Open Access Article
Open Access ArticleLayer-by-layer membrane modification allows scandium recovery by nanofiltration†
Kirsten
Remmen‡
a,
Roman
Schäfer‡
a,
Sebastian
Hedwig
a,
Thomas
Wintgens
*a,
Matthias
Wessling
b and
Markus
Lenz
 ac
ac
aFHNW, School of Life Sciences, Institute for Ecopreneurship, Hofackerstrasse 30, 4132 Muttenz, Switzerland. E-mail: thomas.wintgens@fhnw.ch
bChemical Process Engineering, RWTH Aachen University, Forckenbeckstrasse 51, 52074 Aachen, Germany
cSub-Department of Environmental Technology, Wageningen University, 6700 AA, Wageningen, The Netherlands
First published on 31st July 2019
Abstract
Aluminium scandium (Sc) alloys are stronger, more corrosion resistant and more heat tolerant than classical aluminium alloys and allow for 3D printing. In particular, the aerospace industry benefits from better fuel efficiency due to lighter materials as well as the advantages of additive manufacturing. However, Sc is currently not available in sufficient quantities and has recently been identified as a raw material critical to the economy. Due to the recentness of the demand, technologies for recovery of Sc from secondary sources are in their infancy. In this study, Sc recovery from titanium dioxide pigment production waste by nanofiltration was investigated. Custom-made layer-by-layer (LbL) modified membranes were optimized with regards to their elemental retention (i.e., selectivity towards Sc) as well as their acid resistance. In model solutions, the optimized membrane retained up to 64% ± 4% Sc, removing the major impurity, iron (Fe), efficiently (12% ± 7% retention) while achieving high flux [32 L m−2 h−1] at a low transmembrane pressure of 5 bar. Acid resistance was shown down to a pH of 0.1, which could be even further increased (up to ≤3 M HCl) by adding more bi-layers and changing the coating conditions. In real wastes, the optimized LbL membrane showed higher Sc retention (60% vs. 50%) compared to a commercial acid resistant membrane, while achieving considerably higher fluxes [27 L m−2 h−1versus 1 L m−2 h−1, respectively at 5 bar]. It was possible to operate filtration at low transmembrane pressure with up to 70% permeate recovery and flux that was still high [∼10 L m−2 h−1]. In a nutshell, titanium dioxide pigment wastes contained sufficient amounts to satisfy the growing demand for Sc and can be exploited to their full extent by LbL nanofiltration due to the proven advantages of acid stability, Sc retention and selectivity and high achievable fluxes at low pressures.
Water impactThe paper presents an innovative approach to recover scandium from titanium dioxide pigment production waste by the use of newly produced nanofiltration membranes applying the layer-by-layer method. The novel separation technique offers potential to replace conventional processing routes lowering the environmental impact related to the use of organic solvents, which are a burden to the aquatic environment. |
Introduction
Recovery of resources from waste streams is essential in moving towards a circular economy.1–4 Critical raw materials have an above average economic importance as well as a supply risk. Amongst 27 materials recently identified by the European Commission (similar lists exist for other major economies5), scandium (Sc) is exceptional in terms of complete import reliance and zero end-of-life recycling rates.6 A main interest of Sc is its application in aluminium (Al) alloys, in which its unique properties lead to stronger, weldable, more corrosion resistant, and more heat tolerant Al products.2 Al–Sc alloys may decrease an aircraft weight by as much as 15–20%, making Sc particularly interesting for the aerospace industry. In addition, 3D printable alloys are now available (e.g. Scalmalloy©) that allow for additive manufacturing.Some attempts have been made to recover Sc from secondary sources; in particular, recovery from bauxite residues (so-called red mud) has been found promising.1,2,7–10 Apart from red mud, there are further stockpiled waste materials that may help satisfy the growing global Sc demand, in particular residues from titanium dioxide (TiO2) production. These are available on a millions-of-tonnes-per-annum scale. Currently, an estimated 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of ilmenite and 750
000 metric tons of ilmenite and 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of rutile are mined globally.11 From these, TiO2 is extracted/purified through either the traditional “sulfate” or the “chloride” route.12 However, very few attempts have been made for Sc recovery via the sulphate route,13,14 and to the authors' best knowledge, none via the chloride route.
000 metric tons of rutile are mined globally.11 From these, TiO2 is extracted/purified through either the traditional “sulfate” or the “chloride” route.12 However, very few attempts have been made for Sc recovery via the sulphate route,13,14 and to the authors' best knowledge, none via the chloride route.
Briefly, during TiO2 pigment production via the chloride route, ore is processed with coke and gaseous chlorine in a fluidized bed reactor at high temperatures of 900–1000 °C.8,15,16 The process produces HCl waste containing high dissolved metal concentrations, as well as unreacted ores/coke overblow. Depending on the original ore and processing conditions, varying Sc concentrations may be found in these waste streams. A small level of Sc (0.5%) in Al alloys in only 0.1% of the global annual Al market would result to an annual scandia (Sc2O3) need of ∼350 tonnes2 (∼230 t Sc). Currently, the global supply of Sc is estimated to be between 10–15 tonnes per year only11 and primary Sc deposits above 100 ppm are rarely reported.2 It has been estimated that about 60% of the 4.5 million tons of pigment production world-wide is generated by the chlorine process.15 The volume of aqueous acid waste of the chloride route as well as Sc concentrations will certainly depend on the operational conditions of the respective plant (e.g., type and grade of ore used, dimensions of scrubber, etc.). Nevertheless such wastes may contain sufficient Sc to meet even future demand.
Sc is commonly concentrated and purified by solvent extraction/precipitation.14 For some elements, in particular the solvent extraction process step may contribute considerably to the overall environmental impact of the processing chain (e.g., up to one third in the case of neodymium oxide17). For Sc recovery, it has been demonstrated that a pre-enrichment is needed for sufficiently concentrating Sc for later selective extraction.14 Here, nanofiltration (NF) can offer two crucial advantages: firstly, since it is based on a different separation principle, it may offer selectivity while, secondly, decreasing the volume to be extracted downstream, decreasing the environmental impact of the solvent extraction steps.18 However, a severe limitation of NF is that in high ionic strength solutions satisfactory fluxes can only be achieved via high operational pressures, increasing operational costs.19–21 Furthermore, only a very limited number of commercial membranes can withstand strongly acidic conditions (regularly found in hydrometallurgy).
LbL modified membranes are assembled by depositing several layers of oppositely charged polyelectrolytes (PE) on an ultrafiltration (UF) membrane. This technology allows tailoring of the membrane characteristics (porosity, selectivity, stability, etc.)22–24 towards a target ion and application. This is achieved through selection of a suitable PE, varying the number of layers, and/or varying the coating method (e.g., the ionic strength, the pH).22,25 LbL membranes often have a higher permeability during acidic filtration than conventional NF membranes. For instance, LbL membranes showed 16 times higher permeability in comparison to a commercial membrane (AS 3012, AMS, TelAviv, Israel) in P recovery from acidic leachates26 with increased P yield.25 In consequence, the filtration units can be smaller, or a lower transmembrane pressure (TMP) can be applied, leading to a considerably lower operational (energy consumption) and capital costs.25,26 A limitation to LbL membranes may be their instability in strong acids. So far, studies focused on stability in H3PO4 (ref. 25 and 26) and acid stability in HCl was only shown by immersing the membrane in 1 M HCl.22,27,28 No studies have been carried out post-filtration regarding element retention or flux behaviour, so that it remains unclear if sufficient acid stability can be achieved over the long term.
Thus, this study is the first to apply LbL membranes in concentrated HCl matrixes for Sc recovery. LbL membranes were optimized with regards to their selectivity towards the target ion (Sc) as well as to their acid resistance by modifying coating parameters (number of layers, ionic strength) in model solutions. The best LbL membrane was applied for filtration of real TiO2 wastes and compared to commercial membranes.
Experimental
Membranes
Polyethersulfone (PES) based hollow fibre membranes (type: UFCLE) provided by pentair (Enschede, the Netherlands) were used as a support structure to prepare LbL membranes (see section 1.2). The UF membrane had a molecular weight cut-off of 100 kDa and a pure water permeability of 1100 L m−2 h−1 bar−1. The membranes were potted in modules containing two fibres each. Each hollow fibre had an inner diameter of 0.8 mm, and a length of 300 mm. This resulted in a total membrane surface of 15 cm2 per module. After potting, the bare membranes were rinsed with deionized water for at least 1 h. An acid resistant flat sheet membrane (Duracid KH) with a membrane area of 8 cm2 was purchased from Suez (Ratingen, Germany). The Duracid membrane was cut in to pieces of 100 × 30 mm and immersed in deionized water for 24 h. Compaction took place for at least 12 h in the test cell at 5 bar and a feed flow of 12 L h−1.Layer-by-layer modified membrane preparation
The application of the PE on the membranes was carried out using a custom-made set up which allowed dynamic coating.22,26 The positively charged poly(diallyldimethylammonium chloride) (PDADMAC; MW = 400–500 kDa, 20 wt% in water) and the negatively charged poly(sodiumstyrenesulfonate) (PSS; MW = 1000 kDa, 25 wt% in water) were purchased from Sigma-Aldrich (Buchs, Switzerland). The oppositely charged PE were diluted in an NaCl solution (0.05–1 M), and were alternately coated onto the membrane, starting with the positively charged PE, and always terminated by the PSS. The pH of the coating solution was neutral. Dead-end filtration was applied to concentrate the PE inside the lumen of the membrane. Each filtration cycle was carried out with the same duration and pressure to ensure equal amounts of PE retained in the lumen. After each coating cycle, the membrane was flushed with deionized water until a conductivity of below 3 μS cm−1 was reached. The conductivity was measured using a GMH 3451 conductivity meter from Greisinger (Regenstauf, Germany).Membrane filtration
A custom-made testing device was used for the filtration (see ESI†). At a TMP of 5 bar a BVP-Z gear pump (Cole-Parmer, Wertheim, Germany) was used to establish the desired cross-flow velocity for operation in turbulent mode. Experiments were performed in batch circulation mode with the concentrate fed back into the feed vessel. The feed vessel was placed in an ice bath, maintaining the feed temperature at 0 °C. Elemental retention was determined in cross-flow mode. At a TMP of 5 bars, the flow was 160 mL min−1, resulting in a cross-flow velocity of 2.65 m s−1 and a Reynolds number of >2300 (thus at turbulent flow). Each LbL membrane modification was tested in triplicate.Feed solutions
Model solutions consisted of 1 M HCl, 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 Fe (added as Fe(II)O × 7H2O, 99% purity, Sigma- Aldrich, Buchs, Switzerland) and 90 mg L−1 Sc (added as Sc2(III)O3, 99.9% purity, Sigma-Aldrich, Buchs, Switzerland). When needed, the pH of the model solution was adjusted using NaOH (98.5% purity, Sigma-Aldrich, Buchs, Switzerland). The pH was measured using a WTW inoLab Multi 9310 IDS pH-meter (Weilheim, Germany). In addition, both the optimized LbL membranes and the Duracid membrane were used for filtering a real TiO2 acidic waste stream (see 2.3). The composition of the solution changed depending on the process as determined by elemental analysis (see ESI†). For filtration, the acid waste was diluted at 1
000 mg L−1 Fe (added as Fe(II)O × 7H2O, 99% purity, Sigma- Aldrich, Buchs, Switzerland) and 90 mg L−1 Sc (added as Sc2(III)O3, 99.9% purity, Sigma-Aldrich, Buchs, Switzerland). When needed, the pH of the model solution was adjusted using NaOH (98.5% purity, Sigma-Aldrich, Buchs, Switzerland). The pH was measured using a WTW inoLab Multi 9310 IDS pH-meter (Weilheim, Germany). In addition, both the optimized LbL membranes and the Duracid membrane were used for filtering a real TiO2 acidic waste stream (see 2.3). The composition of the solution changed depending on the process as determined by elemental analysis (see ESI†). For filtration, the acid waste was diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (vol
5 (vol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vol) and the pH set to 1.5. Solids were separated by filtration (0.45 μm).
vol) and the pH set to 1.5. Solids were separated by filtration (0.45 μm).
Results and discussion
Selective Sc recovery using layer-by-layer modified membranes
Ionic strength in the coating solution is known as a major parameter influencing the structure and morphology of the assembled layers. This is due to the fact that net charge neutrality of the PE can be attained either by oppositely charged repeat units of the counter PE (referred to as “intrinsic charge compensation”) or by salt counter ions (referred to as “extrinsic charge compensation”).29 Extrinsic charge compensation by salt ions is expected to yield thicker multilayers, with individual chains having more mobility, resulting in less stable structures.29 Here, high ionic strength during coating (1 M NaCl) resulted in the highest Sc retention (64.3 ± 1.5%; Fig. 1), whereas low ionic strength did not yield membranes with sufficient Sc retention (33.7 ± 1.9% at 0.05 M; Fig. 1). Ionic strength did not influence Fe retention, remaining below 12.3% in all cases. The impact of the applied NaCl concentrations on Sc retention as well as on stability is in accordance with Adusumilli et al.,30 who showed a 10 times thicker PE film when the assembly was performed with 1 M NaCl compared to 0.05 M NaCl. Despite yielding the highest Sc retention, the high ionic strength did indeed result in instable membranes, underlined by the decrease in Sc retention after 60 min of filtration (30.6 ± 10.4%; Fig. 1). It is known that the high ionic strength of the feed solution can irreversibly impact the structure of NF membranes.31–33 During exposure of the LbL membranes to high ionic strength and/or acidic solutions, the intermolecular repulsion effects within the polymer chains can be lessened, leading to a more open and swollen structure.31,34,35 Indeed, the intermediate (0.5 M) NaCl concentration appeared to be the best trade-off between sufficient initial Sc retention (53.5 ± 3.6%) and stability (45.7 ± 7.2 Sc retention after 60 min; Fig. 1).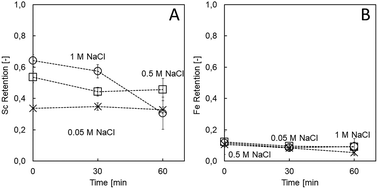 | ||
| Fig. 1 Sc (A) and Fe (B) retention by (PDADMAC/PSS)3 in model solution as a function of coating condition. | ||
Additional bi-layers may result in improved membrane properties with regards to element retention as well as stability,22,27,30 yet come at the price of requiring more time and chemicals for membrane preparation. Increasing the number of bi-layers in PDADMAC/PSS systems impacting filtration has been ascribed to the charge overcompensation of PDADMAC, leading to diffusion of the PE from top layers into lower layers.27,36 Here, the addition of more than three bi-layers did not result in improved Sc retention, and the (PDADMAC/PSS)3 system showed satisfactory acid stability (i.e., 45.7 ± 7.2% Sc retention after 60 min; Fig. 2). The addition of more bi-layers only had a minor beneficial effect on Fe retention (minimal retention of 4.2 ± 1.3% with seven bi-layers, Fig. 2), which is too little to justify additional preparatory work due to the low retention using three bilayers (12.3 ± 1.1%). The results are in line with previous studies that have shown already three bi-layers giving high magnesium retention and thus NF membrane properties.27
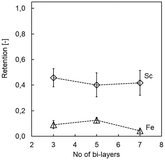 | ||
| Fig. 2 Sc and Fe retention after 60 min of model solution filtration for (PDADMAC/PSS)x membranes as a function of the number of bi-layers applied. | ||
Acidity may influence both chemical (i.e., degradation) and physical (e.g., swelling, structural order, disintegration) integrity of the LbL membranes.23 Regarding the application in hydrometallurgy in general and for TiO2 wastes in particular, high resistivity towards acid is desirable. Considering that a solution with high ionic strength can diminish the electrostatic interaction of the PE, less stable membranes are expected in high acidic environments. Indeed, at low (0.1) pH, the (PDADMAC/PSS)3 system was indicated to be insufficiently stable, underlined by decreased Sc retention before and after filtration (53.5 ± 3.6% to 45.7 ± 7.2%, Fig. 3A and B). Adjusting the pH to 1.5 had a positive effect on Sc retention and membrane stability (63.3 ± 4.4% before to 64.6 ± 10.7% after 60 min of filtration; Fig. 3A and B), while achieving high selectivity towards undesired Fe (14.4 ± 0.9% after filtration). Acid stability down to pH ≥1.5 constitutes an expansion of (PDADMAC/PSS)3 systems that have previously been shown to be stable down to pH 2.5 (PDADMAC/PSS coated Si capillaries for chromatography).28 Improving acid resistivity even further for other hydrometallurgical applications while conserving selectivity for the target ions remains the subject of future work. Certainly, increasing the number of bi-layers and modifying the coating conditions can still considerably improve acid resistivity (e.g., up to 3 M HCl, which is considerably higher than previously reported,28,37 see ESI†). Alternatively, in the future cross-linking of other PE may result in high acid resistivity, however, at the expense of more steps/chemicals necessary and potential loss of selectivity. Here, increasing the pH of the waste had an additional advantage, since some unwanted elements (impurities such as radioactive uranium (U) and thorium (Th)) already precipitate at this value, whereas most Sc (80%) remained in solution (see ESI†). Therefore, acid resistivity towards pH 1.5 solutions was found sufficient for Sc recovery from TiO2 wastes.
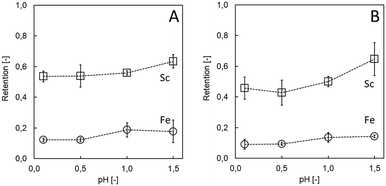 | ||
| Fig. 3 Acid stability in term of Sc and Fe retention as a function pH before (A) and after 60 min (B) of model solution filtration. | ||
Scandium recovery from real wastes of the TiO2 industry
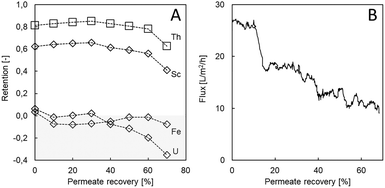
Using real, complex aqueous waste from the TiO2 industry confirmed the good Sc retention and selectivity towards major impurities (Fe). There was little effect of permeate recovery on Sc retention up to 60% permeate recovery, and Sc retention remained high (≥56%; Fig. 4). After, retention decreased to 41%, only. The same trend was observed for Th, an impurity in the TiO2 waste, although with higher initial retention of 81% (Fig. 4). At high permeate recovery, the decrease in element retention can be partly ascribed to higher membrane-shielding effects and hence a decrease of the Donnan effect.31,38,39 Next to these phenomena, the decreased flux led to a lower convective flow, favouring diffusive membrane transport. Initial flux values of 27 L m−2 h−1 were recorded, which decreased successively to 10 L m−2 h−1 at 70% permeate recovery. Since scaling or precipitation of metals is not likely in this acidic solution, the decrease in flux can probably be ascribed solely to an increasing osmotic pressure during permeate recovery. It should be noted that despite the high Th retention, overall Th concentrations were low due to pH adjustment (removing ∼75% of dissolved Th prior filtration) so that Th was not concentrated above the initial value.Other impurities such as Fe and U were efficiently removed by LbL filtration, as underlined by the even negative retentions (Fig. 4). Since no Fe or U are concentrated in the retentate, the Sc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio was shifted in an even more favourable direction. In previous studies, low U retention was observed for NF membranes and a decrease in pH led to lower retention values as well.40,41 Other studies report a decreased influence of Donnan exclusion for anions such as chloride in a lower pH environment.42 Thus, dielectric and steric exclusion play a major role in retention. Two other studies claim that large hydrated ions with a strong hydration shell are retained best.43,44 This seems to apply to Sc and Th in the here presented experiments. In a study presented by Tansel et al. (2012), Fe2+ was categorized as a large hydrated ion but with a weak hydration shell, leading to lower retention values, which might also be the case for U.44
Fe ratio was shifted in an even more favourable direction. In previous studies, low U retention was observed for NF membranes and a decrease in pH led to lower retention values as well.40,41 Other studies report a decreased influence of Donnan exclusion for anions such as chloride in a lower pH environment.42 Thus, dielectric and steric exclusion play a major role in retention. Two other studies claim that large hydrated ions with a strong hydration shell are retained best.43,44 This seems to apply to Sc and Th in the here presented experiments. In a study presented by Tansel et al. (2012), Fe2+ was categorized as a large hydrated ion but with a weak hydration shell, leading to lower retention values, which might also be the case for U.44
To benchmark the obtained membrane performance, a filtration experiment was conducted using a commercially available acid resistant Duracid membrane at 0% permeate recovery. The Duracid membrane achieved less Sc retention (50%) and retained more Fe (4%) and U (31%), whereas less Th was retained (69%). The results are in accordance with a previous study that assigned lower retention for monovalent, divalent, and trivalent ions to HCl used to acidify the feed solution using the same membrane.18 Most importantly, it has to be noted that even at 0% permeate recovery, a flux of as little as 1 L m−2 h−1 was measured, which was 3% of the flux that was reached for the LbL membranes at the same conditions (Fig. 4B). Thus, when aiming at an identical amount of permeate volume, a 28-fold membrane area or notable pressure increase would be needed. In conclusion, the LbL membranes used here offer advantages regarding not only selectivity but also lower operational costs and/or energy demand.
Conclusions
Nanofiltration as alternative separation for critical raw material recovery from acidic wastes
Here, first the separation of Sc from TiO2 waste was demonstrated as a proof of principle. The Sc concentration found here (76–90 mg L−1) qualify TiO2 acid wastes as an ore.2 Some ∼2.5 to ∼3 million m3 of this waste would thus be sufficient to cover the entire projected future Sc demand, a volume that may in fact be available considering the ∼4.5 million tons of pigment produced. This study adds to previous studies that have shown the potential of nanofiltration for recovery of critical raw material from acidic wastes. For instance, NF could be used for recovery of Fe and acid (HNO3, HF) from spent pickling baths,45 Zinc and Fe recovery from acidic electroplating effluents,46 phosphorous recovery from acid leached sewage sludge,38,47 or indium from acid leached photovoltaics,18 to name a few.Recovery of critical raw materials by nanofiltration in general offers the major advantages of relying on a different separation principle than conventional techniques (i.e., ion exchange, solvent extraction, precipitation) as well as generating concentrates depleted in impurities, beneficial for downstream processing by conventional techniques.18 One challenge regarding the filtration of strong acidic solutions is their corrosivity. Since LbL membranes operate at lower pressure ranges (here, 5 bar) but still reach high fluxes [here, up to 27 L m−2 h−1 in real wastes], the filtration units can be built out of corrosion resistant plastic parts. Though the proof of principle for LbL filtration of TiO2 wastes was made here, a challenge for future research remains the acid resistivity and long-term stability23 of the membranes themselves. Although acid resistivity can be improved to some extent by the addition of more layers or changed coating conditions (see ESI†), some wastes may still remain inaccessible without pre-treatment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work presented in this paper was financially supported by Swiss National Science Foundation and the Commission for Innovation and Technology: Bridge – Proof of Concept for the funding under Grant agreement 20B1-1177283. This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 730105 (SCALE: http://www.scale-project.eu/). This work was supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 16.0155. The opinions expressed and arguments employed herein do not necessarily reflect the official views of the Swiss government.Notes and references
- N. T. Nassar, T. E. Graedel and E. M. Harper, Sci. Adv., 2015, 1(3), e1400180 CrossRef CAS PubMed.
- S. Reid, J. Tam, M. Yang and G. Azimi, Sci. Rep., 2017, 7(1), 15252 CrossRef PubMed.
- W. R. Stahel, Nature, 2016, 531(7595), 435–438 CrossRef CAS PubMed.
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven and Y. Pontikes, J. Cleaner Prod., 2015, 99, 17–38 CrossRef CAS.
- T. Hennebel, N. Boon, S. Maes and M. Lenz, New Biotechnol., 2015, 32, 121–127 CrossRef CAS PubMed.
- European Commission, COM/2017/0490, 2017, available at https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52017DC0490 Search PubMed.
- C. R. Borra, B. Blanpain, Y. Pontikes, K. Binnemans and T. Van Gerven, J. Sustain. Metall., 2016, 2, 365–386 CrossRef.
- W. Wang, Y. Pranolo and C. Y. Cheng, Hydrometallurgy, 2011, 100, 100–108 CrossRef.
- É. Ujaczki, V. Feigl, M. Molnár, P. Cusack, T. Curtin, R. Courtney, L. O'Donoghue, P. Davris, C. Hugi, M. W. H. Evangelou, E. Balomenos and M. Lenz, J. Chem. Technol. Biotechnol., 2018, 93, 2498–2510 CrossRef PubMed.
- G. Alkan, B. Yagmurlu, L. Gronen, C. Dittrich, Y. Ma, S. Stopic and B. Friedrich, Hydrometallurgy, 2019, 185, 266–272 CrossRef CAS.
- United States Geological Survey, Mineral commodity summaries, 2019, available at https://www.usgs.gov/centers/nmic/mineral-commodity-summaries Search PubMed.
- G. Buxbaum and G. Pfaff, Industrial Inorganic Pigments, 3rd edn, 2005 Search PubMed.
- Y. Li, Q. Li, G. Zhang, L. Zeng, Z. Cao, W. Guan and L. Wang, Hydrometallurgy, 2018, 178, 1–6 CrossRef CAS.
- H. Qiu, M. Wang, Y. Xie, J. Song, T. Huang, X. M. Li and T. He, Process Saf. Environ. Prot., 2019, 121, 118–124 CrossRef CAS.
- M. J. Gázquez, J. P. Bolívar, R. Garcia-Tenorio and F. Vaca, Mater. Sci. Appl., 2014, 5, 441–458 Search PubMed.
- S. Middlemas, Z. Z. Fang and P. Fan, J. Cleaner Prod., 2015, 89, 137–147 CrossRef CAS.
- E. Vahidi and F. Zhao, J. Environ. Manage., 2017, 203, 255–263 CrossRef CAS PubMed.
- Y.-S. Zimmermann, C. Niewersch, M. Lenz, Z. Z. Kül, P. F.-X. Corvini, A. Schäffer and T. Wintgens, Environ. Sci. Technol., 2014, 48, 13412–134128 CrossRef CAS PubMed.
- A. Agrawal and K. K. Sahu, J. Hazard. Mater., 2009, 171, 61–75 CrossRef CAS PubMed.
- M. Regel-Rosocka, J. Hazard. Mater., 2010, 177, 57–69 CrossRef CAS PubMed.
- M. P. González, R. Navarro, I. Saucedo, M. Avila, J. Revilla and C. Bouchard, Desalination, 2002, 147, 315–320 CrossRef.
- D. Menne, J. Kamp, J. Erik Wong and M. Wessling, J. Membr. Sci., 2016, 499, 396–405 CrossRef CAS.
- J. de Grooth, B. Haakmeester, C. Wever, J. Potreck, W. M. de Vos and K. Nijmeijer, J. Membr. Sci., 2015, 489, 153–159 CrossRef CAS.
- J. de Grooth, R. Oborný, J. Potreck, K. Nijmeijer and W. M. de Vos, J. Membr. Sci., 2015, 475, 311–319 CrossRef CAS.
- L. Paltrinieri, K. Remmen, B. Müller, L. Chu, J. Köser, T. Wintgens, M. Wessling, L. C. P. M. Smet and E. J. R. Sudhölter, J. Membr. Sci., 2019, 587, 117162 CrossRef CAS.
- K. Remmen, B. Müller, J. Köser, M. Wessling and T. Wintgens, J. Membr. Sci., 2019, 582, 254–263 CrossRef CAS.
- D. Menne, RWTH Dissertation, 2017, available at https://publications.rwth-aachen.de/record/684806 Search PubMed.
- R. Nehmé, C. Perrin, H. Cottet, M. D. Blanchin, H. Fabre and J. Chromatogr, A, 2011, 1218, 3537–3544 Search PubMed.
- J. B. Schlenoff, H. Ly and M. Li, J. Am. Chem. Soc., 1998, 120, 7626–7634 CrossRef CAS.
- M. Adusumilli and M. L. Bruening, Langmuir, 2009, 25, 7478–7485 CrossRef CAS PubMed.
- W. Cheng, C. Liu, T. Tong, R. Epsztein, M. Sun, R. Verduzco, J. Ma and M. Elimelech, J. Membr. Sci., 2018, 559, 98–106 CrossRef CAS.
- G. Bargeman, J. B. Westerink, O. Guerra Miguez and M. Wessling, Sep. Purif. Technol., 2014, 134, 46–57 CrossRef CAS.
- C. Liu, L. Shi and R. Wang, React. Funct. Polym., 2015, 86, 154–160 CrossRef CAS.
- L. Ouyang, R. Malaisamy and M. L. Bruening, J. Membr. Sci., 2008, 301, 76–84 CrossRef.
- A. A. Antipov, G. B. Sukhorukov and H. Möhwald, Langmuir, 2003, 19, 2444–2448 CrossRef CAS.
- C. Porcel, P. Lavalle, G. Decher, B. Senger, J. C. Voegel and P. Schaaf, Langmuir, 2007, 23, 1898–1904 CrossRef CAS PubMed.
- D. Menne, C. Üzüm, A. Koppelmann, J. E. Wong, C. van Foeken, F. Borre, L. Dähne, T. Laakso, A. Pihlajamäki and M. Wessling, J. Membr. Sci., 2016, 520, 924–932 CrossRef CAS.
- T. Schütte, C. Niewersch, T. Wintgens and S. Yüce, J. Membr. Sci., 2015, 480, 74–82 CrossRef.
- A. R. Guastalli, J. Labanda and J. Llorens, Desalination, 2009, 243, 218–228 CrossRef CAS.
- M. Ghasemi Torkabad, A. R. Keshtkar and S. J. Safdari, Hydrometallurgy, 2018, 178, 106–115 CrossRef CAS.
- M. Ghasemi Torkabad, A. R. Keshtkar and S. J. Safdari, Prog. Nucl. Energy, 2017, 94, 93–100 CrossRef CAS.
- R. Epsztein, E. Shaulsky, N. Dizge, D. M. Warsinger and M. Elimelech, Environ. Sci. Technol., 2018, 52, 4108–4116 CrossRef CAS PubMed.
- B. Tansel, J. Sager, T. Rector, J. Garland, R. F. Strayer, L. Levine, M. Roberts, M. Hummerick and J. Bauer, Sep. Purif. Technol., 2006, 51, 40–47 CrossRef CAS.
- B. Tansel, Sep. Purif. Technol., 2012, 86, 119–126 CrossRef CAS.
- K. M. Forsberg and Å. C. Rasmuson, Miner. Eng., 2007, 20, 950–955 CrossRef CAS.
- A. G. Boricha and Z. V. P. Murthy, Sep. Purif. Technol., 2009, 65, 282–289 CrossRef CAS.
- C. Blöcher, C. Niewersch and T. Melin, Water Res., 2012, 46, 2009–2019 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00509a |
| ‡ Both first authors made an equal contribution to this publication. |
| This journal is © The Royal Society of Chemistry 2019 |