Open Access Article
Open Access ArticleCs2AgxNa1−xBiyIn1−yCl6 perovskites approaching photoluminescence quantum yields of 100%†
Oleksandr
Stroyuk
 *a,
Oleksandra
Raievska
*a,
Oleksandra
Raievska
 a,
Anastasia
Barabash
b,
Christian
Kupfer
b,
Andres
Osvet
b,
Volodymyr
Dzhagan
a,
Anastasia
Barabash
b,
Christian
Kupfer
b,
Andres
Osvet
b,
Volodymyr
Dzhagan
 cd,
Dietrich R. T.
Zahn
cd,
Dietrich R. T.
Zahn
 ef,
Jens
Hauch
ab and
Christoph J.
Brabec
ab
ef,
Jens
Hauch
ab and
Christoph J.
Brabec
ab
aForschungszentrum Jülich GmbH, Helmholtz-Institut Erlangen Nürnberg für Erneuerbare Energien (HI ERN), 91058 Erlangen, Germany. E-mail: o.stroyuk@fz-juelich.de
bFriedrich-Alexander-Universität Erlangen-Nürnberg, Materials for Electronics and Energy Technology (i-MEET), Martensstrasse 7, 91058 Erlangen, Germany
cV. Lashkaryov Institute of Semiconductors Physics, National Academy of Sciences of Ukraine, 03038 Kyiv, Ukraine
dPhysics Department, Taras Shevchenko National University of Kyiv, 60 Volodymyrs'ka str., 01601 Kyiv, Ukraine
eSemiconductor Physics, Chemnitz University of Technology, 09107 Chemnitz, Germany
fCenter for Materials, Architectures and Integration of Nanomembranes (MAIN), Chemnitz University of Technology, 09107, Chemnitz, Germany
First published on 22nd August 2022
Abstract
A new single-step and green approach for the synthesis of microcrystalline Cs2AgxNa1−xBiyIn1−yCl6 (CANBIC) perovskites in ambient conditions is introduced. The CANBIC powders emit broadband self-trapped excitonic photoluminescence (PL) with a champion PL quantum yield (QY) of 98 ± 2% and a PL lifetime of ca. 2 μs observed for x = 0.40 and y = 0.01–0.02. The study focuses on the dependence of structural, spectral, and photophysical properties of CANBICs on Bi content. CANBICs are solid solutions with isomorphous In-to-Bi substitution with the bandgap and valence band edge energy decreasing gradually with increasing y. The PL QY and the rate constant of the radiative recombination showed volcano-shaped dependences on the Bi content, while the rate of the non-radiative recombination revealed a drastic growth by three orders of magnitude as the Bi fraction y was elevated from 0.01 to 1.0 indicating that BiCl6 units are responsible for non-radiative recombination.
Introduction
The tremendous progress in the field of light conversion using lead-halide perovskite compounds achieved within an unprecedentedly short time period1–4 stimulated a massive advancement in many related areas, including exploration of tandem perovskite-based photovoltaic materials,1,2,4–6 search for low-dimensional (2D, 0D) forms of halide perovskite absorbers,1,4,5,7–12 as well as attempts to develop lead-free perovskite compounds with light harvesting properties comparable with those of lead-halide perovskites.1,3,4,8,12–16 The latter research direction highlighted many promising compounds, either directly stemming from lead-based ancestors, such as tin-halide perovskites,3,8,12–16 or those belonging to a family of double-cation perovskites AI2MIMIIIX6, where A is an alkali cation, X is a halide, and the couple of MI and MIII represents an isovalent substitution of two PbII cations in the lead-halide (APbX3)2 perovskite structure.3,8,12–16The double lead-free compounds combine the variability of A and X sites typical for APbX3 compounds with new possibilities of independent variation of both MI and MIII sites, which has no analogs in the chemistry of lead-halide perovskites and imparts the double perovskites with a unique compositional flexibility.3,8,12–16 In particular, candidates for MI and MIII sites can be selected from MI = Na+, K+, Ag+, Tl+, Au+, etc. and MIII = In3+, Bi3+, Sb3+, Fe3+, Au3+, etc., varied independently, and together with AI and X components yield many thousands of possible compositions.3,8,12–16
Among the double halide perovskites, compounds based on BiIII and InIII with a general formula Cs2AgxNa1−xBiyIn1−yCl6 (abbreviated as CANBIC by the first letters of the elements) occupy an outstanding position due to a combination of the compositional variability, stability, and promising light-conversion properties, in particular, highly efficient broadband photoluminescence (PL).12,13,15,17–42 These compounds typically feature a dome-shaped dependence of many photophysical characteristics (PL efficiency and lifetime, electron–phonon coupling strength, etc.) on the ratios of Ag/Na and Bi/In.17,18,20,21,23,34 For example, CANBIC compounds often show exceptionally strong dependence of the PL quantum yield (QY) and Huang–Rhys factor on the Ag-to-Na ratio x,18,21,25,28–30,32,34 while CANBICs with a fixed x typically reveal a dome-shaped dependence of the PL efficiency on the Bi fraction y.17,18,31,32,34,41
Along with the variable parameters x and y, both Cs and Cl sites can also be varied and potentially substituted by analogs (for example, Cs by Rb or methylammonium). This fact strongly multiplies the number of possible compositions opening a vast field for the search of new effects and unexpected compositional dependences of functional properties relevant for applications in light conversion and emission (bandgap, PL QY, charge carrier density, mobility, lifetime, etc.).
At the same time, the potential and capacities of such a search are considerably limited by the current level of development of synthetic approaches to CANBIC perovskites. Typical synthetic protocols are rather unflexible and energy-consuming, requiring the presence of concentrated HCl8,16,19,21,28,31,32,41,42 or application of prolonged hydrothermal treatments and/or annealings.8,16,18,19,26 Attempts to make the synthesis of CANBICs and related compounds more environmentally friendly by mimicking syntheses of lead-based halide perovskites, such as antisolvent- or oversaturation-induced crystallization, either yield mixtures of multiple phases with a low control over the crystallization dynamics16,32,33,43 or do not cover the entire possible ranges of compositions.32,43 In this view, further search for mild and fast and, at the same time, highly controllable protocols for the synthesis of CANBIC perovskites remains a topic of high relevance.
CANBIC compounds are regarded as a green and sustainable Pb-free representative for the class of halide perovskites. However, green and sustainable should not only refer to the material, but also to the synthesis. Recently, we reported a communication on a relatively green single-step synthesis of luminescent CANBIC perovskites in 2-propanol:water mixtures at ambient conditions without any additional thermal treatments necessary to achieve high PL efficiencies and stability.44 Here, a more detailed and comprehensive report on the structural, spectral, and photophysical characteristics of CANBIC perovskites produced by this approach is provided focusing on the dependence of CANBIC properties on the Bi-to-In ratio, while other possible variables are kept constant. A fine tuning of the synthesis conditions is shown to enhance the PL QY of the most emissive CANBIC samples almost to unity.
Results and discussion
A green synthesis of CANBIC perovskites implies minimizing both the energy input and utilization of aggressive/volatile compounds (concentrated HCl, organic solvents), but still maintaining a reliable control over the composition of the products. In line with these criteria, a protocol for the synthesis of microcrystalline CANBIC compounds was developed allowing the perovskites of any given composition to be precipitated under ambient conditions with no necessity of thermal post-treatment to improve crystallization (hydrothermal treatment, annealing, etc.). The synthesis is a single-step procedure and requires interaction of two precursors containing metal ions in 2-propanol:water mixtures and a minimal amount of HCl necessary to prevent hydrolytic by-reactions of InIII and BiIII.To steer the reaction to the formation of a single perovskite phase with no noticeable admixtures of other possible by-products the metal cations were separated between two precursors and their reactivity equalized. In particular, Bi3+ and In3+ were bound into complexes with HCl in precursor 1, while Cs+, Na+, and Ag+ were separated into precursor 2 where ammonia was added to avoid hydrolysis of Ag+ in a mildly alkaline medium produced by the presence of cesium and sodium acetates. The feasibility and some details on the synthesis were discussed in our recent communication,44 where the maximal PL efficiency of CANBIC perovskites was achieved at 2% content of BiIII and a molar fraction of silver of 0.35.
Here a broader characterization of the structure, spectral, and photophysical properties of CANBIC perovskites produced by such an approach is provided with a focus on the variation of the content of bismuth. The molar Bi fraction y was varied from 0.05% to 100% while tracking the influence of the bismuth-to-indium ratio y on the morphology, structure, spectral, and photophysical properties of CANBIC perovskites.
With the bismuth content varied, all other parameters are maintained identical, in particular by retaining a constant nominal silver-to-sodium ratio x = 0.40, as well as by providing excesses of Cs+ and Na+ during the synthesis and by performing the synthesis at room temperature (22 °C). In the following discussion the CANBICs with a varied Bi content are referred to either by the value of y (implying that x = 0.40) or by abbreviating the perovskites as CANBIC-Y%, where Y = 0.05–100. All the properties of CANBIC perovskites are primarily discussed in the present paper as functions of the molar BiIII fraction y.
The details of the synthesis and purification of CANBIC perovskites as well as methods of structural and optical characterization are provided in ESI.†
In the following discussion we provide an account on the structural, spectral, and photophysical properties of CANBIC perovskites with a varied Bi-to-In ratio investigated with appropriate techniques. First, morphology, composition and structure of CANBICs are probed by a combination of scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). Basing on these results we then discuss spectral characteristics of CANBICs, including light absorption and emission, PL efficiency and factors affecting PL QY, positions of valence band (VB) and conduction band (CB) as functions of the Bi content revealed by X-ray photoelectron spectroscopy (XPS), as well as vibrational properties of CANBICs probed by non-resonant Raman spectroscopy. Finally, the kinetics of the photophysical processes in CANBIC perovskites is addressed by analyzing time-resolved PL decay traces of the samples with a variable Bi-to-In ratio.
1 Composition, morphology, and structure of CANBIC perovskites
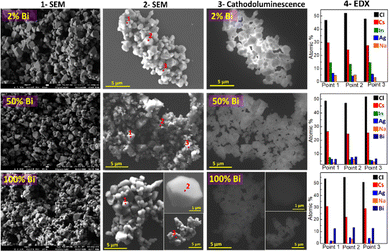
During SEM inspection CANBIC samples were found to emit intense cathodoluminescence (CL) under irradiation with the electron beam. The highest CL intensity was observed at y = 0.02 gradually decreasing again for higher bismuth contents.
The high intensity of CL allows the CANBIC crystals to be clearly observed in SEM using only the CL contrast already at 10 kV accelerating voltage (compare columns 2 and 3 in Fig. 1). At that, the shape of the crystals is identical for SEM and CL images with no dark areas observed in CL images indicating a high structural homogeneity of the tested samples.
Selected probing of the samples in different random points by EDX revealed constant compositions close to the nominal ones set at the synthesis (Fig. 1, column 4) with no noticeable variations among the tested points.
Statistically significant information on the elemental composition of CANBIC samples was collected by probing the samples with y varied from 0 to 1.0 by EDX in 7–10 different areas for each composition (Table 1). The real composition of the CANBIC samples after all treatments and purification was found to be very close to the nominal one (set at the synthesis) showing a good control over the CANBIC composition by tuning the composition of both starting precursors. In particular, the Bi content closely followed the nominal y value, the silver fraction x was found to be quite close to 0.40 varying by about ±5%.
| Nominal y | N EDX | Real y | Real x | Cs-to-M ratio | Cl-to-M ratio |
|---|---|---|---|---|---|
| Notes: NEDX, number of EDX spectra processed for the statistical analysis. | |||||
| 0 | 8 | 0 | 0.35 ± 0.10 | 2.1 ± 0.1 | 5.8 ± 0.3 |
| 0.005 | 8 | 0.02 ± 0.02 | 0.37 ± 0.07 | 2.2 ± 0.2 | 6.0 ± 0.3 |
| 0.02 | 7 | 0.02 ± 0.01 | 0.35 ± 0.10 | 2.1 ± 0.1 | 6.2 ± 0.3 |
| 0.10 | 8 | 0.11 ± 0.02 | 0.42 ± 0.08 | 2.2 ± 0.1 | 5.9 ± 0.1 |
| 0.20 | 9 | 0.23 ± 0.04 | 0.43 ± 0.05 | 2.1 ± 0.1 | 5.8 ± 0.2 |
| 0.30 | 8 | 0.32 ± 0.03 | 0.45 ± 0.05 | 2.2 ± 0.2 | 5.7 ± 0.2 |
| 0.50 | 8 | 0.51 ± 0.05 | 0.43 ± 0.08 | 2.1 ± 0.2 | 5.5 ± 0.2 |
| 0.70 | 10 | 0.70 ± 0.04 | 0.46 ± 0.06 | 2.0 ± 0.1 | 5.4 ± 0.1 |
| 0.90 | 8 | 0.90 ± 0.03 | 0.40 ± 0.07 | 2.1 ± 0.3 | 5.8 ± 0.2 |
| 1.00 | 8 | 0.99 ± 0.01 | 0.47 ± 0.06 | 2.1 ± 0.2 | 5.3 ± 0.4 |
The ratio of Cs to the sum of M = Bi + In was near the expected value of 2 despite the presence of an excess of Cs+ at the synthesis. The ratio of chlorine to the sum of M = Bi + In was close to 6 as expected for the CANBIC stoichiometry (Table 1).
The composition of CANBIC crystals with varied Bi content (y = 0.1) was complementary identified by X-ray photoelectron spectroscopy (see an exemplary survey spectrum and summary of the results in Fig. S2 and Table S1, ESI† respectively), showing a good correspondence with the EDX measurements.
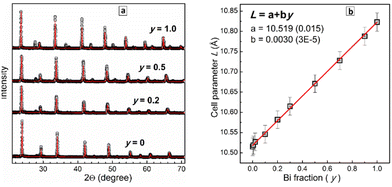
The XRD patterns were subjected to a Rietveld refinement to trace the dependence of the lattice parameter L on the Bi fraction y. This dependence was found to be linear (Fig. 2b) with the lattice parameter increasing from 10.515(2) Å for CANIC (y = 0) to 10.823(2) Å for CANBC (y = 1.0) reflecting the gradual lattice expansion due to the larger ionic radius of Bi3+ as compared to In3+.17,18,20,26,28,32 The linear character of L(y) obeying Vegard's law shows that the CANBIC perovskites are ideal solid solutions and form a single phase for any possible composition.20,21,26,28,29,32 The results of the Rietveld refinement of the XRD patterns of 10 different CANBIC compositions are presented in ESI† (Fig. S3). In Fig. 2b we also provide an empirical linear L(y) relationship allowing to calculate the Bi content in CANBIC perovskites (with x = 0.4) from XRD patterns.
2 Spectral properties of CANBIC perovskites
 | ||
| Fig. 3 (a and b) Absorption, PL excitation (a), and PL (b) spectra of CANBIC perovskites with Bi fraction y = 0 (curve 1), 0.01 (2), 0.05 (3), 0.07 (4), 0.10 (5), 0.15 (6), 0.20 (7), 0.50 (8), 0.90 (9), and 1.00 (10). In (a) solid lines are absorption spectra and circles represent corresponding PL excitation spectra. (c) Band gap of CANBIC perovskites as a function of Bi fraction y. A legend for different symbols in (c) is provided in the caption of Fig. 4. | ||
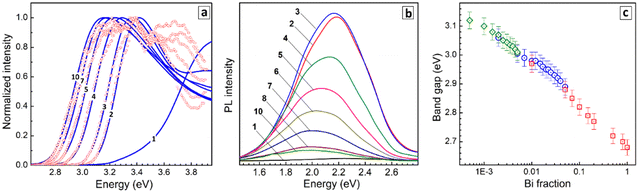
No detectable PL can be found for pure CANIC perovskite under the excitation at 365 nm. When very small amounts of Bi are introduced (y = 0.005–0.01), CANBIC starts to emit PL in a broad band centered at ca. 2.2 eV and encompassing the larger portion of the visible spectra range (Fig. 3b). The highest PL intensity was observed for y = 0.01–0.02 decreasing gradually for higher Bi fractions and shifting to lower energies (Fig. 3b, curves 3–10). The development of intense light absorbance and emission upon introduction of minimal amounts of Bi into the CANIC matrix, which otherwise shows a very high energy of the absorption band edge and no PL, are typically interpreted as a result of the Bi species breaking the symmetry of the CANIC lattice and contributing to the formation of CB edge, thus making interband transitions allowed in both directions (light absorption and radiative recombination).17,18,21,25,26,28,31,32 In terms of this interpretation, BiIII acts as a “key” opening the inherent capacity of the CANIC matrix for light absorption and emission.
The PL excitation (PLE) spectra of CANBICs are very similar to the corresponding absorption spectra, both showing identical positions of band edges depending on y (Fig. 3c). The same energies of band edges derived from absorption and PLE spectra indicate a high structural perfection of the lattice of CANBIC perovskites with no mid-bandgap states originating from defects observed below the edge of the fundamental absorption band. This conclusion is additionally supported by the high quality of the Raman spectra (see discussion below) registered under off-resonance 514.7 nm excitation, showing no PL background despite the very high PL intensity (PLI) of CANBIC samples.
The bandgap Eg of CANBIC perovskites can therefore be evaluated by analyzing both absorption and PLE spectra. Both were found to show linear edges when plotted using the Tauc equation for direct allowed interband electronic transitions, in the case of PLE spectra – as functions of (PLI × hν)2. We followed the dependence of the bandgap on y for three series of CANBIC perovskites with Bi content changing through 3 orders of magnitude (more details are provided below in the discussion of PL QY measurements). The bandgap of CANBIC perovskites was found to decrease monotonously as an almost linear function of the logarithm of the molar BiIII fraction with the Eg(y) dependence overlapping for all three independent sample series (Fig. 3d). Overall, the bandgap of CANBIC perovskites can be precisely tuned between 2.68 eV (y = 1.0) and 3.12 eV (y = 0.005) by changing the BiIII content.
In the present work, the PL efficiency of CANBIC perovskites was further increased by finely tuning the synthesis conditions. As reported,44 the post-synthesis ripening of purified CANBIC perovskites at ambient conditions does not change the PL QY for at least six months of shelf storage. However, the duration of the contact of as-precipitated (that is not separated and not purified) CANBIC perovskites with their parental solution which contains excess of Cs+ and Na+ was found to have a distinct effect on the PL QY of the final perovskites, produced after the separation from the supernatant and purification.
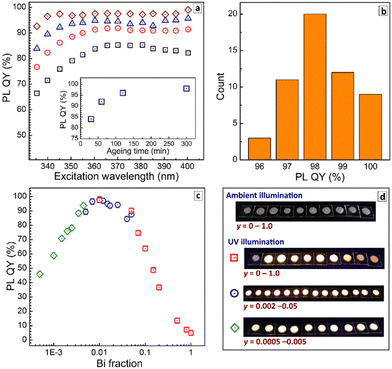
Fig. 4a shows evolution of the PL QY values measured at room temperature (RT) for different excitation wavelengths as a function of the duration of precipitate ripening in the parental solution before the purification. The PL QY was found to be below 80% for freshly deposited CANBIC-2%, increasing to 92% after 60 min ageing and growing further up to 98% when the ageing was prolonged to 300 min (see insert in Fig. 4a).
All probed samples show lower PL QY when excited at 330–340 nm, the emission yield growing to steady values for longer excitation wavelengths. This behavior was attributed to the existence of species absorbing in that spectral range but not contributing to PL emission and resulting, therefore, in lowered PL QYs. A possible candidate for such non-emissive species can be under-crystallized (defective) perovskite on the surface of microcrystals. As the ripening period is increased, the crystallization proceeds and the contribution of these species becomes less pronounced (compare, for example, black squares and brown diamonds in Fig. 4a), while the PL QY grows to a saturation at 98%. The sample aged for 300 min shows a steady PL QY value almost in the entire range of tested excitation wavelengths. No further changes in PL QY were detected for larger ripening times (12 h).
The assumption about incompletely crystallized species affecting the PL QY at excitation in the range of 330–350 nm is additionally supported by a ripening-induced transformation of the absorption spectra (Fig. S4a, ESI†). As the ripening duration is increased from 30 to 300 min the absorbance of the CANBIC sample at 300–340 nm decreases noticeably, while the rest of the spectrum remains mostly unchanged. At the same time, no detectable changes can be observed in the same range in the PLE spectra (not shown). Combining these observations, we can conclude that the ripening results in the extinction of the species contributing to the light absorption but not to the light emission, in accordance with the above arguments.
The reproducibility of the reported synthetic protocol was evaluated by preparing independently four identical batches of CANBIC-2% perovskite and collecting PL QY values for different excitation wavelengths. The samples showed very similar behavior with PL QYs almost converging at the excitation wavelength range of 360–380 nm for all four samples (Fig. S4b, ESI†).
Additionally, samples of ultra-pure BaSO4 powders from three different suppliers (Alfa-Aesar, Sigma-Aldrich, and Thermo-Fisher) were tested as a scattering reference for PL QY measurements. It was found that all PL QY values are very close to each other in the entire excitation wavelength range tested (Fig. S4c, ESI†). An empty cuvette used as a reference expectedly yields much lower PL QY values (Fig. S4c, ESI†) highlighting the importance of a proper scattering reference for the PL QY measurements of highly-luminescent microcrystalline powders.
To evaluate the statistical distribution of PL QYs all values measured for the “champion” CANBIC samples with the highest PL efficiency (x = 0.40, y = 0.01–0.02, at least 300 min of ageing in parental solution) were assembled for several different sample batches. In addition, the PL QY measured at multiple excitation wavelengths (in the range of 350–400 nm) were used for each particular sample as well as data collected using different scattering BaSO4 references. The resulting chart presented in Fig. 4b shows that the champion PL QY at room temperature is 98 ± 2%. We note that this value is, most probably, the highest one reported so far for microcrystalline CANBIC perovskites with no additional dopants. Similarly high PL QYs of 98–99% were also reported for CANBICs of the same composition (x = 0.4, y = 0.01) but additionally doped with 1% Ni or Ce.31
Finally, absolute PL QYs of CANBIC perovskites synthesized with different fractions of BiIII varied from 0 to 1.0 were determined. No noticeable PL was emitted by CANIC sample with y = 0 excited at 365 nm as discussed above while the sample with y = 0.01 showed the highest PL QY of 98%. The PL efficiency gradually decreased almost to zero as the BiIII content is stepwise elevated till y = 1.0 (Fig. 4c, red squares).
To identify more precisely the Bi content at which the maximal PL QY is observed, a second set of samples with y varied from 0.002 to 0.05 was synthesized. This series of CANBICs revealed a high PL QY close to 98% for a rather broad compositional range between 0.8% and 2% BiIII with the PL QY slightly decreasing both for y < 0.008 and y > 0.02 (Fig. 4c, blue circles).
In view of this behavior, and to collect data on the PL behavior of CANBICs with low Bi content, a third series of samples with y varied from 0.0005 to 0.005 was produced. This series showed a gradual decrease of PL QY with decreasing Bi fraction (Fig. 4c, green diamonds). Photographs of all tested samples made under UV illumination (360–370 nm) are provided in Fig. 4d.
All three dependences of PL QYs overlap and form a unified dependence on the Bi content encompassing a range of more than three decades of y. This dependence shows a plateau at y = 0.008–0.02, where the ultimate PL QY of 98% is observed, and decreases for CANBICs with a lower or a higher Bi content.
Density-functional-theory calculations reported by Manna's group showed that incorporation of BiCl6 octahedra into CANIC introduces localized states below the CB minimum.25 Due to differences in parity between Bi and In orbitals their mixing is negligible and the photoexcited electron is strongly localized on BiCl6 centers, while the hole is localized on AgCl6 octahedra.25 Estimations showed that due to the highly localized character of BiCl6 units and typically large lifetime of STE state (>1 μs), the efficiency of the STE emission is expected to be very high even at very low Bi doping level and for a broad range of Bi contents,25 in accordance with the present observations.
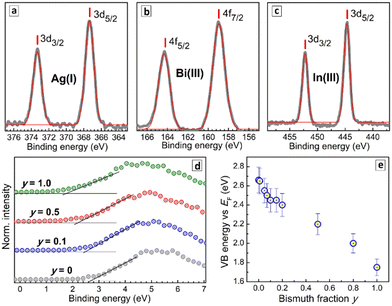
The binding energies of Ag3d and In3d electrons were found to be identical for all CANBIC samples with y varied from 0 to 1.0 (Fig. S5 and Table S2, ESI†). A deconvolution of the Bi4f band into Bi-related components and Cs 4p components (Fig. S5, ESI†) shows bismuth to be present as Bi3+ in all CANBIC samples with the same position of the 4f doublet in Bi-rich samples (Table S2, ESI†).
The VB edge shows a regular shift to lower energies as Bi is introduced into CANIC perovskite and the y fraction increased in CANBICs (Fig. 5d). In particular, the VB edge shifts from 2.66 eV for y = 0 to 1.75 eV for y = 1 with respect to the Fermi level (Table S3, ESI†). The intermediate compositions showed a VB edge energy between these two values (Fig. 5d). The absolute positions of VB and CB edges for all tested CANBIC compositions were calculated by combining the positions of the VB edge recalculated versus vacuum level with Eg values derived from absorption/PLE spectra (Table S3, ESI†). Both VB and CB positions were found to shift gradually to lower energies as the content of Bi was increased (Fig. S6, ESI†).
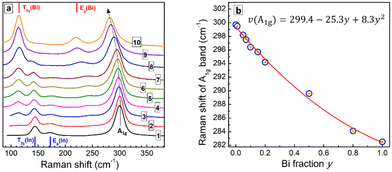
The Raman spectrum of CANIC perovskite (y = 0) shows three major modes, including an A1g LO phonon band at 300 cm−1 and two modes characteristic for vibrations of InCl6 octahedra, namely an Eg(In) at 173 cm−1 and a T2g(In) at 144 cm−1 (Fig. 6a, curve 1; Fig. S7b, ESI†).20,21,24,28,32 The Raman spectrum of CANBC perovskite (y = 1.0) has a similar structure, revealing the A1g LO phonon mode at 282 cm−1 and two modes characteristic for BiCl6 octahedra, the Eg(Bi) at 219 cm−1 and the T2g(Bi) at 114 cm−1 (Fig. 6a, curve 10; Fig. S7b, ESI†).20,21,24,28,32
The spectra of Bi-In mixed CANBIC perovskites show in total five modes, including A1g, Eg(In), Eg(Bi), T2g(In), and T2g(Bi). Their positions and relative intensities are proportional to the molar fraction of Bi (Fig. 6a). A clear compositional dependence of the LO mode and Bi, In-related modes, in combination with the high quality of the Raman spectra (high signal-to-noise ratio, no PL background, and high stability of the samples toward laser excitation, etc.) make Raman spectroscopy a powerful tool for the identification of the composition of CANBIC perovskites directly from Raman spectra, without the need of additional XRD or EDX analysis. For this purpose different parameters of the Raman spectra can be used, for example, the compositional variation of the LO phonon frequency or energy (Fig. S8a, ESI†), the intensity ratios of Eg(Bi)/Eg(In) and T2g(Bi)/T2g(In) features, or other combinations of composition-dependent parameters (Fig. S8b and c, ESI†).
Fig. 6b shows the dependence of the A1g LO phonon peak frequency on the nominal molar Bi fraction y. The A1g frequency decreases with an increase of the Bi content following a polynomial law. The best fit of the “ν(A1g) – y” dependence is a parabolic expression with a moderate bowing (presented in Fig. 6b), allowing the composition of CANBIC to be identified with good precision in the entire y range.
As the Raman spectra can be measured directly during or after the synthesis, the identification of CANBIC composition using Raman spectroscopy is probably the fastest method among all possible alternatives. Potentially, Raman spectroscopy may be used to probe the formation of double perovskites by the characteristic shape of the spectra. For example, Bi-In perovskites always show a characteristic multi-mode shape of the spectrum with a dominating A1g vibrational band for all CANBICs, even if sodium is completely substituted by silver (Fig. S9, ESI†). However, no spectral patterns characteristic for double perovskites can be found for samples produced without Ag or Cs (Fig. S9a, ESI†), in accordance with corresponding XRD patterns showing the absence of double perovskite phases in such samples.
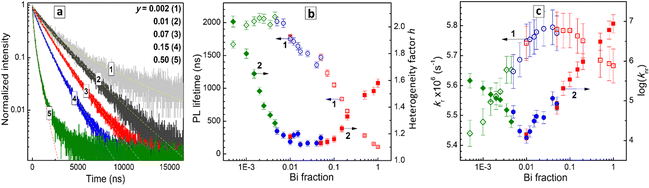
The shape of PL decay curves deviates from a single-exponential one with the degree of deviation depending on the Bi content (Fig. 7a). This behavior is typical for lead-free perovskites with STE emission and is assumed to originate from local fluctuations of the lattice structure resulting in a distribution of possible energies of the STE state.9,10 The transitions between different STE states occur on a fs – ps time scale, i.e. much faster than the PL emission events, resulting in the perovskites acting as single-state emitters (like molecules), but with a certain distribution of PL lifetimes, observed as a deviation of the PL decay curves from the single-exponential character.25,46,47
In order to collect information simultaneously on the PL lifetime and on the PL decay deviation from the single-exponential decay law, the PL decay curves were fitted using a stretched-exponent model with two fitting parameters – PL lifetime and the heterogeneity parameter h as I = I0 × exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ((−t/τ)1/h).23,47,48 The heterogeneity parameter h is equal to 1 for the single-exponential PL decay, and larger than 1 for the cases of deviations from the single-exponential decay due to the structural fluctuations.25,46–48
((−t/τ)1/h).23,47,48 The heterogeneity parameter h is equal to 1 for the single-exponential PL decay, and larger than 1 for the cases of deviations from the single-exponential decay due to the structural fluctuations.25,46–48
Fig. 7b illustrates the dependences of the PL lifetime and heterogeneity factor on the Bi content in a broad composition range covering three orders of magnitude of y variation. The PL lifetime was found to increase from ca. 100 ns for pure CANBC perovskite (y = 1.0) up to ca. 1600–1700 ns for the most luminescent CANBICs with y = 0.01–0.02 and further to ca. 2 μs for compositions with lower Bi content (Fig. 7b, scatter 1). The PL lifetime remains almost constant in the range of y = 0.001–0.008 and decreases for the lowest Bi fraction tested, y = 0.0005, down to ca. 1650 ns.
The heterogeneity factor h was found to be at ca. 1.6 for CANBC perovskite, decreasing to ca. 1.1 for a broad range of compositions encompassing y = 0.01–0.1 (Fig. 7b, scatter 2). At y < 0.01 a steady growth of h is observed, reaching above 2.0 for the lowest Bi content of y = 0.0005. These observations indicate that the highest PL efficiencies are observed for the compositional range with the lowest heterogeneity factor, where the PL decay almost obeys the single-exponential law, indicating the highest homogeneity of the perovskite lattice.
The PL lifetime decreases by a factor of almost 20, when the Bi fraction is increased from 1–2% to 100%, evidencing a high probability of the participation of Bi-related structural units of the perovskite lattice in the non-radiative relaxation processes.
By combining the results of TRPL measurements and PL QY values the rate constants for radiative recombination kr and non-radiative recombination knr as functions of y (see details in SI) were calculated. The kr(y) dependence has a dome shape (Fig. 7c, scatter 1) with the rate constant increasing in the range of y = 0.0005–0.08, reaching a broad plateau at y = 0.02–0.08 and decreasing for Bi-richer CANBIC compositions. We note that no drastic changes in the radiative recombination rate were observed for higher Bi contents. The kr value decreases by only about 25% when y is increased from 0.02 to 1.0. This observation indicates that PL quenching and sharp shortening of the PL lifetime originate from the activation of non-radiative processes in Bi-richer perovskites, rather than from a loss of the emissive capacity.
On the contrary, the rate constant of the non-radiative recombination shows minimal values for the most emissive CANBIC samples and a drastic growth by three orders of magnitude as y is increased from ca. 0.01–0.02 to 1.0 (Fig. 7c, scatter 2). By combining the trends of the kr and knr changes with the composition, the strong decrease of the PL emission capacity for Bi-richer samples can be assumed to originate from a faster extinction of the STE states in non-radiative processes while the probability of the formation of emissive STE states remains mostly unaffected by the compositional variation.
Conclusions
A new green and single-step approach for the synthesis of microcrystalline Cs2AgxNa1−xBiyIn1−yCl6 (CANBIC) perovskites is introduced. The synthesis is performed under ambient conditions, it does not require in situ or post-synthesis thermal treatments, and yields CANBIC powders emitting broadband yellow-white self-trapped excitonic photoluminescence (PL). By using PL as a performance indicator the perovskite composition and the synthesis conditions were tuned to reach the highest possible PL quantum yield (QY) of 98% at room temperature registered for a CANBIC with x = 0.40 and y = 0.01–0.02.Among other parameters, the duration of ageing of freshly prepared CANBICs in the parental solutions containing an excess of sodium and cesium cations was found to be crucial for reaching near-unity PL QY, most probably due to an increase in the crystallinity and lattice perfection of the CANBIC perovskites. Along with bright PL, CANBICs were found to emit strong visible cathodoluminescence when irradiated with an electron beam.
In the search for the highest possible PL QY the present work focuses on the effect of Bi content (molar Bi fraction y) on the structural, spectral, and photophysical properties of CANBIC perovskites. By applying XRD and Raman spectroscopy the CANBIC perovskites were found to be single-phase products showing an ideal solid solution structure in the entire range of 0 < y < 1 with In3+ isomorphously substituted by Bi3+. Due to high sensitivity of the phonon spectrum to perovskite structure and composition, Raman spectroscopy can be used for an express evaluation of the composition and phase of CANBICs directly during or after the synthesis.
The effect of Bi content on the light absorption and PL properties of CANBIC perovskites was probed on several sets of samples with Bi fraction y varied by three orders of magnitude. The bandgap of CANBICs evaluated both from absorption and PL excitation spectra decreases gradually from 3.12 eV (y = 0.0005) to 2.68 eV (y = 1.0). A similar declining trend was found by XPS also for the valence band (VB) edge with the VB energy decreasing from ca. 6.8 eV (versus vacuum level) at y = 0 to ca. 5.9 eV at y = 1.0.
The room-temperature PL QY showed a dome-shaped dependence on the molar Bi fraction increasing from ca. 45% at y = 0.0005 to 97–98% at y = 0.008–0.02 and decreasing at higher Bi contents to a few percent at y = 1.0. For the CANBIC samples with the highest PL efficiency (y = 0.01–0.02) reliable statistics were collected on the absolute PL QY values with several different sample batches, excitation wavelengths, and scattering references, the average value found to be 98 ± 2%.
The room-temperature PL lifetime is close to 2 μs at y < 0.01, rapidly decreasing for higher Bi fractions down to ca. 100 ns for y = 1.0. The rate constant of the radiative recombination shows only a limited dependence on the Bi content at y > 0.01, while the rate constant of non-radiative recombination increases by three orders of magnitude in the same range of 0.01 < y < 1.0 indicating that BiCl6 octahedra are mostly responsible for the non-radiative recombination of self-trapped excitons.
Author contributions
O. Stroyuk: conceptualization (lead), investigation (equal), writing – original draft preparation (lead); O. Raievska: investigation (lead), methodology (lead); A. Barabash: investigation (equal), writing – review & editing (equal); C. Kupfer: investigation (equal), A. Osvet: investigation (equal), writing – review & editing (equal); V. Dzhagan: investigation (equal), writing – review & editing (equal); D. R. T. Zahn: resources (equal), writing – review & editing (equal); J. Hauch: conceptualization (equal), project administration (lead), writing – review & editing (equal); C. J. Brabec: conceptualization (equal), funding acquisition (lead), writing – review & editing (equal).Conflicts of interest
There are no conflicts to declare.Acknowledgements
O. S., O. R., J. H., and C. B. gratefully acknowledge financial support of The German Federal Ministry for Economic Affairs and Climate Action (project Pero4PV, FKZ: 03EE1092A) and The Bavarian State Government (project “ELF-PV-Design and development of solution processed functional materials for the next generations of PV technologies”, No. 44-6521a/20/4). CB and AO gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft under GRK2495/E. VD acknowledges Visiting Scholar program for funding of his research stay at TU Chemnitz. We thank Hamamatsu Photonics GmbH Deutschland (and personally Dr Alexander Kiel) for providing a Quantaurus QY C11347-11 spectrometer for PL QY measurements.Notes and references
- A. Dey, J. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Wang, J. Yin and Y. Wang, et al. , ACS Nano, 2021, 15, 10775 CrossRef CAS PubMed.
- J. Liu, J. Qu, T. Kirchartz and J. Song, J. Mater. Chem. A, 2021, 9, 20919 RSC.
- Z. Xiao, Z. Song and Y. Yan, Adv. Mater., 2019, 31, 1 Search PubMed.
- M. G. Ju, M. Chen, Y. Zhou, J. Dai, L. Ma, N. P. Padture and X. C. Zeng, Joule, 2018, 2, 1231 CrossRef CAS.
- J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Chem. Rev., 2019, 119, 3296 CrossRef CAS PubMed.
- R. Wang, T. Huang, J. Xue, J. Tong, K. Zhu and Y. Yang, Nat. Photonics, 2021, 15, 411 CrossRef CAS.
- E. Shi, Y. Gao, B. P. Finkenauer, A. Akriti, A. H. Coffey and L. Dou, Chem. Soc. Rev., 2018, 47, 6046 RSC.
- N. K. Tailor, S. Kar, P. Mishra, A. These, C. Kupfer, H. Hu, M. Awais, M. Saidaminov, M. I. Dar, C. Brabec and S. Satapathi, ACS Mater. Lett., 2021, 3, 1025 CrossRef CAS.
- Z. Xu, X. Jiang, H. Cai, K. Chen, X. Yao and Y. Feng, J. Phys. Chem. Lett., 2021, 12, 10472 CrossRef CAS PubMed.
- S. Li, J. Luo, J. Liu and J. Tang, J. Phys. Chem. Lett., 2019, 10, 1999 CrossRef CAS PubMed.
- E.-B. Kim, M. S. Akhtar, H.-S. Shin, S. Ameen and M. K. Nazeeruddin, J. Photochem. Photobiol., C, 2021, 48, 100405 CrossRef CAS.
- W. Ning and F. Gao, Adv. Mater., 2019, 31, 1 Search PubMed.
- H. Tang, Y. Xu, X. Hu, Q. Hu, T. Chen, W. Jiang, L. Wang and W. Jiang, Adv. Sci., 2021, 8, 2004118 CrossRef CAS.
- Y. Gao, Y. Pan, F. Zhou, G. Niu and C. Yan, J. Mater. Chem. A, 2021, 9, 11931 RSC.
- P. K. Kung, M. H. Li, P. Y. Lin, J. Y. Jhang, M. Pantaler, D. C. Lupascu, G. Grancini and P. Chen, Sol. RRL, 2020, 4, 1 Search PubMed.
- X. Li, X. Gao, X. Zhang, X. Shen, M. Lu, J. Wu, Z. Shi, V. L. Colvin, J. Hu, X. Bai, W. W. Yu and Y. Zhang, Adv. Sci., 2021, 8, 1 Search PubMed.
- D. Manna, T. K. Das and A. Yella, Chem. Mater., 2019, 31, 10063 CrossRef CAS.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao and Q. Dong, et al. , Nature, 2018, 563, 541 CrossRef CAS.
- E. T. McClure, M. R. Ball, W. Windl and P. M. Woodward, Chem. Mater., 2016, 28, 1348 CrossRef CAS.
- D. Manna, J. Kangsabanik, T. K. Das, D. Das, A. Alam and A. Yella, J. Phys. Chem. Lett., 2020, 11, 2113 CrossRef CAS.
- A. C. Dakshinamurthy and C. Sudakar, J. Phys. Chem. Lett., 2022, 13, 433 CrossRef CAS.
- S. E. Creutz, E. N. Crites, M. C. De Siena and D. R. Gamelin, Nano Lett., 2018, 18, 1118 CrossRef CAS.
- D. Zhu, J. Zito, V. Pinchetti, Z. Dang, A. Olivati, L. Pasquale, A. Tang, M. L. Zaffalon, F. Meinardi, I. Infante, L. De Trizio, L. Manna and S. Brovelli, ACS Energy Lett., 2020, 5, 1840 CrossRef CAS PubMed.
- B. Zhou, Z. Liu, S. Fang, H. Zhong, B. Tian, Y. Wang, H. Li, H. Hu and Y. Shi, ACS Energy Lett., 2021, 6, 3343 CrossRef CAS.
- F. Locardi, E. Sartori, J. Buha, J. Zito, M. Prato, V. Pinchetti, M. L. Zaffalon, M. Ferretti, S. Brovelli, I. Infante, L. De Trizio and L. Manna, ACS Energy Lett., 2019, 4, 1976 CrossRef CAS.
- J. Zhou, X. Rong, P. Zhang, M. S. Molokeev, P. Wei, Q. Liu, X. Zhang and Z. Xia, Adv. Opt. Mater., 2019, 7, 1801435 CrossRef.
- Z. Li, F. Sun, H. Song, H. Zhou, Y. Zhou, Z. Yuan, P. Guo, G. Zhou, Q. Zhuang and X. Yu, Dalton Trans., 2021, 50, 9804 RSC.
- K. Dave, W.-T. Huang, T. Leśniewski, A. Lazarowska, D. Jankowski, S. Mahlik and R.-S. Liu, Dalton Trans., 2022, 51, 2026 RSC.
- P. Vashishtha, B. E. Griffith, Y. Fang, A. Jaiswal, G. V. Nutan, A. P. Bartók, T. White and J. V. Hanna, J. Mater. Chem. A, 2022, 10, 3562 RSC.
- P. Han, X. Mao, S. Yang, F. Zhang, B. Yang, D. Wei, W. Deng and K. Han, Angew. Chem., Int. Ed., 2019, 58, 17231 CrossRef CAS PubMed.
- C.-Y. Wang, P. Liang, R.-J. Xie, Y. Yao, P. Liu, Y. Yang, J. Hu, L. Shao, X. W. Sun, F. Kang and G. Wei, Chem. Mater., 2020, 32, 7814 CrossRef CAS.
- H. Siddique, Z. Xu, X. Li, S. Saeed, W. Liang, X. Wang, C. Gao, R. Dai, Z. Wang and Z. Zhang, J. Phys. Chem. Lett., 2020, 11, 9572 CrossRef CAS PubMed.
- S. Li, H. Wang, P. Yang, L. Wang, X. Cheng and K. Yang, J. Alloys Compd., 2021, 854, 156930 CrossRef CAS.
- Q. Hu, G. Niu, Z. Zheng, S. Li, Y. Zhang, H. Song, T. Zhai and J. Tang, Small, 2019, 15, 1903496 CrossRef CAS PubMed.
- R. S. Lamba, P. Basera, S. Bhattacharya and S. Sapra, J. Phys. Chem. Lett., 2019, 10, 5173 CrossRef CAS PubMed.
- Y. Liu, Y. Jing, J. Zhao, Q. Liu and Z. Xia, Chem. Mater., 2019, 31, 3333 CrossRef CAS.
- Z. Zhang, Y. Zhang, X. Guo, D. Wang, Y. Lao, B. Qu, L. Xiao and Z. Chen, ACS Appl. Energy Mater., 2022, 5, 1169 CrossRef CAS.
- B. Zhang, M. Wang, M. Ghini, A. E. M. Melcherts, J. Zito, L. Goldoni, I. Infante, M. Guizzardi, F. Scotognella, I. Kriegel, L. De Trizio and L. Manna, ACS Mater. Lett., 2020, 2, 1442 CrossRef CAS PubMed.
- L. Zdražil, S. Kalytchuk, M. Langer, R. Ahmad, J. Pospíšil, O. Zmeškal, M. Altomare, A. Osvet, R. Zbořil, P. Schmuki, C. J. Brabec, M. Otyepka and Š. Kment, ACS Appl. Energy Mater., 2021, 4, 6445 CrossRef.
- D. Wu, X. Zhao, Y. Huang, J. Lai, H. Li, J. Yang, C. Tian, P. He, Q. Huang and X. Tang, Chem. Mater., 2021, 33, 4971 CrossRef CAS.
- X. Li, S. Xu, F. Liu, J. Qu, H. Shao, Z. Wang, Y. Cui, D. Ban and C. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 31031 CrossRef CAS.
- D. Huang, H. Xiao, D. Liu, Q. Ouyang, Y. Kong, B. Wang, H. Lian and J. Lin, J. Mater. Chem. C, 2021, 9, 8862 RSC.
- M. Ahmadi, M. Ziatdinov, Y. Zhou, E. A. Lass and S. V. Kalinin, Joule, 2021, 5, 2797 CrossRef CAS.
- O. Stroyuk, O. Raievska, A. Barabash, M. Batentschuk, A. Osvet, S. Fiedler, U. Resch-Genger, J. Hauch and C. J. Brabec, J. Mater. Chem. C, 2022, 10, 9938–9944 RSC.
- A. V. Naumkin, A. Kraut-Vass, S. W. Gaarenstroom and C. J. Powell, NIST X-ray Photoelectron Spectroscopy Database, NIST, 2012 Search PubMed.
- J. E. Thomaz, K. P. Lindquist, H. I. Karunadasa and M. D. Fayer, J. Am. Chem. Soc., 2020, 142, 16622 CrossRef CAS.
- R. Chen, J. Lumin., 2003, 102–103, 510 CrossRef CAS.
- J. Klafter and M. F. Shlesinger, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 848 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed descriptions of the procedures of CANBIC synthesis, purification, and preparation of samples for structural and spectral studies; details of the characterization methods; description of the calculation of recombination rate constants; collections of SEM images and XRD patterns of CANBICs with different Bi content; results of PL QY measurements in different conditions; collection of XPS data for Ag, In, and Bi elements in CANBICs; positions of VB/CB levels; vibrational parameters of CANBIC; composition of CANBICs determined by XPS. See DOI: https://doi.org/10.1039/d2ma00737a |
| This journal is © The Royal Society of Chemistry 2022 |