Controlled capture and release of cardiac fibroblasts using peptide-functionalized alginate gels in microfluidic channels†
Brian D.
Plouffe
a,
Melissa A.
Brown
bc,
Rohin K.
Iyer
b,
Milica
Radisic
*bc and
Shashi K.
Murthy
*a
aDepartment of Chemical Engineering, Northeastern University, Boston, Massachusetts 02115, USA. E-mail: smurthy@coe.neu.edu; Fax: +1 (617) 373-2209; Tel: +1 (617) 373-4017
bDepartment of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, Canada. E-mail: m.radisic@utoronto.ca; Fax: +1 (416) 978- 8605; Tel: +1 (416) 946-5295
cInstitute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, Canada
First published on 12th March 2009
Abstract
The utilization of peptide-functionalized hydrogels in combination with a divalent chelator offers an effective methodology for capture and release of cells within microfluidic channels.
Tissue and stem cell engineering are potential methods of tissue regeneration and repair that have seen major research efforts in recent years, particularly with the discovery of rare stem and progenitor cell populations in tissue niches.1,2 The isolation of these rare cell subpopulations is a challenge, particularly if the separation is to be carried out in clinical point-of-care settings. The ability of microfluidic devices to isolate rare cells was recently demonstrated by Nagrath et al.3 with antibody-coated micropillars that captured circulating tumor cells from whole blood.
Adhesion-based cell separation within microfluidic devices can be carried out by either positive selection, as demonstrated by Nagrath et al.,3 or by negative selection.4 Negative selection is an attractive approach when markers for target cells are not fully known but its implementation poses several challenges. Since this approach requires 100% removal of the non-target cells with minimal target cell capture, extensive characterization of the adhesion profiles of all cell types in the heterogeneous suspension is needed. Positive selection, by contrast, involves direct capture of the target cell type, and is therefore easier to implement. However, a major limitation of this approach is the lack of techniques to recover cells with minimal perturbation from the microfluidic device following isolation. In the aforementioned study, Nagrath et al.3 lysed the captured cells on chip in order to extract genomic and proteomic information. On-chip cell lysis works well for devices designed for diagnostic applications, but is not suitable for applications requiring recovery of viable and functional cells.
Few studies have focused on the detachment of cells after capture in microfluidic devices. The detachment of cells adhered to any surface requires the application of a force that is greater in magnitude to that of adhesion. Fluid shear forces have been shown to be the simplest method for cell detachment.5–7 Although this is a local and simple method of cell release, excessive exposure to fluid shear may result in cell damage and reduction in viability. An alternative approach is to cleave the protein ligand that is bound to the capture surface using enzymes, such as trypsin. However enzymatic exposure can cause morphological changes due to a disruption of the cell membrane and glycocalyx, leading to losses in cellular activity. Furthermore, enzymatic digestion has been shown to directly affect both the behavior and chemical makeup of the cells themselves.8,9 These limitations illustrate the need to establish a general technique to capture and release cells in micro-scale devices without extensive physical or chemical perturbations to the cell environment.
Several recent publications in the literature have described the design of surface coatings that can facilitate cell detachment when an external stimulus is applied, such as an electrical potential or a small temperature change.10–14 An example of the former is a surface coating that consists of ligands bound to the surface via an electroactive chemical functional group.12 The electroactive quinoine ester undergoes a chemical change to lactone upon applying an electrical potential. This approach requires electrode incorporation into the capture device and careful optimization of release parameters. The use of a thermally-responsive polymer, such as poly(N-isopropylacrylamide), which is hydrophobic at 37 °C and hydrophilic at 20 °C, is another recently-described approach.10,11,14 The hydrophobic surface is adhesive to cells and its transformation results in nearly-complete cell release. The shortcomings of this method are the lack of adhesion specificity in a flow regime and potential adverse effects of lowering the temperature below the physiological temperature of 37 °C.
In this communication, we describe the use of peptide-functionalized alginic acid as a method for cell capture and release within microfluidic channels. An alginate complex was adsorbed on the surface of the device and converted into a thin hydrogel layer by means of a rinse with Ca2+ ionic solution. Primary rat cardiac fibroblasts were injected into the device and subsequently captured from the flow stream by the peptide-functionalized gel. Following capture, the hydrogel was dissolved using ethylene diamine tetraacetic acid (EDTA), a strong chelator of divalent ions, thereby releasing the captured cells. This method is simple and amendable to all micro-scale devices that operate in a low shear stress regime. In addition, the carbodiimide conjugation chemistry utilized to functionalize alginic acid can be applied to a wide range of molecules containing primary amines. Furthermore, cell release requires no external forces such as heating/cooling, electrical potential, or irradiation of any kind.
Previous studies by our group4,15 have illustrated the utility of using surface-immobilized tetrapeptides to achieve cell capture within microfluidic devices. Specifically, the arg-gly-asp-ser (RGDS) tetrapeptide has been shown to provide a high degree of fibroblast adhesion within microchannels.4 In the present work, this tetrapeptide was conjugated to sodium alginate using carbodiimide chemistry with a N-hydroxysulfosuccinimide ester (sulfo-NHS) stabilizer.16 The amount of RGDS bound to alginic acid using this protocol was then quantified using Fourier transform infrared spectroscopy (FT-IR). The amide group in RGDS shows a peak at 638 cm−1 where there are no alginate-associated absorptions, as shown in Fig. 1. Comparison of the amide peak area of the alginate–RGDS sample and aqueous RGDS solutions of known concentrations (100 µg mL−1, 50 µg mL−1 and 25 µg mL−1 RGDS in deionized water; open symbols in Fig. 1) allows quantification of the peptide bound to alginate. Specifically, the RGDS content in the alginate solution (solid black symbols in Fig. 1) was determined to be 38 µg of RGDS per milligram of solid modified alginate stock.
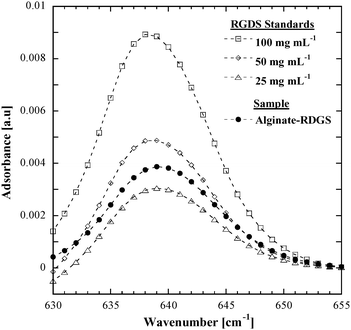 | ||
| Fig. 1 RGDS content in the alginate–RGDS was quantified using FT-IR and a three point calibration. Open symbols represent standards and the solid black symbols represent the alginate–RGDS sample. | ||
Prior to the microfluidic cell adhesion experiments, we first conducted a dose response of cell attachment and detachment in static culture by placing glass slides spin-coated with 2 mg mL−1alginate or alginate–RGDS in tissue culture wells and incubating with the cell suspension of a desired concentration for 45 min. As expected, this study (Fig. 2) indicated that the alginate cell capture and release system was sensitive to the total number of cells seeded. Fig 2(a) illustrates greater cell capture with larger numbers of seeded cells. Importantly, alginate–RGDS supported significantly higher attachment of cells than unmodified alginate at every concentration (as determined by Tukey's test with one-way ANOVA analysis, p < 0.001), whereas bare glass supported the highest cell attachment, in agreement with literature.17
 | ||
| Fig. 2 Dose response of (a) cell attachment and (b) detachment on alginate hydrogel under static conditions on bare glass, native alginate, and alginate–RGDS. Three concentrations of primary rat fibroblasts in serum-free medium were investigated and cells were released using a 50 mM EDTA solution with culture medium rinse. Release percentages determined from images before and after EDTA incubation. Error bars denote standard errors for four replicates of each experiment. | ||
To identify conditions for cell release without loss of cell viability, we incubated suspensions of cardiac fibroblasts in serum-free medium (10 × 104cell mL−1) followed by different EDTA solutions (with concentrations of 0.5, 5, 10, 25, 50 mM) in phosphate buffered saline (PBS) for 15 min on an orbital shaker at 70 rpm. Adhered fibroblasts incubated with PBS or trypsin–EDTA were used as controls. As shown in Fig. S1 in the ESI,† this experiment showed that the cell retrieval was approximately the same, within the margin of error, for all EDTA concentrations in buffer (< 70%). However, a marked difference in cell release was shown for 50 mM EDTA when incubation was followed by a culture medium rinse (82 ± 8%), a release comparable to trypsin–EDTA positive controls (84 ± 5%). Furthermore, the viability of the EDTA released cells (mean: 82%; Fig. S1)† was observed to be comparable to that of cells incubated with PBS (78.0 ± 4.4%) and with trypsin–EDTA (89.3 ± 1.7%). Hence, an EDTA concentration of 50 mM accompanied by a culture medium rinse was used for all subsequent cell detachment studies.
The results of cell-detachment experiments using this optimized release method are shown in Fig. 2(b) for three different concentrations of cells seeded. Fig 2(b) shows that alginate and alginate–RGDS both released over 98% of the cells captured using this optimized solution. Although bare glass showed the highest cell adhesion after plating for all seeding densities (Fig 2(a)), cells could not be completely detached from bare glass using the 50 mM EDTA solution. Moreover, Fig 2(b) illustrates that cell release was inversely proportional to the number of cells in the seeding solution, a result of the higher number of cells attaching to the respective substrate.
The microfluidic device used in this investigation was a 1 mm × 50 mm × 0.07 mm (W × L × H) channel, fabricated by standard soft lithography techniques (as described in the ESI).†Cell adhesion was investigated on two different channel surfaces: channels coated with unconjugated alginate hydrogel, and channels coated with peptide-conjugated alginate hydrogel. Cardiac fibroblasts were used for all experiments and fluid flow rates for all steps (incubation, rinse, cell seeding, and cell release) were controlled to ensure a shear stress of 1 dyn cm−2.
Microchannel surfaces were coated using a 12 mg mL−1 solution of native alginate or peptide-modified alginate. It should be noted that a higher concentration of alginate and alginate–RGDS with respect to static experiments was used in the flow devices as lower concentrations of alginate/alginate–RGDS resulted in no hydrogel coating within the channel. (The presence of hydrogel coating was verified by fluorescence microscopy as described in ESI.)† Per the calibration shown in Fig. 1, the 12 mg mL−1alginate–RGDS has a peptide content of 456 µg RGDS mL−1.
For flow experiments, microfluidic devices were prepared by flowing solutions of alginate or alginate–RGDS into the devices and incubating to allow alginate adsorption. The adsorbed alginate/alginate–RGDS was then converted into a thin hydrogel layer by flowing a 100 mM CaCl2 solution. Suspensions of cardiac fibroblasts (10 × 104cells mL−1) were then injected and cell adhesion was measured by counting cells at select locations along each device. A 50 mM EDTA solution rinse was then performed to release the cells.
Protein conjugation to alginate has been well established in research involving cell encapsulation, cell transplantation, and tissue engineering applications.18–21 By applying these same concepts to microfluidic devices in conjunction with the ability of EDTA to chelate Ca2+ ions,22 capture and release of cells can be achieved. As shown in Fig. 3(a), conjugation of RGDS to the alginate backbone showed a two-fold increase in fibroblast adhesion compared to unconjugated alginate; the unconjugated alginate controls showed 23 ± 1 cells mm−1, whereas 57 ± 1 cells mm−1 adhered onto peptide conjugated alginate hydrogels. Rinsing with a 50 mM EDTA solution resulted in the release of appreciable numbers of these adhered cells (Fig 3(a)). This outcome was reaffirmed by brightfield microscopy as shown in Fig. 3(b,c). These micrographs illustrate that the cells are nearly all removed from the substrate coated with alginate–RGDS upon rinsing with EDTA. Also of note is the difference in the cell release of alginate (69 ± 7%) with respect to alginate–RGDS (97 ± 3%). It is possible that the chemical conjugation to the carboxylate groups reduces the number of hydrogen bonding sites within the hydrogel making the peptide-functionalized alginate more easily soluble by the EDTA chelator, thus releasing a greater number of cells. The cell adhesion values are slightly lower than that of the static studies, but this may be a result of the short incubation time of EDTA with alginate hydrogel. Live/dead assays indicated no significant difference in cell viability after release versus pre-injection.
 | ||
| Fig. 3 Attachment and detachment of cardiac fibroblast at a shear stress of 1 dyn cm−2 in microfluidic channels (a) coated with native alginate gel or RGDS modified alginate gel. Error bars denote standard errors for five replicates of each experiment. Representative region of an alginate–RGDS coated channel (b) pre-EDTA rinse and (c) post-EDTA rinse. The channel width (vertical dimension) is 1 mm. | ||
In summary, this work demonstrates the application of peptide-functionalized alginate hydrogels as a method of capture and release of cells in microfluidic channels. When combined with the capability of microfluidic devices to selectively capture cells of a particular type from heterogeneous suspensions, the cell release methodology described herein could provide viable, functional cells for further application (such as culturing on scaffolds for tissue engineering) or analysis as part of disease diagnostics.
Acknowledgements
We gratefully acknowledge financial support from the National Heart Foundation, a program of the American Health Assistance Foundation (Grant No. H2007-017 to SKM), the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant to MR) and the Heart and Stroke Foundation (Grant-in-Aid to MR).References
- K. L. Laugwitz, A. Moretti, J. Lam, P. Gruber, Y. H. Chen, S. Woodard, L. Z. Lin, C. L. Cai, M. M. Lu, M. Reth, O. Platoshyn, J. X. J. Yuan, S. Evans and K. R. Chien, Nature, 2005, 433, 647–653 CrossRef CAS.
- H. Oshima, A. Rochat, C. Kedzia, K. Kobayashi and Y. Barrandon, Cell, 2001, 104, 233–245 CrossRef CAS.
- S. Nagrath, L. V. Sequist, S. Maheswaran, D. W. Bell, D. Irimia, L. Ulkus, M. R. Smith, E. L. Kwak, S. Digumarthy, A. Muzikansky, P. Ryan, U. J. Balis, R. G. Tompkins, D. A. Haber and M. Toner, Nature, 2007, 450, 1235–1239 CrossRef CAS.
- B. D. Plouffe, M. Radisic and S. K. Murthy, Lab Chip, 2008, 8, 462–472 RSC.
- H. Lu, L. Y. Koo, W. C. M. Wang, D. A. Lauffenburger, L. G. Griffith and K. F. Jensen, Anal. Chem., 2004, 76, 5257–5264 CrossRef CAS.
- S. P. Wankhede, Z. Q. Du, J. M. Berg, M. W. Vaughn, T. Dallas, K. H. Cheng and L. Gollahon, Biotechnol. Progr., 2006, 22, 1426–1433 CrossRef CAS.
- X. Zhang, P. Jones and S. J. Haswell, Chem. Eng. J., 2008, 135, S82–S88 CrossRef CAS.
- N. Fujioka, Y. Morimoto, K. Takeuchi, M. Yoshioka and M. Kikuchi, Appl. Spectrosc., 2003, 57, 241–243 CrossRef CAS.
- K. Jung, G. Hampel, M. Scholz and W. Henke, Cell. Physiol. Biochem., 1995, 5, 353–360 CrossRef.
- O. Ernst, A. Lieske, M. Jager, A. Lankenau and C. Duschl, Lab Chip, 2007, 7, 1322–1329 RSC.
- M. Yamato, C. Konno, M. Utsumi, A. Kikuchi and T. Okano, Biomaterials, 2002, 23, 561–567 CrossRef.
- W. S. Yeo, C. D. Hodneland and M. Mrksich, Chembiochem, 2001, 2, 590–593 CrossRef CAS.
- H. Zhu, J. Yan and A. Revzin, Colloids Surf. B, 2008, 64, 260–268 CrossRef CAS.
- M. Yamato, M. Utsumi, A. Kushida, C. Konno, A. Kikuchi and T. Okano, Tissue Eng., 2001, 7, 473–480 CrossRef CAS.
- B. D. Plouffe, D. N. Njoka, J. Harris, J. H. Liao, N. K. Horick, M. Radisic and S. K. Murthy, Langmuir, 2007, 23, 5050–5055 CrossRef CAS.
- G. T. Hermanson, Bioconjugate Techniques, Academic Press, Boston, MA, 1996 Search PubMed.
- M. Morra and C. Cassinelli, Langmuir, 1999, 15, 4658–4663 CrossRef CAS.
- E. Alsberg, K. W. Anderson, A. Albeiruti, J. A. Rowley and D. J. Mooney, Proc. Natl. Acad. Sci. USA, 2002, 99, 12025–12030 CrossRef CAS.
- P. S. J. Cheetham, K. W. Blunt and C. Bocke, Biotech. Bioeng., 1979, 21, 2155–2168 CrossRef CAS.
- J. L. Drury, T. Boontheeku and D. J. Mooney, J. Biomech. Eng.-Trans. ASME, 2005, 127, 220–228 Search PubMed.
- J. A. Rowley, G. Madlambayan and D. J. Mooney, Biomaterials, 1999, 20, 45–53 CrossRef CAS.
- I. Couperwhite and M. F. McCallum, Arch. Microbio., 1974, 97, 73–80 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed alginate synthesis and experimental protocols. Fig. S1 Concentration dependence on cell release and viability. See DOI: 10.1039/b823523f |
| This journal is © The Royal Society of Chemistry 2009 |
