Tunneling and thermoelectric characteristics of N-heterocyclic carbene-based large-area molecular junctions†
Seohyun
Kang
,
Sohyun
Park
,
Hungu
Kang
,
Soo Jin
Cho
,
Hyunsun
Song
and
Hyo Jae
Yoon
 *
*
Department of Chemistry, Korea University, Seoul, 02841, South Korea. E-mail: hyoon@korea.ac.kr
First published on 25th March 2019
Abstract
Reported herein are tunneling and thermoelectric characteristics of large-area molecular junctions formed with N-heterocyclic carbene (NHC)-based self-assembled monolayers on gold.
Large-area molecular junctions formed with self-assembled monolayers (SAMs) formed via thiol chemistry over coinage metal substrates have been widely harnessed in the study of organic and molecular electronics.1–3 Indeed, molecular junctions incorporating thiol-based SAMs have shown their utility in fundamental understanding of molecular conduction4–11 and various applications including molecular diodes,12–17 photo-switches,18,19 negative differential resistors,20 thermoelectrics,21–23 and plasmonics.24 However, as free thiols and thiolates anchored on a substrate can be easily oxidized and decomposed by oxygen in the air and photoirradiation,25–27 thiol-based SAMs often do not guarantee long-term stability and durability.25 To circumvent this problem, Crudden et al.28 have reported a stimulating work in 2014 that utilizes the N-heterocyclic carbene (NHC) chemistry over gold to form thiol-free, robust SAMs not only under ambient conditions but also under high temperature and chemically/electrochemically active conditions. In 2018, Doud et al.29 reported the first NHC-based single-molecule junction via incorporation of MeS(Ph)nNHC–MCl complexes (M = Au, Ag, and Cu; n = 0, 1, and 2) into scanning tunneling microscope-based break junction (STM-BJ). According to this previous work, the conductance depends on the metal in the precursor (decreasing in the order of M = Au > Ag > Cu). It has been noted that the junctions formed with solutions of free NHCs did not yield reproducible conductance results.
Herein, we show for the first-time fabrication of large-area molecular junctions of NHC-based monolayers constructed directly from solutions of metal-free NHC derivatives and examine their tunneling and thermoelectric characteristics. Oligophenylene-NHC SAMs (denoted as NHC(Ph)n where n = 0, 1, and 2; Fig. 1a) were formed on ultraflat template-stripped gold (AuTS),30 and electrically and thermoelectrically characterized with a liquid metal top-electrode comprising eutectic Ga–In alloy (EGaIn)21,31 covered with a conductive native oxide of ∼1 nm nominal thickness (Fig. 1b). We observed the length dependence of tunneling current density (J, A cm−2) across the junctions, obeying a rectangular energy barrier model, the simplified Simmons model.5,32 Junction measurements at low temperatures indicate the charge transport across the NHC-based large-area junctions occurring in a pure tunneling regime, not relying on a thermal hopping process. Thermoelectric characterization over identical junctions showed that thermopower increased with increasing length of the molecule. The positive sign of the measured Seebeck coefficient values implies that the frontier molecular orbital close to the Fermi level of the electrode is the highest occupied molecular orbital (HOMO) rather than the lowest unoccupied molecular orbital (LUMO), and the molecular conduction is dominated by hole tunneling.
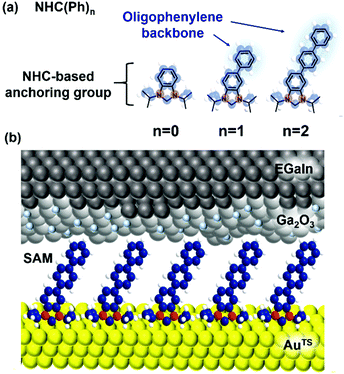 | ||
| Fig. 1 (a) Molecules used in this study. (b) A schematic describing the structure of the large-area molecular junction we used. | ||
We synthesized NHC(Ph)n hydrogen carbonate salts (NHC(Ph)nHCO3) as NHC precursors,33 following the synthetic schemes presented in Scheme S1 in the ESI.† The 1H and 13C NMR spectroscopic analyses and the high resolution mass spectrometric analysis confirmed the desired structures (see the ESI† for details). To form NHC SAMs on AuTS, a freshly prepared AuTS chip was placed in an ethanolic solution of 2 mM NHC(Ph)nHCO3.33 After 3 h incubation at room temperature in the air, the resulting SAM-bound AuTS chip was rinsed with pure ethanol and dried in air (see the ESI† for details). The AuTS/NHC(Ph)n SAMs were characterized with X-ray photoelectron spectroscopy (XPS), and static and dynamic contact angle goniometries. The XPS spectra (Fig. S6–S8 in the ESI†) exhibited the presence of nitrogen, carbon and oxygen atoms in all the SAMs. A single binding energy of N1s was observed at 402.5 eV; the C1s peak was deconvoluted into three signals, which corresponded to C–O, C–N, and C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively.28,29,34 As the number of phenylene units increased, the intensity ratio of C–C/C
C, respectively.28,29,34 As the number of phenylene units increased, the intensity ratio of C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C to C–N signals increased (Table S3 in the ESI†). XPS data showed that all the SAMs have oxygen atoms, and the peak of O1s was observed at 525 eV. This is attributed to the decomposition by-products (CO2 and/or H2O formed upon chemisorption of the NHC precursor) trapped in the monolayers on the substrates.28,33,34 As shown in Fig. S12 in the ESI,† the static contact angle (θs) and the dynamic contact angle (Δcos
C to C–N signals increased (Table S3 in the ESI†). XPS data showed that all the SAMs have oxygen atoms, and the peak of O1s was observed at 525 eV. This is attributed to the decomposition by-products (CO2 and/or H2O formed upon chemisorption of the NHC precursor) trapped in the monolayers on the substrates.28,33,34 As shown in Fig. S12 in the ESI,† the static contact angle (θs) and the dynamic contact angle (Δcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θd: the difference in advancing (cos
θd: the difference in advancing (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θa) and receding (cos
θa) and receding (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θr) contact angles) did not significantly change with the increasing length (n in NHC(Ph)n). These observations indicate that all the SAMs have similar surface energy and roughness regardless of the number of phenylene units.
θr) contact angles) did not significantly change with the increasing length (n in NHC(Ph)n). These observations indicate that all the SAMs have similar surface energy and roughness regardless of the number of phenylene units.
The electronic structure of the NHC(Ph)n SAMs was characterized using ultraviolet photoelectron spectroscopy (UPS) and Kelvin probe force microscopy (KPFM) (Fig. S12 in the ESI†). From the UPS and KPFM studies, the work function (WF) of the AuTS/NHC(Ph)n SAMs was estimated through measurements of secondary electron cutoff (SECO) and contact potential difference (CPD), respectively (see the ESI† for details). The UPS-based WF values ranged from 3.45–4.04 eV, similar to the previous observation.35 The trends of WF estimated by UPS and KPFM were similar although there were some deviations between the absolute values. Values of KPFM-based WF were obtained by relative comparison of target samples with the WF (∼4.5 eV) of highly oriented pyrolytic graphite (HOPG). We did not observe the correlation between the WF and the molecular length.
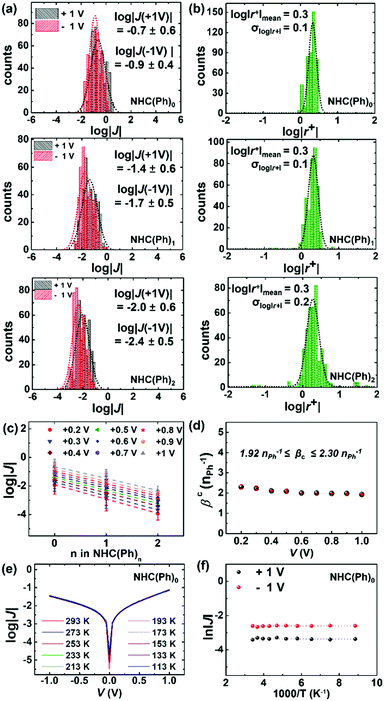
To measure J(V) across junctions, we formed junctions with a cone-shaped EGaIn microelectrode following the previously reported methods.11,31,36 The EGaIn microelectrode permits noninvasive and reversible top-contacts over SAMs. We obtained 374–468 J–V traces from 20–27 separate junctions in six different samples for each molecule. Values of J across AuTS/NHC-(Ph)n//Ga2O3/EGaIn junctions at ±1.0 V were log-normally distributed (Fig. 2a). The degree of asymmetric feature in the J–V curve is quantitatively defined as rectification: |r+| = |J(+V)|/|J(−V)|. The SAMs showed modest rectification ratios (1.9 < |r+| < 2.1 at ±1.0 V; Fig. 2b), similar to the rectification ratios observed in conventional n-alkane and oligophenylene thiolates.5,37 Mean (log|J|mean), median (log|J|median) and standard deviation (σlog|J|) were extracted from fitting of log|J| histograms with single Gaussian curves. Table S1 in the ESI† summarizes the data of junction measurements. The yield of working junctions ranged from 88 to 92%. Fig. 2c shows that log|J| linearly decreased with the increasing length of the molecule. This linear regression indicates that the tunneling current density across junctions well-obeys the simplified Simmons model (eqn (1)):5,6,32
| J = J0 × exp(−βcd) | (1) |
With the identical junction architecture, we measured for the first time the Seebeck coefficient of the NHC-based monolayers (Fig. 3a), following the previously reported procedures.21 Briefly, a SAM-bound AuTS chip was placed on a hot chuck, and exposed to a finite temperature. A microelectrode comprising EGaIn was then brought onto the SAM, and the thermoelectric voltage (ΔV) created by the temperature differential (ΔT) applied to the junction was measured (see the ESI† for details). We measured ΔV values for ΔT = 2, 4 and 6 K (Fig. 3b). Overall, the distribution of ΔV was broadened as the value of ΔT and the number of phenylene units were increased, as shown in ΔV histograms (Fig. S13 in the ESI†). This observation was consistent with the results observed in other thermoelectric junctions.21,40,41 The increased ΔT and the structural complexity of the molecule induce an increase in the degree of freedom (by variation in C–C torsional angles) and internal vibration, thereby leading to largely dispersed data of ΔV.41 The Seebeck coefficient (S, μV K−1; S = −ΔV/ΔT) of the SAM (SSAM) was extracted through the thermopower analysis of the circuit equivalent to the junction (Fig. S5 in the ESI†). Finally, using the following equation, we obtained the SSAM values:
ΔV = −(SSAM − SW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tip) × ΔT tip) × ΔT | (2) |
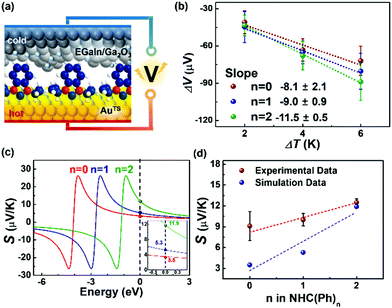 | ||
| Fig. 3 (a) A schematic describing the structure of the large-area thermoelectric junction. (b) Plots of ΔV as a function of ΔT. (c) Simulations of S values based on the transmission function and Landauer formula (eqn (S2) and (S3) in the ESI†). The inset shows the magnification of Seebeck coefficient near the Fermi level of EGaIn. (d) Plots of experimental and theoretical S values as a function of the molecular length. | ||
The data of thermopower measurements are summarized in Table S2 in the ESI.† All the NHC(Ph)n junctions exhibited positive values of SSAM (Table S2, ESI†). This indicates that the transport in our junctions is dominated by the HOMO, not the LUMO. Our finding is against the previous calculation29 that shows that the LUMO is close to the EF of the electrode in the NHC-based single molecule junction. This discrepancy can be explained by the different top-interfaces: our junction has the van der Waals top-contact whereas the junction in the previous work does not. The thermopower in the NHC-based junctions was further investigated by transmission function-based modelling (see the ESI† for details).22,42 As shown in Fig. 3c, the experimentally observed length dependence and sign of SSAM were reproduced, at least qualitatively, by the modelling (Fig. 3d). There were some deviations between experimental and calculated values, which could be attributed to complexities arising from the top-contact and the molecular vibrations that are not reflected in the modelling.22
The linear correlation between the SSAM and the molecular length can be explained by a simplified model (eqn (3)) proposed
| SSAM = SC + n·βS | (3) |
In conclusion, we have investigated for the first-time tunneling and thermoelectric properties of NHC-based large-area junctions through a combined experimental and computational approach. Our studies indicate that the charge tunneling of NHC-based monolayers follows the Simmons model widely used for studies of molecular conduction, and the Seebeck coefficient is positive in sign and increases with molecular length. Our computational studies confirm these empirical findings.
This research was supported by the NRF of Korea (NRF-2017M3A7B8064518).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Vilan, D. Aswal and D. Cahen, Chem. Rev., 2017, 117, 4248 CrossRef CAS PubMed.
- D. Xiang, X. L. Wang, C. C. Jia, T. Lee and X. F. Guo, Chem. Rev., 2016, 116, 4318 CrossRef CAS PubMed.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1169 CrossRef CAS PubMed.
- H. J. Yoon, N. D. Shapiro, K. M. Park, M. M. Thuo, S. Soh and G. M. Whitesides, Angew. Chem., Int. Ed., 2012, 51, 4658 CrossRef CAS PubMed.
- F. C. Simeone, H. J. Yoon, M. M. Thuo, J. R. Barber, B. Smith and G. M. Whitesides, J. Am. Chem. Soc., 2013, 135, 18131 CrossRef CAS PubMed.
- H. J. Yoon, C. M. Bowers, M. Baghbanzadeh and G. M. Whitesides, J. Am. Chem. Soc., 2014, 136, 16 CrossRef CAS PubMed.
- J. Chen, M. Kim, S. Gathiaka, S. J. Cho, S. Kundu, H. J. Yoon and M. M. Thuo, J. Phys. Chem. Lett., 2018, 9, 5078 CrossRef CAS PubMed.
- J. Jin, G. D. Kong and H. J. Yoon, J. Phys. Chem. Lett., 2018, 9, 4578 CrossRef CAS PubMed.
- Z. Xie, I. Bâldea and C. D. Frisbie, Chem. Sci., 2018, 9, 4456 RSC.
- J. M. Beebe, B. Kim, C. D. Frisbie and J. G. Kushmerick, ACS Nano, 2008, 2, 827 CrossRef CAS PubMed.
- G. D. Kong, J. Jin, M. M. Thuo, H. Song, J. F. Joung, S. Park and H. J. Yoon, J. Am. Chem. Soc., 2018, 140, 12303 CrossRef CAS PubMed.
- N. Nerngchamnong, L. Yuan, D. C. Qi, J. Li, D. Thompson and C. A. Nijhuis, Nat. Nanotechnol., 2013, 8, 113 CrossRef CAS PubMed.
- H. J. Yoon, K. C. Liao, M. R. Lockett, S. W. Kwok, M. Baghbanzadeh and G. M. Whitesides, J. Am. Chem. Soc., 2014, 136, 17155 CrossRef CAS PubMed.
- L. Qiu, Y. X. Zhang, T. L. Krijger, X. K. Qiu, P. van't Hof, J. C. Hummelen and R. C. Chiechi, Chem. Sci., 2017, 8, 2365 RSC.
- S. J. Cho, G. D. Kong, S. Park, J. Park, S. E. Byeon, T. Kim and H. J. Yoon, Nano Lett., 2019, 19, 545 CrossRef CAS PubMed.
- G. J. Ashwell and A. Mohib, J. Am. Chem. Soc., 2005, 127, 16238 CrossRef CAS PubMed.
- M. L. Chabinyc, X. X. Chen, R. E. Holmlin, H. Jacobs, H. Skulason, C. D. Frisbie, V. Mujica, M. A. Ratner, M. A. Rampi and G. M. Whitesides, J. Am. Chem. Soc., 2002, 124, 11730 CrossRef CAS PubMed.
- C. C. Jia, A. Migliore, N. Xin, S. Y. Huang, J. Y. Wang, Q. Yang, S. P. Wang, H. L. Chen, D. M. Wang, B. Y. Feng, Z. R. Liu, G. Y. Zhang, D. H. Qu, H. Tian, M. A. Ratner, H. Q. Xu, A. Nitzan and X. F. Guo, Science, 2016, 352, 1443 CrossRef CAS PubMed.
- D. Kim, H. Jeong, H. Lee, W. T. Hwang, J. Wolf, E. Scheer, T. Huhn, H. Jeong and T. Lee, Adv. Mater., 2014, 26, 3968 CrossRef CAS PubMed.
- J. Chen, W. Wang, M. A. Reed, A. M. Rawlett, D. W. Price and J. M. Tour, Appl. Phys. Lett., 2000, 77, 1224 CrossRef CAS.
- S. Park and H. J. Yoon, Nano Lett., 2018, 18, 7715 CrossRef CAS PubMed.
- A. Tan, J. Balachandran, S. Sadat, V. Gavini, B. D. Dunietz, S.-Y. Jang and P. Reddy, J. Am. Chem. Soc., 2011, 133, 8838 CrossRef CAS PubMed.
- L. Cui, R. Miao, K. Wang, D. Thompson, L. A. Zotti, J. C. Cuevas, E. Meyhofer and P. Reddy, Nat. Nanotechnol., 2018, 13, 122 CrossRef CAS PubMed.
- W. Du, T. Wang, H.-S. Chu, L. Wu, R. Liu, S. Sun, W. K. Phua, L. Wang, N. Tomczak and C. A. Nijhuis, Nat. Photonics, 2016, 10, 274 CrossRef CAS.
- G. D. Kong and H. J. Yoon, J. Electrochem. Soc., 2016, 163, G115 CrossRef CAS.
- Y. Li, J. Huang, R. T. McIver Jr. and J. C. Hemminger, J. Am. Chem. Soc., 1992, 114, 2428 CrossRef CAS.
- M. H. Schoenfisch and J. E. Pemberton, J. Am. Chem. Soc., 1998, 120, 4502 CrossRef CAS.
- C. M. Crudden, J. H. Horton, I. I. Ebralidze, O. V. Zenkina, A. B. McLean, B. Drevniok, Z. She, H.-B. Kraatz, N. J. Mosey, T. Seki, E. C. Keske, J. D. Leake, A. Rousina-Webb and G. Wu, Nat. Chem., 2014, 6, 409 CrossRef CAS PubMed.
- E. A. Doud, M. S. Inkpen, G. Lovat, E. Montes, D. W. Paley, M. L. Steigerwald, H. Vázquez, L. Venkataraman and X. Roy, J. Am. Chem. Soc., 2018, 140, 8944 CrossRef CAS PubMed.
- E. A. Weiss, G. K. Kaufman, J. K. Kriebel, Z. Li, R. Schalek and G. M. Whitesides, Langmuir, 2007, 23, 9686 CrossRef CAS PubMed.
- R. C. Chiechi, E. A. Weiss, M. D. Dickey and G. M. Whitesides, Angew. Chem., Int. Ed., 2008, 47, 142 CrossRef CAS PubMed.
- J. G. Simmons, J. Appl. Phys., 1963, 34, 2581 CrossRef.
- C. M. Crudden, J. H. Horton, M. R. Narouz, Z. Li, C. A. Smith, K. Munro, C. J. Baddeley, C. R. Larrea, B. Drevniok, B. Thanabalasingam, A. B. McLean, O. V. Zenkina, I. I. Ebralidze, Z. She, H.-B. Kraatz, N. J. Mosey, L. N. Saunders and A. Yagi, Nat. Commun., 2016, 7, 12654 CrossRef CAS PubMed.
- G. Lovat, E. A. Doud, D. Lu, G. Kladnik, M. S. Inkpen, M. L. Steigerwald, D. Cvetko, M. S. Hybertsen, A. Morgante, X. Roy and L. Venkataraman, Chem. Sci., 2019, 10, 930 RSC.
- H. K. Kim, A. S. Hyla, P. Winget, H. Li, C. M. Wyss, A. J. Jordan, F. A. Larrain, J. P. Sadighi, C. Fuentes-Hernandez, B. Kippelen, J.-L. Bredas, S. Barlow and S. R. Marder, Chem. Mater., 2017, 29, 3403–3411 CrossRef CAS.
- G. D. Kong, M. Kim, S. J. Cho and H. J. Yoon, Angew. Chem., Int. Ed., 2016, 55, 10307 CrossRef CAS PubMed.
- K. C. Liao, H. J. Yoon, C. M. Bowers, F. C. Simeone and G. M. Whitesides, Angew. Chem., Int. Ed., 2014, 53, 3889 CrossRef CAS PubMed.
- S. Y. Quek, H. J. Choi, S. G. Louie and J. B. Neaton, Nano Lett., 2009, 9, 3949 CrossRef CAS PubMed.
- S. E. Byeon, M. Kim and H. J. Yoon, ACS Appl. Mater. Interfaces, 2017, 9, 40556 CrossRef CAS PubMed.
- P. Reddy, S.-Y. Jang, R. A. Segalman and A. Majumdar, Science, 2007, 315, 1568 CrossRef CAS PubMed.
- J. A. Malen, P. Doak, K. Baheti, T. D. Tilley, A. Majumdar and R. A. Segalman, Nano Lett., 2009, 9, 3406 CrossRef CAS PubMed.
- K. Baheti, J. A. Malen, P. Doak, P. Reddy, S.-Y. Jang, T. D. Tilley, A. Majumdar and R. A. Segalman, Nano Lett., 2008, 8, 715 CrossRef CAS PubMed.
- S. Y. Quek, H. J. Choi, S. G. Louie and J. B. Neaton, ACS Nano, 2011, 5, 551 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and further discussions. See DOI: 10.1039/c9cc01585j |
| This journal is © The Royal Society of Chemistry 2019 |