Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transformations, interactions, and acute biological responses of nanoplastics on mixotrophic microalgae Poterioochromonas malhamensis†
Zijiao
Meng
,
Serge
Stoll
 and
Wei
Liu
and
Wei
Liu
 *
*
Department F.-A. Forel for Environmental and Aquatic sciences, University of Geneva, Carl-Vogt 66, CH-1211 Geneva, Switzerland. E-mail: wei.liu@unige.ch; Tel: +41223790314
First published on 28th July 2023
Abstract
Nanoplastics show a high potential risk to aquatic organisms due to their small size, high mobility and large surface area. However, our knowledge about their behavior in different water conditions and their potential effect on freshwater microalgae as well as the influential toxicity factor remains elusive. Here, we study the transformation and toxicity of polystyrene nanoplastics (PS-NPLs) of 20 and 100 nm to a widespread mixotrophic phytoflagellate, Poterioochromonas malhamensis, using Lake Geneva water and cell culture medium. The colloidal stability of PS-NPLs, cellular burden, variety of physiological responses, and the potential of producing diverse high-value biocompounds upon exposure to PS-NPLs under two exposure conditions were evaluated. Significant effects are observed after 24 h of exposure for cell viability and PS-NPLs associated with/taken up by cells at the lowest tested concentration of 0.1 mg L−1. It is also found that in both Lake Geneva water and culture medium, the biological effects of 20 nm PS-NPLs are 1.7–11.5 times higher than those of 100 nm PS-NPLs. PS-NPLs exhibit 2.6–20.5 times higher biological effect in Lake Geneva water compared to the culture medium, demonstrating that the effect of NPLs is concentration-, size- and medium-dependent. Moreover, PS-NPLs induced changes in protein and carbohydrate content within microalgae cells. Principal component analysis of 12 key parameters in both Lake Geneva water and culture medium indicates that aggregation behavior is the most influential factor controlling the biological effects of NPLs. These results highlight a clear mechanistic link between NPLs' intrinsic and water properties in the evaluation of NPL biological responses.
Environmental significanceNanoplastics have received increasing attention in recent years because of their potential risk to aquatic ecosystems. However, how microorganisms interact with nanoplastics in freshwater environments is largely unknown. In this study, we examine the transformation behavior of polystyrene nanoplastics (PS-NPLs) of 20 and 100 nm in traditional culture medium and in natural water environment on freshwater microalgae Poterioochromonas malhamensis to obtain new insights into the effects of size, surface charge, and medium composition on the behavior of NPLs and how freshwater microorganisms exposed to these PS-NPLs respond. We show that a significant biological response of P. malhamensis was observed from the lowest PS-NPL tested concentration of 0.1 mg L−1 in a concentration-, size-, and medium dependent manner. The aggregation behavior is found to be the most influential explanatory factor of PS-NPLs on P. malhamensis under both exposure conditions. These results provide more comprehensive information for the assessment of the fate and toxicity of NPLs according to their characteristics, stability, and interaction in freshwater systems. |
1. Introduction
Plastics are ubiquitous in aquatic environments and are quite persistent due to their chemical inertness.1 However, their size could decrease from macro-/microsized to nanosized particles via physical, chemical, and biological degradation processes.2 Although the toxicity of macro- and microplastics has been relatively well studied, the toxicity of nanoplastics (NPLs) is largely uncharacterized. Recent study has discovered that NPLs may exhibit remarkably different physical and adsorption properties from both the bulk polymer and microplastics.3 It is now an indisputable reality that freshwater compartments are thoroughly exposed to these micro- and nanoparticles and become their main repository.4,5 Thus, the influence of NPL contamination on the freshwater environmental behavior and biological effects needs further investigation.Several studies focused on polystyrene nanoplastics (PS-NPLs) as they are one of the most frequently reported polymers in the aquatic environment,6,7 mainly derived from personal care products and detergents.8 The possible consequences of PS-NPLs on freshwater organisms are progressively discovered such as the induction of oxidative stress,4,9 the effect on individual physiological activities10–12 and population abundance13,14 as well as freshwater community composition and biodiversity.14
Particle size, surface charge, surface functionalization, medium salinity, ionic strength, and natural organic matter (NOM)11,15–17 were found as the influencing factors of PS-NPL toxicity. For example, small-sized PS-NPLs were found to be more toxic than large-sized ones to freshwater fish Danio rerio.18,19 Their small size increases the potential for tissue translocation and systemic distribution in organisms.20 Positively charged PS-NPLs were demonstrated to be more toxic than negatively charged ones to freshwater zooplankton Daphnia magna, Thamnocephalus platyurus, and Brachionus calyciflorus.11,12 In our previous work, the surface charge and aggregation behavior were found to be the most influential toxicity factors of PS-NPLs for freshwater crustacean species D. magna and G. fossarum, respectively.21 However, the possible surface properties and heteroaggregation behavior of PS-NPLs under realistic conditions, their correlation with the overall toxicity and their functional mechanism on freshwater plankton remain an open question.
The interaction of PS-NPLs with freshwater microorganisms such as microalgae should deserve special attention22,23 because lower trophic level organisms are the entrance of anthropogenic pollutants to the freshwater food web and have a vital impact on the biogeochemical cycles in ecosystems.24,25 Unfortunately, only 1.34% of the 2540 exposure studies published until 2020 concerned the adverse effects of nano- and microplastics on microorganisms.23,26,27 Some scattered evidence suggests that microbial taxa with different nutritional modes in freshwater microbial communities can actively interact with NPLs. For example, PS-NPLs reduce the cell viability, biomass and chlorophyll a concentration of freshwater microalgae Scenedesmus obliquus,28Microcystis aeruginosa,29,30Chlorella vulgaris,31,32 and Chlamydomonas reinhardtii33 and altered the metabolic diversity and functional structure of freshwater biofilms.25,34 However, the effects of NPLs on microalgae are not unambiguous. Some studies have concluded that NPLs with sizes less than or equal to 100 nm and at concentrations of even up to 100 mg L−1 have no effects on microalgae.32,35,36 Moreover, these studies were only focused on freshwater microorganisms or NPLs per se and have little relevance to the informative links between them and realistic environment exposure. Therefore, how freshwater microorganisms interact with NPLs in freshwater environments is largely unknown.
The primary goal of the current study is to obtain new insights into the effects of size, concentration, and medium composition on the behavior of NPLs and how freshwater microorganisms exposed to these NPLs respond. More specifically, we examine the behavior of NPLs with 2 specific sizes in traditional culture medium and in natural water environment on freshwater microalgae. The physiological response, cellular burden measurements and the major biocompounds produced within cells are considered to address the following questions. (1) What are the differences in the behavior of PS-NPLs with 2 primary sizes in surface water and in culture medium? (2) Do these behaviors affect the PS-NPL cellular burden and biological response within cells? (3) What is/are the main factor(s) affecting the interaction between PS-NPLs and freshwater microalgae?
To address these questions, the behavior of 20 and 100 nm PS-NPLs in traditional culture medium (Waris-H) and in natural water (Lake Geneva water) was examined. The freshwater microalgae Poterioochromonas malhamensis was selected as a representative biological model. Surviving through a dual strategy of phototrophy and phagocytosis, these mixotrophic microalgae are widely distributed in freshwater and play an important ecological role in the microbial food web and freshwater nutrient cycling.37,38 PS-NPL concentrations used in this work were in the range of 0.1 to 10 mg L−1, which was based on previous studies on the NPL average concentration in freshwater (0.0003–0.563 mg L−1)39,40 and the non-observed effect concentrations on freshwater microorganisms (0.32–22 mg L−1).41,42 We postulate that these microalgae would have complex trilateral interactions with 2 sizes of PS-NPL in culture medium and in Lake Geneva water. Z-average hydrodynamic diameter and ζ-potential as two key parameters for the characterization of colloidal dispersion systems43 were used to discuss the surface properties and stability changes of PS-NPLs over time. The stabilization of PS-NPLs will have an influence on their cellular burden, and bioaccumulation is also fundamental in understanding the biological action of NPLs.44 Cellular burden measurement of PS-NPLs with different sizes and concentrations was performed by alkaline hydrolysis and spectroscopic analysis under the two medium conditions for 24 h. The biological responses of PS-NPLs, including cell growth inhibition, cell membrane damage and production of reactive oxygen species (ROS), on P. malhamensis were assessed. Moreover, the effects of PS-NPLs on the production of diverse biological molecules from cells were also evaluated by infrared spectroscopic analysis. The results highlight new insights into the concentration, size and medium effects that lead to differences in the behavior of PS-NPLs as well as the corresponding biological outcomes of freshwater planktonic microorganisms.
2. Materials and methods
2.1. Characterization of PS-NPLs in exposure media
Surfactant-free aqueous suspensions (4% w/v) of amidine-functionalized –C(–NH2) NH2+ polystyrene nanospheres with primary sizes of 20 and 100 nm were purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). The stock solution was diluted with ultrapure water to prepare 1 g L−1 PS-NPLs. The pH was adjusted to 3.0 using 0.05 M HCl to prevent aggregation and improve stability. The exposure media chemistry of Lake Geneva water (pH 8.0 ± 0.1, conductivity 288 ± 1 μS cm−1) and modified Waris-H culture medium (pH 7.0 ± 0.1, conductivity 675 ± 1 μS cm−1) are listed in the ESI† (Tables S1 and S2). The homogeneous working solution of PS-NPLs was diluted to 0.1, 1 and 10 mg L−1 in culture medium and Lake Geneva water after filtering at 0.2 μm (hydrophilic polycarbonate membrane, GTTP04700, Merck Millipore Ltd., Switzerland). The ζ-potential and z-average hydrodynamic diameter were measured at 25 °C using a Malvern Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) at 0, 0.5, 1, 2, 4, 8, and 24 h. Each sample was immediately analyzed 5 times in the automatic mode (10 runs).2.2. Cellular burden and bioassays of PS-NPLs with P. malhamensis
P. malhamensis (CCAC 3498 strain, Cologne Biocenter, Germany) were cultivated in a modified Waris-H culture medium at 25 °C and with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 light–dark cycle of 5 μmol photons m−2 s−1.45 Cells were collected by centrifugation at 4000 rpm for 5 min in the mid-exponential phase, washed twice with the corresponding mediums, and then resuspended in Lake Geneva water and Waris-H culture medium with a cell density of 5 × 105 cells per mL. For cellular burden and bioassay experiments, PS-NPLs were diluted in the two exposure mediums in culture flasks to a final concentration of 0 to 10 mg L−1. All experiments were conducted in triplicate and repeated three times.
12 light–dark cycle of 5 μmol photons m−2 s−1.45 Cells were collected by centrifugation at 4000 rpm for 5 min in the mid-exponential phase, washed twice with the corresponding mediums, and then resuspended in Lake Geneva water and Waris-H culture medium with a cell density of 5 × 105 cells per mL. For cellular burden and bioassay experiments, PS-NPLs were diluted in the two exposure mediums in culture flasks to a final concentration of 0 to 10 mg L−1. All experiments were conducted in triplicate and repeated three times.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C and then the supernatant was removed. The cell lysate mixture was obtained by vortexing at 300 rpm for 24 h with addition of 1 ml of 10 M NaOH. After filtration using 0.2 μm hydrophilic polycarbonate membranes (GTTP04700, Merck Millipore Ltd., Switzerland), the resulting solution was incubated in an 80 °C water bath for 30 min with 10 ml 100% EtOH. Following centrifugation at 2500 rpm for 10 min, the final bottom liquid was analyzed using a Varian Cary 50 Bio UV-vis spectrometer (McKinley Scientific). The concentrations of PS-NPLs were calculated from standard curves.
000 rpm for 10 min at 4 °C and then the supernatant was removed. The cell lysate mixture was obtained by vortexing at 300 rpm for 24 h with addition of 1 ml of 10 M NaOH. After filtration using 0.2 μm hydrophilic polycarbonate membranes (GTTP04700, Merck Millipore Ltd., Switzerland), the resulting solution was incubated in an 80 °C water bath for 30 min with 10 ml 100% EtOH. Following centrifugation at 2500 rpm for 10 min, the final bottom liquid was analyzed using a Varian Cary 50 Bio UV-vis spectrometer (McKinley Scientific). The concentrations of PS-NPLs were calculated from standard curves.
2.3. P. malhamensis biocomposition monitoring using infrared spectroscopy
Infrared spectra were collected to monitor the interactions between PS-NPLs and microorganisms in different exposure media in transmission mode using a FTIR microscope (Spotlight 200i, PerkinElmer, Beaconsfield, UK). For the spectra acquisition, 10 μL of each tested condition after 30 min of air-drying of samples were deposited on a CaF2 filter (U.Q.G. Milton. England). Infrared spectra acquisition was carried out in triplicate to monitor cell composition in the spectral range from 4000 to 500 cm−1 with an accumulation of 32 scans and resolution of 4 cm−1. The intensities of 4 target biocompounds in cells were then labelled according to their characteristic bands, which were lipids (1742 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), proteins (1650 cm−1, amide I), nucleic acids (1255 cm−1, P
O), proteins (1650 cm−1, amide I), nucleic acids (1255 cm−1, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and carbohydrates (1045 cm −1, C–O).49–51
O) and carbohydrates (1045 cm −1, C–O).49–51
2.4. Statistical analysis
The visualization of PS-NPL behavioral data and P. malhamensis response data was realized using Sheet and Analysis functions in GraphPad Prism 9.4.1 (GraphPad Software, LLC). The data normality was checked using two-way ANOVA with the Geisser–Greenhouse correction. The correlations and principal component analysis (PCA) among behavioral characteristics, environmental variables and associated cellular physiological effects were implemented using the FactoMineR and PerformanceAnalytics package in R version 4.0.3. All spectra datasets were normalized with the D1SG + SNV method (first derivative Savitsky–Golay + standard normal variate) in PeakFit v4.02 software.3. Results and discussion
3.1. Behavior of PS-NPLs in Lake Geneva water and culture medium
To understand the role of concentration, size, solution chemistry and environmental factors in PS-NPL biological effects, detailed evaluation of the stability of PS-NPLs in the different medium conditions was performed using ζ-potential (Fig. 1) and z-average hydrodynamic diameter variations (Fig. 2) from 0 to 24 h.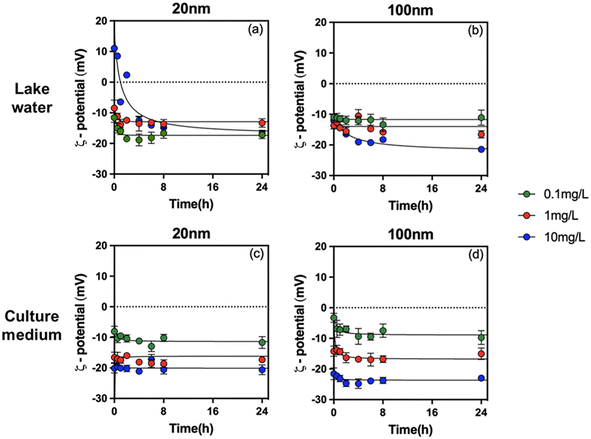 | ||
| Fig. 1 ζ-Potential of PS-NPLs of 20 nm (a and c) and 100 nm (b and d) at 0.1, 1, and 10 mg L−1 in Lake Geneva water and culture medium as a function of time. | ||
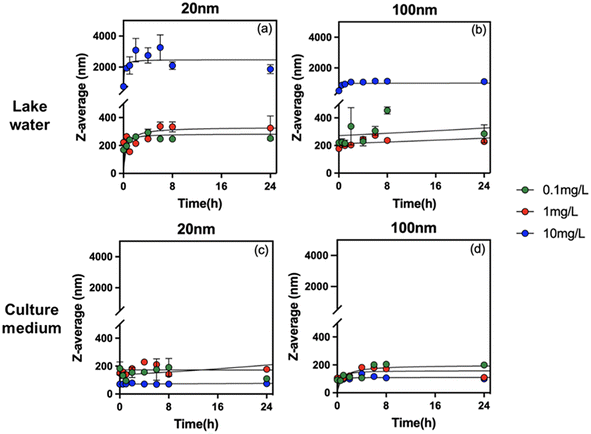 | ||
| Fig. 2 Z-average hydrodynamic diameter of PS-NPLs of 20 nm (a and c) and 100 nm (b and d) at 0.1, 1, and 10 mg L−1in Lake Geneva water and culture medium as a function of time. | ||
After PS-NPL dispersion in Lake Geneva water (pH 8.0 ± 0.1), their ζ-potential rapidly changed to negative values and had a decreasing trend with increasing concentrations (Fig. 1a and b). Some other studies have found similar phenomena.11,16,52 This can be explained by Lake Geneva water mainly containing not only salt ions with positive and negative charges, such as Ca2+, Mg2+, K+, Na+, HCO3−, SO42−, PO43−, and Cl−, but also NOM (0.96 mg L−1 DOC) with negative charges (Table S1†). Electrostatic interactions can occur between the positive charges on the PS-NPL surface and the negative charges on NOM as well as on salt ions.53 At three orders of magnitude test concentrations from 0.1 mg L−1 to 10 mg L−1, the amount of negative charges introduced by salt ions and NOM exceeded the positive charges carried by PS-NPLs, resulting in rapid charge neutralization and reversal.
The change in z-average hydrodynamic diameter confirmed that all tested PS-NPLs were unstable in Lake Geneva water (Fig. 2a and b). Charge neutralization and Brownian motion between particles contributed to the formation of large-diameter heterogeneous aggregates.52,54 The higher the concentration of PS-NPLs, the stronger the collision frequency between particles, and the more negatively charged particles (NOM and salt ions) can adsorb on PS-NPLs and neutralize the surface charge. Furthermore, this aggregation behavior was also affected by the primary size of PS-NPLs. At the same concentration, 20 nm PS-NPLs induced larger-diameter aggregates which were 1.5 times that of 100 nm PS-NPLs. These results are consistent with those reported by previous studies investigating the size and concentration effect on the aggregation behavior of PS-NPLs, which indicate that the smaller PS-NPLs may have a higher aggregation rate compared with larger ones55via electrostatic and steric effects from NOM corona56,57 as well as via interparticle collision due to Brownian motion.52,54,55
In addition to particle concentration and size, exposure medium chemistry is another essential factor which will affect the colloidal stability and the aggregation behavior of NPLs.15,16,52,58 Similar to that found in Lake Geneva water, the ζ-potential of all tested PS-NPLs in culture medium (pH 7.0 ± 0.1) also showed negative values and decreased with increasing concentration. This can be explained by the fact that the culture medium contains a large number of organic macromolecules such as proteins and amino acids. Electrostatic interactions rapidly promote the formation of the negatively charged surface of PS-NPLs in culture medium.59 Moreover, all the z-average hydrodynamic diameters of 2 sizes of PS-NPLs remain approximately constant over the 24-h test period. The stability increased with increasing concentration. This stabilization of NPLs could be due to the presence of organic macromolecules with negative charges in the culture medium, increasing the electrostatic repulsion and steric repulsion.16,60 The steric hindrance was generated by the layer of organic macromolecules adsorbed on the PS-NPLs, which further inhibited the aggregation of PS-NPLs and increased the dispersion among particles.55 It should be mentioned that the primary size effect of PS-NPLs was still present in the culture medium, as observed in Lake Geneva water. The z-average hydrodynamic diameters of 100 nm (199.3 ± 6.4 nm, 110.1 ± 4.3 nm, 99.9 ± 1.9 nm) PS-NPLs were found closer to their primary size compared with that of 20 nm (110.1 ± 5.2 nm, 169.2 ± 10.3 nm, 74.1 ± 0.6 nm) PS-NPLs. This indicates that small-sized NPLs per unit mass have a larger surface area, higher surface charges and more particle numbers, which make them more unstable or more prone to aggregation than the large-sized ones in the culture medium.
3.2. Cellular burden of PS-NPLs in P. malhamensis
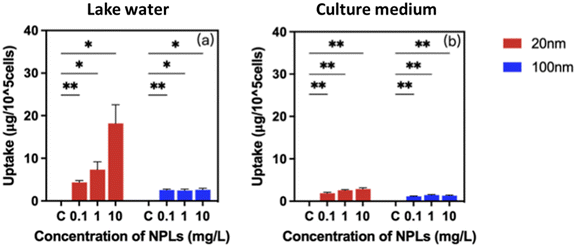
Studies on the bioavailability and cellular burden of nanoparticles are critically essential to link the transformation of nanoparticles to biological responses.44 It is known that the intrinsic properties of nanoparticles in combination with characteristics of the exposure medium govern their bioavailability and bioaccumulation.61 Our results show that P. malhamensis cells treated with 20 nm and 100 nm PS-NPLs at concentrations ranging from 0 to 10 mg L−1 exhibited an increase in PS-NPLs associated with/taken up by cells and showed a concentration-dependent manner (Fig. 3 and Table S3†). A significant difference in cellular burden was observed between 20 and 100 nm PS-NPLs and was exposure medium dependent.In Lake Geneva water, the 20 nm PS-NPLs associated with/taken up by cells were predominant compared to 100 nm PS-NPLs. PS-NPLs of 20 nm showed 1.7–11.5 times higher cellular burden potential than 100 nm PS-NPLs. PS-NPLs of 20 nm displayed a 4.1-fold higher change in the cellular PS-NPL content at 10 mg L−1 compared to 0.1 mg L−1 in Lake Geneva water, while after treatment with 100 nm PS-NPLs, the PS-NPL cellular burden was approximately 2.2 ± 0.5 μg per 105 cells, which was almost independent of the concentration (Fig. 3a).
In cell culture medium, a similar trend of PS-NPL cellular burden level was found, but the quantity was relatively low compared to that in the Lake Geneva water, especially for 20 nm PS-NPLs (Fig. 3b). The cellular content of 20 nm PS-NPLs was 1.9 ± 0.2 μg per 105 cells at 0.1 mg L−1 and increased to 2.9 ± 0.3 μg per 105 cells at 10 mg L−1. Regarding 100 nm PS-NPL treatment, the cellular PS-NPL content remained almost 1.3 ± 0.2 μg per 105 cells within three orders of magnitude concentrations. The cellular content of 20 nm PS-NPLs in culture medium was about 1.6–2.2 times higher than that of 100 nm PS-NPLs. Lake Geneva water enhanced the cellular burden of 20 and 100 nm PS-NPLs by 1.2–6.3-fold compared to culture medium. It is known that P. malhamensis can ingest plankton by phagocytosis,62 and their internalization ability of metallic nanoparticles was also evidenced.45 In the only report on internalization of NPLs in microalgae, it was observed that 75 nm PS-NPLs could be translocated into the cytoplasm and the chloroplast of E. gracilis via endocytosis but did not accumulate on the cell membrane or damage cell membranes and organelles.63 Our results clearly demonstrate that 20 nm and 100 nm PS-NPLs could be significantly associated with/taken up by P. malhamensis cells. Moreover, this interaction was concentration-, size- and exposure medium-dependent. These findings may illustrate how PS-NPLs act as a stressor to affect microalgae growth and physiological functions.
3.3. Biological responses of PS-NPLs on P. malhamensis in Lake Geneva water and culture medium
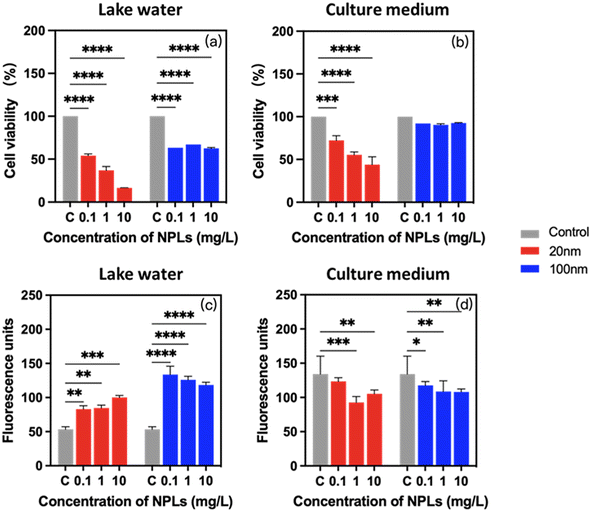
To determine whether the observed differences in PS-NPL stability behavior result in an adverse effect on the physiological responses of P. malhamensis, the cell viability, chlorophyll content, cellular ROS production and cell membrane damage were determined at 24-h incubation. As shown in Fig. 4a and b, 20 nm PS-NPLs induced a statistically significant decrease in cell viability with increasing concentration, while 100 nm PS-NPLs induced a similar cell growth inhibition rate without concentration effect. Moreover, the cell viability of PS-NPLs was found to be size- and medium-dependent (Table S3†). PS-NPLs of 20 nm showed a lower percentage of cell viability against P. malhamensis than 100 nm particles in both exposure media. For example, in Lake Geneva water, the percentage of cell viability of 20 nm PS-NPLs at 0.1, 1 and 10 mg L−1 was 54.0 ± 2.1%, 36.9 ± 4.6%, and 16.6 ± 0.2%, respectively. PS-NPLs of 100 nm caused only 35.7 ± 2.4% decrease in cell viability (Fig. 4a). PS-NPLs of 20 nm exhibited about 1.2–2.2 times cell mortality than 100 nm PS-NPLs. In culture medium, a similar size–toxicity trend was observed. The percentage of cell viability of 20 nm PS-NPLs decreased with increasing concentrations, but was less toxic than that in Lake Geneva water. For example, at the highest exposure concentration of 10 mg L−1, the cell viability was 16.6 ± 0.2% in Lake Geneva water, whereas it was 44.0 ± 9.2% in culture medium. The cell viability of 100 nm PS-NPLs was about 3.5–7.5 times higher than that of 20 nm PS-NPLs in culture medium.These results are consistent with those reported by several authors, which indicate that the acute toxicity of NPLs to individual organisms may be significantly affected by particle size. Smaller-sized NPLs may have more prominent toxic effects than larger-sized NPLs.12,58 Besides the primary size effect, PS-NPLs in Lake Geneva water exhibited 1.4–5.0 times higher toxicity than that in culture medium. This may be due to the fact that the positive charges on the PS-NPL surface could interact with the negative charges on P. malhamensis cells and the NOM in Lake Geneva water to achieve electrical neutralization, which inhibits the mobility of microalgae.63 Moreover, the formation of a protein corona could obstruct the cellular damage caused by NPLs due to the presence of rich biomolecules in the culture medium.64 Smaller-sized NPLs have a greater surface-to-volume ratio and higher surface reactivity than larger-sized NPLs, making them more sensitive to environmental variables.4,58 In our study, 20 nm PS-NPLs at the lowest exposure concentration of 0.1 mg L−1 has caused 45.9 ± 2.1% cell mortality in Lake Geneva water, suggesting that PS-NPLs are not “no effect” or “reversible response” to microorganisms17,36,65 and also indicate that the exposure condition is an important factor for the toxic effects.
Exposure to PS-NPLs resulted in a significant and medium-dependent change in chlorophyll content in P. malhamensis (Fig. 4c and d). Compared with unexposed controls, in Lake Geneva water, the percentage of enhanced chlorophyll synthesis in cells increased to 1.7 ± 0.2-fold with 20 nm PS-NPL treatment and 2.4 ± 0.2-fold with 100 nm treatment, which was evidently size-dependent. In contrast, in culture medium, PS-NPL treatment caused about 0.2 ± 0.1-fold inhibition of chlorophyll a synthesis, which was not obviously size-dependent (Table S3†). Photosynthesis only provides a “marginal contribution” to P. malhamensis with heterotrophic dominance, mainly acting as an adaptive regulator protecting the cells from environmental stress.66,67 Our results with the changes in chlorophyll a synthesis are analogous to a few studies of P. malhamensis and some phytoflagellates in which it was demonstrated that chloroplast development was repressed under conditions supporting heterotrophic growth, and conversely, environmental stress such as carbon source depletion was accompanied by an enhanced chlorophyll a production.66–69 This implies that the photosynthesis regulation of P. malhamensis at 24 h was significantly affected by PS-NPLs, which is size-dependent in realistic environments such as Geneva Lake water. The different synthesis tendency of chlorophyll indicates that the interactions of PS-NPLs with the two environmental media created different environmental stress conditions for P. malhamensis. Subsequently, FTIR monitoring results also confirmed that the interaction between PS-NPLs and certain biocomponents was medium-dependent (Section 3.4).
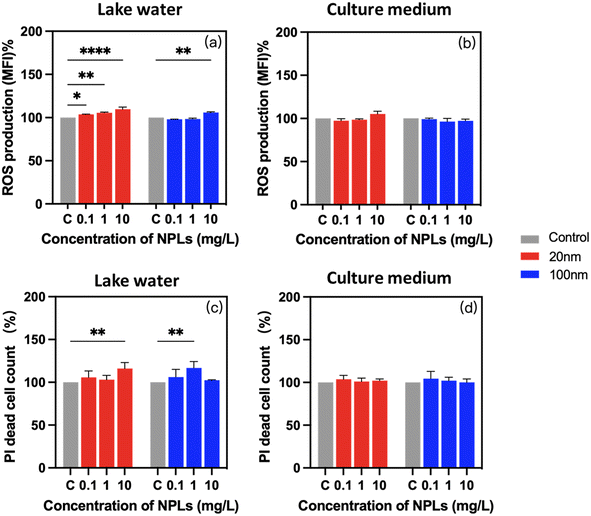
Reactive oxygen species are commonly recognized as detrimental by-products of xenobiotic exposure, which generate the oxidative stress in cells. After 24-h exposure to PS-NPLs, ROS production was significantly increased compared to unexposed controls (Fig. 5a and b). Moreover, these enhanced ROS generations were concentration-, size-, and medium-dependent (Table S1†).
In Lake Geneva water, ROS production was increased from 3.7 ± 0.5% at 0.1 mg L−1 to 9.8 ± 2.4% at 10 mg L−1 in 20 nm PS-NPLs treatment cells, while with 100 nm PS-NPL treatment, the ROS production was comparable with those of the untreated controls at 0.1 mg L−1 and increased to 6.0 ± 0.7% at 10 mg L−1 (Fig. 5a). In culture medium, a similar trend of ROS production increase was found, which was also concentration- and size-dependent (Fig. 5b). ROS generation growth rates were unchanged at 0.1 mg L−1 and increased to 5.3 ± 3.2% at 10 mg L−1 with 20 nm PS-NPL treatment. For 100 nm PS-NPL treatment, the ROS production exhibited unchanged values compared to those of the unexposed controls whatever the concentration. The above findings are consistent with the existing literature showing that significant excessive ROS were observed in C. reinhardtii, E. gracilis and C. vulgaris exposed to PS-NPLs with high concentrations ranging from 25 mg L−1 to ∼500 mg L−1.32,33,70 Interestingly, these previous observations showed adverse effects on microalgae when the NPL concentration was above 5 mg L−1,27,69 but in our study in a realistic freshwater environment (Lake Geneva water), smaller-sized PS-NPLs (20 nm) induced a significant ROS production at fairly low concentration (0.1 mg L−1). Moreover, 20 nm PS-NPLs induced more prominent enhancement of ROS production than larger-sized PS-NPLs (100 nm), and PS-NPLs exhibited 1.9–6.0 times higher ROS-inducing effect in Lake Geneva water than that in culture medium. The results are in accordance with the decrease of cell viability observed (Fig. 4a and b), which indicated that complex organic molecules in the culture medium may adsorb on the surface of PS-NPLs and form the biocorona, and this interaction can reduce the oxidative stress of cells.12,71
PI fluorophores could intercalate in cells and in response to a cellular membrane integrity level.72 Exposure to PS-NPLs led to an obvious and medium-dependent increase of cells with damaged membrane (Fig. 5c and d). The percentage of cells with enhanced PI fluorophore intercalation increased by 8.3 ± 6.4% in Lake Geneva water and 2.2 ± 1.6% in culture medium compared to unexposed controls. Similar effects of PS-NPLs on the membrane integrity of aquatic macroalgae suggest that the interaction of PS-NPLs with the cell wall surface leads to cellular morphological distortion and membrane integrity disruption.33,72–74 Our study illustrated that the percentage of cells with membrane damage increased by 4.9 ± 1.1% even at 0.1 mg L−1 PS-NPL exposure, which was not dependent on size or concentration (Table S3†). Moreover, PS-NPLs caused 1.4 to 8.3 times higher cell membrane damage level in Lake Geneva water than that in culture medium.
In addition, it is important to mention that in our observations, the slight excess of ROS production and low presence of membrane-damaged cells contrasted sharply with high cell inhibition and strong chlorophyll a change. This suggests that the exposure to 20 and 100 nm PS-NPLs for 24 h did not directly lead to apoptosis or necrosis but affected photosynthetic regulation, motility, and growth of P. malhamensis, which is similar to the potential mechanism of PS-NPLs observed in the microalgae E. gracilis.63
The above results are consistent with microscopic observations showing the differences of PS-NPL effects on P. malhamensis in culture medium and in Lake Geneva water (Fig. S1†). After 24-h exposure in Lake Geneva water, a large number of heteroaggregated forms with cells were observed but not in culture medium. Owing to the presence of NOM in Lake Geneva water, cellular photosynthetic regulation and increased immobility appear to be probably co-driven by eco-corona formation11,75,76 and heteroaggregation of cells with NPLs.77 When PS-NPLs were added, they interacted with the negative charges on the surface of P. malhamensis and NOM to generate heteroaggregates, which could not only make P. malhamensis a part of these heteroaggregates but also allow them to accumulate and adhere to the surfaces of these heteroaggregates (Fig. S1†).
3.4. PS-NPL effects on P. malhamensis biocomposition
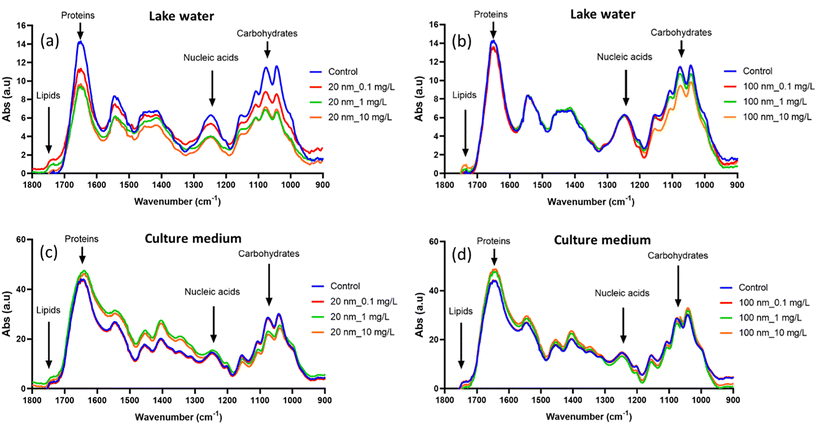
FTIR has recently been described as a useful tool for monitoring the biochemical composition of microalgae under different stress conditions. It was demonstrated that PS-NPL–cell interaction results in intensity changes or spectral shifts of the peaks corresponding to major biochemical groups including proteins, lipids, nucleic acids or carbohydrates on microalgae C. vulgaris32 and C. reinhardtii.51,78 In these studies, the biocomposition of P. malhamensis was monitored by FTIR after 24 h of interaction with 20 nm and 100 nm PS-NPLs in Lake Geneva water and culture medium. Fig. 6a–d present the results. The spectral changes in PS-NPL-exposed microalgae compared to unexposed controls were significant and remained concentration-, size-, and medium-dependent.In Lake Geneva water, the spectral changes of 20 nm PS-NPL-exposed microalgae compared to unexposed controls showed a decrease in peak intensity at 1650 cm–1, 1045 cm−1, and 1255 cm−1 which is a clear indication of the decrease in protein, carbohydrate, and nucleic acid content. The increased peak intensity at 1742 cm−1 is related to an increase in lipid content. Lipids are organic compounds which yield high energy and are usually associated with oxidative imbalance and stress defense mechanisms. The increase in lipids observed in this study is linked to their contribution to stress responses caused by PS-NPLs by providing the cells with the energy needed to counter the unfavorable growth conditions and improve their defense.79 For 100 nm PS-NPL-exposed microalgae, the spectral changes occurred mainly in the carbohydrate-related peaks (1742 cm−1), and the decreases in peak intensity were more pronounced at 10 mg L−1. Our results evidenced that carbohydrate and protein accumulation within the microalgae was suppressed under both 20 and 100 nm PS-NPL stress. Specifically, the spectra of 20 nm-treated microalgae showed a greater drop in peak intensity changes than those of 100 nm-exposed ones. Carbohydrates are also involved in the defense mechanism of algae,80 and protein synthesis is highly influenced under stressful conditions.81 This biochemical composition results are in accordance with the findings obtained from different cellular stress response studies via ROS production, membrane damage and photosynthetic ability for two sizes PS-NPL stress in Lake Geneva water (Fig. 4 and 5).
In culture medium, the spectra of PS-NPL-exposed microalgae exhibited different variation patterns. Specifically, compared to the control, 20 nm PS-NPL-treated microalgae showed mainly an increase in protein-related peak intensities together with peak shifts, but a decrease in carbohydrate-related peak intensities, while the spectral changes of 100 nm PS-NPL-exposed microalgae were more moderate, showing only a slight increase in protein-related peak intensities. Compared to a marginal influence observed under culture medium exposure conditions, the enhanced spectral changes in Lake Geneva water suggest that Lake Geneva water could intensify PS-NPL–cell interaction. In addition, it should be noted that the mode of PS-NPLs–protein interaction in culture medium (increase and shift) was different from that in Lake Geneva water (decrease) as discussed above. This may indicate that PS-NPL–protein interactions are medium-dependent not only in intensity but also in certain bond interaction modes32 on which it was necessary to carry out a further study.
3.5. Correlation between PS-NPL surface properties, exposure medium and biophysiological responses
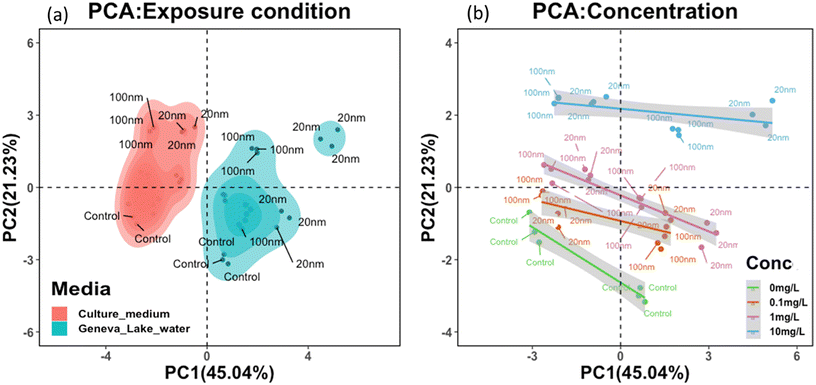
To further examine the trilateral correlation between PS-NPL surface properties, exposure medium conditions and biophysiological response of P. malhamensis, 12 key parameters were selected. The 6 parameters corresponding to PS-NPL behavioral characteristics were ζ-potential, z-average hydrodynamic diameter, conductivity, polydispersity index (PDI), pH, and concentration. The other 6 parameters corresponding to biophysiological responses were ROS, cellular burden, cell count, inhibition, membrane damage and chlorophyll a content in P. malhamensis after 24 h exposure. Considering the 12 key parameters constituting the variables for principal component analysis, the test PS-NPL–cell interactions in Lake Geneva water and in culture medium could be summarized as two different systems (Fig. 7a), which meant that the test PS-NPL–cell interactions in the same medium condition exhibited common characteristics that could be normalized, and these characteristics could be differentiated from those in another medium condition. Fig. 7b clearly shows the separation of the groups corresponding to the different concentrations from one to the others, which implied that the tested PS-NPLs in the same concentration exhibited similar behaviors and led to similar biological responses.Moreover, the correlation analysis between the surface properties and biological responses of the tested PS-NPLs (Fig. S2†) showed that the 24-h biological responses to P. malhamensis and z-average hydrodynamic diameter trend toward statistically significant correlation. This indicates that after PS-NPLs are subjected to the medium conditions, they rapidly interact with other components to form a complex colloidal system composed of heteroaggregates. In this system, the higher the hydrodynamic diameter of the heteroaggregates, the more significant the biological responses are on P. malhamensis. In our study, the biological responses of NPLs remained strongly size-dependent, which are precisely determined by NPL aggregation behavior.
4. Conclusion
Even though the environmental impact of nanoparticulate contaminants is widely studied in terms of intrinsic properties of nanoparticles and environmental variables, few informative links are available describing the interactions between nanoparticles, environments, and organisms. In order to fill this information gap, our multifactorial-driven experimental set-up used two sizes of PS-NPLs, with two exposure media, on typical freshwater microalgae. Their complex trilateral interactions are characterized by 12 key parameters including 6 parameters corresponding to NPL behavior (ζ-potential, z-average hydrodynamic diameter, conductivity, polydispersity index (PDI), pH and concentration) and 6 parameters corresponding to biological responses (cell inhibition/growth, chlorophyll content, ROS production, cell membrane damage, cellular burden). The biochemical composition changes of microalgae in response to NPLs have also been examined. Our results evidenced that the cellular burden of PS-NPLs in P. malhamensis is concentration- and size-dependent.A significant biological response of P. malhamensis was observed from the lowest PS-NPL tested concentration of 0.1 mg L−1 including growth inhibition and stimulation of photosynthetic regulation as well as cellular burden of PS-NPLs, with the modification of stored protein, carbohydrate, nucleic acid and lipid content. Moreover, these biological responses are concentration-, size-, and medium-dependent. Smaller-sized (20 nm) PS-NPLs are found to trigger more prominent responses of P. malhamensis than larger-sized (100 nm) PS-NPLs and exhibit 2.6–20.5 times stronger effect in Lake Geneva water than in culture medium. More importantly, these biological responses are driven by both PS-NPL surface properties and environmental factors. The aggregation behavior is found to be the most influential explanatory factor of PS-NPL effects on P. malhamensis in both culture medium and environmental natural waters. This study helps expand the current knowledge of the ecological impact and fate of NPLs on associated organisms along the freshwater microbial food webs and contributes to better understanding of the interactions between nanoplastic particles, environmental medium, and low-trophic plankton. The findings of the present study reveal a clear mechanistic link between the characterization of the colloidal stability of particle pollutants and biological response assessment in given exposure systems on microalgae. These results provide more comprehensive information for the assessment of the fate and toxicity of NPLs according to their characteristics, stability, and interaction in freshwater systems, which will promote further environmental risk assessment of NPLs.
Author contributions
Zijiao Meng: methodology, investigation, writing – original draft. Serge Stoll: methodology, investigation, supervision. Wei Liu: conceptualization, methodology, investigation, formal analysis, writing – original draft, supervision, validation, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Wei Liu acknowledges the financial support from Foundation Ernst & Lucie Schmidheiny. We acknowledge Angel Negrete Velasco for the FTIR analysis.References
- G. Esposito, M. Prearo, M. Renzi, S. Anselmi, A. Cesarani, D. Barcelò, A. Dondo and P. Pastorino, Occurrence of microplastics in the gastrointestinal tract of benthic by–catches from an eastern Mediterranean deep–sea environment, Mar. Pollut. Bull., 2022, 174, 113231 CrossRef CAS PubMed.
- M. Shen, Y. Zhang, Y. Zhu, B. Song, G. Zeng, D. Hu, X. Wen and X. Ren, Recent advances in toxicological research of nanoplastics in the environment: A review, Environ. Pollut., 2019, 252, 511–521 CrossRef CAS PubMed.
- K. Mattsson, S. Jocic, I. Doverbratt and L.-A. Hansson, Nanoplastics in the aquatic environment, Microplastic contamination in aquatic environments, 2018, pp. 379–399 Search PubMed.
- M. Bundschuh, J. Filser, S. Lüderwald, M. S. McKee, G. Metreveli, G. E. Schaumann, R. Schulz and S. Wagner, Nanoparticles in the environment: where do we come from, where do we go to?, Environ. Sci. Eur., 2018, 30, 6–6 CrossRef PubMed.
- M. Arienzo and L. Ferrara, Environmental Fate of Metal Nanoparticles in Estuarine Environments, Water, 2022, 14, 1297 CrossRef CAS.
- A. A. Koelmans, N. H. Mohamed Nor, E. Hermsen, M. Kooi, S. M. Mintenig and J. De France, Microplastics in freshwaters and drinking water: Critical review and assessment of data quality, Water Res., 2019, 155, 410–422 CrossRef CAS PubMed.
- R. Coyle, G. Hardiman and K. O. Driscoll, Microplastics in the marine environment: A review of their sources, distribution processes, uptake and exchange in ecosystems, Case Stud. Chem. Environ. Eng., 2020, 2, 100010 CrossRef.
- D. Elkhatib and V. Oyanedel-Craver, A Critical Review of Extraction and Identification Methods of Microplastics in Wastewater and Drinking Water, Environ. Sci. Technol., 2020, 54, 7037–7049 CrossRef CAS PubMed.
- Q. Chen, D. Yin, Y. Jia, S. Schiwy, J. Legradi, S. Yang and H. Hollert, Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish, Sci. Total Environ., 2017, 609, 1312–1321 CrossRef CAS PubMed.
- S. Rist, A. Baun and N. B. Hartmann, Ingestion of micro- and nanoplastics in Daphnia magna - Quantification of body burdens and assessment of feeding rates and reproduction, Environ. Pollut., 2017, 228, 398–407 CrossRef CAS PubMed.
- J. Saavedra, S. Stoll and V. I. Slaveykova, Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton, Environ. Pollut., 2019, 252, 715–722 CrossRef CAS PubMed.
- E. Kelpsiene, O. Torstensson, M. T. Ekvall, L.-A. Hansson and T. Cedervall, Long-term exposure to nanoplastics reduces life-time in Daphnia magna, Sci. Rep., 2020, 10, 5979–5979 CrossRef CAS PubMed.
- Y. Li, W. Li, P. Jarvis, W. Zhou, J. Zhang, J. Chen, Q. Tan and Y. Tian, Occurrence, removal and potential threats associated with microplastics in drinking water sources, J. Environ. Chem. Eng., 2020, 8, 104527 CrossRef CAS.
- P. Redondo-Hasselerharm, G. Gort, E. Peeters and A. Koelmans, Nano-and microplastics affect the composition of freshwater benthic communities in the long term, Sci. Adv., 2020, 6, eaay4054 CrossRef CAS PubMed.
- O. Oriekhova and S. Stoll, Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter, Environ. Sci.: Nano, 2018, 5, 792–799 RSC.
- M. Shams, I. Alam and I. Chowdhury, Aggregation and stability of nanoscale plastics in aquatic environment, Water Res., 2020, 171, 115401 CrossRef CAS PubMed.
- M. Heinlaan, K. Kasemets, V. Aruoja, I. Blinova, O. Bondarenko, A. Lukjanova, A. Khosrovyan, I. Kurvet, M. Pullerits, M. Sihtmäe, G. Vasiliev, H. Vija and A. Kahru, Hazard evaluation of polystyrene nanoplastic with nine bioassays did not show particle-specific acute toxicity, Sci. Total Environ., 2020, 707, 136073 CrossRef CAS PubMed.
- Y. Jin, J. Xia, Z. Pan, J. Yang, W. Wang and Z. Fu, Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish, Environ. Pollut., 2018, 235, 322–329 CrossRef CAS PubMed.
- M. Enfrin, J. Lee, Y. Gibert, F. Basheer, L. Kong and L. F. Dumée, Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces, J. Hazard. Mater., 2020, 384, 121393 CrossRef CAS PubMed.
- R. Trevisan, P. Ranasinghe, N. Jayasundara and R. T. Di Giulio, Nanoplastics in Aquatic Environments: Impacts on Aquatic Species and Interactions with Environmental Factors and Pollutants, Toxics, 2022, 10, 326 CrossRef CAS PubMed.
- Z. Meng, R. Recoura-Massaquant, A. Chaumot, S. Stoll and W. Liu, Acute toxicity of nanoplastics on Daphnia and Gammarus neonates: Effects of surface charge, heteroaggregation, and water properties, Sci. Total Environ., 2023, 854, 158763 CrossRef CAS PubMed.
- S. Reichelt and E. Gorokhova, Micro- and Nanoplastic Exposure Effects in Microalgae: A Meta-Analysis of Standard Growth Inhibition Tests, Front. Environ. Sci., 2020, 8, 131 CrossRef.
- A. Kukkola, S. Krause, I. Lynch, G. H. Sambrook Smith and H. Nel, Nano and microplastic interactions with freshwater biota – Current knowledge, challenges and future solutions, Environ. Int., 2021, 152, 106504 CrossRef CAS PubMed.
- K. L. Rogers, J. A. Carreres-Calabuig, E. Gorokhova and N. R. Posth, Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics, Limnol. Oceanogr. Lett., 2020, 5, 18–36 CrossRef CAS.
- L. Miao, S. Guo, J. Wu, T. M. Adyel, Z. Liu, S. Liu and J. Hou, Polystyrene nanoplastics change the functional traits of biofilm communities in freshwater environment revealed by GeoChip 5.0, J. Hazard. Mater., 2022, 423, 127117 CrossRef CAS PubMed.
- A. L. Santos, C. C. Rodrigues, M. Oliveira and T. L. Rocha, Microbiome: A forgotten target of environmental micro(nano)plastics?, Sci. Total Environ., 2022, 822, 153628 CrossRef CAS PubMed.
- R. Qiao, M. Mortimer, J. Richter, B. Rani-Borges, Z. Yu, M. Heinlaan, S. Lin and A. Ivask, Hazard of polystyrene micro-and nanospheres to selected aquatic and terrestrial organisms, Sci. Total Environ., 2022, 853, 158560 CrossRef CAS PubMed.
- E. Besseling, B. Wang, M. Lürling and A. A. Koelmans, Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna, Environ. Sci. Technol., 2014, 48, 12336–12343 CrossRef CAS PubMed.
- L.-J. Feng, X.-D. Sun, F.-P. Zhu, Y. Feng, J.-L. Duan, F. Xiao, X.-Y. Li, Y. Shi, Q. Wang, J.-W. Sun, X.-Y. Liu, J.-Q. Liu, L.-L. Zhou, S.-G. Wang, Z. Ding, H. Tian, T. S. Galloway and X.-Z. Yuan, Nanoplastics Promote Microcystin Synthesis and Release from Cyanobacterial Microcystis aeruginosa, Environ. Sci. Technol., 2020, 54, 3386–3394 CrossRef CAS PubMed.
- Q. Zhang, Q. Qu, T. Lu, M. Ke, Y. Zhu, M. Zhang, Z. Zhang, B. Du, X. Pan, L. Sun and H. Qian, The combined toxicity effect of nanoplastics and glyphosate on Microcystis aeruginosa growth, Environ. Pollut., 2018, 243, 1106–1112 CrossRef CAS PubMed.
- M. Khoshnamvand, P. Hanachi, S. Ashtiani and T. R. Walker, Toxic effects of polystyrene nanoplastics on microalgae Chlorella vulgaris: Changes in biomass, photosynthetic pigments and morphology, Chemosphere, 2021, 280, 130725 CrossRef CAS PubMed.
- L. J. Hazeem, G. Yesilay, M. Bououdina, S. Perna, D. Cetin, Z. Suludere, A. Barras and R. Boukherroub, Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes, Mar. Pollut. Bull., 2020, 156, 111278 CrossRef CAS PubMed.
- Z. Yan, L. Xu, W. Zhang, G. Yang, Z. Zhao, Y. Wang and X. Li, Comparative toxic effects of microplastics and nanoplastics on Chlamydomonas reinhardtii: Growth inhibition, oxidative stress, and cell morphology, J. Water Process. Eng., 2021, 43, 102291 CrossRef.
- L. Miao, S. Guo, Z. Liu, S. Liu, G. You, H. Qu and J. Hou, Effects of Nanoplastics on Freshwater Biofilm Microbial Metabolic Functions as Determined by BIOLOG ECO Microplates, Int. J. Environ. Res. Public Health, 2019, 16, 4639 CrossRef CAS PubMed.
- D. Wu, T. Wang, J. Wang, L. Jiang, Y. Yin and H. Guo, Size-dependent toxic effects of polystyrene microplastic exposure on Microcystis aeruginosa growth and microcystin production, Sci. Total Environ., 2021, 761, 143265 CrossRef CAS PubMed.
- Z. Zhang, M. Zheng, B. Chen, Y. Pan, Z. Yang and H. Qian, Nano-Sized Polystyrene at 1 mg/L Concentrations Does Not Show Strong Disturbance on the Freshwater Microbial Community, Bull. Environ. Contam. Toxicol., 2021, 107, 610–615 CrossRef CAS PubMed.
- D. K. Stoecker, Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications, Protistologica, 1998, 34, 281–290 Search PubMed.
- M. Ivankovic, R. Ptacnik and M. M. Bengtsson, Top-down structuring of freshwater bacterial communities by mixotrophic and heterotrophic protists, bioRxiv, 2022, preprint, DOI:10.1101/2022.08.12.503741.
- Y. Xu, Q. Ou, M. Jiao, G. Liu and J. P. Van Der Hoek, Identification and quantification of nanoplastics in surface water and groundwater by pyrolysis gas chromatography–mass spectrometry, Environ. Sci. Technol., 2022, 56, 4988–4997 CrossRef CAS PubMed.
- D. Materić, M. Peacock, J. Dean, M. Futter, T. Maximov, F. Moldan, T. Röckmann and R. Holzinger, Presence of nanoplastics in rural and remote surface waters, Environ. Res. Lett., 2022, 17, 054036 CrossRef.
- T. Yang and B. Nowack, A meta-analysis of ecotoxicological hazard data for nanoplastics in marine and freshwater systems, Environ. Toxicol. Chem., 2020, 39, 2588–2598 CrossRef CAS PubMed.
- B. Zhang, J. Chao, L. Chen, L. Liu, X. Yang and Q. Wang, Research progress of nanoplastics in freshwater, Sci. Total Environ., 2021, 757, 143791 CrossRef CAS PubMed.
- S. Mourdikoudis, R. M. Pallares and N. T. K. Thanh, Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties, Nanoscale, 2018, 10, 12871–12934 RSC.
- T. Wang and W. Liu, Emerging investigator series: metal nanoparticles in freshwater: transformation, bioavailability and effects on invertebrates, Environ. Sci.: Nano, 2022, 9, 2237–2263 RSC.
- W. Liu, S. Majumdar, W. Li, A. A. Keller and V. I. Slaveykova, Metabolomics for early detection of stress in freshwater alga Poterioochromonas malhamensis exposed to silver nanoparticles, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed.
- H. C. Birnboim and J. Doly, A rapid alkaline extraction procedure for screening recombinant plasmid DNA, Nucleic Acids Res., 1979, 7, 1513–1523 CrossRef CAS PubMed.
- N. Wasmund, I. Topp and D. Schories, Optimising the storage and extraction of chlorophyll samples, Oceanologia, 2006, 48, 125–144 Search PubMed.
- Z. Lu, C. Zhang, C. Han, Q. An, Y. Cheng, Y. Chen, R. Meng, Y. Zhang and J. Su, Plasticizer Bis(2-ethylhexyl) Phthalate Causes Meiosis Defects and Decreases Fertilization Ability of Mouse Oocytes in Vivo, J. Agric. Food Chem., 2019, 67, 3459–3468 CrossRef CAS PubMed.
- A. P. Dean, J. M. Nicholson and D. C. Sigee, Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: an FTIR microspectroscopy study, Eur. J. Phycol., 2008, 43, 345–354 CrossRef CAS.
- Y. Meng, C. Yao, S. Xue and H. Yang, Application of Fourier transform infrared (FT-IR) spectroscopy in determination of microalgal compositions, Bioresour. Technol., 2014, 151, 347–354 CrossRef CAS PubMed.
- M. Déniel, F. Lagarde, A. Caruso and N. Errien, Infrared spectroscopy as a tool to monitor interactions between nanoplastics and microalgae, Anal. Bioanal. Chem., 2020, 412, 4413–4422 CrossRef PubMed.
- C.-H. Lee and J. K.-H. Fang, Effects of temperature and particle concentration on aggregation of nanoplastics in freshwater and seawater, Sci. Total Environ., 2022, 817, 152562 CrossRef CAS PubMed.
- J. Wu, R. Jiang, W. Lin and G. Ouyang, Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges, Environ. Pollut., 2019, 245, 836–843 CrossRef CAS PubMed.
- H. Sun, R. Jiao and D. Wang, The difference of aggregation mechanism between microplastics and nanoplastics: Role of Brownian motion and structural layer force, Environ. Pollut., 2021, 268, 115942 CrossRef CAS PubMed.
- X. Li, E. He, B. Xia, Y. Liu, P. Zhang, X. Cao, L. Zhao, X. Xu and H. Qiu, Protein corona-induced aggregation of differently sized nanoplastics: impacts of protein type and concentration, Environ. Sci.: Nano, 2021, 8, 1560–1570 RSC.
- J. Liu, S. Legros, F. von der Kammer and T. Hofmann, Natural Organic Matter Concentration and Hydrochemistry Influence Aggregation Kinetics of Functionalized Engineered Nanoparticles, Environ. Sci. Technol., 2013, 47, 4113–4120 CrossRef CAS PubMed.
- X. Li, E. He, K. Jiang, W. J. G. M. Peijnenburg and H. Qiu, The crucial role of a protein corona in determining the aggregation kinetics and colloidal stability of polystyrene nanoplastics, Water Res., 2021, 190, 116742 CrossRef CAS PubMed.
- A. Pochelon, S. Stoll and V. I. Slaveykova, Polystyrene Nanoplastic Behavior and Toxicity on Crustacean Daphnia magna: Media Composition, Size, and Surface Charge Effects, Environments, 2021, 8, 101 CrossRef.
- Y. Chen, W. Liu, X. Leng and S. Stoll, Toxicity of selenium nanoparticles on Poterioochromonas malhamensis algae in Waris-H culture medium and Lake Geneva water: Effect of nanoparticle coating, dissolution, and aggregation, Sci. Total Environ., 2022, 808, 152010 CrossRef CAS PubMed.
- A. Pradel, S. Ferreres, C. Veclin, H. El Hadri, M. Gautier, B. Grassl and J. Gigault, Stabilization of Fragmental Polystyrene Nanoplastic by Natural Organic Matter: Insight into Mechanisms, ACS ES&T Water, 2021, 1, 1198–1208 Search PubMed.
- K. L. Garner, Y. Qin, S. Cucurachi, S. Suh and A. A. Keller, Linking exposure and kinetic bioaccumulation models for metallic engineered nanomaterials in freshwater ecosystems, ACS Sustainable Chem. Eng., 2018, 6, 12684–12694 CrossRef CAS.
- M. Ma, Y. Gong and Q. Hu, Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture, Algal Res., 2018, 29, 142–153 CrossRef.
- L. Sun, S. Sun, M. Bai, Z. Wang, Y. Zhao, Q. Huang, C. Hu and X. Li, Internalization of polystyrene microplastics in Euglena gracilis and its effects on the protozoan photosynthesis and motility, Aquat. Toxicol., 2021, 236, 105840 CrossRef CAS PubMed.
- S. Kihara, A. Ashenden, M. Kaur, J. Glasson, S. Ghosh, N. van der Heijden, A. E. S. Brooks, J. P. Mata, S. Holt, L. J. Domigan, I. Köper and D. J. McGillivray, Cellular interactions with polystyrene nanoplastics—The role of particle size and protein corona, Biointerphases, 2021, 16, 41001 CrossRef CAS PubMed.
- Q. Kong, Y. Li, X. Xu, J. Cheng, Y. Sun, L. Zhang, Y. Huang and Z. Yang, Microplastics interfere with mixotrophic Ochromonas eliminating toxic Microcystis, Chemosphere, 2021, 265, 129030 CrossRef CAS PubMed.
- A. J. Lewitus and D. A. Caron, Physiological responses of phytoflagellates to dissolved organic substrate additions. 1. Dominant role of heterotrophic nutrition in Poterioochromonas malhamensis (Chrysophyceae), Plant Cell Physiol., 1991, 32, 671–680 CrossRef CAS.
- D. A. Holen, Effects of prey abundance and light intensity on the mixotrophic chrysophyte Poterioochromonas malhamensis from a mesotrophic lake, Freshwater Biol., 1999, 42, 445–455 CrossRef.
- Y. Xiao, X. Jiang, Y. Liao, W. Zhao, P. Zhao and M. Li, Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae, Chemosphere, 2020, 255, 126914 CrossRef CAS PubMed.
- X. Li, Z. Wang, M. Bai, Z. Chen, G. Gu, X. Li, C. Hu and X. Zhang, Effects of polystyrene microplastics on copper toxicity to the protozoan Euglena gracilis: emphasis on different evaluation methods, photosynthesis, and metal accumulation, Environ. Sci. Pollut. Res., 2022, 29, 23461–23473 CrossRef CAS PubMed.
- Y. Liao, X. Jiang, Y. Xiao and M. Li, Exposure of microalgae Euglena gracilis to polystyrene microbeads and cadmium: Perspective from the physiological and transcriptional responses, Aquat. Toxicol., 2020, 228, 105650 CrossRef CAS PubMed.
- Y. Tan, X. Zhu, D. Wu, E. Song and Y. Song, Compromised autophagic effect of polystyrene nanoplastics mediated by protein corona was recovered after lysosomal degradation of corona, Environ. Sci. Technol., 2020, 54, 11485–11493 CrossRef CAS PubMed.
- M. Déniel, N. Errien, P. Daniel, A. Caruso and F. Lagarde, Current methods to monitor microalgae-nanoparticle interaction and associated effects, Aquat. Toxicol., 2019, 217, 105311 CrossRef PubMed.
- X. You, M. You, Y. Lyu, G. Peng and W. Sun, Single and combined exposure to micro (nano) plastics and azithromycin disturbing the photosynthetic carbon fixation of Synechocystis sp, Environ. Sci.: Nano, 2022, 4354–4366 RSC.
- M. Tamayo-Belda, J. J. Vargas-Guerrero, K. Martin-Betancor, G. Pulido-Reyes, M. González-Pleiter, F. Leganés, R. Rosal and F. Fernández-Piñas, Understanding nanoplastic toxicity and their interaction with engineered cationic nanopolymers in microalgae by physiological and proteomic approaches, Environ. Sci.: Nano, 2021, 8, 2277–2296 RSC.
- F. Nasser and I. Lynch, Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna, J. Proteomics, 2016, 137, 45–51 CrossRef CAS PubMed.
- M. Junaid and J. Wang, Interaction of nanoplastics with extracellular polymeric substances (EPS) in the aquatic environment: A special reference to eco-corona formation and associated impacts, Water Res., 2021, 201, 117319 CrossRef CAS PubMed.
- C. J. Dedman, J. A. Christie-Oleza, V. Fernández-Juárez and P. Echeveste, Cell size matters: Nano- and micro-plastics preferentially drive declines of large marine phytoplankton due to co-aggregation, J. Hazard. Mater., 2022, 424, 127488 CrossRef CAS PubMed.
- M. Déniel, N. Errien, F. Lagarde, M. Zanella and A. Caruso, Interactions between polystyrene nanoparticles and Chlamydomonas reinhardtii monitored by infrared spectroscopy combined with molecular biology, Environ. Pollut., 2020, 266, 115227 CrossRef PubMed.
- M. Kajikawa, Y. Sawaragi, H. Shinkawa, T. Yamano, A. Ando, M. Kato, M. Hirono, N. Sato and H. Fukuzawa, Algal dual-specificity tyrosine phosphorylation-regulated kinase, triacylglycerol accumulation regulator1, regulates accumulation of triacylglycerol in nitrogen or sulfur deficiency, Plant Physiol., 2015, 168, 752–764 CrossRef CAS PubMed.
- P. Wang, B. Zhang, H. Zhang, Y. He, C. N. Ong and J. Yang, Metabolites change of Scenedesmus obliquus exerted by AgNPs, J. Environ. Sci., 2019, 76, 310–318 CrossRef CAS PubMed.
- C. H. Tan, P. L. Show, M. K. Lam, X. Fu, T. C. Ling, C.-Y. Chen and J.-S. Chang, Examination of indigenous microalgal species for maximal protein synthesis, Biochem. Eng. J., 2020, 154, 107425 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00324h |
| This journal is © The Royal Society of Chemistry 2023 |