Open Access Article
Open Access ArticleVitrimer composites: current status and future challenges
Vincent
Schenk
abc,
Karine
Labastie
a,
Mathias
Destarac
 ac,
Philippe
Olivier
*b and
Marc
Guerre
ac,
Philippe
Olivier
*b and
Marc
Guerre
 *c
*c
aIRT Saint Exupéry, Bâtiment B612 3 rue Tarfaya, 31405 Toulouse cedex 4, France
bICA, Université de Toulouse, UT3, CNRS UMR 5312, Espace C. Ader, 3 Rue Caroline Aigle, 3140 Toulouse, France. E-mail: philippe.olivier@iut-tlse3.fr
cLaboratoire des IMRCP, Université de Toulouse, CNRS UMR 5623, Université Paul Sabatier, 118 route de Narbonne, 31062 Toulouse Cedex 9, France. E-mail: marc.guerre@cnrs.fr
First published on 23rd September 2022
Abstract
Thermosets dominate the composite industry owing to their outstanding stiffness to weight ratio and fatigue resistance. Nevertheless, the possibilities of recycling these materials are limited due to the irreversible chemical bonds created during the curing process which cause the materials to be set in their final form. The available recycling strategies generally degrade the polymer matrix either by burning off (pyrolysis) or chemically dissolving (solvolysis) the resins in order to recover the fibres. But methodologies to reprocess or fully recycle (i.e. resin + fibres) these composite materials are rare. Thus, the development of more sustainable approaches is now increasing importantly and is seen as a decisive challenge for the further development of composite materials. Vitrimer materials, which combine the mechanical resilience of thermosets with reprocessability of “glass” at high temperatures, appear as a very promising alternative towards recyclable thermoset composites. This review gathers the recent progress in the domain of vitrimer composites and points out the next future challenges to be tackled. A brief section discussing the first industrial initiatives is also presented.
1. Introduction
Industry uses more and more thermoset matrix composites for high-demand applications (e.g. from wind turbines to structural parts of aircraft, such as fuselage or wings) owing to their outstanding mechanical properties, good fatigue, creep resistance and ease of production.1 Nevertheless, these important materials raise a major problem regarding their recyclability as they are intrinsically insoluble and infusible. Although these properties are considered as major assets for thermoset matrix composites during their lifetime, they become important drawbacks at the end of their life as they are very difficult to recycle.2To improve the mechanical properties, thermoset polymers are generally combined with reinforcements such as carbon or glass fibres, carbon nanotubes, graphene or many others. As a result, thermoset composites are even more difficult to reprocess, reshape or recycle. One of the challenges regarding the recycling process consists of separating the reinforcement from the matrix. Current processes use thermolysis (the matrix is degraded at high temperatures),3,4 solvolysis5 or other methods6 enabling reinforcement recovery (carbon or glass fibre-focused) but mostly with altered mechanical properties. Therefore, these fibres cannot be recycled several times and used for the same applications they were initially designed for.5,7,8
The most popular reinforcement in the aeronautics industry is carbon fibre and the reasons are simple: low weight to strength ratio, high tensile strength, high chemical resistance and many others.9 For these reasons, carbon fibre reinforced thermoset polymers are also increasingly used for a wide range of other applications including land and sea vehicles, storage tanks, wind turbines and sports equipment. This soaring demand for carbon fibre reinforced polymers (CFRPs) is driven by new targets for lower CO2 emission and the need for light-weight and high strength vehicular structures. Therefore, the production of CFRPs is expected to grow drastically with an estimated projection of more than 190 kt by 2050. Concerning global CFRP wastes comprising both thermosets and thermoplastics, it is important to note that this will increase up to 20 kt annually by 2025 and even more by the end of 2030 especially because around 8000 commercial aircrafts will reach their end-of-life.7 This is in part why there is a clear need for an economically-sustainable waste management and recycling techniques for composites (Fig. 1).10
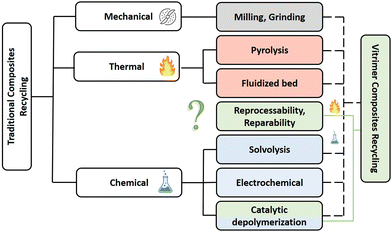 | ||
| Fig. 1 Classification of the major recycling methods of traditional composites with possible alternatives offered by vitrimer composites. | ||
An interesting way to recycle thermoset composites would be to customize the chemical structure of the thermoset polymer matrix before they are produced in their final form. Many efforts have been made in the past two decades to produce reprocessable, healable and recyclable thermosets. For instance, reversible bonds were introduced into polymeric networks, including reversible non-covalent interactions (e.g. ionic interaction,11 hydrogen bonding12 and metal–ligand coordination13) and reversible covalent exchanges. Thermosets issued from non-covalent interactions often exhibit weak mechanical characteristics and could not withstand large stress, making them not suitable for structural applications.
Alternatively, polymer networks with reversible covalent bonds, known as covalent adaptable networks (CANs), offer an attractive way to make thermosets with good mechanical integrity combined with thermoplastics-like behaviour at high temperatures.14,15 CANs are generally divided into two groups depending on their exchange mechanism: dissociative CANs and associative CANs. The first group, dissociative CANs, is constituted of reversible chemical reactions based on dissociative exchange mechanisms, meaning that the cross-linked bonds break upon heating and reform at lower temperatures resulting in a decrease of network connectivity and modification of the cross-linking degree during network rearrangement.16 Hence, such exchange reactions lead to networks that cannot maintain their dimensional integrity at high temperatures. In the second group, associative CANs, the cross-link density is maintained with a constant number of chemical bonds during reprocessing.17
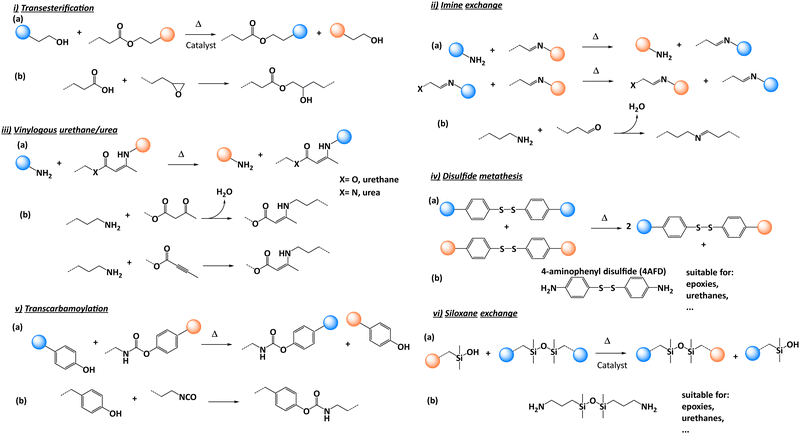
In 2011, Leibler and co-workers coined this new type of polymeric material based on associative CANs, “vitrimer”.18 The first vitrimer was obtained by adding a transesterification catalyst to an epoxy/acid polyester network. This catalyst promoted the transesterification reactions with control possible over the exchange kinetics by varying the quantity of catalyst initially introduced. Vitrimers are now commonly considered as the third class of polymer materials alongside thermoplastics and thermosets. At the service temperature, vitrimers behave like traditional thermosets, with good mechanical characteristics. However, when heated above a specific temperature, the exchangeable reaction (such as transesterification) occurs. In the past decade, various chemistries have been implemented for vitrimer purposes including transesterification,19,20 transamination of vinylogous urethanes,21,22 transalkylation,23,24 disulfide exchange25,26 or imine exchange,27,28 to name a few.17,29–31
These vitrimers have quickly been picked up by the composite community and studied as matrices in different classes of materials.32,33 By replacing thermoset matrices with vitrimer counterparts, new capabilities emerged such as healability, reprocessability and recyclability (Fig. 1).33 This review reports an overview of vitrimer composites with an emphasis on the type of reinforcement, composite manufacturing processes and recyclability potential. A brief description of the used chemistries, composite processing techniques and recent industry-oriented developments in the field of vitrimer composite materials is also presented.
2. Vitrimer chemistries in composite materials
Since the seminal work of Leibler on vitrimers, various chemical platforms have been developed. The reader will find detailed information on the chemistry of vitrimers in recent review and perspective articles.29,31 Among the many chemistries, only a few were selected for composite purposes.32 The reason for this poor diversity is likely related to the limited large-scale commercial availability of dynamic chemical precursors. Composite manufacturing requires large quantities of matter restraining the possibilities to use precursors from multi-step syntheses or sophisticated chemical platforms. The selected dynamic chemistry must be integrated with the overall chemical crosslinking procedure with preferably no release of volatile molecules forming defects, such as water. The processing parameters such as viscosity must be compatible with the manufacturing process spread across the composite industry such as resin transfer moulding, infusion and impregnation. Finally, the selected chemistry should withstand high temperatures as these conditions can be regularly met during structural composite manufacturing or during their use in real application. In this context the glass transition temperature (Tg) is a key parameter and must be located above the in-service temperature of the composites. In addition, the reprocessing temperature (Tr) and degradation temperature (Td) of the polymer matrix also need to be determined as they are paramount for establishing the vitrimer reprocessing conditions. Indeed, Tr is usually set at 50 °C above Tg to introduce enough segmental motion into polymer chains allowing dynamic exchanges to occur. But care should be taken for high Tg polymer matrices as the reprocessing temperature range could be rather narrow, imposing a subtle compromise to achieve between sufficient polymer mobility and avoidance of degradation reactions. For all these reasons, only a few chemical platforms were used for preparing vitrimer composites.2.1. Transesterification
In the pioneering studies of Leibler and co-workers,19 zinc acetate [Zn(ac)2] catalyst was used to promote transesterification reactions (Fig. 2i). In conventional epoxy/acid polymer networks, the abundance of both free hydroxyl and ester functions is guaranteed by simply mixing stoichiometric amounts of the monomers. However, under off-stoichiometric conditions, the polymerisation is more complex as anionic ring opening polymerization occurs simultaneously with acid-epoxy addition requiring a careful design of the stoichiometry. Indeed, non-exchangeable ether functions are formed all through the network with the formation of non-dynamic clusters which impair the properties of the vitrimers.34 Another interesting feature of transesterification-based vitrimers is the relatively straightforward control of the exchange reaction kinetics through catalysis. By changing the amount or the nature of the catalyst, the activation energy can be easily tuned.35,362.2. Imine exchange
Imine bonds exhibit good stability owing to the high bond dissociation energy of 147 kcal mol−1 compared to 87.9 kcal mol−1 for regular H3C–CH3 bond.27 They can be formed by a simple condensation reaction of an amine with an aldehyde (Fig. 2ii). This dynamic moiety can undergo rapid degenerative bond exchange with a free amine through a hemi-aminal intermediate or via two different imines following a metathesis mechanism (Fig. 2ii(a)). Overall, the chemistry is robust with little or no side reactions except a possible oxidation of free amines (if present) at high temperatures or hydrolysis in the presence of water. Reports on polymer networks composed of imine linkages are abundant considering the diverse range of primary amines and aldehydes that are readily available. Some recent studies showed the excellent mechanical and dynamical properties of thermosets consisting of imine linkages. Other studies of small model imine compounds in solution showed that the imine bond exchange is catalysed by the presence of a primary amine and the rate of exchange is dependent on the solvent, temperature, and imine structure making this chemistry very much dependent on the chemical environment. However, the release of water during imine formation (Fig. 2ii) leads to material defects requiring a preliminary step with water elimination or a post-reprocessing procedure.37 This chemistry has been combined with different reinforcements that will be described in the following sections.2.3. Vinylogous urethane/urea
Du Prez and co-workers21,38,39 developed a new chemistry based on the transamination of vinylogous urethane moieties. They can be prepared via bulk polymerisation using the spontaneous condensation reaction between acetoacetate and amine monomers with the release of water, or via the direct addition of an amine onto an alkyne ester function (Fig. 2iii(b)).21 The dynamic exchanges are catalyst-free and can exchange through two distinct mechanisms: activated iminium intermediate or direct Michael addition.38,39 These mechanisms can be selectively triggered using an external catalyst offering important control over dynamic exchanges. From a thermodynamic point of view, the exchangeable vinylogous urethane bond is comparable to an urethane bond. Thus, vinylogous urethanes are more stable than imine linkages towards hydrolysis and can even be formed quantitatively in water as solvent. The presence of free amines is mandatory to allow swift exchange reactions, but this can easily be done using slightly off-stoichiometric conditions. Overall, the resulting materials presented good stability similar to those of epoxy/acid vitrimers described previously but with shorter relaxation times. Despite these interesting features, the stability of the small quantity of amines throughout the network needs to be considered for long-term applications, in view of possible oxidative damages.21Similar to imines, the formation of vinylogous urethanes releases water which can be considered as an important drawback although this has been partially circumvented in a recent study.40 A pre-curing step can be conducted or the use of alkyne ester functions could replace acetoacetate moieties (Fig. 2iii(b) bottom).
2.4. Disulfide exchange
Disulfide exchange (Fig. 2iv) is a well-known chemistry which shows many advantages such as easy implementation and relatively easy access to precursors which make this chemistry very popular.25,41–44 Furthermore, this chemistry has been extensively studied as it is commonly used in vulcanized rubber manufacturing. Recent reports show that the behaviour of covalently exchanging disulfide bonds is a rather complex process, and involves several mechanisms with the most likely to happen being the [2+1] radical-mediated mechanism which depends on the used conditions and substitution patterns of the disulfides.45 Although it is a widespread chemistry, oxidation of free thiols easily occurs under air often showing marked deterioration of the dynamic properties over time. In addition, the thermal stability of the S–S bond needs to be monitored as this bond is sensitive to temperature, also leading to the deterioration of dynamic properties over time.2.5. Transcarbamoylation
Transcarbamoylation of urethanes can occur with both aliphatic and aromatic polyurethanes. The main advantage of an aromatic structure is that the reaction can occur without the need of a catalyst between phenols and urethanes (Fig. 2v(a)). Urethane bonds can be formed under mild conditions using commercial hydroxyl compounds and isocyanates available in large quantities (Fig. 2v(b)).46,472.6. Siloxane exchange
Siloxane bonds can be found in many materials ranging from typical silicone rubber such as polydimethylsiloxane (PDMS), to inorganic glass. It is a very appealing chemistry for dynamic materials as it has intrinsic characteristics such as high chemical and thermal stability. Although a typical silyl ether material exhibits slow exchange,48 a recent discovery by Debsharma and co-workers reported much faster exchanges based on a new pathway involving siloxanes and a base catalyst: 1,5,7-triazabicyclo[4.4.0] dec-5-ene49 (Fig. 2vi(a)). This opens new perspectives for this chemistry in the context of composite materials as it can easily be implemented through the commercially available 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyl disiloxane (BAS) (Fig. 2vi(b)).3. Processing techniques in composite manufacturing
The use of vitrimer matrices for composite manufacturing depends on the ability of these matrices to be compliant with the requirements and parameters of the composite manufacturing processes.1 The most representative ones are: contact moulding, pultrusion, resin transfer moulding (RTM), infusion, prepegging, vacuum bagging, autoclave curing and out-of-autoclave curing. To date, the focus was put on the synthesis and evaluation of vitrimer composite properties and these processing parameters were often not investigated although it is essential for the further development of this new class of materials.The choice of a manufacturing process for the production of composite parts depends of several criteria such as (i) constitutive materials (fibres and matrix), forms (roving, short fibres, unidirectional fibres, textiles,…), (ii) expected mechanical performances, (iii) production rates (e.g. one part a day, one thousand part a day,…) and (iv) cost.55 For these reasons, the later chapter has been segmented based on the type of reinforcements.
4. Reinforcements in vitrimer matrix composites
4.1. Carbon fibres
Carbon fibres have been used for decades in the industry thanks to their high stiffness, high tensile strength, high chemical resistance, high strength to weight ratio, etc. Nevertheless, they are relatively expensive compared to glass fibres and therefore mainly used for high performance and structural applications.The pioneering paper on CFRP vitrimers was reported with bisphenol A diglycidyl ether (DGEBA), a common epoxy resin which was cured with 4-aminophenyl disulfide (4AFD, a relatively expensive hardener compared to permanent analogues but commercially available on a multikilogram scale) as dynamic crosslinker (Fig. 2iv(b)).44 Compared to conventional epoxy systems, the dynamic network (i.e. with a vitrimer curing agent) exhibited similar mechanical characteristics except for the degradation temperature which decreased from 350 °C down to 300 °C. The resulting matrix was then used to prepare thin carbon fibres/vitrimer matrix laminates either by manual impregnation or RTM. The CFRP vitrimer showed equivalent mechanical properties to the epoxy reference, while exhibiting new features such as (re)processability, reparability and recyclability. For instance, a thin carbon fibre/vitrimer matrix laminated plate was reshaped by compression moulding in order to get a thermoformed zig-zag-shaped part (Fig. 3).
 | ||
| Fig. 3 Thermoforming of a cured composite laminate. A 2 mm thick carbon fibre reinforced dynamic epoxy laminate (a) was compression-moulded in a zig-zag shaped mould (b), rendering a thermoformed wavy 3D part (c). Reproduced with permission from ref. 43. Copyright 2016 Royal Society of Chemistry. | ||
CFRPs were also fully recycled using two different methods: (i) chemical recycling with disruption and dissolution of the matrix network in a thiol-containing solution in order to reuse the carbon fibres; and (ii) mechanical recycling by grinding the carbon/vitrimer composites and transferring the powder into a mould subjected to a 20 MPa pressure at 210 °C. The carbon/vitrimer composites presented here were obtained using standard manufacturing processes by simply substituting the conventional hardener with the industrially available 4AFD. Thus, this system constitutes a step towards the implementation of vitrimers in industrial applications, and offers the possibility of obtaining a new generation of recyclable CFRP materials.43 Following the same chemistry, Aranberri et al. formulated an epoxy vitrimer matrix suitable for pultrusion.50 The substitution of the anhydride crosslinker with 4AFD slowed down the polymerization kinetics, modifying the overall curing process. Nonetheless, the pultruded profile exhibited competitive thermomechanical properties and could be successfully reprocessed and reshaped by thermoforming.
The reprocessing time is very often long or requires high temperatures. To reduce this drawback, Si et al. introduced a higher content of exchangeable aromatic disulfide crosslinks. To do so, a bis-epoxy with embedded S–S bonds was synthesised and used with 4AFD as a crosslinker, increasing the overall number of dynamic exchanges within the network.24 The dual disulfide vitrimer released stress more rapidly in comparison to the reference with only 4AFD as the dynamic hardener. The resulting materials were thus reprocessable and malleable at lower temperatures and could be more easily degraded by dithiothreitol (DTT) solution (Fig. 4).
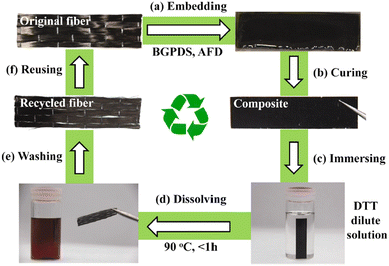 | ||
| Fig. 4 The recycling process of CFRP composites based on a dual disulfide vitrimer: (a) embedding the carbon fibres in the epoxy matrix; (b) obtaining CFRP composites by curing; (c) immersing the CFRP composites in a dilute solution of DTT (0.1 mg ml−1); (d) dissolving the epoxy matrix at 90 °C for 1 h; (e) washing and drying the recovered carbon fibres; and (f) reusing the recycled carbon fibres to form new composites. Reproduced with permission from ref. 51. Copyright 2020 Elsevier Ltd. | ||
After removing the epoxy matrix efficiently, the carbon fibres were successfully recycled and reused to form new CFRP composites. This study is particularly interesting as it illustrates the influence of disulfide bond contents on recycling kinetics.
Another fabrication method of fibre-reinforced polymer composites based on hot-pressing woven carbon fibres and vitrimer powder was also reported.52 It is inspired by the powder-based reprocessing of vitrimers and they demonstrated that vitrimer matrix composite samples can be fabricated within a few minutes (as short as 1 min) at different temperatures and pressures, which is remarkably shorter than the time required for typical liquid-state manufacturing methods with conventional thermoset matrices (Fig. 5).
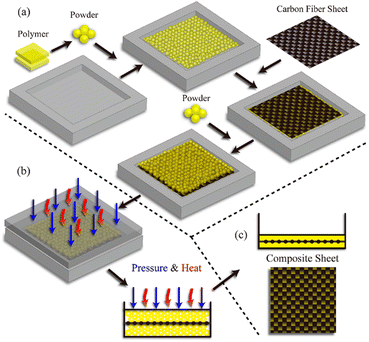 | ||
| Fig. 5 Fabrication process of composite vitrimers from vitrimer powder. (a) Vitrimer powder (matrix) and carbon fibre mat (filler) are deposited in the mould. (b) Pressure and heat are applied for a prescribed duration to induce the fusion of vitrimer powder. (c) A composite sample is extracted from the mould after the heat press process. Reproduced with permission from ref. 52. Copyright 2019 American Chemical Society. | ||
The cured vitrimer matrix composed of DGEBA with transesterification as dynamic chemistry was converted into powder through mechanical abrasion. The malleability of the vitrimer composites was also evaluated with possibilities for the material to be reprocessed through thermoforming, which can greatly expand the manufacturing capability of fibre-reinforced composites. The effect of processing conditions such as temperature and pressure was the key factor to obtain good mechanical properties, although it seems that the microstructure and size of the grinded particles are also a really important parameter which has not been investigated. Indeed, Li and co-workers showed that the effect of particle size is a key parameter to achieve efficient healing and thus full recovery of mechanical properties.53 The particle size changes healing conditions such as temperature, time, and pressure which must be adapted to obtain full recovery of properties. For instance, the lowest particle size required the highest values of temperature, time and pressure. If optimized, the presented fabrication method seems to have the potential for high volume manufacturing of fibre-reinforced composites in industrial sectors. Nonetheless, the relative high viscosity of vitrimers might constrain the further development of this technique. For instance, the thermoformability of vitrimer composites was also compared to that of carbon/PA66 composite (known to offer good thermoformability). The carbon/vitrimer composite showed ply wrinkling and delamination occurring during the thermoforming process due to the high viscosity of the vitrimer matrix above its Tg compared to PA66 matrix. Indeed, low viscosity is an important process prerequisite to obtain better thermoforming behaviour.54 This feature was also evidenced in vanillin-based CFRP composites prepared by the conventional hot-press process. Two different vitrimer matrix composite materials were prepared and strictly compared: (i) CFRP-1, fabricated with soft and tacky prepregs as typically used for thermosetting prepregs, and (ii) CFRP-2, prepared from stacked fully cured prepregs which have been consolidated together by hot press. The mechanical tests showed lower flexural strength and modulus for CFRP-2 compared to CFRP-1 (Fig. 6).
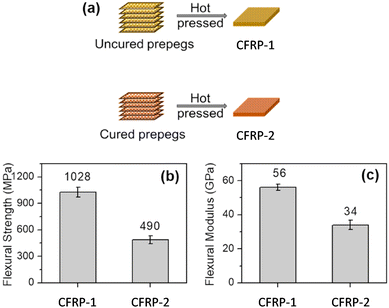 | ||
| Fig. 6 Schematic fabrication of CFRP-1 and CFRP-2 composites (a); Comparison of the flexural strength (b) and modulus (c) of CFRC-1 and CFRC-2 composites. Modified with the permission of ref. 55. Copyright 2020 Elsevier Ltd. | ||
This decrease in properties is likely due to the restrained mobility arising from carbon fibres preventing sufficient bond exchanges at the prepreg interface. As a result, the polymer chain entanglement at the interface might not be sufficient to compete with the mechanical properties resulting from a tacky prepreg. Besides, the epoxy vitrimer matrix enabled the carbon fibre reinforced composites to be repairable as evidenced by 92% strength recovery after interlaminar shear failure. Carbon fibres were recovered from composites by degrading the matrix resin in an amine solvent, and the degradation products can be reused to prepare new vitrimers, thus achieving a full recycling process. These results highlight the possible limitation of CFRPs prepared from fully cured prepregs, although this limitation could be potentially overpassed with further investigation.55
Recently, the interlaminar properties of CFR epoxy-dicarboxylic acid vitrimer have been studied in detail, through mode I interlaminar fracture toughness and flexural experiments. Different CFR vitrimers were prepared with various epoxy/acid ratios and manually laid-up and hot-pressed. While the flexural stiffness and strength were comparable to those of conventional anhydride epoxy CFRPs, interlaminar fracture energy under mode I loading outperformed the latter at the initiation and propagation stages. Furthermore, SEM micrographs of fracture surfaces after double cantilever beam test showed matrix residues on carbon fibres indicating a satisfying interfacial adhesion.56
Considering the societal incentives towards bio-based resins, the use and development of such matrices in combination with vitrimers also attracted considerable attention.57 Based on renewable resources, Wang et al. prepared a CFRP composite composed of a Schiff base epoxy matrix with a high Tg of 172 °C and good mechanical properties.58 It was obtained from a vanillin-based monoepoxide and a diamine via in situ formation of the Schiff base structure. The composite could be recycled under mild acidic conditions allowing the recycling of its carbon fibres, but the release of water during network formation as a result of the amine/aldehyde reaction generated trapped bubbles requiring an additional thermoforming step. Later, Liu et al. reported the use of a vanillin-based imine crosslinker with epoxidized soybean oil.59 The bio-based epoxy vitrimer showed good malleability, recyclability, and mechanical properties, making it suitable as a matrix to fabricate CFRP composites. The vanillin-based composites have been recycled by depolymerisation of the Schiff base bond under soft acidic conditions and using temperature through traditional processing techniques such as compression moulding. Lately, the soybean epoxy resin was replaced by glycerol triglycidyl ether. Similar to the soybean resin, the CF fabric could be recycled after degrading the matrix in an amine solution.60 Then, after recombining the degraded resin with a recycled CF fabric, a “regenerated” CF vitrimer matrix composite with similar mechanical properties to those of the original composite could be obtained, achieving full recycling of the CF vitrimer matrix composite. Nevertheless, similar studies have been previously performed focusing on the reuse of the depolymerised matrix based on CAN or thermoset networks presenting some limitations regarding the repolymerising step to produce new polymer composites. Indeed, they noticed a slight decrease of the new composite's mechanical properties compared to the initial composite's properties probably due to the residual presence of solvent within the network.61,62 Hence, this should also be investigated for vitrimer composites in order to apply similar recycling processes and reuse of the matrix to achieve fully recyclable composite materials.
Fully bio-based catalyst-free epoxy vitrimers for CFRPs were synthesized from a bio-based itaconic acid-based epoxy monomer, maleic anhydride and glycerin.63 The dynamic behaviour based on transesterification reactions occurs readily without a catalyst thanks to the large number of carboxylate and hydroxyl groups, accelerating the matrix relaxation and degradation times. The CF vitrimers were recycled and reprocessed several times without degradation of their mechanical properties.
4.2. Carbon nanotubes (CNTs)
Carbon nanotubes exhibit remarkable electrical conductivity, tensile strength and thermal conductivity thanks to their nanostructure. Therefore, CNTs are valuable in many areas like electronics, composite materials and nanotechnology. Additionally, CNTs absorb light of almost all wavelengths and transform the energy into heat, resulting in fast and precise local heating which could be really useful for vitrimer matrix composites. However, homogeneous dispersion of CNTs in composites is difficult due to their tendency to agglomerate. Care should be taken especially in high CNT loading to prevent CNT agglomeration since it can induce significant changes of material properties.For example, Yang et al.64 raised the problem that assembling classical epoxy materials by welding with remote control was extremely difficult as epoxies cannot melt or be dissolved. They presented a simple but highly efficient strategy by exploring the photothermal effect of CNTs to manipulate the transesterification reaction in vitrimers. The epoxy vitrimer was synthesized by reacting the DGEBA resin with adipic acid in the presence of triazobicyclodecene as a transesterification catalyst and CNTs at a concentration of 1 wt%. The resulting CNT-filled epoxy vitrimer could be welded by light within a few minutes. Indeed, transmission welding could be used to weld CNT vitrimers with different kinds of epoxy or thermoplastic polymers, which is not applicable to welding by direct heating. Thus, CNT vitrimer composites were successfully welded with non-CNT vitrimers via irradiation with an infrared laser (Fig. 7).
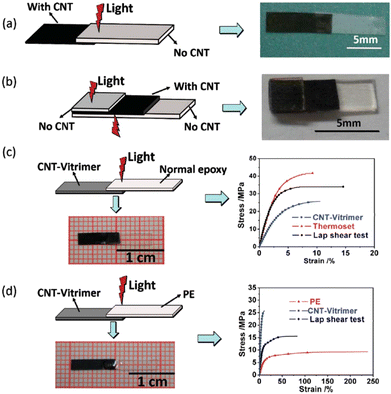 | ||
| Fig. 7 Transmission welding (a) joining a non-CNT vitrimer with a CNT-vitrimer. (b) Joining two pieces of a non-CNT vitrimer using a CNT-vitrimer as an “adhesive”. (c) Joining normal epoxy with a CNT-vitrimer. (d) Joining thermoplastic PE with a CNT-vitrimer. Reproduced with permission from ref. 64. Copyright The Royal Society of Chemistry 2014. | ||
Their results have demonstrated that the photo-thermal effect of CNTs is strong enough to activate the transesterification reaction. This makes it possible to weld covalently cross-linked epoxy networks using light, with versatile remote control on the selected areas at any time as required. Such photo-modulating welding without glues and moulds could be suitable for processing complex contours or the in situ joining and repairing of epoxy networks that have been integrated into high value sensitive objects. CNTs can also convert electric or magnetic energy into heat. With appropriate modification of the composite reported in this article, electrical and magnetic fields could also be employed to manipulate the welding and healing of epoxy vitrimer composites.64
CNTs were also implemented in conductive polymers to facilitate the transesterification and endow vitrimers with enhanced electrical conductivity. It is important to mention that transesterification rates and electrical properties of standard vitrimers are inadequate for many practical applications. The transesterification reaction performance has been evaluated by stress relaxation and showed a 3.6 times faster relaxation rate after doping it with only 3 wt% of CNT/polypyrrole (PPy). The improved transesterification in stress relaxation benefited from the upper thermal conductivity of CNTs and the interfacial interaction between CNT/PPy and vitrimer matrix. Unlike CNT/PPy vitrimer matrix composites, pure CNTs as dopants resulted in little enhancement suffering from strong agglomeration in the matrix. In addition, CNT/PPy doping improved the conductivity by several orders of magnitude.20
Polyimine vitrimers containing multiwalled carbon nanotube (MWCNT) fillers that allow bending, stretching, healing, and closed-loop recycling were also reported. These composites have been prepared by dispersing less than 10 wt% MWCNTs in a solution of terephthalaldehyde (top left), diethylenetriamine (top middle) and the cross-linker tris(2-aminoethyl)amine (top right) (Fig. 8).
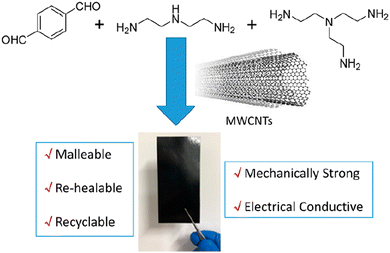 | ||
| Fig. 8 Polyimine–MWCNT composite for flexible electronics. Reproduced with permission from ref. 65. Copyright 2020 American Chemical Society. | ||
The resulting composites combine both the advantages of the polyimine vitrimer (i.e. dynamic covalent bond exchange) and carbon nanotubes (i.e. electron conducting) but the MWCNTs also improved the mechanical properties. Moreover, after bending, reshaping, breaking/healing and reuse, the electrical conductivity of the composite could remain almost the same thus making it a potentially good candidate for flexible electronics. In addition, thin film polyimine composites could also be fully recycled, which would reduce the overall production cost and the generation of environmentally hazardous electronic waste.65
The increase of exchangeable aromatic disulfide content as previously reported in a CFRP,51 and in dual covalent adaptable networks based on disulfide metathesis and transesterification, was also used in the context of MWCNTs.66,67 The relaxation time of the composites at 200 °C was approximately 9 s without any catalyst, which is remarkably low and 3 cycles of continuous breaking/compression moulding did not alter the properties (Fig. 9). The electric resistance of MWCNT/PPy/vitrimer composites was decreased from 1016 down to 109 Ω with only 1 wt% MWCNTs, which could be a promising candidate for self-repairing materials in the field of antistatic materials as it requires to have a surface resistance ≤109 Ω for antistatic coatings but also for electromagnetic shielding and microwave absorption. In a similar way to carbon fibre composites, the conductive fillers could be easily recovered in a solution of DTT and DMF after filtration, indicating that this could be applicable for all types of reinforcements in the recycling routes of vitrimer composites.66
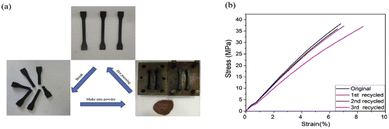 | ||
| Fig. 9 Recycling of the ground polymer network by hot pressing. (b) Stress–strain curves of the recycled samples demonstrated full recovery of the mechanical properties. Reproduced with permission from ref. 66. Copyright 2018 Elsevier Ltd. | ||
4.3. Graphene
Graphene is a single layer of carbon atoms (single layer of graphite) arranged in a two-dimensional honeycomb lattice. It is considered to be the world's strongest material and can be used to enhance the strength of other materials (e.g. polymers). It also has a good capability to conduct electricity and heat, making it very valuable for specific applications such as energy storage or electronics.In order to fully exploit the potential of graphene in polymer composites, its alignment is a key factor. Nevertheless, it is still a challenge to orientate graphene in thermosets due to its insoluble and infusible character. To do so, Chen et al. reported an easy and scalable hot press method to fabricate aligned graphene nanoplate (GnP)/epoxy composites by utilizing the dynamic character of epoxy vitrimers. The bond exchange and topological rearrangement associated with the viscous flow of the epoxy vitrimer during the hot pressing process allowed spontaneous orientation of GnP in the vitrimer matrix because of the graphite 2D structure and volume exclusion effect (Fig. 10).
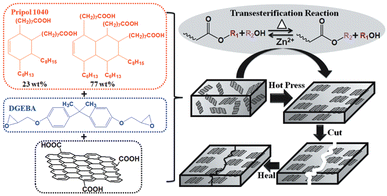 | ||
| Fig. 10 Chemical structure of the monomers and the fabrication process of the aligned and healable graphene/epoxy composites. Reproduced with permission from ref. 68. Copyright 2019 Frontiers in Chemistry. | ||
The correct orientation of GnP was confirmed by SEM images and tensile tests revealed the significantly increased reinforcement effect of GnP. The dynamic reactions within the epoxy vitrimer matrix conferred a good healability and recyclability to the aligned composites as confirmed by the nearly fully recovered mechanical properties of the healed sample after cutting and the recycled sample after grinding.68
To introduce good malleability, enhanced mechanical properties and multi-stimuli response, graphene was introduced into a styrene-butadiene rubber (SBR). The crosslinked networks were able to modify their topologies via transimination reactions in both the bulk network and SBR–graphene interphase in the absence of a catalyst, enabling them to be recycled and reshaped under heating or IR irradiation. The incorporation of graphene into the SBR network improved the mechanical properties of vitrimer composites. Also, the mechanical properties of the samples (containing different percentages of graphene) after multiple generations of recycling (cut and hot-pressed) were almost identical to those of the first reprocessed samples (Fig. 11).
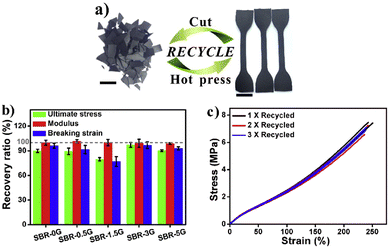 | ||
| Fig. 11 (a) Optical images showing the recycling of SBR-3G, and the scale bar represents 1 cm; (b) recovery ratio of the mechanical performance of SBR-xG between the first and second generations of recycling; and (c) stress–strain curves of SBR-3G after multiple cycles of recycling. Reproduced with permission from ref. 69. Copyright 2018 Elsevier Ltd. | ||
The incorporation of graphene could allow the vitrimer to be readily wrought into geometrically complex shapes under heating or IR irradiation.69
Following the same strategy, Krishnakumar et al. designed a catalyst-free self-healable vitrimer/graphene oxide (GO) nanocomposite, based on disulfide exchanges. This study found that the GO nanosheets enhanced the self-healing properties but also the shape memory and flexural strength of the vitrimer composites. The vitrimer nanocomposites demonstrate 88% and 80% self-healing for the first and second cycles in comparison to the neat epoxy vitrimers which resulted in 73% and 60% self-healing recovery, respectively (Fig. 12).70
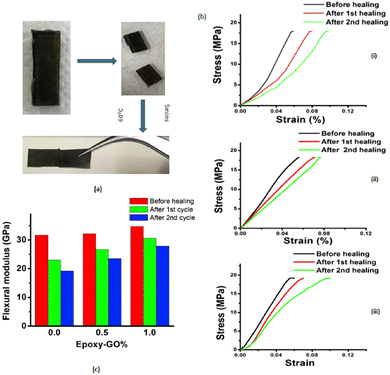 | ||
| Fig. 12 (a) Optical images of uncut, cut and healed EP-1% specimen. (b) Healing represented stress–strain curves for (i) EP-pristine (ii) EP-0.5% and (iii) EP-1%. (c) Flexural modulus changes after healing cycles for different samples. Reproduced with permission from ref. 70. Copyright 2019 Elsevier Ltd. | ||
The enhancing effect of graphene was recently confirmed by molecular dynamics simulations between a GO/vitrimer nanocomposite and pristine vitrimers using a self-healing simulation.71 The simulations were based on DGEBA epoxy and 2-AFD as the hardener. The results revealed that the incorporation of GO reduced the Tg of vitrimers. Also, the self-healing properties of the nanocomposite were found to be better than those of vitrimers at all temperature ranges, in accordance with a previous article on GO vitrimers.70 In addition, atomistic investigations demonstrated that the number of new disulfide bonds that exchange during the self-healing simulation increased in GO/vitrimer nanocomposites, which confirmed that adding GO to the vitrimer stimulated the bond exchange reaction. It is interesting to note that the Tg reduction in polymeric nanocomposites is a general phenomenon observed in various filler/matrix compositions and these simulation results imply that diverse nanofillers could be adopted for the same purpose.
4.4. Carbon black, nanodots and activated carbons
Carbon black, nanodots and activated carbons were also used as fillers to prepare vitrimer composites. Niu et al. used an epoxidized polyisoprene (EPI) and carboxylated carbon nanodots (CNDs, carbon nanoparticles less than 10 nm in size) used as both a cross-linker and a reinforcement for the construction of the dynamic network with exchangeable β-hydroxyl ester bonds. These dynamic exchanges at the interface between EPI and CNDs improved the mechanical properties and dispersion of CNDs in the rubber matrix (Fig. 13). The CND vitrimer composite could be reshaped and reprocessed, showing also good shape memory performance.72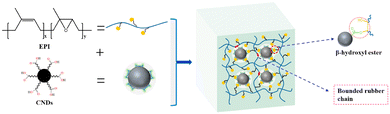 | ||
| Fig. 13 CND vitrimer composite network. Reproduced with permission from ref. 72. Copyright 2021 Elsevier Ltd. | ||
A vitrimer composite using a conventional DGEBA epoxy resin with a biomass-derived activated carbon (AC) filler to achieve enhanced thermal and mechanical properties was also reported. The fact that AC was prepared from sugarcane refuses makes it therefore sustainable. Adding AC to the epoxy vitrimers enabled the self-healing temperature of the material to be decreased. The main advantage of AC is its high surface area which allows progressive chain exchanges with the matrix. The healing properties of the vitrimer composite were evaluated during flexural tests and showed for a 1 wt% AC recoveries of 85% and 70%, respectively, after the first and second healing. In the future, it would be interesting to use a biomass-derived epoxy matrix in order to synthesize a fully sustainable vitrimer composite.73 Vitrimer composites could also be used to produce advanced and recyclable functional materials. Wang and co-workers introduced carbon black into the Schiff-based epoxy vitrimer to prepare thermal-sensitive sensors. Thermal sensitivity of the material could be easily tuned by adjusting the vitrimer matrix or CB content.74
As discussed in the previous sections, the recycling of reinforcement in vitrimer composites could be generalised and this was successfully achieved and described by Poutrel et al. for different graphene nanoparticles such as GNP, GO or rGO. Nevertheless, some chemical changes were observed afterwards including change of functionality.75
4.5. Glass fibres
For almost a century, glass fibres have been widely used to manufacture composite parts for applications in energy (wind turbines) or transports (boats). According to the JEC Observer, they represent more than 90% of the reinforcement fibre world market.54 Although they exhibit weaker mechanical performance in comparison to carbon fibres, there are still commonly used for structural applications because of their much more affordable cost. They also exhibit superior insulating features and high chemical resistance but with drawbacks to be considered such as low tensile modulus, relatively high density and low fatigue resistance compared to carbon fibres.9In addition to CFR vitrimers, Luzuriaga et al. also described the use of glass fibres to produce vitrimer composites.43 Single prepreg sheets were prepared using epoxy vitrimer matrices and glass fibre fabrics, and then laid up and hot pressed. This manufacturing process led to the formation of a perfectly compact multilayer composite with better mechanical properties compared to those of the manual lay-up. A question arises regarding the welding ability of the matrix when it is reinforced with a high content of fibres. Soon after, Chabert et al. studied the welding properties of vitrimer composites with a fibre volume content greater than 50 vol%.76 Glass fibre reinforced epoxy vitrimers based on transesterification reactions were produced using RTM and they could be repeatedly welded, allowing the joining of composite parts without fasteners or adhesives. Cohesive fracture in the same order that typical structural adhesive such as epoxy glue was obtained. This new alternative method of assembly could be of interest in industries such as aeronautics where large parts and/or hollow structures cannot be obtained via moulding techniques.
More recently and following a similar approach, single glass fibre sheets were manually impregnated with vinylogous urea vitrimers based on dynamic amine exchanges. Next, they were fully cured and used to prepare multilayer composites by stacking and hot-pressing. Pressing parameters such as pressure and temperature are crucial to achieve the desired properties and to avoid porosities within the multi-layered composite. The mechanical properties obtained were similar to those of the reference glass fabric epoxy composite displaying the efficiency of fully cured vitrimer prepregs.77
Another aspect concerning the high flammability of epoxy resins was investigated by Markwart et al. They synthesized glass fibre reinforced vitrimers containing a phosphonate-based flame-retardant additive. Pyrolysis combustion flow calorimetry measurements were conducted proving the flame-retardant effect and they demonstrated the reprocessability of the vitrimer composites.78
Post et al. reported the development of a healing glass fibre reinforced polymer (GFRP) composite based on a disulphide-containing organic–inorganic thermoset matrix.79 Owing to the vitrimer properties, multiple thermally induced healing delamination was possible between 70 and 85 °C. Regarding the composite processing, they were prepared by conventional vacuum assisted resin infusion moulding. Afterwards, the vitrimer composite mechanical properties and the extent of healing were determined by flexural, fracture and low-velocity impact testing. Small sized (<cm2) damage could be partially healed multiple times using a minimal healing pressure (0.02 MPa) to ensure a good alignment of the damaged interfaces (Fig. 14).
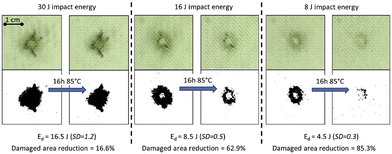 | ||
| Fig. 14 Image analysis of the healing of low-velocity impact at 3 different levels of impact energy. Healing is performed at 85 °C and under 0.02 MPa for 16 h. Top images show the real damaged composite whereas the bottom pictures show the same pictures treated with a binary filter to facilitate characterization. Reproduced with permission from ref. 79. Copyright 2017 Elsevier Ltd. | ||
In addition, the level of healing could be enhanced even for large (>cm2) damages, by increasing the healing pressure onto the location of the primary damage if concentrated in the matrix phase. Overall, this study showed a healable vitrimer with mechanical properties adequate for medium-tech applications and opens the path towards the development of intrinsic low-temperature healing composites with high performance applications.79
Viscosity is a key parameter in the liquid composite moulding processes and the introduction of vitrimer properties can considerably modify this characteristic. Recently, a low-viscosity resin (300 mPa s) based on siloxane exchanges was especially designed for the preparation of glass fibre-reinforced composites.49 The composite was prepared via infusion using the VARI technique (vacuum assisted resin infusion), which is a widely used technique for the manufacturing of wind turbine blades. The produced composite was fully transparent and appeared defect-free but some fibre wrinkling was observed in the longitudinal section. Interestingly, these wrinkling defects were greatly minimized via thermoforming under high pressure (3 MPa). The formation of wrinkles is a common drawback in the manufacturing of composites and the specific properties brought by vitrimers such as reshapability could offer a long way solution to this issue.
4.6. Cellulose-based reinforcements
The crystalline regions extracted from cellulose microfibrils, mainly by strong acid hydrolysis at elevated temperatures, are called cellulose nanocrystals (CNCs). CNCs possess the properties of high aspect ratio, high surface area and high mechanical strength making them low-cost, sustainable and eco-friendly materials for various applications.These CNCs were recently used in a novel concept for converting permanently crosslinked networks of thermoset polymers into dynamic exchangeable networks, called “vitrimerization”.80 The concept is based on a planetary ball mill processing of thermosets with are mechanochemically ground with a catalyst allowing recycling and reprocessing. The hydroxyl functions at the interface achieve proper dynamic exchange transforming the initial permanently crosslinked network into a vitrimer.
Yue et al. demonstrated that introducing CNCs as a feedstock of external hydroxyl groups into the mechanochemical vitrimerization process could improve the exchange reaction rate as well as the thermomechanical properties of the epoxy vitrimer.81 After being processed, the new epoxy vitrimer exhibited typical features of vitrimer polymers such as network topology rearrangement. Network reforming and property recovery are based on the transesterification exchange reaction-induced welding. In addition to the enhanced transesterification exchange reactions, the bio-based CNCs allowed better thermomechanical properties of the nanocomposites but also mechanical repair and recycling (Fig. 15). Instead of synthesizing recyclable vitrimers, this method enables the fabrication of vitrimer polymers from thermoset wastes and could be suitable for industrial applications.81
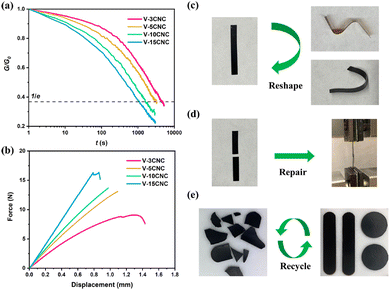 | ||
| Fig. 15 (a) Stress relaxation curves of reprocessed epoxy/CNC nanocomposites at 200 °C. (b) Force–displacement curves (lap-shear test) of repaired epoxy/CNC nanocomposites after 30 min of welding at 200 °C. (c) Reshaping of V-15CNC subjected to different deformations at 200 °C for 10 min. (d) Repairing of broken epoxy/CNC nanocomposites after 30 min of welding at 200 °C. (e) Recycling of epoxy/CNC. Reproduced with permission from ref. 81. Copyright 2021 American Chemical Society. | ||
Regenerated cellulose (RC) was also reported as a reinforcement to improve the mechanical properties of the vitrimer composites (tensile, flexural and impact strengths, scratch healing and reprocessability).82 Cellulose paper was used in combination with vitrimers introducing a series of properties such as shape-memory, reshaping, self-healing, and reprocessing.83
4.7. Silica and aluminium-oxide-based fillers
In addition to fibres, typical fillers such as silica (SiO2) or aluminium oxide (Al2O3) were implemented. This is particularly true for elastomers and they were extensively studied as one of the first reinforcements in vitrimers. The influence of silica nanoparticles (NPs) on the mechanical and viscoelastic properties of vitrimer nanocomposites was investigated by Soulié-Ziakovic et al.84 Results showed that the silica NPs had a negative effect on stress relaxation compared to an unfilled epoxy vitrimer matrix, although it could relax completely while achieving higher tensile modulus. Later, the interactions between the filler surface and the matrix network which can influence the dynamic exchanges were investigated in polyhydroxyurethane nanocomposites.85 Similarly, the non-reactive nanofillers slowed the stress relaxation but the vitrimer nanocomposite could still fully recover its initial properties. In contrast, the insertion of reactive NPs within the vitrimer matrix resulted in faster stress relaxation compared to non-reactive NPs but with a loss of the initial properties, likely due to side-reactions between silica functionalities and the vitrimer chemistry affecting also the cross-link density.The modification of cross-link density was reported in nanocomposites in comparison to non-reinforced networks, such as the gradual decrease of stress relaxation related to the amount of added covalently bound fillers. An increase of the concentration of silica NPs induces a denser network preventing the overall macroscopic flow which decreases the stress relaxation time.86 More recently, Spiesschaert et al. demonstrated that adding silica or aluminium oxide-based fillers to polydimethylsiloxane (PDMS) vinylogous urethane vitrimers can be a good solution to control the viscoelastic properties of vitrimers.87
In another paper, surface modified silica was dynamically crosslinked in vinylogous urethane vitrimers.88 The introduction of Zn(II) ions could enhance the mechanical properties of composites significantly by sacrificial bonds. The samples have been cut with a width of 2 mm and an healing of 84% was obtained after heated at 150 °C for 30 min, while only 17% was recovered at 110 °C for 30 min. Also, vitrimers have been cut into pieces then remoulded in order to assess their reprocessability (Fig. 16).
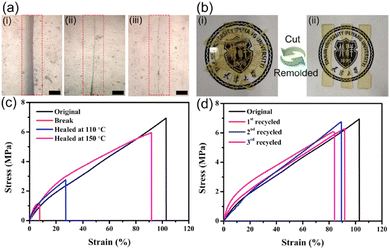 | ||
| Fig. 16 (a) Optical microscope photographs showing a crack surface (i) before healing, (ii) after healing at 110 °C for 30 min, and (iii) after healing at 150 °C for 30 min. The bar is 100 μm. (b) Photographs showing the reprocessing of a vitrimer composite. The sample was cut into pieces and reprocessed by hot-pressing (150 °C, 10 MPa) for 30 min. (c) Typical stress–strain curves of original and healed composites under different healing conditions. (d) Typical stress–strain curves of original and reprocessed composites after different cycles of reprocessing (150 °C, 10 MPa, 30 min). Reproduced with permission from ref. 88. Copyright 2020 Elsevier Ltd. | ||
Liu et al. used Al2O3 particles to produce vitrimer composites based on a phenolic resin matrix with dynamic urethane bonds promoting transcarbamoylation reactions. Al2O3 particles could be well dispersed due to hydroxyl groups located at the surface of the particles and their affinity with isocyanates contained in the cross-linking agent. Different percentages of Al2O3 increased the breaking strength and flexural strength although after breaking and hot pressed only 60% of its original flexural strength could be recovered. It did not fully recover probably because of irreversible covalent bonds forming between Al2O3 particles and the released isocyanates during the dynamic exchange. Hence, Al2O3 particles can also have a negative effect on the dynamic properties of vitrimers depending on the selected chemistry.47
4.8. POSS fillers
Polyhedral oligomeric silsesquioxane nanostructures, also known as POSS, are very promising materials for several applications from biomedical to aerospace technologies. For instance, they can be used as reinforcing agents in polymer nanocomposites so that these composites exhibit lower mass densities and greater stiffness, and are also capable of withstanding high temperatures as well as high levels of radiation. Regarding their composition, they have a cage nanostructure composed of siloxane motifs Si–O–Si with a possibility to insert functional groups inside the cage.In their paper, Hajiali et al. developed vitrimers with a relatively high biosourced content. These vitrimers are catalyst-free and based on vinylogous urethane chemistry.89 Different loadings (0, 5, 10 and 20 wt%) of NH2-functionalized POSS were incorporated and mechanical and thermal analyses of these nanocomposites showed that POSS reinforcement improved the tensile modulus and strength but also the degradation temperature compared to the parent vitrimer (Fig. 17).
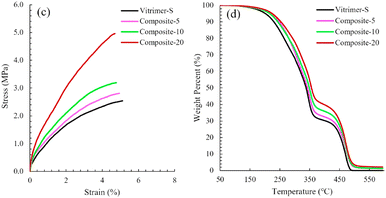 | ||
| Fig. 17 (c) Tensile stress–strain curves and (d) TGA curves of Vitrimer-S and nanocomposites. Reproduced with permission from ref. 89. Copyright 2020 Elsevier Ltd. | ||
In addition, POSS reinforcement slowed down the relaxation rate and increased the apparent activation energy of stress relaxation. Finally, they demonstrated the recyclability and reprocessability of their vitrimers by hot pressing at 125 °C. Then, they showed that the vitrimers studied and the nanocomposites containing vinylogous urethane cross-linking networks could be un-cross-linked by dissolving in excess monofunctional amine at 65 °C or recycled by grinding and remoulding at 125 °C without compromising the mechanical properties.
These catalyst-free vinylogous urethane bio-based vitrimers and nanocomposites have potential applications in coatings and insulating materials. However, in order to further improve the mechanical properties of their materials in the future, the authors could target higher Tgs for the synthesized polymer as well as using multifunctional amines or multifunctional POSS in the vitrimer.
More recently, silicon elastomer vitrimer composites for thermal conductivity were investigated, also using POSS. This silicon vitrimer using 4AFD as the cross-linking agent was used with octaglycidyl POSS to increase the mechanical properties and functionalized boron nitride nanosheets (fBNNS) to enhance thermal conductivity. The repairing efficiency was slightly reduced with the addition of 66 wt% of fBNNS but had no negative effect on the thermal conductivity even after 6 healing cycles. The main application for these materials could be a thermal interface for electronic devices.90
5. Emerging actions towards industry
Many laboratories across the world focused their research activities around vitrimers since their discovery one decade ago. Nevertheless, researchers are just at the start of a new age on vitrimer composites, many aspects of which still remain to be discovered.Over the years, vitrimer composites have awoken an increasing interest in industry; this trend is reflected in a growing number of industrial R&D activities, public–private research partnerships and start-up companies. In the following sections, the dynamism of this emerging sector will be illustrated with the activities of two start-up companies and several EU-funded projects.
5.1. 2 start-ups: ATSP Innovations and Mallinda
ATSP Innovations was founded in 2010 at the University of Illinois (UIUC) to design and produce new resin systems for demanding applications in severe thermal, mechanical, and tribological environments. They commercialized the first discovered vitrimers based on aromatic thermosetting copolyesters that was reported by James Economy's group at UIUC already in the 1990s.91,92These new so-called Aromatic Thermosetting coPolyesters (ATSP) Estherm™ resins combine many benefits such as high modulus (>3 GPa), high working temperature (Tg > 250 °C), low moisture pickup, and non-flammability, among others.93 The dynamic exchanges of this vitrimer are based on transesterification reactions. Estherm™ can be bought in different forms such as resin, bulk sheets or composite laminates (Fig. 18).
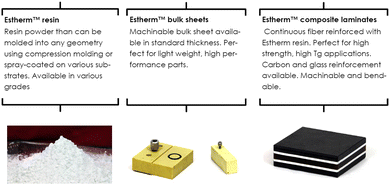 | ||
| Fig. 18 Various available forms of Estherm™.94 | ||
The vitrimers developed by ATSP Innovations are focused on structural composite applications ranging from launch vehicles to commercial automobiles, as well as rigid structural foams, ablative material for re-entry vehicles (space applications) and fast-bonding high temperature adhesives.94,95
Mallinda was created in 2014 at the University of Colorado (CU-Boulder). The company develops vitrimer formulations based on dynamic imine chemistry with Tgs ranging from 20 °C up to 240 °C. They launched two vitrimer resins named VITRIMAX™ T100 and T130 (corresponding to Tgs of 100 and 130 °C, respectively).96 As the imine chemistry is based on a condensation reaction, Mallinda circumvented this issue by formulating NH2-terminated oligomers with embedded imines used as the dynamic hardener. This strategy is an effective technical solution to prevent the release of water during network formation enabling the formation of porosity-free networks. Mallinda's patented97,98 vitrimer resin platform is used to manufacture vitrimer composites and eliminates the slow infusion and long curing cycles of today's resins which enables compression-moulding of products in a few minutes for high-throughput and high-volume production of structural composites. Additionally, their prepreg is pre-cured, shelf-stable and therefore does not require any refrigeration during transport or storage. This platform is designed for fibre reinforced vitrimer production using rapid compression moulding techniques with similarities to sheet metal stamping (Fig. 19).
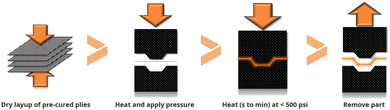 | ||
| Fig. 19 Pre-cured vitrimer matrix composite in mould consolidation cycle.99 | ||
Another advantage of Mallinda's resin is that it can be recovered using reagents utilized in the resin synthesis although this requires an isolation step to enable full reusability. In addition, the vitrimer matrix may also be ground into a powder and reformed into new shapes under heat and pressure (Fig. 20).
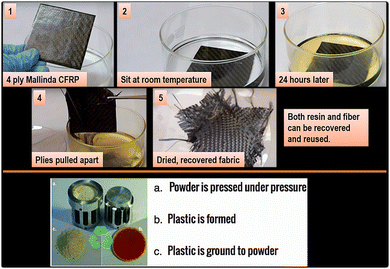 | ||
| Fig. 20 Recycling of Mallinda's CFRP vitrimer.99 | ||
These recycling methods increase the input lifetime recycling and reduce the energy required during reprocessing. Finally, Mallinda's ability to implement solid state thermoforming could really improve the production of powder-based composite materials.96,99,100
5.2. EU-funded projects
AIRPOXY (2018-2022) is a European collaborative project (11 partners in 6 countries) funded by the EU Framework Programme for Research and Innovation. AIRPOXY stands for thermoformable, repairable and bondable smart epoxy-based composites for aero structures. The aim is to replace epoxy thermoset matrices currently used in composites with vitrimer matrices in order to make the new composites Reprocessable, Reparable and Recyclable (3R). CIDETEC, a Basque private non-profit organization for applied research which developed and patented101–104 aromatic disulfide dynamic crosslinkers (e.g. 4AFD), acts as the coordinator of the project.The primary objectives for the new generation of 3R epoxy vitrimer composites are:
– high production rates thanks to the new 3R-thermoforming processes
– easy cost-efficient repairing methods for damaged or rejected composite parts
– reprocessability and recyclability in order to achieve true sustainability
Their thermoforming processes should reduce the manufacturing cost of CFRP parts by 35% compared to autoclave manufacturing and also reduce processing times from hours to minutes thanks to the dynamic chemistry of vitrimers.105,106 Earlier this year, Builes Cárdenas et al. reported the study of a low viscosity epoxy resin/aromatic disulfide hardener formulation especially designed for aeronautics and RTM processes. The report was mainly technical and the mechanical properties of the CFR epoxy vitrimer presented were comparable to those of reference CFR epoxy thermosets.107
ECOXY (2017–2020, Fig. 21)108 was a consortium of 13 partners from 8 different European countries that was formed to develop new thermosets and sustainable materials. The project was coordinated by CITEDEC and thus based on disulfide exchanges. The objectives were manifold and oriented towards 3R bio-based materials for construction and transportation industries. The main objectives were the following:
 | ||
| Fig. 21 AIRPOXY 3R resin.106 | ||
– development of bio-based fibres and vitrimer resins with the ambition to manufacture composites;
– evaluation of different composite manufacturing processes such as pultrusion and RTM; and
– technical, economic and environmental assessment.
The investigation has concluded that bio-based materials with competitive advantages such as repairability, reprocessability and recyclability can be prepared. The composite materials were successfully manufactured using two processes: pultrusion and wet compression moulding. The project members published numerous articles but only one dealing with composites. Flax fibres or poly(lactic acid) woven fibers were used as a reinforcement in various epoxidized vegetable oil thermosets.109
VITRIMAT (Training in VITRImers: high performance MAterials and Trainees for cutting-edge industrial applications, 2020–2024) is an ongoing project which aims at reinforcing the link of 6 academic partners – pioneers in vitrimers and advanced composite materials – with 1 national technical centre and 7 industrial partners that are world leaders in chemistry, adhesives, thermosets and composites for consumer goods, construction and automotive applications. So far, only one article related to composites has been published, referring to the use of siloxane exchange for making fibre-reinforced composites.49
VIT (polymer engineering via molecular design: embedding electrical and optical properties into VITrimers) proposes to endow vitrimers with desirable optical and electrical properties which retain their properties after recycling, fulfilling the circular economy paradigm. Coordinated by the University of Parma, the project gathers 10 academic research groups from 3 different continents (Europe, America, and Asia) and 2 highly innovative companies. The project is recent and started in September 2021 and will end in 2026.
6. Conclusions and perspectives
Owing to their numerous advantages including reprocessability, reparability and recyclability, the development of vitrimer composites is continually growing. This sudden interest opens new green perspectives for the composite industry such as crack repairing, correction of moulding defects and depolymerisation/recycling under soft conditions. These new possibilities are often investigated on a small scale and it could be interesting in the near future to prove these capabilities on a much wider scale through impact resistance tests on composite parts (and healability of the damage afterwards) or via correction of important gauche defects obtained during composite manufacturing. This will provide valuable information on the real potential of vitrimer composites from an industrial perspective. The type of process and process parameters are also key elements for composite manufacturing. Some related examples are given in this review, involving compression moulding/hot-press, RTM, pultrusion, hot-press of prepregs and ball-milling. All these processes resulted in composite materials with good characteristics, but the processing parameters were often not optimized especially in view of potential industrial prospects. Yet, this aspect is crucial as it could restrain the further development of vitrimer composites. This gap is currently being filled by emerging reports fully dedicated to the implementation of vitrimers in the industrial manufacturing process and will surely be further extended to other processes in the future. In addition, the introduction of vitrimer properties into a well-known resin may induce considerable modifications of the intrinsic parameters such as viscosity, gel time, and curing procedure, to name a few. These aspects are often overlooked or not fully applicable towards industrial manufacturing, and will be very likely the subject of future studies. Similarly, the chosen dynamic chemistry must be carefully selected to be perfectly compatible with the chemistry of curing and should be based on commercially available monomers. So far, the development of new chemistries seems no longer relevant in the context of composites and effort must be put into evaluating the limits of already reported systems. The introduction of dynamic chemistry very often decreases the thermal stability of materials, which could potentially restrain vitrimer composites from certain applications. This is especially true for composite materials exhibiting a high glass transition temperature (Tg >180 °C). In this situation, the temperature range which can be used for reprocessing/healing could be narrow and very close to the degradation temperature. Due to this limitation, the mobility and time of healing might not be enough to fully repair or reprocess a composite material. So far, most of the reports focused on DGEBA-based materials with a Tg of about 130 °C. Therefore, this limitation was never encountered so far but this will be for sure an important drawback to consider for high performance epoxy resin as for instance RTM6, widespread in aeronautical applications and with a Tg > 200 °C.110 In addition, important composite characteristics must also be considered such as water uptake, resistance to hydrolysis and creep resistance. As also discussed, the fibres or particles may positively or negatively interact with the dynamic chemistry and this should be carefully taken into consideration. In general, the introduction of particles, if non-functional or without the proper surface functionality, tends to slow down the overall macroscopic flow. This effect may represent an important drawback especially for thermo-conducting resins which can contain up to 80% filler. Finally, maintaining the properties after recycling will also represent a significant challenge in the future. Depolymerisation procedures can modify the surface functionality of fibres impairing the overall properties of composites and more specifically the matrix/fibre interactions. Repolymerisation often occurs in the presence of additional precursors changing the structure, crosslink density and thus general composite properties. For all these reasons, every system (resin + fibres + dynamic chemistry) is different and must be carefully examined independently. Despite all these drawbacks, vitrimer composites represent an important alternative toward durable and recyclable thermoset composites with expected bright future for industrial application. There are still many challenges for the researchers to explore and the successful development of market-competitive products for real-world applications will need extensive collaborative efforts from scientists and engineers in chemistry, materials science and engineering.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank IRT Saint Exupéry for funding the PhD thesis of V.S.Notes and references
- D. Ratna, in Recent Advances and Applications of Thermoset Resins, ed. D. Ratna, Elsevier, 2nd edn, 2022, pp. 371–418 Search PubMed.
- S. Utekar, V. K. Suriya, N. More and A. Rao, Compos. Part B Eng., 2021, 207, 108596 CrossRef CAS.
- M. Boulanghien, M. R’Mili, G. Bernhart, F. Berthet and Y. Soudais, in ICCM 19–19th International Conference on Composite Materials, Montreal, Canada, 2013, pp. 9087–9094.
- M. Boulanghien, M. R’Mili, G. Bernhart, F. Berthet and Y. Soudais, Adv. Mater. Sci. Eng., 2018, 2018, e8630232 Search PubMed.
- G. Oliveux, J.-L. Bailleul, A. Gillet, O. Mantaux and G. A. Leeke, Compos. Sci. Technol., 2017, 139, 99–108 CrossRef CAS.
- S. Jlassi, F. Berthet and G. Bernhart, in ECCM18 – 18th European Conference on Composite Materials, 2018, vol. hal-01862432.
- J. Zhang, V. S. Chevali, H. Wang and C.-H. Wang, Composites, Part B, 2020, 193, 108053 CrossRef CAS.
- S. Verma, B. Balasubramaniam and R. K. Gupta, Curr. Opin. Green Sustainable Chem., 2018, 13, 86–90 CrossRef.
- S. A. Mirdehghan, in Engineered Polymeric Fibrous Materials, ed. M. Latifi, Woodhead Publishing, 2021, pp. 1–58 Search PubMed.
- L. Giorgini, T. Benelli, G. Brancolini and L. Mazzocchetti, Curr. Opin. Green Sustainable Chem., 2020, 26, 100368 CrossRef.
- J. N. Hunt, K. E. Feldman, N. A. Lynd, J. Deek, L. M. Campos, J. M. Spruell, B. M. Hernandez, E. J. Kramer and C. J. Hawker, Adv. Mater., 2011, 23, 2327–2331 CrossRef CAS PubMed.
- K. Chino and M. Ashiura, Macromolecules, 2001, 34, 9201–9204 CrossRef CAS.
- Z. Tang, J. Huang, B. Guo, L. Zhang and F. Liu, Macromolecules, 2016, 49, 1781–1789 CrossRef CAS.
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643–2653 CrossRef CAS PubMed.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- Y. Cao, M. Z. Rong and M. Q. Zhang, Composites, Part A, 2021, 151, 106647 CrossRef CAS.
- W. Denissen, J. M. Winne and F. E. D. Prez, Chem. Sci., 2015, 7, 30–38 RSC.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, J. Am. Chem. Soc., 2012, 134, 7664–7667 CrossRef CAS PubMed.
- H. Zhang and X. Xu, Composites, Part A, 2017, 99, 15–22 CrossRef CAS.
- W. Denissen, G. Rivero, R. Nicolaÿ, L. Leibler, J. M. Winne and F. E. Du Prez, Adv. Funct. Mater., 2015, 25, 2451–2457 CrossRef CAS.
- J. Tellers, R. Pinalli, M. Soliman, J. Vachon and E. Dalcanale, Polym. Chem., 2019, 10, 5534–5542 RSC.
- M. M. Obadia, B. P. Mudraboyina, A. Serghei, D. Montarnal and E. Drockenmuller, J. Am. Chem. Soc., 2015, 137, 6078–6083 CrossRef CAS PubMed.
- B. Hendriks, J. Waelkens, J. M. Winne and F. E. Du Prez, ACS Macro Lett., 2017, 6, 930–934 CrossRef CAS PubMed.
- I. Azcune and I. Odriozola, Eur. Polym. J., 2016, 84, 147–160 CrossRef CAS.
- M. Chen, L. Zhou, Y. Wu, X. Zhao and Y. Zhang, ACS Macro Lett., 2019, 255–260 CrossRef PubMed.
- A. Chao, I. Negulescu and D. Zhang, Macromolecules, 2016, 49, 6277–6284 CrossRef CAS.
- S. Zhao and M. M. Abu-Omar, Macromolecules, 2018, 51, 9816–9824 CrossRef CAS.
- N. J. Van Zee and R. Nicolaÿ, Prog. Polym. Sci., 2020, 104, 101233 CrossRef CAS.
- W. Alabiso and S. Schlögl, Polymers, 2020, 12, 1660 CrossRef CAS PubMed.
- J. Zheng, Z. M. Png, S. H. Ng, G. X. Tham, E. Ye, S. S. Goh, X. J. Loh and Z. Li, Mater. Today, 2021, 51, 586–625 CrossRef CAS.
- Y. Zhang, L. Zhang, G. Yang, Y. Yao, X. Wei, T. Pan, J. Wu, M. Tian and P. Yin, J. Mater. Sci. Technol., 2021, 92, 75–87 CrossRef CAS.
- Y. Yang, Y. Xu, Y. Ji and Y. Wei, Prog. Mater. Sci., 2020, 100710 Search PubMed.
- Q.-A. Poutrel, J. J. Blaker, C. Soutis, F. Tournilhac and M. Gresil, Polym. Chem., 2020, 11, 5327–5338 RSC.
- M. Capelot, M. M. Unterlass, F. Tournilhac and L. Leibler, ACS Macro Lett., 2012, 1, 789–792 CrossRef CAS PubMed.
- J. L. Self, N. D. Dolinski, M. S. Zayas, J. Read de Alaniz and C. M. Bates, ACS Macro Lett., 2018, 7, 817–821 CrossRef CAS PubMed.
- P. Taynton, K. Yu, R. K. Shoemaker, Y. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2014, 26, 3938–3942 CrossRef CAS PubMed.
- W. Denissen, M. Droesbeke, R. Nicolay, L. Leibler, J. M. Winne and F. E. Du Prez, Nat. Commun., 2017, 8, 14857 CrossRef CAS PubMed.
- C. Taplan, M. Guerre, J. M. Winne and F. E. D. Prez, Mater. Horiz., 2020, 7, 104–110 RSC.
- Y. Spiesschaert, J. Danneels, N. Van Herck, M. Guerre, G. Acke, J. Winne and F. Du Prez, Macromolecules, 2021, 54, 7931–7942 CrossRef CAS.
- Z. Q. Lei, H. P. Xiang, Y. J. Yuan, M. Z. Rong and M. Q. Zhang, Chem. Mater., 2014, 26, 2038–2046 CrossRef CAS.
- A. Rekondo, R. Martin, A. Ruiz de Luzuriaga, G. Cabanero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC.
- A. R. de Luzuriaga, R. Martin, N. Markaide, A. Rekondo, G. Cabañero, J. Rodríguez and I. Odriozola, Mater. Horiz., 2016, 3, 241–247 RSC.
- I. Azcune, A. Huegun, A. Ruiz de Luzuriaga, E. Saiz and A. Rekondo, Eur. Polym. J., 2021, 148, 110362 CrossRef CAS.
- J. M. Matxain, J. M. Asua and F. Ruipérez, Phys. Chem. Chem. Phys., 2016, 18, 1758–1770 RSC.
- P. Yan, W. Zhao, X. Fu, Z. Liu, W. Kong, C. Zhou and J. Lei, RSC Adv., 2017, 7, 26858–26866 RSC.
- X. Liu, Y. Li, X. Xing, G. Zhang and X. Jing, Polymer, 2021, 229, 124022 CrossRef CAS.
- Y. Nishimura, J. Chung, H. Muradyan and Z. Guan, J. Am. Chem. Soc., 2017, 139, 14881–14884 CrossRef CAS PubMed.
- T. Debsharma, V. Amfilochiou, A. A. Wróblewska, I. De Baere, W. Van Paepegem and F. E. Du Prez, J. Am. Chem. Soc., 2022, 144, 12280–12289 CrossRef CAS PubMed.
- I. Aranberri, M. Landa, E. Elorza, A. M. Salaberria and A. Rekondo, Polym. Test., 2021, 93, 106931 CrossRef CAS.
- H. Si, L. Zhou, Y. Wu, L. Song, M. Kang, X. Zhao and M. Chen, Composites, Part B, 2020, 199, 108278 CrossRef CAS.
- L. Yu, C. Zhu, X.-H. Sun, J. Salter, H. Wu, Y. Jin, W. Zhang and R. Long, ACS Appl. Polym. Mater., 2019, 1, 2535–2542 CrossRef CAS.
- H. Li, B. Zhang, K. Yu, C. Yuan, C. Zhou, M. L. Dunn, H. J. Qi, Q. Shi, Q.-H. Wei, J. Liu and Q. Ge, Soft Matter, 2020, 16, 1668–1677 RSC.
- S. Weidmann, P. Volk, P. Mitschang and N. Markaide, Composites, Part A, 2022, 154, 106791 CrossRef CAS.
- H. Memon, Y. Wei, L. Zhang, Q. Jiang and W. Liu, Compos. Sci. Technol., 2020, 199, 108314 CrossRef CAS.
- K. Tangthana-umrung and M. Gresil, Compos. Commun., 2022, 32, 101182 CrossRef.
- T. Vidil and A. Llevot, Macromol. Chem. Phys., 2022, 2100494 CrossRef CAS.
- S. Wang, S. Ma, Q. Li, X. Xu, B. Wang, W. Yuan, S. Zhou, S. You and J. Zhu, Green Chem., 2019, 21, 1484–1497 RSC.
- Y.-Y. Liu, J. He, Y.-D. Li, X.-L. Zhao and J.-B. Zeng, Compos. Commun., 2020, 22, 100445 CrossRef.
- Y.-Y. Liu, G.-L. Liu, Y.-D. Li, Y. Weng and J.-B. Zeng, ACS Sustainable Chem. Eng., 2021, 9, 4638–4647 CrossRef CAS.
- K. Yu, Q. Shi, M. L. Dunn, T. Wang and H. J. Qi, Adv. Funct. Mater., 2016, 26, 6098–6106 CrossRef CAS.
- X. Kuang, Y. Zhou, Q. Shi, T. Wang and H. J. Qi, ACS Sustainable Chem. Eng., 2018, 6, 9189–9197 CrossRef CAS.
- Y. Liu, B. Wang, S. Ma, T. Yu, X. Xu, Q. Li, S. Wang, Y. Han, Z. Yu and J. Zhu, Composites, Part B, 2021, 211, 108654 CrossRef CAS.
- Y. Yang, Z. Pei, X. Zhang, L. Tao, Y. Wei and Y. Ji, Chem. Sci., 2014, 5, 3486–3492 RSC.
- J. Zhang, Z. Lei, S. Luo, Y. Jin, L. Qiu and W. Zhang, ACS Appl. Nano Mater., 2020, 3, 4845–4850 CrossRef CAS.
- F. Zhou, Z. Guo, W. Wang, X. Lei, B. Zhang, H. Zhang and Q. Zhang, Compos. Sci. Technol., 2018, 167, 79–85 CrossRef CAS.
- Z. Guo, W. Wang, Z. Liu, Y. Xue, H. Zheng, K. Majeed, B. Zhang, F. Zhou and Q. Zhang, Polymer, 2021, 235, 124280 CrossRef CAS.
- J. Chen, H. Huang, J. Fan, Y. Wang, J. Yu, J. Zhu and Z. Hu, Front. Chem., 2019, 7, 632 CrossRef CAS PubMed.
- Y. Liu, Z. Tang, Y. Chen, S. Wu and B. Guo, Compos. Sci. Technol., 2018, 168, 214–223 CrossRef CAS.
- B. Krishnakumar, R. V. S. Prasanna Sanka, W. H. Binder, C. Park, J. Jung, V. Parthasarthy, S. Rana and G. J. Yun, Composites, Part B, 2020, 184, 107647 CrossRef CAS.
- C. Park, G. Kim, J. Jung, B. Krishnakumar, S. Rana and G. J. Yun, Polymer, 2020, 206, 122862 CrossRef CAS.
- Z. Niu, R. Wu, Y. Yang, L. Huang, W. Fan, Q. Dai, L. Cui, J. He and C. Bai, Polymer, 2021, 228, 123864 CrossRef CAS.
- B. Krishnakumar, D. Bose, M. Singh, R. V. S. P. Sanka, V. V. S. S. Gurunadh, S. Singhal, V. Parthasarthy, L. Guadagno, P. Vijayan P, S. Thomas and S. Rana, Int. J. Polym. Sci., 2021, 2021, e5561755 Search PubMed.
- S. Wang, S. Ma, L. Cao, Q. Li, Q. Ji, J. Huang, N. Lu, X. Xu, Y. Liu and J. Zhu, J. Mater. Chem. C, 2020, 8, 11681–11686 RSC.
- Q.-A. Poutrel, Y. Baghdadi, A. Souvignet and M. Gresil, Compos. Sci. Technol., 2021, 216, 109072 CrossRef CAS.
- E. Chabert, J. Vial, J.-P. Cauchois, M. Mihaluta and F. Tournilhac, Soft Matter, 2016, 12, 4838–4845 RSC.
- W. Denissen, I. De Baere, W. Van Paepegem, L. Leibler, J. Winne and F. E. Du Prez, Macromolecules, 2018, 51, 2054–2064 CrossRef CAS.
- J. C. Markwart, A. Battig, T. Urbaniak, K. Haag, K. Koschek, B. Schartel and F. R. Wurm, Polym. Chem., 2020, 11, 4933–4941 RSC.
- W. Post, A. Cohades, V. Michaud, S. van der Zwaag and S. J. Garcia, Compos. Sci. Technol., 2017, 152, 85–93 CrossRef CAS.
- L. Yue, H. Guo, A. Kennedy, A. Patel, X. Gong, T. Ju, T. Gray and I. Manas-Zloczower, ACS Macro Lett., 2020, 9, 836–842 CrossRef CAS PubMed.
- L. Yue, M. Amirkhosravi, K. Ke, T. G. Gray and I. Manas-Zloczower, ACS Appl. Mater. Interfaces, 2021, 13, 3419–3425 CrossRef CAS PubMed.
- B. Huang, H. He, A. Dufresne, X. He and S. Wang, Ind. Crops Prod., 2021, 170, 113804 CrossRef CAS.
- W. Zhao, Z. Feng, Z. Liang, Y. Lv, F. Xiang, C. Xiong, C. Duan, L. Dai and Y. Ni, ACS Appl. Mater. Interfaces, 2019, 11, 36090–36099 CrossRef CAS PubMed.
- A. Legrand and C. Soulié-Ziakovic, Macromolecules, 2016, 49, 5893–5902 CrossRef CAS.
- X. Chen, L. Li, T. Wei, D. C. Venerus and J. M. Torkelson, ACS Appl. Mater. Interfaces, 2019, 11, 2398–2407 CrossRef CAS PubMed.
- Y. Liu, Z. Tang, Y. Chen, C. Zhang and B. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 2992–3001 CrossRef CAS PubMed.
- Y. Spiesschaert, M. Guerre, L. Imbernon, J. M. Winne and F. Du Prez, Polymer, 2019, 172, 239–246 CrossRef CAS.
- L. Bai and J. Zheng, Compos. Sci. Technol., 2020, 190, 108062 CrossRef CAS.
- F. Hajiali, S. Tajbakhsh and M. Marić, Polymer, 2021, 212, 123126 CrossRef CAS.
- C. Yue, L. Zhao, L. Guan, X. Zhang, C. Qu, D. Wang and L. Weng, J. Colloid Interface Sci., 2022, 620, 273–283 CrossRef CAS PubMed.
- D. Frich, K. Goranov, L. Schneggenburger and J. Economy, Macromolecules, 1996, 29, 7734–7739 CrossRef CAS.
- P. A. O’Connell and G. B. McKenna, Polym. Eng. Sci., 1997, 37, 1485–1495 CrossRef.
- J. L. Meyer, M. Bakir, P. Lan, J. Economy, I. Jasiuk, G. Bonhomme and A. A. Polycarpou, Macromol. Mater. Eng., 2019, 304, 1800647 CrossRef.
- ATSP, https://www.atspinnovations.com/, accessed 30 August 2021.
- J. L. Meyer, Z. Parkar and P. Lan, Reinf. Plast., 2021, 65, 190–194 CrossRef.
- D. A. Kissounko, P. Taynton and C. Kaffer, Reinf. Plast., 2018, 62, 162–166 CrossRef.
- W. Zhang and P. Taynton, WO2015138804 (A1), Univerity of Colorado a Body Corporate, 2015.
- P. Taynton, Y. Luo, H. Rubin, D. Kissounko, S. Loob and S. Sadowski, Mallinda, WO2020051506 (A1), 2020 Search PubMed.
- ABOUT|Mallinda.com|United States, https://www.mallinda.com/the-technology, accessed 30 August 2021.
- P. Taynton, H. Ni, C. Zhu, K. Yu, S. Loob, Y. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2016, 28, 2904–2909 CrossRef CAS PubMed.
- I. Odriozola, A. Rekondo, R. Martin and G. Cabanero, WO2013127989 (A1), Fundacion Cidetec, 2013.
- I. Odriozola, A. Rekondo, R. Martin, D. Ruiz, G. Cabanero and H.-J. Grande, WO2015127981 (A1), Fundación Cidetec, 2015.
- I. Odriozola, D. Ruiz, A. Rekondo, R. Martin, N. Markaide, G. Cabanero and H.-J. Grande, WO2015181054 (A1), Fundación Cidetec, 2015.
- I. Odriozola, D. Ruiz, A. Rekondo, R. Martin, G. Cabanero and H.-J. Grande, WO2016046135 (A1), Fundación Cidetec, 2016.
- Cidetec, AIRPOXY|Cidetec, https://www.cidetec.es/en/projects/surface-engineering-6/airpoxy-2, accessed 2 September, 2021.
- AIRPOXY – Smart Epoxy-based Composites for Aeronautics, https://www.airpoxy.eu/, accessed 2 September 2021.
- C. Builes Cárdenas, V. Gayraud, M. E. Rodriguez, J. Costa, A. M. Salaberria, A. Ruiz de Luzuriaga, N. Markaide, P. Dasan Keeryadath and D. Calderón Zapatería, Polymers, 2022, 14, 1223 CrossRef PubMed.
- Ecoxy Project – Biobased, reciclable, reshapable & repairable fiber, https://ecoxy.eu/, (accessed 3 June 2022).
- C. Di Mauro, A. Genua, M. Rymarczyk, C. Dobbels, S. Malburet, A. Graillot and A. Mija, Compos. Sci. Technol., 2021, 205, 108678 CrossRef CAS.
- A. Ruiz de Luzuriaga, N. Markaide, A. M. Salaberria, I. Azcune, A. Rekondo and H. J. Grande, Polymers, 2022, 14, 3180 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |