Improving the relevance and efficiency of human exposure assessments within the process of regulatory risk assessment
Chris
Money

Cynara Consulting, Brockenhurst, UK. E-mail: chrismoneyuk@gmail.com
First published on 9th November 2017
Abstract
The process for undertaking exposure assessments varies dependent on its purpose. But for exposure assessments to be relevant and accurate, they are reliant on access to reliable information on key exposure determinants. Acquiring such information is seldom straightforward and can take significant time and resources. This articles examines how the application of tiered and targeted approaches to information acquisition, within the context of European human health risk assessments, can not only lead to improvements in the efficiency and effectiveness of the process but also in the confidence of stakeholders in its outputs. The article explores how the benefits might be further improved through the coordination of such activities, as well as those areas that represent barriers to wider international harmonisation.
Environmental significanceAcquiring exposure information sufficient to yield reliable exposure assessments is seldom straightforward. Not only must the information be relevant for the problem, but the process for acquiring it needs to be relevant and efficient. Tiered strategies for exposure assessment have been advocated as an efficient mechanism for achieving such aims. But tiering alone would appear insufficient to provide the necessary focus. Combining tiering and targeting within the process would appear best able to deliver the necessary focus and efficiency. Using examples derived from recent activities supporting the EU REACH regulation, the paper outlines a framework for tiering and targeting the acquisition of exposure information and discusses key considerations that also need to be respected. |
Background
A scientifically based risk assessment is, by definition, factually based. Over the past 40 years, much hazard and exposure data have been developed to support such assessments. However, while the process of hazard assessment is now structured (at least for those hazard assessments determined by regulation e.g. REACH), with different levels of understanding being expected at different stages (tiers) of the risk assessment process, such a comparability does not currently exist for the process of exposure assessment (EA). This weakens the resulting risk assessments as the confidence that can be invested in them is heavily affected by the uncertainties surrounding the associated EA. To compensate for this, conservative values that will result in over-predictions of exposure are often intentionally chosen, so that even if the absolute value is uncertain it can still be used as an upper bound exposure estimate. Thus, the assessment can still be used in a manner that should provide confidence that a substance/product can be safely used within these assumptions.While hazard assessments are invariably based upon established sets of quality assured animal data (as well as the findings from human experience), no such codification of data expectations exists for EAs. By contrast, the range of types of exposure that different populations (human and environmental, including different target populations) may be subject to varies widely. While the broad concept of tiering the risk assessment process has been advocated for many years in order to improve the relevance and accuracy of EAs,1–4 there has been no accompanying consensus on what the different tiers might constitute and when they might be implemented. For example, the US National Academies of Science5 has suggested that Tier 1 is used to prioritise chemicals, that Tier 2 is used to screen them further with Tier 3 being retained for comprehensive evaluations. This is similar to approaches such as the EU REACH Regulation6 where potential regulatory importance is first assessed using manufactured or imported tonnage as an exposure proxy, but a preliminary evaluation of use exposures only first occurs at Tier 1. However, no matter how many tiers there are or how they are named, what is accepted is that the increased accuracy of the EA comes at a price: the assessment is inevitably more complex; requires more information on which it can be based and as a consequence costs more in time and effort.
In contrast to exemplifying how a tiered approach might be optimised and practically implemented, much investment has been made in recent years in exploring how existing data sources might be more effectively utilized to improve the EA process.7–9 However, in the main, these efforts are focused on screening chemicals for their potential health and environmental risks in order that resources can be targeted towards the application of traditional RA methods.
This leaves exposure assessors in a partial conundrum. First stage assessments would currently appear to be too coarse for everyday use and hence are probably best associated with governmental or industry prioritisation schemes. On the other hand, higher tier assessments appear too resource intensive for general application. The most workable solutions would therefore appear to reside at the Tier 1 level. The challenge though is what might these solutions look like and how might they be enacted in practice?
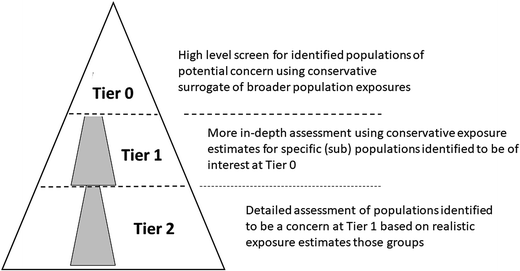
These considerations, when combined, suggest that any EA process is not necessarily linear but is shaped by the manner in which the EA develops, together with those factors that are most likely to influence the associated uncertainties surrounding it. Fig. 1 illustrates this conceptually. Lower tier assessments (in this case termed Tier 0) should be capable of being undertaken using a restricted set of determinants in order to establish populations of potential concern. Where these have been identified, then more detailed assessments can be undertaken using appropriate methods, but where the required data are informed by the findings of the lower tier assessment i.e. a more detailed examination of the populations of potential concern. Thus, a benefit of the Tier 0 concept is that the data required to refine the assessment is identified, and provides a focus for further work. When the Tier 1 (intermediate level) assessment is unable to deliver a definitive outcome then higher tier assessments, focused on those remaining situations of concern, can be undertaken. Here again, the work already undertaken serves to focus and inform additional data collection or modelling efforts.
Considerations affecting the exposure assessment process
In an ideal world, detailed information on exposure will exist for all uses of all substances, covering all possible populations of interest. While this ambition may be a reality for a few substances (often of historical regulatory interest), it is not the case for the vast majority of industrial chemicals. Accepting that the acquisition of exposure information for all substances and uses to support the risk assessment process is, at least in the short term, unrealistic, the WHO10 suggested that the acquisition and application of exposure information be adapted for specific/targeted situations. While such targeted approaches for acquiring information have the advantage that they are resource and cost efficient, they may not however meet the challenge of risk assessment demands such as those presented by REACH, requiring appropriate information throughout the substance life cycle. For this reason it is appropriate to evaluate the type of exposure information needed to support substance risk assessments and how such information might be efficiently obtained.Much has already been written on the different approaches available to conduct a risk assessment,11–13 including improving the efficiency and effectiveness of the overall process through the application of screening level assessments as a first stage.4 But achieving the required levels of confidence in any risk assessment outcome can be data demanding and resource intensive. So the question that has to be posed is whether the efficiency of the process can be improved without compromising the quality of the output.
The traditional focus for EAs has lain in the accuracy of the exposure estimate for the population of interest and the question at hand. But this only views the EA in isolation whereas they are often applied as part of a process in which risk based decisions are used to inform wider considerations, for example, the extent to which a particular use of the chemical might be considered safe or otherwise. This means that not only should the EA be accurate in itself for the population of relevance, but it should be capable of achieving other objectives. For example, for REACH objectives also include that the assessment be: readily reproduced (across different assessors wishing to undertake comparable assessments), capable of ready iteration (for example to determine the consequence of different emission pathways or the relative effectiveness of alternative exposure control strategies); being understandable to non-experts; transparent; transferable (across geographies and different forms of EA), ideally using electronic formats; and, on occasion, for the outcome to be capable of being suitably communicated e.g. as part of a safety data sheet.
Potential strategies for acquiring exposure information
Obtaining data demands resources and past experience has demonstrated that the uncoordinated and untargeted collection of data is both invariably inefficient and deficient. In the case of EAs, data is not simply confined to exposure measurements but also extends to the range of different exposure factors that can be relevant when modelling exposure. From an EA process perspective, it is clearly beneficial therefore that any strategy which seeks to acquire exposure information neither fails to describe key determinants nor focuses on information that has little or no bearing on the EA. In an ideal world, the various exposure information needs that exist at the different uses and tiers would be codified and harmonised and this would enable information acquisition strategies to be optimised and coordinated. However, while some attempts have been made to describe the information needs that accompany higher tier EAs e.g. workers14–16 and consumers,17 such work is lacking when seen in the broader context of tiered acquisition strategies. Clearly, this represents an obstacle to any efficient process. In the absence of such a consensus, an alternative approach could be to use the information needs of ‘key’ exposure models to inform such a strategy.But even then, the process applied to support such a strategy can be further enhanced by the recognition that many of the available EA models will utilise common determinants. This introduces an opportunity for improving the efficiency of the information collection process by the use of generic schemes. Such schemes focus on improving the understandings of a defined set of determinants that are accepted as having the most influence on the EA outcome. These will necessarily differ with the population/scenario of interest. However, they have been successfully employed at different levels within the context of workplace EAs18–20 as well as for human and environmental assessments under the Canadian Environmental Protection Act21 and REACH.22
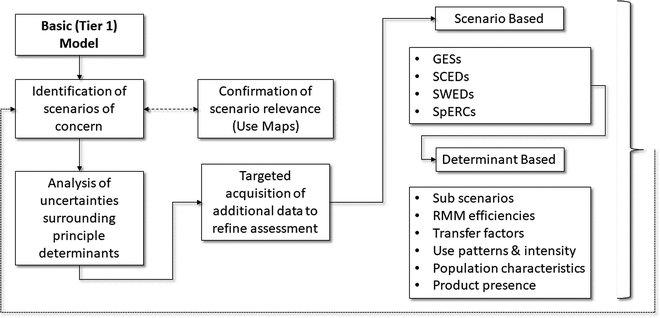
Fig. 2 outlines how these concepts might be combined and applied within the context of how EAs are undertaken in support of the EU REACH regulation. The first step is to recognise that a preliminary tier may not be necessary (for example, the incorporation of tonnage information based triggers within REACH is seen as a proxy for Tier 0 screening, albeit one that in the absence of use profiling can have a poor correlation with actual exposures). Therefore, the real process of information acquisition can begin by focusing on the expectations of Tier 1. The first step is to ensure that there is an understanding of the basic nature of exposures and emissions throughout the substance life cycle (which includes uses of formulated products containing the substance). It is only by having some form of mapping of where and under what conditions human health exposures and environmental emissions are likely to occur, that it is possible to be confident that key uses have not been overlooked or omitted when undertaking the EA. The Use Maps, developed initially by industry in 2009/10, contain information on such conditions, but they only exist for certain sectors and supply chains.23 Of note is the fact that different sectors can vary in their description of uses having similar exposure or emission profiles. The application of the REACH use descriptors to these situations can help to bring some consistency, but constitute only a partial ontology. For this reason, some industrial sectors have used Generic Exposure Scenarios (GESs) to develop their Use Maps. However, the CEFIC Use Maps do represent the most current and comprehensive listing of intended uses of chemical products for those sectors but they have not been independently reviewed regarding their content or accuracy.
Once potential scenarios of concern can be identified, then exposure models such as the ECETOC TRA24 and ECHA Chesar tool25 allow the user to iterate the exposure estimates such that it is then possible to target the acquisition of additional exposure information to help refine the assessment and reduce associated uncertainties. In terms of targeting, this can either be focused on particular situations/scenarios of interest (e.g. those commonly encountered and/or those considered to be of particular interest) or those exposure determinants that may have a critical role in affecting the nature of the exposure estimate. Using as inspiration the OECD work on Emission Scenario Documents, ECETOC first outlined the basis by which such a targeting could benefit environmental EAs under REACH,26 the information being described in the Specific Environmental Release Category (SPERC). The Specific Consumer Exposure Determinant (SCED) was subsequently proposed for describing consumer exposures.23 More recently, the Specific Worker Exposure Description (SWED) has been proposed by ENES with the purpose of reporting worker exposures.27 The ECHA ENES activity has recently introduced more comprehensive Use Maps, which summarise all the information developed by a sector on life cycle exposures, together with a supporting process for stakeholder review prior to their posting.27
Fig. 3 illustrates how the European oil industry (Concawe) has applied such ideas when refining its understanding of consumer exposures to petroleum products. The first stage is to complete a comprehensive use map which addresses all those exposure situations which can be expected to result from the marketing of fuels and lubricants. As the knowledge or expectations of industry do not always equate to how consumers actually use such products, surveys of consumer use patterns can help confirm assumptions or refine understandings in this area.28 Based on the extent of understanding of each use (using as a reference point the SCED templates), it is then possible to identify those areas/determinants where knowledge is either low or lacking. This can be then used to initiate further targeted actions aimed at refining understandings of key exposure determinants. In the case of petroleum products, the major uncertainties related to the nature of dermal contact and transfer.29 Once this information is available, for example via laboratory simulations of actual exposure events, then this can be shared for wider use in exposure and risk assessments.30
An alternative approach to Fig. 2 can be to focus on steps aimed at improving stakeholder confidence in the output. The starting conditions are identical i.e. initially identifying scenarios of concern. The approach, however, diverges at the next step in that rather than looking at the nature of uncertainties surrounding the principal determinants, it instead addresses those aspects which have been known to devalue the integrity of the core estimate. For example, collecting exposure measurement data for the scenario in question or obtaining a more detailed knowledge of the degree to which specific subpopulations may be subject to more elevated exposures, can both help to reinforce key assumptions and bound residual uncertainties. Recognising the fact that most human and environmental exposures are to multiple substances/stresses rather than a single chemical, the ECHA ENES activity has begun to ‘chart’ the extent to which different uses may be associated with predominant exposures to a single chemical or not. This activity, in part, is aimed at ensuring that any EA can be assured to be focusing on the substance(s) of potential interest and hence serves to reduce any concern that the target of the EA is misplaced.
Such a ‘softer’ approach should be seen as complementary and not mutually exclusive as it is intended to address different needs in the EA process. There is little benefit in having models which deliver accurate exposure estimates but which are not, for whatever reason, accepted by stakeholders. At the same time, one must question the wisdom of having a model which is accepted by stakeholders yet which provides uncertain exposure estimates.
Discussion
Exposure is not an intrinsic property of a substance as it is determined very much by how any substances is used. Moreover, uses in themselves can vary quite considerably, depending not only upon the conditions of how a substance is manufactured and the type of product that it may be present in, but also individual behaviours and use environments (e.g. industrial workplace vs. residential setting). This leads to the need for information on these factors and the associated discussion about how it might be obtained in a manner that is both efficient and relevant.Before such information is collected, however, there is a need for some accepted standardisation in the terminology used to describe the determinants of exposure in order to introduce some form of standardisation for those engaged in the process, as in the absence of such harmonisation the resulting descriptions are likely to vary and be inconsistent. Higher level definitions are available at the international level31,32 but these are not sufficiently exhaustive to be of practical value at the operational level. Moreover, their language appears more aimed towards the technical community. Schemes such as the EU's use description system33 that aim to allow uses to be systematically described and are capable of being practically applied by a range of interests, demonstrate that practically useful approaches can be developed, in addition to those other considerations that also serve to help reduce uncertainty.34 International cooperation in this area would seem therefore appear beneficial; much ground work has already been undertaken, the need being for consensus on the scope, content and definition of relevant terms. However, it is to be noted that progress may not be rapid: even the United Nations globally harmonised system (GHS) for hazard classification and labelling, despite being agreed in principle in 1992, has still to be implemented in full globally.
Another consideration that is important in the development of any tiered process is for the underpinning templates to be aligned with some accepted base set of exposure determinants for the target population(s). Recently, the concept of the Aggregate Exposure Pathway (AEP) has been proposed35 as a mechanism that can be applied to compliment the use of the Adverse Outcome Pathway (AOP). The AEP serves as a framework to organize exposure data within individual units of (external and internal) prediction. In this respect, the AEP is broader in its scope and ambition than those approaches being developed in Europe. For example, the structure and content of SCEDs, SPERCs etc. are informed in part because of the widespread use of the ECETOC TRA and EUSES models within the EU REACH RA process (and their incorporation into the ECHA Chesar tool). But the TRA and EUSES are only two examples of the range of models available which seek to characterise human and environmental exposures to chemicals. Indeed it is possible to identify such models within every regional or geographical boundary. However although there are geographical differences in the nature of some exposure determinants, there is also a high degree of commonality. It would therefore appear beneficial if a basic standardisation of ‘input needs’ could be identified and agreed in order that the information obtained through regional initiatives is able to be more widely utilised than at present.
The introduction of structured data-driven and scenario-based approaches such as SPERCs and SCEDs also demonstrates that it is possible for industry to contribute valuable information to the EA process. However, in order that this can be accomplished, not only must any such scheme incorporate a transparent basis on which to judge the relevance and veracity of the information, but it also requires coordination and for submitted information to undergo appropriate review. Indeed, the ENES experiences demonstrate that not all sectors of industry are equal in the number or quality of their contributions. Therefore, if such approaches are also to have wider utility via the harmonisation of international efforts, then not only is consensus required on the core input parameters, but they also need mechanisms that encourage such contributions, as well as for coordination and review.
A key consideration for Tier 1 is to make complicated problems tractable i.e. the application of fit for purpose models (models that are no more complicated than they need to be) for the tier/scenario in question. This represents a challenge for model developers as well as the stakeholder community that is tasked with ‘accepting’ the available models, as the likelihood is that their performance may well vary across scenarios i.e. it is probably optimistic to assume that their performance will be uniform across the range of situations that they might be expected to cover. One question that should always be asked is ‘what is the purpose of the EA?’ (which could be seen to be an integral part of the ‘problem definition’ phase proposed by WHO/IPCS),4 as this will affect the choice of model and hence the required level of understanding of the underlying exposure determinants that support it. For example, identifying which chemicals in commerce might present a risk from their use can be presumed to require a different degree of discrimination and sensitivity to evaluating a specific use of a known chemical. In this respect, some sort of system that enables the domain of such models to be more readily described and understood by non-experts might help in this respect e.g. a scoring or colour coding in key areas of required performance.
Linked to the collection of information is the need to somehow evaluate the representativity and reliability of it. If exposure data are to be better harmonised (and hence capable of being more widely shared), then one element that also requires attention is the extent to which exposure data/information ‘quality criteria’ might be developed and applied. Schemes already exist and are widely (and internationally) applied for hazard data.36,37 Related schemes have also of been proposed for the application of quality criteria to human data, including its weighting in the RA process.38–40 But while suggestions have previously been made concerning how available worker exposure data might be integrated into the EA process accounting for considerations of quality,41,42 these do not exist as far as the classification of exposure data quality.
Using as examples many of the exposure-driven activities currently underway in support of REACH (shown in italics), Fig. 4 summarises some of the above considerations. It illustrates that although the concepts of tiering may be simple, establishing the necessary steps for its successful implementation is often more challenging and complex. While clear benefits at each step can be identified, their success is partly dependent on the establishment of necessary supporting structures and activities. For example, without a universal set of use descriptors that allow use situations to be fully described, then it becomes difficult to develop mechanisms for sharing relevant exposure conditions. However, agreeing on such a set of descriptors is not straightforward, as different stakeholders will have their own perspectives on what is required or not, together with how any descriptor might be phrased. Moreover, when some or all of these conditions need to be communicated to stakeholders (such as is the case with safety data sheets), then it is also necessary to develop supporting standard phrases that can be applied multi-lingually, with all the possibilities for debate that such an activity creates. Similarly, although they may exist, applying complex EA models at early Tiers of the RA process is not necessarily pertinent. What may be more relevant is the availability of fit-for-purpose models, covering key human health and environmental populations, which are capable of ready iteration across the range of stakeholders. In this respect, the use of more data intensive and technically demanding models is best reserved for later in the process.
Tiered and targeted approaches to information acquisition in support of chemicals RA processes can succeed and generate new understandings on the nature of current exposures. However, achieving such an objective is challenging and is reliant on the availability of a significant number of parallel activities and structures. While exposure information can be unique to a particular geography, much can also be common across similar use experiences. The OECD IUCLID platform has demonstrated that hazard information can be shared globally once considerations of protocols and quality are implemented and respected. With many of the necessary elements now available, the challenge for regulatory exposure science is to build off this work and to facilitate the wider sharing of human and environmental exposure information, for example under the umbrella of the activities overseen by the OECD Task Force on Exposure Assessment.
The field of exposure science will continue to evolve and new directions and concepts will consequently emerge that need to be evaluated and, where appropriate, suitably integrated into existing EA schemes and approaches.43 The challenge of the exposure science community, together with its supporting stakeholders, will be to distinguish which of those areas and ideas represent substantive contributions that are essential for refining our understandings. In this respect, it will be important to monitor the findings of the current research programmes seeking to evaluate how the exposome44 might be practically described and applied, as they not only need to constitute a reliable technology for characterising the totality of exposure, but their use will need to be optimised through their tiered and targeted deployment.
Conclusions
There is common agreement on the importance of exposure estimation within the context of risk assessment, yet most historic regulatory approaches to risk assessment have tended to place more attention to hazard characterisation than EA. This is unfortunate as the wider uncertainties are generally associated with the EA.There would seem to be an imperative to obtain better and fuller understandings of the nature of human and environmental exposures in order to deliver the necessary balance within regulatory RAs. However, obtaining exposure information is invariably resource intensive. Tiered and targeted strategies that lead to the co-ordinated and harmonised collection of this information would appear to represent an efficient basis through which gaps in our understandings can be filled. The development of standardised data collection templates, such as those being applied within ENES, constitute opportunities for broadening and strengthening such actions beyond Europe. Much exposure information is transferable across borders and regions and international coordination of such activities would appear desirable. Indeed, the experiences of the OECD emissions scenario document activity demonstrate that international harmonisation is possible.
The development of templates alone is unlikely to be insufficient, however. Because human and environmental exposure is experienced across all levels of society, these templates must be sufficiently understandable and practical such that they are capable of being populated by those without high-level technical expertise. In this respect, it appears important to agree a common and international EA ontology, perhaps using the experiences of the REACH use descriptors as a start point. It would also appear desirable to develop supporting guidance that ensures the integrity of such information in order to maintain the necessary stakeholder confidence as well as process efficiencies.
In this respect, industry has a major role to play in supporting and contributing to these initiatives as much of the uncertainty resides in the use information that is often most under the control of (and hence presumably understood by) companies and industrial sectors. In order for such initiatives to be successful, mechanisms need to be identified that encourage this information to be shared and harvested while respecting legitimate business confidentiality concerns.
Conflicts of interest
There are no conflicts of interest to declare.References
- EPA, Guidelines for exposure assessment, US Environmental Protection Agency, Washington D.C, 1992 Search PubMed.
- ECETOC, Targeted risk assessment, Technical Report No. 93, ECETOC, Brussels, 2004 Search PubMed.
- M. E. Meek and V. C. Armstrong, in Risk Assessment of Chemicals, ed. C. J. Van Leeuwen and T. G. Vermeire, Springer, Dordrecht, The Netherlands, 2007 Search PubMed.
- M. E. Meek, A. R. Boobis, K. M. Crofton, G. Heinemeyer, M. van Raaij and C. Vickers, Regul. Toxicol. Pharmacol., 2011, 60, S1–S14 CAS.
- NAS, Using 21st century science to improve risk related evaluations, US National Academy of Sciences, Washington DC, 2017 Search PubMed.
- European Parliament Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No. 793/93 and Commission Regulation (EC) No. 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
- D. H. Bennett, M. D. Margni, T. E. McKone and O. Jolliet, Risk Anal., 2002, 22, 905–918 CrossRef PubMed.
- J. F. Wambaugh, R. W. Setzer, D. M. Reif, S. Gangwal, J. Mitchell-Blackwood, J. A. Arnot, O. Joliet, A. Frame, J. Rabinowitz, T. B. Knudsen, R. S. Judson, P. Egeghy, D. Vallero and E. A. Cohen Hubal, Environ. Sci. Technol., 2013, 47, 8479–8488 CAS.
- M. R. Goldsmith, C. M. Grulke, R. D. Brooks, T. R. Transue, Y. M. Tan, A. Frame, P. P. Egeghy, R. Edwards, D. T. Chang, R. Tornero-Velez, K. Isaacs, A. Wang, J. Johnson, K. Holm, M. Reich, J. Mitchell, D. A. Vallero, L. Phillips, M. Phillips, J. F. Wambaugh, R. S. Judson, T. J. Buckley and C. C. Dary, Food Chem. Toxicol., 2014, 65, 269–279 CrossRef CAS PubMed.
- WHO, Human Exposure Assessment, Environmental Health Criteria 214, World Health Organisation, Geneva, 2000 Search PubMed.
- IPCS, Human health risks: Principles for the assessment of risks to human health from exposure to chemicals (EHC No. 210), World Health Organisation, Geneva, 1999 Search PubMed.
- OECD, Environmental Exposure Assessment Strategies for Existing Industrial Chemicals in OECD Member Countries, OECD, Paris, 1999 Search PubMed.
- Risk Assessment of Chemicals: An Introduction, ed. C. J. Van Leuwen and T. G. Vermeire, Springer, Dordrecht, The Netherlands, 2007 Search PubMed.
- M. Lippmann, M. R. Gomez and G. M. Rawls, Appl. Occup. Environ. Hyg., 1996, 11, 1294–1311 CrossRef CAS.
- B. Rajan, R. Alesbury, B. Carton, M. Gérin, H. Litske, H. Marquart, E. Olsen, T. Scheffers, R. Stamm and T. Woldbaek, Appl. Occup. Environ. Hyg., 1997, 12, 31–39 CrossRef CAS.
- H. Marquart, T. Schneider, H. Goede, M. Tischer, J. Schinkel, N. Warren, W. Fransman, S. Spaan, M. Van Tongeren, H. Kromhout, E. Tielemans and J. W. Cherrie, Ann. Occup. Hyg., 2011, 55, 989–1005 CAS.
- B. Hall, S. Tozer, B. Safford, M. Coroama, W. Steiling, M. C. Leneveu-Duchemin, C. McNamara and M. Gibney, Food Chem. Toxicol., 2007, 45, 2097–2108 CrossRef CAS PubMed.
- CIA, Safe Handling of Potentially Carcinogenic Aromatic Amines and Nitro Compounds, Chemical Industries Association, London, 1992 Search PubMed.
- C. Money, Ann. Occup. Hyg., 1992, 36, 601–607 CrossRef CAS PubMed.
- S. P. Binks, Occup. Med., 2003, 53, 363–370 CrossRef CAS PubMed.
- K. Hughes, J. Paterson and M. E. Meek, Regul. Toxicol. Pharmacol., 2009, 55, 382–393 CrossRef CAS PubMed.
- C. Money, A. Margary, D. Noij and K. Hommes, Ann. Occup. Hyg., 2011, 55, 451–464 CAS.
- CEFIC, Overview table of association activities on Use Maps, 2016, http://www.cefic.org/Industry-support/Implementing-reach/Guidances-and-Tools1/, date accessed 30.10.17 Search PubMed.
- ECETOC, ECETOC TRA version3: Background and rationale for the improvements, Technical Report No. 114, ECETOC, Brussels, 2012 Search PubMed.
- European Chemicals Agency (ECHA), Chemical Safety Assessment and Reporting tool, 2017, http://chesar.echa.europa.eu/, date accessed 30.10.17 Search PubMed.
- ECETOC, Addendum to ECETOC Targeted Risk Assessment Report No.93, Technical Report No. 107, ECETOC, Brussels, 2009 Search PubMed.
- ECHA, ENES Use Maps activity, 2017, http://echa.europa.eu/csr-es-roadmap/use-maps/use-maps-library, date accessed 30.10.17 Search PubMed.
- Concawe, Use of motor fuels and lubricants: habits and practices of consumers in Europe, Report 4/14, Concawe, Brussels, 2014 Search PubMed.
- K. S. Galea, A. Davis, D. Todd, L. MacCalman, C. McGonagle and J. W. Cherrie, J. Exposure Sci. Environ. Epidemiol., 2014, 24, 665–672 CrossRef CAS PubMed.
- Concawe, Specific Consumer Exposure Determinants (SCEDs) for key uses of fuels and lubricants, Report 15/14, Concawe, Brussels, 2015 Search PubMed.
- OECD, Descriptions of selected key generic terms used in chemical hazard/risk assessment, OECD, Paris, 2003 Search PubMed.
- IPCS, Risk assessment terminology, International Programme for Chemical Safety, WHO, Geneva, 2004 Search PubMed.
- ECHA, Guidance on Information Requirements and Chemical Safety Assessment, European Chemicals Agency, Helsinki, 2015, ch. R.12, Use description Search PubMed.
- WHO, Guidance Document on Characterizing and Communicating Uncertainty in Exposure Assessment, World Health Organisation, Geneva, 2008 Search PubMed.
- J. G. Teeguarden, Y. M. Tan, S. W. Edwards, J. A. Leonard, K. A. Anderson, R. A. Corley, M. L. Kile, S. M. Simonich, D. Stone, R. L. Tanguay, K. M. Waters, S. L. Harper and D. E. Williams, Environ. Sci. Technol., 2016, 50, 4579–4586 CrossRef CAS PubMed.
- H. J. Klimisch, M. Andreae and U. Tillmann, Regul. Toxicol. Pharmacol., 1997, 25, 1–5 CrossRef CAS PubMed.
- OECD, Guidance document on the validation and international acceptance of new or updated test methods for hazard assessment, OECD, Paris, 2005 Search PubMed.
- H. O. Adami, S. C. Berry, C. B. Breckenridge, L. L. Smith, J. A. Swenberg, D. Trichopoulos, N. S. Weiss and T. P. Pastoor, Toxicol. Sci., 2011, 122, 223–234 CrossRef CAS PubMed.
- K. Lavelle, R. Schnatter, K. Travis, G. Swaen, D. Pallapies, C. Money, P. Priem and H. Vrijhof, Regul. Toxicol. Pharmacol., 2012, 62, 302–312 CrossRef CAS PubMed.
- T. P. Pastoor, A. N. Bachman, D. R. Bell, S. M. Cohen, M. Dellarco, I. C. Dewhurst, J. E. Doe, N. G. Doerrer, M. R. Embry, R. N. Hines, A. Moretto, R. D. Phillips, J. C. Rowlands, J. Y. Tanir, D. C. Wolf and A. R. Boobis, Crit Rev Tox., 2014, 44, 1–5 CrossRef CAS PubMed.
- E. Tielemans, H. Marquart, J. De Cock, M. Groenewold and J. Van Hemmen, Ann. Occup. Hyg., 2002, 46, 287–297 CrossRef PubMed.
- C. D. Money and S. A. Margary, Ann. Occup. Hyg., 2002, 46, 279–285 CAS.
- NAS, Exposure science in the 21st century science: a vision and a strategy, US National Academy of Sciences, Washington DC, 2012 Search PubMed.
- C. J. Wild, Cancer Epidemiol., Biomarkers Prev., 2005, 14, 1847–1850 CAS.
| This journal is © The Royal Society of Chemistry 2018 |