Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The anti-diarrhea activity of red algae-originated sulphated polysaccharides on ETEC-K88 infected mice
Bo Liu,
Qing-Mei Liu,
Gui-Ling Li,
Le-Chang Sun,
Yuan-Yuan Gao,
Ya-Fen Zhang,
Hong Liu,
Min-Jie Cao and
Guang-Ming Liu *
*
College of Food and Biological Engineering, Xiamen Key Laboratory of Marine Functional Food, Fujian Provincial Engineering Technology Research Center of Marine Functional Food, Fujian Collaborative Innovation Center for Exploitation and Utilization of Marine Biological Resources, Jimei University, 43 Yindou Road, Xiamen 361021, Fujian, P. R. China. E-mail: gmliu@jmu.edu.cn; Fax: +86-592-6180470; Tel: +86-592-6180378
First published on 18th January 2019
Abstract
Polysaccharides from red algae Porphyra haitanensis and Gracilaria lemaneiformis possess various bioactive functions, however, their anti-diarrhea activity remains incompletely defined. In the current study, sulphated polysaccharides were extracted by high pressure treatment plus ethanol precipitation from these two algae, and named PHSP(hp) and GLSP(hp), respectively. PHSP(hp) and GLSP(hp) showed decreased viscosity and molecular weight. Meanwhile, they have a certain immunomodulatory effect on wound healing and migration of RAW264.7 cells. Moreover, they significantly increased the secretion of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). A BALB/c model infected by enterotoxigenic Escherichia coli (ETEC)-K88 was also established to evaluate the anti-diarrhea activity of PHSP(hp) and GLSP(hp). The results showed that PHSP(hp) and GLSP(hp) were able to alleviate mice diarrhea symptoms. Meanwhile, they inhibited the release of pro-inflammatory cytokines and suppressed the secretion of immunoglobulin A via reducing the population of B cells. In addition, the nitroblue tetrazolium levels of mouse serum were decreased. Taken together, PHSP(hp) and GLSP(hp) alleviated the inflammatory response of ETEC-K88-induced diarrhea through both specific and non-specific immunity. Sulphated polysaccharides from red algae may be used as functional food components for remitting diarrhea.
Introduction
Diarrhea is a digestive disorder disease generally characterized by abnormally frequent defaecation (three times or more per day), and/or blood and poorly fecal matter in feces, as well as electrolyte imbalance.1 According to epidemiological analysis, diarrhea is one of the most common diseases with global incidence greater than one billion per year.2 Although nowadays its mortality rate has been reduced due to improved medical care, it still remained the second leading lethal cause for children under age five, particularly in the developing countries, as reported in 2012.3,4 Among various types of infectious diarrhea, bacterial infection can cause a cute diarrhea which is characterized by fever, vomiting, abdominal pain, nausea and anorexia.5 The most common bacterial dysentery pathogens include Escherichia coli, Salmonella, Shigella and Staphylococcus aureus.6 Some strains of E. coli cause infections in the gastrointestinal tract system, while others may lead to non-gastrointestinal infections such as bacteremia, hospital pneumonia and neonatal meningitis.7The enterotoxigenic Escherichia coli (ETEC) expresses one or more fimbrial adhesins, including F4 (K88), F5 (K99), F6 (987P), F7 (F41) and F18, that tightly bind to glycoprotein receptors on the enterocyte brush border to enable bacteria colonization in the small intestine. The bacteria then secrete heat-labile enterotoxins and/or heat-stable enterotoxins that alter the tight junction integrity and disrupt the paracellular passages of ions, solutes and water, leading to diarrhea.8–10
Current main therapies for diarrhea consist of oral rehydration solutions (ORS) and medications such as antibiotics. ORS is the first-line treatment for diarrhea worldwide, however, it is unable to reduce diarrhea's duration and severity.11 Antibiotic therapy was considered as one of the most effective diarrhea treatments in the past. However, it might develop antibiotic resistance in bacteria, which counteracts diarrhea treatment.11 Up to date, there has been no vaccine available against ETEC-induced diarrhea of children or travelers. Therefore treatment with bioactive molecule from marine resource with therapeutic potential for infectious diarrhea has gained growing research interest.12
Among bioactive components, the polysaccharides from seaweed are of special interest due to their great biological activities. It has been reported that sulphated polysaccharides from red algae have different biological activities, such as anti-inflammatory,13 anti-microbial,14,15 antiviral,16 anti-cancer,17 and antioxidant18 bioactivities. Porphyra haitanensis (P. haitanensis) and Gracilaria lemaneiformis (G. lemaneiformis) are the two major species of red algae cultured in China, with annual product of 115![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 and 270
875 and 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 tons, respectively in 2016.19 Our previous work showed that sulphated polysaccharides extracted from P. haitanensis and G. lemaneiformis exhibited a certain anti-allergic activity.20,21 However, the anti-diarrhea activity of red algae-originated sulphated polysaccharides is rarely reported.
149 tons, respectively in 2016.19 Our previous work showed that sulphated polysaccharides extracted from P. haitanensis and G. lemaneiformis exhibited a certain anti-allergic activity.20,21 However, the anti-diarrhea activity of red algae-originated sulphated polysaccharides is rarely reported.
This study aims to investigate the immunomodulatory and anti-diarrhea activity of sulphated polysaccharides isolated from P. haitanensis and G. lemaneiformis in RAW264.7 cells and a mouse model with ETEC-K88-induced diarrhea. The present research may provide insights into future exploration for anti-diarrhea functional foods and/or their feed.
Materials and methods
Reagents
Fetal bovine serum (FBS) was purchased from Gibco (Grand Island, NY, USA). DMEM was obtained from HyClone Co. (Logan, UT, USA). The enzyme-linked immunosorbent assay (ELISA) kits for immunoglobulin A (IgA), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-6 (IL-6) and monocyte chemotactic protein 1 (MCP-1) were purchased from R&D Systems (Minneapolis, MN, USA). The antibodies, including APC-conjugated CD3, PerCP-Cy5.5-conjugated CD4 and PE-conjugated CD19, were obtained from Biolegend (San Diego, CA, USA). Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and are all analytical grade.Extraction of the polysaccharides from red algae
The red algae P. haitanensis and G. lemaneiformis were purchased from a local market of Dadeng Island (Xiamen, Fujian, China). Polysaccharide extraction was performed according to the method of Shi et al. with slight modifications.20 Briefly, the grated dry algae tissue (10 g) is mixed with 400 mL of distilled water and autoclaved under high pressure at 121 °C for 5 h. Being filtered with a nylon membrane, the polysaccharides were precipitated with 4 volumes of ethanol and kept at 4 °C for 24 h. The filtrate was filtered again with a nylon membrane, naturally dried at room temperature, and re-dissolved in a small amount of water followed by freeze-dry under −50 °C. The sulphated polysaccharides extracted from P. haitanensis and G. lemaneiformis with high pressure were named PHSP(hp) and GLSP(hp), respectively. The yield of polysaccharides was calculated as the following formula: polysaccharides recovery rate (%) = mass (g) of dried material obtained/mass (g) of original dried microalgae × 100.Characterization of the polysaccharide
The viscosity of polysaccharide was determined using an Ubbelohde viscometer. Protein content of the polysaccharide fraction was measured by the Bradford method as previously described.22 The total carbohydrate and sulfate content in PHSP(hp) and GLSP(hp) were determined as previously described.20 The molecular weight distribution of polysaccharides was determined by high performance gel-permeation chromatography (HPGPC) (Agilent-1100, Santa Clara, U.S.A.) equipped with a ZORBAX Eclipse XDB-C18 column (250 × 4.6 mm2, column temperature 30 °C). The Fourier transform infrared spectra of polysaccharide was analyzed with a Fourier transformed infrared spectrometer (FT-IR) (Vector-22, Bruker, Switzerland) in the wave number range of 4000–400 cm−1 using the KBr-disk method.RAW264.7 cell culture, wound healing assay and transwell migration assay
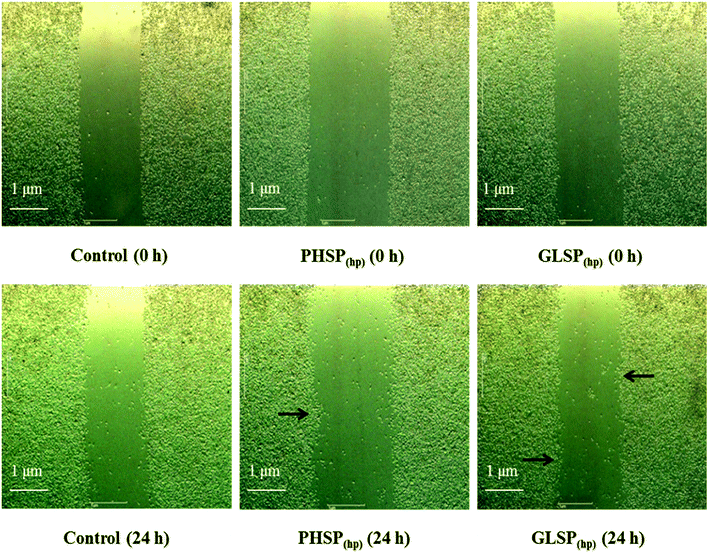
The murine monocyte-macrophage cell line RAW264.7 from Wuhan Beinglay Biotech Co. (Wuhan, Hubei, China) was grown in DMEM medium supplemented with 10% FBS, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin at 37 °C in an incubator with a humidified atmosphere of 5% CO2.The wound healing assay was carried out to investigate the immunomodulation activity of PHSP(hp) and GLSP(hp) on RAW264.7 macrophages in 6-well culture plate.23 Briefly, after the cells adhere and the density was greater than 95%, they were scratched by small tips and continued to incubate with 200 μg mL−1 PHSP(hp) or GLSP(hp) for 24 h. Images were taken at 0 h and 24 h. The degree of healing (three wells per set) at 24 h was determined as compared to that of cell at 0 h.
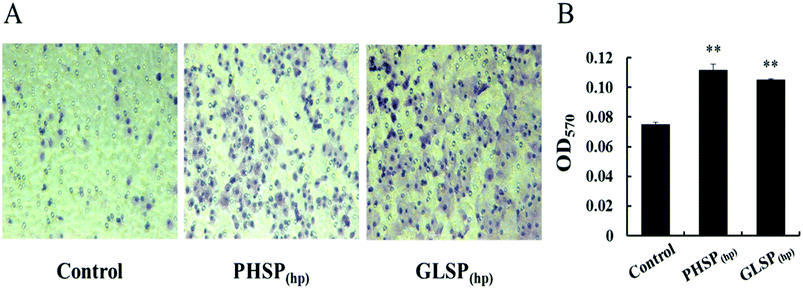
The migration assay was carried out as described before.24 RAW264.7 cells (1 × 105) were added to the upper chamber of transwell inserts (8 μm pores, 6.5 mm polycarbonate membranes, Costar, Corning, NY, USA) that were placed in the 24-well culture plate. Six hundred microliter of DMEM complete medium together with 200 μg mL−1 PHSP(hp) or GLSP(hp) was added to the lower chamber. Twenty four hours after incubation, the cells on the topside of upper chamber were removed with a cotton swab, followed by washing with PBS to remove the residual medium. Cells adherent the opposite surface of the insert was fixed with paraformaldehyde and stained with 0.1% crystal violet for 20 min, and washed 2–3 times with tap water. The cell migration was observed under microscope (three wells per group). Finally, the bound dye was released with 30% glacial acetic acid, and its optical density was measured at 570 nm using an automated ELISA plate reader.
Measurement of released TNF-α and IL-6
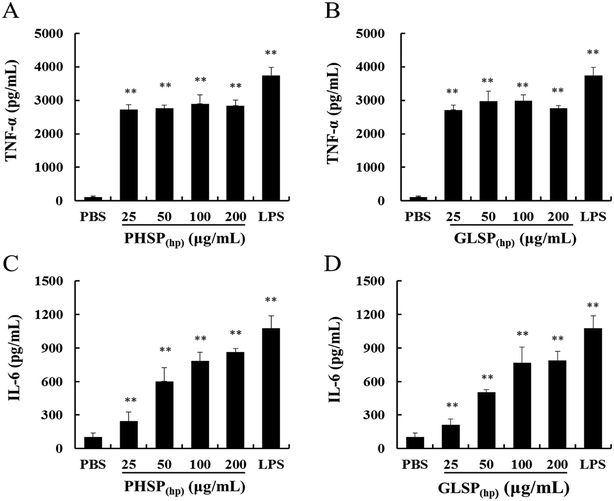
The RAW264.7 cells (1 × 105 cells per well) were seeded in 48-well plates with different concentrations of PHSP(hp) and GLSP(hp) (0, 25, 50, 100 and 200 μg mL−1) or lipopolysaccharide (LPS) (2 μg mL−1) as a positive control. After 24 h incubation, the culture supernatant was collected and assayed using the ELISA kits for TNF-α and IL-6, following the manufacturer's instructions (R&D, Minneapolis, MN, USA).Establishment of ETEC-K88 bacteria induced-diarrhea mouse model
The 6 week-old specific-pathogen-free (SPF) female BALB/c mice were purchased from the Shanghai Laboratory, Animal Center of the Chinese Academy of Sciences (Shanghai, China). Mice were maintained in a SPF environment at 22 ± 1 °C with relative 55 ± 10% humidity. All animal procedures were performed in accordance with the guidelines for care and use of Laboratory Animals of Jimei University and experiments were approved by the Animal Ethics Committee of Jimei University (Xiamen, Fujian, China), SCXK 2012-0005. Mice were fed with standard chows (Beijing Keao Biological Pharmaceutical Co., Ltd, Beijing, China) and water ad libitum throughout the experiments, except for fast prior to ETEC-K88 infection.Standard strains of the Gram-negative enterotoxigenic bacteria Escherichia coli (ETEC-K88) freeze-drying powder (bio-10666) was purchased from Beijing Biobw Biotechnology Co., Ltd. (Biobw, Beijing, China). The bacteria were grown in Luria–Bertani (LB) medium, and incubated at 37 °C with shaking for 12 h. Cultures were harvested by centrifugation at 5000g for 10 min, and the pellet was washed twice and re-suspended in sterile phosphate buffer saline (PBS). The counting of bacteria was carried out by means of inverted plate and their OD600 value.
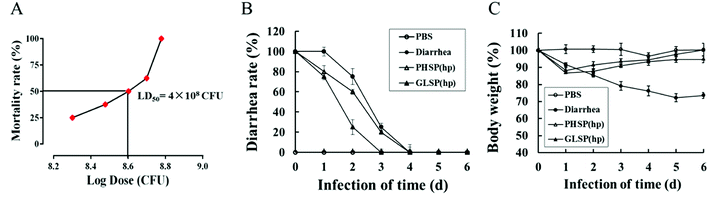
The 50% lethal dose (LD50) of ETEC-K88 was determined using a dose–response method.25 Thirty six female BALB/c mice were randomly divided into six groups. Mice were fasted for 12 h before infection, except for access to water ad libitum. The next day, mice in different groups were administrated in intraperitoneal injection with approximately 2 × 108, 3 × 108, 4 × 108, 5 × 108, 6 × 108 CFU of ETEC-K88 in PBS at a volume of 200 μL per mouse. The control group was administrated in intraperitoneal injection with sterile PBS alone. Food was then reintroduced ad libitum. Animals were observed four times per day for clinical signs and mortality for a period of 72 h. Mice that were still alive 3 days after bacterial challenge were recorded as survivors. The total number of dead mice at each dose level was recorded and the LD50 was determined from a dose lethality curve using the Prism 6 statistical software of GraphPad Prism (San Diego, CA, USA).
Forty female BALB/c mice were randomly divided into four groups, including PBS control group, diarrhea group, PHSP(hp) group and GLSP(hp) group. To induce diarrhea, the mice were injected intraperitoneally with 0.2 mL of ETEC-K88 at the level of LD50. Three hours later, two groups were orally administered PHSP(hp) and GLSP(hp) (10 mg per mice), respectively.26,27 The other two groups were orally administered PBS (200 μL per mice). After that, all doses were given once every 24 h for one week.
The animals were observed 30 min after treatment and periodically during the first 24 h, with special attention given during the first 4 h, and daily thereafter, for a total of 7 days. The symptoms of illness or abnormal behavior were recorded as Leódido described.28 Furthermore, the body weight and diarrhea rate of mice were monitored throughout the study period.
Measurement of the levels of cytokines and IgA in mouse serum
The blood samples were collected from orbital venous plexus 24 h after the last administration and centrifuged at 4000g for 10 min. The sera were obtained and the levels of MCP-1, TNF-α, IFN-γ, IL-6 and IgA were measured by ELISA according to the manufacturer's instructions.Flow cytometry analysis of T/B cell subpopulation
A small portion of the spleen was used for splenocytes preparation. The splenocytes were freed of red blood cells upon treatment with lysis buffer (Solabio Co., Beijing, China), stained with CD3-APC, CD4-PerCP-Cy5.5 and CD19-PE for 30 min at 4 °C and the T and B lymphocyte subpopulation was analyzed by flow cytometry. A Guava easyCyte 6-2L system were used for the analysis with the GuavaSoft 3.1.1 software (Millipore, MA, USA), following the manufacturer's instructions.Determination of nitroblue tetrazolium (NBT) activity
The NBT assay was performed according to Anderson and Siwicki (1995) with slight modification.29 The blood (50 μL) was transferred into a glass tube containing equal amount of 0.2% (w/v) NBT solution (prepared in PBS), or equal amount of PBS as the control. The tubes were incubated at room temperature for 30 min before addition of 1.5 mL N,N-dimethyl formamide (DMF). The tubes were then centrifuged at 3000g for 5 min at room temperature. The supernatant was collected and the sample absorbance at 540 nm was determined on an automated ELISA plate reader.Statistical analysis
The data were expressed as mean ± standard deviation (SD) and the group difference was analyzed by one-way ANOVA of Duncan test using the SPSS 17.0 software package (IBM, USA), and considered significant with P < 0.05. Regarding the animal model assays, samples from individual mouse were processed and analyzed separately. Each experiment was repeated at least 3 times.Results
Polysaccharide extraction and property analysis
Using high pressure treatment and ethanol precipitation, 4.11 ± 0.36 g and 2.77 ± 0.19 g of polysaccharides were obtained from 10 g P. haitanensis and G. lemaneiformis, respectively. The microstructures of polysaccharides were also determined under a scanning electron microscope (SEM), as show in Fig. 1A. The microstructure of PHSP(hp) has an irregular geometrical shape with a certain gap on the surface and loosely superimpose. However, the surface structure of GLSP(hp) was smooth and flat, showing a continuous sheet.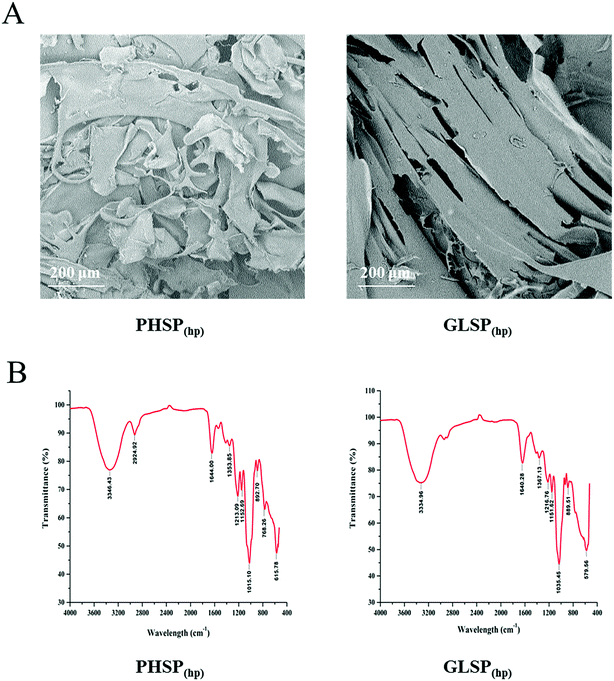 | ||
| Fig. 1 SEM and FT-IR spectrum of PHSP(hp) and GLSP(hp). (A) SEM of PHSP(hp) and GLSP(hp). (B) FT-IR spectra of PHSP(hp) and GLSP(hp). | ||
The total sugar content of the extracted substance was above 98% and few protein residues were detected in the polysaccharides fraction. As shown in Table 1, PHSP(hp) and GLSP(hp) have viscosity of 0.17 dL g−1 and 0.10 dL g−1. The average molecular weight of PHSP(hp) was 165.45 kDa, while GLSP(hp) have two components, 71.87 kDa (accounting for 96.36%) and 1641.95 kDa (3.64%). The FT-IR spectrum showed that PHSP(hp) and GLSP(hp) exhibited absorption peaks at 3330 cm−1 and 1640 cm−1, representing –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group, respectively (Fig. 1B). Furthermore, the absorption peaks at 1216 and 890 cm−1 for sulfated components were present. The sulfate content in PHSP(hp) and GLSP(hp) were further determined as 10.94% and 11.26%, respectively.
C group, respectively (Fig. 1B). Furthermore, the absorption peaks at 1216 and 890 cm−1 for sulfated components were present. The sulfate content in PHSP(hp) and GLSP(hp) were further determined as 10.94% and 11.26%, respectively.
| Physicochemical properties | PHSP(hp) | GLSP(hp) |
|---|---|---|
| a Represents the major component (>96.0%). | ||
| Total sugar (%) | 99.12 ± 0.36 | 98.85 ± 0.25 |
| Protein content (%) | 0.64 ± 0.08 | 0.39 ± 0.06 |
| Viscosity (dL g−1) | 0.17 ± 0.04 | 0.10 ± 0.02 |
| Sulfate content (%) | 10.94 ± 0.43 | 11.26 ± 0.39 |
| Average molecular weight (kDa)a | 165.45 | 71.87 |
Effects of PHSP(hp) and GLSP(hp) on RAW264.7 wound healing and migration
To investigate the effects of PHSP(hp) and GLSP(hp) on the inflammatory cells, their wound healing stimulating activity on RAW264.7 cells were evaluated using the scratch assay. Compared with the control, the number of cells in the middle wound area was increased upon treatment with PHSP(hp) or GLSP(hp) for 24 h (Fig. 2). The cells in the marginal area had a tendency to heal in the middle. Therefore PHSP(hp) and GLSP(hp) had effective healing capability for the scratched RAW264.7 cells.The influence of PHSP(hp) and GLSP(hp) on RAW264.7 migration was also determined. As shown in Fig. 3A, 200 μg mL−1 PHSP(hp) and GLSP(hp) significantly promoted the migration of RAW264.7 from the upper chamber of transwell to its bottom side. The number of migrated cells in PHSP(hp) and GLSP(hp) groups were significantly higher than that in the control group (Fig. 3A). The absorbance values (OD570) of eluted crystal violet also showed that PHSP(hp) and GLSP(hp) significantly promoted RAW264.7 migration, as shown in Fig. 3B.
Effects of PHSP(hp) and GLSP(hp) on the production of TNF-α and IL-6 in RAW264.7 cells
The inflammatory factors of RAW264.7 cells were further explored. As shown in Fig. 4, exposure of RAW264.7 cells to PHSP(hp) and GLSP(hp) for 24 h resulted in significant increases in the secretion of TNF-α and IL-6, compared to PBS. The levels of TNF-α were significantly increased to 2897.75 pg mL−1 and 2983.13 pg mL−1, respectively, in the PHSP(hp) and GLSP(hp) (100 μg mL−1) treated group (Fig. 4A and B). However, the secretion level of IL-6 increased in a dose-dependent manner in PHSP(hp) and GLSP(hp) treated RAW264.7 cells (Fig. 4C and D), which were 861.48 pg mL−1 and 788.23 pg mL−1, respectively, in 200 μg mL−1 PHSP(hp) and GLSP(hp) treated cells. These findings suggested that PHSP(hp) and GLSP(hp) regulated the immune system by enhancing the secretion of pro-inflammatory cytokines.Alleviation of PHSP(hp) and GLSP(hp) on diarrhea symptoms
To investigate the anti-diarrhea activity of PHSP(hp) and GLSP(hp) in vivo, an ETEC-K88 induced mice model were established in the present study. The study found that the ETEC-K88 at all dose caused death, and mice of the highest dose group died after two days. The LD50 of the ETEC-K88 was determined as 4 × 108 CFU in female BALB/c mice (Fig. 5A).The mice infected with ETEC-K88 (LD50) could suffer diarrhea after 30 min (data not shown), excreting dilute stool like water. Compared with the diarrhea group, the PHSP(hp) and GLSP(hp) treated mice showed reduced infectious diarrhea rate in the first four days (Fig. 5B). On day 4, all the diarrhea mice returned to normal. The weight of mice infected with ETEC-K88 was significantly reduced on the first day. In addition, the weight of diarrhea group mice decreased for 5 days in a row, while the weight of PHSP(hp) or GLSP(hp) treated mice rebounded on the second day (Fig. 5C). These results suggest that PHSP(hp) and GLSP(hp) can reduce diarrhea rate and put on weight to alleviate diarrhea symptoms.
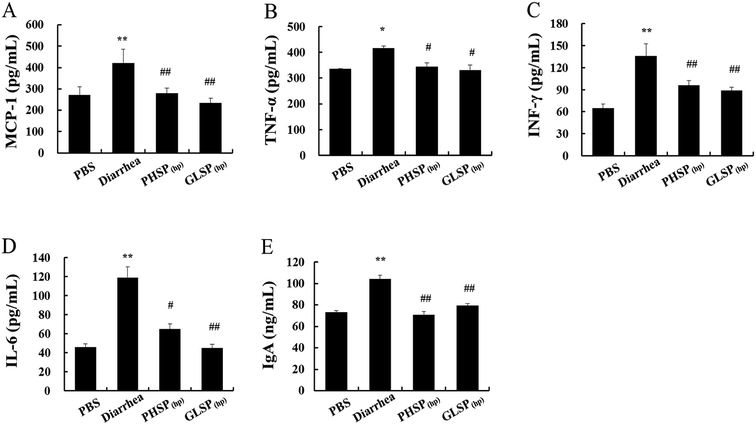
Effects of PHSP(hp) and GLSP(hp) on cytokines and IgA antibody levels in mice serum
The previous study reported that ETEC-K88-induced mice diarrhea caused inflammatory response with highly secretion of pro-inflammation mediators.30 As shown in Fig. 6A–D, the levels of pro-inflammatory cytokines (MCP-1, TNF-α, IFN-γ, IL-6) were all significantly reduced in PHSP(hp) and GLSP(hp) treated mice serum.The changes in total IgA levels in the serum in different groups were also measured and the data were shown in Fig. 6E. The serum IgA concentration was 104.21 ng mL−1 in the diarrhea group, which was significantly higher than that of the control PBS group at 73.04 ng mL−1 (P < 0.01), indicating increased inflammatory response. Moreover, serum IgA levels in the PHSP(hp) and GLSP(hp) groups were decreased to 70.54 ng mL−1 and 79.20 ng mL−1, respectively. These results suggested that PHSP(hp) and GLSP(hp) remitted inflammatory response in ETEC-K88 induced mice diarrhea.
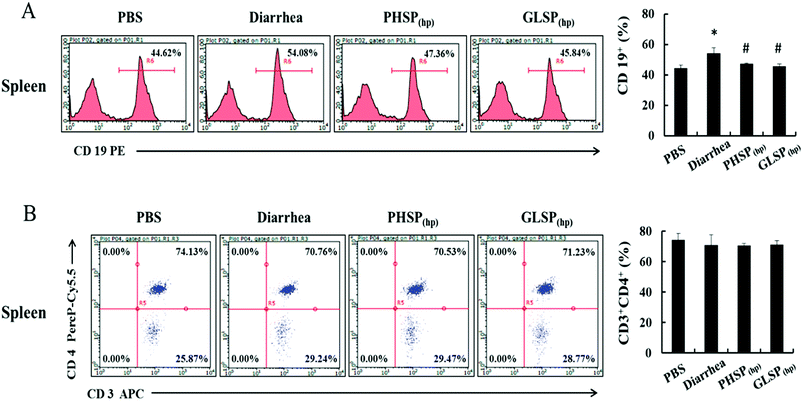
Effects of PHSP(hp) and GLSP(hp) on T/B cell subpopulation of mice splenocytes
Considering the suppression effects of PHSP(hp) and GLSP(hp) on serum IgA, the classification of mice spleen lymphocytes was further performed by flow cytometry. Compared with PBS group, the CD19+ B cells population in the diarrhea group was increased about 10% to 54.08%, indicating that the B cell subpopulation was increased upon ETEC-K88 infection (Fig. 7A). And the CD19+ B cells population were 47.36% and 45.84% in PHSP(hp) and GLSP(hp) (10 mg d−1) treatment mice spleen, respectively, suggesting that PHSP(hp) and GLSP(hp) (10 mg d−1) down-regulated B cell population. Moreover, PHSP(hp) and GLSP(hp) showed no effect on CD3+CD4+ Th cell population (Fig. 7B). Taken together, it was tempting to speculate that PHSP(hp) and GLSP(hp) reduce B cells subpopulation, which suppress IgA expression in mice serum and contribute to the anti-inflammatory activities in ETEC-K88-induced mice diarrhea.Effects of PHSP(hp) and GLSP(hp) on the NBT activity in mice serum
The NBT assay was used to determine the neutrophil activity as nonspecific immune factor.25 As shown in Table 2, compared with PBS group, the OD540 of the diarrhea group was significantly increased (P < 0.01) upon infection by ETEC-K88, which indicated the neutrophilic granulocyte function was enhanced and the nonspecific immune balance was broken. Nevertheless, the OD540 was reduced in PHSP(hp) and GLSP(hp)-treated mice serum. Thus, PHSP(hp) and GLSP(hp) may not only suppress the inflammatory response but also regulate nonspecific immunity.| Nitroblue tetrazolium activity (OD540) | |
|---|---|
| a Data are present as mean ± SD (n = 3). *P < 0.05 versus the PBS group, and **P < 0.01 versus the PBS group. #P < 0.05 versus the diarrhea group, and ##P < 0.01 versus the diarrhea group. | |
| PBS | 0.1284 ± 0.0082 |
| Diarrhea | 0.1940 ± 0.0122** |
| PHSP(hp) | 0.1757 ± 0.0165# |
| GLSP(hp) | 0.1422 ± 0.0073## |
Discussion
In this study, two sulphated polysaccharides, PHSP(hp) and GLSP(hp), were extracted from P. haitanensis and G. lemaneiformis, respectively, by high pressure treatment and ethanol precipitation. MTT assay showed that PHSP(hp) and GLSP(hp) were not cytotoxic to the normal spleen lymphocytes of mouse (P > 0.05). The infrared spectrum results showed that hot water treatment and ethanol precipitation did not change the active ingredients of these polysaccharides. PHSP(hp) and GLSP(hp) have absorption peaks at 1030 cm−1 and 890 cm−1, indicating they are typical agar type polysaccharides.31–33 The infrared spectrogram data also shows some important functional groups of the polysaccharides. The absorption peaks at approximately 3340 cm−1, 2920 cm−1 and 1640 cm−1 represent –OH, C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional groups, respectively. In addition, the peak at about 1210 cm−1 was attributed to the sulphated ester groups, and the peak at about 1020 cm−1 corresponds to galactan backbone. However, altered physicochemical properties were also observed. For example, PHSP(hp) and GLSP(hp) showed more loose structure compared with polysaccharides extracted by hot water, as monitored by SEM. This result was consistent with previous finding that the changes of viscosity upon high pressure treatments and ethanol precipitation.27 The sulphated polysaccharides extracted from P. haitanensis and G. lemaneiformis showed average molecular weight of 2960 kDa and 152 kDa,20,21 with viscosity of 1.76 dL g−1 and 0.63 dL g−1, respectively, while the viscosity of PHSP(hp) and GLSP(hp) were 0.17 dL g−1 and 0.10 dL g−1, respectively. Decreased molecular weight and viscosity of PHSP(hp) and GLSP(hp) that underwent high pressure treatment may be beneficial to the digestion and absorption in the intestine of mice.
C functional groups, respectively. In addition, the peak at about 1210 cm−1 was attributed to the sulphated ester groups, and the peak at about 1020 cm−1 corresponds to galactan backbone. However, altered physicochemical properties were also observed. For example, PHSP(hp) and GLSP(hp) showed more loose structure compared with polysaccharides extracted by hot water, as monitored by SEM. This result was consistent with previous finding that the changes of viscosity upon high pressure treatments and ethanol precipitation.27 The sulphated polysaccharides extracted from P. haitanensis and G. lemaneiformis showed average molecular weight of 2960 kDa and 152 kDa,20,21 with viscosity of 1.76 dL g−1 and 0.63 dL g−1, respectively, while the viscosity of PHSP(hp) and GLSP(hp) were 0.17 dL g−1 and 0.10 dL g−1, respectively. Decreased molecular weight and viscosity of PHSP(hp) and GLSP(hp) that underwent high pressure treatment may be beneficial to the digestion and absorption in the intestine of mice.
Macrophages are immune cells that actively participate in the inflammatory immune response by releasing inflammatory factors and pro-inflammatory cytokines.34 During inflammation, activated macrophages can secrete nitric oxide (NO) and many pro-inflammatory cytokines, such as IL-6, TNF-α and IL-1β.35 PHSP was shown to significantly increase the levels of TNF-α, IL-6, IL-10 and iNOS/NO in RAW264.7 cells.27 In this study, the immune regulation by PHSP(hp) and GLSP(hp) were demonstrated in vitro, including induced RAW264.7 cell migration, enhanced wound healing, as well as increased secretion of TNF-α and IL-6. The phagocytosis of pathogens by macrophages plays an important role in immune regulation, which involves the migration and adhesion of macrophages. PHSP(hp) and GLSP(hp) may act as chemokines to recruit macrophages and induce pathogen phagocytosis. Upon macrophage recruitment, they migrate to pathogens and sites of inflammation, and eventually exert their antibacterial effects.23
Bacteria intraperitoneal injection of has been a classic way to induce intestinal infection.36,37 To examine the anti-bacterial diarrhea activity of PHSP(hp) and GLSP(hp), BALB/c mice were infected with ETEC-K88 (LD50 = 4 × 108 CFU) and then orally supplied with PHSP(hp) and GLSP(hp). The infected mice showed severe diarrhea, piloerection, lethargy and weight loss. However, PHSP(hp) and GLSP(hp) not only reduced the total amount of mouse diarrheal stools, but also prevented the loss of weight. Moreover, the levels of pro-inflammatory factors and IgA were significantly decreased (P < 0.05) upon PHSP(hp) and GLSP(hp) treatment. The flow cytometry results showed that B cells subpopulation was significantly increased after infection, which suggested that toxin antigen-attacked mice stimulate B cells to produce antibodies to engulf pathogens. This is consistent with high IgA content in the diarrhea group. And decreased IgA levels in PHSP(hp) and GLSP(hp) treated mice may resulted from the reduced B cell population. The reduction of pro-inflammatory factors, IgA, and B cell population suggested that PHSP(hp) and GLSP(hp) show anti-diarrhea activity through specific immune regulation in mice.
The neutrophil activity was considered a nonspecific immune parameter and determined by the NBT assay.25 The current study indicated that PHSP(hp) and GLSP(hp) reduced NBT level in ETEC-K88 infected BALB/c mice. Sulphated polysaccharides may stimulate the microbicidal activity of polymorphonuclear leukocytes and leucocytosis, which involves different nonspecific immune regulation mechanisms.25 The further effects of PHSP(hp) and GLSP(hp) on these leukocytes remains to be explored later.
Binding of toxins to ganglioside (GM) is the first step in toxins-induced diarrhea, which has become an attractive drug developing target for the treatment and prophylaxis of cholera toxins.38 Many studies have shown that galactose analogues interfere with the binding of toxin to GM1.38 The basic constituents of sulphated polysaccharides extracted from P. haitanensis and G. lemaneiformis are sulphated D-galactose monomers. PHSP(hp) and GLSP(hp) can alleviate ETEC-K88 bacteria diarrhea. This may be due to that the galactose monomers of sulphated polysaccharide block the toxin binding site and interfere with the binding of LT (heat-labile toxin) toxin to GM1. ETEC subtype STbP is involved in the induction of secretary diarrhea in animals, including humans. This toxin binds to sulphatide (3′-sulphogalactosyl-ceramide) receptors containing regions distributed on the intestinal epithelium cell wall.39 Inhibition of STbP binding to its receptor was achieved by type λ-carrageenan, a polymer extracted from seaweed and consisting of sulphated galactose units.40 It is speculated that PHSP(hp) and GLSP(hp) could alleviate diarrhea by mimicking this toxin to compete with the receptor.
Studies have reported that ganglioside receptors exhibit high affinity for carbohydrates, because toxins bind to ganglioside receptors in host epithelial cells in the form of glycoproteins and exhibit greater affinity for GM1.41 However, the galactoside acted as a high-affinity inhibitor of toxins-GM1 interaction. PHSP(hp) and GLSP(hp) comprises high-molecular-weight sulfated galactans, with high sugar content; galactose accounts for the majority of the sugars. It may be able to explain the interaction of PHSP(hp) and GLSP(hp) with GM1, preventing toxins binding, which interferes in the key step of transport of the toxin into the enterocytes.
Conclusions
In this study, sulphated polysaccharides from Porphyra haitanensis (PHSP(hp)) and Gracilaria lemaneiformis (GLSP(hp)) were obtained by high pressure treatment plus ethanol precipitation, with the molecular weight of 165.45 kDa and 71.87 kDa, respectively. The wound healing and cell migration results indicated that PHSP(hp) and GLSP(hp) had immunomodulatory effects. They also alleviated diarrhea symptoms in mice via dietary intervention. Meanwhile, PHSP(hp) and GLSP(hp) inhibited the release of pro-inflammatory cytokines and IgA by reducing B cell population. In addition, they decreased nitroblue tetrazolium level in the ETEC-K88 infected mice. In summary, PHSP(hp) and GLSP(hp) were effectively against ETEC-K88-induced secretary diarrhea, probably by both specific and non-specific immunities. These findings may provide substantial support for the use of functional food as sulphated polysaccharides from red algae to alleviate diarrhea.Conflicts of interest
There are no conflicts to declare.Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| ETEC | Enterotoxigenic Escherichia coli |
| FT-IR | Fourier transformed infrared spectrometer |
| IFN-γ | Interferon-γ |
| IgA | Immunoglobulin A |
| IL-6 | Interleukin-6 |
| LD50 | 50% lethal dose |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemotactic protein 1 |
| NBT | Nitroblue tetrazolium |
| PHSP(hp) and GLSP(hp) | Sulphated polysaccharides extracted from Porphyra haitanensis and Gracilaria lemaneiformis with high pressure |
| TNF-α | Tumor necrosis factor-α |
Acknowledgements
This work was supported by the grants from the National Natural Scientific Foundation of China (31871720), the Science and Technology Program of Fujian province (2018N5009, 2018R0071), and the Marine Scientific Research Special Foundation for Public Sector Program (DY135-B2-07, 201505026-03).References
- M. M. Suleiman, T. Dzenda and C. A. Sani, Antidiarrhoeal activity of the methanol stem-bark extract of Annona senegalensis, Pers. (Annonaceae), J. Ethnopharmacol., 2008, 116, 125–130 CrossRef CAS PubMed.
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015, Lancet, 2016, 388, 1545–1602 Search PubMed.
- A. I. English, New frontiers in the development of vaccines against enterotoxinogenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections. Part II, Wkly. Epidemiol. Rec., 1999, 74, 105–111 Search PubMed.
- C. L. Walker, I. Rudan, L. Liu, H. Nair, E. Theodoratou, Z. A. Bhutta, K. L. O'Brien and H. Campbell, Global burden of childhood pneumonia and diarrhea, Lancet, 2013, 381, 1405–1416 CrossRef.
- M. Qu, Y. Deng, X. Zhang, G. Liu, Y. Huang, C. Lin, J. Li and H. Yan, Etiology of acute diarrhea due to enteropathogenic bacteria in Beijing, China, J. Infect., 2016, 65, 214–222 CrossRef PubMed.
- O. G. Gómezduarte, J. Bai and E. Newel, Detection of E. coli, Salmonella spp. Shigella spp. Yersinia enterocolitica, Vibrio cholerae, and Campylobacter spp. enteropathogens by three-reaction multiplex PCR, Diagn. Microbiol. Infect. Dis., 2009, 63, 1–9 CrossRef PubMed.
- J. B. Kaper, J. P. Nataro and H. L. Mobley, Pathogenic Escherichia coli, Nat. Rev. Microbiol., 2004, 2, 123–140 CrossRef CAS PubMed.
- R. A. Wilson and D. H. Francis, Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis, Am. J. Vet. Res., 1986, 47, 213–217 CAS.
- X. Ruan and W. Zhang, Oral immunization of a live attenuated Escherichia coli strain expressing a holotoxin-structured adhesin-toxoid fusion (1FaeG-FedF-LTA2:5LTB) protected young pigs against enterotoxigenic E. coli (ETEC) infection, Vaccine, 2013, 31, 1458–1463 CrossRef CAS PubMed.
- R. I. Walker, An assessment of enterotoxigenic Escherichia coli, and Shigella, vaccine candidates for infants and children, Vaccine, 2015, 33, 954–965 CrossRef CAS PubMed.
- J. S. Suh, W. H. Hahn and B. S. Cho, Recent advances of oral rehydration therapy (ORT), Electrolyte Blood Pressure, 2010, 8, 82–86 CrossRef PubMed.
- K. Reddy, G. K. Mohan, S. Satla and S. Gaikwad, Natural polysaccharides: versatile excipients for controlled drug delivery systems, Asian J. Pharm. Sci., 2011, 6, 275–286 Search PubMed.
- A. I. F. Vázquez, C. M. D. Sánchez, N. G. Delgado, A. M. S. Alfonso, Y. S. Ortega and H. C. Sánchez, Anti-inflammatory and analgesic activities of red seaweed Dichotomaria obtusata, Braz. J. Pharm. Sci., 2011, 47, 111–118 Search PubMed.
- S. Kumaran, B. Deivasiganiani, K. Alagappan, M. Sakthivel and R. Karthikeyan, Antibiotic resistant Esherichia coli strains from seafood and its susceptibility to seaweed extracts, Asian Pac. J. Trop. Med., 2010, 3, 977–981 CrossRef CAS.
- T. Sweeney, S. Dillon, J. Fanning, J. Egan, C. J. O'Shea and S. Figat, Evaluation of seaweed-derived polysaccharides on indices of gastrointestinal fermentation and selected populations of microbiota in newly weaned pigs challenged with Salmonella typhimurium, Anim. Feed Sci. Technol., 2011, 165, 85–94 CrossRef CAS.
- R. Bouhlal, C. Haslin, J. C. Chermann, S. Colliec-Jouault, C. Sinquin and G. Simon, Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales), Mar. Drugs, 2011, 9, 1187–1209 CrossRef CAS PubMed.
- E. Hussain, L. J. Wang, B. Jiang, S. Riaz, G. Y. Butt and D. Y. Shi, A review of the components of brown seaweeds as potential candidates in cancer therapy, RSC Adv., 2016, 6, 12592–12610 RSC.
- Z. J. Wang, J. H. Xie, S. P. Nie and M. Y. Xie, Review on cell models to evaluate the potential antioxidant activity of polysaccharides, Food Funct., 2017, 8, 915–926 RSC.
- Anonymous China fisheries yearbook, China Agricultural Press, 2016 Search PubMed.
- C. L. Shi, T. M. Pan, M. J. Cao, Q. M. Liu, L. J. Zhang and G. M. Liu, Suppression of Th2 immune responses by the sulfated polysaccharide from Porphyra haitanensis in tropomyosin-sensitized mice, Int. Immunopharmacol., 2015, 24, 211–218 CrossRef CAS PubMed.
- Q. M. Liu, Y. Yang, S. J. Maleki, M. Alcocer, S. S. Xu, C. L. Shi, M. J. Cao and G. M. Liu, The anti-food allergic activity of sulfated polysaccharide from Gracilaria lemaneiformis is dependent on immunosuppression and inhibition of p38 MAPK, J. Agric. Food Chem., 2016, 64, 4536–4544 CrossRef CAS PubMed.
- M. M. Bradford, A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- Y. Geng, L. Xing, M. Sun and F. Su, Immunomodulatory effects of sulfated polysaccharides of pine pollen on mouse macrophages, Int. J. Biol. Macromol., 2016, 91, 846–855 CrossRef CAS PubMed.
- J. Qian, L. Xu, X. Sun, Y. Wang, W. Xuan and Q. Zhang, Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis, Arthritis Res. Ther., 2018, 20, 26–36 CrossRef PubMed.
- H. K. Yakob, A. M. Uyub and S. F. Sulaiman, Immune-stimulating properties of 80% methanolic extract of Ludwigia octovalvis against Shiga toxin-producing E. coli O157:H7 in Balb/c mice following experimental infection, J. Ethnopharmacol., 2015, 172, 30–37 CrossRef PubMed.
- Healthy-lifestyle counselling, World Health Organization, 2018, http://120.52.51.13/apps.who.int/iris/bitstream/handle/10665/260422/WHO-NMH-NVI-18.1-eng.pdf?sequence=1-<?pdb_no 166k?>166k<?pdb END?> Search PubMed.
- Q. M. Liu, S. S. Xu, L. Li, T. M. Pan, C. L. Shi, H. Liu, M. J. Cao, W. J. Su and G. M. Liu, In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis, Carbohydr. Polym., 2017, 165, 189–196 CrossRef CAS PubMed.
- A. C. Leódido, L. E. Costa, T. S. Araújo, D. S. Costa, N. A. Sousa and L. K. Souza, Anti-diarrhoeal therapeutic potential and safety assessment of sulphated polysaccharide fraction from Gracilaria intermedia seaweed in mice, Int. J. Biol. Macromol., 2017, 97, 34–45 CrossRef PubMed.
- D. Anderson and A. Siwicki, Basic haematology and serology for fish health programs, in Diseases in Asian Aquaculture II, ed. M. Shariff, J. R. Auther and R. P. Subasinghe, Asian Fisheries Society, Philippines, 1995, pp. 185–202 Search PubMed.
- R. Yuhan, A. Koutsouris, S. D. Savkovic and G. Hecht, Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability, Gastroenterology, 1997, 113, 1873–1882 CrossRef CAS.
- J. S. Maciel, L. S. Chaves, S. Bws, B. W. S. Souza, D. I. A. Teixeira, A. L. P. Freitas and J. P. A. Feitosa, Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae, Carbohydr. Polym., 2008, 71, 559–565 CrossRef CAS.
- J. C. Mollet, A. Rahaoui and Y. Lemoine, Yield, chemical composition and gel strength of agarocolloids of Gracilaria gracilis, Gracilariopsis longissima and the newly reported Gracilaria cf. vermiculophylla from Roscoff (Brittany, France), J. Appl. Phycol., 1998, 10, 59–66 CrossRef CAS.
- C. Rochas, M. Lahaye and W. Yaphe, Sulfate content of carrageenan and agar determined by infrared spectroscopy, Bot. Mar., 1986, 29, 335–340 CAS.
- S. P. Gilmore, A. Gonye, E. C. Li, S. D. L. R. Espinosa, J. T. Gupton and O. A. Quintero, Effects of a novel microtubule-depolymerizer on pro-inflammatory signaling in RAW264.7 macrophages, Chem.-Biol. Interact., 2018, 280, 109–116 CrossRef CAS PubMed.
- A. H. Cruz, R. Z. Mendonça and V. L. Petricevich, Crotalus durissus terrificus venom interferes with morphological, functional, and biochemical changes in murine macrophage, Mediators Inflammation, 2014, 2005, 349–359 CrossRef PubMed.
- O. Cirioni, A. Giacometti, R. Ghiselli, F. Mocchegiani, A. Fineo and F. Orlando, Single-dose intraperitoneal magainins improve survival in a Gram-negative-pathogen septic shock rat model, Antimicrob. Agents Chemother., 2002, 46, 101–104 CrossRef CAS PubMed.
- M. L. Kruzel, Y. Harari, D. Mailman, J. K. Actor and M. Zimecki, Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice, Clin. Exp. Immunol., 2002, 130, 25–31 CrossRef CAS PubMed.
- P. M. Becker, H. C. Widjajagreefkes and P. G. van Wikselaar, Inhibition of binding of the AB5-type enterotoxins LT-I and cholera toxin to ganglioside GM1 by galactose-rich dietary components, Foodborne Pathog. Dis., 2010, 7, 225–233 CrossRef CAS PubMed.
- F. Alsaedi, D. P. Vaz, D. H. Stones and A. M. Krachler, 3-Sulfogalactosyl-dependent adhesion of Escherichia coli HS multivalent adhesion molecule is attenuated by sulfatase activity, J. Biol. Chem., 2017, 292, 19792–19803 CrossRef CAS PubMed.
- C. Gonçalves, F. Berthiaume, M. Mourez and J. D. Dubreuil, Escherichia coli, STb toxin binding to sulfatide and its inhibition by carragenan, FEMS Microbiol. Lett., 2008, 281, 30–35 CrossRef PubMed.
- H. R. Sinclair, C. W. Smejkal, C. Glister, F. Kemp, H. E. Van Den, J. Slegte, G. R. Gibson and R. A. Rastall, Sialyloligosaccharides inhibit cholera toxin binding to the GM1 receptor, Carbohydr. Res., 2008, 343, 2589–2594 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |