Open Access Article
Open Access ArticleImproved photostability in ternary blend organic solar cells: the role of [70]PCBM†
Nutifafa Y.
Doumon
 *a,
Félix V.
Houard
*a,
Félix V.
Houard
 a,
Jingjin
Dong
b,
Panagiotis
Christodoulis
a,
Mikhail V.
Dryzhov
a,
Giuseppe
Portale
a,
Jingjin
Dong
b,
Panagiotis
Christodoulis
a,
Mikhail V.
Dryzhov
a,
Giuseppe
Portale
 b and
L. Jan Anton
Koster
b and
L. Jan Anton
Koster
 *a
*a
aPhotophysics and Optoelectronics, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, NL-9747 AG, Groningen, The Netherlands. E-mail: n.y.doumon@rug.nl; l.j.a.koster@rug.nl
bMacromolecular Chemistry and New Polymeric Materials, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, NL-9747 AG, Groningen, The Netherlands
First published on 11th April 2019
Abstract
Polymer solar cells are potentially key contributors to the next-generation organic photovoltaics for sustainable green sources of energy. In the past few years, ternary organic solar cells have emerged with promising characteristics. They have proven to yield high efficiency at about 15% for single junction donor:acceptor (D:A) solar cells. However, the low stability of organic solar cells is a hindrance to the commercialisation of this technology, and thus, needs more attention. Here, we show that with the right ratio of D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A1
A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2, ternary blend solar cells can be more efficient and more photostable than their D:A binary blend solar cells. We add [70]PCBM to PBDB-T:ITIC and PTB7-Th:ITIC binary blend solar cells in various ratios to fabricate ternary solar cells. The ternary solar cells outperform all binary cells in terms of efficiency and photostability with only a 10% average loss in efficiency under continuous illumination irrespective of the device structure. We identify changes in the molecular structure of the active layer blends as the main reason behind the observed degradation behaviour of the solar cells. The ternary blends are the most resilient to photo-induced molecular structural changes. This finding suggests that ternary organic solar cells could be a way to achieve photostable devices.
A2, ternary blend solar cells can be more efficient and more photostable than their D:A binary blend solar cells. We add [70]PCBM to PBDB-T:ITIC and PTB7-Th:ITIC binary blend solar cells in various ratios to fabricate ternary solar cells. The ternary solar cells outperform all binary cells in terms of efficiency and photostability with only a 10% average loss in efficiency under continuous illumination irrespective of the device structure. We identify changes in the molecular structure of the active layer blends as the main reason behind the observed degradation behaviour of the solar cells. The ternary blends are the most resilient to photo-induced molecular structural changes. This finding suggests that ternary organic solar cells could be a way to achieve photostable devices.
Introduction
Bulk heterojunction (BHJ) organic solar cells (OSCs) have attracted much attention in the past decades as they offer the possibility to achieve high power conversion efficiency (PCE). This is due to the opportunities offered for easy processing of the active layer namely the ability for solution processing and the mixture of (novel) organic materials readily soluble in various solvents.1 The latter brought into conception the ternary blend OSCs. The ternary blend OSCs differ from the well-known binary blend OSCs by one additional component in the mixture of their active layer organic materials. This means one can migrate from a donor:acceptor (D:A) binary system to either a D:A:A2–5 or a D:D:A6–9 ternary system. The ternary blends have helped achieve over the last few years a PCE of more than 14%10–14 in single junction OSCs and lately a record 17.3%15 in tandem structure serving as the rear sub-cell. This is because of the flexibility in choices of the additional D or A material such that a wider range of the solar spectrum is covered due to the complementarity of the additional material absorption profile to the parent cell's active layer absorption spectrum. The result could be an extension to the near infrared region or an improvement in the absorption strength for shorter wavelengths.16,17 The additional component serves as a bridge in the energy levels of the parent materials and creates an energetic cascade effect in the ternary blends by either facilitating charge transport as a charge relay component or as an agent of energy transfer, an antenna, and thus, improving the overall cell performance.17 This improvement in PCE is not only limited to these electronic effects. There could also be a positive structural effect. Thus, the third component could also enhance the nanomorphology3,18–20 of the active layer to help in better charge dissociation and transport. However, this is not always the case; the addition of a third component could also negatively affect the nanostructure of the blend. Therefore, there should be a trade-off in the ratio of the three components depending on the materials used. A third proposed working mechanism of the ternary blend is termed the parallel-like model, in which the device works as two intermixed binary or tandem like solar cell. This working mechanism is also called the alloy model, in which it is thought that either the donors or the acceptors form a kind of electronic alloy. Thus, the new HOMO and LUMO levels are considered to be the average of the individual energy levels. It is suggested that in the alloy concept, the Voc of the ternary device linearly scales with the blend ratios as opposed to pinning it to the difference in energy levels of the D and A,17,21–24 a point of view which is still under debate in the literature. The studies in the performance of the ternary blend solar cells have so far been only limited to the PCE. Then again, the same is true for the new class of binary OSCs, the non-fullerene solar cells.8,25–27 Only a few studies have truly tried to understand the stability issues related to these types of OSCs, and thus, study the degradation mechanisms in such cells.2,28,29 Few reports suggested the non-fullerene OSCs are relatively more stable than their fullerene counterparts, but no comparative studies have been carried out under the same conditions.30–32Here, we propose a systematic study under the same experimental and environmental conditions into the photostability of two binary OSCs namely, fullerene and non-fullerene OSCs, and D:A1:A2 ternary OSCs. The A1:A2 component of the ternary OSC is made up with the same fullerene and non-fullerene acceptors of the binary blends. Our study mainly employed the workhorse polymer PBDB-T as the donor material, [70]PCBM and ITIC as the fullerene and non-fullerene acceptor materials respectively, as shown in Fig. 1. To ascertain our finding, PTB7-Th workhorse low bandgap polymer33–35 was also employed in place of PBDB-T. Under the same experimental conditions, the ternary blend OSCs outperform by far the [70]PCBM binary blend OSC in terms of PCE and are slightly better in PCE than the ITIC binary blend OSC. More interestingly, we show that the ternary solar cells are the most efficient, followed by ITIC binary and then [70]PCBM binary solar cells. It is equally the most photostable over the period of the study, followed by [70]PCBM binary and then ITIC binary solar cells. This makes the non-fullerene binary OSCs the least photostable in the series. Additionally, through experimental techniques such as absorption spectroscopy, atomic force microscopy (AFM), grazing incidence wide-angle X-ray scattering (GIWAXS), and charge transport studies we explain the reasons behind the observed trend, thus, a decrease in photostability under continuous illumination: from the ternary blend to the fullerene binary blend with the least photostable being the non-fullerene binary blend solar cells. This study suggests that ternary blends could be a way to more efficient as well as photostable OSCs. Thus, more attention is to be invested in studies in this direction to ascertain this claim.
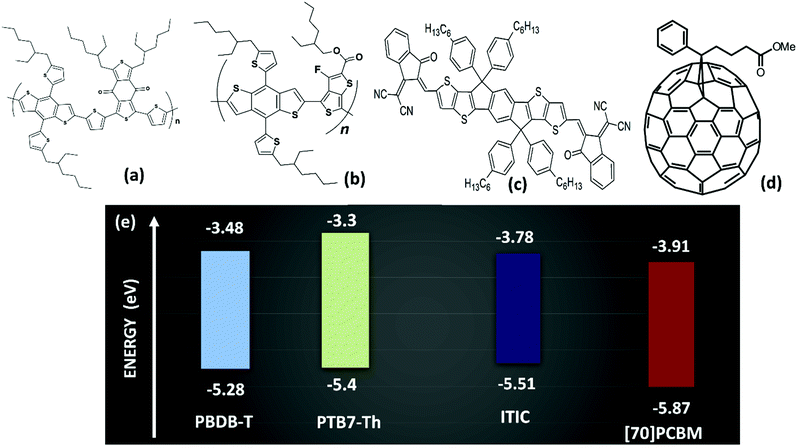 | ||
| Fig. 1 Semiconductor materials employed: PBDB-T (a), PTB7-Th (b), ITIC (c), [70]PCBM (d) with their corresponding HOMO–LUMO energy levels (e). | ||
Results & discussion
We fabricated both conventional and inverted structure OSCs of binary blends on the one hand and ternary blends on the other hand by varying the acceptor ratio (A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2) from 0 to 1 for PBDBT-based blends (or 0
A2) from 0 to 1 for PBDBT-based blends (or 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 for PTB7-Th-based blends) in a ratio of 1
1.5 for PTB7-Th-based blends) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dissolved in anhydrous chlorobenzene (CB) at a concentration of 20 mg mL−1. The cells are exposed continuously to 1 sun illumination for two hours at open-circuit, keeping them at room temperature in an inert atmosphere.
1 dissolved in anhydrous chlorobenzene (CB) at a concentration of 20 mg mL−1. The cells are exposed continuously to 1 sun illumination for two hours at open-circuit, keeping them at room temperature in an inert atmosphere.
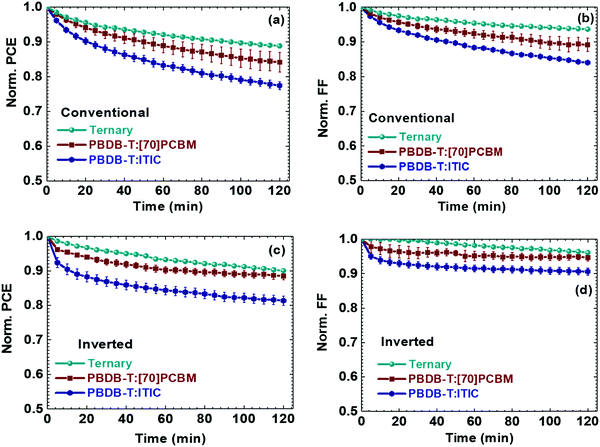
Fig. 2a reveals the average degradation pathway of the studied conventional OSCs (minimum of 10 devices) for their photostability while Fig. 2c shows the photodegradation behaviour of the inverted cells (minimum of 10 devices). The PBDB-T:ITIC binary blend OSCs suffer the most in degradation with more than 20% efficiency loss after 2 hours of illumination, regardless of the configuration (−23% for the conventional OSCs, and −20% for the inverted ones). In contrast, [70]PCBM-based devices take advantage of the inverted configuration, with an enhancement in stability from −16% to −12%. The most stable blend is the ternary blend with a limited degradation rate around −10% for both conventional and inverted configurations. Clearly, the ternary blend solar cells are more photostable than the binary blend solar cells.
Fig. 2b, d, and Fig. S1 (ESI†) display the corresponding degradation behaviour of the rest of the J–V parameters. Generally, as observed in Fig. S1 (ESI†) the Jsc and Voc remain fairly constant, excluding a potential photo-bleaching phenomenon (a loss in absorption) or changes in the HOMO and LUMO energy levels. However, the FF, as seen in Fig. 2b and d show the largest loss, and thus, remains the main contributor to the observed PCE loss in all three devices. Graphically, the loss behaviour of the PCEs is very similar to that of the FFs. The most significant finding is that the ternary blend solar cells are substantially more photostable than the ITIC based binary solar cells. To ascertain this finding, PBDB-T is substituted with PTB7-Th, and the experiments are repeated for two best ratios of the ternary blends. Fig. S2 (ESI†) distinctively displays the PCE decay behaviour of the single devices. Fig. S2c and d (ESI†) show clearly that the observation is true for both polymers and the different ratios. It is important to note that the degradation trends of the three solar cells are preserved irrespective of the device configuration, which allows excluding a possible impact of the different interfacial layers such as PEDOT:PSS, ZnO, MoO3, or LiF on the evolution of the photovoltaic performances over time. These changes are hence inherent to the active layer. The single degradation plots of the devices, conventional and inverted, in Fig. S2a and b (ESI†) reveal the real degradation behaviour of the three types of solar. The binary devices show the “burn-in” degradation behaviour known for solar cells under continuous illumination while the ternary ones only show a steady, gradual degradation behaviour. These findings do not imply that there is photostability beyond a couple of hours. We are limited within 2 hours in our study as we wanted to absolutely make sure that the devices are measured under the same conditions. Thus, more testing is needed to establish the long-term behaviour. However, there is a clear difference in the first two hours.
Additionally, the ternary blends outperform the binary blends in terms of PCE. Table 1 shows the J–V parameters of the selected OSCs in each category, fabricated under the same conditions, while Fig. S3 (ESI†) displays the J–V curves of the best performing OSCs, their external quantum efficiency (EQE), the absorption spectra of their films, and the device statistics. Before conducting the photodegradation experiments on the studied OSCs, we first work out the ratio of the binary and ternary blends in order to have them practically under the same conditions. Previously, the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has been reported to be the best for PBDB-T
1 has been reported to be the best for PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC binary devices. To have the same concentration for all blend solutions, the same ratio was used to gauge the ternary blends. While keeping constant the ratio of PBDB-T in the ternary blends, the ratio of the A1
ITIC binary devices. To have the same concentration for all blend solutions, the same ratio was used to gauge the ternary blends. While keeping constant the ratio of PBDB-T in the ternary blends, the ratio of the A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2 part of the D
A2 part of the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A1
A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2 ([D]
A2 ([D]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [A1]
[A1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [A2] = 1
[A2] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [A1]
[A1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-[A1]) is changed to optimize efficiency and stability. Tables S1 and S2 (ESI†) show the full range of the ratios covered for the conventional ternary blend OSCs as an example while Fig. S4 (ESI†) shows the dependency of the J–V parameters on the blend ratios. It is clear that our ternary blend devices do not conform with the alloy model22–24 as the Voc shows no linear dependency on the blending ratio, as is the case for the rest of the parameters. From Table S1 (ESI†), there are three ratios of PBDB-T
1-[A1]) is changed to optimize efficiency and stability. Tables S1 and S2 (ESI†) show the full range of the ratios covered for the conventional ternary blend OSCs as an example while Fig. S4 (ESI†) shows the dependency of the J–V parameters on the blend ratios. It is clear that our ternary blend devices do not conform with the alloy model22–24 as the Voc shows no linear dependency on the blending ratio, as is the case for the rest of the parameters. From Table S1 (ESI†), there are three ratios of PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM that recorded similar but highest PCEs, namely 1
[70]PCBM that recorded similar but highest PCEs, namely 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1
0.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3. Thus, the photodegradation experiments are primarily conducted for these ratios together with the binary blends. Fig. S2c (ESI†) shows this primary result, revealing similar photo-degradation behaviour for all three ratios with 1
0.3. Thus, the photodegradation experiments are primarily conducted for these ratios together with the binary blends. Fig. S2c (ESI†) shows this primary result, revealing similar photo-degradation behaviour for all three ratios with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 being slightly better. Our study mainly considered the 1
0.3 being slightly better. Our study mainly considered the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 ratio for PBDB-T
0.3 ratio for PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM as it yielded the best average PCE. Very recently, it was found by Wang et al. that the addition of [70]PCBM as a third component in PBDB-T:ITIC binary cells improves the efficiency, with 1
[70]PCBM as it yielded the best average PCE. Very recently, it was found by Wang et al. that the addition of [70]PCBM as a third component in PBDB-T:ITIC binary cells improves the efficiency, with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 ratio yielding the best efficiency in their device structure,36 as is the case also in our study (see Table S1, ESI†). Here, we show that [70]PCBM does not only improve the PCE of PBDB-T:ITIC binary cells, but also that of PTB7-Th:ITIC binary cells (with ratios of 1
0.2 ratio yielding the best efficiency in their device structure,36 as is the case also in our study (see Table S1, ESI†). Here, we show that [70]PCBM does not only improve the PCE of PBDB-T:ITIC binary cells, but also that of PTB7-Th:ITIC binary cells (with ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75) as well as the photostability of the parent binary cells.
0.75) as well as the photostability of the parent binary cells.
| Devices | Structure | Calc. Jsc (mA cm−2) | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) (PCEmn ± SD) |
|---|---|---|---|---|---|---|
| a 27 devices. b 11 devices. c 13 devices. d 18 devices. e 10 devices. f 19 devices. | ||||||
| Ternary | Conv.a | 14.4 | 15.0 (14.3 ± 0.4) | 0.879 (0.876 ± 0.006) | 65.0 (65.0 ± 1.2) | 8.6 (7.6 ± 0.5) |
| Inv.b | 14.1 (13.8 ± 0.4) | 0.836 (0.837 ± 0.008) | 70.5 (67.8 ± 2.4) | 8.3 (8.0 ± 0.3) | ||
| PBDB-T:ITIC | Conv.c | 13.6 | 13.6 (13.4 ± 0.6) | 0.895 (0.876 ± 0.012) | 64.1 (63.9 ± 2.2) | 7.8 (6.9 ± 0.5) |
| Inv.d | 14.1 (13.9 ± 0.7) | 0.842 (0.837 ± 0.011) | 68.2 (66.1 ± 2.0) | 8.1 (7.7 ± 0.3) | ||
| PBDB-T:[70]PCBM | Conv.e | 10.2 | 10.9 (10.4 ± 0.8) | 0.864 (0.855 ± 0.005) | 66.1 (67.8 ± 1.4) | 6.4 (5.9 ± 0.4) |
| Inv.f | 12.8 (11.6 ± 0.8) | 0.766 (0.813 ± 0.029) | 65.9 (62.1 ± 2.6) | 6.5 (5.6 ± 0.3) | ||
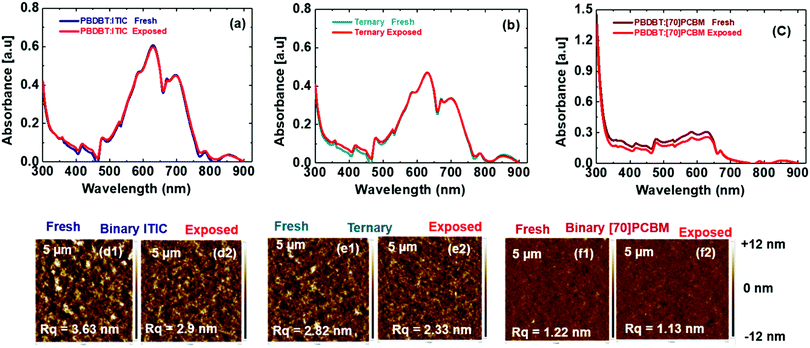
The superior performance of the ternary blends in terms of PCE has been sparingly touted in the literature. However, the reason why they perform better in terms of photodegradation than the binary blends has not been thoroughly investigated and remained largely unclear. Indeed, ternary blend solar cells lead to high efficiency in few cases,12,15 but in general, the efficiency of these cells are largely poor. We believe it is in the interest of the scientific community to study the few that show promising results so to understand their working mechanisms. It is in this need that we set out to investigate the aforementioned observed behaviours by studying all three blends in details: PBDB-T:ITIC, PBDB-T:[70]PCBM and PBDB-T:ITIC:[70]PCBM. We perform a series of experiments including UV-vis absorption spectroscopy and atomic force microscopy on their respective films before (fresh) and after exposure (exposed) to simulated sunlight for 2 hours, under the same condition as the solar cells. The absorption spectra of the fresh films are displayed in Fig. S3b (ESI†). Just as depicted in the EQE spectra in Fig. S3c (ESI†) the ternary film shows a slight improvement at lower wavelengths compared to the ITIC binary film while displaying similar contribution as ITIC binary at the longer wavelengths. Also, the AFM images show the ternary films as an intermediate morphology with the mean roughness of 2.8 nm between the ITIC binary with the roughest morphology (3.6 nm) and the [70]PCBM binary with the smoothest morphology (1.2 nm). This may explain in part the better performance of the ternary cells as they benefit from enhanced nanomorphology3,18 as compared to the ITIC binary blends. However, when we compare the absorption spectra of the fresh to the exposed films in Fig. 3a–c, no significant changes were detected as earlier speculated to suggest photo-bleaching as the cause of the degradation. The AFM images of the fresh and exposed films in Fig. 3d–f also show no significant changes capable of explaining the observed photodegradation behaviour of these blends over the period of exposure, though morphological changes cannot be totally discounted.
Next, we performed Jsc and Voc light intensity dependence measurements on the fresh and exposed (1 h and 2 h) solar cells to monitor the changes in recombination within the device. The presence of bimolecular recombination is highlighted by a sublinear behaviour of the Jsc with the light intensity. Thus, Jsc is proportional to Iα with α below or close to unity. No changes in α values are observed before and after exposure for the PBDB-T:ITIC (α = 0.96), PBDB-T:[70]PCBM (α = 0.97) and the ternary blends (α = 0.96) in Fig. S4a–c (ESI†). In addition to bimolecular recombination, trap-assisted recombination also contributes to losses in solar cells, as highlighted by the calculated ideality factors, n, above unity. It is especially the case for the fullerene blend solar cell, which exhibits a slightly higher density of traps with its n of 1.5, more than the 1.2 for the ITIC blend, and 1.29 for the ternary blend solar cells. Fig. S4d–f (ESI†) shows the variation of the Voc with light intensity. We can observe that the Voc of the fresh ternary blend device is pinned around that of the ITIC binary device. The extracted n from Fig. S4d–f (ESI†) reveal no change in n for ITIC binary devices which remained constant at around 1.20 and only minute increases in n for the [70]PCBM binary cells from 1.5 to 1.6, and for the ternary devices from 1.29 to 1.33. Thus, trap-assisted recombination cannot be ascribed to the observed degradation pattern; at least that is not the case for ITIC binary devices.
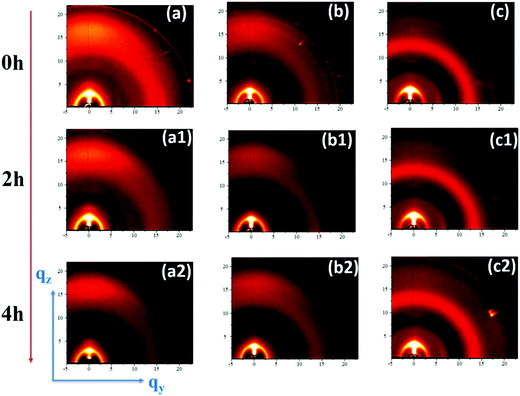
To elucidate the degradation process, we resort to studying the differences in molecular packing that may occur in the films at the fresh (0 h) and exposed (after 2 h and 4 h) states. The structure of the PBDB-T:ITIC, PBDB-T:PCBM and PBDB-T:ITIC:PCBM films and their time-dependence behaviour during exposure are studied by grazing incidence wide-angle X-ray scattering (GIWAXS). Fig. 4 shows the GIWAXS patterns and Fig. S6 (ESI†) their corresponding horizontal and vertical intensity cuts. As shown in Fig. 4, the image of PBDB-T:ITIC fresh film shows an intense (010) peak located at qz = 17.1 nm−1, associated to a molecule π–π stacking distance of 0.38 nm and concentrated along the vertical direction (see Fig. S6d, ESI†), indicating a dominantly face-on orientation of the PBDB-T crystallites (see Fig. 4a). Conversely, the (010) peak of PBDB-T is not present in the GIWAXS pattern of the fresh PBDB-T:[70]PCBM film. Instead, the intensity of the (100) peak at qz = 2.9 nm−1 is much higher than the PBDB-T:ITIC fresh film which means the PBDB-T:[70]PCBM film is mainly packed as edge-on. The PBDB-T:ITIC:[70]PCBM ternary blend fresh film shows a mixture of both binary structures; thus, a mixture of face-on and edge-on orientation.
The degradation upon light exposure was also assessed by GIWAXS. At different times during the exposure, the structural changes experienced by the three systems are quite different. Upon exposure, the (010) peak along the out-of-plane vertical direction of PBDB-T:ITIC film notably decreases over time together with a decrease of the (100) peak in the in-plain horizontal direction. This intensity decrease reported in Fig. S6a and d (ESI†) suggests that the face-on packed crystallites significantly degrade and the molecular packing is negatively affected by light exposure. However, the edge-on packed crystallites also degraded dramatically as shown in the in-plane plots (see Fig. S6a, ESI†) of PBDB-T:ITIC films with the (010) peak at qy = 15.4 nm−1 disappearing after 2 hours and the out-of-plane (100) peak decreasing with time. The changes in the structure of the PBDB-T:ITIC films point clearly to a decrease in crystallinity and could impede charge mobility, creating unbalanced charges as already revealed in our previous study29 explaining why the FF dropped the fastest. Thus, the PBDB-T:ITIC binary cells recorded the fastest PCE decay among the three cells. In contrast, both the PBDB-T:[70]PCBM and the PBDB-T:ITIC:[70]PCBM ternary blend films are less affected by the exposure, with minimal structural changes upon exposure. This can explain the relatively good stability of these two blends with respect to the PBDB-T:ITIC binary solar cells. As stated earlier, the structural changes experienced by the PBDB-T:ITIC cells, linked to the unbalanced charge carrier mobilities in their active layers, is the main reason behind the losses observed in the ITIC binary devices. However, the [70]PCBM binary devices with their more balanced mobilities remain more photostable than ITIC despite the slight increase in trap-assisted recombination. We can thus conclude that the preserved crystallinity upon illumination is the reason why the PBDB-T:ITIC:[70]PCBM ternary blend film remains the most efficient. Key in this preservation of the crystallinity upon illumination seems to be the incorporation of [70]PCBM in the PBDB-T:ITIC-based blends, that helps to enhance the system's photostability.
Conclusion
The goal of the current study is to find a way to make the non-fullerene acceptor ITIC-based solar cells more photostable without compromising the power conversion efficiency. This study has shown that this is achievable by means of ternary blends by carefully tuning the ratio of the acceptors in the D:A:A blends to maximise the output of the resulting ternary blend organic solar cells. In our case the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 of PBDB-T
0.3 of PBDB-T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM blend helped in achieving our goal. Thus, the resulting ternary blend solar cells are more photostable over the period of study than the two binary solar cells, especially the PBDB-T:ITIC binary cell, while keeping a higher efficiency than both. The finding of the photostability of the ternary blend solar cells, in this particular case, is also not only valid for the ratios of 1
[70]PCBM blend helped in achieving our goal. Thus, the resulting ternary blend solar cells are more photostable over the period of study than the two binary solar cells, especially the PBDB-T:ITIC binary cell, while keeping a higher efficiency than both. The finding of the photostability of the ternary blend solar cells, in this particular case, is also not only valid for the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 but also for a different donor polymer, PTB7-Th. The ternary blend resilience to photodegradation is explained by the fact that they retain more their crystallinity and molecular packing structure over time compared to the binary blends, especially the ITIC binary organic solar cells. These findings have significant implications for the understanding of the photodegradation mechanisms in [70]PCBM and ITIC-based solar cells and suggest that a better understanding into the ternary blend solar cells could pave a way to more photostable devices.
0.1 but also for a different donor polymer, PTB7-Th. The ternary blend resilience to photodegradation is explained by the fact that they retain more their crystallinity and molecular packing structure over time compared to the binary blends, especially the ITIC binary organic solar cells. These findings have significant implications for the understanding of the photodegradation mechanisms in [70]PCBM and ITIC-based solar cells and suggest that a better understanding into the ternary blend solar cells could pave a way to more photostable devices.
Experimental methods
Materials
All materials used are commercially available: PBDB-T and ITIC were purchased from Solarmer Energy Inc., while [70]PCBM was supplied by Solenne BV. All the solvents and anhydrous zinc acetate (Zn(OAc)2) were obtained from Sigma-Aldrich and used without further purification.Solutions preparation
For the polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC and polymer
ITIC and polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM solutions, blends in a ratio of 1
[70]PCBM solutions, blends in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are dissolved in anhydrous chlorobenzene (CB) at a concentration of 20 mg mL−1 and stirred at 40 °C overnight in a glovebox. The same concentrations are used for the ternary devices, by varying the acceptor ratio (A1
1 are dissolved in anhydrous chlorobenzene (CB) at a concentration of 20 mg mL−1 and stirred at 40 °C overnight in a glovebox. The same concentrations are used for the ternary devices, by varying the acceptor ratio (A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A2) from 0 to 1. The ratio was varied between 1
A2) from 0 to 1. The ratio was varied between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to the best known 1
1 to the best known 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 as used before for PBDT-TT polymer with [70]PCBM34,35 when PTB7-Th is used.
1.5 as used before for PBDT-TT polymer with [70]PCBM34,35 when PTB7-Th is used.
Zinc oxide solution was prepared by dissolving zinc acetate (109.67 mg) in 2-methoxyethanol (1 mL) and ethanolamine (30.2 μL). The solution was then stirred at room temperature for a few hours.
Device fabrication
The following steps of the device fabrication were performed in a cleanroom environment. All devices were prepared on pre-patterned ITO glass substrates. All substrates were carefully and successively cleaned in soap, ultraclean water, acetone, and isopropanol (IPA) with sonication for 10 minutes, then spin-dried. Additional drying steps were carried out in an oven at 140 °C for 10 minutes and in UV-ozone steriliser for 20 minutes. For conventional OSCs, PEDOT:PSS layer (50 nm) was spin-coated on the ITO substrate in ambient and annealed at 140 °C for 10 minutes to remove the excess water. For inverted devices, zinc oxide solution was spin-coated instead, at 4000 rpm for 40 seconds then annealed at 170 °C for 30 minutes in air. Then substrates were transferred into a glovebox under inert conditions for the active layer spin-coating. Spin-coating was performed at 1500 rpm for 5 seconds, followed by active spin-drying at 400 rpm for 60 seconds to reach a thickness of 100 nm. Finally, the top electrodes were thermally deposited in an evaporator under vacuum at <1 × 10−7 mbar: LiF (1 nm) and Al (100 nm) for conventional configuration, or MoO3 (10 nm) and Al or Ag (100 nm) for inverted devices.Characterisations
Conflicts of interest
The authors declare no competing interests.Acknowledgements
The work is funded by the Zernike Bonus Incentive Scheme Grant. This is a publication by the FOM Focus Group “Next Generation Organic Photovoltaics”, participating in the Dutch Institute for Fundamental Energy Research (DIFFER). N. Y. D. would like to acknowledge A. Kamp and T. Zaharia for technical support.References
- J.-H. Kim, A. Gadisa, C. Schaefer, H. Yao, B. R. Gautam, N. Balar, M. Ghasemi, I. Constantinou, F. So, B. T. O’Connor, K. Gundogdu, J. Hou and H. Ade, J. Mater. Chem. A, 2017, 5, 13176–13188 RSC
.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Röhr, S. Holliday, A. Wadsworth, S. Lockett, M. Neophytou, C. J. M. Emmott, J. Nelson, C. J. Brabec, A. Amassian, A. Salleo, T. Kirchartz, J. R. Durrant and I. McCulloch, Nat. Mater., 2017, 16, 363–369 CrossRef CAS PubMed
.
- R. Yu, S. Zhang, H. Yao, B. Guo, S. Li, H. Zhang, M. Zhang and J. Hou, Adv. Mater., 2017, 29, 1–6 Search PubMed
.
- W. Zhong, J. Cui, B. Fan, L. Ying, Y. Wang, X. Wang, G. Zhang, X. F. Jiang, F. Huang and Y. Cao, Chem. Mater., 2017, 29, 8177–8186 CrossRef CAS
.
- H. Lu, J. Zhang, J. Chen, Q. Liu, X. Gong, S. Feng, X. Xu, W. Ma and Z. Bo, Adv. Mater., 2016, 28, 9559–9566 CrossRef CAS PubMed
.
- Y. J. Hwang, H. Li, B. A. E. Courtright, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2016, 28, 124–131 CrossRef CAS PubMed
.
- L. Zhong, L. Gao, H. Bin, Q. Hu, Z. G. Zhang, F. Liu, T. P. Russell, Z. Zhang and Y. Li, Adv. Energy Mater., 2017, 7, 1–7 Search PubMed
.
- J. W. Lee, Y. S. Choi, H. Ahn and W. H. Jo, ACS Appl. Mater. Interfaces, 2016, 8, 10961–10967 CrossRef CAS PubMed
.
- N. Gasparini, X. Jiao, T. Heumueller, D. Baran, G. J. Matt, S. Fladischer, E. Spiecker, H. Ade, C. J. Brabec and T. Ameri, Nat. Energy, 2016, 1, 16118 CrossRef CAS
.
- N. Gasparini, L. Lucera, M. Salvador, M. Prosa, G. D. Spyropoulos, P. Kubis, H. J. Egelhaaf, C. J. Brabec and T. Ameri, Energy Environ. Sci., 2017, 10, 885–892 RSC
.
- P. Cheng, J. Wang, Q. Zhang, W. Huang, J. Zhu, R. Wang, S. Y. Chang, P. Sun, L. Meng, H. Zhao, H. W. Cheng, T. Huang, Y. Liu, C. Wang, C. Zhu, W. You, X. Zhan and Y. Yang, Adv. Mater., 2018, 30, 1–8 Search PubMed
.
- Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562–1564 CrossRef CAS
.
- Y. Xie, F. Yang, Y. Li, M. A. Uddin, P. Bi, B. Fan, Y. Cai, X. Hao, H. Y. Woo, W. Li, F. Liu and Y. Sun, Adv. Mater., 2018, 1803045 CrossRef PubMed
.
- T. Kumari, S. M. Lee, S. H. Kang, S. Chen and C. Yang, Energy Environ. Sci., 2017, 10, 258–265 RSC
.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H. Yip, Y. Cao and Y. Chen, Science, 2018, 2612, 1–10 Search PubMed
.
- J. You, L. Dou, Z. Hong, G. Li and Y. Yang, Prog. Polym. Sci., 2013, 38, 1909–1928 CrossRef CAS
.
- L. Lu, M. A. Kelly, W. You and L. Yu, Nat. Photonics, 2015, 9, 491–500 CrossRef CAS
.
- M. Campoy-Quiles, Y. Kanai, A. El-Basaty, H. Sakai and H. Murata, Org. Electron., 2009, 10, 1120–1132 CrossRef CAS
.
- C. Kästner, S. Rathgeber, D. A. M. Egbe and H. Hoppe, J. Mater. Chem. A, 2013, 1, 3961 RSC
.
- R. Bechara, N. Leclerc, P. Lévêque, F. Richard, T. Heiser and G. Hadziioannou, Appl. Phys. Lett., 2008, 93, 013306 CrossRef
.
- A. D. de Zerio and C. Müller, Adv. Energy Mater., 2018, 1702741, 1–18 Search PubMed
.
- T. Liu, Y. Guo, Y. Yi, L. Huo, X. Xue, X. Sun, H. Fu, W. Xiong, D. Meng, Z. Wang, F. Liu, T. P. Russell and Y. Sun, Adv. Mater., 2016, 28, 10008–10015 CrossRef CAS PubMed
.
- R. A. Street, D. Davies, P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2013, 135, 986–989 CrossRef CAS PubMed
.
- P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2012, 134, 9074–9077 CrossRef CAS PubMed
.
- H. Cha, S. Wheeler, S. Holliday, S. D. Dimitrov, A. Wadsworth, H. H. Lee, D. Baran, I. McCulloch and J. R. Durrant, Adv. Funct. Mater., 2018, 28, 1704389 CrossRef
.
- H. Li, Y.-J. Hwang, B. A. E. Courtright, F. N. Eberle, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2015, 27, 3266–3272 CrossRef CAS PubMed
.
- N. Gasparini, M. Salvador, T. Heumueller, M. Richter, A. Classen, S. Shrestha, G. J. Matt, S. Holliday, S. Strohm, H.-J. Egelhaaf, A. Wadsworth, D. Baran, I. McCulloch and C. J. Brabec, Adv. Energy Mater., 2017, 7, 1701561 CrossRef
.
- Y. Wang, M. J. Jafari, N. Wang, D. Qian, F. Zhang, T. Ederth, E. Moons, J. Wang, O. Inganäs, W. Huang and F. Gao, J. Mater. Chem. A, 2018, 6, 11884–11889 RSC
.
- N. Y. Doumon, M. V. Dryzhov, F. V. Houard, V. M. Le Corre, A. Rahimi Chatri, P. Christodoulis and L. J. A. Koster, ACS Appl. Mater. Interfaces, 2019 DOI:10.1021/acsami.8b20493
.
- D. Baran, N. Gasparini, A. Wadsworth, C. H. Tan, N. Wehbe, X. Song, Z. Hamid, W. Zhang, M. Neophytou, T. Kirchartz, C. J. Brabec, J. R. Durrant and I. Mcculloch, Nat. Commun., 2018, 9, 2059 CrossRef PubMed
.
- H. Cha, J. Wu, A. Wadsworth, J. Nagitta, S. Limbu, S. Pont, Z. Li, J. Searle, M. F. Wyatt, D. Baran, J. S. Kim, I. McCulloch and J. R. Durrant, Adv. Mater., 2017, 29, 1–8 Search PubMed
.
- N. Gasparini, M. Salvador, S. Strohm, T. Heumueller, I. Levchuk, A. Wadsworth, J. H. Bannock, J. C. de Mello, H. J. Egelhaaf, D. Baran, I. Mcculloch and C. J. Brabec, Adv. Energy Mater., 2017, 1700770, 1–7 Search PubMed
.
- S. H. Liao, H. J. Jhuo, Y. S. Cheng and S. A. Chen, Adv. Mater., 2013, 25, 4766–4771 CrossRef CAS PubMed
.
- N. Y. Doumon, G. Wang, R. C. Chiechi and L. J. A. Koster, J. Mater. Chem. C, 2017, 5, 6611–6619 RSC
.
- N. Y. Doumon and L. J. A. Koster, Sol. RRL, 2019, 1800301 CrossRef
.
- B. Wang, Y. Fu, C. Yan, R. Zhang, Q. Yang, Y. Han and Z. Xie, Front. Chem., 2018, 6, 1–8 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Time evolution of J–V parameters of the studied solar cells, J–V parameters of the studied solar cells, ratio-dependent photovoltaic performance of PBDB-T based solar cells, light intensity dependence measurements, and GIWAXS peak intensity plots. See DOI: 10.1039/c8tc06621c |
| This journal is © The Royal Society of Chemistry 2019 |