Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The impact of plug-in fragrance diffusers on residential indoor VOC concentrations†
Thomas
Warburton
 a,
Stuart K.
Grange
a,
Stuart K.
Grange
 ab,
James R.
Hopkins
ac,
Stephen J.
Andrews
ac,
Alastair C.
Lewis
ab,
James R.
Hopkins
ac,
Stephen J.
Andrews
ac,
Alastair C.
Lewis
 *ac,
Neil
Owen
d,
Caroline
Jordan
d,
Greg
Adamson
e and
Bin
Xia
f
*ac,
Neil
Owen
d,
Caroline
Jordan
d,
Greg
Adamson
e and
Bin
Xia
f
aWolfson Atmospheric Chemistry Laboratories, University of York, York, YO10 5DD, UK. E-mail: ally.lewis@ncas.ac.uk
bEmpa, Swiss Federal Laboratories for Materials Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland
cNational Centre for Atmospheric Science, University of York, York, YO10 5DD, UK
dGivaudan UK Ltd, Kennington Road, Ashford, TN24 0LT, UK
eGivaudan Fragrances Corp., 717 Ridgedale Ave, East Hanover, New Jersey 07936, USA
fBath & Body Works Inc., 8885 Smiths Mill Road, New Albany, OH 43054, USA
First published on 8th March 2023
Abstract
Plug-in fragrance diffusers are one of myriad volatile organic compound-containing consumer products that are commonly found in homes. The perturbing effects of using a commercial diffuser indoors were evaluated using a study group of 60 homes in Ashford, UK. Air samples were taken over 3 day periods with the diffuser switched on and in a parallel set of control homes where it was off. At least four measurements were taken in each home using vacuum-release into 6 L silica-coated canisters and with >40 VOCs quantified using gas chromatography with FID and MS (GC-FID-QMS). Occupants self-reported their use of other VOC-containing products. The variability between homes was very high with the 72 hour sum of all measured VOCs ranging between 30 and >5000 μg m−3, dominated by n/i-butane, propane, and ethanol. For those homes in the lowest quartile of air exchange rate (identified using CO2 and TVOC sensors as proxies) the use of a diffuser led to a statistically significant increase (p-value < 0.02) in the summed concentration of detectable fragrance VOCs and some individual species, e.g. alpha pinene rising from a median of 9 μg m−3 to 15 μg m−3 (p-value < 0.02). The observed increments were broadly in line with model-calculated estimates based on fragrance weight loss, room sizes and air exchange rates.
Environmental significanceThe effects of using of a single VOC-emitting domestic product – a plug-in fragrance diffuser – on changing indoor concentrations of VOCs were evaluated in 60 homes. Its use led to detectable increases in α-pinene of a few μg m−3 in homes with the lowest air exchange rates (inferred from indoor CO2). A model was developed that estimated indoor increments in VOC concentrations for a given product emission rate, room size and air changes per hour. |
1. Introduction
The use of room fragrance products is an acknowledged source of emissions of a range of different volatile organic compounds (VOCs) indoors. Quantifying the emission rate characteristics and speciation of fragranced products is central to understanding how they may impact indoor air in real-world settings.1 The wider health effects of VOCs indoors are well explored and documented; it is clear that long-term exposure to high concentrations of certain VOCs may carry with it an increased likelihood of experiencing negative health effects.2 In 2019, Public Health England (PHE) released a set of recommended exposure limits for selected VOCs in indoor settings, based on literature data collated by Shrubsole et al.3,4 Not all VOCs are included in these guidelines, unsurprising given that many thousands of different VOCs exist. For many VOCs there are still some basic uncertainties related to what are representative concentrations indoors, the variability between different indoor spaces, and the emission sources that control concentrations. VOCs such as monoterpenes (including α-pinene and limonene) have particularly complex emissions. They are released from natural sources such as building materials, plants and food products and are also included as fragrance ingredients in many cleaning and personal care products. There is limited information on individual source strengths and a general lack of quantitative assessment of the contributions made to indoor VOCs from individual commercial products. This limits the development of regulatory and/or industry guidance and best practice that might be effective in managing VOC emissions and overall exposure.Previous work evaluating the relationship between air fresheners, room fragrances and indoor air quality has been mixed, with only limited relationships found between air fresheners/room fragrance use and elevated VOC concentrations in real-world indoor residential settings.5,6 This is in contrast to a larger number of laboratory studies which have evaluated emissions from air fresheners/room fragrances in (often smaller) test chambers, with marked increases in VOC concentrations after product use.7,8 It has also been shown in test chambers that removal of room fragrance products, such as plug-in diffusers, results in a rapid decay in concentration of those VOCs derived from the fragrance formulation.8 This is in contrast to real-world studies which have shown a lingering of VOCs indoors after products have been removed.9,10 A possible reason for this discrepancy is the absence of representative surface materials such as building materials and room furnishings within test facilities – materials which may act as temporary reservoirs for VOCs. This surface sink effect can also be considered as a mechanism for VOC removal.11
The effects of exposure to high VOC concentrations are complex and varied, ranging from mild-to-moderate exacerbation of respiratory symptoms to cardiac rhythm interruption.12–14 While ambient concentrations typically found in residential settings are unlikely to contribute to widespread acute health effects, long-term exposure to VOCs in residential settings is less well evidenced in terms of impacts. Previous studies exploring links between the exacerbation of asthma symptoms and allergic reactions to VOC exposure have been inconsistent in their conclusions, and sometimes of poor design quality.15 There are studies, however, which highlight the importance of monitoring VOC concentrations to mitigate potential exacerbation of respiratory symptoms in vulnerable people.16,17 Additionally, a recent study has shown a correlation between VOC metabolites, indicative of personal VOC exposure, and atopic dermatitis.18
Analytical approaches to sampling VOCs can vary in indoor air studies. Diffusive air sampling using sorbent-packed tubes remains a popular and low-cost option allowing for bulk testing. Sorbent tube sampling followed by thermal desorption (TD) and gas chromatographic (GC) methods remains the international standard for VOC identification in indoor and test chamber facilities, ISO 16000-6: 2021. However, there are limits to the VOCs that can be sampled, which are dependent on the sorbent materials used, as well as potential interferences and reduced species sensitivity from the co-adsorption of water and ozone.19,20 Whole air sampling using silica-treated inert canisters is another possible technique and is used in this study. This uses a canister evacuated to vacuum, then exposed to either the test chamber, target room, or connected to a sampling gas line. With sorbent tubes air can enter through natural diffusion or be actively pumped. In vacuum-treated canisters air enters due to a differential in pressure, often through a fixed flow or critical orifice restrictor. Active sampling, either onto tubes, or using vacuum-treated inert canisters generally requires more expensive equipment however it offers a quantitative route to measuring the most volatile VOCs.21
The relationships between amount/frequency of chemical product use and total volatile organic compound (TVOC) concentrations found indoors are complex, with literature results varying between no relationships seen, to significant correlation between the two variables.5,22,23 This is likely influenced by factors such as testing conditions, locations and sampling methods as well as being impacted by a lack of common methodology and speciation. Intuition would suggest that using VOC-containing products such as cleaning products or personal care products should increase VOC concentrations within the room the product was used in. Indeed, it has been found that VOCs typically found in cleaning products and VOCs emitted through cooking tend to dominate indoor air samples and include some fragrance VOCs.24–26 Increases in frequency of cleaning and cooking activities in indoor settings might therefore be reflected in an increase in concentrations of specific marker VOCs. Plug-in diffusers belong to the class of air fresheners, differing from most products in that fragrance output is kept constant over a period of time. These devices use mains electricity to deliver a constant low heat to aid in the diffusion of the fragrance oil through a wick. Some devices may have variable output settings, resulting in a varied emission rate. In this study the device used had a fixed output rate.
The effect of ventilation rate (commonly expressed either as air exchange rates (AER) or air changes per hour (ACH)) on VOC concentrations is well established, with the relationship between the two inversely proportional; as AER increases, indoor VOC concentrations decrease for constant indoor emission rates.6,27 The relative influence of variable ventilation rates compared with varying usage of VOC-containing products is uncertain, in large part because individual activity- and product-based emission rates are not well defined and AER is building- and occupant-specific. There has been some investigation of the relationships between temperatures, sink effects and ventilation rates on VOC concentrations, with results showing a dominance of ventilation over temperature and sink effects for short periods.28 The measurement of AER in real settings requires key information for its calculation, such as internal and external carbon dioxide mole fractions/ppm, room occupancy, room volume, as well as CO2 generation within the room.29 This data can prove difficult to obtain for large scale residential indoor air studies, which necessitates often the use of proxy indicators for AER.
CO2 has long been used as an indicator for ventilation rates, both in terms of indoor pollutant exposure and risk to airborne-disease infection, which has been particularly prominent during the SARS-CoV-2 pandemic.23,30,31 The work of Jia et al. (2021)32 demonstrated a link between CO2 and total VOC concentration in highly-occupied university lecture theatres, as well as a link between room occupancy levels and TVOC concentrations. This is potentially helpful as it means AER, CO2 concentrations and TVOC may be inferred from one another, if only a subset of these data were available. We note however that the terminology and definition of TVOC is problematic in so much as it is operationally defined by the method of measurement and is rarely calibrated or directly comparable between studies. Whilst there is no single definition of what TVOC means, it remains a widely quoted metric in indoor air studies, and despite the lack of comparability it has utility as a means for any study to express the totality of VOC behaviours. The methods used in this paper differ from Jia et al., who used photoionisation detectors (PID) to evaluate TVOC concentrations, and nondispersive infrared (NDIR) sensors to measure CO2 concentrations continuously. Here TVOC concentrations are calculated by summing all individually measured VOCs, and CO2 determined through using laser absorption spectroscopy, both based on a 3 day sampling period. While the method in this paper does not show real-time changes in atmospheric VOC composition related to changes in room occupancy, activities or product use, it does give a representative insight into summed VOC/CO2 concentrations over time, and it illuminates whether the relationship shown by Jia et al. is seen in residential settings using an alternative methodology. A study by Murakami et al. (2019)33 showed a weak positive correlation between TVOC and CO2 concentrations in classrooms with air conditioning, however, the TVOC definition in the work of Murakami et al. differed again to that of Jia et al. These differences in measurement methods and operational definitions of TVOC does make comparison between studies difficult.
1.1 Objectives
The purpose of this study was to identify in real-world home settings the incremental impacts on ambient indoor VOC concentrations arising from using a single well-controlled VOC-emitting consumer product with known emission rate. The study was conducted across a group of 60 homes in Ashford, UK. Having established a baseline set of indoor VOC observations in all homes,5 a commercially available plug-in diffuser was used releasing VOCs from a modified commercial fragrance formulation. The study split homes into those that would use the product (switched on), and those where it was switched off, the latter acting as a parallel control group. The use throughout of a control group was to account for any systematic and large-scale changes to ventilation rates arising from regional scale meteorological effects acting across the cohort as a whole, for example high windspeeds systematically increasing AER or high/low temperatures leading to changes in window opening. The study explored the relationships between use of the plug-in diffuser, observed VOCs (both individual VOCs and summed VOC amount), indoor CO2 mole fraction (as a proxy for AER) and wider self-reported chemical product use statistics from occupants in the homes.2. Methodology
2.1 Experimental methodology
Homes were selected from a pre-existing non-trained fragrance industry panel cohort based in the Ashford area, UK. Each homeowner was given a commercially available mains-powered fragrance diffuser (referred to as ‘the diffuser’) with known liquid fragrance formulation. The fragrance was contained in a dipropylene glycol carrier liquid. Homes were split into two groups of 30 homes, each group studied over a four-to-five-week period. The first 30 homes were sampled between October and December 2021, and the second 30 homes between January and March 2022. Sampling was into 6 Litre stainless-steel canisters internally treated with silica (Restek, PA, USA and Entech, CA, USA) attached to a flow restrictor to limit sample into the canister to 1 Litre every 8 hours (∼2 mL min−1). Heeley-Hill et al. found these flow restrictors to sample linearly over a 48 hour period, with a reduced rate between 48 hours and 72 hours, achieving the full 6 L sample at ambient pressure after 72 hours.5 Canisters were tagged with unique identifiers evacuated to at least 170 Pa then sent for dispersal among the participants. Samples were taken over weekend periods, starting at 7 am on Friday and finishing at 7 am the following Monday. Filled samples were then returned for processing and analysis. Canisters were then evacuated once again, and the process repeated. Personal details relating to the participants and their homes were fully blinded to the University of York and anonymity of the participants maintained throughout the study.At least four consecutive weekend samples were taken from each home:
(1) an initial sample with the diffuser switched off (baseline sample),
(2) a sample taken after the diffuser had been switched on at the start of the in-use period,
(3) a further sample taken a week later from (2) where the diffuser remained switched on throughout and,
(4) a final sample taken several days after the diffuser had been switched off (post-diffuser sample).
This gave as a minimum two ‘diffuser off’ samples and two ‘diffuser on’ samples for each home. 15 houses from each group provided a second post-diffuser sample two weeks after the diffuser was switched off.
On return to the lab the canisters were pressurised and thus diluted to 1 bar (gauge pressure) with highly purified air, free of VOCs. This diluent air was purified by passing through a bed of platinum beads heated to 400 °C to fully oxidise any VOCs present. The same purified air was directly analysed by the instrument in order to quantify any impurities or interfering compounds contained therein (none were found). Samples were analysed using a custom thermal desorption unit (TDU) coupled to an Agilent 7890A gas chromatograph (GC) fitted with flame ionisation detectors (FID) and an Agilent 5977A quadrupole mass spectrometer (QMS) manufactured by Agilent Technologies, CA, USA (GCFID-QMS). A flow chart for the system can be seen in ESI Fig. 1.†
Sample canisters and blank samples were connected to the TDU through a 16-port Valco microelectric actuator multi-position valve (VICI Valco Instruments Co. Inc., TX, USA). 500 mL was withdrawn from each sample and first passed through a water trap, comprising a 30 cm length of 1/16′′ silica-coated stainless-steel tube held at −40 °C, which removed moisture from the samples. The dried samples were then passed through a pre-concentration trap comprising a 30 cm length of 1/16′′ silica-coated stainless-steel tube packed with both Carbopack™ X 40–60 mesh and Carbopack™ B 60–80 mesh adsorbents (Sigma-Aldrich, subsidiary of Merck KGaA, Darmstadt, Germany) fritted with glass wool and held at the lowest achievable temperature the unit could maintain, always less than −120 °C. After sample collection, the pre-concentration trap was warmed to −80 °C and purged with helium carrier gas (in the same direction of flow) for 4 minutes to remove CO2 from the trap which would otherwise interfere with the analytical method. The trap was then heated to 190 °C for 3 minutes in a flow of helium in the opposite direction of flow to desorb the volatile gases onto the focus trap. The focus trap comprised a 20 cm length of 1/32′′ silica-coated stainless-steel tube packed with Carbopack™ X 40–60 mesh and Carbopack™ B 60–80 mesh adsorbents and held at the lowest achievable temperature the unit could maintain, always less than −120 °C. Upon complete transfer of the analytes of interest, the focus trap was purged with helium and rapidly heated to 200 °C for 2 minutes during transfer to the GC oven for separation and analysis. During the GC analysis phase, the pre-concentration and focus traps were back-flushed with carrier gas and heated to 220 °C to remove any remaining organic material, in preparation for following samples, while the water trap was back-flushed and heated to 100 °C to remove any water.
Upon transfer into the GC oven, the analytes were initially passed onto a 60 m long, 150 μm internal diameter (ID) VF-WAX column with a film thickness of 0.50 μm (Agilent Technologies, CA, USA) at a flow rate of 1.6 mL min−1 (carrier gas pressure of 35 psi). Initially, the unresolved analytes (C2–C8 NMHCs) eluting from the WAX column were passed onto a Na2SO4-deactivated Al2O3 porous-layer open tubular (PLOT) column (50 m × 320 μm ID, with a film thickness of 5 μm), via a Deans switch, for separation and detection by FID. After 8.3 minutes, the Deans switch was actuated to divert the analytes onto a length of fused silica (2 m × 150 μm ID) to balance column flows at the Deans switch and subsequently split between the second FID and the QMS for simultaneous detection via sections of 150 μm ID fused silica of length 0.91 m and 2.1 m, respectively. Quantification of VOCs was mostly completed using FID peak integration, however for some species QMS data was used when mass resolution was required to deconvolve overlapping peaks. MS quantification was limited to benzene, benzaldehyde, and all speciated monoterpenes and monoterpenoids. A thirty-component mix of NMHCs in nitrogen (in the region of 4 ppb) provided by the National Physical Laboratory, Teddington, UK, cylinder number D933515 (hereafter referred to as ‘NPL 30’), was used for quantification of the components contained therein while equivalent carbon responses were used to quantify all other species. A table of which species were directly calibrated, and which used equivalent carbon numbers for quantification is shown in ESI Fig. 2.† Direct calibration of monoterpene species was not employed in this study, owing to time constraints given the sequential timeline of the study and logistical implications.
Canisters were randomly selected for blank canister tests to ensure there was no carry-over between samples, and blank tests (using the highly purified air used to dilute canister samples) were interspersed in the sample sequence, along with NPL 30 calibrations. A typical sequence would see a group of 15 canisters run consecutively, followed by three blank runs, five NPL 30 calibration runs, and then further blank gas samples between sequences. All blank gas and NPL 30 tests also used 500 mL of gas. A selection of households from the second cohort of houses (n = 22) were chosen after samples had been processed through GC-FID-QMS for analysis for carbon dioxide (CO2) mole fraction (measured in ppm). This was done by flowing the sample at 600 mL min−1 into a laser absorption spectrometer (Ultraportable Greenhouse Gas Analyser, Los Gatos Research Inc., CA, USA).
Chromatograms were integrated using GCWerks software (GC Soft Inc., CA, USA) and using the NPL 30 standards, concentrations for each species were determined in μg m−3. In total, 47 VOC species were quantified, of which six were known to be contained in the diffuser fragrance formulation. In this study, TVOC was defined as being the sum of all individual species found in a sample, although on many occasions only a subset of 47 VOC species were present in detectable quantities. As discussed in the introduction, it is important to note that TVOC as defined above is not a true representation of the absolute amount of reactive carbon content of the indoor air sampled, but is used here as a helpful metric that is reflective of the sum total concentration of the species quantified. Summing concentrations of all 47 species quantified might be better expressed as ‘sum VOC concentration’, however due to the pervasiveness of use of the term ‘TVOC’, it will continue to be referred to as such in this paper.
Occasionally chromatograms obtained from samples would present with disrupted elution of ethane, ethene and propane peaks, shown in ESI Fig. 3(a).† This effect was seen multiple times in each batch of 15 canisters, anecdotally associated with high CO2 mole fractions. When this occurred, samples were re-run at lower canister pressures until a good chromatogram was produced. ESI Fig. 3(b)† shows the same sample canister which produced the data in ESI Fig. 3(a),† but after being reanalysed. While this effect added time it was not detrimental to the overall data capture. A small number of samples (n = 3) were discounted due to canisters not having taken a full sample (indicated by the canister not being at ambient pressure upon arrival at University of York). This was likely due to participants (all from the same home) not successfully opening the sampling valve. A small number of samples (n = 2) were deemed spoiled as the canisters had been exposed to a leak from the diffuser during transport. After these samples were removed, this left a total of n = 259 individual useable samples. An image of the plug-in diffuser used in this study is shown in ESI Fig. 4.†
2.2 Statistical approach
All data processing and analysis was completed using the R programming language (v.4.1.3), through RStudio Software. Data manipulation was completed using packages contained within the Tidyverse collection of packages, mainly ggplot2 and dplyr. Boxplots show values in the order of (from bottom-to-top): lower outliers, 5th percentile, 25th percentile, median value, 75th percentile, 95th percentile, and upper outliers, along with mean values displayed as a red diamond on select boxplots. Statistical testing was completed using Wilcoxon signed-rank tests, using a confidence interval of α = 0.95. Statistical significance using this approach was defined as a p-value yielded from a Wilcoxon signed-rank test lower than 0.05. Unless stated otherwise in the paper, it should be assumed this is our threshold for significance for all statistical testing done. Mean values were not used in data visualisation but were used in statistical testing. Tests were completed using the house the sample originated from as the identifier, so observations were aggregated into groups typically of 1 or 2, which would not yield a difference whether mean or median values were used in statistical testing. Sensitivity tests were completed using median values for statistical analyses however, which confirmed that both methods gave the same conclusions. Important to note is that while a change in species concentration may be of statistical significance, this does not necessarily mean the change in species concentration was large in absolute terms of μg m−3. Correlation matrices were produced using the corrplot package using visual aids to help show correlations: a forward slanting blue line indicates a positive correlation, a full circle indicates no correlation, and a backward slanting red line indicates a negative correlation. A more intense colour and a narrower line indicates a stronger correlation (both for positive and negative correlations). Covariance analysis was completed through initially rescaling concentration and product use data from 0 to 1. Covariance values were then calculated and assigned, then rescaled from 0 to 100 using BBMisc. The matrix was then displayed graphically using the tile function in ggplot2. Diffuser increment plots were produced using the raster and contour_filled functions in ggplot2, with additional contour lines and contour line labels added using the contour2 and geom_text_contour functions in the metR package.TVOC concentrations were calculated as being the sum of all individually quantified VOC in each sample. These included C2 to C8 hydrocarbons, and a range of monoterpenes and oxygenated VOCs (oVOCs) such as esters and alcohols. An additional metric of ‘fragrance TVOC’ was defined as the sum of individual concentrations for α-pinene, β-pinene, γ-terpinene, benzaldehyde, p-cymene and eucalyptol. Important to note however is that these six species do not reflect the total fragrance formulation, and there were many species within the fragrance which were not detectable in ambient air, owing to limits with the analytical method and often very low compound vapour pressures. For transparency, all data gathered, including survey answers, species concentrations and statistical test results, have been uploaded to the Centre for Environmental Data and Analysis (CEDA) repository.
2.3 Participant panel and survey
Each participant was provided with a tablet-based questionnaire which included questions on property and construction details, occupancy, participant activities and household VOC product use. This is shown in ESI Fig. 5.† Product use information was gathered on a cumulative basis over the 3 day sampling period. Product use statistics were limited to frequency of use of product types (e.g. paints, air fresheners, sealants etc.), and not brands or sub-types, or absolute quantities. Instructions on use of the sampler (how to open and close the sampling valve, and when) and where to place it were given in written form to the participants. The canisters were placed in the main living space within the house, in most cases the living room. Approximate distances between the diffuser and sampling canisters were reported by each participant. There was a 100% response rate to the survey.3. Results
3.1 Individual VOCs and TVOC
Table 1 shows the median concentration, 5th percentile, 95th percentile and standard deviation for each of the 47 species, separated into diffuser off and diffuser on values for all 60 homes combined. Running Wilcoxon signed-rank tests were conducted on the aggregated data paired by the house the sample came from. Statistically significant increases (p-values for all following species were less than 0.05) in mean concentration were seen for 20 species, including α-pinene, eucalyptol, ethanol, benzaldehyde and TVOC with the diffuser turned on. There were also statistically significant decreases (p-value for all following species were less than 0.05) in aggregated mean concentrations, again paired by house, when the diffuser was turned on: acetone, δ-terpinene, γ-terpinene and o-xylene. Fig. 1(a) shows TVOC values graphically through boxplots, differentiated by diffuser off and diffuser on values. α-Pinene constituted ∼5% of the fragrance formulation and was the most volatile aroma compound by vapour pressure. The diffuser off and diffuser on values for α-pinene can be seen in Fig. 1(b), alongside the emission-estimated diffuser α-pinene increment. This increment is elaborated on later in the paper and compared to estimated values based on emissions rates, AER and room sizes. To confirm that the diffuser was delivering a detectable fragrance, occupants were asked to note whether there was a perceivable odour. 55 homes had at least one occupant report a detectable fragrance attributable to use of the diffuser. For occupants that reported being unable to detect the fragrance, diffuser operation was alternatively confirmed by a negative mass-change (range of 5 g to 7 g of liquid fragrance lost), indicating that the lack of perceived fragrance may have arisen due to those individuals' odour detection response, or other factors such as a high AER, or presence of other more dominant fragrance-emitting sources. All diffusers used in the study were confirmed to work before deployment. Statistical mean weight loss across all diffusers with available data, excluding those which leaked, was 6.84 g with a standard deviation of 1.93 g. Boxplots for other species as well as fragrance TVOC are shown in ESI Fig. 6.†| Species | Median concentration | 5th Percentile | 95th Percentile | Standard deviation | ||||
|---|---|---|---|---|---|---|---|---|
| Diffuser off | Diffuser on | Diffuser off | Diffuser on | Diffuser off | Diffuser on | Diffuser off | Diffuser on | |
| Ethane | 6.9 | 8.5 | 1.9 | 3.4 | 160 | 140 | 100 | 94 |
| Ethene | 1.2 | 1.3 | 0.34 | 0.45 | 4.1 | 4 | 4.8 | 6.5 |
| Propane | 99 | 150 | 4.3 | 5.3 | 960 | 1300 | 330 | 400 |
| Propene | 0.4 | 0.58 | 0.13 | 0.19 | 1.6 | 1.7 | 7.9 | 11 |
| Iso-butane | 120 | 210 | 2.1 | 4.2 | 1200 | 1300 | 380 | 390 |
| n-Butane | 230 | 310 | 6.1 | 9.3 | 1300 | 1300 | 460 | 500 |
| Acetylene | 0.35 | 0.47 | 0.17 | 0.24 | 1.3 | 1.7 | 0.81 | 1 |
| But-1-ene | 0.15 | 0.22 | <0.1 | <0.1 | 0.61 | 0.68 | 0.24 | 0.37 |
| cis-But-2-ene | 0.67 | 1.6 | 0.2 | 0.19 | 5.6 | 12 | 2.4 | 3.9 |
| Isopentane | 4.1 | 7.8 | 0.79 | 1.3 | 42 | 48 | 13 | 20 |
| n-Pentane | 1.4 | 1.5 | 0.42 | 0.61 | 11 | 8 | 5.1 | 2.6 |
| cis-Pent-2-ene | <0.1 | 0.1 | <0.1 | <0.1 | 0.29 | 0.45 | <0.1 | 0.18 |
| n-Hexane | 0.23 | 0.29 | 0.12 | 0.15 | 1.2 | 0.92 | 0.83 | 0.28 |
| Isoprene | 2.1 | 2.2 | 0.49 | 0.57 | 6.4 | 5.7 | 2 | 1.9 |
| n-Heptane | 1.1 | 0.42 | <0.1 | 0.12 | 6.4 | 3 | 2.3 | 1.1 |
| n-Octane | 0.21 | 0.29 | <0.1 | 0.11 | 1.6 | 4.7 | 1.3 | 1.9 |
| Ethylbenzene | 0.63 | 0.61 | <0.1 | 0.12 | 3.4 | 4.8 | 6.7 | 1.9 |
| m-Xylene | 0.62 | 0.73 | 0.17 | 0.21 | 2.7 | 4.5 | 4.5 | 2.1 |
| o-Xylene | 1.5 | 0.68 | 0.18 | 0.1 | 23 | 4.4 | 8.1 | 19 |
| 1,3,5-Trimethylbenzene | 0.17 | 0.25 | <0.1 | <0.1 | 0.94 | 2.9 | 1 | 1.7 |
| 1,2,4-Trimethylbenzene | 0.76 | 1.1 | 0.28 | 0.2 | 3.1 | 3.8 | 1.6 | 1.9 |
| 1,2,3-Trimethylbenzene | 0.16 | 0.25 | <0.1 | <0.1 | 1.3 | 1.1 | 0.95 | 0.83 |
| Benzene | 0.46 | 0.44 | 0.15 | 0.15 | 1.2 | 1.3 | 0.42 | 0.43 |
| Toluene | 2.4 | 2.5 | 0.72 | 0.8 | 11 | 11 | 3.2 | 4.6 |
| Styrene | 0.18 | 0.16 | <0.1 | <0.1 | 0.82 | 0.47 | 0.47 | 0.16 |
| Acetone | 36 | 36 | 13 | 14 | 150 | 130 | 81 | 150 |
| Acetaldehyde | 11 | 11 | 3.7 | 3.8 | 28 | 24 | 52 | 7.3 |
| Hexanal | 5.3 | 5 | <0.1 | <0.1 | 13 | 14 | 4.4 | 4.5 |
| Butan-2-one | 4 | 4.5 | 0.85 | 0.86 | 21 | 31 | 8.5 | 11 |
| Methanol | 48 | 49 | 16 | 11 | 160 | 170 | 49 | 58 |
| Benzaldehyde | 0.17 | 0.18 | <0.1 | <0.1 | 0.36 | 0.38 | <0.1 | 0.11 |
| Ethanol | 730 | 1000 | 130 | 250 | 3100 | 2700 | 960 | 980 |
| Ethyl acetate | 5.3 | 4.6 | 0.43 | 0.48 | 50 | 56 | 39 | 23 |
| Butyl acetate | 1.4 | 1.5 | 0.21 | 0.15 | 20 | 18 | 20 | 15 |
| Propyl acetate | 1.9 | 2.1 | 0.15 | 0.21 | 14 | 50 | 10 | 20 |
| Acetonitrile | 7.5 | 6.1 | 1.6 | 0.32 | 16 | 37 | 10 | 18 |
| Dichloromethane | 0.35 | 0.41 | 0.11 | 0.15 | 2.6 | 3.4 | 1.5 | 2.3 |
| α-Pinene | 5.8 | 8.7 | 1.2 | 2.5 | 32 | 27 | 12 | 8.8 |
| β-Pinene | 1.5 | 1.8 | 0.4 | 0.46 | 8.2 | 5.3 | 4.8 | 1.9 |
| D-Limonene | 8.9 | 10 | 1.4 | 1.6 | 38 | 40 | 14 | 18 |
| Eucalyptol | <0.1 | <0.1 | <0.1 | <0.1 | 0.39 | 0.53 | 0.62 | 0.18 |
| β-Terpinene | 0.12 | <0.1 | <0.1 | <0.1 | 0.64 | 0.64 | 0.46 | 0.23 |
| γ-Terpinene | 0.3 | 0.26 | <0.1 | <0.1 | 2.5 | 1.8 | 0.99 | 0.53 |
| δ-Terpinene | <0.1 | <0.1 | <0.1 | <0.1 | 0.82 | 0.58 | 0.32 | 0.2 |
| 3-Carene | 0.63 | 0.61 | 0.13 | 0.13 | 3.3 | 2.7 | 1.2 | 1.4 |
| β-Myrcene | 0.18 | 0.19 | <0.1 | <0.1 | 0.72 | 0.7 | 0.25 | 0.46 |
| p-Cymene | 0.78 | 0.68 | 0.23 | 0.24 | 2.9 | 2.5 | 1 | 0.84 |
There are often common patterns for VOCs related to common emission sources, time of day year−1, temperature, building materials and many other variables.34–36 To study any correlation between quantified species, correlation matrices were constructed between all quantified species, shown in ESI Fig. 7(a) and (b)† for diffuser off and diffuser on correlations, respectively. Inter-fragrance VOC correlations were generally strengthened when the diffuser was turned on. Most fragrance VOCs exhibited a positive correlation with each other both with the diffuser off and when on. There were mostly positive correlations between other non-methane hydrocarbons (NMHCs) C2 to C6, seen most strongly between C2 to C4 NMHCs. Species which show stronger correlations with each other mostly arise from similar sources, for example C3 and C4 hydrocarbons commonly derive from aerosols, monoterpenes from natural and fragranced products, and monoaromatic compounds from solvents and combustion of petroleum-derived fuels.37–39
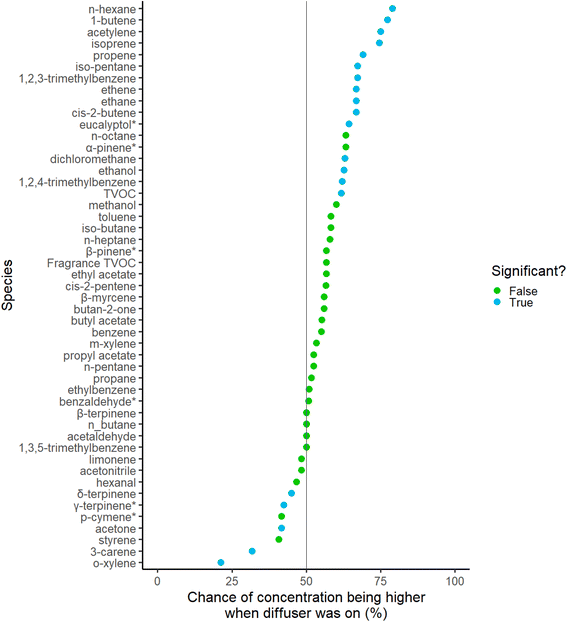
To assess the changes in individual species concentration when the diffuser was on versus off, Wilcoxon signed-rank tests were performed. These tests were initially conducted on aggregated values, differentiated by diffuser status and statistics were paired by the house the sample was taken from. Individual species observations are shown in ESI Fig. 8.† Taking observations on a home-by-home basis – that is treating each house as a stand-alone series of four/five samples – a further test can be made evaluating individually whether a simple increment or decrement in concentration was observed when the diffuser was turned on. Aggregating this simple binary outcome (VOC = higher or lower with the diffuser on) across all homes it is possible to evaluate whether the occurrence of increments (or decrements) systematically varies by more than would be expected from random chance. This is shown for each VOC in Fig. 2, along with a marker to indicate whether the deviation from chance is statistically significant. As discussed earlier, the tests completed to yield Fig. 2 used matched mean values, rather than median values. Most VOCs that showed an increment in concentration with the diffuser switched on by this simple binary metric were NMHCs C2 to C6, and only eucalyptol from the fragrance species showed a statistically significant probability of being higher in a home with the diffuser on. Changes to all the other fragrance VOCs, other than γ-terpinene which showed a statistically significant decrease in probability of being higher in a home with the diffuser on, did not deviate by more than would be expected via random chance, however any changes seen in Fig. 2, whether of significance or otherwise, cannot be satisfactorily assessed to originate through diffuser use alone. Given the highly changeable nature of indoor air seen in this study, there may be background fluctuations which could influence the sampled concentration of a VOC. As such, changes of significance should not be presumed to arise through diffuser use alone.
3.2 Influence of CO2/ventilation on VOC exposure
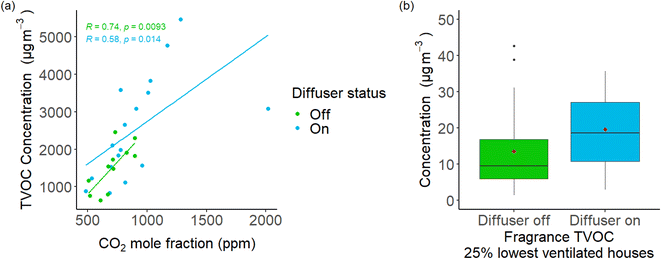
72 hour averaged CO2 mole fraction was quantified from a sub-set of houses (n = 28) in the second cohort, ranging from 482 ppm to 2019 ppm. CO2 mole fraction was compared against TVOC values from the same sample, shown in Fig. 3(a). Lines of regression were calculated using a linear model, with R and p values being calculated using Pearson's R. A positive correlation between CO2 fraction and TVOC concentration is seen in both ‘diffuser off’ and ‘diffuser on’ samples. However, in the ‘diffuser on’ subset there was one outlier with a CO2 fraction of 2019 ppm and a TVOC concentration of 3084 μg m−3, which deviates the regression line for ‘diffuser on’ samples away from ‘diffuser off’ samples. While the use of CO2-emitting products such as candles during this sampling window was not extraordinary, it should be noted that ambient outdoor temperatures in locality to the sampling cohort were lower than previous or following weeks. Indeed, most higher CO2 fractions were recorded during this sampling period. This may have resulted in lower ventilation rates due to closing of windows and internal doors, or more-than-normal use of appliances such as gas fires. Removal of this outlier still produces a plot which exhibits a positive relationship between CO2 fraction and TVOC concentration. Since CO2 data was only available for a subset of samples, but TVOC concentrations were available for all samples, quartiles were constructed using baseline TVOC concentration as an indicator of AER, based on the relationship seen in Fig. 3(a) and with CO2 used as the AER proxy. Only baseline data were used to infer AER to remove any potential circular influence of the diffuser itself. To construct the quantiles, samples were ranked from highest to lowest baseline TVOC: Q1 (highest 25% baseline TVOC concentrations) to Q4 (lowest 25% baseline TVOC concentrations). The change in fragrance TVOC concentration when the diffuser was turned on for Q1, the 25% of homes with notionally the lowest AER are shown in Fig. 3(b). For homes in the lowest AER quantile, the sum of fragrance VOCs doubled for ‘diffuser on’ samples, a statistically significant increase (p-value < 0.02), and more pronounced than when evaluated for all homes. A statistically significant increase in α-pinene concentration was also seen for homes in this quantile (p-value < 0.02). For the other quartiles however, the differences between diffuser on and off reduced as baseline TVOC values lowered, in line with Fig. 1(b).3.3 Participant effects and product use data
Each participant self-reported the distance between the diffuser and the sampling canister, specific to each sample taken. To observe how well the fragrance diffused into the airspace in the room, the sum of VOCs was plotted against the distance between the sampling canister and the diffuser, shown in Fig. 4. Distances between sampling canister and diffuser ranged from between 0.5 m to 6 m. No relationship was observed with proximity of sampler to diffuser, implying the diffuser emission was well mixed in the room.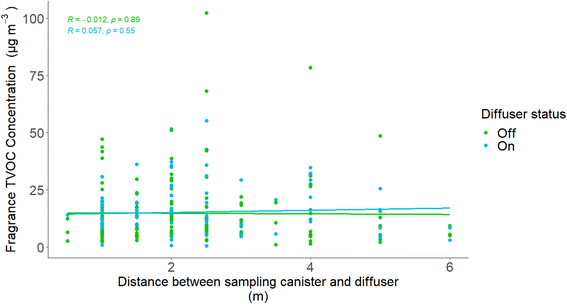 | ||
| Fig. 4 The relationship between the sum of fragrance VOCs and the distance between the sampling canister and the fragrance diffuser. Regression statistics were calculated using a linear model. | ||
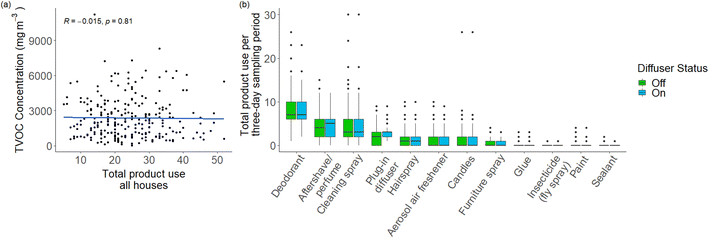
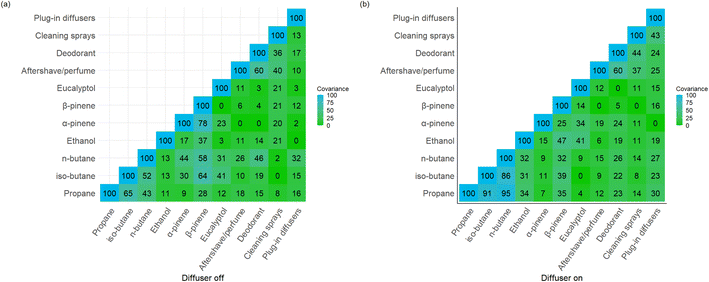
Cumulative product use statistics, limited to product type and number of uses within the 72 hour period, were self-reported by occupants. These values were then plotted against TVOC concentration shown in Fig. 5(a). Total product use was further broken down into the quantiles used previously based on baseline TVOC concentration, and this is shown in ESI Fig. 9.†Fig. 5(b) shows individual product use data for the three-day sampling period, with deodorants being the most used VOC-containing product. To test for any relationship between the frequency of use of particular VOC-containing products and variability observed in individual VOCs, covariance matrices were produced with the four most abundant contributors to TVOC (propane, n-butane, iso-butane and ethanol) along with three fragrance species, α-pinene, β-pinene and eucalyptol. α-Pinene contributed the most to the fragrance formulation; β-pinene is typically used in other fragranced products along with α-pinene, and eucalyptol was included owing to its relatively low concentrations found in the baseline samples. Deodorant, cleaning sprays and aftershave were chosen as the products for comparison since they were the most frequently used. Plug-in diffuser was included as it was known that the use value for this product use increased by exactly 1 per day during the ‘diffuser on’ sampling period. The matrices produced are seen in Fig. 6(a) for the diffuser off matrix, and Fig. 6(b) for the diffuser on matrix. It is clear that few significant relationships exist between frequency of usage of individual products and variability in specific VOC concentrations indoors.
3.4 Bottom-up estimates of increments in concentration
Diffusers were chosen for this study because their emission rates are well controlled and are not affected by user behaviour. By weighing each diffuser before and after use the mass of fragrance and VOC emitted can be quantified. If a constant rate of loss is assumed, then this can be converted into an emission rate in units of μg h−1. An assumption is made that the profile of VOCs emitted from the diffuser does not substantially change over the 72 hour period (or indeed over the study period). Industry norms of technical performance for products of this type would aim to meet this expectation in order to deliver a consistent fragrance to consumers.Using weight loss data for each diffuser along with the fragrance formulation, the emission rate for each fragrance VOC could be calculated. Additionally, for samples with known CO2 mole fractions, AER was calculated as an expression of air changes per hour (ACH). These values are shown in Table 2. To calculate this metric, some conservative assumptions were made: where the volume of the room the sample was obtained from was not reported by the homeowner, a room volume of 30 m3 was used derived from data available from the Royal Institution of British Architects (RIBA);40 that the room experienced air exchange only with (cleaner) outdoor air and not the rest of the house; external CO2 mole fractions were assumed to be 450 ppm for all samples; natural CO2 generation was estimated to be 0.46 L per min per person, as per the work of Batterman (2017),29 and the recorded number of occupants in the house were present in the room being sampled. ACH was calculated using a one-compartment box model. This was justified as Fig. 4 indicated that there appeared to be no ‘personal cloud’ of higher fragrance concentrations in the immediate proximity to the diffuser. ACH was calculated according to eqn (1):
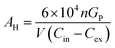 | (1) |
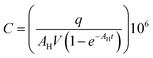 | (2) |
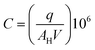 | (3) |
| Species | Diffuser output (μg h−1) | Fragrance species concentration increment (μg m−3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | 5th Percentile | 95th Percentile | Standard deviation | Median | 5th Percentile | 95th Percentile | Standard deviation | |
| α-Pinene | 1270 | 680 | 1830 | 360 | 4.69 | 1.1 | 17.4 | 7.15 |
| β-Pinene | 24.5 | 13.2 | 35.4 | 6.96 | 0.0907 | 0.0213 | 0.336 | 0.138 |
| Benzaldehyde | 39.6 | 21.2 | 57.2 | 11.2 | 0.265 | 0.0624 | 0.982 | 0.404 |
| p-Cymene | 5.42 | 2.91 | 7.83 | 1.54 | 0.0201 | 0.00472 | 0.0743 | 0.0306 |
| Eucalyptol | 71.6 | 38.4 | 103 | 20.3 | 0.146 | 0.0345 | 0.543 | 0.224 |
| γ-Terpinene | 2.83 | 1.52 | 4.08 | 0.803 | 0.0105 | 0.00246 | 0.0388 | 0.016 |
Eqn (3) was used in the calculation of fragrance VOC concentration increase within the modelled room. The bottom-up estimated increment in concentrations shown in Table 2 fall broadly within the range of observed α-pinene concentration increments, shown as the third boxplot in Fig. 1(b). It should be recognised that these increment values do not account for simultaneous removal through oxidative reaction pathways or any surface loss of fragrance species into VOC reservoirs or sinks.
3.5 Diffuser increment estimates
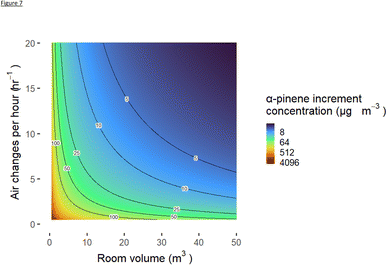
Using a variety of room volumes and ventilation rates, as well as a constant diffuser output rate based on values from this study, a spread of diffuser increments in VOC concentrations can be calculated for all combinations of room volume and ACH. Plotting these values with room volume on the x-axis, ventilation rate on the y-axis, and with a colour gradient of species concentration as a z-axis variable, a contour plot can be produced for any species included in the diffuser formulation. The contour plot of increments in α-pinene from a single diffuser are shown in Fig. 7. Ventilation rates and room volumes were capped at 20 h−1 and 50 m3 respectively, with the vast majority of physically plausible combinations of AER and room sizes experiencing a predicted increment of no more than 100 μg m−3. Whilst this model cannot replicate exactly homes tested in the real world, the bottom-up calculation is helpful in demonstrating that the modest increases in VOCs such as α-pinene that were measured when a single diffuser was used are of the same order of magnitude as would be predicted using a bottom-up methodology. For reference the α-pinene 1 day exposure limit was recommended at 4500 μg m−3 by PHE; this does not however necessarily constitute a long-term exposure recommendation. A single diffuser used in a typical domestic room volume of 30 m3 (RIBA average room and house sizes for south-east England) and a recommended minimum ventilation rate of 6 ACH would be predicted to give rise to a room increment of around 10 μg m−3 in α-pinene, again broadly in line with the observations. We note that the loss of VOC from a room is assumed to be solely from ventilation and dilution and that oxidation or deposition are ignored, hence Fig. 7 is likely to represent an upper bound of increment.3.6 Generation of secondary oxidation products from diffuser use
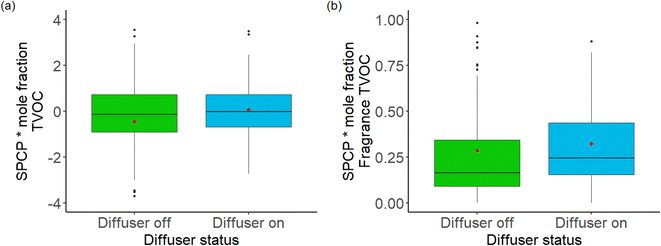
Carslaw and Shaw (2019) described an operational metric to indicate the potential of a VOC to undergo further reactions within indoor airspaces and create secondary products, named the secondary product creation potential (SPCP).41 The SPCP is defined as the sum of a range of secondary products created from degradation of a VOC, divided by the mixing ratio of the primary species added, in units of ppb of secondary products per ppb of VOC. It is of relevance for health since secondary products derived from VOC oxidation can play a role in the formation of secondary aerosols42 and include other more directly harmful products such as formaldehyde.43–45 It is useful to apply the metric here since indoor air in the homes studied were often dominated in mass terms by relatively unreactive species such as propane and butane that have low SPCP multipliers. Monoterpenes are known to have higher reactivities and SPCP, hence use of this metric ensures that the potential wider effects of increments in by-products are also evaluated. Similar to Fig. 1, calculated SPCP values were differentiated by the diffuser status for TVOC and for the sum of the fragrance VOCs where there is SPCP data (α-pinene, β-pinene and benzaldehyde). This is shown in Fig. 8(a) and (b). There was no discernible change for SPCP* mole fraction in Fig. 8(a), but a slightly larger increase in SPCP* mole fraction product for fragrance species in Fig. 8(b). Noteworthy, however, is the increase in relative contribution of fragrance species data to the overall SPCP values, when compared to their mass contributions, reflecting a higher potential for monoterpene species to lead secondary products compared to high mass contributors such as propane, i-butane, n-butane and ethanol.4. Conclusions
The use of room fragrance products such as plug-in diffusers undoubtedly impacts on the airspace composition within the area the product is used. Clearly using any VOC-containing products in poorly ventilated spaces will result in a higher VOC concentration and higher exposure to the user than would occur in a well-ventilated space. In this study virtually all participants reported that they could detect the presence of fragrance when the diffusers were switched on indicating that in a purely functional sense the devices were working as intended by the manufacturer. However quantitatively determining by how much VOCs were incrementally raised by using the diffuser was not straightforward since the diffuser emitted VOCs into an already highly congested indoor atmosphere containing many VOCs at higher concentrations, including some species that were also in fragrance formulation. Future inclusion of appliance use data in surveys may prove insightful, as the frequency of use of appliances such as gas fires may help with interpreting the presence of high CO2 fractions that may not be from respiration.When the study homes were segmented by air exchange rate, inferred from concurrent CO2 measurements acting as a proxy, then a small increment in VOCs could be detected. For homes in the lowest ventilation quartile, α-pinene concentrations increased on average from 9 to 14.5 μg m−3, with the median value increasing from 6 μg m−3 to 14.1 μg m−3. This increment in α-pinene was set against a background mean TVOC concentration in that quartile of >3500 μg m−3. By using the weight loss of fragrance from the diffuser, a bottom up estimate of increment increase in α-pinene was calculated to be around 10 μg m−3, assuming 6 ACH, a typical room volume of 30 m3 and no oxidative/depositional losses. In this regard the use of a fragrance diffuser for studies in real-world settings is helpful since the emission rate is well controlled, formulation is known, and amounts released are not subject to end-user variability. Using a more targeted metric related to secondary product creation potential did not lead to any substantial change in conclusions. Whilst fragrance-related VOCs made up a larger fractional contribution to the SPCP metric there was no statistically significant increase in calculated SPCP across all homes when diffusers were used. A bottom-up model for estimating the incremental increases in a VOC when a single diffuser is used indicated that increments of >450 μg m−3 (a high safety margin of 1/10th the 24 hour exposure recommendation) are physically plausible but would require a combination of very small room volumes (below 5 m3) and very low air exchange rates. It is possible that additional user instruction might provide guidance against use in such unusual situations.
Author contributions
ACL, TW, NO, CJ, BX and GA designed the original experiment. JRH and SJA developed the analytical instruments and methods and TW, JRH and SJA performed the laboratory analysis. TW undertook the data analysis and visualisations and SKG and ACL guided the statistical and data methods. All authors contributed to the writing of the manuscript and the development of its conclusions.Conflicts of interest
NO, CJ, and GA are employees of Givaudan UK Ltd, Givaudan Fragrances Corp. and BX is an employee of Bath & Body Works, Inc. who are industrial suppliers of chemicals and finished household and personal care products. To support independence all analytical work and data analysis was undertaken by University of York and no restrictions placed on freedoms to publish. To support transparency in research, data collected in this study is freely available from the Centre for Environment Data and Analysis (https://www.ceda.ac.uk).Acknowledgements
Funding for this research was provided by Givaudan UK Ltd and Bath & Body Works Inc. ACL, JRH and SJA receive support from the National Centre for Atmospheric Science National Capability research programme, via funding provided by the Natural Environment Research Council. TW's PhD studentship is part supported by UKRI grant number NE/W002256/1.References
- E. Uhde and N. Schulz, Atmos. Environ., 2015, 106, 492–502 CrossRef CAS
.
- D. A. Sarigiannis, S. P. Karakitsios, A. Gotti, I. L. Liakos and A. Katsoyiannis, Environ. Int., 2011, 37, 743–765 CrossRef CAS PubMed
.
- S. Dimitroulopoulou, C. Shrubsole, K. Foxall, B. Gadeberg and A. Doutsi, 2019, pp. 1–9, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/831319/VO__statement_Final_12092019_CS__1_.pdf.
- C. Shrubsole, S. Dimitroulopoulou, K. Foxall, B. Gadeberg and A. Doutsi, Build. Environ., 2019, 165, 106382 CrossRef
.
- A. C. Heeley-Hill, S. K. Grange, M. W. Ward, A. C. Lewis, N. Owen, C. Jordan, G. Hodgson and G. Adamson, Environ. Sci.: Processes Impacts, 2021, 23, 699–713 RSC
.
- C. Jia, S. Batterman and C. Godwin, Atmos. Environ., 2008, 42, 2101–2116 CrossRef CAS
.
- E. Höllbacher, T. Ters, C. Rieder-Gradinger and E. Srebotnik, Atmos. Environ., 2017, 150, 389–394 CrossRef
.
- X. Liu, M. Mason, K. Krebs and L. Sparks, Environ. Sci. Technol., 2004, 38, 2802–2812 CrossRef CAS PubMed
.
- N. Goodman, N. Nematollahi, G. Agosti and A. Steinemann, Air Qual., Atmos. Health, 2020, 13, 1–4 CrossRef CAS
.
- N. B. Goodman, A. J. Wheeler, P. J. Paevere, G. Agosti, N. Nematollahi and A. Steinemann, Air Qual., Atmos. Health, 2019, 12, 289–295 CrossRef CAS
.
- A. Lamplugh, A. Nguyen and L. D. Montoya, Build. Environ., 2020, 176, 106784 CrossRef
.
- S. Senthilkumaran, R. Meenakshisundaram, A. D. Michaels, N. Balamurgan and P. Thirumalaikolundusubramanian, J. Electrocardiol., 2012, 45, 164–166 CrossRef PubMed
.
- D. Vethanayagam, H. Vliagoftis, D. Mah, J. Beach, L. Smith and R. Moqbel, J. Asthma, 2013, 50, 975–982 CrossRef
.
- J. E. M. Gibbs, J. Asthma Allergy, 2019, 12, 169–182 CrossRef CAS PubMed
.
- U. B. Nurmatov, N. Tagiyeva, S. Semple, G. Devereux and A. Sheikh, Eur. Respir. Rev., 2015, 24, 92–101 CrossRef PubMed
.
- J. H. Lee, H. S. Lee, M. R. Park, S. W. Lee, E. H. Kim, J. B. Cho, J. Kim, Y. Han, K. Jung, H. K. Cheong, S. I. Lee and K. Ahn, Allergy, Asthma Immunol. Res., 2014, 6, 517 CrossRef PubMed
.
- J.-Y. Chin, C. Godwin, E. Parker, T. Robins, T. Lewis, P. Harbin and S. Batterman, Indoor Air, 2014, 24, 403–415 CrossRef CAS PubMed
.
- E. K. Ha, J. H. Kim, D. Park, E. Lee, S. W. Lee, H. M. Jee, Y. H. Shin and M. Y. Han, J. Korean Med. Sci., 2022, 37, e63 CrossRef CAS PubMed
.
- E. Woolfenden, J. Air Waste Manage. Assoc., 1997, 47, 20–36 CrossRef CAS
.
- M. Even, E. Juritsch and M. Richter, TrAC, Trends Anal. Chem., 2021, 140, 116265 CrossRef CAS
.
-
G. V. Silva, E. O. Fernandes, M. J. Samudio, T. Tirkkonen, H. Jarnstrom, K. Villberg, K. Saarela, C. Cox, C. Aizlewood, D. Crump, V. Brown and S. Dimitroulopoulou, Health & Environmental Research Online, 2005, vol. 2443022, pp. 816–820 Search PubMed
.
- K. Pytel, R. Marcinkowska and B. Zabiegała, Environ. Sci. Pollut. Res., 2021, 28, 59214–59232 CrossRef CAS PubMed
.
- S. Mentese and D. Tasdibi, Global NEST J., 2017, 19, 726–732 CAS
.
- R. D. Edwards, J. Jurvelin, K. Koistinen, K. Saarela and M. Jantunen, Atmos. Environ., 2001, 35, 4829–4841 CrossRef CAS
.
- C. Arata, P. K. Misztal, Y. Tian, D. M. Lunderberg, K. Kristensen, A. Novoselac, M. E. Vance, D. K. Farmer, W. W. Nazaroff and A. H. Goldstein, Indoor Air, 2021, 31, 2099–2117 CrossRef CAS PubMed
.
- M. Cheng, I. E. Galbally, S. B. Molloy, P. W. Selleck, M. D. Keywood, S. J. Lawson, J. C. Powell, R. W. Gillett and E. Dunne, Indoor Air, 2016, 26, 219–230 CrossRef CAS PubMed
.
- Ľ. Mečiarová, S. Vilčeková, E. Krídlová Burdová and J. Kiselák, Int. J. Environ. Res. Public Health, 2017, 14, 1443 CrossRef PubMed
.
-
R. Otson, P. Fellin, C. S. Davis, Healthy Materials, 1995, vol. 3, pp. 11–12 Search PubMed
.
- S. Batterman, Int. J. Environ. Res. Public Health, 2017, 14, 145 CrossRef
.
- L. Chatzidiakou, D. Mumovic and A. Summerfield, Build. Serv. Eng. Res. Technol., 2015, 36, 129–161 CrossRef
.
- Z. Peng and J. L. Jimenez, Environ. Sci. Technol. Lett., 2021, 8, 392–397 CrossRef CAS
.
- Y. Jia, X. Zheng, J. Guan, X. Tan, S. Chen, B. Qi and H. Zheng, Indoor Built Environ., 2021, 30, 838–850 CrossRef CAS
.
- K. Murakami, K. Sakai, D. Nakamura, H. Ishikawa, S. Tsushima and S.-I. Tanabe, E3S Web Conf., 2019, 111, 01020 CrossRef CAS
.
- Y. P. Zhang and Y. Xu, Int. J. Heat Mass Transfer, 2003, 46, 4877–4883 CrossRef CAS
.
- N. Fu, P. Wei, Y. B. Jia, X. Zheng and J. Guan, Build. Environ., 2021, 191, 107599 CrossRef
.
- C. Lutes, C. Holton, B. Schumacher, J. Zimmerman, A. Kondash and R. Truesdale, Groundwater Monit. Rem., 2021, 41, 99–111 CrossRef PubMed
.
- F. Klein, N. J. Farren, C. Bozzetti, K. R. Daellenbach, D. Kilic, N. K. Kumar, S. M. Pieber, J. G. Slowik, R. N. Tuthill, J. F. Hamilton, U. Baltensperger, A. S. H. Prévôt and I. El Haddad, Sci. Rep., 2016, 6, 36623 CrossRef CAS PubMed
.
- T. Barboni and N. Chiaramonti, Combust. Sci. Technol., 2010, 182, 1193–1200 CrossRef CAS
.
- B. D. Škrbić, V. B. Marinković and S. Spaić, Fuel, 2020, 282, 118824 CrossRef
.
-
M. Crosby, Space Standards for Homes, Royal Institute of British Architects, London, 2015 Search PubMed
.
- N. Carslaw and D. Shaw, Environ. Sci.: Processes Impacts, 2019, 21, 1313–1322 RSC
.
- X. Niu, Y. Huang, S. C. Lee, J. Sun and K. F. Ho, Aerosol Air Qual. Res., 2020, 20, 1675–1685 CAS
.
- T. Liu, Z. Wang, D. D. Huang, X. Wang and C. K. Chan, Environ. Sci. Technol. Lett., 2018, 5, 32–37 CrossRef CAS
.
- M. Takhar, Y. Li and A. W. H. Chan, Atmos. Chem. Phys., 2021, 21, 5137–5149 CrossRef CAS
.
- Z. Zhang, W. Zhu, M. Hu, K. Liu, H. Wang, R. Tang, R. Shen, Y. Yu, R. Tan, K. Song, Y. Li, W. Zhang, Z. Zhang, H. Xu, S. Shuai, S. Li, Y. Chen, J. Li, Y. Wang and S. Guo, Atmos. Chem. Phys., 2021, 21, 15221–15237 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2em00444e |
| This journal is © The Royal Society of Chemistry 2023 |

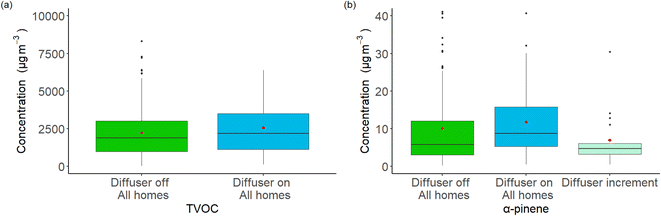
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg m−3 and 40 μg m−3 for (a) and (b) respectively are removed from graphic to give equal presentation but are included in calculations.
000 μg m−3 and 40 μg m−3 for (a) and (b) respectively are removed from graphic to give equal presentation but are included in calculations.