A simple hydrogen peroxide-activatable Bodipy for tumor imaging and type I/II photodynamic therapy†
Fangqing
Ge‡
 a,
Yujie
Sun‡
b,
Yu
Wang
b,
Dan
Yu
b,
Zhijia
Wang
a,
Yujie
Sun‡
b,
Yu
Wang
b,
Dan
Yu
b,
Zhijia
Wang
 *ab,
Fabiao
Yu
*ab,
Fabiao
Yu
 *c,
Bingran
Yu
*c,
Bingran
Yu
 *b and
Hongbing
Fu
*b and
Hongbing
Fu
 a
a
aBeijing Key Laboratory for Optical Materials and Photonic Devices, Department of Chemistry, Capital Normal University, Beijing 100048, P. R. China. E-mail: wangzhj@cnu.edu.cn
bLaboratory of Biomedical Materials and Key Lab of Biomedical Materials of Natural Macromolecules, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: yubr@mail.buct.edu.cn
cKey Laboratory of Emergency and Trauma, Ministry of Education, Key Laboratory of Haikou Trauma, Key Laboratory of Hainan Trauma and Disaster Rescue, Engineering Research Centre for Hainan Bio-Smart Materials and Bio-Medical Devices, Key Laboratory of Hainan Functional Materials and Molecular Imaging, The First Affiliated Hospital of Hainan Medical University, Hainan Medical University, Haikou 571199, China. E-mail: yufabiao@muhn.edu.cn
First published on 26th September 2024
Abstract
Tumor microenvironment-activatable photosensitizers have gained significant attention for cancer theranostics. Considering the hypoxic environment of solid tumors, activatable phototheranostic agents with type I PDT are desired to obtain improved cancer treatment efficiency. Herein, we report a simple, effective and multifunctional Bodipy photosensitizer for tumor imaging and type I/II photodynamic therapy. The photosensitizer featuring a methylphenylboronic acid pinacol ester group at the meso-position of Bodipy specifically responds to tumor-abundant H2O2. Its photophysical properties were characterized using steady-state and time-resolved transient optical spectroscopies. The fluorescence (ΦF = 0.09%) and singlet oxygen efficacy (ΦΔ = 10.2%) of the Bodipy units were suppressed in the caged dyads but significantly enhanced (ΦF = 0.72%, ΦΔ = 20.3%) upon H2O2 activation. Fluorescence emission spectroscopy and continuous wave electron paramagnetic resonance (EPR) spectroscopy confirmed that the Bodipy photosensitizer generates reactive oxygen species (ROS) via both electron transfer-mediated type I and energy transfer-mediated type II mechanisms. In vitro experiments demonstrated rapid internalization into tumor cells, enhanced brightness stimulated by tumor microenvironments, and tumor cell death (phototoxicity, IC50 = 0.5 μM). In vivo fluorescence imaging indicated preferential accumulation of this Bodipy photosensitizer in tumor sites, followed by decaging by tumor-abundant H2O2, further elevating the signal-to-background ratio (SBR) of imaging. Besides outstanding performance in tumor imaging, a prominent inhibition of tumor growth was observed. Given its simple molecular skeleton, this Bodipy photosensitizer is a competitive candidate for cancer theranostics.
Introduction
Phototheranostics is crucial for surgical navigation, therapeutic effect evaluation, and therapeutic strategy optimization, therefore it has attracted much attention.1 Recent advancements have focused on developing activatable phototheranostic agents that respond to disease-related biomarkers.2–8 In contrast to conventional agents that continuously emit signals and exhibit phototoxicity, these activatable phototheranostic agents generate optical signals and therapeutic effects only in the presence of disease-specific biomarkers. Consequently, an enhanced signal-to-background ratio (SBR), reduced side effects and improved accuracy of imaging-guided treatments are achieved.1Photodynamic therapy (PDT) offers high temporal–spatial resolution and low systemic toxicity, holding great potential for cancer therapy.9–15 Triplet photosensitizers are pivotal ingredients that undergo intersystem crossing (ISC) to their triplet excited state.16,17 Compared to the short-lived singlet excited state, the long-lived triplet state18,19 benefits subsequent electron transfer (type I reaction) and energy transfer (type II reaction) processes, yielding superoxide anion/hydroxyl radicals and singlet oxygen, respectively. These reactive oxygen species (ROS) induce cancer cell death. In recent times, numerous activatable phototheranostic agents have been developed to enable imaging-guided PDT upon the detection of tumor-specific biomarkers, including hydrogen peroxide, glutathione (GSH), various enzymes, etc.11,20,21
However, the efficacy of PDT is often limited by the hypoxic nature of the tumor microenvironment because the prevalent activatable phototheranostic agents function through a type II reaction mechanism via the energy transfer and the production of singlet oxygen depends on an adequate oxygen supply. Therefore, the reliance of type II photosensitizers on oxygen to produce cytotoxic ROS significantly limited its therapeutic efficacy. In contrast, type I photosensitizers can overcome the limitations of the hypoxic tumor environment, achieving minimal therapeutic effect difference between hypoxic and normoxic conditions.22–29 This is because type I photosensitizers generate radicals such as superoxide anions (O2˙−) through electron transfer, which can be effectively produced in low-oxygen environments and are less dependent on oxygen since subsequent dismutation reactions or Haber–Weiss/Fenton reactions compensate for oxygen consumption.30–32 This characteristic makes type I photosensitizers more promising in clinical applications, especially in treating hypoxic tumors.33
Few organic dyes are proved to be type I PDT agents,34–36 but are still rare and much room is left. In particular, Bodipy, a structurally similar ‘little sister’ of porphyrin, is a promising new generation of PDT agents due to its strong absorption in the visible-NIR region and easily derivatized chemical structures.37–39 Some Bodipy-based photosensitizers are reported to be capable of PDT via a type I mechanism.40–44 However, they usually have no tumor targeting ability. Moreover, the optical signals and therapeutic effects are not activatable by disease-specific biomarkers. Activatable phototheranostic agents with type I PDT based on Bodipy are desired to obtain improved cancer treatment efficiency.
On the other hand, simple yet efficient activatable phototheranostic agents are highly desirable and have potential for clinical translation. Two prevalent strategies for constructing activable triplet photosensitizers are (1) self-quenching due to aggregation and (2) quenching by inhibitors.42 Tumor microenvironment-responsive nanoparticles, polymers, and conjugated molecules have been reported.45,46 However, the properties of photosensitizer–polymer amphiphiles depend heavily on the polymer backbone substitution degree, leading to poor reproducibility. Nanoparticle systems encapsulated with photosensitizers and quenchers risk high levels of leakage.47,48 The current conjugated molecules are usually complex and synthetically demanding.49 Therefore, structurally simple but effective activatable phototheranostic agents are in high demand.
Herein, we synthesized a simple, effective, and multifunctional H2O2-responsive Bodipy photosensitizer (BpinBDP, Fig. 1), which incorporates a boronic acid pinacol ester group at the meso-position, for cancer imaging and type I/II PDT. The fluorescence (ΦF = 0.09%) and singlet oxygen efficacy (ΦΔ = 10.2%) of the Bodipy units were suppressed in the caged dyads but significantly enhanced (ΦF = 0.72%, ΦΔ = 20.3%) upon H2O2 activation. The photosensitizer generates ROS through both electron transfer-mediated type I and energy transfer-mediated type II mechanisms. This dual action ensures a robust anticancer effect on solid tumors. In vitro and in vivo experiments demonstrated its preferential accumulation in tumor and decaging by tumor-abundant H2O2, which significantly boost the imaging quality and therapeutic efficacy, making it a powerful candidate for cancer theranostics. The simplicity in molecular skeleton facilitates its synthesis and modification, which further enhances its potential for clinical translation.
 | ||
| Fig. 1 Schematic illustration of the reaction mechanism of BpinBDP with hydrogen peroxide, and subsequent PDT via type I and II mechanisms. | ||
Results and discussion
All animal experiments were conducted in conformity with the National Guidelines for the Care and Use of National Guidelines for the Laboratory Animals, and the experimental protocols were approved by the Institutional Animal Care and Use Committee in Hainan Medical University, Haikou, China (Approval No. HYLL-2022-358). The synthetic route of BpinBDP is depicted in Fig. 1. Compound 1, synthesized according to the literature,42 reacted with two different aldehydes via one-pot Knoevenagel condensation to produce compound 2, with one side of the Bodipy decorated with poly(ethylene glycol) to enhance water solubility and extend body retention time, while the other side was left available for future functional derivatization. Hydrolyzation of 2 generated the carboxylic acid derivative 3, which was subsequently caged with a methylphenylboronic acid pinacol ester group, yielding compound 4. Finally, the azide group of compound 4 was functionalized with alkyne-functionalized poly(ethylene glycol) (Alk-PEG2k) via a click reaction to afford the water-soluble target compound BpinBDP. Its structure, including the successful attachment of poly(ethylene glycol) to the Bodipy dye, was verified by 1H-NMR and MALDI-TOF (≈3200 Da, Fig. S12 and S13, ESI†).The response of BpinBDP to H2O2 was recorded by UV-visible absorption and fluorescence emission spectroscopies (Fig. 2a and b). BpinBDP presented maximum absorption at 696 nm and dim fluorescence emission; uncaged by H2O2, the Bodipy moiety underwent a hypsochromic shift in absorption and a burst of fluorescence quantum yield from 0.09% to 0.72%, due to the relief of the photon-induced electron transfer (PET) effect. The strongest responses to H2O2 and ONOO− (12.0-fold and 11.2-fold fluorescence enhancement, respectively), yet negligible responses to other RONS and metal ions, were detected (Fig. 2c).
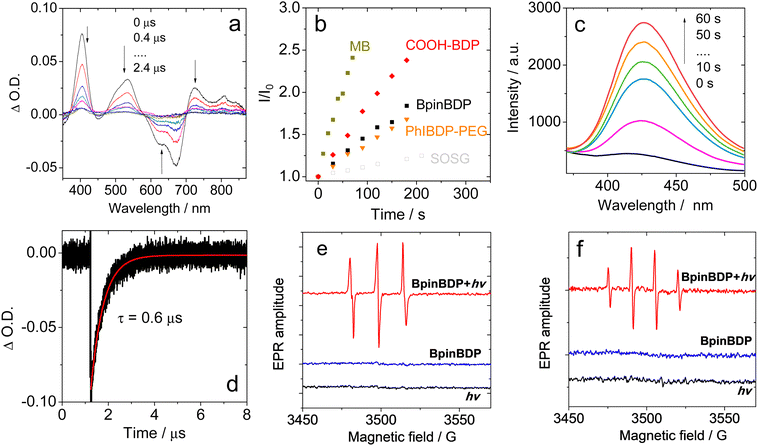
A comprehensive photophysical study of the new triplet photosensitizer is essential. The triplet state properties (such as triplet lifetime) and ROS production capability and ROS species, often overlooked in previous studies, are crucial for photosensitizers in PDT. Herein, the absorption spectrum and decay kinetics of the triplet state were investigated by nanosecond transient absorption spectroscopy (Fig. 3a and b). The negative band in the range of 600–700 nm and positive bands in the ranges of 375–430 nm and 480–575 nm were attributed to the ground state bleaching band and the excited state absorption bands (T1 → Tn transition), respectively. Monitoring the decay of the ground state bleaching signal at 688 nm determined the triplet excited state lifetime to be 0.6 μs in deaerated solution (Fig. 3b), comparable to previously reported styryl-diiodoBodipy (τT ≈ 1 μs).49
The ROS production capability in PBS was evaluated using singlet oxygen sensor green (SOSG) as the 1O2 indicator and methylene blue (MB) as the standard. The 1O2 quantum yields of COOH-BDP, BpinBDP, and PhBDP were determined to be 20.3%, 10.2%, and 7.7%, respectively. Similarly, the generated hydroxyl radical (Fig. 3e) and superoxide anion (Fig. S3, ESI†) were probed by terephthalate (TA) and dihydrorhodamine 123 (DHR 123), respectively.
Consistent with the fluorescence emission results, the EPR analysis (Fig. 3d and f, Fig. S4, ESI†) further verified the ROS generation, including 1O2, ˙OH, and O2−˙. Both electron transfer-based type I (producing O2−˙ and subsequently ˙OH) and energy transfer-based type II (producing 1O2) photoreactions are involved. Type I Bodipy photosensitizers are rare.34,48,49
The response to H2O2 and ROS production capability of BpinBDPin vitro were examined (Fig. 4). Confocal laser scanning microscopy (CLSM) images (Fig. 4a) visually displayed successful endocytosis of BpinBDP into 4T1 cancer cells. Flow cytometry statistically presented a more robust brightness of H2O2-preincubated 4T1 cells than unincubated ones (Fig. 4b), illustrating that BpinBDP can distinguish the cells with distinct H2O2 levels, e.g., tumor and normal cells. To digress, the endocytosis and H2O2-response processes occurred within 6 hours and approached saturation (Fig. S5, ESI†), showing remarkable efficiency even at low concentrations (Fig. S6, ESI†). Next, the in vitro phototoxicity and dark toxicity of BpinBDP were evaluated with the MTT assay (Fig. 4d) and the ROS indicator DCF-DA (Fig. 4c). Intracellular ROS mediated by light irradiation were responsible for a high phototoxicity to the 4T1 tumor cell line (IC50 = 0.5 μM) and cell death, while no noticeable dark toxicity was detected even at extremely high concentrations (c = 30.0–50.0 μM) for negligible ROS in the dark. The above results indicate that BpinBDP is a promising H2O2 activatable imaging and PDT reagent.
BpinBDP serves as both a fluorophore and a photosensitizer, with its radiative transitions and intersystem crossing processes competing with each other. To check whether such a brightness is sufficient to indicate tumor, in vivo fluorescence images of BpinBDP and PhBDP at different time points post i.v. injection were recorded (Fig. 5a). The amphipathic structure of BpinBDP facilitated its self-assembly into nanoparticles with a particle size of 25 nm and accumulation and retention in the tumor. More importantly, the methylphenylboronic acid pinacol ester group is sensitive to H2O2 while the phenyl moiety is not. Reasonably, BpinBDP presented a more intensified fluorescence signal in the tumor site as compared with PhBDP (Fig. 5a and b) due to an additive contribution of the activation of tumor abundant H2O2 (ranging from 100 μM to 1 mM),50 enabling a higher tumor-to-background ratio of BpinBDP compared to PhBDP. Similar to conventional PDT reagents, the main metabolic pathway of BpinBDP, as indicated by the ex vivo fluorescence images of main organs, is through the liver and kidneys. The more remarkable fluorescence signal of the tumor site than those of the main organs verified BpinBDP as a superior tumor indicator.
The antitumor effect of BpinBDPin vivo was examined (Fig. 6) and the 4T1 tumor-bearing mice were randomly divided into 3 groups: G1 – injection of PBS + hv; G2 – injection of BpinBDP merely; and G3 – injection of BpinBDP + hv. The therapeutic efficacies of the above three treatment groups, evaluated by measuring the tumor volume (Fig. 6a), indicate a remarkable tumor inhibition of the photodynamic therapy group (G3 in Fig. 6a and c). To evaluate the biocompatibility of BpinBDP, both the body weights (Fig. 6b) and the hematoxylin and eosin (H&E) stained images of the major organs (Fig. 6d) of the mice from each group were collected. None of the groups displayed observable organ damage, which suggests that no obvious heart, liver, spleen, lung, or kidney dysfunctions of the mice were induced by the photoactivation process of BpinBDP. Given its excellent biocompatibility and simple molecular structure, BpinBDP holds significant promise as a reagent for cancer therapy.
Conclusions
In summary, a simple, effective and multifunctional Bodipy photosensitizer for tumor imaging and type I/II photodynamic therapy (PDT) was reported. With the methylphenylboronic acid pinacol ester group caged in the meso-position, both the fluorescence and singlet oxygen quantum yields of the Bodipy moiety were depressed (ΦF = 0.09%, ΦΔ = 10.2%). The response to H2O2 leads to the uncaged form of Bodipy that exhibited increases in the fluorescence and singlet oxygen quantum yields (ΦF = 0.72%, ΦΔ = 20.3%). Nanosecond time-resolved transient absorption spectroscopies revealed that the shape of the triplet excited state absorption and the decay of the triplet state lifetime of ca. 0.6 μs resembled its analogues. Interestingly, the Bodipy photosensitizer generates reactive oxygen species (ROS) via both electron transfer mediated type I and energy transfer mediated type II mechanisms, as both the superoxide anion/hydroxyl radical and singlet oxygen were detected via fluorescence spectroscopy and continuous wave EPR spectroscopy. The endocytosis of the Bodipy photosensitizer into the 4T1 tumor cells and subsequently response to H2O2 occurred in the first 6 h period, causing a brighter fluorescence emission in H2O2-pretreated tumor cells as compared with untreated ones, which were probed by confocal laser scanning microscopy (CLSM) and flow cytometry analysis. High phototoxicity (IC50 = 0.5 μM) inducing tumor cell death, yet negligible dark toxicity, was proved. In vivo fluorescence imaging indicates a preferable accumulation of the Bodipy photosensitizer in tumor tissues owing to its amphiphilic molecular structure that is beneficial for self-assembly into nanoparticles with an appropriate size (ca. 25 nm). A brighter tumor fluorescence imaging and a higher signal-to-background ratio (SBR), because of the tumor abundant H2O2 activation, were observed for BpinBDP as compared with the reference PhBDP. Tumor growth inhibition and negligible main organ destruction were detected with the implement of in vivo PDT. Regarding the simple molecular structure, outstanding tumor imaging performance, moderate PDT efficacy and good biocompatibility, this skeleton is a competitive candidate for cancer theranostics.Author contributions
Fangqing Ge and Yujie Sun contributed equally to this work. Fangqing Ge was responsible for photophysical data testing. Yujie Sun and Dan Yu performed the cell experiments. Zhijia Wang and Yu Wang performed the animal experiments. Zhijia Wang and Bingran Yu devised the project and performed the data analysis. Fabiao Yu and Hongbing Fu dealt with the manuscript writing and data analysis.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the NSFC (22303056, 22150005, 22090022, 22275125, and 22264013), the R&D Program of Beijing Municipal Education Commission (KM202410028016) and the State Key Laboratory of Fine Chemicals, Dalian University of Technology (KF2313) for financial support.Notes and references
- Y.-Y. Zhao, H. Kim, V.-N. Nguyen, S. Jang, W. Jun Jang and J. Yoon, Coord. Chem. Rev., 2024, 501, 215560 CrossRef CAS.
- G. Feng, G.-Q. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC.
- Z. Wang, J. Huang, J. Huang, B. Yu, K. Pu and F.-J. Xu, Aggregate, 2021, 2, e140 CrossRef CAS.
- S. Zeng, Z. Guo, Y. Hao, Y. S. Kafuti, Z. Yang, Q. Yao, J. Wang, X. Peng and H. Li, Coord. Chem. Rev., 2024, 509, 215786 CrossRef CAS.
- Y. Liu, H. Zhao, L. Li, B. Yang, Y. Yue, M. Li, X. Shi, B. Zhang, L. Wang, C. Qi, Y. Liu, S. Ren, K. Zhang and J. Yoon, Sens. Actuators, B, 2023, 374, 132836 CrossRef CAS.
- H. Zhu, X. Yin, Y. Zhou, S. Xu, T. D. James and L. Wang, Chem, 2022, 8, 2498–2513 CAS.
- J. Huang, L. Su, C. Xu, X. Ge, R. Zhang, J. Song and K. Pu, Nat. Mater., 2023, 22, 1421–1429 CrossRef PubMed.
- T. Yang, Q. Zhang, Y. Miao, Y. Lyu, Y. Xu, M. Yang and C. Mao, Adv. Mater., 2024, 2403756 CrossRef.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS.
- R. Lincoln, L. Kohler, S. Monro, H. Yin, M. Stephenson, R. Zong, A. Chouai, C. Dorsey, R. Hennigar, R. P. Thummel and S. A. McFarland, J. Am. Chem. Soc., 2013, 135, 17161–17175 CrossRef CAS.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- A. M. Bugaj, Photochem. Photobiol. Sci., 2011, 10, 1097–1109 CrossRef CAS PubMed.
- J. Tian, L. Ding, H.-J. Xu, Z. Shen, H. Ju, L. Jia, L. Bao and J.-S. Yu, J. Am. Chem. Soc., 2013, 135, 18850–18858 CrossRef CAS.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. Peng, Chem. Soc. Rev., 2021, 50, 4185–4219 RSC.
- Á. Juarranz, P. Jaén, F. Sanz-Rodríguez, J. Cuevas and S. González, Clin. Transl. Oncol., 2008, 10, 148–154 CrossRef PubMed.
- V.-N. Nguyen, Y. Yan, J. Zhao and J. Yoon, Acc. Chem. Res., 2021, 54, 207–220 CrossRef CAS.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
- T. Takemura, N. Ohta, S. Nakajima and I. Sakata, Photochem. Photobiol., 1989, 50, 339–344 CrossRef CAS PubMed.
- Z. Wang, L. Huang, Y. Yan, A. M. El-Zohry, A. Toffoletti, J. Zhao, A. Barbon, B. Dick, O. F. Mohammed and G. Han, Angew. Chem., Int. Ed., 2020, 59, 16114–16121 CrossRef CAS PubMed.
- R. He, D. Tang, N. Xu, H. Liu, K. Dou, X. Zhou and F. Yu, Chin. Chem. Lett., 2024, 35, 108658 CrossRef CAS.
- M. He, Z. Cheng, Z. Wang, M. Li, H. Liang, H. Liu, L. Yu, L. Zhao and F. Yu, Adv. Healthcare Mater., 2023, 12, 2300752 CrossRef CAS PubMed.
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2018, 140, 14851–14859 CrossRef CAS.
- X. Li, D. Lee, J.-D. Huang and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 9885–9890 CrossRef CAS PubMed.
- J. Tian, B. Li, F. Zhang, Z. Yao, W. Song, Y. Tang, Y. Ping and B. Liu, Angew. Chem., Int. Ed., 2023, 62, e202307288 CrossRef CAS.
- R. Lin, J. Liu, W. Xu, Z. Liu, X. He, C. Zheng, M. Kang, X. Li, Z. Zhang, H.-T. Feng, J. W. Y. Lam, D. Wang, M. Chen and B. Z. Tang, Adv. Mater., 2023, 35, 2303212 CrossRef CAS.
- S. Zeng, Y. Wang, C. Chen, H. Kim, X. Liu, M. Jiang, Y. Yu, Y. S. Kafuti, Q. Chen, J. Wang, X. Peng, H. Li and J. Yoon, Angew. Chem., Int. Ed., 2024, 63, e202316487 CrossRef CAS PubMed.
- Z. Lv, H. Wei, Q. Li, X. Su, S. Liu, K. Y. Zhang, W. Lv, Q. Zhao, X. Li and W. Huang, Chem. Sci., 2018, 9, 502–512 RSC.
- Y. Jiang, W. Zhu, Z. Xu, Z. Zhang, S. Tang, M. Fan, Z. Li, J. Zhang, C. Yang, W.-C. Law, K.-T. Yong, D. Wang, G. Xu and B. Zhong Tang, Chem. Eng. J., 2022, 448, 137604 CrossRef CAS.
- W. Zhang, X. Li, M. Kang, Z. Zhang, Y. Pei, M. Fan, D. Yan, Y. Zhang, C. Yang, G. Xu, D. Wang, Z. Xu and B. Z. Tang, ACS Mater. Lett., 2024, 6, 2174 CrossRef CAS.
- J. Sun, X. Cai, C. Wang, K. Du, W. Chen, F. Feng and S. Wang, J. Am. Chem. Soc., 2021, 143, 868–878 CrossRef CAS PubMed.
- Y.-Y. Wang, Y.-C. Liu, H. Sun and D.-S. Guo, Coord. Chem. Rev., 2019, 395, 46–62 CrossRef CAS.
- K. Wen, H. Tan, Q. Peng, H. Chen, H. Ma, L. Wang, A. Peng, Q. Shi, X. Cai and H. Huang, Adv. Mater., 2022, 34, 2108146 CrossRef CAS.
- Z. Liu, Q. Wang, W. Qiu, Y. Lyu, Z. Zhu, X. Zhao and W.-H. Zhu, Chem. Sci., 2022, 13, 3599–3608 RSC.
- W. Zhen, D. W. Kang, Y. Fan, Z. Wang, T. Germanas, G. T. Nash, Q. Shen, R. Leech, J. Li, G. S. Engel, R. R. Weichselbaum and W. Lin, J. Am. Chem. Soc., 2024, 146, 16609–16618 CrossRef CAS.
- H. Li, J. Wang, H. Kim, X. Peng and J. Yoon, Angew. Chem., Int. Ed., 2024, 63, e202311764 CrossRef CAS PubMed.
- M. Kang, Z. Zhang, W. Xu, H. Wen, W. Zhu, Q. Wu, H. Wu, J. Gong, Z. Wang, D. Wang and B. Z. Tang, Adv. Sci., 2021, 8, 2100524 CrossRef CAS.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- K.-X. Teng, W.-K. Chen, L.-Y. Niu, W.-H. Fang, G. Cui and Q.-Z. Yang, Angew. Chem., Int. Ed., 2021, 60, 19912–19920 CrossRef CAS PubMed.
- D. Chen, Q. Yu, X. Huang, H. Dai, T. Luo, J. Shao, P. Chen, J. Chen, W. Huang and X. Dong, Small, 2020, 16, 2001059 CrossRef CAS.
- Y. Zhu, F. Wu, B. Zheng, Y. Yang, J. Yang and H. Xiong, Nano Lett., 2024, 24, 8287–8295 CrossRef CAS PubMed.
- X. Song, S. Bai, N. He, R. Wang, Y. Xing, C. Lv and F. Yu, ACS Sens., 2021, 6, 1228–1239 CrossRef CAS.
- Y.-c Liu, G.-j Liu, W. Zhou, G.-l Feng, Q.-y Ma, Y. Zhang and G.-w Xing, Angew. Chem., Int. Ed., 2023, 62, e202309786 CrossRef CAS PubMed.
- H. Shi, W. Sun, C. Liu, G. Gu, B. Ma, W. Si, N. Fu, Q. Zhang, W. Huang and X. Dong, J. Mater. Chem. B, 2016, 4, 113–120 RSC.
- I.-h Oh, H. S. Min, L. Li, T. H. Tran, Y.-k Lee, I. C. Kwon, K. Choi, K. Kim and K. M. Huh, Biomaterials, 2013, 34, 6454–6463 CrossRef CAS PubMed.
- B. J. Liang, M. Pigula, Y. Baglo, D. Najafali, T. Hasan and H.-C. Huang, J. Nanobiotechnol., 2020, 18, 1 CrossRef CAS PubMed.
- Y. Shen, Q. Tian, Y. Sun, J.-J. Xu, D. Ye and H.-Y. Chen, Anal. Chem., 2017, 89, 13610–13617 CrossRef CAS PubMed.
- Y. Peng, X. Da, W. Zhou, Y. Xu, X. Liu, X. Wang and Q. Zhou, Dalton Trans., 2024, 53, 3579–3588 RSC.
- C. S. Jin, J. F. Lovell, J. Chen and G. Zheng, ACS Nano, 2013, 7, 2541–2550 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01650e |
| ‡ Fangqing Ge and Yujie Sun contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |