Timm Lankau and Chin-Hui Yu
Green Chem., 2016,18, 1590-1596
DOI:
10.1039/C5GC02192H,
Paper
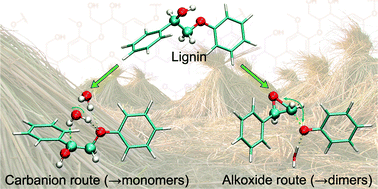
Monomer yields from the base catalysed depolymerisation (BCD), which has recently evolved as a promising first step in the valorisation of lignin, are severely limited by the repolymerisation of the monomers. DFT calculations with β-phenoxy-α-phenylethanol as a model for the βO4′ link in lignin are used to explore various routes for the BCD process and the repolymerisation of its monomers. The depolymerisation proceeds via a resonance-stabilised carbanion, while intermediate oxiranes and formaldehyde, which originates from a second reaction path, are the key compounds in the repolymerisation. It is concluded that controlling the reactive intermediates such as the oxirane in the BCD process will increase monomer yields.