge, D. L. Kays, N. D. Coombs, A. Rossin, D. J. Willock, J. K. Day, C. Jones and L.-L. Ooi, Organometallics, 2005, 24, 5891 CrossRef CAS.
- R. C. Burns, R. J. Gillespie and W.-C. Luk, Inorg. Chem., 1978, 17, 3596 CrossRef CAS.
- D. Alberti and K.-R. Pörschke, Organometallics, 2004, 23, 1459 CrossRef CAS.
- P. K. Hurlburt, O. P. Anderson and S. H. Strauss, Can. J. Chem., 1992, 70, 726 CrossRef CAS.
- T. J. Barbarich, S. M. Miller, O. P. Anderson and S. H. Strauss, J. Mol. Catal. A: Chem., 1998, 128, 289 CrossRef CAS.
- M. Gonsior, I. Krossing and N. Mitzel, Z. Anorg. Allg. Chem., 2002, 628, 1821 CrossRef CAS.
- R. S. Mathur, T. Drovetskaya and C. A. Reed, Acta Crystallogr., 1997, C53, 881 CAS.
- M. D. Noirot, O. P. Anderson and S. H. Strauss, Inorg. Chem., 1987, 26, 2216 CrossRef CAS.
- Y. Sarazin, D. L. Hughes, N. Kaltsoyannis, J. A. Wright and M. Bochmann, J. Am. Chem. Soc., 2007, 129, 881 CrossRef CAS PubMed.
- Y. Sarazin, N. Kaltsoyannis, J. A. Wright and M. Bochmann, Organometallics, 2007, 26, 1811 CrossRef CAS.
- H. Nakai, Y. Tang, P. Gantzel and K. Meyer, Chem. Commun., 2003, 24 RSC.
- T. Jurca, I. Korobkov, S. I. Gorelsky and D. S. Richeson, Inorg. Chem., 2013, 52, 5749 CrossRef CAS PubMed.
- S. Welsch, L. J. Gregoriades, M. Sierka, M. Zabel, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2007, 46, 9323 CrossRef CAS PubMed.
- O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369 CrossRef CAS PubMed.
- N. K. Szymczak, F. Han and D. R. Tyler, Dalton Trans., 2004, 3941 RSC.
- C.-T. Shen, Y.-H. Liu, S.-M. Peng and C.-W. Chiu, Angew. Chem., Int. Ed., 2013, 52, 13293 CrossRef CAS PubMed; C.-T. Shen, Y.-H. Liu, S.-M. Peng and C.-W. Chiu, Angew. Chem., 2013, 125, 13535 CrossRef.
- H. Hartl, J. Nowicki and R. Minkwitz, Angew. Chem., Int. Ed., 1991, 30, 328 CrossRef.
- B. Bentivegna, C. I. Mariani, J. R. Smith, S. Ma, A. L. Rheingold and T. J. Brunker, Organometallics, 2014, 33, 2820 CrossRef CAS.
- S. Dagorne, I. Janowska, R. Welter, J. Zakrzewski and G. Jaouen, Organometallics, 2004, 23, 4706 CrossRef CAS.
- S. Dagorne, C. R. Chim., 2006, 9, 1143 CrossRef CAS.
- F. Cosledan, P. B. Hitchcock and M. F. Lappert, Chem. Commun., 1999, 705 RSC.
- J. D. Masuda, D. M. Walsh, P. Wei and D. W. Stephan, Organometallics, 2004, 23, 1819 CrossRef CAS.
- M. Haddad, M. Laghzaoui, R. Welter and S. Dagorne, Organometallics, 2009, 28, 4584 CrossRef CAS.
- S. Welsch, C. Lescop, R. Reau and M. Scheer, Dalton Trans., 2009, 2683 RSC.
- D. L. Reger, J. E. Collins, R. Layland and R. D. Adams, Inorg. Chem., 1996, 35, 1372 CrossRef CAS PubMed.
- D. L. Coombs, S. Aldridge, C. Jones and D. J. Willock, J. Am. Chem. Soc., 2003, 125, 6356 CrossRef CAS PubMed; H. Braunschweig and D. Rais, Heteroat. Chem., 2005, 16, 566 CrossRef.
- J. Niemeyer, D. A. Addy, I. Riddlestone, M. Kelly, A. L. Thompson, D. Vidovic and S. Aldridge, Angew. Chem., Int. Ed., 2011, 50, 8908 CrossRef CAS PubMed; J. Niemeyer, D. A. Addy, I. Riddlestone, M. Kelly, A. L. Thompson, D. Vidovic and S. Aldridge, Angew. Chem., 2011, 123, 9070 CrossRef.
- H. Braunschweig, K. Kraft, T. Kupfer, K. Radacki and F. Seeler, Angew. Chem., Int. Ed., 2008, 47, 4931 CrossRef CAS PubMed.
- J. Chen, R. A. Lalancette and F. Jakle, Chem. Commun., 2013, 49, 4893 RSC.
- D. L. Kays, J. K. Day, L.-L. Ooi and S. Aldridge, Angew. Chem., Int. Ed., 2005, 44, 7457 CrossRef CAS PubMed; D. L. Kays, A. Rossin, J. K. Day, L.-L. Ooi and S. Aldridge, Dalton Trans., 2006, 399 RSC.
- D. L. Kays, J. K. Day, S. Aldridge, R. W. Harrington and W. Clegg, Angew. Chem., Int. Ed., 2006, 45, 3513 CrossRef CAS PubMed.
- P. Bissinger, H. Braunschweig, A. Damme, R. D. Dewhurst, K. Kraft, T. Kramer and K. Radacki, Chem. – Eur. J., 2013, 19, 13402 CrossRef CAS PubMed.
- E. Firinci, J. I. Bates, I. M. Riddlestone, N. Phillips and S. Aldridge, Chem. Commun., 2013, 49, 1509 RSC.
- G. A. Pierce, S. Aldridge, C. Jones, T. Gans-Eichler, A. Stasch, N. D. Coombs and D. J. Willock, Angew. Chem., Int. Ed., 2007, 46, 2043 CrossRef CAS PubMed.
- H. Braunschweig, K. Radacki, D. Rais and D. Scheschkewitz, Angew. Chem., Int. Ed., 2005, 44, 5651 CrossRef CAS PubMed; H. Braunschweig, K. Radacki, D. Rais and D. Scheschkewitz, Angew. Chem., 2005, 117, 5796 CrossRef.
- N. Arnold, H. Braunschweig, P. Brenner, J. O. C. Jimenez-Halla, T. Kupfer and K. Radacki, Organometallics, 2012, 31, 1897 CrossRef CAS.
- H. Braunschweig, K. Radacki and K. Uttinger, Chem. – Eur. J., 2008, 14, 7858 CrossRef CAS PubMed.
- K. D. Hesp, F. O. Kannemann, M. A. Rankin, R. McDonald, M. J. Ferguson and M. Stradiotto, Inorg. Chem., 2011, 50, 2431 CrossRef CAS PubMed.
- N. D. Coombs, W. Clegg, A. L. Thompson, D. J. Willock and S. Aldridge, J. Am. Chem. Soc., 2008, 130, 5449 CrossRef CAS PubMed.
- N. D. Coombs, D. Vidovic, J. K. Day, A. L. Thompson, D. D. Le Pevelen, A. Stasch, W. Clegg, L. Russo, L. Male, M. B. Hursthouse, D. J. Willock and S. Aldridge, J. Am. Chem. Soc., 2008, 130, 16111 CrossRef CAS PubMed.
- J. Beck and K.-J. Schlitt, Chem. Ber., 1995, 128, 763 CrossRef CAS.
- A. Hershaft and J. D. Corbett, Inorg. Chem., 1963, 2, 979 CrossRef CAS.
- A. Prokofjevs and E. Vedejs, J. Am. Chem. Soc., 2011, 133, 20056 CrossRef CAS PubMed.
- S. Dagorne, L. Lavanant, R. Welter, C. Chassenieux, P. Haquette and G. Jaouen, Organometallics, 2003, 22, 3732 CrossRef CAS.
- E. Ihara, V. G. Young and R. F. Jordan, J. Am. Chem. Soc., 1998, 120, 8277 CrossRef CAS.
- C. Cui, G. R. Giesbrecht, J. A. R. Schmidt and J. Arnold, Inorg. Chim. Acta, 2003, 351, 404 CrossRef CAS.
- M. S. Hill and D. A. Atwood, Eur. J. Inorg. Chem., 1998, 67 CrossRef CAS.
- H. Braunschweig, K. Radacki and A. Schneider, Angew. Chem., Int. Ed., 2010, 49, 5993 CrossRef CAS PubMed.
- T. Bollermann, A. Puls, C. Gemel, T. Cadenbach and R. A. Fischer, Dalton Trans., 2009, 1372 RSC.
- K. Freitag, H. Banh, C. Gemel, P. Jerabek, R. W. Seidel, G. Frenking and R. A. Fischer, Inorg. Chem., 2015, 54, 352 CrossRef CAS PubMed.
- T. Cadenbach, T. Bollermann, C. Gemel and R. A. Fischer, Dalton Trans., 2009, 322 RSC.
- G. G. Henderson, J. Chem. Soc., Trans., 1887, 51, 224 RSC.
- A. Baeyer and V. Villiger, Ber. Dtsch. Chem. Ges., 1902, 35, 1189 CrossRef.
- A. H. Gomes de Mesquita, C. H. MacGillavry and K. Eriks, Acta Crystallogr., 1965, 18, 437 CrossRef CAS.
- J. Krauße, G. Heublein, G. Rudakoff, P. Leibnitz and G. Reck, J. Crystallogr. Spectrosc. Res., 1991, 21, 45 CrossRef CAS; I. Krossing, H. Brands, R. Feuerhake and S. Koenig, J. Fluorine Chem., 2001, 112, 83 CrossRef; J. Zhou, S. J. Lancaster, D. A. Walker, S. Beck, M. Thornton-Pett and M. Bochmann, J. Am. Chem. Soc., 2001, 123, 223 CrossRef PubMed.
- M. Sundaralingam and L. H. Jensen, J. Am. Chem. Soc., 1963, 85, 3302 CrossRef CAS.
- J. B. Lambert, L. Kania and S. Zhang, Chem. Rev., 1995, 95, 1191 CrossRef CAS.
- T. Müller, Cations of Group 14 Organometallics, Elsevier, 2005, vol. 53, pp. 155–215 Search PubMed.
- J. Parr, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2005, 101, 74 RSC.
- H. F. T. Klare and M. Oestreich, Dalton Trans., 2010, 39, 9176 RSC.
- C. A. Reed, Acc. Chem. Res., 2010, 43, 121 CrossRef CAS PubMed.
- M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354 CrossRef CAS PubMed.
- C. Marschner and J. Hlina, Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 83–117 Search PubMed.
- V. S. V. S. N. Swamy, S. Pal, S. Khan and S. S. Sen, Dalton Trans., 2015, 44, 12903 RSC.
- C. A. Reed, Science, 2000, 289, 101 CrossRef CAS PubMed.
- M. Riccò, D. Pontiroli, M. Mazzani, F. Gianferrari, M. Pagliari, A. Goffredi, M. Brunelli, G. Zandomeneghi, B. H. Meier and T. Shiroka, J. Am. Chem. Soc., 2010, 132, 2064 CrossRef PubMed.
- K.-C. Kim, F. Hauke, A. Hirsch, P. D. W. Boyd, E. Carter, R. S. Armstrong, P. A. Lay and C. A. Reed, J. Am. Chem. Soc., 2003, 125, 4024 CrossRef CAS PubMed.
- P. von Ragué Schleyer, W. E. Watts, R. C. Fort, M. B. Comisarow and G. A. Olah, J. Am. Chem. Soc., 1964, 86, 5679 CrossRef CAS; M. Saunders, P. von Ragué Schleyer and G. A. Olah, J. Am. Chem. Soc., 1964, 86, 5680 CrossRef.
- L. K. Montgomery, M. P. Grendze and J. C. Huffman, J. Am. Chem. Soc., 1987, 109, 4749 CrossRef CAS; T. Laube, J. Am. Chem. Soc., 1989, 111, 9224 CrossRef; T. Laube, Angew. Chem., Int. Ed. Engl., 1987, 26, 560 CrossRef; T. Laube, Angew. Chem., Int. Ed. Engl., 1986, 25, 349 CrossRef.
- S. Hollenstein and T. Laube, J. Am. Chem. Soc., 1993, 115, 7240 CrossRef CAS.
- T. Kato and C. A. Reed, Angew. Chem., Int. Ed., 2004, 43, 2908 CrossRef CAS PubMed.
- F. Scholz, D. Himmel, H. Scherer and I. Krossing, Chem. – Eur. J., 2013, 19, 109 CrossRef CAS PubMed.
- E. S. Stoyanov, I. V. Stoyanova, F. S. Tham and C. A. Reed, Angew. Chem., Int. Ed., 2012, 51, 9149 CrossRef CAS PubMed.
- K. O. Christe, X. Zhang, R. Bau, J. Hegge, G. A. Olah, G. K. S. Prakash and J. A. Sheehy, J. Am. Chem. Soc., 2000, 122, 481 CrossRef CAS.
- I. Krossing, A. Bihlmeier, I. Raabe and N. Trapp, Angew. Chem., Int. Ed., 2003, 42, 1531 CrossRef CAS PubMed.
- H. P. A. Mercier, M. D. Moran, G. J. Schrobilgen, C. Steinberg and R. J. Suontamo, J. Am. Chem. Soc., 2004, 126, 5533 CrossRef CAS PubMed.
- A. J. Lehner, N. Trapp, H. Scherer and I. Krossing, Dalton Trans., 2011, 40, 1448 RSC.
- R. Minkwitz and F. Neikes, Eur. J. Inorg. Chem., 2000, 2283 CrossRef CAS.
- T. Laube, J. Am. Chem. Soc., 2004, 126, 10904 CrossRef CAS PubMed.
- I. Raabe, C. Röhr and I. Krossing, Dalton Trans., 2007, 5376 RSC.
- T. Müller, M. Juhasz and C. A. Reed, Angew. Chem., Int. Ed., 2004, 43, 1543 CrossRef PubMed.
- A. Klaer, W. Saak, D. Haase and T. Müller, J. Am. Chem. Soc., 2008, 130, 14956 CrossRef CAS PubMed.
- N. C. Baenziger and A. D. Nelson, J. Am. Chem. Soc., 1968, 90, 6602 CrossRef CAS.
- H. Shorafa, D. Mollenhauer, B. Paulus and K. Seppelt, Angew. Chem., Int. Ed., 2009, 48, 5845 CrossRef CAS PubMed.
- M. J. Molski, D. Mollenhauer, S. Gohr, B. Paulus, M. A. Khanfar, H. Shorafa, S. H. Strauss and K. Seppelt, Chem. – Eur. J., 2012, 18, 6644 CrossRef CAS PubMed.
- M. J. Molski, M. A. Khanfar, H. Shorafa and K. Seppelt, Eur. J. Org. Chem., 2013, 3131 CrossRef CAS.
- F. Marchetti, C. Pinzino, S. Zacchini and G. Pampaloni, Angew. Chem., Int. Ed., 2010, 49, 5268 CrossRef CAS PubMed.
- X. Chen, X. Wang, Y. Sui, Y. Li, J. Ma, J. Zuo and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 11878 CrossRef CAS PubMed.
- R. A. Moss, S. Shen, K. Krogh-Jespersen, J. A. Potenza, H. J. Schugar and R. C. Munjal, J. Am. Chem. Soc., 1986, 108, 134 CrossRef CAS.
- S. K. Chadda, R. F. Childs, R. Faggiani and C. J. L. Lock, J. Am. Chem. Soc., 1986, 108, 1694 CrossRef CAS.
- J. B. Lambert, L. Lin and V. Rassolov, Angew. Chem., Int. Ed., 2002, 41, 1429 CrossRef CAS; M. Otto, D. Scheschkewitz, T. Kato, M. M. Midland, J. B. Lambert and G. Bertrand, Angew. Chem., Int. Ed., 2002, 41, 2275 CrossRef.
- S. Duttwyler, Y. Zhang, A. Linden, C. A. Reed, K. K. Baldridge and J. S. Siegel, Angew. Chem., Int. Ed., 2009, 48, 3787 CrossRef CAS PubMed.
- T. Kato, E. Stoyanov, J. Geier, H. Grützmacher and C. A. Reed, J. Am. Chem. Soc., 2004, 126, 12451 CrossRef CAS PubMed.
- C. Bolli, J. Derendorf, M. Keßler, C. Knapp, H. Scherer, C. Schulz and J. Warneke, Angew. Chem., Int. Ed., 2010, 49, 3536 CrossRef CAS PubMed.
- J. B. Lambert, S. Zhang, C. L. Stern and J. C. Huffman, Science, 1993, 260, 1917 CAS.
- A. Schäfer, M. Reißmann, S. Jung, A. Schäfer, W. Saak, E. Brendler and T. Müller, Organometallics, 2013, 32, 4713 CrossRef.
- M. Reißmann, PhD dissertation, University of Oldenburg, 2014.
- A. Sekiguchi, T. Matsuno and M. Ichinohe, J. Am. Chem. Soc., 2000, 122, 11250 CrossRef CAS.
- M. Ichinohe, M. Igarashi, K. Sanuki and A. Sekiguchi, J. Am. Chem. Soc., 2005, 127, 9978 CrossRef CAS PubMed.
- S. Inoue, J. D. Epping, E. Irran and M. Driess, J. Am. Chem. Soc., 2011, 133, 8514 CrossRef CAS PubMed.
- M. Driess, S. Yao, M. Brym and C. van Wüllen, Angew. Chem., Int. Ed., 2006, 45, 6730 CrossRef CAS PubMed.
- K. Hensen, T. Zengerly, P. Pickel and G. Klebe, Angew. Chem., Int. Ed., 1983, 22, 725 CrossRef.
- Z. Xie, D. J. Liston, T. Jelínek, V. Mitro, R. Bau and C. A. Reed, J. Chem. Soc., Chem. Commun., 1993, 384 RSC.
- M. Ichinohe, H. Fukui and A. Sekiguchi, Chem. Lett., 2000, 600 CrossRef CAS.
- C. Gerdes, W. Saak, D. Haase and T. Müller, J. Am. Chem. Soc., 2013, 135, 10353 CrossRef CAS PubMed.
- M. J. MacLachlan, S. C. Bourke, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2000, 122, 2126 CrossRef CAS.
- Z. Xie, R. Bau and C. A. Reed, J. Chem. Soc., Chem. Commun., 1994, 2519 RSC.
- A. P. M. Robertson, J. N. Friedmann, H. A. Jenkins and N. Burford, Chem. Commun., 2014, 50, 7979 RSC.
- P. Romanato, S. Duttwyler, A. Linden, K. K. Baldridge and J. S. Siegel, J. Am. Chem. Soc., 2010, 132, 7828 CrossRef CAS PubMed.
- M. F. Ibad, P. Langer, A. Schulz and A. Villinger, J. Am. Chem. Soc., 2011, 133, 21016 CrossRef CAS PubMed.
- J. B. Lambert and S. Zhang, J. Chem. Soc., Chem. Commun., 1993, 383 RSC; M. Nava and C. A. Reed, Organometallics, 2011, 30, 4798 CrossRef CAS PubMed.
- S. J. Connelly, W. Kaminsky and D. M. Heinekey, Organometallics, 2013, 32, 7478 CrossRef CAS.
- M. Lehmann, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2009, 48, 7444 CrossRef CAS PubMed.
- A. Schulz, J. Thomas and A. Villinger, Chem. Commun., 2010, 46, 3696 RSC.
- A. Schulz and A. Villinger, Chem. – Eur. J., 2010, 16, 7276 CrossRef CAS PubMed.
- K. O. Christe, W. W. Wilson, D. A. Dixon, S. I. Khan, R. Bau, T. Metzenthin and R. Lu, J. Am. Chem. Soc., 1993, 115, 1836 CrossRef CAS.
- R. Panisch, M. Bolte and T. Müller, J. Am. Chem. Soc., 2006, 128, 9676 CrossRef CAS PubMed.
- R. Panisch, M. Bolte and T. Müller, Organometallics, 2007, 26, 3524 CrossRef CAS.
- N. Choi, P. D. Lickiss, M. McPartlin, P. C. Masangane and G. L. Veneziani, Chem. Commun., 2005, 6023 RSC.
- A. Schäfer, M. Reißmann, A. Schäfer, M. Schmidtmann and T. Müller, Chem. – Eur. J., 2014, 20, 9381 CrossRef PubMed.
- A. Sekiguchi, Y. Murakami, N. Fukaya and Y. Kabe, Chem. Lett., 2004, 33, 530 CrossRef CAS.
- K. Müther, R. Fröhlich, C. Mück-Lichtenfeld, S. Grimme and M. Oestreich, J. Am. Chem. Soc., 2011, 133, 12442 CrossRef PubMed.
- A. Bockholt, P. Jutzi, A. Mix, B. Neumann, A. Stammler and H.-G. Stammler, Z. Anorg. Allg. Chem., 2009, 635, 1326 CrossRef CAS.
- Y. Xiong, S. Yao, S. Inoue, E. Irran and M. Driess, Angew. Chem., Int. Ed., 2012, 51, 10074 CrossRef CAS PubMed.
- H.-X. Yeong, H.-W. Xi, Y. Li, K. H. Lim and C.-W. So, Chem. – Eur. J., 2013, 19, 11786 CrossRef CAS PubMed.
- Y. Xiong, S. Yao, S. Inoue, J. D. Epping and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 7147 CrossRef CAS PubMed.
- A. C. Filippou, Y. N. Lebedev, O. Chernov, M. Straßmann and G. Schnakenburg, Angew. Chem., Int. Ed., 2013, 52, 6974 CrossRef CAS PubMed.
- P. Jutzi, Science, 2004, 305, 849 CrossRef CAS PubMed.
- P. Jutzi, A. Mix, B. Neumann, B. Rummel and H.-G. Stammler, Chem. Commun., 2006, 3519 RSC.
- K. Leszczynska, A. Mix, R. J. F. Berger, B. Rummel, B. Neumann, H.-G. Stammler and P. Jutzi, Angew. Chem., Int. Ed., 2011, 50, 6843 CrossRef CAS PubMed.
- C. A. Reed, Z. Xie, R. Bau and A. Benesi, Science, 1993, 262, 402 CAS.
- T. Küppers, E. Bernhardt, R. Eujen, H. Willner and C. W. Lehmann, Angew. Chem., Int. Ed., 2007, 46, 6346 CrossRef PubMed.
- M. Rohde, L. O. Müller, D. Himmel, H. Scherer and I. Krossing, Chem. – Eur. J., 2014, 20, 1218 CrossRef CAS PubMed.
- Z. Xie, R. Bau, A. Benesi and C. A. Reed, Organometallics, 1995, 14, 3933 CrossRef CAS.
- Z. Xie, J. Manning, R. W. Reed, R. Mathur, P. D. W. Boyd, A. Benesi and C. A. Reed, J. Am. Chem. Soc., 1996, 118, 2922 CrossRef CAS.
- A. Budanow, M. Bolte, M. Wagner and H.-W. Lerner, Eur. J. Inorg. Chem., 2015, 2524 CrossRef CAS.
- S. C. Bourke, M. J. MacLachlan, A. J. Lough and I. Manners, Chem. – Eur. J., 2005, 11, 1989 CrossRef CAS PubMed.
- A. Sekiguchi, Y. Ishida, Y. Kabe and M. Ichinohe, J. Am. Chem. Soc., 2002, 124, 8776 CrossRef CAS PubMed.
- I. Zharov, B. T. King, Z. Havlas, A. Pardi and J. Michl, J. Am. Chem. Soc., 2000, 122, 10253 CrossRef CAS.
- A. Sekiguchi, T. Fukawa, V. Y. Lee, M. Nakamoto and M. Ichinohe, Angew. Chem., Int. Ed., 2003, 42, 1143 CrossRef CAS PubMed.
- A. Sekiguchi, T. Fukawa, V. Y. Lee and M. Nakamoto, J. Am. Chem. Soc., 2003, 125, 9250 CrossRef CAS PubMed.
- C. Schenk, C. Drost and A. Schnepf, Dalton Trans., 2009, 773 RSC.
- A. Schäfer, W. Saak, D. Haase and T. Müller, J. Am. Chem. Soc., 2011, 133, 14562 CrossRef PubMed.
- J. H. Wright, G. W. Mueck, F. S. Tham and C. A. Reed, Organometallics, 2010, 29, 4066 CrossRef CAS.
- A. Sekiguchi, Science, 1997, 275, 60 CrossRef CAS PubMed.
- M. Ichinohe, N. Fukaya and A. Sekiguchi, Chem. Lett., 1998, 1045 CrossRef CAS.
- A. Sekiguchi, N. Fukaya, M. Ichinohe and Y. Ishida, Eur. J. Inorg. Chem., 2000, 1155 CAS.
- I. Lange, J. Krahl, P. G. Jones and A. Blaschette, J. Organomet. Chem., 1994, 474, 97 CrossRef CAS.
- N. Kordts, C. Borner, R. Panisch, W. Saak and T. Müller, Organometallics, 2014, 33, 1492 CrossRef CAS.
- S. Khan, G. Gopakumar, W. Thiel and M. Alcarazo, Angew. Chem., Int. Ed., 2013, 52, 5644 CrossRef CAS PubMed.
- M. Wagner, T. Zöller, W. Hiller, M. H. Prosenc and K. Jurkschat, Chem. – Eur. J., 2013, 19, 9463 CrossRef CAS PubMed.
- T. Müller, C. Bauch, M. Ostermeier, M. Bolte and N. Auner, J. Am. Chem. Soc., 2003, 125, 2158 CrossRef PubMed.
- Y. Ishida, A. Sekiguchi and Y. Kabe, J. Am. Chem. Soc., 2003, 125, 11468 CrossRef CAS PubMed.
- T. Müller, C. Bauch, M. Bolte and N. Auner, Chem. – Eur. J., 2003, 9, 1746 CrossRef PubMed.
- Y. Xiong, T. Szilvási, S. Yao, G. Tan and M. Driess, J. Am. Chem. Soc., 2014, 136, 11300 CrossRef CAS PubMed.
- E. Hough, D. G. Nicholson and A. K. Vasudevan, J. Chem. Soc., Dalton Trans., 1989, 2155 RSC.
- R. D. Rogers and A. H. Bond, Inorg. Chim. Acta, 1992, 192, 163 CrossRef CAS.
- R. Bandyopadhyay, B. F. Cooper, A. J. Rossini, R. W. Schurko and C. L. Macdonald, J. Organomet. Chem., 2010, 695, 1012 CrossRef CAS.
- C. Beattie, P. Farina, W. Levason and G. Reid, Dalton Trans., 2013, 42, 15183 RSC.
- P. A. Rupar, V. N. Staroverov, P. J. Ragogna and K. M. Baines, J. Am. Chem. Soc., 2007, 129, 15138 CrossRef CAS PubMed.
- P. A. Rupar, V. N. Staroverov and K. M. Baines, Science, 2008, 322, 1360 CrossRef CAS PubMed.
- J. C. Avery, M. A. Hanson, R. H. Herber, K. J. Bladek, P. A. Rupar, I. Nowik, Y. Huang and K. M. Baines, Inorg. Chem., 2012, 51, 7306 CrossRef CAS PubMed.
- P. A. Rupar, R. Bandyopadhyay, B. Cooper, M. R. Stinchcombe, P. J. Ragogna, C. Macdonald and K. M. Baines, Angew. Chem., Int. Ed., 2009, 48, 5155 CrossRef CAS PubMed.
- F. Cheng, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Angew. Chem., Int. Ed., 2009, 48, 5152 CrossRef CAS PubMed.
- F. Cheng, J. M. Dyke, F. Ferrante, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Dalton Trans., 2010, 39, 847 RSC.
- A. P. Singh, H. W. Roesky, E. Carl, D. Stalke, J.-P. Demers and A. Lange, J. Am. Chem. Soc., 2012, 134, 4998 CrossRef CAS PubMed.
- M. Bouška, L. Dostál, A. Růžička and R. Jambor, Organometallics, 2013, 32, 1995 CrossRef.
- T. Jurca, L. K. Hiscock, I. Korobkov, C. N. Rowley and D. S. Richeson, Dalton Trans., 2014, 43, 690 RSC.
- Y. Xiong, S. Yao, S. Inoue, A. Berkefeld and M. Driess, Chem. Commun., 2012, 48, 12198 RSC.
- Y. Xiong, S. Yao, G. Tan, S. Inoue and M. Driess, J. Am. Chem. Soc., 2013, 135, 5004 CrossRef CAS PubMed.
- A. Schäfer, W. Saak, D. Haase and T. Müller, Chem. – Eur. J., 2009, 15, 3945 CrossRef PubMed.
- J. Li, C. Schenk, F. Winter, H. Scherer, N. Trapp, A. Higelin, S. Keller, R. Pöttgen, I. Krossing and C. Jones, Angew. Chem., Int. Ed., 2012, 51, 9557 CrossRef CAS PubMed.
- M. J. Taylor, A. J. Saunders, M. P. Coles and J. R. Fulton, Organometallics, 2011, 30, 1334 CrossRef CAS.
- H. Arii, M. Matsuo, F. Nakadate, K. Mochida and T. Kawashima, Dalton Trans., 2012, 41, 11195 RSC.
- M. Stender, A. D. Phillips and P. P. Power, Inorg. Chem., 2001, 40, 5314 CrossRef CAS PubMed.
- S. Hino, M. Brynda, A. D. Phillips and P. P. Power, Angew. Chem., Int. Ed., 2004, 43, 2655 CrossRef CAS.
- A. Schäfer, F. Winter, W. Saak, D. Haase, R. Pöttgen and T. Müller, Chem. – Eur. J., 2011, 17, 10979 CrossRef PubMed.
- T. Probst, O. Steigelmann, J. Riede and H. Schmidbaur, Angew. Chem., Int. Ed., 1990, 29, 1397 CrossRef.
- P. Jutzi, F. Kohl and C. Krüger, Angew. Chem., Int. Ed. Engl., 1979, 18, 59 CrossRef.
- P. Jutzi, U. Holtmann, H. Bögge and A. Müller, J. Chem. Soc., Chem. Commun., 1988, 305 RSC.
- J. N. Jones, J. A. Moore, A. H. Cowley and C. L. B. Macdonald, Dalton Trans., 2005, 3846 RSC.
- J. G. Winter, P. Portius, G. Kociok-Köhn, R. Steck and A. C. Filippou, Organometallics, 1998, 17, 4176 CrossRef CAS.
- J. Rouzaud, M. Joudat, A. Castel, F. Delpech, P. Rivière, H. Gornitzka, J. Manriquez and I. Chavez, J. Organomet. Chem., 2002, 651, 44 CrossRef CAS.
- A. H. Cowley, C. L. B. Macdonald, J. S. Silverman, J. D. Gorden and A. Voigt, Chem. Commun., 2001, 175 RSC.
- A. C. Filippou, A. I. Philippopoulos and G. Schnakenburg, Organometallics, 2003, 22, 3339 CrossRef CAS.
- A. C. Filippou, A. I. Philippopoulos, P. Portius and G. Schnakenburg, Organometallics, 2004, 23, 4503 CrossRef CAS.
- Y. Cabon, H. Kleijn, M. A. Siegler, A. L. Spek, R. J. M. Klein Gebbink and B.-J. Deelman, Dalton Trans., 2010, 39, 2423 RSC.
- R. Dostálová, L. Dostál, A. Růžička and R. Jambor, Organometallics, 2011, 30, 2405 CrossRef.
- M. Wagner, M. Henn, C. Dietz, M. Schürmann, M. H. Prosenc and K. Jurkschat, Organometallics, 2013, 32, 2406 CrossRef CAS.
- S. Krabbe, M. Wagner, C. Löw, C. Dietz, M. Schürmann, A. Hoffmann, S. Herres-Pawlis, M. Lutter and K. Jurkschat, Organometallics, 2014, 33, 4433 CrossRef CAS.
- H. Braunschweig, M. A. Celik, R. D. Dewhurst, M. Heid, F. Hupp and S. S. Sen, Chem. Sci., 2015, 6, 425 RSC.
- D. Stasko and C. A. Reed, J. Am. Chem. Soc., 2002, 124, 1148 CrossRef CAS PubMed.
- C. A. Reed, K.-C. Kim, E. S. Stoyanov, D. Stasko, F. S. Tham, L. J. Mueller and P. D. W. Boyd, J. Am. Chem. Soc., 2003, 125, 1796 CrossRef CAS PubMed.
- C. A. Reed, N. L. P. Fackler, K.-C. Kim, D. Stasko, D. R. Evans, P. D. W. Boyd and C. E. F. Rickard, J. Am. Chem. Soc., 1999, 121, 6314 CrossRef CAS.
- I. Objartel, H. Ott and D. Stalke, Z. Anorg. Allg. Chem., 2008, 634, 2373 CrossRef CAS.
- N. Burford, P. J. Ragogna, R. McDonald and M. J. Ferguson, J. Am. Chem. Soc., 2003, 125, 14404 CrossRef CAS PubMed.
- J. J. Weigand and N. Burford, Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 119–149 Search PubMed.
- C. A. Dyker and N. Burford, Chem. – Asian J., 2008, 3, 28 CrossRef CAS PubMed.
- A. P. M. Robertson, P. A. Gray and N. Burford, Angew. Chem., Int. Ed., 2014, 53, 6050 CrossRef CAS PubMed.
- I. Krossing, M. Driess and H. Nöth, Molecular Clusters of the Main Group Elements, Wiley-VCH, 2004, p. 223 Search PubMed.
- K.-O. Feldmann and J. J. Weigand, Angew. Chem., Int. Ed., 2012, 51, 6566 CrossRef CAS PubMed.
- H. J. Breunig and C. I. Raţ, Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 151–178 Search PubMed.
- M. H. Holthausen and J. J. Weigand, Chem. Soc. Rev., 2014, 43, 6639 RSC.
- M. Ruck and F. Locherer, Coord. Chem. Rev., 2015, 297–298, 208 CrossRef CAS.
- M. Ruck and F. Locherer, Coord. Chem. Rev., 2015, 285, 1 CrossRef CAS.
- K. O. Christe, W. W. Wilson, J. A. Sheehy and J. A. Boatz, Angew. Chem., Int. Ed., 1999, 38, 2004 CrossRef CAS.
- A. Vij, W. W. Wilson, V. Vij, F. S. Tham, J. A. Sheehy and K. O. Christe, J. Am. Chem. Soc., 2001, 123, 6308 CrossRef CAS PubMed.
- M. Lindsjö, A. Fischer and L. Kloo, Angew. Chem., Int. Ed., 2004, 43, 2540 CrossRef PubMed.
- E. Ahmed, D. Köhler and M. Ruck, Z. Anorg. Allg. Chem., 2009, 635, 297 CrossRef CAS.
- A. Decken, G. B. Nikiforov and J. Passmore, Polyhedron, 2005, 24, 2994 CrossRef CAS.
- C. Schwarzmaier, M. Sierka and M. Scheer, Angew. Chem., Int. Ed., 2013, 52, 858 CrossRef CAS PubMed.
- R. Glaser and C. J. Horan, Can. J. Chem., 1996, 74, 1200 CrossRef CAS.
- E. Niecke, M. Nieger and F. Reichert, Angew. Chem., Int. Ed., 1988, 27, 1715 CrossRef.
- M. Kuprat, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2013, 52, 7126 CrossRef CAS PubMed.
- E. Ahmed, A. Isaeva, A. Fiedler, M. Haft and M. Ruck, Chem. – Eur. J., 2011, 17, 6847 CrossRef CAS PubMed.
- A. Eich, S. Schlüter, G. Schnakenburg and J. Beck, Z. Anorg. Allg. Chem., 2013, 639, 375 CrossRef CAS.
- K.-O. Feldmann, T. Wiegand, J. Ren, H. Eckert, J. Breternitz, M. F. Groh, U. Müller, M. Ruck, B. Maryasin, C. Ochsenfeld, O. Schön, K. Karaghiosoff and J. J. Weigand, Chem. – Eur. J., 2015, 21, 9577 CrossRef CAS.
- K.-O. Feldmann, T. Wiegand, J. Ren, H. Eckert, J. Breternitz, M. F. Groh, U. Müller, M. Ruck, B. Maryasin, C. Ochsenfeld, O. Schön, K. Karaghiosoff and J. J. Weigand, Chem. – Eur. J., 2015, 21, 9697 CrossRef CAS PubMed.
- J. Beck, S. Schlüter and N. Zotov, Z. Anorg. Allg. Chem., 2004, 630, 2512 CrossRef CAS.
- J. Beck, M. Dolg and S. Schlüter, Angew. Chem., Int. Ed., 2001, 40, 2287 CrossRef CAS.
- N. Burford, T. M. Parks, B. W. Royan, B. Borecka, T. S. Cameron, J. F. Richardson, E. J. Gabe and R. Hynes, J. Am. Chem. Soc., 1992, 114, 8147 CrossRef CAS.
- R. Kinjo, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 5930 CrossRef CAS PubMed.
- M. Y. Abraham, Y. Wang, Y. Xie, R. J. Gilliard, P. Wei, B. J. Vaccaro, M. K. Johnson, H. F. Schaefer, P. von Ragué Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2013, 135, 2486 CrossRef CAS PubMed.
- X. Pan, X. Chen, T. Li, Y. Li and X. Wang, J. Am. Chem. Soc., 2013, 135, 3414 CrossRef CAS PubMed.
- X. Pan, Y. Su, X. Chen, Y. Zhao, Y. Li, J. Zuo and X. Wang, J. Am. Chem. Soc., 2013, 135, 5561 CrossRef CAS PubMed.
- Y. Su, X. Zheng, X. Wang, X. Zhang, Y. Sui and X. Wang, J. Am. Chem. Soc., 2014, 136, 6251 CrossRef CAS PubMed.
- A. Schmidpeter, S. Lochschmidt and W. S. Sheldrick, Angew. Chem., Int. Ed., 1985, 24, 226 CrossRef.
- R. J. Barnham, R. M. K. Deng, K. B. Dillon, A. E. Goeta, J. A. K. Howard and H. Puschmann, Heteroat. Chem., 2001, 12, 501 CrossRef CAS.
- G. Reeske and A. H. Cowley, Chem. Commun., 2006, 1784 RSC.
- J. M. Slattery and S. Hussein, Dalton Trans., 2012, 41, 1808 RSC.
- A. P. M. Robertson, N. Burford, R. McDonald and M. J. Ferguson, Angew. Chem., Int. Ed., 2014, 53, 3480 CrossRef CAS PubMed.
- A. D. Hendsbee, N. A. Giffin, Y. Zhang, C. C. Pye and J. D. Masuda, Angew. Chem., Int. Ed., 2012, 51, 10836 CrossRef CAS PubMed.
- M. H. Holthausen, M. Mehta and D. W. Stephan, Angew. Chem., Int. Ed. Engl., 2014, 53, 6538 CrossRef CAS PubMed.
- R. Minkwitz and S. Schneider, Angew. Chem., Int. Ed., 1999, 38, 210 CrossRef CAS.
- B. Wahl and M. Ruck, Z. Anorg. Allg. Chem., 2008, 634, 2873 CrossRef CAS.
- M. Ruck, Z. Anorg. Allg. Chem., 1998, 624, 521 CrossRef CAS.
- B. Krebs, M. Mummert and C. Brendel, J. Less-Common Met., 1986, 116, 159 CrossRef CAS.
- M. Ruck and F. Steden, Z. Anorg. Allg. Chem., 2007, 633, 1556 CrossRef CAS.
- B. Krebs, M. Hucke and C. J. Brendel, Angew. Chem., Int. Ed. Engl., 1982, 21, 1108 CrossRef.
- J. Beck and T. Hilbert, Eur. J. Inorg. Chem., 2004, 2019 CrossRef CAS.
- A. Hershaft and J. D. Corbett, J. Chem. Phys., 1962, 36, 551 CrossRef CAS.
- R. M. Friedman and J. D. Corbett, Inorg. Chem., 1973, 12, 1134 CrossRef CAS.
- B. Wahl and M. Ruck, Z. Anorg. Allg. Chem., 2010, 636, 337 CrossRef CAS.
- G. Santiso-Quinones, A. Reisinger, J. Slattery and I. Krossing, Chem. Commun., 2007, 5046 RSC.
- L. C. Forfar, T. J. Clark, M. Green, S. M. Mansell, C. A. Russell, R. A. Sanguramath and J. M. Slattery, Chem. Commun., 2012, 48, 1970 RSC.
- I. de los Rios, J.-R. Hamon, P. Hamon, C. Lapinte, L. Toupet, A. Romerosa and M. Peruzzini, Angew. Chem., Int. Ed., 2001, 40, 3910 CrossRef CAS.
- M. Caporali, M. Di Vaira, M. Peruzzini, S. Seniori Costantini, P. Stoppioni and F. Zanobini, Eur. J. Inorg. Chem., 2010, 152 CrossRef CAS.
- P. Barbaro, M. Di Vaira, M. Peruzzini, S. Seniori Costantini and P. Stoppioni, Chem. – Eur. J., 2007, 13, 6682 CrossRef CAS PubMed.
- C. Schwarzmaier, A. Y. Timoshkin and M. Scheer, Angew. Chem., Int. Ed., 2013, 52, 7600 CrossRef CAS PubMed.
- A. Adolf, M. Gonsior and I. Krossing, J. Am. Chem. Soc., 2002, 124, 7111 CrossRef CAS PubMed.
- M. Cygler, M. Przybylska and R. M. Elofson, Can. J. Chem., 1982, 60, 2852 CrossRef CAS.
- A. Kütt, F. Werner, I. Kaljurand, I. Leito and I. A. Koppel, ChemPlusChem, 2013, 78, 932 CrossRef.
- B. S. McGilligan, J. Arnold, G. Wilkinson, B. Hussain-Bates and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 1990, 2465 RSC.
- W. Baumann, D. Michalik, F. Reiß, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2014, 53, 3250 CrossRef CAS PubMed.
- C. Hering, M. Hertrich, A. Schulz and A. Villinger, Inorg. Chem., 2014, 53, 3880 CrossRef CAS PubMed.
- Y. K. Loh, C. Gurnani, R. Ganguly and D. Vidović, Inorg. Chem., 2015, 54, 3087 CrossRef CAS PubMed.
- N. Burford, T. S. Cameron, P. J. Ragogna, E. Ocando-Mavarez, M. Gee, R. McDonald and R. E. Wasylishen, J. Am. Chem. Soc., 2001, 123, 7947 CrossRef CAS PubMed.
- J. J. Weigand, S. D. Riegel, N. Burford and A. Decken, J. Am. Chem. Soc., 2007, 129, 7969 CrossRef CAS PubMed.
- M. Gonsior, I. Krossing, L. Müller, I. Raabe, M. Jansen and L. van Wüllen, Chem. – Eur. J., 2002, 8, 4475 CrossRef CAS.
- S. Pohl, Z. Anorg. Allg. Chem., 1983, 498, 20 CrossRef CAS.
- N. Burford, C. A. Dyker and A. Decken, Angew. Chem., Int. Ed., 2005, 44, 2364 CrossRef CAS PubMed.
- A. Schmidpeter, S. Lochschmidt, K. Karaghiosoff and W. S. Sheldrick, J. Chem. Soc., Chem. Commun., 1985, 1447 RSC.
- I. Krossing, J. Chem. Soc., Chem. Commun., 2002, 500 CAS.
- E. Conrad, N. Burford, R. McDonald and M. J. Ferguson, Inorg. Chem., 2008, 47, 2952 CrossRef CAS PubMed.
- C. A. Dyker, N. Burford, M. D. Lumsden and A. Decken, J. Am. Chem. Soc., 2006, 128, 9632 CrossRef CAS PubMed.
- J. Bresien, K. Faust, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2015, 54, 6926 CrossRef CAS PubMed.
- E. Conrad, N. Burford, R. McDonald and M. J. Ferguson, J. Am. Chem. Soc., 2009, 131, 17000 CrossRef CAS PubMed.
- I. Krossing and I. Raabe, Angew. Chem., Int. Ed., 2001, 40, 4406 CrossRef CAS.
- M. H. Holthausen, K.-O. Feldmann, S. Schulz, A. Hepp and J. J. Weigand, Inorg. Chem., 2012, 51, 3374 CrossRef CAS PubMed.
- M. H. Holthausen, A. Hepp and J. J. Weigand, Chem. – Eur. J., 2013, 19, 9895 CrossRef CAS PubMed.
- M. H. Holthausen and J. J. Weigand, Z. Anorg. Allg. Chem., 2012, 638, 1103 CrossRef CAS.
- M. Donath, E. Conrad, P. Jerabek, G. Frenking, R. Fröhlich, N. Burford and J. J. Weigand, Angew. Chem., Int. Ed., 2012, 51, 2964 CrossRef CAS PubMed.
- M. Donath, M. Bodensteiner and J. J. Weigand, Chem. – Eur. J., 2014, 20, 17306 CrossRef CAS PubMed.
- N. Burford, J. C. Landry, M. J. Ferguson and R. McDonald, Inorg. Chem., 2005, 44, 5897 CrossRef CAS PubMed.
- B. H. Christian, R. J. Gillespie and J. F. Sawyer, Inorg. Chem., 1981, 20, 3410 CrossRef CAS.
- J. Beck, S. Schlüter and N. Zotov, Z. Anorg. Allg. Chem., 2005, 631, 2450 CrossRef CAS.
- J. Beck, M. Hengstmann and S. Schlüter, Z. Kristallogr., 2005, 220, 147 CAS.
- M. Ruck, V. Dubenskyy and T. Söhnel, Angew. Chem., Int. Ed., 2003, 42, 2978 CrossRef CAS PubMed.
- D. Michalik, A. Schulz, A. Villinger and N. Weding, Angew. Chem., Int. Ed., 2008, 47, 6465 CrossRef CAS PubMed.
- A. Schulz and A. Villinger, Inorg. Chem., 2009, 48, 7359 CrossRef CAS PubMed.
- M. Lehmann, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2012, 51, 8087 CrossRef CAS PubMed.
- A. Brückner, A. Hinz, J. B. Priebe, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2015, 54, 7426 CrossRef PubMed.
- O. Back, M. A. Celik, G. Frenking, M. Melaimi, B. Donnadieu and G. Bertrand, J. Am. Chem. Soc., 2010, 132, 10262 CrossRef CAS PubMed.
- N. Burford, P. Losier, P. K. Bakshi and T. S. Cameron, J. Chem. Soc., Dalton Trans., 1993, 201 RSC.
- N. Burford, P. Losier, C. Macdonald, V. Kyrimis, P. K. Bakshi and T. S. Cameron, Inorg. Chem., 1994, 33, 1434 CrossRef CAS.
- C. Hering, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2012, 51, 6241 CrossRef CAS PubMed.
- C. Hering, A. Schulz and A. Villinger, Inorg. Chem., 2013, 52, 5214 CrossRef CAS PubMed.
- A. Kraft, J. Beck and I. Krossing, Chem. – Eur. J., 2011, 17, 12975 CrossRef CAS.
- E. Conrad, N. Burford, R. McDonald and M. J. Ferguson, J. Am. Chem. Soc., 2009, 131, 5066 CrossRef CAS PubMed.
- E. Conrad, N. Burford, R. McDonald and M. J. Ferguson, Chem. Commun., 2010, 46, 4598 RSC.
- W. Baumann, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2008, 47, 9530 CrossRef CAS PubMed.
- D. Gudat, A. Haghverdi, H. Hupfer and M. Nieger, Chem. – Eur. J., 2000, 6, 3414 CrossRef CAS.
- M. K. Denk, S. Gupta and A. J. Lough, Eur. J. Inorg. Chem., 1999, 41 CrossRef CAS.
- C. J. Carmalt and V. Lomeli, Chem. Commun., 1997, 2095 RSC.
- H. A. Spinney, I. Korobkov and D. S. Richeson, Chem. Commun., 2007, 1647 RSC.
- N. Burford, T. M. Parks, B. W. Royan, J. F. Richardson and P. S. White, Can. J. Chem., 1992, 70, 703 CrossRef CAS.
- N. Burford, C. L. Macdonald, T. M. Parks, G. Wu, B. Borecka, W. Kwiatkowski and T. S. Cameron, Can. J. Chem., 1996, 74, 2209 CrossRef CAS.
- D. Gudat, T. Gans-Eichler and M. Nieger, Chem. Commun., 2004, 2434 RSC.
- K. O. Christe, M. D. Lind, N. Thorup, D. R. Russell, J. Fawcett and R. Bau, Inorg. Chem., 1988, 27, 2450 CrossRef CAS.
- G. S. H. Chen and J. Passmore, J. Chem. Soc., Dalton Trans., 1979, 1251 RSC.
- C. Aubauer, M. Kaupp, T. M. Klapotke, H. Noth, H. Piotrowski, W. Schnick, J. Senker and M. Suter, J. Chem. Soc., Dalton Trans., 2001, 1880 RSC.
- J. Shamir, S. Luski, A. Bino, S. Cohen and D. Gibson, Inorg. Chem., 1985, 24, 2301 CrossRef CAS.
- S. Pohl, Z. Anorg. Allg. Chem., 1983, 498, 15 CrossRef CAS.
- R. Minkwitz, J. Nowicki and H. Borrmann, Z. Anorg. Allg. Chem., 1991, 596, 93 CrossRef CAS.
- M. Gerken, P. Kolb, A. Wegner, H. P. A. Mercier, H. Borrmann, D. A. Dixon and G. J. Schrobilgen, Inorg. Chem., 2000, 39, 2813 CrossRef CAS PubMed.
- M. Gonsior and I. Krossing, Dalton Trans., 2005, 1203 RSC.
- H. B. Miller, H. W. Baird, C. L. Bramlett and W. K. Templeton, J. Chem. Soc., Chem. Commun., 1972, 262 RSC.
- W. J. Casteel, P. Kolb, N. LeBlond, H. P. A. Mercier and G. J. Schrobilgen, Inorg. Chem., 1996, 35, 929 CrossRef CAS PubMed.
- S. Brownridge, I. Krossing, J. Passmore, H. Jenkins and H. K. Roobottom, Coord. Chem. Rev., 2000, 197, 397 CrossRef CAS.
- I. Krossing, Top. Curr. Chem., 2003, 230, 135 CrossRef CAS.
- W. S. Sheldrick, Molecular Clusters of the Main Group Elements, Wiley-VCH, 2004, p. 230 Search PubMed.
- A. V. Zakharov, I. D. Sadekov and V. I. Minkin, Russ. Chem. Rev., 2006, 75, 207 CrossRef CAS.
- P. F. Kelly and R. King, Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 179–196 Search PubMed.
- R. S. Laitinen and R. Oilunkaniemi, Comprehensive Inorganic Chemistry II, Elsevier, 2013, pp. 197–231 Search PubMed.
- K. V. Shuvaev, J. Passmore, Recent Developments in Main Group Chemistry, 2013, vol. 257, p. 1067 Search PubMed.
- N. Bartlett and D. H. Lohmann, Proc. Chem. Soc., 1962, 115 CAS.
- W. Hujo and S. Grimme, J. Chem. Theory Comput., 2013, 9, 308 CrossRef CAS PubMed.
- G. Santiso-Quiñones, R. Brückner, C. Knapp, I. Dionne, J. Passmore and I. Krossing, Angew. Chem., Int. Ed., 2009, 48, 1133 CrossRef PubMed.
- D. Aris, J. Beck, A. Decken, I. Dionne, I. Krossing, J. Passmore, E. Rivard, F. Steden and X. Wang, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179, 859 CrossRef CAS.
- A. Apblett, A. J. Banister, D. Biron, A. G. Kendrick, J. Passmore, M. Schriver and M. Stojanac, Inorg. Chem., 1986, 25, 4451 CrossRef CAS.
- W. V. F. Brooks, T. S. Cameron, S. Parsons, J. Passmore and M. J. Schriver, Inorg. Chem., 1994, 33, 6230 CrossRef CAS.
- E. G. Awere, J. Passmore and P. S. White, J. Chem. Soc., Dalton Trans., 1993, 299 RSC.
- A. Apblett, T. Chivers and J. F. Fait, J. Chem. Soc., Chem. Commun., 1989, 1596 RSC.
- A. Apblett, T. Chivers and J. F. Fait, Inorg. Chem., 1990, 29, 1643 CrossRef CAS.
- H.-G. Stammler and J. Weiß, Z. Naturforsch., 1989, 44b, 1483 Search PubMed.
- J. Passmore, G. Sutherland, T. Whidden and P. S. White, J. Chem. Soc., Chem. Commun., 1980, 289 RSC.
- W. A. Shantha Nandana, J. Passmore, P. S. White and C.-M. Wong, J. Chem. Soc., Chem. Commun., 1982, 1098 RSC.
- P. D. Boyle, S. Parsons, J. Passmore and D. J. Wood, J. Chem. Soc., Chem. Commun., 1993, 199 RSC.
- T. S. Cameron, A. Decken, F. Grein, C. Knapp, J. Passmore, J. M. Rautiainen, K. V. Shuvaev, R. C. Thompson and D. J. Wood, Inorg. Chem., 2010, 49, 7861 CrossRef CAS PubMed.
- H. Fujihara, H. Mima and N. Furukawa, J. Am. Chem. Soc., 1995, 117, 10153 CrossRef CAS.
- J. L. Dutton, H. M. Tuononen and P. J. Ragogna, Angew. Chem., Int. Ed., 2009, 48, 4409 CrossRef CAS PubMed.
- H. Poleschner and K. Seppelt, Angew. Chem., Int. Ed., 2008, 47, 6461 CrossRef CAS PubMed.
- T. Chivers and J. Konu, Angew. Chem., Int. Ed., 2009, 48, 3025 CrossRef CAS PubMed.
- C. D. Martin, M. C. Jennings, M. J. Ferguson and P. J. Ragogna, Angew. Chem., Int. Ed., 2009, 48, 2210 CrossRef CAS PubMed.
- J. L. Dutton, H. M. Tuononen, M. C. Jennings and P. J. Ragogna, J. Am. Chem. Soc., 2006, 128, 12624 CrossRef CAS PubMed.
- H. Gerding and H. Houtgraaf, Recl. Trav. Chim. Pays-Bas, 1954, 73, 759 CrossRef CAS.
- B. H. Christian, M. J. Collins, R. J. Gillespie and J. F. Sawyer, Inorg. Chem., 1986, 25, 777 CrossRef CAS.
- M. Minoura, T. Mukuda, T. Sagami and K.-y. Akiba, J. Am. Chem. Soc., 1999, 121, 10852 CrossRef CAS.
- D. Naumann, W. Tyrra, R. Herrmann, I. Pantenburg and M. S. Wickleder, Z. Anorg. Allg. Chem., 2002, 628, 833 CrossRef CAS.
- J. P. Johnson, G. K. MacLean, J. Passmore and P. S. White, Can. J. Chem., 1989, 67, 1687 CrossRef CAS.
- M. Minoura, T. Mukuda, T. Sagami and K.-Y. Akiba, Heteroat. Chem., 2001, 12, 380 CrossRef CAS.
- T. Soltner, N. R. Goetz and A. Kornath, Eur. J. Inorg. Chem., 2011, 3076 CrossRef CAS.
- R. Minkwitz and S. Schneider, Angew. Chem., Int. Ed., 1999, 38, 714 CrossRef CAS.
- R. Minkwitz, R. Seelbinder and R. Schöbel, Angew. Chem., Int. Ed., 2002, 41, 111 CrossRef CAS.
- J. A. Ibers, J. Chem. Phys., 1966, 44, 1748 CrossRef CAS.
- J. Passmore, G. Sutherland and P. S. White, J. Chem. Soc., Chem. Commun., 1980, 330 RSC.
- J. Passmore, G. Sutherland and P. S. White, Inorg. Chem., 1982, 21, 2717 CrossRef CAS.
- R. Faggiani, R. J. Gillespie, J. F. Sawyer and J. E. Vekris, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1989, 45, 1847 CrossRef.
- T. S. Cameron, I. Dionne, H. D. B. Jenkins, S. Parsons, J. Passmore and H. K. Roobottom, Inorg. Chem., 2000, 39, 2042 CrossRef CAS PubMed.
- C. G. Davies, R. J. Gillespie, J. J. Park and J. Passmore, Inorg. Chem., 1971, 10, 2781 CrossRef CAS.
- R. C. Burns, R. J. Gillespie and J. F. Sawyer, Inorg. Chem., 1980, 19, 1423 CrossRef CAS.
- G. Cardinal, R. J. Gillespie, J. F. Sawyer and J. E. Vekris, J. Chem. Soc., Dalton Trans., 1982, 765 RSC.
- R. Minkwitz, H. Borrmann and J. Nowicki, Z. Naturforsch., 1991, 46b, 629 Search PubMed.
- J. Beck, Z. Anorg. Allg. Chem., 1995, 621, 131 CrossRef CAS.
- J. Beck, M. Kellner and M. Kreuzinger, Z. Anorg. Allg. Chem., 2002, 628, 2656 CrossRef CAS.
- J. D. Corbett, R. K. McMullan and D. J. Prince, Inorg. Chem., 1971, 10, 1749 CrossRef CAS; B. Buchin, C. Gemel, T. Cadenbach, R. Schmid and R. A. Fischer, Angew. Chem., 2006, 118, 1091 CrossRef.
- M. J. Collins, R. J. Gillespie and J. F. Sawyer, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 405 CrossRef.
- R. C. Burns, W.-L. Chan, R. J. Gillespie, W.-C. Luk, J. F. Sawyer and D. R. Slim, Inorg. Chem., 1980, 19, 1432 CrossRef CAS.
- M. J. Collins, R. J. Gillespie, J. F. Sawyer and G. J. Schrobilgen, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1986, 42, 13 CrossRef.
- J. Beck and S. J. Eck, Z. Anorg. Allg. Chem., 2010, 636, 1910 CrossRef CAS.
- J. Beck and J. Wetterau, Inorg. Chem., 1995, 34, 6202 CrossRef CAS.
- J. Beck and A. Fischer, Z. Anorg. Allg. Chem., 1997, 623, 780 CrossRef CAS.
- T. W. Couch, D. A. Lokken and J. D. Corbett, Inorg. Chem., 1972, 11, 357 CrossRef CAS.
- J. Beck, Z. Naturforsch., 1990, 45b, 413 Search PubMed.
- J. Beck, Z. Naturforsch., 1994, 49b, 1159 Search PubMed.
- J. Beck, Chem. Ber., 1991, 124, 677 CrossRef CAS.
- J. Beck and G. Bock, Z. Naturforsch., 1996, 51b, 119 Search PubMed.
- J. Beck, M. Kasper and A. Stankowski, Chem. Ber., 1997, 130, 1189 CrossRef CAS.
- M. J. Collins, R. J. Gillespie, J. W. Kolis and J. F. Sawyer, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1987, 43, 2033 CrossRef.
- J. Beck, Z. Naturforsch., 1990, 45b, 1610 Search PubMed.
- J. Beck, A. Fischer and A. Stankowski, Z. Anorg. Allg. Chem., 2002, 628, 2542 CrossRef CAS.
- J. Beck, Chem. Ber., 1995, 128, 23 CrossRef CAS.
- J. Beck and G. Bock, Z. Anorg. Allg. Chem., 1996, 622, 823 CrossRef CAS.
- R. J. Gillespie, W. Luk and D. R. Slim, J. Chem. Soc., Chem. Commun., 1976, 791 RSC; R. C. Burns, R. J. Gillespie, W.-C. Luk and D. R. Slim, Inorg. Chem., 1979, 18, 3086 CrossRef CAS.
- G. W. Drake, G. L. Schimek and J. W. Kolis, Inorg. Chem., 1996, 35, 1740 CrossRef CAS PubMed.
- J. Beck, Angew. Chem., Int. Ed., 1991, 30, 1128 CrossRef.
- J. Beck, Z. Anorg. Allg. Chem., 1993, 619, 237 CrossRef CAS.
- J. Beck, Angew. Chem., Int. Ed., 1990, 29, 293 CrossRef.
- J. Beck and K. Müller-Buschbaum, Z. Anorg. Allg. Chem., 1997, 623, 409 CrossRef CAS.
- J. Beck and A. Stankowski, Z. Naturforsch., 2001, 56b, 453 Search PubMed.
- J. Beck and A. Fischer, Z. Anorg. Allg. Chem., 2002, 628, 369 CrossRef CAS.
- D. Freudenmann and C. Feldmann, Z. Anorg. Allg. Chem., 2011, 637, 1481 CrossRef CAS.
- J. Beck and G. Bock, Angew. Chem., Int. Ed., 1995, 34, 2559 CrossRef CAS.
- G. Santiso-Quiñones, A. Higelin, J. Schaefer, R. Brückner, C. Knapp and I. Krossing, Chem. – Eur. J., 2009, 15, 6663 CrossRef PubMed.
- T. S. Cameron, A. Decken, I. Dionne, M. Fang, I. Krossing and J. Passmore, Chem. – Eur. J., 2002, 8, 3386 CrossRef CAS.
- D. Aris, J. Beck, A. Decken, I. Dionne, J. Schmedt auf der Günne, W. Hoffbauer, T. Köchner, I. Krossing, J. Passmore, E. Rivard, F. Steden and X. Wang, Dalton Trans., 2011, 40, 5865 RSC.
- T. Köchner, N. Trapp, T. A. Engesser, A. J. Lehner, C. Röhr, S. Riedel, C. Knapp, H. Scherer and I. Krossing, Angew. Chem., Int. Ed., 2011, 50, 11253 CrossRef PubMed.
- U. Thewalt, K. Berhalter and P. Müller, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1982, 38, 1280 Search PubMed.
- R. J. Gillespie, D. R. Slim and J. D. Tyrer, J. Chem. Soc., Chem. Commun., 1977, 253 RSC.
- R. J. Gillespie, J. F. Sawyer, D. R. Slim and J. D. Tyrer, Inorg. Chem., 1982, 21, 1296 CrossRef CAS.
- P. Bakshi, P. D. Boyle, T. S. Cameron, J. Passmore, G. Schatte and G. W. Sutherland, Inorg. Chem., 1994, 33, 3849 CrossRef CAS.
- S. Parsons, J. Passmore and P. S. White, J. Chem. Soc., Dalton Trans., 1993, 1499 RSC.
- R. Faggiani, R. J. Gillespie and J. E. Vekris, J. Chem. Soc., Chem. Commun., 1988, 902 RSC.
- R. J. Gillespie, W. Luk, E. Maharajh and D. R. Slim, Inorg. Chem., 1977, 16, 892 CrossRef CAS.
- R. Faggiani, R. J. Gillespie and J. W. Kolis, J. Chem. Soc., Chem. Commun., 1987, 592 RSC.
- W. A. Shantha Nandana, J. Passmore, P. S. White and C. M. Wong, Inorg. Chem., 1989, 28, 3320 CrossRef CAS; J. Passmore, P. S. White and C.-M. Wong, J. Chem. Soc., Chem. Commun., 1985, 1178 RSC.
- J. Beck, J. Richter, M. A. Pell and J. A. Ibers, Z. Anorg. Allg. Chem., 1996, 622, 473 CrossRef CAS.
- J. Beck and T. Schlörb, Phosphorus, Sulfur Silicon Relat. Elem., 1997, 124, 305 Search PubMed.
- M. J. Collins, R. J. Gillespie and J. F. Sawyer, Inorg. Chem., 1987, 26, 1476 CrossRef CAS.
- P. Boldrini, I. D. Brown, R. J. Gillespie, P. R. Ireland, W. Luk, D. R. Slim and J. E. Vekris, Inorg. Chem., 1976, 15, 765 CrossRef CAS.
- S. Herler, P. Mayer, H. Nöth, A. Schulz, M. Suter and M. Vogt, Angew. Chem., Int. Ed., 2001, 40, 3173 CrossRef CAS.
- M. J. Collins, R. J. Gillespie, J. F. Sawyer and G. J. Schrobilgen, Inorg. Chem., 1986, 25, 2053 CrossRef CAS.
- E. G. Awere, J. Passmore and P. S. White, J. Chem. Soc., Dalton Trans., 1992, 1267 RSC; E. G. Awere, J. Passmore, P. S. White and T. Klapotke, J. Chem. Soc., Chem. Commun., 1989, 1415 RSC.
- P. Boldrini, I. D. Brown, M. J. Collins, R. J. Gillespie, E. Maharajh, D. R. Slim and J. F. Sawyer, Inorg. Chem., 1985, 24, 4302 CrossRef CAS.
- R. J. Gillespie, P. R. Ireland and J. E. Vekris, Can. J. Chem., 1975, 53, 3147 CrossRef CAS.
- R. J. Gillespie, J. P. Kent and J. F. Sawyer, Inorg. Chem., 1981, 20, 3784 CrossRef CAS.
- R. J. Gillespie, J. P. Kent and J. F. Sawyer, Inorg. Chem., 1981, 20, 4053 CrossRef CAS.
- B. Mueller, H. Poleschner and K. Seppelt, Dalton Trans., 2008, 4424 RSC.
- A. Wakamiya, T. Nishinaga and K. Komatsu, J. Am. Chem. Soc., 2002, 124, 15038 CrossRef CAS PubMed.
- B. Mueller, T. T. Takaluoma, R. S. Laitinen and K. Seppelt, Eur. J. Inorg. Chem., 2011, 4970 CrossRef CAS.
- S. Zhang, X. Wang, Y. Sui and X. Wang, J. Am. Chem. Soc., 2014, 136, 14666 CrossRef CAS PubMed.
- O. Mallow, M. A. Khanfar, M. Malischewski, P. Finke, M. Hesse, E. Lork, T. Augenstein, F. Breher, J. R. Harmer, N. V. Vasilieva, A. Zibarev, A. S. Bogomyakov, K. Seppelt and J. Beckmann, Chem. Sci., 2015, 6, 497 RSC.
- R. Laitinen, R. Steudel and R. Weiss, J. Chem. Soc., Dalton Trans., 1986, 1095 RSC.
- R. Destro, V. Lucchini, G. Modena and L. Pasquato, J. Org. Chem., 2000, 65, 3367 CrossRef CAS PubMed.
- H. Fujihara, H. Mima, T. Erata and N. Furukawa, J. Am. Chem. Soc., 1992, 114, 3117 CrossRef CAS.
- D. D. Gibler, C. J. Adams, M. Fischer, A. Zalkin and N. Bartlett, Inorg. Chem., 1972, 11, 2325 CrossRef CAS.
- T. E. Mallouk, G. L. Rosenthal, G. Mueller, R. Brusasco and N. Bartlett, Inorg. Chem., 1984, 23, 3167 CrossRef CAS.
- A. J. Edwards, J. Chem. Soc., Dalton Trans., 1978, 1723 RSC.
- A. Finch, P. N. Gates, T. H. Page, K. B. Dillon and T. C. Waddington, J. Chem. Soc., Dalton Trans., 1980, 2401 RSC.
- W. Sawodny, K. Rediess and U. Thewalt, Z. Anorg. Allg. Chem., 1983, 499, 81 CrossRef CAS.
- S. I. Trojanow, L. Kolditz and A. Radde, Z. Chem., 1983, 23, 136 CrossRef CAS.
- J. Beck and M. Hengstmann, Z. Naturforsch., 1996, 51b, 1415 Search PubMed.
- J. P. Johnson, M. Murchie, J. Passmore, M. Tajik, P. S. White and C.-M. Wong, Can. J. Chem., 1988, 66, 2671 CrossRef.
- J. P. Johnson, M. Murchie, J. Passmore, M. Tajik, P. S. White and C.-M. Wong, Can. J. Chem., 1987, 65, 2744 CrossRef CAS.
- W. V. F. Brooks, G. K. MacLean, J. Passmore, P. S. White and C.-M. Wong, J. Chem. Soc., Dalton Trans., 1983, 1961 RSC.
- A. J. Edwards and G. R. Jones, J. Chem. Soc. A, 1970, 1491 RSC; A. J. Edwards and G. R. Jones, J. Chem. Soc. A, 1970, 1891 RSC; A. J. Edwards and G. R. Jones, Chem. Commun., 1968, 346 RSC.
- B. A. Stork-Blaisse and C. Romers, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1971, 27, 386 CrossRef CAS.
- A. Baumann and J. Beck, Z. Anorg. Allg. Chem., 1998, 624, 1725 CrossRef CAS.
- P. G. Jones, R. Schelbach and E. Schwarzmann, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1987, 43, 607 CrossRef.
- J. Passmore, E. K. Richardson, T. K. Whidden and P. S. White, Can. J. Chem., 1980, 58, 851 CrossRef CAS.
- J. Beck and A. Fischer, Z. Anorg. Allg. Chem., 1995, 621, 1042 CrossRef CAS.
- A. J. Edwards and P. Taylor, J. Chem. Soc., Dalton Trans., 1973, 2150 RSC.
- B. Krebs, B. Buss and D. Altena, Z. Anorg. Allg. Chem., 1971, 386, 257 CrossRef CAS.
- P. G. Jones, D. Jentsch and E. Schwarzmann, Z. Naturforsch., 1986, 41b, 1483 CAS.
- J. Beck, Z. Naturforsch., 1991, 46b, 183 Search PubMed.
- J. Beck, P. Biedenkopf and K. Müller-Buschbaum, Z. Naturforsch., 1996, 51b, 727 Search PubMed.
- J. Beck, Z. Naturforsch., 1996, 51b, 1127 Search PubMed.
- J. Beck and T. Schlörb, Z. Kristallogr., 1999, 214, 780 CAS.
- C. Freire-Erdbrügger, D. Jentsch, P. G. Jones and E. Schwarzmann, Z. Naturforsch., 1987, 42b, 1553 Search PubMed.
- J. Passmore, G. Sutherland and P. S. White, Can. J. Chem., 1981, 59, 2876 CrossRef CAS.
- E. Schulz Lang, U. Abram, J. Strähle and E. M. Vazquez Lopez, Z. Anorg. Allg. Chem., 1998, 624, 999 CrossRef CAS.
- J. Beck and F. Steden, Z. Naturforsch., 2003, 58b, 711 Search PubMed.
- T. A. Engesser, P. Hrobárik, N. Trapp, P. Eiden, H. Scherer, M. Kaupp and I. Krossing, ChemPlusChem, 2012, 77, 643 CrossRef CAS.
- A. B. Bergholdt, K. Kobayashi, E. Horn, O. Takahashi, S. Sato, N. Furukawa, M. Yokoyama and K. Yamaguchi, J. Am. Chem. Soc., 1998, 120, 1230 CrossRef CAS.
- M. Hopfinger, K. Lux and A. Kornath, ChemPlusChem, 2012, 77, 476 CrossRef CAS.
- H.-J. Frohn, M. E. Hirschberg, A. Wenda and V. V. Bardin, J. Fluorine Chem., 2008, 129, 459 CrossRef CAS.
- R. P. Tuckett, A. R. Dale, D. M. Jaffey, P. S. Jarrett and T. Kelly, Mol. Phys., 1983, 49, 475 CrossRef CAS; T. L. Porter, J. Chem. Phys., 1968, 48, 2071 CrossRef.
- T. Schlöder and S. Riedel, RSC Adv., 2012, 2, 876 RSC.
- R. P. Singh and J. M. Shreeve, Acc. Chem. Res., 2004, 37, 31 CrossRef CAS PubMed.
- S. Seidel and K. Seppelt, Angew. Chem., Int. Ed., 2000, 39, 3923 CrossRef CAS.
- K. O. Christe, R. Bau and D. Zhao, Z. Anorg. Allg. Chem., 1991, 593, 46 CrossRef CAS.
- T. S. Cameron, J. Passmore and X. Wang, Angew. Chem., Int. Ed., 2004, 43, 1995 CrossRef CAS PubMed.
- A. Y. Rogachev and R. Hoffmann, J. Am. Chem. Soc., 2013, 135, 3262 CrossRef CAS PubMed; F. A. Cotton, E. V. Dikarev and M. A. Petrukhina, Angew. Chem., Int. Ed., 2000, 39, 2362 CrossRef.
- P. Malinowski, D. Himmel and I. Krossing, 2015, to be submitted.
- M.-J. Crawford, M. Göbel, K. Karaghiosoff, T. M. Klapötke and J. M. Welch, Inorg. Chem., 2009, 48, 9983 CrossRef CAS PubMed.
- F. J. Fañanás, M. Alvarez-Pérez and F. Rodríguez, Chem. – Eur. J., 2005, 11, 5938 CrossRef PubMed.
- R. J. Gillespie and M. J. Morton, Inorg. Chem., 1970, 9, 811 CrossRef CAS; M. Schmeißer, W. Ludovici, D. Naumann, P. Sartori and E. Scharf, Chem. Ber., 1968, 101, 4214 CrossRef.
- N. Thorup and J. Shamir, Inorg. Nucl. Chem. Lett., 1981, 17, 193 CrossRef CAS.
- S. Pohl and W. Saak, Z. Naturforsch., 1981, 36b, 83 Search PubMed.
- T. Birchall and R. D. Myers, Inorg. Chem., 1982, 21, 213 CrossRef CAS.
- E. S. Stoyanov, I. V. Stoyanova, F. S. Tham and C. A. Reed, J. Am. Chem. Soc., 2010, 132, 4062 CrossRef CAS PubMed.
- R. S. Brown, R. W. Nagorski, A. J. Bennet, R. E. D. McClung, G. H. M. Aarts, M. Klobukowski, R. McDonald and B. D. Santarsiero, J. Am. Chem. Soc., 1994, 116, 2448 CrossRef CAS.
- H.-J. Frohn, A. Wenda and U. Flörke, Z. Anorg. Allg. Chem., 2008, 634, 764 CrossRef CAS.
- K. O. Christe, E. C. Curtis and C. J. Schack, Inorg. Chem., 1972, 11, 2212 CrossRef CAS; C. J. Schack, C. B. Lindahl, D. Pilipovich and K. O. Christe, Inorg. Chem., 1972, 11, 2201 CrossRef.
- M. Adelhelm and E. Jacob, Angew. Chem., Int. Ed., 1977, 16, 461 CrossRef.
- J. F. Lehmann, G. J. Schrobilgen, K. O. Christe, A. Kornath and R. J. Suontamo, Inorg. Chem., 2004, 43, 6905 CrossRef CAS PubMed.
- K. O. Christe, X. Zhang, J. A. Sheehy and R. Bau, J. Am. Chem. Soc., 2001, 123, 6338 CrossRef CAS PubMed.
- A. Vij, F. S. Tham, V. Vij, W. W. Wilson and K. O. Christe, Inorg. Chem., 2002, 41, 6397 CrossRef CAS PubMed.
- A. J. Edwards, G. R. Jones and R. J. C. Sills, Chem. Commun., 1968, 1527 RSC; A. J. Edwards and G. R. Jones, J. Chem. Soc. A, 1971, 2318 RSC.
- A. Apblett, F. Grein, J. P. Johnson, J. Passmore and P. S. White, Inorg. Chem., 1986, 25, 422 CrossRef CAS.
- R. J. Gillespie, R. Kapoor, R. Faggiani, C. J. L. Lock, M. Murchie and J. Passmore, J. Chem. Soc., Chem. Commun., 1983, 8 RSC; R. Faggiani, R. J. Gillespie, R. Kapoor, C. J. L. Lock and J. E. Vekris, Inorg. Chem., 1988, 27, 4350 CrossRef CAS.
- H. Lynton and J. Passmore, Can. J. Chem., 1971, 49, 2539 CrossRef CAS.
- A. J. Edwards and R. J. C. Sills, J. Chem. Soc. A, 1970, 2697 RSC.
- R. Bougon, W. V. Cicha, M. Lance, L. Meublat, M. Nierlich and J. Vigner, Inorg. Chem., 1991, 30, 102 CrossRef CAS.
- A. J. Edwards and G. R. Jones, Chem. Commun., 1967, 1304 RSC; A. J. Edwards and G. R. Jones, J. Chem. Soc. A, 1969, 1467 RSC.
- H.-J. Frohn, M. Giesen and D. Welting, Eur. J. Solid State Inorg. Chem., 1996, 33, 841 CAS.
- T. Birchall and R. D. Myers, Inorg. Chem., 1981, 20, 2207 CrossRef CAS.
- T. Birchall and R. D. Myers, Inorg. Chem., 1983, 22, 1751 CrossRef CAS.
- F. Bailly, P. Barthen, H.-J. Frohn and M. Köckerling, Z. Anorg. Allg. Chem., 2000, 626, 2419 CrossRef CAS.
- J. F. Lehmann, S. Riedel and G. J. Schrobilgen, Inorg. Chem., 2008, 47, 8343 CrossRef CAS PubMed.
- A. J. Edwards and R. J. C. Sills, J. Chem. Soc., Dalton Trans., 1974, 1726 RSC.
- T. E. Mallouk, B. Desbat and N. Bartlett, Inorg. Chem., 1984, 23, 3160 CrossRef CAS.
- K. M. Tobias and M. Jansen, Angew. Chem., Int. Ed., 1986, 25, 993 CrossRef.
- M. D. Lind and K. O. Christe, Inorg. Chem., 1972, 11, 608 CrossRef CAS.
- H. W. Baird and H. F. Giles, Acta Crystallogr., 1969, A25, 115 Search PubMed.
- A. J. Edwards and P. Taylor, J. Chem. Soc., Dalton Trans., 1975, 2174 RSC.
- L. Graham, O. Graudejus, N. K. Jha and N. Bartlett, Coord. Chem. Rev., 2000, 197, 321 CrossRef CAS.
- J. F. Lehmann and G. J. Schrobilgen, J. Fluorine Chem., 2003, 119, 109 CrossRef CAS.
- R. J. Gillespie and G. J. Schrobilgen, Inorg. Chem., 1974, 13, 765 CrossRef CAS.
- K. O. Christe, W. W. Wilson and E. C. Curtis, Inorg. Chem., 1983, 22, 3056 CrossRef CAS.
- R. J. Gillespie and G. J. Schrobilgen, J. Chem. Soc., Chem. Commun., 1974, 90 RSC; R. J. Gillespie and G. J. Schrobilgen, Inorg. Chem., 1974, 13, 1230 CrossRef CAS.
- J. H. Holloway and G. J. Schrobilgen, J. Chem. Soc., Chem. Commun., 1975, 623 RSC.
- V. M. McRae, R. D. Peacock and D. R. Russell, J. Chem. Soc. D, 1969, 62 RSC.
- N. Bartlett, F. Einstein, D. F. Stewart and J. Trotter, J. Chem. Soc. A, 1967, 1190 RSC.
- N. Bartlett, F. Einstein, D. F. Stewart and J. Trotter, Chem. Commun., 1966, 550 RSC.
- M. J. Hughes, H. P. A. Mercier and G. J. Schrobilgen, Inorg. Chem., 2010, 49, 3501 CrossRef CAS PubMed.
- H. S. A. Elliott, J. F. Lehmann, H. P. A. Mercier, H. D. B. Jenkins and G. J. Schrobilgen, Inorg. Chem., 2010, 49, 8504 CrossRef CAS PubMed.
- Z. Mazej and E. A. Goreshnik, J. Fluorine Chem., 2015, 175, 47 CrossRef CAS.
- H. J. Frohn, S. Jakobs and G. Henkel, Angew. Chem., Int. Ed., 1989, 28, 1506 CrossRef.
- B. E. Pointner, R. J. Suontamo and G. J. Schrobilgen, Inorg. Chem., 2006, 45, 1517 CrossRef CAS PubMed.
- T. Drews, S. Seidel and K. Seppelt, Angew. Chem., Int. Ed., 2002, 41, 454 CrossRef CAS.
- S. Seidel, K. Seppelt, C. van Wüllen and X. Y. Sun, Angew. Chem., Int. Ed., 2007, 46, 6717 CrossRef CAS PubMed.
- S. Seidel and K. Seppelt, Science, 2000, 290, 117 CrossRef CAS PubMed.
- I.-C. Hwang, S. Seidel and K. Seppelt, Angew. Chem., Int. Ed., 2003, 42, 4392 CrossRef CAS PubMed.
- J. F. Lehmann, D. A. Dixon and G. J. Schrobilgen, Inorg. Chem., 2001, 40, 3002 CrossRef CAS PubMed.
- N. Bartlett, M. Gennis, D. D. Gibler, B. K. Morrell and A. Zalkin, Inorg. Chem., 1973, 12, 1717 CrossRef CAS.
- A. Zalkin, D. L. Ward, R. N. Biagioni, D. H. Templeton and N. Bartlett, Inorg. Chem., 1978, 17, 1318 CrossRef CAS.
- J. F. Sawyer, G. J. Schrobilgen and S. J. Sutherland, Inorg. Chem., 1982, 21, 4064 CrossRef CAS.
- F. O. Sladky, P. A. Bulliner, N. Bartlett, B. G. DeBoer and A. Zalkin, Chem. Commun., 1968, 1048 RSC.
- N. Bartlett, B. G. DeBoer, F. J. Hollander, F. O. Sladky, D. H. Templeton and A. Zalkin, Inorg. Chem., 1974, 13, 780 CrossRef CAS.
- B. A. Fir, M. Gerken, B. E. Pointner, H. P. A. Mercier, D. A. Dixon and G. J. Schrobilgen, J. Fluorine Chem., 2000, 105, 159 CrossRef CAS.
- R. Faggiani, D. K. Kennepohl, C. J. L. Lock and G. J. Schrobilgen, Inorg. Chem., 1986, 25, 563 CrossRef CAS.
- K. Koppe, H.-J. Frohn, H. P. A. Mercier and G. J. Schrobilgen, Inorg. Chem., 2008, 47, 3205 CrossRef CAS PubMed.
- H.-J. Frohn, T. Schroer and G. Henkel, Angew. Chem., Int. Ed., 1999, 38, 2554 CrossRef CAS.
- B. A. Fir, H. P. A. Mercier, J. C. P. Sanders, D. A. Dixon and G. J. Schrobilgen, J. Fluorine Chem., 2001, 110, 89 CrossRef CAS.
- H. P. A. Mercier, M. D. Moran, J. C. P. Sanders, G. J. Schrobilgen and R. J. Suontamo, Inorg. Chem., 2005, 44, 49 CrossRef CAS PubMed.
- S. Seidel and K. Seppelt, Angew. Chem., Int. Ed., 2001, 40, 4225 CrossRef CAS.
- B. Fir, J. M. Whalen, H. P. A. Mercier, D. A. Dixon and G. J. Schrobilgen, Inorg. Chem., 2006, 45, 1978 CrossRef CAS PubMed.
- G. L. Smith, H. P. A. Mercier and G. J. Schrobilgen, Inorg. Chem., 2007, 46, 1369 CrossRef CAS PubMed.
- G. L. Smith, H. P. A. Mercier and G. J. Schrobilgen, J. Am. Chem. Soc., 2009, 131, 7272 CrossRef CAS PubMed.
- G. L. Smith and G. J. Schrobilgen, Inorg. Chem., 2009, 48, 7714 CrossRef CAS PubMed.
- M. Gerken, M. D. Moran, H. P. A. Mercier, B. E. Pointner, G. J. Schrobilgen, B. Hoge, K. O. Christe and J. A. Boatz, J. Am. Chem. Soc., 2009, 131, 13474 CrossRef CAS PubMed.
- D. E. McKee, A. Zalkin and N. Bartlett, Inorg. Chem., 1973, 12, 1713 CrossRef CAS.
- R. J. Gillespie, D. Martin, G. J. Schrobilgen and D. R. Slim, J. Chem. Soc., Dalton Trans., 1977, 2234 RSC.
- D. S. Brock, H. P. A. Mercier and G. J. Schrobilgen, J. Am. Chem. Soc., 2013, 135, 5089 CrossRef CAS PubMed.
- K. Leary, D. H. Templeton, A. Zalkin and N. Bartlett, Inorg. Chem., 1973, 12, 1726 CrossRef CAS.
- Z. Mazej and E. Goreshnik, Eur. J. Inorg. Chem., 2009, 4503 CrossRef CAS.
- K. Lutar, A. Jesih, I. Leban, B. Zemva and N. Bartlett, Inorg. Chem., 1989, 28, 3467 CrossRef CAS.
- B. Zemva, A. Jesih, D. H. Templeton, A. Zalkin, A. K. Cheetham and N. Bartlett, J. Am. Chem. Soc., 1987, 109, 7420 CrossRef CAS.
- K. Leary, A. Zalkin and N. Bartlett, Inorg. Chem., 1974, 13, 775 CrossRef CAS.
Footnote |
| † The names of the co-authors are ordered alphabetically. |
| This journal is © The Royal Society of Chemistry 2016 |