Open Access Article
Open Access ArticleProgress in process parameters and mechanism research of polymer emulsion preparation
Shuncheng Xiang a,
Zhijian Chenga,
Wei Shi*b,
Tingxiang Zhenga,
Yingli gaoa,
Jiake Zhangc and
Liangjun Huangd
a,
Zhijian Chenga,
Wei Shi*b,
Tingxiang Zhenga,
Yingli gaoa,
Jiake Zhangc and
Liangjun Huangd
aHunan Provincial Engineering Technology Research Center for Novel and Carbon Neutral Road Material, Changsha University of Science and Technology, Changsha 410114, China
bScience and Technology Affairs Center of Hunan Province, Changsha, 410082, China. E-mail: 2919596471@qq.com
cCollege of Transportation Engineering, Tongji University, Shanghai 200092, China
dHuaihua Dongxing Concrete Co., Ltd, Huaihua 418000, China. E-mail: huang0408tx@yeah.net
First published on 17th May 2024
Abstract
As a new type of concrete admixture, polymer emulsion is mainly used to strengthen the properties of concrete by adhesion and physical and chemical crosslinking with cement in concrete. Under the background of construction in the new era, it is of great significance to elucidate all aspects of concrete performance under the action of polymer emulsion. In this paper, the main formation process of polymer emulsion is reviewed, the influence of synthetic materials required for polymerization on the polymerization process is discussed, and the regulating effects of reaction temperature, reaction time, admixtures, and treatment methods on the synthesis process of polymer emulsion are analyzed. The action mechanism of polymer emulsion on concrete was deeply investigated, and the synthesis method was studied to provide an important experimental and theoretical basis for the preparation of new emulsion materials and the process of emulsion polymerization. The problems of polymer emulsion raw materials, synthetic conditions, and synthetic methods are introduced. The future development trend of polymer emulsion is predicted and the future research ideas are put forward.
1 Introduction
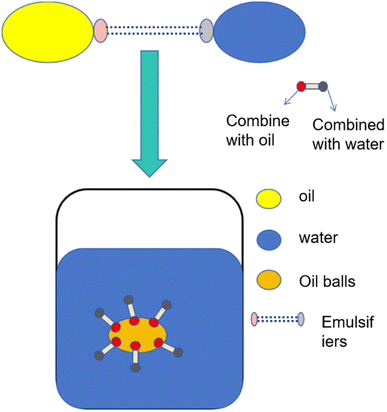
As a chemical admixture, emulsion has gradually become one of the key research objects for improving concrete properties. An emulsion is a stable mixture formed by two or more non-phase solution bodies through the action of an emulsifier, usually to effectively mix two insoluble components, and the properties and stability of the emulsion can be adjusted by controlling the type and concentration of the emulsifier. In the process of polymer emulsion polymerization, the polymer is stably dispersed in the water phase with nano or micron-sized particles to form a stable emulsion system. The controllability of the formation of the emulsion system makes the polymer particle size, molecular weight, molecular structure, introduction of functional groups and adjustment of raw materials can be precisely controlled to meet the specific application needs. A stable emulsion system can also resist the trend of agglomeration and settlement, extend the service life of the emulsion, and have excellent dispersion, so that the polymer can be highly dispersed in the system and provide a uniform performance distribution for cement concrete.1 In addition, polymer emulsion, as a highly efficient admixture, can effectively avoid problems such as high durability of concrete when using ordinary emulsions, and show advantages such as improving water resistance and ductility of concrete.2The purpose of studying polymer emulsions is to deeply understand their formation, structure, and properties to develop emulsion systems with specific functions and properties. This research is helpful to optimize the stability, rheology, and application characteristics of the emulsion, and expand its application range in various fields. Exploring the preparation method of polymer emulsion, emulsifier selection, polymer structure regulation, and other aspects, can provide scientific basis and technical support for industrial production and application, and promote the development and application innovation of emulsion technology. This paper reviews the synthesis of polymer emulsion parameters, focusing on the selection of raw materials and polymerization methods on the characteristics, advantages, and future development of concrete properties. Compared to other emulsions, polymer emulsions have the following characteristics:
(1) Wide range of applications.3,4 Polymer emulsions as admixtures in cement-based materials have better effects than other common emulsions, ensuring the effect of water reduction and improving the strength, compactness, and ductility of cement concrete.
(2) Good stability.5 Polymer emulsions exist in the form of particles in the emulsion, have a smaller size and higher surface activity, resist the tendency to aggregate and settle, demonstrate higher stability compared to other emulsions, and extend the service life of the concrete.
(3) Strong dispersion.6 By suspending the polymer in the form of tiny particles in water or other solvents to form an emulsion, the polymer is highly dispersed in the system. The highly dispersed properties can effectively reduce the surface tension of cement concrete, providing better flow and pumpability while providing a uniform performance distribution.
(4) The molecular structure is more designable.7 By adjusting the polymer composition and reaction conditions in the polymer emulsion, the particle size, molecular weight, molecular structure, and functional group of the polymer can be precisely controlled. This allows polymer emulsions to meet specific application requirements, obtain products with different properties, and have a wider regulatory range and more precise action than ordinary emulsions.
At present, based on developing polymer emulsions with higher water reduction rates, better performance, and greater adaptability, many researchers are expanding raw material sources and synthesis processes, reducing preparation costs and improving quality stability.8–10 This paper aims to comprehensively discuss the research progress of polymer emulsion synthesis technology, mainly from the selection of raw materials and action mechanism, the influence of reaction conditions on emulsion properties, and the development trend of emulsion polymerization technology in the future and put forward the prospect of polymer emulsion research progress at home and abroad in recent years. It provides scientific guidance and engineering references for future research in emulsion polymerization technology.
2. Synthetic materials
Emulsion polymerization is carried out by dissolving large alkenyl alkyl monomer with a terminal double bond and small unsaturated carboxylic acid monomer in an appropriate solvent under the action of an initiator, catalyst, and chain transfer agent. Macromonomers provide the building blocks of polymerization, The small monomers bring functionality and special properties, the initiator is to start the polymerization reaction, the catalyst accelerates the reaction rate, and the chain transfer agent regulates the molecular weight distribution of the polymer. By reasonable selection and control of these raw materials, polymer emulsions with specific properties and functions can be prepared.2.1 Monomers
Common macromonomers are classified according to functional groups, as shown in Table 1.
| Category | Compound |
|---|---|
| Alkyl group | Methyl methacrylate,11 acrylate,12 styrene,13 methyl vinyl glycolate,14 butene,15 etc. |
| Ester group | 2-Hydroxyethyl methacrylate,16 hydroxypropyl acrylate,17 hydroxyethyl acrylate,18 glyceryl acrylate,19 ethylene–ethylene glycol triacrylate,20 etc. |
| Hydroxyl group | Glycol-lactide,21 poly(ethylene glycol) methyl ether methacrylate,22 etc. |
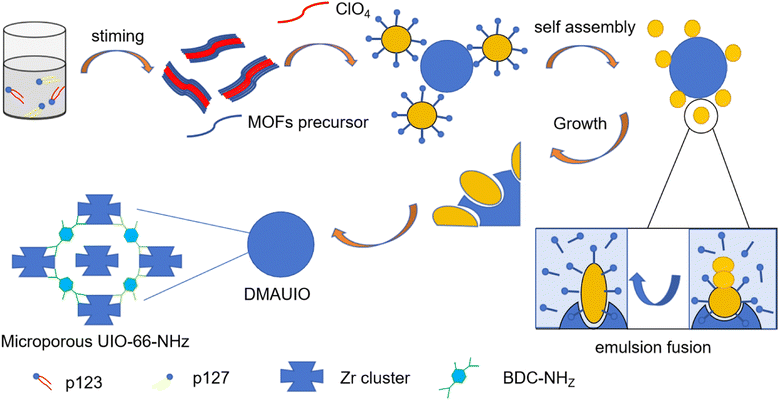
The preparation methods of macromonomer mainly include microemulsion free radical polymerization, sequential free radical polymerization, copolymerization, ring-opening transposition polymerization, and counter-emulsion polymerization, among which the most widely used is free radical polymerization. Booth J. R.23 using hexadecane as a hydrophobic agent and ω-unsaturated methacrylate-based monomers as reaction stabilizers, synthesized poly(benzyl methacrylate) (P(BzMA)) polymer latex monomers via emulsion free-radical polymerization. Through catalytic chain transfer emulsion polymerization (CCTP), they were able to synthesize amphiphilic macromolecular monomers. Rengifo Joel24 through sequential free-radical polymerization in an aqueous medium, synthesized various IPN hydrogel monomers in the presence of N,N′-methylenebisacrylamide as a crosslinking agent. Luo S.25 in the copolymerization reaction of lactic acid (LA) and maleic anhydride (MAH), generated poly(lactic acid-co-maleic anhydride) (PLAM) macromolecular monomers with reactive double bonds. The copolymerization of different acrylic esters with PLAM via free radical polymerization demonstrated that acrylic esters with substituted methyl groups exhibited stable polymerization performance. Lu Hao26 synthesized macromolecular monomers composed of chemically protected oligonucleotides (prot DNA) and polyethylene glycol (PEG) chains, using ring-opening metathesis polymerization (ROMP). By employing phosphoramidite containing norbornene, linear norbornene-prot DNA-PEG structures and Y-shaped structures of macromolecular monomers were obtained. Dai Baimei27 synthesized polymer microsphere latex macromolecular monomers via inverse emulsion polymerization, using acrylamide, 2-acrylamido-2-methylpropanesulfonic acid, and acryloyl morpholine as monomers, N,N′-methylene bisacrylamide as a crosslinker, Span-80 and Tween-60 as emulsifiers, and 2,2′-azobis(2-methylpropionamidine) dihydrochloride as an initiator. New macromonomers are prepared by introducing new groups based on compound models or molecular macromonomers. Chen Lunliang28 investigated the synthesis of 77 different chain-length AB-type polystyrene macromolecular monomers using alkyne-PS-azide (l-PS-N3) as a model macromolecular monomer via the free radical polymerization mechanism (ATRP). Ganguly Ritabrata29 achieved high-loaded polyzwitterionic chains based on poly(methyl methacryloyloxyethyl phosphorylcholine) polymer (PMPC) macromolecular monomers. Through a three-step reaction involving controlled radical polymerization (RAFT) polymerization, thiol group introduction, and polymerizable double bond introduction, PMPC-based macromolecular monomers were synthesized. As shown in Fig. 1 and 2, microgels were synthesized by copolymerization of different molar masses and molar ratios of macromolecular monomers with N-vinylcaprolactam (NVCL). Bendrea AncaDana30 studied a thienyl macromolecular monomer containing oligo(2-methyl-2-oxazoline) (OMeOx) units (Th-OMeOx) as a reactive precursor, which was used to synthesize polymerizable 2,2′-3-OMeOx-substituted terthiophenes through Suzuki coupling reactions. The research findings indicate that Th-OMeOx exhibits various functional properties such as water self-dispersibility, self-assembling structures, and the formation of micelles in water, which enhance the strength of concrete.
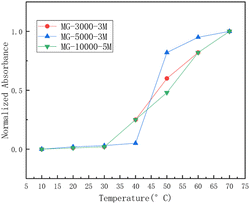 | ||
| Fig. 1 The relationship between normalized absorbance and temperature for three types of microemulsions: MG-3000-3M, MG-5000-3M, and MG-10000-5M.29 | ||
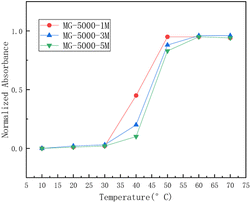 | ||
| Fig. 2 The relationship between temperature and normalized absorbance of three large monomers: MG-5000-1M, MG-5000-3M, and MG-5000-5M.29 | ||
There are also some special macromonomers such as hyperbranched polyether macromonomers, which have a highly branched structure different from linear polymers, mostly three-dimensional spherical molecular structures, and contain many active groups. Liu Hai31 synthesized carboxylic acid-terminated poly(ethylene glycol) macromolecular monomers (HPES) using polyethylene glycol monomer (HPEG) and succinic anhydride as raw materials, with 4-dimethylaminopyridine (DMAP) as a catalyst, through esterification as depicted in Fig. 3.
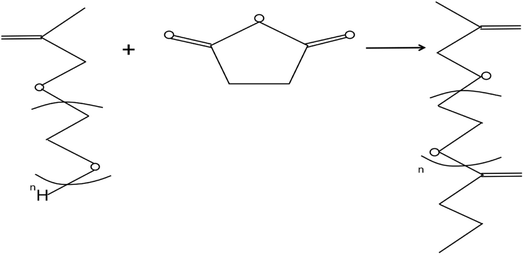 | ||
| Fig. 3 Synthesis of HPES monomer.31 | ||
Using HPEG and malonic acid as raw materials and DMAP as a catalyst, dicarboxylate terminated polyoxyethylene ether macromonomers (HPET, HPET500) with different molecular weights were prepared by esterification. The synthesis is shown in Fig. 4.
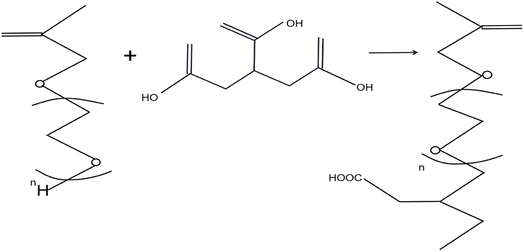 | ||
| Fig. 4 Synthesis of HPET monomer.31 | ||
HPET macromonomer and HPEG macromonomer will produce steric hindrance, as shown in Fig. 5.
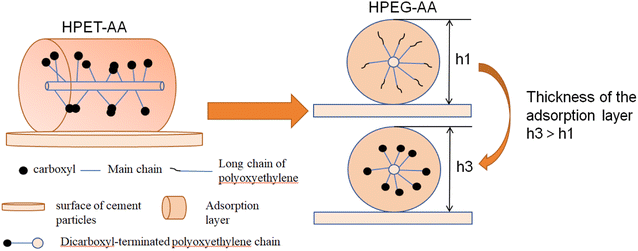 | ||
| Fig. 5 Schematic representation of the steric hindrance effect between HPET-AA and HPEG-AA.31 | ||
The emulsion polymerization method provides flexibility and control to regulate polymer emulsion stability, molecular weight control, pH sensitivity, and complex structure construction. The application potential of polymer emulsion macromonomers in specific environments was demonstrated in terms of contractility, stability enhancement, and thiophene's multiple functional properties. In the stage of synthesis of macromonomers, emulsion polymerization has a great influence on synthetic products, and there are complicated problems such as the separation and purification of intermediates. Steric hindrance will also occur between the prepared macromonomers, as shown in Fig. 3. It is very important to use appropriate macromonomers to prepare emulsions. Reduce production costs.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) to form polymer chains. Such as acrylic acid, methacrylate, and other small monomers, these small monomers have reactivity and good solubility, can be uniformly dispersed in the water phase, and form a stable emulsion system, while by introducing different functional groups and copolymerization reaction, you can adjust the chemical properties of the polymer.
C) to form polymer chains. Such as acrylic acid, methacrylate, and other small monomers, these small monomers have reactivity and good solubility, can be uniformly dispersed in the water phase, and form a stable emulsion system, while by introducing different functional groups and copolymerization reaction, you can adjust the chemical properties of the polymer.Commonly used small monomers are classified according to functional groups, as shown in Table 2.
The main methods for the synthesis of small monomers include free radical polymerization and esterification, etc. Usually, the presence of small monomers can improve the adaptability between polymer emulsion and cement. Zhuojun Jiang40 synthesized high conversion rate polycarboxylate superplasticizer monomers using methyl methacrylate, methoxy polyethylene glycol ester, 3-methyl-3-buten-1-ol polyethylene ether, acrylic acid, ester-type monomer A, sulfonic acid-type monomer B, amide-type monomer C, phosphoric acid-type monomer D, and silane-type monomer E. The following year, Zhuojun Jiang41 used ethanesulfonic acid as a catalyst and hydroquinone as a polymerization inhibitor. Acrylic acid and p-hydroxybenzenesulfonic acid were esterified at 90 °C for 3 hours. After esterification, a low-sensitive stable polycarboxylate superplasticizer monomer with a double-layer dispersion structure was obtained. Bakhshali Massoumi42 synthesized bromine-terminated polyacrylic ester (PEA-Br) via atom transfer radical polymerization of ethyl acrylate using 2-bromopropionic acid methyl ester and a catalyst/initiator system of copper chloride/pyridine. By morphological transformation into its reduced form, polyaniline (PANI) was successfully grafted onto polyacrylic ester (PEA) monomers. In conclusion, small monomers of acrylate are one of the important components of emulsion admixture. The synthesis pathway of acrylate is shown in Fig. 6.
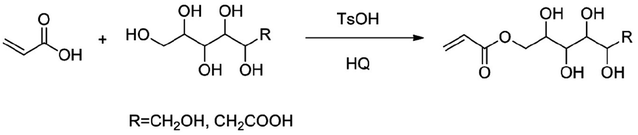 | ||
| Fig. 6 The synthesis of acrylic esters.42 | ||
The preparation of small monomers can not only improve the adaptability of cement in polymer emulsion but also is a very important raw material to improve the performance of cement concrete. Liu Yuanyuan43 proposed the preparation of a monomer with a catalytic effect. The results showed that monomers prepared using this new strategy, combining maleimide (MAL) as a raw material, exhibited over 80% conversion rate and methyl acrylic acid (MAA) selectivity at a hydrothermal temperature of 120 °C for 4 hours. Janusz K.44 studied the cationic emulsion polymerization of octamethylcyclotetrasiloxane (D4) with methyltriethoxysilane (MTES) and vinyltriethoxysilane (VTES) mixed monomers. When D4 was polymerized in the mixture with alkoxysilanes, the crosslinked polymer exhibited the fastest polymerization rate.
Small acrylate monomers are usually used in polymer emulsion polymerization, as shown in Fig. 6. Small acrylate monomers are volatile, and it is difficult to control particle size distribution and emulsion stability during emulsion preparation, which will affect the quality and performance of polymer emulsions. Polymerized small acrylate monomers will produce volatile organic compounds, posing potential risks to the environment and human health. However, it plays an important role in improving the stability and rheological properties of cement paste. Catalysts are also used to inhibit polymerization and esterification of specific small monomers, and polymers are grafted to other base compounds to prepare new water-reducing agents by modifying adsorption groups in the molecular structure of small monomers.
2.2 Initiator
Initiators drive polymerization by catalyzing the formation of chemical bonds between monomers. Common initiators include free radical initiators, ionic initiators, and composite initiators. Composite initiators are mainly used in emulsion polymerization, which is composed of multiple components and catalyzed polymerization through multiple reaction paths to achieve efficient polymerization and obtain emulsion properties and stability.The commonly used initiators are classified as shown in Table 3.
The selection of an initiator should consider the activity of the reaction monomer, the temperature range of the reaction system, and other factors, in the selection of appropriate and easy to dissolve initiator. So Jae I.51 researched the utilization of quaternary ammonium salt (QA) anion tetramethylammonium silanol (TMAS) as an initiator to synthesize fluoromethyl vinyl silicone (FVMQ) and fluorohydrogen methyl silicone (FHMQ) via anionic ring-opening polymerization, resulting in the preparation of fluorinated colloidal silicone rubber (F-LSR). Yuan Ming52 investigated the use of four silane hydride compounds (diethylsilane (DES), triethylsilane (TES), phenylsilane (PSH), and diphenylsilane (DPS)) as initiators for the preparation of poly(methyl methacrylate) (PMMA).
When the properties of copolymer cannot be satisfied by the common initiator, a new initiator is synthesized based on the common initiator. Mistry Bhavita53 synthesized a halogen-terminated macromolecular initiator with good grafting ability by reacting vinyl-terminated polydimethylsiloxane (PDMS) with 3-chloropropyl mercaptan, for the synthesis of ABA-type block copolymers. Coban Zehra Gul54 investigated the use of diphenyliodonium hexafluorophosphate (DPI) as an initiator to initiate the cationic ring-opening polymerization (ROP) of octamethylcyclotetrasiloxane (D4), resulting in the preparation of cationic emulsions. Przesławski Grzegorz55 studied the use of benzoyl peroxide (BPO) as an oxidizing agent (initiator) and N,N-dimethyl-aniline (DMA) as a reducing agent (co-initiator) to investigate the properties of poly(methyl methacrylate) (PMMA) cement. It was found that increasing the concentration of the oxidizing agent accelerated the polymerization rate and reduced the setting time. DeoghareC56 studied three isomeric alkyl bromide initiators: N-phenyl succinimide, N-phenyl succinimide, and N-phenyl succinimide. The research indicated that bromine can replace succinimide as an initiator for various monomers in appropriate solvent systems.
In the process of synthesizing copolymers, it is very important to choose the appropriate initiator, through the synthesis or improvement of the initiator method to improve the performance of the copolymer, a specific material needs a specific initiator. In addition, appropriate use of initiator, temperature control, and adjustment of mix ratio are also critical. In emulsion polymerization, the initiator mainly promotes the formation of emulsion-layered micelles, as shown in Fig. 7. Therefore, macromolecular initiators with special structures and functions should be developed during the preparation of emulsion copolymers to improve the compatibility of emulsion copolymers.
2.3 Surfactant
Surfactants play a key role in emulsions through specific chemical bonds and functional groups, regulating interface properties and reducing surface tension, thus forming micellar structures and giving the surfactants hydrophobic and hydrophilic properties. The hydrophobic alkyl chain forms a hydrophobic core, while the charged carboxylic acid and sulfonic acid groups form electrostatic interactions with the water molecules, increasing the hydrophilicity of the emulsion. The phosphate group has the dual properties of hydrophilic and hydrophobic, which means the surfactant can effectively increase the dispersion, emulsification, and stability of the emulsion.Surfactants can be classified as follows as shown in Table 4.
Surfactants play a major role in regulating and optimizing the properties of emulsion polymerization, which is mainly synthesized by free radical copolymerization of monomers such as acrylic acid, methylpropenesulfonic acid, and acrylamide. Hydrogen peroxide is usually used as the initiator. The addition of surfactants can make the polymer have the characteristics of high stability and can significantly improve the hydration rate. Liyu Zhang64 investigated cationic Gemini surfactants with biphenyl and dianhydride groups as rigid spacer units. It was found that the Gemini surfactants exhibited a lower critical micelle concentration and excellent surface tension in aqueous solutions. Guo Lingxiao65 synthesized high-amine value amino-propyl polydimethylsiloxane (APSO) surfactants via amination reactions. It was found that APSO exhibited a low surface tension at the critical aggregation concentration (CAC) and possessed good solubility and excellent wetting properties. Wang Bo66 synthesized two surfactants (Naph-GS and Naph-MS) through the reaction of dichloromethyl naphthalene and chloromethyl naphthalene with N,N-dimethyl long-chain alkyl tertiary amines. Wu Wenxiang67 synthesized a monoester product, sodium bisulfite, through esterification of long-chain fatty alcohol polyoxyethylene ether with maleic anhydride. The research results indicate that sodium bisulfite was used as a sulfonation agent to design and synthesize anionic–nonionic Gemini surfactants via sulfonation reactions, as shown in Fig. 8.
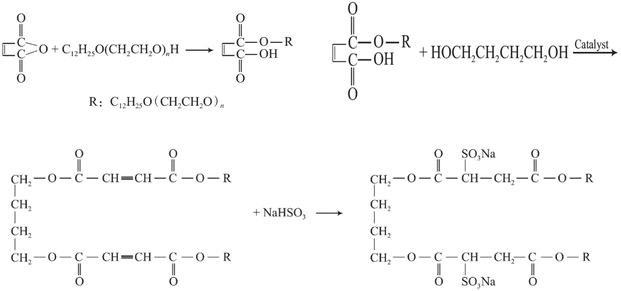 | ||
| Fig. 8 The synthesis of various anionic–nonionic Gemini surfactants.67 | ||
When the surfactant is added to the emulsion, it will form a stable film at the two-phase interface, and the formation of this film can effectively reduce the interfacial tension. This effect of reducing the interfacial tension helps to reduce the total free energy of the system and promotes the formation and stability of the emulsion. At the same time, the adsorption of surfactant molecules on the interface can also enhance the affinity between the two phases, making the emulsion more stable, but if the addition of too much surfactant, it will lead to excessive coating, the formation of an overly stable emulsion, will affect the rheological properties of the emulsion, making it more viscous or difficult to flow. In addition, anionic, cationic, or non-ionic surfactants will form different charge distributions on the interface, which will affect the charge balance and stability of the emulsion, and changes in temperature and pH value will also affect the behavior of the surfactant in the emulsion and the size of the interface forces, thus further affecting the stability of the emulsion.
During the emulsion polymerization process, surfactants were synthesized through the free radical copolymerization of monomers such as acrylic acid, methyl methacrylate, and acrylamide. Peroxides, including hydrogen peroxide, were employed as initiators. Increasing the concentration of oxidants shortened the setting time and enhanced compressive strength. Furthermore, the presence of surfactants primarily addressed the interconnection between the oil phase and the water phase, as illustrated in Fig. 9. The design and synthesis of cationic Gemini surfactants and amine-functionalized polysiloxanes with specific structural properties improved the performance of polymer emulsions, reducing the critical micelle concentration and surface tension. Gemini surfactants exhibited excellent catalytic properties, making them a focal point for future research.
2.4 Chain transfer agent
The commonly used dispersing media for emulsion polymerization included water, organic solvents, oils, alcohols, and polymer solutions, among others. The selection of dispersing media aimed at improving the dispersion of the emulsion and maintaining the stability of organic solvents. Usually, the stability of polymer emulsions was improved by introducing polymer chains with high molecular weights. In addition, during the emulsion polymerization process, chain transfer agents were used to adjust the molecular weight and molecular weight distribution of the polymer, facilitating the generation of polymer chains while controlling the particle size, dispersion, and rheological properties of the polymer in the emulsion.Wei Jiahui68 investigated the preparation of metastable suspensions of natural cellulose nanocrystals (CNCs) using volatile liquid trifluoroacetic acid (TFA) as a dispersing medium. Safronova Ekaterina Yu69 investigated the dispersibility of Nafion in different ion forms and dispersing liquids (ethylene glycol, N,N-dimethylformamide, N-methyl-2-pyrrolidone, and isopropanol-water mixture). The research has shown that dispersing media could exist in the form of membranes within the polymer to improve its performance. Zhornik V.70 studied the structural characteristics of biodegradable lubricating greases formed by considering a mixture of dispersing media and a heterogeneous lithium-calcium soap-like dispersed phase. The research showed that during the thermal deformation process, it is possible to minimize the negative effects of water and high temperatures on the dispersing media to the maximum extent. Khakhin L.71 utilized synthetic high VI (viscosity index) low pour point poly-alpha-olefin base oil based on L-octene to produce the lighter (by-product) fraction. The study demonstrated that the dimer fraction of L-octene meets the requirements of emulsion-dispersing media.
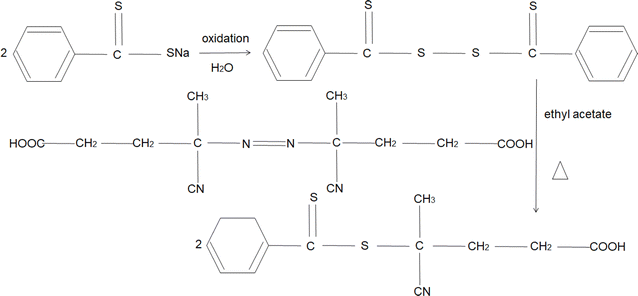
Chain transfer agents not only improved the stability and dispersibility of the emulsion in the dispersing medium but also enhanced the rheological properties of the emulsion. Spring Scott W.72 The cationic reversible addition–fragmentation chain transfer (RAFT) polymerization of vinyl ethers was introduced. The research results indicated that the cationic polymerization of vinyl ethers provided a sustainable and efficient method. The resulting solid, colorless CTA demonstrated excellent performance and could be applied on a large scale. Furthermore, this promoted the preparation and application of bio-renewable materials. Mandal Indradip73 A mono-substituted conjugated diene derivative was discovered to function as a large molecular chain transfer agent attached to the polymer. The research results demonstrated that the synthesized large molecular chain transfer agent enabled the preparation of poly(1,3-propylene oxide)-based diblock copolymers with different chain lengths, poly(ethylene oxide)-based diblock copolymers, and triblock (ABA-type) copolymers, all exhibiting favorable outcomes. Mandal Ankita74 The ROMP–ROMP method was employed for the synthesis of block copolymers, and ATRP was utilized with ROMP macromolecular initiators and active ROMP for PEG macromolecular chain transfer agents. The research results indicated that the synthesis of block copolymers from degradable polymers became economically efficient, sustainable, and environmentally friendly. This approach holds broad prospects for applications in the field of materials science and technology. Mtesukam75 The preparation of 4-cyanopentanoic acid dithiobenzoate as a chain transfer agent was carried out using the patented technology by Le T. P.76 It was successfully applied to the synthesis of styrene sulfonic acid salt homopolymers under aqueous conditions, as illustrated in Fig. 10. The molecule of 4-cyanopentanoic acid dithiobenzoate contains polar functional groups, carboxyl, and cyanide, ensuring good water solubility of the chain transfer agent. The benzene group within it plays a stabilizing role, determining a higher chain transfer efficiency in the RAFT polymerization reaction.
The dispersing medium could be employed for the preparation of stable suspensions and, at the same time, could be combined with discarded material polyethylene terephthalate (PET). For Nafion materials, the ion form and properties of the dispersing medium had a significant impact on dispersibility, polymer morphology, and characteristics. Excellent performance and selectivity were achieved by using a dispersant in a non-proton polar solvent. External factors also had a notable influence on the application of the dispersing medium. Further optimization of the selection of the dispersing medium and the regulation of external factors contributed to improving material dispersibility, enhancing the quality, and effectiveness of related applications.
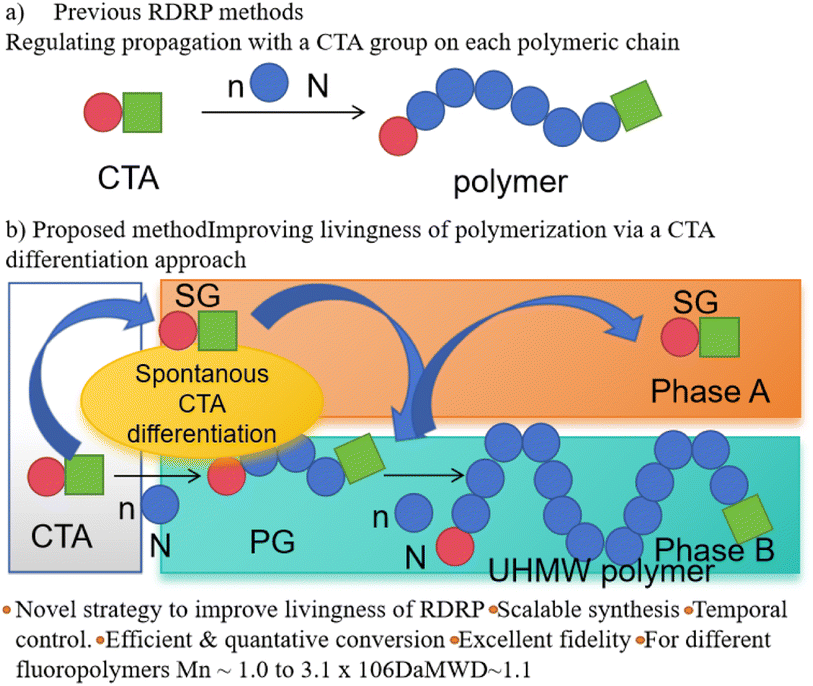
In the emulsion polymerization process, chain transfer agents could regulate the polymer's molecular weight and molecular weight distribution, and control the particle size, dispersion, and rheological properties of the polymer in the emulsion. Different types of chain transfer agents, such as cationic reversible addition–fragmentation chain transfer (RAFT) polymerization and macroinitiators, have been successfully applied in emulsion polymerization reactions. In the emulsion polymerization process, the strategy of chain transfer agent differentiation was applied to the polymer emulsion, as illustrated in Fig. 11. By precisely controlling the amount of chain transfer agent and reaction conditions, customization of polymer properties could be achieved, contributing to the optimization of emulsion stability, dispersion, and rheological behavior.
3. Synthesis conditions
3.1 Temperature
In the polymer emulsion polymerization process, the free radical polymerization principle was employed, and the polymerization temperature was typically controlled within the range of 50–80 °C. Different initiators have varying activation energies, and the suitable polymerization temperature needs to be determined based on the nature of the initiator. Furthermore, the polymerization temperature needed to be determined based on the characteristics of the polymer. Different polymer systems had distinct temperature requirements, requiring a balance between polymerization rate and polymer properties.M. Daneshgar77 In suspension polymerization, the particle size distribution of polymers and external temperature conditions resulted from the dynamic equilibrium between polymer breakage and coagulation. The impact of stabilizer temperature on the average particle size and its distribution in the suspension polymerization of styrene was experimentally investigated. The research results indicated that reducing the polymerization temperature resulted in a decrease in the average particle size and a narrowing of the distribution. L. Anusha78 Porous conductive epoxy materials were prepared using a single emulsion method. Under a stirring speed of 1000 rpm, the epoxy mixture was dropped into heated corn oil at temperatures of 140, 160, and 180 °C, respectively. The porous conductive epoxy material prepared at an emulsion temperature of 160 °C exhibited the smallest size and the highest electrical performance. Dai Baimei79 A copolymer microsphere emulsion with optimal comprehensive properties was successfully synthesized by utilizing acrylamide, 2-acrylamido-2-methylpropane sulfonic acid, and acryloyl morpholine as monomers, with N,N′-methylene bisacrylamide serving as a crosslinking agent. The emulsifiers Span-80 and Tween-60, along with 2,2′-azobis(2-methylpropionamidine) dihydrochloride as an initiator, were employed in the synthesis process. The monomer ratio of acrylamide, 2-acrylamido-2-methylpropane sulfonic acid, and acryloyl morpholine was set at 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, with an emulsifier dosage of 8%. The reaction was carried out at a temperature of 55 °C. during the synthesis of the copolymer microsphere emulsion. Liu Xiaopei.80 A NMA-modified acrylic ester emulsion was prepared using a semi-continuous seed emulsion polymerization process with methyl methacrylate (MMA) and isobutyl methacrylate (IBMA) as polymerization monomers. The impact of polymerization temperature on the emulsion stability and particle size was investigated. The research results indicated that, at a reaction temperature of 80 °C, a non-ionic Tween-40/cationic CTAC combination was used as emulsifiers, with a mass fraction (relative to monomer mass) of 4.5%, and NMA mass fraction (relative to monomer mass) of 5%, the average particle size was 69 nm, as shown in Fig. 12–15.
15, with an emulsifier dosage of 8%. The reaction was carried out at a temperature of 55 °C. during the synthesis of the copolymer microsphere emulsion. Liu Xiaopei.80 A NMA-modified acrylic ester emulsion was prepared using a semi-continuous seed emulsion polymerization process with methyl methacrylate (MMA) and isobutyl methacrylate (IBMA) as polymerization monomers. The impact of polymerization temperature on the emulsion stability and particle size was investigated. The research results indicated that, at a reaction temperature of 80 °C, a non-ionic Tween-40/cationic CTAC combination was used as emulsifiers, with a mass fraction (relative to monomer mass) of 4.5%, and NMA mass fraction (relative to monomer mass) of 5%, the average particle size was 69 nm, as shown in Fig. 12–15.
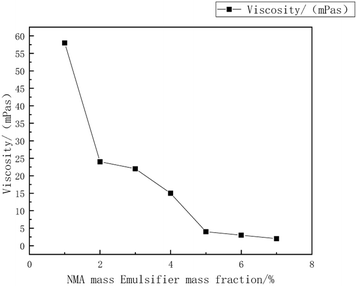 | ||
| Fig. 12 The relationship between the mass fraction of NMA emulsifiers and viscosity.80 | ||
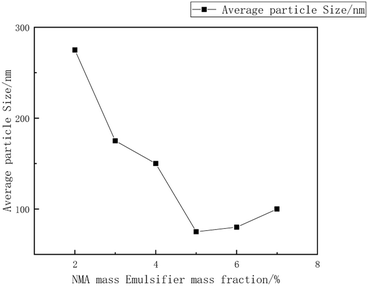 | ||
| Fig. 13 The relationship between the mass fraction of NMA emulsifiers and the average particle size.80 | ||
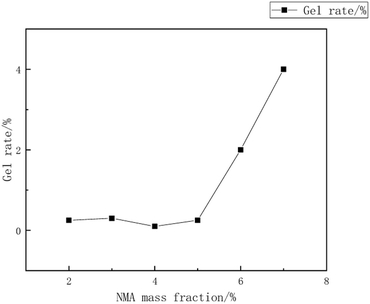 | ||
| Fig. 14 Relationship between mass fraction of NMA and gel yield.80 | ||
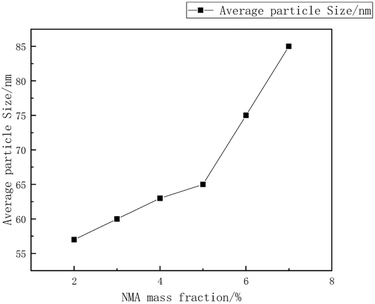 | ||
| Fig. 15 The relationship between the mass fraction of NMA and the average particle size.80 | ||
Emulsion polymerization was an important method for synthesizing polymers with specific properties. The temperature of stabilizers and initiators had a crucial impact on controlling the particle size distribution and properties of the polymer. High-molecular-weight stabilizers could effectively reduce coagulation phenomena, resulting in a narrower particle size distribution. Generally, increasing the temperature of the stabilizer tends to reduce the average particle size and narrow the size distribution of the polymer, while increasing the initiator temperature tends to increase both the average particle size and distribution. In the exploration of specific applications of porous materials in emulsion polymerization, the monomer ratio, emulsifier dosage, and reaction temperature showed the most noticeable impact on the properties of the polymer emulsion.
3.2 Time
In the emulsion polymerization process, the half-life of the free radical initiator should be within the range of 0.5–500 hours, and the optimal duration of the polymerization reaction generally falls between 1 and 5 hours. Deviating from this range, either exceeding or falling below, may result in a decrease in polymerization rate and quality issues. Based on the characteristics of the emulsion, the free radical polymerization reaction was typically completed within 2–4 hours. This ensured the full utilization of the radicals released by the free radical initiator, enabling an efficient polymerization process and the desired product quality.Huang Qiang81 investigated the preparation of small granule starch emulsifiers using corn starch as the raw material. The study examined the effect of acid hydrolysis time on the small granule starch emulsifiers and the stability of Pickering emulsions, as shown in Fig. 16. With the extension of acid hydrolysis time, the particle size of small granule starch decreased from 15.6 μm of the original starch to 5.8 μm, resulting in enhanced stability of the Pickering emulsion. Liu Chang82 studied the influence of the copolymerization reaction time of methyl methacrylate (n-BMA) and ethylene glycol dimethacrylate (EGDMA) on the gel content. Copolymerization for 2 hours resulted in a 30% gel content. Liu83 utilized sodium acrylate and methoxy poly(ethylene glycol)-acrylate monomers, sodium persulfate as the initiator, and sodium bisulfite as the activator in solution, conducting a 5 hour copolymerization reaction under a nitrogen atmosphere to synthesize a series of water-reducing agents consisting of poly(acrylic acid)-g-poly(ethylene glycol) comb-shaped copolymers with varying lengths of poly(ethylene glycol) side chains and graft densities.
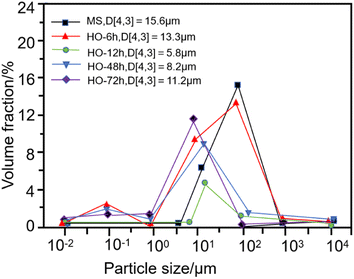 | ||
| Fig. 16 Particle size distribution of corn starch and small particle starch under different acid hydrolysis times.81 | ||
Based on the comprehensive research results mentioned above, control over time allowed for the regulation and improvement of material properties. The hydrolysis time significantly influenced the emulsion stability. Generally, with the gradual increase in polymerization rate, polymerization temperature, and the amounts of emulsifier and initiator in copolymerization, the effectiveness of the polymer water reducer decreased. Additionally, if the reaction occurred in an activator, a reaction with excessively high activity required a reaction under inert gases such as nitrogen. Choosing an appropriate time allowed for precise control over material structure characteristics, achieving regulation and improvement of material properties.
3.3 Additives
Additives regulated the stability of the emulsion, preventing the precipitation or settling of emulsified materials and maintaining the uniform dispersed state of the emulsion. Additives could also control the size and distribution of polymer particles, achieving control over particle size distribution and particle size by selecting the appropriate type and amount. Additionally, additives regulated the morphology and appearance of polymers, influencing their performance and functionality in applications.Lu Yuting84 investigated the influence of the addition amount of three additives, namely, silane coupling agent KH-550 (3-aminopropyltrimethoxysilane), polyether-modified silicone defoamer (polyether-modified polysiloxane), and polycarboxylate water reducer (PCE), on the properties of chloroprene–acrylate composite emulsions shown in Fig. 17–20. The study indicated that the tensile strength increased first and then decreased, reaching its maximum at 0.3%, while the elongation at break decreased first and then increased, reaching its minimum at 0.3%. The tensile strength, adhesion, and hardness of the composite emulsion significantly improved. Additionally, further enhancements in the coating's tensile properties, adhesion to cement mortar substrate, and reduction in the emulsion's water absorption were achieved by adding 0.30% polyether-modified silicone defoamer and 1.00% polycarboxylate water reducer. Steinerová Denisa85 studied the embedding of metal oxide nanoparticles as additives (namely MgO, ZnO, La2O3, and combinations of MgO and ZnO) into the self-crosslinking acrylic emulsion to enhance the stability of the emulsion. The metal oxide nanoparticles all showed promising application prospects.
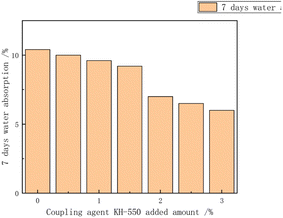 | ||
| Fig. 17 Effect of the amount of coupling agent added on the 7 day water absorption.84 | ||
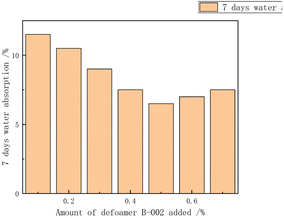 | ||
| Fig. 18 Effect of the amount of antifoam added on the water absorption rate at 7day.84 | ||
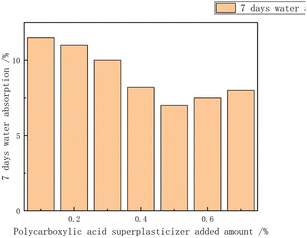 | ||
| Fig. 19 Effect of water-reducing agent addition on 7 day water absorption.84 | ||
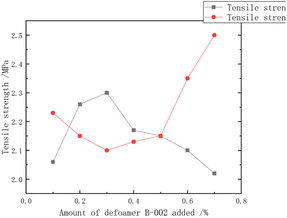 | ||
| Fig. 20 Effect of the amount of antifoam added on the tensile properties.84 | ||
The addition of a silane coupling agent, polyether-modified silicone defoamer, and polycarboxylate water reducer can enhance the tensile strength, adhesion, and hardness of polymer emulsion while reducing water absorption. Further addition of polyether-modified silicone defoamer and polycarboxylate water reducer can further improve the tensile performance of polymer emulsion and its adhesion to cement mortar base while reducing water absorption. In addition, organic small molecules exhibited inhibitory or promoting effects on cement setting, while carboxylate polymers inhibited the cement hydration process by adsorbing on the surface of cement particles, revealing the significant role of different additives in the cement hydration process.
4. Synthesis methods
4.1 Free radical polymerization
The free radical polymerization method addressed issues related to reaction rate, control of molecular structure, reaction conditions, and industrial-scale production. It featured flexible reaction conditions and efficient polymerization reaction rates, allowing precise control of polymer molecular structures by adjusting reaction conditions and monomer ratios. Additionally, the free radical polymerization method was operationally straightforward, suitable for large-scale production, and exhibited high scalability.Michelas Maxime86 investigated the synthesis and characterization of complexes of two tridentate tetradentate bisphenol ligands (1 and 2). The results showed that during the free radical polymerization process of methyl methacrylate and ethyl acrylate, the tridentate tetradentate bisphenol ligands (1 and 2) acted as effective regulators for the polymerization of active styrene, tert-butyl acrylate, and non-active ethyl acetate monomers. L. García-Uriostegui87 developed a free-radical emulsion polymerization formulation for the preparation of colloidal terpolymer of acrylamide (AAm)/2-acrylamido-2-methylpropanesulfonic acid sodium salt (AMPSNa)/N-vinylpyrrolidone (VP). Whitely Michael E.88 utilized tetrafunctional thiol, pentaerythritol tetrakis(3-mercaptopropionate), and biodegradable macromonomer fumaric acid di(methyl)allyl ester to prepare polymerized high internal phase emulsions with enhanced oxygen resistance via free-radical polymerization. Stenzel89 synthesized trithiocarbonate latex using mercaptoacetic acid, potassium hydroxide, carbon disulfide, and benzyl bromide as raw materials via free-radical polymerization. Controlling free-radical polymerization is not only applicable to emulsions but also catalyzes polymer emulsions. Bing Zhu90 polymerized 2,2′-azobis(2-methylpropionitrile) and styrene via free-radical polymerization to form the soap-free emulsion. The research findings indicate that in soap-free emulsions, the free radicals are isolated by dried polymer chains. Keling91 described a method and its application for the mild organic catalysis of living free-radical polymerization of high-molecular-weight polymers (HMWPs). The research findings indicate that using mild organic catalysts achieves controlled polymerization of HMWPs, resulting in low dispersity linear and star-shaped polymers. Segmental polymerization with functional methacrylate monomers was achieved, forming linear A–B diblock, linear B–A–B triblock, and 3-arm star-shaped A–B diblock copolymers.
The key research included the synthesis of ligand–cobalt complexes with determined structures, the development of emulsion polymerization formulations to enhance the stability and conversion rate of copolymers, the improvement of the performance of high internal phase emulsion polymerization by increasing the concentration of thiol additives, and the initiation of controlled free radical polymerization reactions using Metal–Organic Frameworks (MOFs), as illustrated in Fig. 21. Additionally, a synthesis strategy for amphiphilic diblock copolymers based on the RAFT (Reversible Addition–Fragmentation Chain Transfer) living radical polymerization method was developed. Furthermore, precise control over high molecular weight polymers was achieved using mild organic catalysts.
4.2 Microemulsion polymerization
The microemulsion polymerization method had advantages such as particle size control, energy savings, good flowability, and the preparation of high-performance materials. Within polymers, the microemulsion polymerization method allowed for the microscopic rearrangement of polymer molecules to achieve improved performance. This included achieving a uniform distribution within the matrix to enhance dispersion performance and adjusting the molecular arrangement of unit chains to improve thermal performance. Additionally, the microemulsion polymerization process was easy to control, enabling the production of smaller colloidal particles.Wang Yong92 successfully synthesized poly(acrylonitrile-co-butadiene) nanoparticles using a semi-continuous microemulsion polymerization method. The research indicates that during the polymerization process when the mass ratio of surfactant to monomer is 7.9%, latex with a minimum particle size of 34.2 nm can be obtained. Wu Li-Guang93 studied the construction of high-performance mixed matrix membranes (MMMs) and prepared MMMs emulsions through microemulsion polymerization. The research indicates that the protective effect of microemulsion micelles during polymerization enables uniform distribution of nanoparticles in the polymer matrix. Additionally, in MMMs, well-dispersed nanoparticles interact with benzene molecules through π–π interactions, enhancing the swelling and pervaporation separation performance of MMMs towards benzene. Pakdel Amir Saeid94 investigated the copolymerization of hydrophobic cellulose nanocrystals (CNCs) using microemulsion polymerization (MEP) method (butyl acrylate/ethyl acetate/acrylic acid). The research shows an increase in particle size and a decrease in polymerization rate. The polymerization method employed is illustrated in Fig. 18. Initially, solution polymerization was conducted in water, followed by the addition of monomers to form an emulsion until the synthesis reaction was completed.
Emulsion polymerization was employed to generate colloidal particles during the polymerization process, utilized for enhancing the performance of nano-composite materials. It was also employed for the synthesis and characterization of copolymers, altering the arrangement of unit chains. Hend S. Magar95 copolymerized (methyl methacrylate/N,N-dimethylaminoethyl methacrylate/acrylic acid) with nanoparticles of cadmium oxide (CdO), copper oxide (CuO), manganese oxide (MnO2), and selenium dioxide (SeO2) via microemulsion polymerization. The stability of the nanoparticles was significantly enhanced. Alvarado Abraham G.96 studied the synthesis and characterization of poly(butyl acrylate) (BA) and poly(ethylene glycol) methacrylate (PEGDMA) copolymers using the microemulsion method. The research indicates that the stability of the latex depends on the PEGDMA content, which must be less than 30%, as PEGDMA content exceeding 30% leads to latex instability and the formation of lumps.
Different types of nanoparticles were successfully synthesized through microemulsion polymerization by controlling reaction conditions, surfactant concentration, monomer feeding time, and other factors. The method achieved control over particle size, allowing for the rearrangement of particle size distribution. These nanoparticles were applied in the preparation of nano-composite materials and the controlled release of zinc phthalocyanine, as illustrated in Fig. 22. Furthermore, for the preparation of nano-composite materials, the emulsion polymerization method provided an effective pathway. By optimizing reaction conditions and component concentrations, uniform dispersion of nanoparticles and enhanced material properties could be achieved.
4.3 Active emulsion polymerization
Active emulsion polymerization was a polymerization method with high reactivity and selectivity, enabling the rapid and efficient completion of polymerization reactions. It possessed excellent reaction rates and monomer conversion rates, allowing for fast production and time/cost savings. Furthermore, active emulsion polymerization could achieve directed synthesis and precise control, allowing for accurate regulation of the structure and functionality of polymers. This polymerization method had a wide range of application prospects and could be used to prepare polymers with specific structures and functions. It was also capable of producing complex multilayer structures and nano-composite materials.Hidetaka Tobita97 investigated the branched structures formed when chain transfer reactions coexist with polymerization reactions in conventional free radical polymerization (FRP) and atom transfer radical polymerization (AR-P). The research indicated that the mean square radius of gyration (Rg2) and the maximum span (maximum segment length) of branched polymers are independent of polymer transfer constants and main chain length distributions. Hidetaka Tobita98 investigated the response of conventional free radical polymerization and atom transfer radical polymerization to this unique reaction environment. The research indicated that, for a given molecular weight, the expected radius of gyration is smaller compared to randomly branched polymers. Lin Zhou99 prepared chloromethylated polystyrene latex via activators regenerated by electron transfer atom transfer radical polymerization (ARGET ATRP) and observed that excessive monomer addition disrupts the conditions required for latex polymerization, leading to the detachment of large molecules from the latex interior. These findings guide the rational design and synthesis of high-performance catalyst latex in the future.
Emulsion polymerization comprises two studies: branched polymer structure and active free radical polymerization. The research revealed that the structural characteristics of branched polymers were independent of the polymer transfer constant and main chain length distribution. Additionally, an active emulsion polymerization method was employed to synthesize emulsion copolymers, demonstrating excellent mechanical properties in reaction with concrete, as depicted in Fig. 23. The study uncovered differences between traditional free radical polymerization and randomly branched polymers. Furthermore, polymers with iodine-terminated ends of varying molecular weights were successfully synthesized, showcasing faster reaction rates in copolymer synthesis.
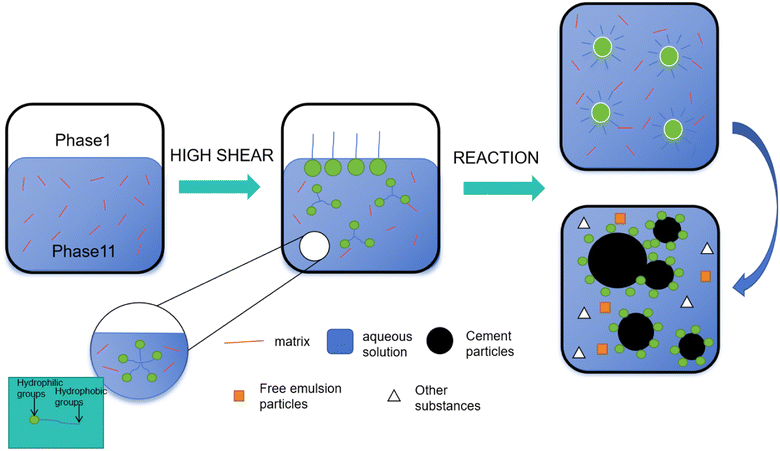 | ||
| Fig. 23 Schematic diagram of the application of active emulsion polymerization in the concrete process. | ||
4.4 Other emulsion polymerization methods
In addition, there are other emulsion preparation methods such as high-pressure homogenization, phacoemulsification, and microfluidization. In the process of emulsion preparation, the high-pressure homogenization method can achieve homogenization by squeezing the liquid through the aperture at high pressure, which is simple and stable. Phacoemulsification uses the high temperature and high pressure generated by the rupture and collapse of tiny bubbles generated by ultrasound to realize the emulsification process, maintain the quality of raw materials, fast speed, and no external heat source; Microfluidization technology can precisely control particle size and distribution, energy saving and environmental protection, and the preparation process is highly controllable.ShiWan100 prepared Pickering emulsions using the high-pressure homogenization method. The research results indicated that under the influence of different pH, temperature, and ion strengths, all emulsions prepared by high-pressure homogenization exhibited excellent stability. Maria C.101 has focused on investigating the physical stability of several oil-in-water nanoemulsions obtained by ultra-high-pressure homogenization (UHPH). The results have shown that UHPH has led to decreased viscosity, reduced droplet size, and a more uniform system. Loncke Jonas102 combined continuous ultrasonic emulsification with nano-polymerization processes to obtain latex nanoparticles from methyl methacrylate and ethylene glycol dimethacrylate. The research results have shown that continuous ultrasonic emulsification could reduce production costs and energy consumption. Wang Tengyu103 employed ultrasonic emulsification to prepare a stable double-layer emulsion system. The research results have indicated that the double-layer emulsion exhibited better storage stability, oxidation stability, and environmental stability compared to the single-layer emulsion. Santos Jenifer104 utilized microfluidization to prepare emulsions with submicron droplet sizes. The research findings have demonstrated that employing microfluidization technology has enhanced the rheological gel properties of the emulsion and improved its physical stability. Yaowei Liu105 prepared whey protein-stabilized gel-like emulsions through microfluidization and studied the influence of oil phase components on the physical properties of the emulsion. The research indicated that the viscosity of the gel-like emulsion increased exponentially with increasing oil phase fraction and exhibited a good correlation coefficient.
High-pressure homogenization, phacoemulsification, and microfluidization can improve the stability and physical properties of the emulsion. High-pressure homogenization and ultra-high-pressure homogenization can reduce the viscosity of the emulsion, reduce the droplet size, and improve the uniformity of the system. The combination of continuous phacoemulsification and nano-polymerization can reduce the production cost and energy consumption. The double-layer emulsion prepared by the phacoemulsification method has better storage, oxidation, and environmental stability. Microfluidization technology can improve the rheological gel properties and physical stability of the emulsion. These results provide an important reference for the selection and optimization of emulsion preparation methods.
5. Mechanism analysis
The feasibility of emulsion in cement-based materials was demonstrated through internal mixing and coating methods, employing macroscopic experiments and microscopic analyses. The influence of the functional groups in the emulsion on cement-based materials was comprehensively understood. Xushilin106 The research investigated the mechanism of the impact of silane composite emulsion on the crack resistance of cement-based materials. The results indicated that when the emulsion content was 3%, the total crack area per unit area of C50 concrete decreased by 84.0% compared to concrete without internal admixture. Additionally, the composite emulsion could be applied to cement-based materials through both internal mixing and coating methods. Zhou107 In the study of polyvinyl alcohol (PVA) emulsion's effect on cement-based materials, exploring the possibility of PVA intercalation involved integrating independently generated PVA structures into the interlayer regions of the C–S–H model. Molecular dynamics results indicated that organic entities could interact with the inorganic matrix on different length scales, and PVA emulsion molecules could be intercalated within the interlayer spaces of C–S–H. Weak hydrogen bond connections between PVA hydroxyl groups and non-bridging oxygen on the C–S–H surface accelerated the contraction of the interlayer regions. Furthermore, at larger length scales (>50 nm), large-sized PVA chains could be positioned between C–S–H nano-particles, reducing porosity and densifying particle packing, as illustrated in Fig. 24.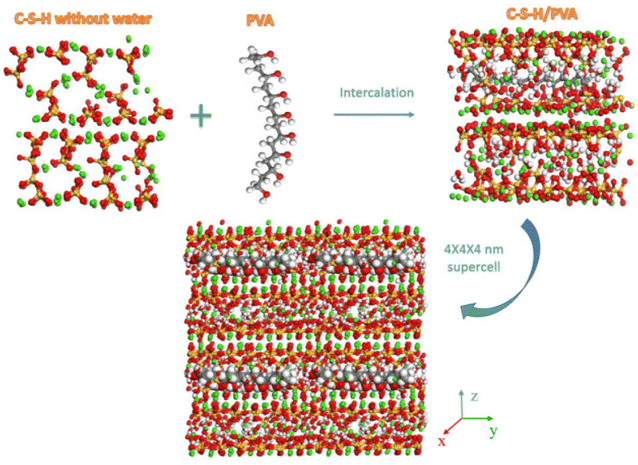 | ||
| Fig. 24 Molecular dynamics simulation of the interlayer incorporation of PVA emulsion structure into the C–S–H model.107 | ||
The silane composite emulsion achieves precise wetting and bonding of cement particles through its precise-designed interfacial film, thus effectively reducing crack formation. This thin film structure not only provides a stable interfacial environment but also regulates the structure of the cement-based material at the microscopic scale, thereby significantly improving its mechanical properties and durability. Secondly, the polyvinyl alcohol (PVA) emulsion achieves a high degree of integration with the cement matrix through its molecular-level insertion and interaction. PVA molecules can not only interact with C–S–H structures at the nanoscale but also realize micro-regulation of cement matrix by forming a complex hydrogen bond network. This micro-level control not only optimizes the pore structure of the cement-based material but also improves its overall density and mechanical strength. Therefore, these studies not only reveal the complex interaction between the interface of emulsion and cement-based materials but also provide theoretical guidance and practical basis for the design and development of cement concrete materials with excellent properties.
6. Conclusions and outlook
Based on the above review analysis and discussion, the following conclusions can be obtained:(1) Polymer emulsion is a widely used material, the most important synthetic materials are colloidal macromolecules and acrylate small monomers, free radical aqueous solution polymerization is preferred to synthesize, with excellent dispersion properties and rheological properties, and can be used in cement paste, suspension, catalytic templating silica, and many other fields.
(2) The polymerization process of polymer emulsion is affected by the initiator and stabilizer polymerization temperature. Increasing the stabilizer temperature reduces the average particle size of the polymer while increasing the initiator temperature increases the average particle size and its distribution. In addition, decreasing the polymerization temperature helps to control the size of the particle size and the narrowness of the distribution.
(3) If the activity of the reactive material is large, it is necessary to react in an inert gas atmosphere such as nitrogen, argon, etc., and if the activity is small, it is necessary to add a catalyst for the reaction. Strategies for the synthesis of amphiphilic block polymers based on the RAFT reactive radical polymerization method and the use of mild organic catalysts to achieve precise control of high molecular weight polymers and to improve the stability and conversion of copolymers were also developed.
(4) The stable emulsion can maintain its particle size distribution and dispersion state, thus ensuring uniform dispersion in the concrete and maintaining long-term stability. The viscosity and structure of the emulsion directly affect its dispersion in concrete and interaction with cement-based materials. The high-viscosity emulsion may lead to a decrease in concrete fluidity and affect the construction performance. The emulsion with reasonable structure and moderate particle size can realize an effective combination with cement-based materials, and improve the mechanical properties and durability of concrete.
To solve the problems arising from the production of polymer emulsions and to better meet the requirements related to concrete development, the synthesis and development of related polymer emulsions are mainly in the following areas:
(1) New monomer development
There are many synthetic processes for the development of macromonomers in China, and the most commonly used is the introduction of a single group or a single chain, which has simpler synthetic conditions and is easy to control, but the optimized performance of macromonomers is relatively single. So on this basis, according to the need to introduce two or more different groups at the same time to change the structure of the macromonomer, the development of new types of excellent macromonomers, and constantly optimize the synthesis process or develop new synthetic processes to improve the monomer conversion rate and product quality.
(2) Optimization of initiator
Aiming at different application requirements, we are committed to developing more efficient and stable initiators to improve the polymer emulsion polymerization rate and product properties, polymer emulsion will continue to develop in the direction of high dispersion, high slump-proof, and other high performance. Hyper-branched structure of the polymer molecules can better control the rheological properties of fresh concrete, but also has better adaptability and low viscosity, in addition, the introduction of “amphoteric ions” in the main chain of the molecule can reduce the saturated mixing of admixtures, to achieve its high dispersibility.
(3) Functionalization
Designing and synthesizing molecular structures with special functions, so that the polymer emulsion has various functions such as super early strength, shrinkage and crack reduction, and rust inhibition, is conducive to further promoting the technological progress and sustainable development of concrete. The grafting of polyethers with the above effects into the main chain of copolymers can further realize the functionalization of polymer emulsion molecules.
(4) Optimization of emulsifier
Fine-regulate the structure and molecular weight of the emulsifier, achieve high stability of the emulsion components, and expand its application in different cement-based materials. Polymers with high molecular weight and cross-linked structure provide greater emulsification and stability for high-performance concrete and special environment applications. In the future, emulsifiers will achieve high-efficiency emulsification at low concentrations, reducing their use and cost. In addition, the emulsifier will not only have the emulsification function but also may have a variety of performance improvement functions such as enhancement, crack resistance, durability, etc., to achieve integrated performance optimization. It will focus more on the use of biobased or renewable resources to reduce environmental impact and carbon footprint and achieve sustainable development.
Polymer emulsions have a wide range of practical applications in real life. One of them is as an additive to improve the performance and durability of concrete. By adding polymer emulsions, the bonding properties of concrete can be improved, its cracking resistance can be enhanced, and cracks caused by age shrinkage and temperature change can be reduced. In addition, polymer emulsion can also improve the impermeability and frost resistance of concrete, extend the service life of concrete, especially suitable for underground engineering, hydraulic engineering and road repair, and other fields. These applications allow polymer emulsions to play an important role in construction and infrastructure engineering, providing an effective guarantee for the reliability and durability of engineered structures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (General Program: 52209154, 52278239), Chunhui Project Foundation of the Education Department of China (HZKY20220358), Natural Science Foundation of Hunan Province (2022JJ30042, 2023JJ30040), Hunan Provincial Youth Science and Technology Talent Support Plan (2022RC1175), Hunan Provincial Innovation Foundation for Postgraduate (CX20230859).References
- S. Wang and X. Li, The influence of carboxylated styrene butadiene lotion content on the microstructure of modified concrete, Int. J. Microstruct. Mater. Prop., 2024, 17(1), 17–31 CAS.
- A. Pasandín and I. Pérez, Mechanical properties of hot-mix asphalt made with recycled concrete aggregates coated with bitumen emulsion, Constr. Build. Mater., 2014, 55350–55358 Search PubMed.
- L. SuJin, S. HyungJin and P. Changi, Strength and Durability of Hybrid Fiber-Reinforced Latex-Modified Rapid-Set Cement Preplaced Concrete for Emergency Concrete Pavement Repair, Appl. Sci., 2021, 11(10), 4595 CrossRef.
- Z. Hongfei, Z. Jiupeng and Y. Yang, et al., Effects of asphalt emulsion on the durability of self-compacting concrete, Constr. Build. Mater., 2021, 292, 123322 CrossRef.
- Z. Qian, Z. Guozheng and Y. Jun, et al., Highly Penetrable Silicone Emulsion for Water-Repellent Concrete with Reserved Surface Recoatability, J. Wuhan Univ. Technol.-Mater. Sci. Ed., 2022, 37(1), 58–65 CrossRef.
- L. Dafei, W. Yingni and Y. Haoran, et al., Panax Notoginseng polysaccharide stabilized gel-like Pickering emulsions: Stability and mechanism, Int. J. Biol. Macromol., 2023, 249, 125893 CrossRef PubMed.
- K. B. Sharu, G. P. Simon and C. Wenlong, et al., Effect of Organic Modification on Multiwalled Carbon Nanotube Dispersions in Highly Concentrated Emulsions, ACS Omega, 2019, 4(4), 6647–6659 CrossRef PubMed.
- Y. Zhang and X. Kong, Influences of superplasticizer, polymer latexes, and asphalt emulsions on the pore structure and impermeability of hardened cementitious materials, Constr. Build. Mater., 2014, 53392–53402 Search PubMed.
- V. V. C. Himakar, V. C. Khed and A. Musa, et al., Investigating properties of concrete containing cationic bitumen emulsion, Innov. Infrastruct. Solut., 2022, 7, 218 CrossRef.
- S. Yada, K. Matsuoka and Y. Nagai Kanasaki, et al., Emulsification, solubilization, and detergency behaviors of homogeneous polypropylene-polyoxyethylene alkyl ether type nonionic surfactants, Colloids Surf., A, 2019, 564, 51–58 CrossRef CAS.
- K. Ko, J. D. Lundberg and M. A. Johnson, et al., Mechanism-Guided Discovery of Cleavable Comonomers for Backbone Deconstructable Poly(methyl methacrylate), J. Am. Chem. Soc., 2024, 22–25 Search PubMed.
- S. Kwon, K. Oh and J. S. Shin, et al., Effects of hydroxyethyl methacrylate comonomer in styrene/acrylate latex on coating structure and printability, Prog. Org. Coat., 2020, 147, 105862 CrossRef CAS.
- H. Zheng, Z. Qiu and D. Li, et al., Advance on nickel- and palladium-catalyzed insertion copolymerization of ethylene and acrylate monomers, J. Polym. Sci., 2023, 61(23), 2987–3021 CrossRef CAS.
- D. Andrea, S. Claas and B. Eszter, et al., New bifunctional monomers from methyl vinyl glycolate, Chem. Commun. (Cambridge, U. K.), 2022, 58(94), 13091–13094 RSC.
- M. Hisano and K. Takeda, et al., Sequence-controlled radical copolymerization of N-substituted maleimides with olefins and polyisobutene macromonomers to fabricate thermally stable and transparent maleimide copolymers with tunable glass transition temperatures and viscoelastic properties, Macromolecules, 2013, 46(19), 7733–7744 CrossRef CAS.
- M. Chowdhury, P. Poddar and A. Kashem, et al., Surface modification of polycarbonate films with 2-hydroxyethyl methacrylate (HEMA) and N-vinyl pyrrolidone (NVP) monomers by radiation techniques, Results Eng., 2024, 22, 101981–101993 CrossRef CAS.
- L. A. Campos, S. A. Santos and F. H. Franco, Influence of the hydroxypropyl acrylate and hydroxypropyl methacrylate monomer concentration on the emulsion copolymerization of styrene with n-butyl acrylate and acrylonitrile to produce adhesives, Matéria, 2019, 24(3), 9347–9367 Search PubMed.
- I. S. Tsagkalias and D. S. Achilias, Role of Functional Groups in the Monomer Molecule on the Radical Polymerization in the Presence of Graphene Oxide. Polymerization of Hydroxyethyl Acrylate under Isothermal and Non-Isothermal Conditions, Molecules, 2022, 27(2), 345 CrossRef CAS PubMed.
- L. Zongying, C. Xin and Z. Feifang, et al., A strong anion exchanger of poly(glycidyl methacrylate-divinylbenzene) substrate functionalized with cationic quaternary ammonium monomer, J. Separ. Sci., 2022, 45(21), 3995–4000 CrossRef PubMed.
- R. Onozuka, C. Piedrahita and Y. Yanagida, et al., Ion conductive polymer electrolyte membranes based on star -branched poly(ethylene-glycol tri-acrylate) and polysulfide macromonomers, Solid State Ionics, 2020, 346, 115182 CrossRef CAS.
- M. Seyedsina and J. Esmaiel, Mesoscale simulation of the effect of a lactide segment on the nanostructure of star poly(ethylene glycol-co-lactide)-acrylate macromonomers in aqueous solution, J. Phys. Chem. B, 2012, 116(5), 1536–1543 CrossRef PubMed.
- P. Kusch, C. Rieser, G. Knupp and T. Mang, Alkenes; Report Summarizes Alkenes Study Findings from University of Applied Sciences [Characterization of copolymers of methacrylic acid with poly(ethylene glycol) methyl ether methacrylate macromonomers by analytical pyrolysis-gas chromatography/mass], Sci. Lett., 2015, 21–24 Search PubMed.
- J. R. Booth, J. D. Davies and S. A. F. Bon, ω-Unsaturated methacrylate macromonomers as reactive polymeric stabilizers in mini-emulsion polymerization, Polym. Chem., 2022, 13(10), 1335–1349 RSC.
- R. Joel, Z. Stefan, V. Brigitte and C. R. Juan, Synthesis and characterization of new interpenetrated hydrogels from N-isopropyl acrylamide, 2-oxazoline macromonomer, and acrylamide, Eur. Polym. J., 2022, 177, 111456 CrossRef.
- S.-H. Luo, Y. Xiao, J.-Y. Lin, Z.-H. Chen, S.-T. Lin and Z.-Y. Wang, Preparation, characterization, and application of maleic anhydride-modified polylactic acid macromonomer based on direct melt polymerization, Mater. Today Chem., 2022, 25, 100986 CrossRef CAS.
- H. Lu, J. Cai, Y. Fang, M. Ren, X. Tan, F. Jia, D. Wang and K. Zhang, Exploring the Structural Diversity of DNA Bottlebrush Polymers Using an Oligonucleotide Macromonomer Approach, Macromolecules, 2022, 55(6), 2235–2242 CrossRef CAS PubMed.
- B. Dai, P. Xu, M. Xu, Q. Jiang, Q. Liu and S. Wang, Synthesis and plugging effect of inverse emulsion polymerization microspheres (OPME) for oil-based drilling fluids, Arabian J. Chem., 2023, 16(4), 104577 CrossRef CAS.
- L. Chen, L. Li and J. Yang, Effects of macromonomer chain length, solvent, and concentration on the cyclization kinetics during AB-type step-growth polymerization, Polymer, 2023, 269, 125730 CrossRef CAS.
- R. Ganguly, P. Saha, L. A. Kringe, A. Pich and N. K. Singha, Thermoresponsive Microgels with High Loading of Zwitterions Exhibiting Superior Performance: A Macromonomer Approach, Macromol. Chem. Phys., 2022, 224, 200349 Search PubMed.
- A. D. Bendrea, L. Cianga, G. L. Ailiesei, D. Göen Colak, I. Popescu and I. Cianga, Thiophene α-Chain-End-Functionalized Oligo(2-methyl-2-oxazoline) as Precursor Amphiphilic Macromonomer for Grafted Conjugated Oligomers/Polymers and as a Multifunctional Material with Relevant Properties for Biomedical Applications, Int. J. Mol. Sci., 2022, 23(14), 7495 CrossRef CAS PubMed.
- K. Ko, J. D. Lundberg and M. A. Johnson, et al., Mechanism-Guided Discovery of Cleavable Comonomers for Backbone Deconstructable Poly(methyl methacrylate), J. Am. Chem. Soc., 2024, 146(13), 9142–9154 CrossRef CAS PubMed.
- D. Muqian and J. T. Welch, Synthesis of a novel fluorinated polymer: poly(2-(tetrafluoro(trifluoromethyl)-λ6-sulfanyl)ethyl acrylate), J. Polym. Res., 2023, 30(12), 443 CrossRef.
- S. G. Castellanos, V. V. A. Fernández-Escamilla and M. A. Corona-River, et al., Coagulative Nucleation in the Copolymerization of Methyl Methacrylate-Butyl Acrylate under Monomer-Starved Conditions, Polymers, 2023, 15(7), 1628 CrossRef CAS PubMed.
- A. Migdal, M. Dudkiewicz and K. Jacek, et al., Synthesis of acrylic monomers based on renewable raw materials. Part I. Synthesis of acrolein from glycerol, Polimery, 2012, 57(5), 369–374 CrossRef CAS.
- H. K. Lee, P. Chantanapongvanij and R. R. Schmidt, et al., Master Equation Studies of the Unimolecular Decay of Thermalized Methacrolein Oxide: The Impact of Atmospheric Conditions, J. Phys. Chem. A, 2023, 127(20), 4492 CrossRef CAS PubMed.
- W. Xiaoming, F. S. William and N. Kyoko, Synthesis of Polyethylene with In-Chain α,β-Unsaturated Ketone, and Isolated Ketone Units: Pd-Catalyzed Ring-Opening Copolymerization of Cyclopropenone with Ethylene, Angew. Chem., 2019, 58(37), 12955–12959 CrossRef PubMed.
- K. Olga, K. Philippos and A. Maria, et al., Statistical Copolymers of N-Vinylpyrrolidone and Isobornyl Methacrylate via Free Radical and RAFT Polymerization: Monomer Reactivity Ratios, Thermal Properties, and Kinetics of Thermal Decomposition, Polymers, 2021, 13(5), 778 CrossRef PubMed.
- T. Ryo, S. Akane and O. Riki, et al., Selective synthesis of α-vinyl-ω-vinylidene-oligoisoprene as a macromonomer via ethenolysis of polyisoprene, Polym. J., 2024, 56(4), 335–342 CrossRef.
- J. Li, M. Shi and Y. Li, et al., Research on Strengthening Fragile Paper with Polyvinylamine, Polymers, 2024, 16(5), 619 CrossRef CAS PubMed.
- Z. Jiang, Study on Synthesis and Properties of Low Sensitive Type Polycarboxylate Superplasticizer, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 392(3), 032001 Search PubMed.
- Z. Jiang, R. You and X. Guo, Study on Preparation of Low Sensitive and Stable Type Polycarboxylate Superplasticizer Small Monomers, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 631(2), 022030 CAS.
- B. Massoumi, M. Abdollahi, J. S. Somayyeh and A. A. Entezami, Preparation and characterization of polyaniline N-grafted with poly(ethyl acrylate) synthesized via atom transfer radical polymerization, J. Appl. Polym. Sci., 2013, 128(1), 47–53 CrossRef CAS.
- Y. Liu, S. Wang, Y. Li, X. Cai, M. Zhu and X. Han, (NH4)Cu0.2H2.8PMo11VO40 via a hydrothermal homogeneous precipitation method for selective oxidation of methacrolein to methacrylic acid, Appl. Catal., A, 2022, 643(5), 118789 CrossRef CAS.
- Y. Tang, J. Duan, C. Zhang, L. Feng and X. Gu, Preparation of polydimethyl-methylvinylsiloxane catalyzed by high-activity phosphazene base, Organosilicon Materials, 2023, vol. 36(03), pp. 9–15 Search PubMed.
- X. D. Yun, S. Juncai and Z. Jing, et al., Generation of a carbon dots/ammonium persulfate redox initiator couple for free radical frontal polymerization, Polym. Chem., 2018, 9(4), 420–427 RSC.
- X. Xiuhang, X. Yunfei and Q. Jiajia, et al., Roles of Proton in the Formation of Particles: Soap-free Emulsion Polymerization of Styrene using AIBN and Potassium Persulfate as Initiators, ChemistrySelect, 2021, 6(4), 557–568 CrossRef.
- X. N. Wang, Y. L. Zhang and H. Y. Wu, et al., C(sp3)–H Bond Functionalization of Alcohols, Ketones, Nitriles, Ethers and Amides using tert-Butyl Hydroperoxide as a Radical Initiator, Synlett, 2021, 32(01), 23–29 CrossRef.
- N. Vinay, C. Soumia and Y. Kaige, et al., Preparation of open tubular capillary column covalently coated with polystyrene sulfonate with 4,4′-azobis (4-cyanopentanoyl chloride) as polymerization initiator for electrochromatographic separation of alkaloids, sulfonamides, and peptides, J. Sep. Sci., 2023, 46(10), e2200711 CrossRef PubMed.
- C. L. Faria Guilherme and P. Lyra Emerson, Exploring solution ICAR ATRP of methyl acrylate with AIBN as azo initiator: a kinetic modeling and simulation study, Results Mater., 2022, 15, 100313 CrossRef.
- W. Jiaoli, L. Fangyan and H. Zeyu, et al., The improvement of polymer gel electrolyte properties by the compound initiator, Mater. Lett., 2022, 324, 132744 CrossRef.
- J. I. So, C. S. Lee, J. Y. Jung, J. Lee, J. K. Choi, S. E. Shim and Y. Qian, Optimization and Characterization of the F-LSR Manufacturing Process Using Quaternary Ammonium Silanolate as an Initiator for Synthesizing Fluorosilicone, Polymers, 2022, 14(24), 5502 CrossRef CAS PubMed.
- Y. Ming, W. Huan and H. Dayun, et al., Synthesis of ultrahigh molecular weight poly(butyl acrylate) in the presence of palladium nanoparticles, J. Polym. Res., 2023, 30(11), 427 CrossRef.
- B. Mistry and C. N. Murthy, Synthesis and properties of ABA type triblock copolymer from poly(dimethylsiloxane) macroinitiator: Development of novel attachable initiators for atom transfer radical polymerization, J. Macromol. Sci., Part A: Pure Appl. Chem, 2022, 59(12), 786–797 CrossRef CAS.
- Z. C. Gul, H. K. Cem and Y. Yusuf, Photoinitiated Cationic Ring-Opening Polymerization of Octamethylcyclotetrasiloxane, Molecules, 2023, 28(3), 1299 CrossRef PubMed.
- P. Grzegorz, S. Katarzyna, G. Piotr and M. Agnieszka, Influence of Initiator Concentration on the Polymerization Course of Methacrylate Bone Cement, Polymers, 2022, 14(22), 11–18 Search PubMed.
- C. Deoghare, Experimental determination of activation rate constant and equilibrium constant for bromo substituted succinimide initiators for an atom transfer radical polymerization process, Pure Appl. Chem., 2022, 94(7), 839–858 CrossRef CAS.
- R. Zumpano, D. A. Giudice and S. Resta, et al., Sodium lauryl ether sulfates, pivotal surfactants for formulations: Rationalization of their assembly properties, Colloids Surf., A, 2024, 686, 133375 CrossRef CAS.
- S. Yiyue, L. Fahui and D. Yupei, et al., Experimental and theoretical studies on foam features of dodecyl benzene sulfonate-based ionic liquid surfactants: alkyl chain length of the ester group of imidazolium cations, Colloid Polym. Sci., 2023, 301(12), 1421–1436 CrossRef.
- M. Farzaneh, Synthesis of Tetrahydrobenzo[;b;]pyrans Promoted by Sodium Stearate as a Lewis Base-Surfactant Combined Catalyst in an Aqueous Micellar Medium, Org. Prep. Proced. Int., 2023, 55(4), 345–350 CrossRef.
- P. R. Singh, S. Shee and M. Soni, et al., One pot synthesis of carboxylic acid, ester, and amide derivatives from iodoarenes in aqueous solubilized systems using sodium oleate as a surfactant at room temperature, Tetrahedron Lett., 2024, 137, 154930 CrossRef.
- J. Cao and H. Guo, et al., Effect of polyvinyl pyrrolidone/sodium polyacrylate compound surfactants on slurry properties of lithium iron phosphate and electrochemical performance of the battery, Ionics, 2022, 28(4), 1–12 CrossRef.
- A. O. Ezzat, H. A. Al-Lohedan and A. M. Tawfeek, et al., One-Step Synthesis of New Amphiphilic Nonionic Surfactants Based on Alkylamine and Poly(ethylene glycol) Dimethacrylate for Demulsification of Arabian Heavy Crude Oil Emulsions, ACS Omega, 2023, 8(6), 6030–6039 CrossRef CAS PubMed.
- V. Grossi, F. Giannotti and D. Pedreschi, Science - Applied Sciences; Data on Applied Sciences Detailed by Researchers at Seoul National University of Science and Technology [Inhibition of Droplet Growth in Model Beverage Emulsions Stabilized Using Poly (ethylene glycol) Alkyl Ether Surfactants Having Various], Sci. Lett., 2020, 19(11), 123–278 Search PubMed.
- L. Zhang, L. Jia, Z. Zhao, R. Yang, J. Wang and X. Guo, Synthesis, physiochemical properties, and antimicrobial activities of novel gemini surfactants with biphenyl and multiple amide groups, Colloids Surf., A, 2020, 593(6), 170–178 Search PubMed.
- L. Guo, Y. Bai, D. Zhang and G. Wang, Synthesis and properties of a glucono-δ-lactone-modified silicone surfactant from high-amine-value amodimethicone, Phosphorus, Sulfur, Silicon Relat. Elem., 2020, 195(5), 392–398 CrossRef CAS.
- B. Wang, F. Yuan, J. Wang, Q. Wang, Z. Li, W. Zhao, Y. Li, J. Feng and W. Li, Synthesis of pomegranate-structured Si/C microspheres using P123 as a surfactant for high-energy lithium-ion batteries, J. Electroanal. Chem., 2020, 864(1), 114102 CrossRef CAS.
- W. Wu, H. Luan and W. Ding, et al., Micellar thermodynamic properties of Gemini surfactants, Petrochem. Ind., 2013, 42(1), 39–46 CAS.
- J. Wei, A. J. Lara and M. Mascal, Trifluoroacetic acid as an effective dispersing medium for cellulose nanocrystals, Carbohydr. Polym. Technol. Appl., 2022, 5(3), 100277 Search PubMed.
- E. Y. Safronova, D. Y. Voropaeva, D. V. Safronov, N. Stretton, A. V. Parshina and A. B. Yaroslavtsev, Correlation between Nafion Morphology in Various Dispersion Liquids and Properties of the Cast Membranes, Membranes, 2023, 13(1), 13 CrossRef CAS PubMed.
- V. I. Zhornik, A. V. Zapolsky and A. V. Ivakhnik, The Structure and Properties of a Biodegradable Grease with a Mixed Dispersion Medium and a Heterogeneous Lithium–Calcium Dispersed Phase, J. Frict. Wear, 2022, 43(4), 229–235 CrossRef.
- L. A. Khakhin, I. A. Arutyunov, V. L. Zavorotnyi, S. N. Potapova, D. V. Svetikov, E. V. Korolev and A. V. Kulik, Prospects for the Use of Synthetic Hydrocarbon Bases – The Dispersion Media of Nonaqueous Drilling Fluids, Chem. Technol. Fuels Oils, 2022, 58(5), 768–771 CrossRef CAS.
- S. W. Spring, C. S. Cerione, J. H. Hsu, S. L. Shankel and B. P. Fors, Scalable, Green Chain Transfer Agent for Cationic RAFT Polymerizations, Chin. J. Chem., 2022, 41(4), 399–404 CrossRef.
- I. Mandal, A. Mandal and A. F. M. Kilbinger, Macrochain Transfer Agents for Catalytic Ring-Opening Metathesis Polymerization, ACS Macro Lett., 2022, 11(12), 1384–1389 CrossRef CAS PubMed.
- A. Mandal, I. Mandal and A. F. M. Kilbinger, Catalytic Living Ring-Opening Metathesis Polymerization Using Vinyl Ethers as Effective Chain-Transfer Agents, Angew. Chem., 2022, 135(4), e202211842 CrossRef.
- Y. Mitsukami, M. S. Donovan, A. B. lowe and C. L. McCormick, Macromolecules, 2001, 34, 2244–2256 CrossRef.
- T. P. Le, G. Moad, E. Rizzardo, and S. H. A. U. Thang, WO1997US12540, 1997.
- M. Daneshgar, V. E. Farahani and N. M. Haghighi, The Effects of Temperature Initiator and Stabilizer on Polymer Particle Size in Suspension Polymerization of Styrene, Iran. J. Polym. Sci. Technol., 2010, 23(6), 499–507 Search PubMed.
- L. Anusha, Z. Zakaria, A. W. M. Kahar and D. N. U. Lan, Effect of Emulsion Temperature on Properties of Conductive Epoxy Porous Prepared by Single Emulsion Technique, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 429(01), 2061 Search PubMed.
- B. Dai, P. Xu, M. Xu, Q. Jiang, Q. Liu and S. Wang, Synthesis and plugging effect of inverse emulsion polymerization microspheres (OPME) for oil-based drilling fluids, Arab. J. Chem., 2023, 16(4), 104577 CrossRef CAS.
- X. Liu, Z. Li and Y. Song, et al., Synthesis and characterization of NMA-modified acrylate microemulsion for fabric darkening, Printing and Dyeing, 2023, 49(5), 5–9 CAS.
- H. Qiang, Y. Yinzhou, W. Chan and F. Kang, Effect of acid hydrolysis time on properties of small starch particles and stability of Pickering emulsion, J. South China Univ. Technol., Nat. Sci., 2017, 45(03), 104–110 Search PubMed.
- C. Liu, A. K. Tripathi and W. Gao, et al., Crosslinking in Semi-Batch Seeded Emulsion Polymerization: Effect of Linear and Non-Linear Monomer Feeding Rate Profiles on Gel Formation, Polymers, 2021, 13(4), 596 CrossRef CAS PubMed.
- J. Liu, Q. Ran and C. Miao, Synthesis and Characterization of Comb-Like Copolymer Dispersant with Methoxy Poly (Ethylene Oxide) Side Chains, Polym.-Plast. Technol. Eng., 2011, 50(1), 59–66 CrossRef CAS.
- Y. Lu, W. Fu, H. Ren, S. Wu and J. Liu, Study on the effect of admixtures on the properties of JS composite emulsion waterproof coating, Constr. Build. Mater., 2023, 408, 133825 CrossRef.
- S. Denisa, K. Andréa and M. Jana, et al., Influence of Metal Oxide Nanoparticles as Antimicrobial Additives Embedded in Waterborne Coating Binders Based on Self-Crosslinking Acrylic Latex, Coatings, 2022, 12(10), 1445 CrossRef.
- M. Michelas, Y. K. Redjel, J.-C. Daran, M. Benslimane, R. Poli and C. Fliedel, Cobalt(II) and cobalt(III) complexes of tripodal tetradentate diamino-bis(phenolate) ligands: Synthesis, characterization, crystal structures and evaluation in radical polymerization processes, Inorg. Chim. Acta, 2023, 549, 121408 CrossRef CAS.
- L. García-Uriostegui, G. Pineda-Torres, S. López-Ramírez, J. Barragán-Aroche and C. Durán-Valencia, Inverse emulsion free-radical polymerization of acrylamide terpolymer for enhanced oil recovery application in harsh reservoir conditions, Polym. Eng. Sci., 2017, 57(11), 1214–1223 CrossRef.
- M. E. Whitely, J. L. Robinson, M. C. Stuebben, H. A. Pearce, A. P. McEnery Madison and E. Cosgriff-Hernandez, Prevention of Oxygen Inhibition of PolyHIPE Radical Polymerization using a Thiol-based Crosslinker, ACS Biomater. Sci. Eng., 2017, 3(3), 409–419 CrossRef CAS PubMed.
- M. Stenzel-Rosenbaum, T. P. Davis and A. G. Fane, J. Mater. Chem., 2003, 13, 2093–2097 Search PubMed.
- B. Zhu and H. Ni, Evidence of gel effect and living free radicals in particles: Soap-free emulsion polymerization of styrene by using 2,2′-azobis(isobutyronitrile), J. Appl. Polym. Sci., 2023, 141(5) Search PubMed.
- K. Hu, S. Liu, Z. Zhao and Y. Zhang, Synthesis of High Molecular Weight Polymers by Organocatalyzed Living Radical Polymerization and Self-assembly, Macromol. Rapid Commun., 2023, 44(8), e2300005–e2300005 CrossRef PubMed.
- Y. Wang, F. Cao, Y. Fu, H. Chen, Y. Zhang, C. Wang, Y. Li and H. Wang, Preparation of diene-based nanoparticles by semi-batch microemulsion polymerization and their catalytic hydrogenation, Catal. Today, 2023, 407, 156–164 CrossRef CAS.
- L.-G. Wu, Y.-T. Liao, T. Wang, D.-F. Zhu, G.-Q. Wang and X.-J. Jiang, Synthesis of Co(OH)2/poly(MMA-St-APEG) mixed matrix membranes by in-situ microemulsion polymerization for pervaporation separation of benzene/cyclohexane mixture, Colloids Surf., A, 2022, 651, 129712 CrossRef CAS.
- A. S. Pakdel, E. D. Cranston and M. A. Dubé, Incorporating Hydrophobic Cellulose Nanocrystals inside Latex Particles via Mini-Emulsion Polymerization, Macromol. React. Eng., 2021, 15(5), 2100023 CrossRef CAS.
- S. H. Magar, S. M. Hashem and A. R. Sobh, Design of metal oxide nanoparticles-embedded polymeric nanocomposites for hydrogen peroxide chronoamperometric sensor, Polym. Compos., 2024, 45(4), 3653–3665 CrossRef.
- A. G. Alvarado, M. Rabelero, J. Aguilar, M. J. Flores and S. F. J. Moscoso, Synthesis and characterization of butyl acrylate-co-poly (ethylene glycol) methacrylate obtained by microemulsion polymerization, Des. Monomers Polym., 2020, 23(1), 40–49 CrossRef CAS PubMed.
- H. Tobita, Universal Relationships in Branched Architecture Formed in Conventional and Living Emulsion Polymerization, Macromol. Theory Simul., 2019, 28(5), 00018 CrossRef.
- H. Tobita, Effect of Chain Transfer to Polymer in Conventional and Living Emulsion Polymerization Process, Processes, 2018, 6(2), 14 CrossRef.
- L. Zhou and Y. Lu, Chloromethylated magnetic polystyrene composite nanoparticles prepared via RAFT-mediated surfactant-free emulsion polymerization, Colloids Surf., A, 2024, 684, 133144 CrossRef CAS.
- N. ShiWan, C. WaiTing and S. YeeTheng, et al., Pickering emulsion stabilized by palm-pressed fiber cellulose nanocrystal extracted by acid hydrolysis-assisted high-pressure homogenization, PLoS One, 2022, 17(8), e0271512 CrossRef PubMed.
- C. M. Rave, D. J. Echeverri and H. C. Salamanca, Improvement of the physical stability of oil-in-water nanoemulsions elaborated with Sacha inchi oil employing ultra-high-pressure homogenization, J. Food Eng., 2020, 273, 109801 CrossRef.
- L. Jonas, V. Arne and D. Wim, et al., Development and characterization of a continuous ultrasound emulsification and nano-emulsion polymerization process, Tetrahedron Green Chem, 2023, 2, 100023 CrossRef.
- W. Tengyu, W. Shirang and Z. Lijuan, et al., Fabrication of bilayer emulsion by ultrasonic emulsification: Effects of chitosan on the interfacial stability of emulsion, Ultrason. Sonochem., 2023, 93, 106296 CrossRef PubMed.
- J. Santos, L. A. Trujillo-Cayado and M. D. Alcaide, et al., Impact of Microfluidization on the Emulsifying Properties of Zein-Based Emulsions: Influence of Diutan Gum Concentration, Materials, 2021, 14(13), 3695 CrossRef CAS PubMed.
- Y. Liu and W. Zhang, et al., Fabrication of Gel-Like Emulsions with Whey Protein Isolate Using Microfluidization: Rheological Properties and 3D Printing Performance, Food Bioprocess Technol., 2019, 12(6), 1967–1979 CrossRef CAS.
- S. Xu, Effect of Silane Composite Emulsion on the Crack Resistance of Cement-Based Materials and its Mechanism, Qingdao University of Technology, 2022 Search PubMed.
- Y. Zhou, L. Tang and J. Liu, et al., Interaction mechanisms between organic and inorganic phases in calcium silicate hydrates/poly (vinyl alcohol) composites, Cem. Concr. Res., 2019, 125, 105891 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |