Ginsenoside Rg1 modulates vesicular dopamine storage and release during exocytosis revealed with single-vesicle electrochemistry†
Junlan
Zhou‡
ab,
Jing
Zhang‡
ab,
Lijiao
Cao
ab,
Yuying
Liu
abc,
Luyao
Liu
ab,
Chunlan
Liu
*ab and
Xianchan
Li
 *abc
*abc
aKey Laboratory of Mass Spectrometry Imaging and Metabolomics (Minzu University of China), National Ethnic Affairs Commission, Beijing 100081, China. E-mail: xcli@hsc.pku.edu.cn; liuchunlan@muc.edu.cn
bCenter for Imaging and Systems Biology, College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China
cState Key Laboratory of Natural and Biomimetic Drugs and Department of Pharmaceutical Analysis, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China
First published on 14th February 2023
Abstract
Ginsenoside Rg1, a tetracyclic triterpenoid derivative extracted from the roots of Panax ginseng C. A. Meyer, can enhance learning and memory and improve cognitive impairment. However, whether or how it affects vesicular dopamine storage and its release during exocytosis remains unknown. By using single-vesicle electrochemistry, we for the first time find out that Rg1 not only upregulates vesicular dopamine content but also increases exocytosis frequency and modulates dopamine release during exocytosis in PC12 cells, which may relate to the activation of protein kinases, causing a series of biological cascades. This finding offers the possible link between Rg1 and vesicular chemical storage and exocytotic release, which is of significance for understanding the nootropic role of Rg1 from the perspective of neurotransmission.
Ginsenoside Rg1 (structure shown in Fig. 1a) is a tetracyclic triterpenoid derivative extracted from the roots of Panax ginseng C. A. Meyer. This compound exhibits diverse bioactivities, including neurogenesis promotion, anti-aging, anti-fatigue, immunity enhancement, and adjuvant anti-tumor effect.1 As a small molecule, ginsenoside Rg1 can cross the blood-brain barrier and affect various molecules, such as growth factors, adenosine triphosphate (ATP), and different enzymes, thereby regulating signalling pathways in the brain.2 Recent studies have shown that ginsenoside Rg1 can improve cognitive impairment by inducing long-time potentiation, improving synaptic plasticity, increasing neurogenesis, and inhibiting neuronal apoptosis.3 This implies that ginsenoside Rg1 may be a potential drug for neurodegenerative diseases.1 Growing evidence proves that cognitive impairment in neurodegenerative diseases is inextricably linked to neurotransmitter disorders in the brain.4–6 However, whether and how ginsenoside Rg1 affects neurotransmitter storage and release in the nervous system is still unknown.
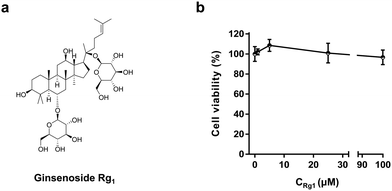 | ||
| Fig. 1 Chemical structure of ginsenoside Rg1 (a) and MTT assay for PC12 cells treated with 1, 5, 25, and 100 μM Rg1 for 3 h (b). | ||
It is known that exocytosis of neurotransmitters occurs at the nanometer spatial scale and millisecond time scale. Therefore, a high spatiotemporal resolution approach is required to quantitatively monitor the dynamics of neurotransmitter storage and release in real-time. Single-vesicle electrochemistry, including the single cell amperometry (SCA)7–11 and intracellular vesicle impact electrochemical cytometry (IVIEC),12–14 have been developed to monitor the neurotransmitter release during exocytosis and quantify the neurotransmitter content in single vesicles in living cells, respectively. Growing evidence proves the coupling of SCA and IVIEC can provide more information on neurotransmitter metabolism and transportation in the nervous system and help us understand neurotransmission.12,15–19
The exploration of whether and how ginsenoside Rg1 acts on neurotransmitter storage and release will largely contribute to our understanding of its neuroprotective effects at the single vesicle level. In this study, we used rat pheochromocytoma (PC12) cells, a kind of neuroendocrine cell, as a model cell to investigate the effect of Rg1 on neurotransmitter storage and release.
Ginsenoside Rg1 has been reported to suppress the proliferation of tumor cells. To test the effect of Rg1 on PC12 cells, the MTT cell viability assay was taken to examine the cell viability over a concentration range of Rg1.12,15 As shown in Fig. 1b, in the concentration range of 1–100 μM,20 the cell viability did not change significantly upon Rg1 treatment for 3 h although 5 μM Rg1 seems to slightly increase the PC12 cell viability. In the following study, 25 μM Rg1 was used.21
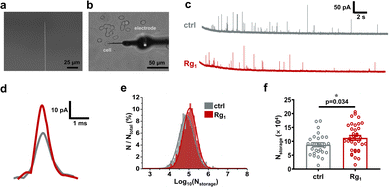
IVIEC using nano-tip conical electrodes (Fig. 2a, b and Fig. S1a, ESI†) was used to quantify the vesicular dopamine content of PC12 cells.12,13 Representative i–t traces obtained from the amperometric recording of vesicular dopamine storage in PC12 cells are displayed in Fig. 2c. Each amperometric spike in the i–t trace represents the electrooxidation of dopamine in one vesicle to dopamine-quinone at the nano-tip conical electrode surface. Typical spikes showing the single events of Rg1-treated and untreated PC12 cells are shown in Fig. 2d.
Fig. 2e compares the normalized frequency histogram of the dopamine content in single vesicles (Nstorage) in Rg1-treated PC12 cells and control cells. Obviously, Nstorage of Rg1-treated PC12 cells is much higher than control cells. Further statistical analysis (Fig. 2f) proves a significant difference between two groups (i.e. (8.76 ± 0.73) × 104 for control, (11.25 ± 0.84) × 104 for 25 μM Rg1 treatment). It reveals that 25 μM Rg1 treatment significantly upregulates the vesicular dopamine storage in PC12 cells. This might relate to the upregulation of tyrosine hydroxylase activity by the protein kinase, in turn promoting dopamine synthesis in cytosol (vide infra).22
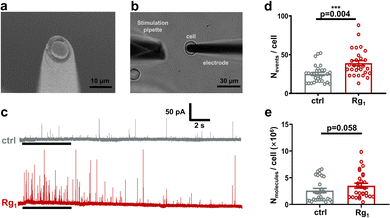
We then used SCA with a disk carbon fiber microelectrode to investigate the effect of 25 μM Rg1 on neurotransmitter release during exocytosis (Fig. 3a, b and Fig. S1b, ESI†). Due to its high sensitivity and high spatial-temporal resolution, SCA has successfully been used to quantify neurotransmitter released during exocytosis and monitor the kinetics of exocytosis in various cell models.23
While stimulating with 70 mM K+ solution, a train of amperometric spikes was obtained (Fig. 3c). Different from the IVIEC, each amperometric spike in SCA traces represents the electrooxidation of dopamine released during an exocytotic event to dopamine-quinone. Apparently, 25 μM Rg1 treatment stimulates the dopamine release by increasing both the number of exocytotic events and the spike current. As shown in Fig. 3d, the statistical comparison displays significant increase of the number of exocytotic events per cell (Nevents/cell) by Rg1 treatment (i.e. 39 ± 3 for Rg1-treated group, 25 ± 2 for control group, p = 0.004), indicating Rg1 treatment elevates exocytotic frequency. To investigate if Rg1 elevates dopamine amount released from single cells, we cumulated the amount of dopamine molecule released in each PC12 cell upon once high K+ stimulation (Nmolecules/cell). Although not significantly, Nmolecules per cell in Rg1 group is slightly higher than control group (i.e., (3.51 ± 0.46) × 106 molecules for Rg1 group vs. (2.60 ± 0.41) × 106 molecules for control group). The elevation of exocytosis frequency and dopamine release may be relevant to the enhancement of Ca2+ influx by Rg1 (vide infra).
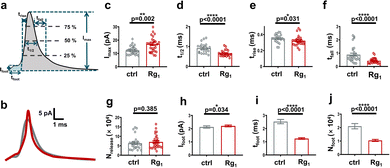
It has been reported that amperometric spikes parameters demonstrate several vital processes of exocytosis (Fig. 4a).24 To further explore the effect of Rg1 on cell exocytosis, we compared the exocytotic spike parameters obtained from control cells and Rg1-treated cells (Fig. 4b–j and Fig. S2 and S3, ESI†).
As shown in Fig. 4c and Fig. S2d (ESI†), Imax of Rg1-treated cells (i.e. 17.20 ± 1.28 pA) is higher than the untreated cells (i.e. 12.29 ± 0.58 pA) significantly, suggesting that the size of fusion pore of Rg1-treated cells is larger than the control group. Meanwhile, a noteworthy decrease of t1/2 in Rg1-treated cells (i.e. 0.65 ± 0.03 ms for Rg1-treated cells vs. 0.92 ± 0.05 ms for control group) was observed as depicted in Fig. 4d and Fig. S2a (ESI†), revealing that Rg1 treatment virtually affects the exocytosis kinetics besides fusion pore size. The adverse effects of Rg1 on Imax and t1/2 lead to no obvious change on dopamine number released in single exocytotic event (Fig. 4g and Fig. S2e (ESI†), Nrelease, (7.07 ± 0.68) × 104 for Rg1-treated vs. (6.33 ± 0.68) × 104 for control cells). This indicates Rg1 treatment does not affect the neurotransmitter amount released by single vesicles during exocytosis.
Besides t1/2, the duration of exocytosis could be demonstrated with trise and tfall, the opening and closing time of the fusion pore, respectively.24 As shown in Fig. 4e and Fig. S2b (ESI†), there is a slightly significant decrease of trise in the Rg1 treatment group compared with control (i.e. 0.32 ± 0.01 ms for Rg1-treated cells vs. 0.35 ± 0.01 ms for untreated cells, p = 0.031), suggesting that the enlarging time of fusion pore gets shorten after Rg1 treatment. Further analysis of pre-spike foot (schemed in Fig. 4a) has been widely used to study the geometry and stability of the initial fusion pore of exocytosis. As same as the trend of trise, Fig. 4i shows that Rg1 treatment significantly decreases the duration of foot (tfoot). Subsequently, it decreases the number of dopamine released via the initial fusion pore (Fig. 4j). These results suggest that Rg1 treatment accelerates the forming of the initial fusion pore and the enlarging of fusion pore.
Next, we analyzed the closing time of the fusion pore (i.e. tfall). As demonstrated in Fig. 4f, there is a significant reduction of tfall after Rg1-treatment compared with control cells (i.e. 0.46 ± 0.04 ms for Rg1 group vs. 0.89 ± 0.10 ms for control, p < 0.0001). To provide more insights into the shortened decay time (tfall) induced by Rg1 treatment, further mathematical analysis was carried out. Previously studies have shown that the decay phase of exocytic amperometric peaks can be fitted with either single exponential or double exponential.15,25 In the full fusion mode, exocytosis is largely controlled by the pure diffusion of the neurotransmitter through the fusion pore, thus its descent fraction can be well fitted with single exponential. Reversely, vesicle releases only a portion of neurotransmitters before the fusion pore closes in the partial release mode, in which the fusion pore's contraction must be faster than the neurotransmitter diffusion from the vesicle. Therefore, in the partial release mode of exocytosis, the decaying phase of exocytotic peaks is more appropriate to fit with double exponential. According to the previously published mathematical model,26 we fitted the descent fraction of all peaks with double exponential (eqn (S1), ESI†) and obtained T1 and T2, which are associated with the pore contraction and neurotransmitter diffusion, respectively.
The standardized d values were calculated according to eqn (S2) and Fig. S4a (ESI†). The spikes with d > 0.5 were previously considered more suitable for double exponential fitting.26 Fig. S4b (ESI†) shows that Rg1 treatment shifts the curve to the left (nd>0.5/nd<0.5 = 651/787 for Rg1 group vs. nd>0.5/nd<0.5 = 330/468 for control group). This means the number of spikes with d > 0.5 slightly increases after Rg1 treatment (shown in Fig. S4c (ESI†), 45.3% for Rg1 group vs. 41.4% for control, when d = 0.5). This result suggests that Rg1 treatment promotes the collapse of fusion pores and more vesicles tend to secrete with the partial release mode. This finding is consistent with the phenomenon that a slightly lower percentage of vesicular dopamine is released during exocytosis in PC12 cells treated with Rg1 (62.8%) than that from the control group (72.3%) when comparing the results shown in Fig. 2f with Fig. 4g.
Generally, secretory vesicles are categorized into two types: one is small synaptic vesicles (SSVs, ∼50 nm in diameter), and the other is large dense core vesicles (LDCVs, 50–300 nm in diameter), to which most vesicles in PC12 cells belong. Except the difference in size and distribution, LDCVs consist of a dense core and a lucent solution called halo. The dense core, which is composed of an assortment of soluble materials inward, can reduce the osmolality of the vesicle, giving the green light for loading a high concentration of transmitters into vesicles.27,28 Once the volume of the dense core or halo changes, the vesicular neurotransmitter concentration changes together.29 To investigate the influence of vesicle size by Rg1 treatment, transmission electron microscopy (TEM) imaging was performed. As depicted in Fig. S5 (ESI†), neither size of vesicle nor its compartments, including the dense core and halo changes significantly by Rg1 treatment. Therefore, the influence of vesicular dopamine storage and release caused by Rg1 treatment is irrelevant to the vesicle size.
Ginsenoside Rg1 may influence neurotransmission through two signalling pathways. One is probably the direct hindrance of phosphodiesterase (PDE) activity by Rg1, which would upregulate the concentration of intracellular cAMP, activating protein kinase A (PKA).30 And the other may be related to the upregulating the concentration of extracellular glutamate which could elevate calcium internal stream via oblique activation of intracellular N-Methyl-D-aspartic acid (NMDA) receptors.31,32 As depicted in Fig. S6 (ESI†), a higher calcium influx is observed following 25 μM Rg1 treatment, confirming that Rg1 could boot calcium influx, which tends to facilitate the occurrence of exocytosis (Fig. 3d). And the elevated intracellular Ca2+ appears to activate phospholipase C-protein kinase C (PLC-PKC) pathway and calmodulin-dependent protein kinase (CaMK II).33
Together with PKA activated through another pathway, PKC and CaMK II might phosphorylate tyrosine hydroxylase and SNARE proteins and other proteins or enzymes. The phosphorylation of tyrosine hydroxylase could elevate the synthesis of dopamine,22 thus promoting dopamine storage in single vesicles (Fig. 2f). The formation of SNAREs complex is the core leading to membrane fusion in exocytosis. Once any change of the SNAREs protein occurs, it may have a dramatic impact on extracellular secretion. It has been reported that PKC could phosphorylate syntaxin and/or SNAP-25, attenuating their adhesion with synaptobrevin,34 and thus shorten the opening and closing time of the exocytotic fusion pore, through which less fraction of transmitter released (Fig. S7, ESI†).
In conclusion, by using single-vesicle electrochemistry, we investigate the influence of ginsenoside Rg1 on vesicular transmitter storage and its release during exocytosis. Our results show that the Rg1 treatment up-regulates the dopamine content in single vesicles. Besides, Rg1 can significantly increase the exocytosis frequency of PC12 cells, which may be related to the promotion of Ca2+ influx by Rg1. Moreover, Rg1 significantly shortens the duration time of exocytosis, including the opening and closing time of exocytotic fusion pores. Further analysis suggests that Rg1 not only affects the fusion pore at the initial forming stage and its enlarging process, but also promotes the collapse of the fusion pore, which may correlate with the activation of CaMK II, PKA, and PKC, subsequently phosphorylating SNAREs proteins. Taking the different aspects from the current study of Rg1 effect on transmitter synthesis, storage and release during exocytosis, our results are helpful for better understanding the nootropic effect of Rg1 from the perspective of neurotransmission.
The authors are grateful for the financial support from the National Key Research and Development Program of China (2022YFA1200067), the National Natural Science Foundation of China (21974154), Recruitment Program of Global Experts (Youth).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Rajabian, M. Rameshrad and H. Hosseinzadeh, Expert Opin. Ther. Pat., 2019, 29, 55–72 CrossRef CAS PubMed.
- T. Huang, F. Fang, L. Chen, Y. Zhu, J. Zhang, X. Chen and S. S. Yan, Curr. Alzheimer Res., 2012, 9, 388–395 CrossRef CAS PubMed.
- F. Li, X. Wu, J. Li and Q. Niu, Mol. Med. Rep., 2016, 13, 4904–4910 CrossRef CAS PubMed.
- R. I. Teleanu, A. G. Niculescu, E. Roza, O. Vladâcenco, A. M. Grumezescu and D. M. Teleanu, Int. J. Mol. Sci., 2022, 23, 5954 CrossRef CAS PubMed.
- C. Xu, Y. Jiang, P. Yu and L. Mao, Int. J. Electrochem., 2022, 28, 2108551 Search PubMed.
- L. Shi, M. Liu, L. Zhang and Y. Tian, Angew. Chem., Int. Ed., 2022, 61, e202117125 CAS.
- B. B. Anderson, S. E. Zerby and A. G. Ewing, J. Neurosci. Methods, 1999, 88, 163–170 CrossRef CAS PubMed.
- R. M. Wightman, J. A. Jankowski, R. T. Kennedy, K. T. Kawagoe, T. J. Schroeder, D. J. Leszczyszyn, J. A. Near, E. J. Diliberto Jr and O. H. Viveros, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 10754–10758 CrossRef CAS PubMed.
- K. Wang, X. Zhao, B. Li, K. Wang, X. Zhang, L. Mao, A. Ewing and Y. Lin, Anal. Chem., 2017, 89, 8683–8688 CrossRef CAS PubMed.
- C. Gu, X. Zhang and A. G. Ewing, Anal. Chem., 2020, 92, 10268–10273 CrossRef CAS PubMed.
- L. Ren, A. Oleinick, I. Svir, C. Amatore and A. G. Ewing, Angew. Chem., Int. Ed., 2020, 59, 3083–3087 CrossRef CAS PubMed.
- X. Li, J. Dunevall and A. G. Ewing, Angew. Chem., Int. Ed., 2016, 55, 9041–9044 CrossRef CAS PubMed.
- X. Li, S. Majdi, J. Dunevall, H. Fathali and A. G. Ewing, Angew. Chem., Int. Ed., 2015, 54, 11978–11982 CrossRef CAS PubMed.
- J. G. Roberts, E. C. Mitchell, L. E. Dunaway, G. S. McCarty and L. A. Sombers, ACS Nano, 2020, 14, 2917–2926 CrossRef CAS PubMed.
- X. Li, A. S. Mohammadi and A. G. Ewing, J. Electroanal. Chem., 2016, 781, 30–35 CrossRef CAS PubMed.
- X. He and A. G. Ewing, Chem. Sci., 2022, 13, 1815–1822 RSC.
- Q. Yue, X. Li, F. Wu, W. Ji, Y. Zhang, P. Yu, M. Zhang, W. Ma, M. Wang and L. Mao, Angew. Chem., Int. Ed., 2020, 59, 11061–11065 CrossRef CAS PubMed.
- Q. Yue, K. Wang, M. Guan, Z. Zhao, X. Li, P. Yu and L. Mao, Angew. Chem., Int. Ed., 2022, 61, e202117596 CAS.
- Y. Liu, J. Du, M. Wang, J. Zhang, C. Liu and X. Li, Front. Chem., 2021, 8, 591311 CrossRef PubMed.
- J. Wang, J. Hou, H. Lei, J. Fu, Y. Pan and J. Liu, Mol. Med. Rep., 2015, 12, 5328–5334 CrossRef CAS PubMed.
- H. Miao, Y. Zhen, G. Ding, F. Hong, Z. Xie and M. Tian, Biomed. Environ. Sci., 2015, 28, 116–126 CAS.
- K. A. Albert, E. Helmer-Matyjek, A. C. Nairn, T. H. Müller, J. W. Haycock, L. A. Greene, M. Goldstein and P. Greengard, Proc. Natl. Acad. Sci. U.S.A., 1984, 81, 7713–7717 CrossRef CAS PubMed.
- A. Larsson, S. Majdi, A. Oleinick, I. Svir, J. Dunevall, C. Amatore and A. G. Ewing, Angew. Chem., Int. Ed., 2020, 59, 6711–6714 CrossRef CAS PubMed.
- E. V. Mosharov and D. Sulzer, Nat. Methods, 2005, 2, 651–658 CrossRef CAS PubMed.
- C. T. Wang, R. Grishanin, C. A. Earles, P. Y. Chang, T. F. Martin, E. R. Chapman and M. B. Jackson, Science, 2001, 294, 1111–1115 CrossRef CAS PubMed.
- R. Trouillon and A. G. Ewing, ACS Chem. Biol., 2014, 9, 812–820 CrossRef CAS PubMed.
- X. Li, J. Dunevall and A. G. Ewing, Acc. Chem. Res., 2016, 49, 2347–2354 CrossRef CAS PubMed.
- N. T. N. Phan, X. Li and A. G. Ewing, Nat. Rev. Chem., 2017, 1, 0048 CrossRef CAS.
- D. M. Omiatek, Y. Dong, M. L. Heien and A. G. Ewing, ACS Chem. Neurosci., 2010, 1, 234–245 CrossRef CAS PubMed.
- Y. Jiang, R. Wei, K. Tang, Z. Wang and N. Tan, J. Ginseng Res., 2022, 177 Search PubMed.
- Z. Liu, M. Zhao, Y. Zhang, J. Xue and N. Chen, Brain Res., 2010, 1333, 1–8 CrossRef CAS PubMed.
- S. Bardo, M. G. Cavazzini and N. Emptage, Trends Pharmacol. Sci., 2006, 27, 78–84 CrossRef CAS PubMed.
- L. K. Kaczmarek, Trends Neurosci., 1987, 10, 30–34 CrossRef CAS.
- D. A. Snyder, M. L. Kelly and D. J. Woodbury, Cell Biochem. Biophys., 2006, 45, 111–123 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06950d |
| ‡ Junlan Zhou and Jing Zhang contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |