Open Access Article
Open Access ArticleModified polyurethane nanofibers as antibacterial filters for air and water purification†
G. Ungur * and
J. Hrůza
* and
J. Hrůza
Institute for Nanomaterials, Advanced Technologies and Innovation, Technical University of Liberec, Bendlova 1409/7, Liberec, 46001, Czech Republic. E-mail: ganna.ungur@tul.cz; Tel: +420 775537609
First published on 20th October 2017
Abstract
In the present research, we aimed to produce polymer nanofibrous filters for antibacterial purification of air and water and prove their efficiency and stability under simulated filtration conditions. Polyurethane solutions were modified by microparticles (700 nm to 1 μm) and nanoparticles (≈50 nm) of copper oxide (CuO) in order to compare the influence of the dimensional characteristics of modifier on the properties of composite filters. Antimicrobial additives (used concentrations 5; 7; 9.5 and 12%) were introduced directly into the pre-electrospinning solutions and then were thoroughly intermingled. The rheological behaviour of such solutions was studied before the electrospinning process. Then composite layers were prepared by the industrial Nanospider technique. SED-EDX results confirmed a smooth and well-oriented structure and the presence of CuO for all of the modified samples. The antibacterial efficiency of the nanofibrous mats with micro- and nanoparticles was studied using the model microorganisms Escherichia coli and Staphylococcus gallinarum. The stability of particle fixation into the fiber structure was determined under the simulated conditions of water filtration. Moreover, a special device AMFIT-13 was designed and used to characterize the bacterial filtration efficiency of nanofibers for air purification. A very important result of this research is a proven fact that microparticles of CuO are a more suitable additive for the selected method of antibacterial modification of polyurethane filters than nanoparticles from technological and economic points of view.
Introduction
The microbial contamination of air and drinking water remains a significant threat and the constant vigilance is important.1,2 So the development of effective materials for combating the bacterial contamination of air and water is an important task of the modern science and industry.3 Metals and metallic oxides have been widely studied due to their antimicrobial activity. The nanosized state of these substances attracts special attention and interest in the scientific world.Copper oxide due to its unique biological, chemical and physical properties, antimicrobial activities as well as the low cost of preparation is of the great interest to the scientists.4 Moreover the elemental copper and its compounds have been recognized as antimicrobial materials by the US Environmental Protection Agency (EPA).5
In order to make possible the use of CuO particles for the air and water purification it is necessary to choose a suitable and stable “carrier”. One of the way to solve this task is an incorporation of particles into the polymer matrix.6 Electrospun nanofibers (NFs) are a promising variant of such a matrix. Due to their important properties, such as a high specific area, small diameters, highly porous structure with excellent pore interconnectivity, nanofibers are attractive materials for different advanced applications including water and air filtration.7 Therefore, nanofibers modified by particles of CuO may become perspective multifunctional materials for the purification of water and air contaminated by particulate matters and/or harmful microorganisms. Electrospinning (ES) is the most suitable technique for the production of nanofibers.8 The suspension of particles directly into the pre-electrospinning polymer solution is the simplest and most commonly used method for the combining metals particles with electrospun nanofibers.9,10
Polyurethane (PU) was chosen as an appropriate polymer matrix for the incorporation of copper oxide due to its excellent elastomeric and mechanical properties, tensile strength, durability, and water insolubility.11–13 There are only few researches about the modification of PU NFs by CuO nanoparticles (NPs). Sheikh et al. have produced PU nanofibers containing NPs of copper oxide using the blending modification approach with further electrospinning by simple laboratory technique. Antibacterial activity of the produced nanofibrous substrates was successfully confirmed.9 In another study CuO particles were mixed with the polymer solution to prepare the composite PU nanofibers by ES from the plastic syringe. The electrical conductivity of the PU/CuO NFs was markedly improved in comparison with pristine PU nanolayers.10
The using of industrial ES methods for the production of nanofibrous filters with antibacterial properties and the efficient ways to confirm their efficiency under the simulated conditions of air and water filtration have not been represented and discussed in the literature. We have tried to contribute to this little-known area of production and investigation of composite antimicrobial nanofibers. In the presented research the modified PU nanolayers with particles of CuO were produced by the industrial Nanospider technique.
Despite the fact that nowadays many researchers use and study the nanoparticles of metals and their oxides for the experiments in different scientific areas, there are still important problems without clear solutions. These are the toxicity of nanoparticles and their tendency to aggregate. It is proven that NPs exhibit greater toxicity than micro ones with the same composition, and the various-sized NPs induce different levels of cytotoxicity and DNA damage.14 Moreover, the nanoparticles have a stronger tendency to undergo agglomeration followed by insufficient dispersal in the polymer matrix, degrading the functional properties of the nanocomposites.15 Our serious fears are also caused by the fact that a certain amount of nanoparticles will be placed inside the polymer matrix because the diameter of the nanoparticles is less than the diameters of nanofibers. Consequently some part of the nanosized modifier won't be available for the contact with bacteria. Therefore we used both micro- and nanoparticles of CuO for the modification of PU solutions in order to compare the influence of different dimensions of the additive on antibacterial properties and on the stability of particles fixation into the structure of fibers.
In the presented study the composite nanofibrous mats with micro- and NPs of CuO have been successfully produced. Antimicrobial activity of pristine and modified PU nanofibers was confirmed against both Gram negative (Escherichia coli) and Gram positive (Staphylococcus gallinarum) bacterial strains. The perspective application of the presented samples is the antibacterial purification of water and air. Therefore the stability of particles fixation into the fibrous structure was tested under the simulated conditions (which corresponded to real) of water filtration. The efficiency of composite nanofibers was also studied under the simulated conditions of bacterial air filtration. The laboratory testing device for the evaluation of antimicrobial properties of the nanofibrous layers (and other textiles) under the filtration of bacterially contaminated air was developed and certificated for this particular purpose. We experimentally confirmed that PU nanofibers with microparticles (MPs) of copper oxide are a better antibacterial filtration material than nano-modified samples from the point of view of the stability of particle fixation in the structure of fibers. The stable particle fixation ensures durable antibacterial efficiency and safe utilization of the modified nanofibrous filters. Our results have a great practical importance and show the real possibility of “transferring” of the production of antibacterial composite filters form the laboratory to industrial level.
Experimental
Materials
In this work, polyurethane (Larithane LS 1086, aliphatic elastomer based on 2000 g mol−1, linear polycarbonated diol, isophorone diisocyanate and extended isophorone diamine) was used as a polymer. Larithane LS 1086 was dissolved in dimethylformamide (DMF). Polyurethane was obtained from Larithane Company. Dimethylformamide and microparticles of copper oxide with a size distribution of 700 nm to 1 μm were purchased from Penta. Nanoparticles of CuO with an average diameter of 50 nm were purchased from Sigma Aldrich. Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus gallinarum) strains were utilized as model organisms to check the antimicrobial properties of the produced nanofibres. The bacteria were obtained from the Czech Collection of Microorganisms (Masaryk University in Brno). The nutrient medium Tryptone Soya Broth (TSB) and sterile Tryptone Soya Agar (TSA) from Oxoid CZ s.r.o. were used for the inoculation and the incubation of the bacteria.Electrospinning process
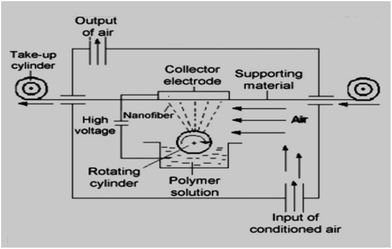
The solution of polyurethane was prepared. Then microparticles (MPs) of CuO were added to the PU to obtain modified solutions with different concentrations of antibacterial agent (5%; 7%; 9.5%; 12 wt%). The same approach and particle concentrations were used for modification of PU solutions by the nano-sized CuO. The obtained colloidal systems were mixed using magnetic stirrers for 12 hours. The ability to produce filtration materials to an industrial scale is very important. In fact, the real practical application of these or other materials is not feasible if their production in the required quantity using an affordable and implemented technology has not been proven. Therefore, we produced composite polyurethane nanofibers using the industrial Nanospider technique (Fig. 1). Nanospider consists of a rotating cylinder (spinning electrode), which spins fibres directly from the polymer solution. In the present research, the PU was filled into a polypropylene dish and a cylindrical rotary electrode with a needle surface was partly immersed into the polymer solution.The electrode with the needle surface was selected in order to ensure the mixing of colloidal solutions and to prevent the deposition of particles at the bottom of the dish with the PU.
A high voltage source is connected to the rotating roller. As the solvent evaporates, the jets of polymer solution are transformed and solid nanofibers are obtained before reaching the collector electrode. The nanofibers were collected on a polypropylene spun bond nonwoven antistatic material. The parameters of the electrospinning process were as follows: voltage = 67 kV; temperature (T °C) = 18 °C; humidity in the spinning chamber = 20%; speed of collecting material = 0.05 m min−1; distance between the rotating cylinder and collector electrode = 16 cm.
Measurement of viscosity
In this study the viscosity of polymer solutions was influenced by the presence of CuO particles. Therefore, it is important to estimate and compare the behaviour of pristine and modified polyurethane solutions. The comparison of the viscosities of solutions modified by micro- and nanoparticles of CuO in order to analyse the influence of dimensional characteristics of additives on the structure of future electrospun samples is also particularly noteworthy. The rheological properties of the solutions were measured using Rheometer HAAKE Roto Visco 1 at 23 °C.Structure of the produced nanofibers
The morphology of the nanofiber layers was analysed using a scanning electron microscope (TESCAN VEGA3 SEM). The average diameter of the fibers and the net diameter distribution of the samples with different CuO concentrations were measured and calculated from SEM photos using Lucie 32G computer software. Fiber uniformity was determined using number and weight average calculations. The number average is known as an arithmetic mean in mathematics. The method for calculating the uniformity coefficient has the same principle as molar mass distribution in chemistry. We calculated both of these values using the formulas (1) (number average or average diameter) and (2) (weight average), which are given below:
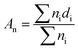 | (1) |
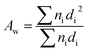 | (2) |
Antibacterial tests
The antimicrobial activity of pristine and composite nanofibers was determined against Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus gallinarum (St. Gal.) bacterial strains according to the Cornell test (ASTM E2149). This is a standard quantitative test method for determining the antibacterial efficiency (%) of immobilized antimicrobial agents under dynamic contact conditions. This method is used to test samples with modified surfaces (fabrics, papers, granular materials, ceramics, plastics, and glass). The method provides a good contact between the bacteria and the tested sample through the constant stirring of the sample in a bacterial suspension. The antibacterial efficiency was studied depending on the contact time (1 min; 1; 2; 3; 4 and 24 hours) between the bacterial solution and the sample. At the appropriate time, 1 ml of the bacterial solution was transferred from the test-tube with a sample onto the surface of an agar. Then the agars were incubated at 37 °C for 24 hours. Finally, viable bacteria were monitored by counting the number of colony-forming units on nutrient agar plates.Measurement of antibacterial filtration efficiency
This part of the experiment is particularly important from the point of view of an evaluation of the practical application of the samples under real conditions of the bacterial air filtration. The bacterial filtration efficiency of pristine and modified nanofibers was tested using a special AMFIT 13 (Anti-Microbial Filtration Tester) device. The AMFIT 13 (Fig. 2) was used to verify the extent to which the filter is able to prevent the penetration of aerosolized inoculum with bacteria to the purifying area.This measurement is obtained by simulating a passage of the aerosolized contaminated inoculum through the tested sample. The presence of bacteria, which were injected into the testing apparatus and which passed through the filter media, is analysed. Petri dishes with agars were placed at the end of the apparatus to determine the number of bacteria in the device.
The bacteria were captured on the surface of the agars and detected after incubation for 24 hours at 37 °C. The bacterial filtration efficiency (% BFE) is defined similarly to the case of particulate filtration according to the eqn (3):
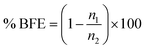 | (3) |
Stability of antibacterial properties
All of the produced samples were tested under simulated water filtration conditions. We decided to determine the stability of the antibacterial activity of filters based on the results of the water filtration test because these conditions are more aggressive than air filtration and the probability of washing-out the bed-fixed particles is higher. A constant flow of water was passed through each sample at a flow rate of 3 l min−1 for duration of 8 hours (1440 l through each sample). The quantitative antibacterial test (Cornell) was repeated after the water filtration test with the aim to verify stability of the antibacterial properties and fixation of CuO particles into the structure of the nanofibers. The EDX analysis was also iterated after this filtration test to make sure that content of the modifier at the surface of samples hadn't changed.Results and discussions
Viscous properties of modified polymer solutions
The first step in our research was to compare the viscosities of a pristine PU solution with solutions modified with micro- or nanoparticles. This is important because a major increase in viscosity may serve as an initial indication of the fact that the solution is not suitable for processing using the electrospinning technique. According to the results of our measurements, the average viscosities of the solutions with modifiers are higher than the viscosity of the non-modified polyurethane solution (first column in Fig. 3, denoted as 0%). Comparison of the effect of micro- and nanoparticles on the rheological behaviour of polymer solutions has a special significance in terms of the influence of these additives on the structural and dimensional characteristics of the future composite nanofibers. As it is shown in Fig. 3, the measured values of viscosity for the solutions with NPs exceed the respective values for the solutions with microparticles. A NPs concentration of 2.5% does not lead to a considerable change in the viscosity value compared with the solution with microparticles. But from a concentration of 5%, the viscosity values for solutions with NPs significantly exceed the corresponding measurements of solutions with microparticles. This indicates a greater tendency of the nano-sized additive to form aggregates in the polymer solution due to its high surface area-to-volume ratio. Therefore, suspicion arises that solutions with NPs are not suitable for processing into NFs by ES.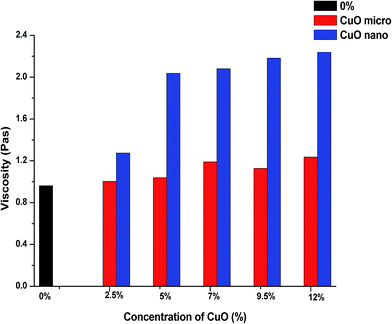 | ||
| Fig. 3 Comparison of the average viscosities of the pristine PU solution and solutions with micro- and nanoparticles of CuO. | ||
But running a few steps ahead we can say that technological problems with the processing of all of the modified solutions were not revealed despite such high values of viscosities of the solutions with NPs.
Structure of the produced nanofibers
The SEM analysis provided the internal morphology of the produced mats. Fig. 4 shows images of the nanofibers. Particular attention should be paid to the photos of fibers with the content of nano-sized copper oxide (Fig. 4c, e, g and i). It is clear that the nanoparticles form quite large aggregates. The higher viscosity of the solutions with nanoparticles has already indicated the same tendency. The unique functional properties (including antibacterial) of nanoparticles are caused by their small size, which provides them with an extensive surface area. Therefore, the formation of aggregates can be an obstacle to the manifestation of the antibacterial properties of the nanoparticles in full. In addition, there are doubts about the stability of such aggregates under further filtration application of the samples.The produced nanofibrous layers exhibited a smooth surface with a distribution of diameters in the range of 75–650 nm. We observed a visible brown colour, which became more intensive with increasing concentrations of CuO. The average fiber diameter was calculated for each sample. The use of copper oxide led to an insignificant increasing in the average diameter of polyurethane nanofibers (Table 1).
| Sample | Number average An (nm) | 95% confidence | Weight average Aw, (nm) | Fibre uniformity coefficient K (Aw/An) |
|---|---|---|---|---|
| Pristine PU | 182 | 5.4 | 194.5 | 1.07 |
| PU + 5% CuO μm | 226 | 6.2 | 239 | 1.06 |
| PU + 5% CuO nm | 228 | 6.04 | 239 | 1.05 |
| PU + 7% CuO μm | 278 | 8.5 | 298 | 1.07 |
| PU + 7% CuO nm | 262 | 5.97 | 270 | 1.03 |
| PU + 9.5% CuO μm | 242 | 6.9 | 257 | 1.06 |
| PU + 9.5% CuO nm | 237 | 6.1 | 249 | 1.05 |
| PU + 12% CuO μm | 231 | 5.7 | 249 | 1.08 |
| PU + 12% CuO nm | 226 | 6.9 | 240 | 1.06 |
The surface density of the prepared nanofibrous layers was calculated to compare the spinning performance depending on the concentrations and size characteristics of copper oxide (Table 2). The obtained data showed that both micro- and nanoparticles have contributed to an increase in the surface density of all of the modified nanofibers in comparison with the pristine PU mat. This effect is explained by well-known conductive properties of copper. We can observe (Table 2) that the lowest concentration (5%) of CuO MPs provides an almost 5-fold improvement to this characteristic. The positive impact of nm CuO persists to a concentration of 7%. The surface density index of the sample with 12% of CuO NPs decreased and became approximately equal to the respective index of the nanofibrous layer with 5% of nanoscale modifier. Higher concentrations of nanoparticles (9.5 and 12%) lead to the formation of larger amounts and/or sizes of aggregates. This may lead to deterioration of the functional properties of the nanoparticles, including their electrical conductivity. Nevertheless, the key conclusion is that micro- and nanoparticles of copper oxide are not merely additives used to impart antibacterial properties but they also contribute to a significant improvement of the electrospinning performance in production of the polyurethane nanofibers. Usually, even small concentrations of additives for the increase of the ES performance promote a substantial thickening of the fibers diameters. However, this was not observed in the case of comparatively high concentrations (5–12%) of copper oxide.
| Size and concentration of CuO | Surface density of fibres (g m−2) |
|---|---|
| PU pristine | 2.5 |
| PU + 5% CuO μm | 12.28 |
| PU + 5% CuO nm | 4.56 |
| PU + 7% CuO μm | 13.05 |
| PU + 7% CuO nm | 9.89 |
| PU + 9.5% CuO μm | 13.93 |
| PU + 9.5% CuO nm | 7.41 |
| PU + 12% CuO μm | 19.46 |
| PU + 12% CuO nm | 5.38 |
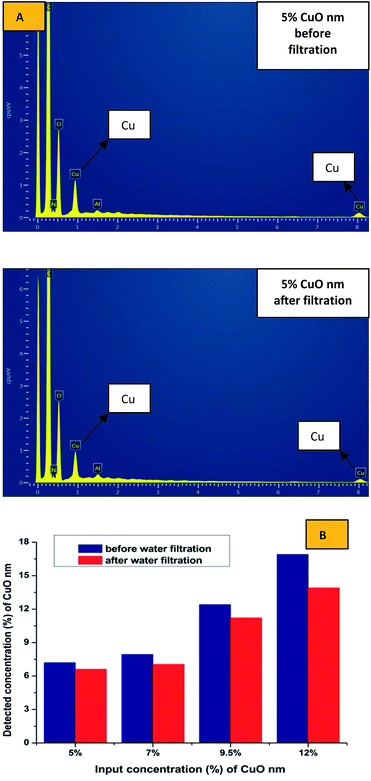
SEM-EDX analysis was performed to confirm the presence and approximate percentage content of micro- and nanoparticles of CuO in the structure of the nanofibers. Extra peaks responsible for Cu appeared for all of the produced samples, except pristine PU nanofibers (Fig. 5).
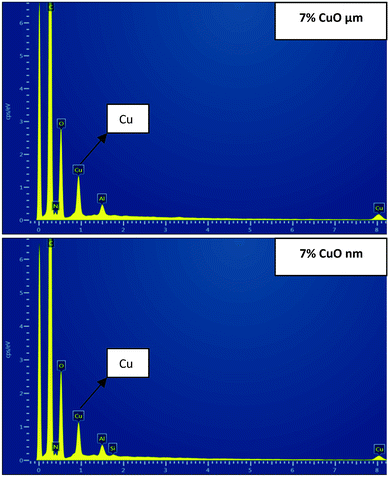 | ||
| Fig. 5 SEM-EDX images: areas of electrospun polyurethane nanofibers with 7% of micro- and nanoparticles of CuO. | ||
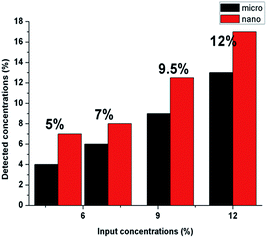
The detected amounts of CuO microparticles better correspond to the introduced concentrations. But it was determined that detected concentrations of CuO nanoparticles for all of the samples are higher than the introduced amounts of the modifier (Fig. 6). This may indicate an uneven distribution of the nanoparticles in the structure of the fibers for the whole concentration range. Such result can be explained by the tendency to aggregate and corresponds to the high viscosity values of the PU solutions with CuO NPs. The tendency of nano-sized modifiers to aggregate is logical enough from the point of view of their high surface energy. But this may negatively influence their antibacterial properties.
Antibacterial efficiency of composite layers
The values of antimicrobial efficiency for all of the modified fibers after 24 hour's contact between the bacterial solutions and tested samples are included in Table 3. It is possible to observe that the antibacterial activity grew with an increase in CuO concentrations for both sizes of particles. There is no particular difference between the antibacterial properties of the samples with micro- and nanoparticles against the E. coli strain. We can conclude that all of the produced composite layers with a content of CuO particles in the concentration range from 7 to 12% demonstrated excellent activity against the Gram-negative strain. The test results for the samples with micro- and nanoparticles against Staphylococcus gallinarum are slightly different. Nanofibers with microparticles showed higher activity against Gram-positive strain, but the negative distinction is evident only for the nanofibrous substrates with 5% of CuO NPs (Table 3).| Sample | Efficiency (%) – Escherichia coli | Efficiency (%) – Staphylococcus gallinarum | ||
|---|---|---|---|---|
| μm | nm | μm | nm | |
| PU + 5% CuO | 97 | 96.8 | 98.8 | 62.7 |
| PU + 7% CuO | 99.7 | 99.8 | 100 | 98.2 |
| PU + 9.5% CuO | 100 | 100 | 100 | 98.8 |
| PU + 12% CuO | 100 | 100 | 100 | 99.6 |
The change of antibacterial efficiency over time (from the minimum contact time between the sample and the bacteria (1 min) to the maximum contact time (24 hours)), is graphically represented in Fig. 7.
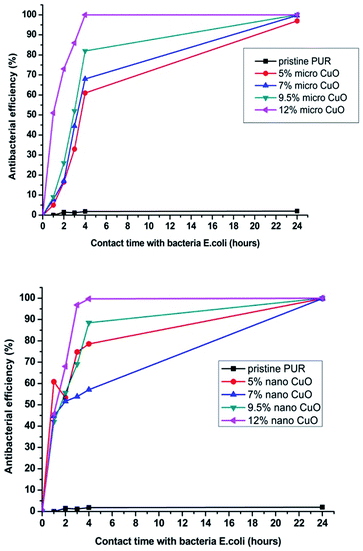 | ||
| Fig. 7 Change of antibacterial efficiency against E. coli over time for samples with micro- or nanoparticles of CuO. | ||
First of all, we can conclude that pristine PU nanofibers did not exhibit activity after a prolonged contact time. This means that the selected polymeric material without proper modification is inert to bacteria. The second important conclusion is that nanoparticles started to exhibit their antibacterial efficiency faster (after a 1 hour contact time) than microparticles (after 4 hours, Fig. 7). However, in terms of the filtration application there is no fundamental difference if the captured bacteria start to perish within 1 or 4 hours after being captured on the filter. But it is important that antimicrobial properties of the modified layers with both particles sizes are almost identical after 24 hour contact between the bacteria and the samples (Table 3 and Fig. 7 and 8).
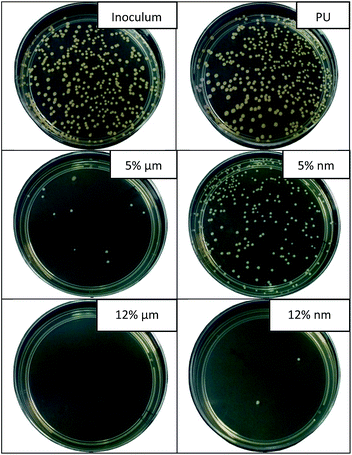 | ||
| Fig. 8 Images of agar plates showing the results of antibacterial tests against Staphylococcus gallinarum (contact time 24 hours). | ||
Fig. 8 provides the confirmation of the results of the antibacterial test with St. Gal. for samples with 5 and 12% of MPs and NPs of CuO, respectively (contact time 24 hours). The number of grown bacterial colonies for the inoculum (reference test without the sample) and for non-modified fibers is similar. We observe that the concentration of 5% of CuO NPs isn't sufficient to impart PU nanofibers with good antimicrobial properties against St. Gal. The results of viscous measurements and SEM/EDX analysis show that NPs form large aggregates in the polymer solution and in the structure of the fibers. This leads to a loss of the unique properties caused by the nanoscale characteristics of the particles. This statement was confirmed by the results of the antibacterial tests, where the significant advantages of the nanoparticles were not observed.
Antibacterial filtration efficiency
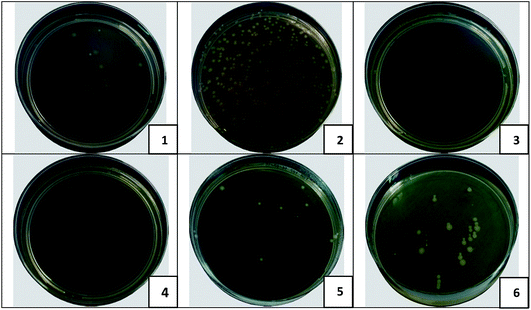
The results of the bacterial filtration test correspond to the values of the surface density for all prepared samples. As it was mentioned before, the surface density of the nanofibers modified by MPs was higher in comparison with layers containing NPs. These results may lead us to the assumption that it is sufficient to use nanofibers with high surface density for the bacterial filtration and it is not necessary to pay attention to the antibacterial modification of the nanolayers. However, this assumption is erroneous. The capture of bacteria on the surface of the filter is only the first task to be solved. The second important objective is to eliminate the trapped bacteria, and it is at this stage that the antibacterial agents will play a key role.We can observe in Table 4 that results of the “smear test” confirmed the antibacterial activity of all of the modified nanofibers in eliminating the captured bacteria after the bacterial filtration test. The samples with 9.5 and 12% of MPs demonstrated the most impressive results as the complete elimination of trapped bacteria was observed. Fig. 9 is provided to illustrate the difference in the behaviour of the pristine and modified (with 9.5% of μm and nm CuO) PU layers after bacterial filtration and “smear” tests.
| Sample | Number of bacteria passing through the sample | BFE (%) | Number of survived bacteria after the “smear-test” |
|---|---|---|---|
| Inoculum | 320 | — | — |
| PU pristine | 17 | 95 | 278 |
| PU + 5% CuO μm | 5 | 98 | 6 |
| PU + 5% CuO nm | 15 | 95 | 13 |
| PU + 7% CuO μm | 0 | 100 | 3 |
| PU + 7% CuO nm | 9 | 97 | 45 |
| PU + 9.5% CuO μm | 0 | 100 | 0 |
| PU + 9.5% CuO nm | 11 | 96.6 | 30 |
| PU + 12% CuO μm | 0 | 100 | 0 |
| PU + 12% CuO nm | 11 | 96.6 | 19 |
Nanofibers with content of CuO microparticles have proven to be more efficient for bacterial air purification. The micro-modified nanofibrous layers are able to capture more bacterial units due to their higher surface density. Homogeneous distribution of MPs, without the formation of large aggregates, into the structure of the NFs provides more efficient elimination of the captured bacteria.
Stability of the antibacterial properties of modified nanofibers
The most important aim of this research was to confirm whether the particles of CuO were securely fixed into the structure of the nanofibrous matrix. Therefore, each sample was treated under the simulated conditions of water filtration. EDX analysis and antibacterial tests were repeated to compare the amount of copper on the surface of the fibres and the antimicrobial efficiencies of samples before and after the water treatment test (Table 5 and Fig. 10).| Tested sample | Efficiency (%) – Escherichia coli | Efficiency (%) – Staphylococcus gallinarum | ||
|---|---|---|---|---|
| Before filtration | After filtration | Before filtration | After filtration | |
| PU + 5% CuO μm | 97 | 97.2 | 98.8 | 98.5 |
| PU + 5% CuO nm | 96.8 | 86.9 | 62.7 | 30.9 |
| PU + 7% CuO μm | 99.7 | 99.3 | 100 | 100 |
| PU + 7% CuO nm | 99.8 | 91 | 98.2 | 80 |
| PU + 9.5% CuO μm | 100 | 100 | 100 | 100 |
| PU + 9.5% CuO nm | 100 | 96.8 | 98.8 | 78 |
| PU + 12% CuO μm | 100 | 99.8 | 100 | 99.7 |
| PU + 12% CuO nm | 100 | 88.7 | 99.6 | 79 |
No difference was found between results of the EDX analysis of the composite samples with microparticles before and after the water filtration test. As we can see in the Fig. 10B, some number of the nanoparticles of copper oxide was poorly fixed into the structure of the produced layers; and as we previously established, the nanoparticles formed sufficiently large aggregates in the fibrous structure. Some particles in the structure of these aggregates are not immobilized inside of the polymer matrix and may be associated with the neighbouring NPs only by the physical interaction. This makes it possible to explain the observed tendency of the nano-sized additive to wash out.
In another study it was determined that CuO NPs antibacterial effect originated from both the released Cu2+ ions and the CuO nanoparticles themselves.17 We suppose that our results have indirectly confirmed this idea. Deterioration of the antibacterial efficiency of the nanofibers with NPs and reduction of the nano-sized CuO concentration after water filtration test can be explained by the release of Cu2+ ions into the aqueous medium. Microparticles don't possess sufficient surface energy for release of the metallic ions. So the micro-sized additive provides antibacterial effect due to the contact of CuO molecules with bacterial cells. In such case the damage of cellular wall requires longer contact time between bacteria and CuO. This assumption was also experimentally confirmed (Fig. 7).
Nevertheless, the results of the EDX analysis from the standpoint of the percentage ratio of the detected compounds are only approximate. A more demonstrative criterion of particle fixation will be the results of the repeated antibacterial tests for samples, which were used for water filtration. For this purpose, the nanofibers with micro- and nanoparticles were investigated using the Cornell test to determine their efficiency against E. coli and St. Gal. after the test under simulated conditions of the water filtration (Table 5).
The change in antibacterial properties after the water filtration test is the most important criterion for the selection of an appropriate material for water filtration application. Only filters with stable and long-term antibacterial activity can be considered as suitable for this use, and PU nanofibers with CuO nanoparticles do not fulfil these requirements. The release of NPs into the environment is potentially dangerous and should be strongly controlled even on an experimental level. So from a technological point of view, the used modification method did not sufficiently prevent the aggregation of nanoparticles, which leads to a decrease in their antibacterial activity and to problems with fixation into the structure of the fibers.
But on the other hand, it can be concluded that microparticles are an appropriate additive for the selected method of nanofiber modification than NPs. Due to the water filtration tests it was proven that the modified nanolayers with microparticles of copper oxide have stable and durable antibacterial properties. The results of the presented research allow to state that produced composite nanofibrous filters with micro-sized additive are a perspective candidate for the antibacterial water purification.
Conclusions
Polyurethane nanofibers were modified by micro- and nanoparticles of copper oxide in order to impart them antibacterial properties and to compare the influence of the dimensional characteristics of modifiers on the structure of nanofibrous layers, antibacterial efficiency and stability of particle fixation.No apparent advantages of nanoparticles in relation to microparticles were found in terms of the future application of our samples for the antibacterial filtration. The inhibitory effect of nanoparticles appears a little faster. But the aim of antimicrobial modification of filters is the need to ensure the elimination of the captured bacteria. So there is no principal difference if the vital functions of bacteria will be suppressed in an hour or in four hours after the capturing on the surface of filter. We think that the explanation of the obtained results lies in the tendency of NPs to aggregation. Due to the formation of big aggregates, nanoparticles lose their major advantage – a larger surface area in relation to the volume. So the deterioration of their functional properties as a result of aggregation is quite expected.
The key indicator of the successful antibacterial modification of filters is the stability of the fixation of the used antimicrobial substances. The water filtration test had demonstrated the washing-out of NPs whereas microparticles were securely fastened in the structure of the nanofibers.
The samples with microparticles demonstrated higher values of the BFE under the conditions of air filtration. It was expected because the surface density of these filters was higher. However these samples have been proven to be more effective in the elimination of captured bacteria than the nanofibers with nanoparticles. Such results are explained by the aggregation of NPs in the structure of fibers. So we can conclude that microparticles of CuO are more appropriate additives for the antibacterial modification of PU nanofibers for the filtration application than NPs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic in the framework of targeted support “National Programme for Sustainability I”.References
- J. Fawell and M. J. Nieuwenhuijsen, Contaminants in drinking water, Br. Med. Bull., 2003, 68, 199–208 CrossRef CAS PubMed.
- C. Pasquarella, O. Pitzurra and A. Savino, The index of microbial air contamination, J. Hosp. Infect., 2000, 46(4), 241–256 CrossRef CAS PubMed.
- N. Beyth, Y. Houri-Haddad, A. Domb, W. Khan and R. Hazan, Alternative antimicrobial approach: Nano-antimicrobial materials, J. Evidence-Based Complementary Altern. Med., 2015, 2015, 246012 Search PubMed.
- S. M. Dizaj, F. Lotfipour, M. Barzegar-Jalali, M. H. Zarrintan and K. Adibkia, Antimicrobial activity of the metals and metal oxide nanoparticles, Mater. Sci. Eng., C, 2014, 44, 278–284 CrossRef CAS PubMed.
- M. Hans, A. Erbe, S. Mathews, Y. Chen, M. Solioz and F. Mücklich, Role of copper oxides in contact killing of bacteria, Langmuir, 2013, 29(52), 16160–16166 CrossRef CAS PubMed.
- H. Palza, Antimicrobial polymers with metal nanoparticles, Int. J. Mol. Sci., 2015, 16(1), 2099–2116 CrossRef CAS PubMed.
- J. Fang, X. Wang and T. Lin, Nanofibers – production, properties and functional applications, InTech, Rijeka, Croatia, 2011, vol. 14, pp. 287–326 Search PubMed.
- K. Graham, M. Ouyang, T. Raether, T. Grafe, B. Mcdonald and P. Knauf, Polymeric Nanofibers in Air Filtration Applications, Fifteenth Annu Tech Conf Expo Am Filtr Sep Soc., 2002, pp. 9–12 Search PubMed.
- F. A. Sheikh, M. A. Kanjwal, S. Saran, W.-J. Chung and H. Kim, Polyurethane nanofibers containing copper nanoparticles as future materials, Appl. Surf. Sci., 2011, 257(7), 3020–3026 CrossRef CAS.
- R. Nirmala, K. S. Jeon, B. H. Lim, R. Navamathavan and H. Y. Kim, Preparation and characterization of copper oxide particles incorporated polyurethane composite nanofibers by electrospinning, Ceram. Int., 2013, 39(8), 9651–9658, DOI:10.1016/j.ceramint.2013.05.087.
- H.-J. Choi, S. B. Kim, S. H. Kim and M.-H. Lee, Preparation of electrospun polyurethane filter media and their collection mechanisms for ultrafine particles, J. Air Waste Manage. Assoc., 2014, 64(3), 322–329 CAS.
- Y. K. Kang, C. H. Park, J. Kim and T. J. Kang, Application of electrospun polyurethane web to breathable water-proof fabrics, Fibers Polym., 2007, 8(5), 564–570 CrossRef CAS.
- H. Daemi and M. Barikani, Molecular engineering of manipulated alginate-based polyurethanes, Carbohydr. Polym., 2014, 112, 638–647, DOI:10.1016/j.carbpol.2014.06.023.
- Y. Chang, M. Zhang, L. Xia, J. Zhang and G. Xing, The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles, Materials, 2012, 2850–2871 CrossRef CAS.
- S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi and R. Kumar, Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites - A review, Prog. Polym. Sci., 2013, 38(8), 1232–1261, DOI:10.1016/j.progpolymsci.2013.02.003.
- F. Cengiz and O. Jirsak, The effect of salt on the roller electrospinning of polyurethane nanofibers, Fibers Polym., 2009, 10(2), 177–184 CrossRef CAS.
- D. Wang, Z. Lin, T. Wang, Z. Yao, M. Qin and S. Zheng, et al., Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both?, J. Hazard. Mater., 2016, 308, 328–334, DOI:10.1016/j.jhazmat.2016.01.066.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06317b |
| This journal is © The Royal Society of Chemistry 2017 |

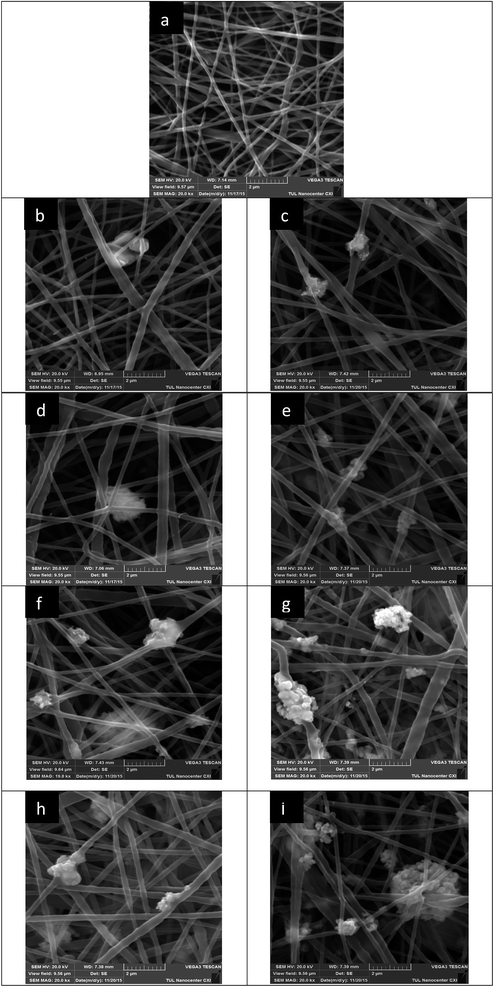
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of nanofibers with different concentrations of micro- or nanoparticles of CuO: (a) 0%; (b) 5% μm; (c) 5% nm; (d) 7% μm; (e) 7% nm; (f) 9.5% μm; (g) 9.5% nm; (h) 12% μm; (i) 12% nm.
000) of nanofibers with different concentrations of micro- or nanoparticles of CuO: (a) 0%; (b) 5% μm; (c) 5% nm; (d) 7% μm; (e) 7% nm; (f) 9.5% μm; (g) 9.5% nm; (h) 12% μm; (i) 12% nm.