Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
NAG-targeting fluorescence based probe for precision diagnosis of kidney injury†
Fei
Yan‡
a,
Xiangge
Tian‡
a,
Zhilin
Luan
a,
Lei
Feng
*abc,
Xiaochi
Ma
 *a and
Tony D.
James
*a and
Tony D.
James
 *b
*b
aAcademy of Integrative Medicine, College of Pharmacy, Advanced Institute for Medical Sciences, Dalian Medical University, Lvshun South Road No. 9, Dalian 116044, P. R. China. E-mail: maxc1978@163.com; leifeng@mail.dlut.edu.cn
bDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
cState Key Laboratory of Fine Chemicals, Dalian University of Technology, Ganjingzi District, Linggong Road No. 2, Dalian 116024, P. R. China
First published on 14th January 2019
Abstract
We report an enzyme-activated fluorescence based probe (NHPO) for the selective detection of N-acetyl-β-D-glucosaminidase (NAG) activity in a drug-induced kidney injury mice model and NAG level in crude human urine. We used NHPO to explore the biological functions of NAG and provide a reference for the prognosis and prediction of proximal renal tubular dysfunction associated with various kidney diseases.
Chronic kidney disease (CKD), acute renal failure (ARF) and terminal-stage renal disease are common in hospitalized patients and increase the risks of morbidity and mortality. Most kidney injury is associated with complications of type 2 diabetes mellitus (DM)1 or induced kidney injury by drugs2 such as cisplatin.3 Therefore, there is an urgent unmet need for the early diagnosis of both drug-induced kidney injury and that caused by disease complications such as diabetes mellitus, hypertension4etc. Over 20 biomarkers as an early clinical index of kidney injury have been studied both in blood and urine,5 such as urinary albumin, neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1) and liver-type fatty acid-binding protein. However, these biomarkers still face numerous challenges as clinical indicators for the diagnosis of renal injury due to difficult quantification and detection. The proximal tubule (PT) part of the kidney nephron, is a vital portion of the duct system that can efficiently regulate pH values of the urine by exchanging hydrogen ions in the interstitium for bicarbonate ions. It is also responsible for secreting organic acids, such as creatinine and other bases, into the urine filtrate. The proximal tubular epithelial cells (PTECs) participate in the progression of tubulointerstitial injury with a pivotal role in various kidney diseases. Therefore, it is essential to detect in real-time PTECs during the development and progression of kidney injury in a clinical environment.
N-Acetyl-β-D-glucosaminidase (NAG) has gained significant attention since its clinical implications in the sensitive detection and accurate prediction of kidney injury.6 NAG is mainly distributed in the cytoplasm of the proximal tubular cell and plasma NAG cannot be filtered through the glomerulus due to its high molecular weight (130 kDa). However, during renal tubule injury, the proximal tubular cell exclusively secretes NAG resulting in a significant increase of NAG in the urine.7
A commercial assay kit has been developed to assay the NAG activity in the clinic using a colorimetric method.8 However, in many cases this assay method is infeasible due to low sensitivity, significant background interference and laborious measurement procedure. Therefore, it is essential to develop an effective method for real-time NAG activity monitoring in complex biological systems. Over recent years fluorescent probes have received considerable attention for rapid and sensitive diseases diagnosis due to their numerous advantages, such as high sensitivity, real-time detection, high spatiotemporal resolution, non-invasive monitoring and use in living systems.9 Relatively few fluorescent probes had been developed for the detection of NAG in tumours.10 Additionally, a fluorescence based NAG assay for precision diagnosis of kidney injury is not currently available.
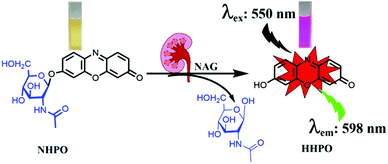
In our current research, we developed an enzyme-activated fluorescent probe NHPO possessing excellent selectivity and sensitivity toward NAG. Resorufin (HHPO) was chosen as the fluorophore due to its long wavelength emission, high fluorescence quantum yield, good water solubility, and efficient tuning of the electrondonating ability via alkylation of the 7-hydroxy group. Due to favourable ICT (intramolecular charge transfer) properties, HHPO derivatives always display extremely low background, and the fluorescence intensity can be modulated by removing substituents from the hydroxyl group, affording high detection sensitivity for enzyme activity.11 Furthermore, NHPO can be successfully applied to sense endogenous NAG in a cisplatin-induced kidney injury mice model,12 and for detecting NAG levels in crude human urine. More importantly, we evaluated the importance of NAG for the early diagnosis of renal injury and proximal tubule cells, as a diagnostic marker that can be used as a practical molecular tool for precision diagnostics in the kidney injury unit.
The fluorescence changes of NHPO after incubating with NAG were observed, as shown in Fig. S1 (ESI†) and compared with the negative control (without NAG), a dramatic spectral increase at 575 nm and distinct colour change from light yellow to pink (Scheme 1), were observed after addition of the NAG isoform, in addition a prominent fluorescence emission was also detected at 598 nm. The influence of pH on the fluorescence intensity of NHPO and its metabolite HHPO was investigated, as shown in Fig. S2 (ESI†), the metabolite HHPO has a stable fluorescence intensity from pH 7.0–12.0; additionally, we investigated the effects of pH on the fluorescence of NHPO reacting with NAG (Fig. S3, ESI†), the results indicate that NHPO has excellent compatibility with NAG under physiological conditions (about pH 7.4 and 37 °C). Furthermore, the fluorescence intensity was linear with increasing NAG enzyme from 0–7 μg mL−1. (Fig. S1c and d, ESI†). Additionally, as shown in Fig. S4 (ESI†), a new chromatographic peak in the HPLC was detected after incubating with NAG which was identified as resorufin (HHPO) using HPLC and ESI-MS/MS analysis. These results confirmed that NHPO could act as a turn-on fluorescence based probe for the rapid detection of NAG under physiological conditions.
The selectivity of NHPO towards NAG was investigated, as shown in Fig. S5 (ESI†), only NAG could trigger the hydrolysis reaction to produce a significant fluorescence emission at 598 nm, other hydrolase enzymes including Ls, α-GLC, Cas, PaK, CE1b, CE1c, CE2, BSA, β-Gla, GLU and β-GLC have no influence on the fluorescence intensity of NHPO. Additionally, some common endogenous substance and metal ions including myristic acid, Ser, Trp, Glu, Gly, Cys, Arg, Cys, Lys, Gln, GSH, glucose, Mn2+, Ca2+, Mg2+, Ni2+, Zn2+, Sn4+, K+, Cu2+, Fe3+, Na+ and Ba2+ all have no effect on the fluorescence intensity of NHPO. These results demonstrate that NHPO can serve as a highly sensitive and selective probe to detect the bioactivity of NAG in complex biological systems.
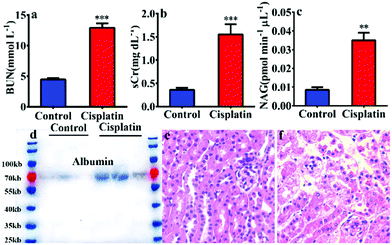
In order to reveal the key role of NAG in the diagnosis of kidney injury, a kidney injury model was developed in C57 BL/6 mice using cisplatin. As shown in Fig. 1, after pre-treatment with cisplatin, the urea nitrogen (BUN) and serum creatinine (sCr) levels exhibited a significantly larger increase than that of a control group (BUN 2.9 fold and sCr 4.3 fold). Furthermore, the western blot detected albumin in the mouse urine after treatment with cisplatin (Fig. 1d), all of which suggested that cisplatin could induce significant kidney injury.
Therefore, the NAG activity in urine was assayed using NHPO, as shown in Fig. 1c, and the results indicated a significant increase in NAG activity for the urine of the cisplatin-treated group, which was in agreement with the classic evaluation method (BUN and sCr) for kidney injury. Lastly, kidney specimens at a thickness of 10 μm were subjected to hematoxylin–eosin staining and Periodic Acid Schiff (PAS) staining.13 As shown in Fig. 1e, f and Fig. S6 (ESI†), after treatment with cisplatin, significant kidney damage in the proximal glomerular tubule was observed. All of the above results clearly indicated that NAG could serve as a biomarker in urine for evaluating kidney injury and the function change of kidney proximal glomerular tubule, with NHPO acting as a rapid screening tool that could efficiently and sensitively diagnose kidney injury by measuring urine NAG activity.
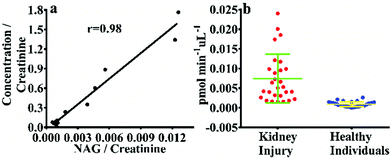
In order to confirm that NHPO can be used to monitor the activity of endogenous NAG in human urine, a correlation study was performed between NHPO and the commercial kit, as shown in Fig. 2, NAG activity in 12 human urine samples measured by NHPO was consistent with the commercial kit with an r value of 0.98, which also indicates that NHPO exhibited excellent selectivity for human NAG. Next, 58 urine samples from renal injury patients (n = 28) and healthy individuals (n = 30) were measured by NHPO, NAG activity of patients with varying degrees of kidney injury all exhibited much higher (about 8.2 fold, calculated by the mean values) in urine than that of healthy individuals. These results indicated NHPO could serve as a novel tool for clinical diagnosis of human kidney injury using real-time monitoring of the endogenous NAG activity in urine.
Fluorescence imaging of endogenous NAG in living kidney cells was evaluated. Firstly, NHPO displayed no cytotoxicity at various concentrations up to 50 μM using a CCK-8 assay. Next, as shown in Fig. S7d (ESI†), after incubation of NHPO with HK-2 cells for 1 h at 37 °C, the cells were imaged at an excitation wavelength of 561 nm and an obvious signal was detected in the 570–620 nm channel, by contrast, the control cells exhibited no fluorescence (Fig. S7a, ESI†). These results indicated that NHPO had good cell permeability and exhibits a hydrolysis process triggered by NAG. Identical fluorescence images were also obtained with NRK-52e (rat renal proximal tubule epithelial cell line) and MCT (mouse renal proximal tubule cell line), respectively (Fig. S8 and S9, ESI†).
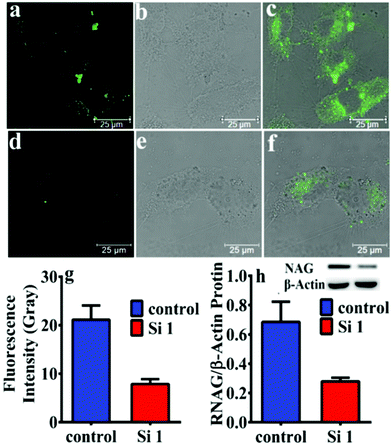
Moreover, after knocking down the expression of NAG in HK-2 cells by siNAG transfection, the expression of intracellular NAG was evaluated by western blotting and NHPO, respectively. As shown in Fig. 3, the expression of NAG in siNAG transfected HK-2 cells had a sharp decrease; compared to the control group, the siNAG transfected group exhibited a significantly suppressed fluorescence signal after incubation with NHPO. This fluorescence decrease was due to the down-regulation of NAG expression, and further illustrated that NHPO is capable of real-time imaging of endogenous NAG in living systems.
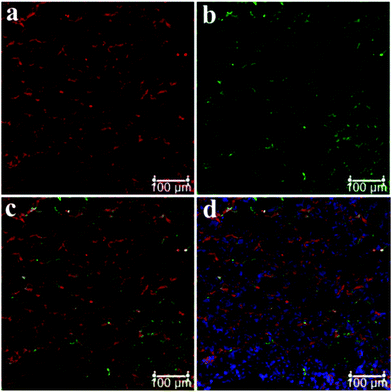
Fresh kidney specimens were collected and prepared at a thickness of 10 μm using a cryostat microtome, and subjected to NHPO for imaging of NAG, additionally, an immunohistochemical staining with Aquaporin 1 (AQP1, a well-known marker of the proximal tubule) was also performed, as shown in Fig. 4a, the red signal produced by NHPO was distributed around the proximal tubule which was consistent with the immuno-histochemical staining by AQP1 (Fig. 4b). A whole tube was observed in the red channel after incubation with NHPO, and these results indicate that NAG is specifically expressed in the proximal tubule. Therefore, these findings confirm that NHPO can serve as a useful approach for imaging NAG activity in specific kidney units (proximal tubule, PT), and might be used for clinical diagnosis of PT damage in various kidney diseases.
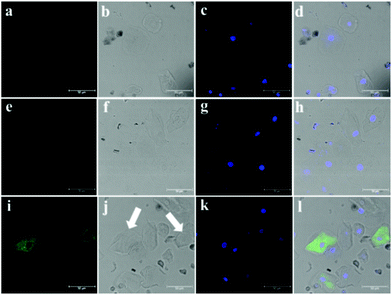
According to the experimental observation mentioned above, we collected and prepared the cells in urine from kidney injury patients and healthy individuals, NHPO was used for fluorescence imaging and to diagnose whether proximal tubule injury had occurred in the patients who had kidney disease. In our work, very few cells were detected for the healthy individual (Fig. S10, ESI†); however, a lot of different cell types were detected in the patients’ urine. Furthermore, as shown in Fig. 5a–c, no fluorescence background was detected for the cells separated from urine. Interestingly after incubating with NHPO, ill-1 displayed no fluorescence response in the fluorescence channel (570–620 nm), however, for ill-2, some cells exhibited a significant fluorescence signal in the 570–620 nm channel. Based on the above results, these cells were confirmed to be proximal tubule epithelial cells. Thus, patient ill-2 might have a proximal tubule injury as part of their kidney disease. These results indicate that NHPO could serve as an efficient tool to diagnose and discover PT injury in kidney disease.
In summary, a new enzyme-activated probe NHPO exhibiting excellent selectivity and sensitivity for endogenous NAG activity has been developed. The results indicate that NHPO could be used for detecting and imaging endogenous NAG in a cisplatin-induced kidney injury mice model with low cytotoxicity and preferred cell penetration. Importantly, using this probe, we revealed the much higher levels of NAG in urine of renal injury patients than from a healthy group, which strongly suggests that NAG could be used as a clinical marker for the early diagnosis of renal injury. Furthermore, NHPO could successfully image proximal tubule cells in order to achieve real-time precision diagnostics of the dysfunction of the proximal renal tubules in kidney injury.
The authors thank the National Natural Science Foundation of China (No. 81622047, 81503201 and 21572029), Distinguished Professor of Liaoning Province program and State Key Laboratory of Fine Chemicals (KF1803) for financial support. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. R. Kim, Y. H. Lee, S. G. Lee, E. S. Kang, B. S. Cha, J. H. Kim and B. W. Lee, Medicine, 2016, 95, e4114 CrossRef CAS PubMed; (b) A. M. Sharifi, B. Zare, M. Keshavarz, M. Rahmani, B. Zaeefy and B. Larijani, Int. J. Diabetes Dev. Countries, 2015, 1–6 Search PubMed; (c) G. H. Tesch, Clin. Sci., 2017, 131, 2183–2199 CrossRef CAS PubMed.
- (a) G. C. Gobe, J. S. Coombes, R. G. Fassett and Z. H. Endre, Expert Opin. Drug Metab. Toxicol., 2015, 11, 1683–1694 CrossRef CAS PubMed; (b) N. Goel, J. M. Pullman and M. Coco, Clin. Kidney J., 2014, 7, 513–517 CrossRef CAS PubMed.
- (a) R. Safirstein, J. Winston, D. Moel, S. Dikman and J. Guttenplan, Int. J. Androl., 1987, 10, 325–346 CrossRef CAS PubMed; (b) H. Soni, D. Kaminski, R. Gangaraju and A. Adebiyi, Ren. Fail., 2018, 40, 314–322 CrossRef CAS PubMed; (c) A. E. Vickers, K. Rose, R. Fisher, M. Saulnier, P. Sahota and P. Bentley, Toxicol. Pathol., 2004, 32, 577–590 CrossRef CAS PubMed.
- G. Simon, S. Morioka and D. K. Snyder, Clin. Exp. Hypertens., 1984, 6, 879–896 CAS.
- (a) M. Abdelsalam, E. Elmorsy, H. Abdelwahab, O. Algohary, M. Naguib, A. A. El Wahab, A. Eldeeb, E. Eltoraby, A. Abdelsalam, A. Sabry, M. El-Metwally, M. Akl, N. Anber, M. El Sayed Zaki, F. Almutairi and T. Mansour, BMC Nephrol., 2018, 19, 219 CrossRef PubMed; (b) P. Devarajan, Curr. Opin. Pediatr., 2011, 23, 194–200 CrossRef CAS PubMed; (c) C. L. Edelstein, Biomarkers of Kidney Disease, 2nd edn, 2017, ch. 6, pp. 241–315 Search PubMed.
- (a) R. L. Sherman, D. E. Drayer, B. R. Leyland-Jones and M. M. Reidenberg, Arch. Intern. Med., 1983, 143, 1183–1185 CrossRef CAS PubMed; (b) M. Nishida, H. Kawakatsu, H. Komatsu, K. Ishiwari, M. Tamai, K. Tsunamoto, Y. Kasubuchi and T. Sawada, Acta Paediatr. Jpn., 1998, 40, 424–426 CrossRef CAS PubMed.
- D. N. Patel and K. Kalia, Int. J. Diabetes Dev. Countries, 2015, 35, 1–9 Search PubMed.
- A. Noto, Y. Ogawa, S. Mori, M. Yoshioka, T. Kitakaze, T. Hori, M. Nakamura and T. Miyake, Clin. Chem., 1983, 29, 1713–1716 CAS.
- (a) L. Feng, Y. Yang, X. Huo, X. Tian, Y. Feng, H. Yuan, L. Zhao, C. Wang, P. Chu, F. Long, W. Wang and X. Ma, ACS Sens., 2018, 3, 1727–1734 CrossRef CAS PubMed; (b) X. Han, X. Song, F. Yu and L. Chen, Chem. Sci., 2017, 8, 6991–7002 RSC; (c) X. Huo, X. Tian, Y. Li, L. Feng, Y. Cui, C. Wang, J. Cui, C. Sun, K. Liu and X. Ma, Sens. Actuators, B, 2018, 262, 508–515 CrossRef CAS; (d) Y. Jin, X. Tian, L. Jin, Y. Cui, T. Liu, Z. Yu, X. Huo, J. Cui, C. Sun, C. Wang, J. Ning, B. Zhang, L. Feng and X. Ma, Anal. Chem., 2018, 90, 3276–3283 CrossRef CAS PubMed; (e) H. W. Liu, L. Chen, C. Xu, Z. Li, H. Zhang, X. B. Zhang and W. Tan, Chem. Soc. Rev., 2018, 47, 7140–7180 RSC; (f) J. Ning, T. Liu, P. Dong, W. Wang, G. Ge, B. Wang, Z. Yu, L. Shi, X. Tian, X. Huo, L. Feng, C. Wang, C. Sun, J. N. Cui, T. D. James and X. Ma, J. Am. Chem. Soc., 2018 DOI:10.1021/jacs.8b12136; (g) J. Zhang, X. Chai, X. P. He, H. J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2018 10.1039/c7cs00907k.
- (a) E. J. Kim, D. O. Kang, D. C. Love and J. A. Hanover, Carbohydr. Res., 2006, 341, 971–982 CrossRef CAS PubMed; (b) H. Matsuzaki, M. Kamiya, R. J. Iwatate, D. Asanuma, T. Watanabe and Y. Urano, Bioconjugate Chem., 2016, 27, 973–981 CrossRef CAS PubMed.
- (a) A. E. Albers, K. A. Rawls and C. J. Chang, Chem. Commun., 2007, 4647–4649 RSC; (b) X. Wu, W. Shi, X. Li and H. Ma, Angew. Chem., Int. Ed., 2017, 56, 15319–15323 CrossRef CAS PubMed.
- (a) R. Galgamuwa, K. Hardy, J. E. Dahlstrom, A. C. Blackburn, E. Wium, M. Rooke, J. Y. Cappello, P. Tummala, H. R. Patel, A. Chuah, L. Tian, L. McMorrow, P. G. Board and A. Theodoratos, J. Am. Soc. Nephrol., 2016, 27, 3331–3344 CrossRef CAS PubMed; (b) Y. Miyasato, T. Yoshizawa, Y. Sato, T. Nakagawa, Y. Miyasato, Y. Kakizoe, T. Kuwabara, M. Adachi, A. Ianni and T. Braun, Sci. Rep., 2018, 8, 5927 CrossRef PubMed.
- (a) H. Pan, K. Shen, X. Wang, H. Meng, C. Wang and B. Jin, PLoS One, 2014, 9, e86057 CrossRef PubMed; (b) F. Li, Y. Yao, H. Huang, H. Hao and M. Ying, Int. Immunopharmacol., 2018, 61, 277–282 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc10311a |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2019 |