Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering inorganic nanozyme architectures for decomposition of reactive oxygen species
Tibor G.
Halmagyi
,
Laila
Noureen
,
Adél
Szerlauth
 and
Istvan
Szilagyi
and
Istvan
Szilagyi
 *
*
MTA-SZTE Momentum Biocolloids Research Group, Department of Physical Chemistry and Materials Science, Interdisciplinary Centre of Excellence, University of Szeged, 6720 Szeged, Hungary. E-mail: szistvan@chem.u-szeged.hu
First published on 31st July 2024
Abstract
Enzyme-mimicking nanomaterials (nanozymes) with antioxidant activity are at the forefront of research efforts towards biomedical and industrial applications. The selection of enzymatically active substances and their incorporation into novel inorganic nanozyme structures is critically important for this field of research. To this end, the fabrication of composites can be desirable as these can either exhibit multiple enzyme-like activities in a single material or show increased activity compared to the nanozyme components. Conversely, by modifying the structure of a nanomaterial, enzyme-like activities can be induced in formerly inert particles. We identify herein the three main routes of composite nanozyme synthesis, namely, surface functionalization of a particle with another compound, heteroaggregation of individual nanozymes, and modification of the bulk nanozyme structure to achieve optimal antioxidant activity. We discuss in particular the different inorganic support materials used in the synthesis of nanozyme architectures and the advantages brought forth by the use of composites.
1. Introduction
Nanomaterials exhibit distinct chemical and physical properties as a direct consequence of their small particle size and high specific surface area.1 In nanomaterial-based catalysis and thus, in nanozyme (nanomaterials with enzyme-like function) research,2 the control of size, shape and structure is paramount.3,4 In addition, appropriate colloidal and chemical stability as well as the possibility for facile modification of the particle composition is required to obtain efficient nanozyme systems.5 Inorganic materials such as clays, metal–organic frameworks (MOFs) and zeolites usually meet these requirements. Such nanozymes are being applied as efficient agents to decompose reactive oxygen species (ROS) via their superoxide dismutase (SOD, converts superoxide radical anions to molecular oxygen and hydrogen peroxide), catalase (CAT, decomposes hydrogen peroxide to molecular oxygen and water) and peroxidase (POD, uses hydrogen peroxide in oxidation of various compounds) mimicking abilities.Low dimensional clay nanomaterials are built up of cations coordinated by oxygen atoms in tetrahedral or octahedral geometry (Fig. 1A).6,7 They possess a lamellar structure and the layers can be either positively or negatively charged, which is compensated by hydrated counterions in the interlayer gallery. The tuneable structure of clays offers the possibility to modify the surface, the interlamellar space or even the composition of the metal ions by isomorphous substitution.8 These modifications can endow clay nanomaterials with enzyme-like properties to be applied as nanozymes.
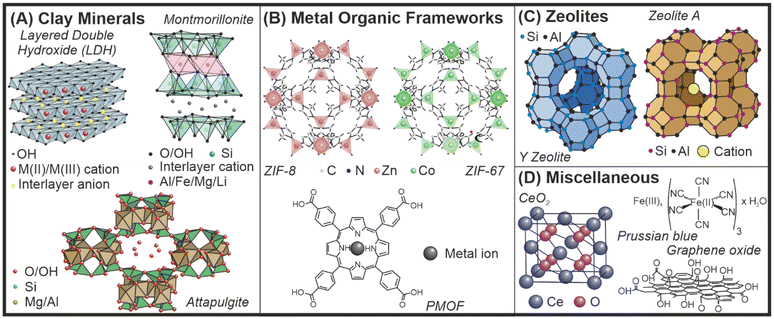 | ||
| Fig. 1 Schematic structure of clays (A), MOFs (B), zeolites (C) and other materials (D) as potential nanozymes. | ||
Similarly, nanozymes based on MOF structures (Fig. 1B) have shown favourable properties in a wide range of biocatalytic applications owing to their advantageous mesoporous structure with enclosed active sites.9 The antioxidant potential of MOFs is particularly noteworthy among other valuable features leading to the opportunity to cure diseases associated with oxidative stress.10,11 Various attempts were made to enhance the enzyme-like activity of MOF-based nanozymes applying materials chemistry approaches such as surface functionalization and structural engineering.12,13
Another class of inorganic porous materials are zeolites, crystalline aluminosilicate minerals with a microporous structure (Fig. 1C), which have already found numerous industrial and scientific applications.14 Zeolites possess metal ion exchange properties as well as size selectivity in catalytic reactions, while surface and structural modifications are promising tools to fabricate zeolite-based catalysts including nanozymes.15 Besides, there are inorganic nanomaterials that do not belong to any of the three categories outlined above, but can be engineered and used as nanozymes or support materials in nanozyme-based composites (Fig. 1D).
The present frontier article aims to explore the influence of recently developed fabrication methods including heteroaggregation and modification of the surface or composition on the biocatalytic performance and stability of nanozyme composites based on the abovementioned inorganic materials (Fig. 2), which were utilized to decompose ROS in various applications. Recent developments in the field will be discussed, while new avenues for future research will be outlined. Note that ROS-scavenging enzymes immobilized on nanoparticulate supports as well as details of biomedical antioxidant studies will not be discussed here.
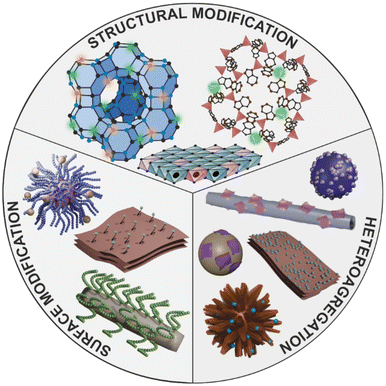 | ||
| Fig. 2 Schematic representation of the modification options for producing enzyme-like nanostructures. | ||
2. Surface modification
The surface of materials constitutes the contact area with substrates in biocatalytic applications and thus, surface modification of carrier materials with nanozymes is a promising approach to obtain composites with enzyme-like activities. Surface-adsorbed or surface-bound enzymatically active materials on inorganic supports are therefore widely researched.Accordingly, a superoxide dismutase (SOD)-like nanozyme was developed by surface modification of layered double hydroxide (an anionic clay, LDH) nanoparticles with polyelectrolytes and enzyme-mimicking copper(II)- and iron(II)-containing metal complexes to achieve the enzymatic activity.16 The co-immobilization of the coordination compounds led to a synergistic effect, which resulted in a higher apparent activity than that of the natural enzyme. Similar results were obtained when immobilizing copper(II)-citrate complexes on polyelectrolyte coated halloysite clay nanotubes. The complex showed remarkable superoxide scavenging activity after immobilization due to the formation of a dimeric structure, in which the metal centres are connected by coordinating carboxylate or hydroxyl bridges.17 In the research of Wang et al., kaolinite nanoclays were first delaminated in molten salts and then functionalized with manganese ferrite nanoparticles. The bare kaolinite nanoplatelets showed no antioxidant activity, while the catalase (CAT), peroxidase (POD) and oxidase-like function intensified significantly after immobilization of manganese ferrite on the kaolinite surface. The higher enzymatic activities were explained with the optimized geometrical configuration of the iron cations, which resulted in a greater binding affinity to H2O2 substrate molecules.18
Likewise, surface alterations were performed on MOF-based nanozymes to enhance their catalytic properties. For instance, Zhang et al. designed a MOF@COF (COF refers to covalent organic framework) composite with improved antioxidant potential via a sequential solvothermal protocol, in which the COF was attached to the surface of the nanozyme covalently.19 The as-fabricated MOF@COF was then exposed to the physiological environment to investigate its antioxidant potential. A significant increase in the antioxidant activity was observed that could be ascribed to the improved surface morphology upon composite formation through covalent linkage between the particles. Similarly, Ling et al. reported a strategy for preparing MOFs and decorating them with platinum nanoparticles for improved biomimetic catalysis. The MOF structure was composed of metalloporphyrin and zirconium(IV) ions. The noble metal nanoparticles were deposited on the surface of the carrier MOF to obtain a complex nanozyme structure, which exhibited remarkable CAT-like activity and functional stability.20 Another study reported a hybrid nanozyme prepared through sequential sonication of gold nanoparticles and Apt-DNAenzyme with a copper-based MOF (Cu-ZIF) followed by a freeze drying process.21 The obtained material possessed multienzyme-like activities that could be attributed to the advantageous surface features formed upon functionalization of Cu-ZIF.
Linde Type A (LTA) zeolites have been used as support materials for immobilization of Co3O4/TiO2 nanocomposites for application in sonophotocatalysis utilizing the POD-like activity of the composite obtained. The resulting Co3O4/TiO2@LTA nanozyme retains the POD-like activity for both TMB and H2O2 substrates.22
Surface modification of an enzymatically active material with another one is also a feasible strategy to achieve superior activities. For example, antioxidant ceria (CeO2) nanozymes can act as support materials,23 as shown with multi-metal coordinated ceria nanorods with POD-like activity. The findings revealed that the surface-coordinated metal ions exhibit synergistic activity with ceria, especially the manganese(II)/CeO2 composite with the highest POD-like activity among the tested materials. In another study, manganese(II)-doped Prussian Blue (PB) nanoparticles possessed both CAT and SOD activity,24 while doping of PB with zinc(II) induced copper(II) separation ability and CAT activity.25
3. Structural modification
By incorporating the catalytic unit – usually transition metal ions – into the structure of the carrier material, the support itself becomes the nanozyme (Fig. 2). In the case of lamellar carriers like clays or porous materials such as MOFs and zeolites, this approach exploits the advantages of the unique structural features, while improving the catalytic activity of the enzymatically active material.In one case, divalent metal ions were partially replaced in the LDH structure by incorporating copper(II) ions into Mg/Al LDH to induce both SOD and CAT function. The metal ion exchange caused superb enzyme-like properties, which was demonstrated in biochemical and intracellular tests.26 The antioxidant activity can also be increased by creating vacancies in the LDH structure. Co/Al LDH nanoplatelets were synthesized by a simple in situ growth procedure that resulted in ultrathin LDH nanoparticles and the number of vacancies increased with the decrease of the thickness. More vacancies led to enhanced superoxide radical anion scavenging ability, as they provided more binding sites for the radicals.27 Structural modification also improved the POD-like features of vermiculite nanoclays (VMT). The molten salt-assisted synthesis allowed lithium(I) ions to penetrate into the vermiculite structure giving rise to the formation of two new phases (LiFeO2 and Li4SiO4) and to an exfoliated structure. The LiFeO2–VMT nanoplatelets showed 10-times higher POD activity than the bare VMT sheets and also had excellent antibacterial properties.7
Achieving good chemical and colloidal stability, while retaining the enzyme-like activity, is an important challenge, especially in dispersions under physiological conditions, as in the practical biomedical applications of nanozymes.13,28 In due course, the confinement and porous feature of MOFs could favour the mass transport efficiency to improve the catalytic performance. To achieve this, tailoring the special conformation of the nanozyme into 3D active structures can give a clearer understanding of the catalytic activity retention of the nanozymes. As an example, Zhu et al. proposed a hybrid biomimetic nanozyme composed of hemin and bovine serum albumin (BSA) in zeolite imidazolate frameworks (ZIF-8) for improved POD-like activities. The nanozyme, denoted as hemin@BSA@ZIF-8, was developed via a facile conventional biomimetic mineralization process. The as-synthesized hemin@BSA@ZIF-8 exhibited better catalytic performance together with remarkable stability under harsh conditions. These features were attributed to the tailored intrinsic properties of the nanozyme.29 Another study reported a facile synthetic route to develop a multiple metal ion-doped zeolite nanozyme, denoted as CoM@NC, through a high temperature pyrolysis process. The CoM@NC has proven as a multifunctional antioxidant nanozyme for biosensing and pollutant degradation. The excellent catalytic performance of CoM@NC was ascribed to the doping with metal ions that induced a synergistic effect between the catalytic centres.30 More recently, Pan et al. reported a novel nanozyme, denoted as Pt@ZIF-8@La, with great antioxidant activities (Fig. 3A).31 The Pt@ZIF-8@La was synthesized by in situ encapsulation of platinum nanozymes into the ZIF-8 support followed by ion exchange. The Pt@ZIF-8@La nanozyme exhibited improved antioxidant activity due to the presence of mutilative elements in the system.
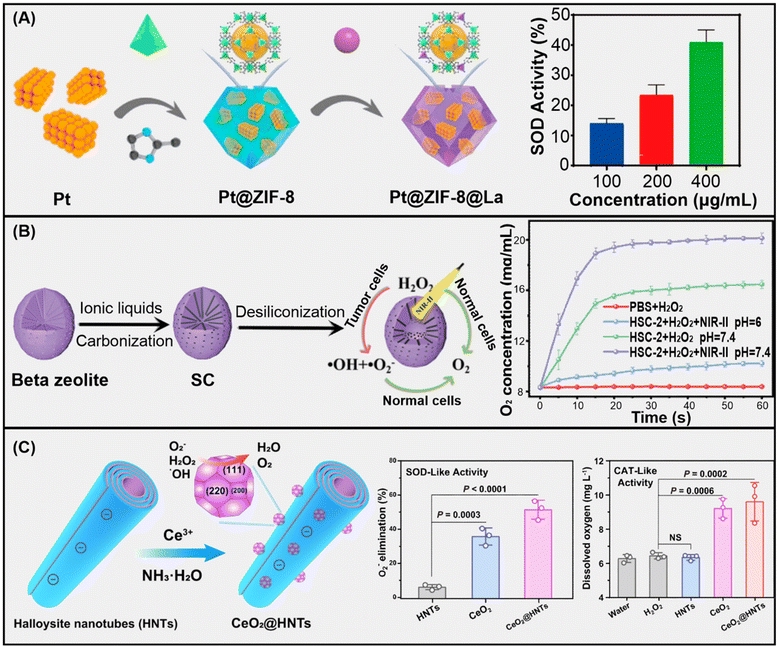 | ||
| Fig. 3 (A) Pt@ZIF-8@La MOF-based nanozyme with SOD-like activity. Adapted from ref. 31 copyright (2023) American Chemical Society. (B) Beta-zeolite-based HSC-2 nanozyme with CAT-like activity. Adapted from ref. 32 copyright (2021) John Wiley & Sons, Inc. (C) Ceria-decorated halloysite nanotubes with SOD- and CAT-like activity. Adapted from ref. 33 with permission from Springer Nature (2023). | ||
LTA zeolites doped with cerium were used to fabricate multifunctional mesoporous nanozymes (Ce@Zeo-NMs) for zinc ion removal – important for the treatment of ischaemic stroke – and ROS elimination.34 The in situ synthesized Ce@Zeo-NMs, sized between 28.2 and 142 nm, showed 90% zinc(II) uptake efficiency after 1 h and 100% within 12 h owing to the microporous zeolite structure, while the mixed-valence cerium(III)/(IV) content provided SOD and CAT enzyme-like activities to the composite.
Nano-sized high-silica faujasite (nZY) was structurally modified through aqueous copper(II) ion exchange processes by Moradpour et al. The resulting nZY–Cu compound showed POD-like activity in contrast to the precursor nZY material.35 In a follow-up article by the same authors, nZY was doped with multiple metal species such as gold, copper and zinc.36 The composites were prepared through aqueous ion exchange with the corresponding metal nitrates at elevated temperature and possessed improved POD activity with a 4-fold increase in the maximum reaction rate at an 8-times lower substrate concentration during degradation of H2O2 compared to nZY–Cu.
Beta-type zeolites synthesized via a hydrothermal method were doped with carbon through functionalization with an ionic liquid and subsequent calcination, resulting in carbon moieties incorporated directly into the aluminosilicate framework (Fig. 3B).32 The resulting zeolite–carbon composite possessed both CAT and POD function. This material also exhibits a photothermal effect, which increases the POD-like activity of the nanozyme, as confirmed in vivo in ROS-regulated tumour treatment applications.
Ceria, aside from possessing intrinsic nanozyme activities, is a potent platform for structural modification as its structure accepts a variety of dopants. Gold–ceria nanoparticles synthesized via NaBH4-driven reduction of gold(III) ions in a ceria-containing medium exhibit gold-content-dependent SOD and POD-like activities.37 Optimal POD-like activity was achieved at 5% gold-doping with a 1.5-fold enhancement in activity compared to the non-doped ceria. In contrast, the best SOD activity was shown at 10% gold content with a 1.6-fold enhancement compared to the ceria precursor. Besides, Jiang et al. shed light on that by anchoring copper single-sites on ceria nanostructures; the POD-like function can be improved significantly.38 At an optimum copper content of 5%, the reaction rate was increased 5-fold, while an approximately 2-fold increase in the turnover rate of individual catalytic sites has been observed. Oxides of other metals can also be modified to yield potent nanozymes. In one instance, mixed metal oxides were prepared via a hydrothermal method and the structure was optimized to achieve the highest POD activity.39 The ratio of Bi0.3Fe1.7MoO6 resulted in greater affinity to the TMB substrate than that of the native HRP enzyme.
In addition to the support materials outlined above, inert substances such as silica and carbon-based inorganic carriers (e.g., graphene oxide (GO)) have been used for immobilization of nanozymes. By incorporating oxygen-coordinated copper(II) ion active sites into the GO structure,40 the formed Cu/GO nanozyme exhibited remarkable SOD-like activity. In a similar way, nitrogen-doped carbon (N–C) supports were further modified with N-coordinated copper(II) ions to develop Cu–SA–zyme nanozymes that mimic the structure of the SOD5 enzyme with inherent SOD-like activity.41
4. Heteroaggregation
Highly charged particles can form stable colloidal dispersions under normal conditions. However, nanoparticles of opposite surface charges are liable to undergo heteroaggregation due to the electrostatic attractive forces between a positively and a negatively charged surface.42 Besides, the growth of nanozyme particles on the surface of a support material may result in similar structures to the one obtained with heteroaggregation. These approaches can be used to develop nanocomposites with a heterogeneous structure and improved enzyme-like properties (Fig. 2).As an example for the use of classical charge-driven heteroaggregation for nanozyme composite formation, Alsharif et al. developed protocols for the controlled assembly of ceria and manganese oxide nanozymes.43 In this method, polyelectrolytes were used to tune the surface charge of the nanoparticles and the carefully selected experimental conditions allowed the formation of stable nanocomposite dispersions with a broad-spectrum antioxidant effect. As per the in situ growth of a nanozyme on the surface of another material, ceria nanoparticles were grown on montmorillonite clay sheets, resulting in a monodisperse size distribution compared to the ceria nanoparticles formed in the absence of the clay template. The nanozyme composite was used in the treatment of inflammatory bowel disease, in which elimination of ROS molecules is a crucial step.44
Furthermore, nanotubular halloysite clays were also applied as carriers for ceria nanoparticles as a result of heteroaggregation between these particles (Fig. 3C). Further structural modifications were made by loading the lumen with the deferiprone drug. The final composite was then stabilized in a Pickering emulsion containing vitamin E. The system obtained was able to cure radiation colitis disease utilizing the advantageous radical scavenging feature of the nanomaterial.33
Single-atom nanozymes (SAEs) attract widespread contemporary interest from the scientific and industrial research communities.45 LDH doped with the ruthenium SAE was synthesized with as-prepared Mg/Al LDH nanoparticles and the SAE was homogeneously dispersed on the LDH support, endowing the nanoparticles with superb ROS and reactive nitrogen species scavenging activities.46 A stable, robust and reusable nanozyme was prepared by heteroaggregation of copper and iron oxide nanoparticles on attapulgite clay nanorods. The composite showed remarkable POD-like activity and significant antibacterial properties. Due to its advantageous properties, it could be used in medical devices to alter H2O2 consumption.6
The intrinsic and extrinsic properties of MOF-based supports have significantly improved nanozyme efficiencies in biomedical applications. However, harsh synthetic routes such as high temperature pyrolysis may lead to the inactivation of the inherent properties of the nanozymes. Therefore, more facile and environmentally friendly techniques, such as heteroaggregation, for the development of MOF-based nanozymes are highly desired. In this regard, Liu et al. reported a tumor-activated nanozyme generator (ZIF@GOx/GQDs) with improved antioxidant activities.18 The ZIF@GOx/GQDs was developed by the simultaneous incorporation of ultrasmall graphene quantum dots (GQDs) with POD-like activity into pH-sensitive ZIF-8. The newly developed nanozyme showed great intratumoral penetration and could directly alter the exogenous ROS concentration without any external energy input. Moreover, the possible inactivation of traditional ROS-based nanoagents in the extremely hypoxic tumor centre did not occur and thus, a notable joint catalytic performance took place.
Silica nanoparticles can also serve as nanozyme carriers in heteroaggregated systems as shown for silica-immobilized gold particles.47 Following the in situ synthesis and immobilization of gold nanoparticles on silica, the resulting silica@gold composites presented excellent POD-like activity. In another study, manganese oxide needles were heteroaggregated with silica nanoparticles and the formed substance possessed improved SOD and CAT activities, as compared to bare manganese oxide.48 These reports evidence that the nanozyme-like potential of silica nanoparticles can be exploited by heteroaggregation with appropriate nanomaterials.
5. Conclusions and outlook
In conclusion, design strategies for inorganic carrier supported nanozymes range from chemical and physical surface modification and heteroaggregation to various structural alteration procedures. The approach taken is largely dependent on the size, charge and composition of the nanozyme, as well as the properties of the carrier material chosen. Nanostructured inorganic supports often enhance enzyme-like activity, but intelligent design principles must be applied to ensure compatibility of the active component with the carrier. Clay minerals and especially LDHs can be utilized to immobilize nanozymes on the surface as well as in the interlayer space, while MOFs and zeolites are optimal choices for the incorporation of transition metal catalysts.One of the most important challenges in nanozyme research is to optimize colloidal stability, while retaining the activity of the nanozyme components (Fig. 4). Since most of the biomedical and industrial applications of these biocatalytic systems take place in liquid media, particle aggregation must be carefully prevented to avoid phase separation and loss of enzyme-like activity. Such a tuning of colloidal stability can be achieved during engineering nanozyme architectures with the three methods discussed above, in parallel with the synthetic processes. Nevertheless, it is particularly difficult to stabilize zeolites as colloids and thus, many nanozyme applications take advantage of the surface modification of relatively larger zeolite particles to achieve acceptable results. Similarly, several issues with MOF-based nanozymes restrict their practical use as colloids. For instance, their long-term biosafety remains undetermined, since colloidal stability and catalytic behaviour are unknown when exposed to various physiochemical conditions relevant to applications. While research on zeolite-based nanozymes is still a nascent field, possible solutions to tackle the challenges posed by these materials do exist. Accordingly, the optimization of colloidal stability with surface modification and utilizing aqueous ion exchange methods to create inherent enzymatically active materials should make zeolite nanozymes more viable in the future. Another possibility to achieve better dispersion stability is to prepare zeolites and MOFs in the nanoscale size range with low polydispersity in microfluidic chips with controllable channels and vortexes.
The structural integration of the above nanozymes with other materials may lead to antioxidant multifunctionality, i.e., scavenging various ROS simultaneously. However, this can be also achieved by designing nanozyme cocktails containing individual nanozymes with various enzyme-like functions as well as with appropriate structural and colloidal stability (Fig. 4). In addition to the application areas mentioned so far, the use of antioxidant nanozymes or their derivatives can also represent a potential advance in biomedical treatments such as nanozyme enabled protective therapy for neurological diseases49 or in complicated environmental processes, e.g., degradation of micro- and nanoplastic (MNPL) pollutants in oxidoreductive processes (Fig. 4).50 Finally, careful selection of individual nanozymes and formation of their composites employing any of the methods discussed in this frontier article can lead to the development of multifunctional systems,51 which may be able to act as antioxidants along with other types (e.g., hydrolytic or oxidative) of catalytic features.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this frontier article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the Hungarian Academy of Sciences through the Momentum program (project LP2022-16/2022) and the European Union's Horizon Europe Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement no. 101086226 is gratefully acknowledged. The support from the University of Szeged Open Access Fund (7170) is gratefully acknowledged.References
- L. A. Kolahalam, I. V. K. Viswanath, B. S. Diwakar, B. Govindh, V. Reddy and Y. L. N. Murthy, Mater. Today: Proc., 2019, 18, 2182–2190 Search PubMed.
- L. Z. Gao, H. Wei, S. J. Dong and X. Y. Yan, Adv. Mater., 2024, 36, 2305249 CrossRef CAS PubMed.
- M. Zandieh and J. W. Liu, Adv. Mater., 2023, 36, 2211041 CrossRef PubMed.
- H. Q. Zhao, R. F. Zhang, X. Y. Yan and K. L. Fan, J. Mater. Chem. B, 2021, 9, 6939–6957 RSC.
- H. Wang, K. W. Wan and X. H. Shi, Adv. Mater., 2019, 31, 1805368 CrossRef CAS PubMed.
- F. Feng, X. Zhang, B. Mu, P. X. Wang, Z. S. Chen, J. H. Zhang, H. F. Zhang, J. L. Zhuang, L. Zhao, Q. An and Y. H. Zhang, ACS Appl. Nano Mater., 2022, 5, 16720–16730 CrossRef CAS.
- L. Y. Tian, Y. Y. Qian, H. Wang, G. Q. Zhao, A. D. Tang and H. M. Yang, Nano Lett., 2023, 24, 386–393 CrossRef PubMed.
- G. Mishra, B. Dash and S. Pandey, Appl. Clay Sci., 2018, 153, 172–186 CrossRef CAS.
- X. Huang, S. T. Zhang, Y. J. Tang, X. Y. Zhang, Y. Bai and H. Pang, Coord. Chem. Rev., 2021, 449, 214216 CrossRef CAS.
- A. A. El-Bindary, E. A. Toson, K. R. Shoueir, H. A. Aljohani and M. M. Abo-Ser, Appl. Organomet. Chem., 2020, 34, e5905 CrossRef CAS.
- J. C. Guo, Z. Z. Yang, Y. Z. Lu, C. Y. Du, C. Cao, B. Wang, X. T. Yue, Z. L. Zhang, Y. Y. Xu, Z. Qin, T. T. Huang, W. Wang, W. Jiang, J. Y. Zhang and J. N. Tang, Bioact. Mater., 2022, 10, 56–67 CAS.
- H. M. Zhong, C. Jiang, J. H. Zou, G. C. Zhu and Y. Y. Huang, Inorg. Chem., 2023, 62, 13168–13172 CrossRef CAS PubMed.
- D. Y. Chao, Q. Dong, Z. X. Yu, D. S. Qi, M. H. Li, L. L. Xu, L. Liu, Y. X. Fang and S. J. Dong, J. Am. Chem. Soc., 2022, 144, 23438–23447 CrossRef CAS PubMed.
- Q. M. Sun, N. Wang and J. H. Yu, Adv. Mater., 2021, 33, 2104442 CrossRef CAS PubMed.
- H. Serati-Nouri, A. Jafari, L. Roshangar, M. Dadashpour, Y. Pilehvar-Soltanahmadi and N. Zarghami, Mater. Sci. Eng., C, 2020, 116, 111225 CrossRef CAS PubMed.
- Z. Somosi, N. V. May, D. Sebok, I. Palinko and I. Szilagyi, Dalton Trans., 2021, 50, 2426–2435 RSC.
- B. Katana, N. V. Nagy, G. Varga and I. Szilagyi, J. Mol. Struct., 2022, 1256, 132492 CrossRef CAS.
- X. P. Liu, Z. W. Liu, K. Dong, S. Wu, Y. J. Sang, T. T. Cui, Y. Zhou, J. S. Ren and X. G. Qu, Biomaterials, 2020, 258, 120263 CrossRef CAS PubMed.
- L. Zhang, Z. W. Liu, Q. Q. Deng, Y. J. Sang, K. Dong, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2021, 60, 3469–3474 CrossRef CAS PubMed.
- P. H. Ling, S. Cheng, N. Chen, C. H. Qian and F. Gao, ACS Appl. Mater. Interfaces, 2020, 12, 17185–17192 CrossRef CAS PubMed.
- F. Y. Mo, S. Zhong, T. H. You, J. Lu and D. P. Sun, ACS Appl. Mater. Interfaces, 2023, 15, 52114–52127 CAS.
- S. Rashtbari, G. Dehghan, A. Marefat, S. Khataee and A. Khataee, Ultrason. Sonochem., 2024, 102, 106740 CrossRef CAS PubMed.
- Y. Yue, H. Y. Wei, J. X. Guo and Y. Z. Yang, Colloids Surf., A, 2021, 610, 125715 CrossRef CAS.
- C. Q. Chen, H. T. Wu, Q. H. Li, M. H. Liu, F. Yin, M. M. Wu, X. L. Wei, H. Wang, Z. B. Zha and F. Wang, Biomater. Sci., 2023, 11, 2348–2358 RSC.
- Y. Zhang, X. Yuan, X. Y. Guo, H. Xu, D. X. Zhang, Z. Y. Wu and J. Zhang, Small, 2024, 20, 2306961 CrossRef CAS PubMed.
- A. Szerlauth, T. Madácsy, G. F. Samu, P. Bíró, M. Erdélyi, G. Varga, Z. P. Xu, J. Maléth and I. Szilágyi, Chem. Commun., 2024, 60, 1325–1328 RSC.
- S. Zhang, J. Chen, W. S. Yang and X. Chen, Nano Res., 2022, 15, 7940–7950 CrossRef CAS.
- Y. Duan, L. Liang, F. G. Ye and S. L. Zhao, Nanoscale, 2023, 15, 13475–13844 RSC.
- N. F. Zhu, C. B. Liu, R. Liu, X. H. Niu, D. H. Xiong, K. Wang, D. Q. Yin and Z. Zhang, Anal. Chem., 2022, 94, 4821–4830 CrossRef CAS PubMed.
- Y. W. Guan, Y. X. Lu, J. Y. Zhao, W. Huang and Y. Y. Liu, Chem. Eng. J., 2023, 465, 142703 CrossRef CAS.
- H. J. Pan, X. X. Miao, J. J. Deng, C. Z. Pan, X. G. Cheng and X. L. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 4935–4946 CrossRef CAS PubMed.
- Z. L. Zheng, Z. Jia, Y. F. Qin, R. Dai, X. J. Chen, Y. C. Ma, X. M. Xie and R. P. Zhang, Small, 2021, 17, 2103252 CrossRef CAS PubMed.
- Y. Feng, X. Luo, Z. C. Li, X. J. Fan, Y. T. Wang, R. R. He and M. X. Liu, Nat. Commun., 2023, 14, 5083 CrossRef CAS PubMed.
- Z. X. Huang, K. Qian, J. Chen, Y. Qi, E. Yifeng, J. Liang and L. Zhao, Acta Biomater., 2022, 144, 142–156 CrossRef CAS PubMed.
- Z. Moradpour, M. H. Kohnehshahri, M. V. Shekarloo, V. Jalili and R. Zendehdel, Colloid Polym. Sci., 2021, 299, 1567–1575 CrossRef CAS.
- F. Ravannakhjavani, S. F. Dehghan, D. Panahi, Z. Moradpour and R. Zendehdel, J. Inorg. Organomet. Polym. Mater., 2024, 34, 818–826 CrossRef CAS.
- Q. Xin, L. Wang, H. Y. Ruan, L. Liu, S. Sun, J. Zhang, M. L. Wu, X. Y. Mu, H. Wang, S. F. Zhang and X. D. Zhang, Part. Part. Syst. Charact., 2023, 40, 2200203 CrossRef CAS.
- P. Jiang, L. D. Zhang, X. L. Liu, C. L. Ye, P. Zhu, T. Tan, D. S. Wang and Y. G. Wang, Nat. Commun., 2024, 15, 1010 CrossRef CAS PubMed.
- J. Dan, Z. H. Su, B. Y. Sun, J. L. Wang and W. T. Zhang, Microchem. J., 2023, 185, 108302 CrossRef CAS.
- M. J. Lu, J. L. Wang, G. Y. Ren, F. J. Qin, Z. Q. Zhao, K. Li, W. X. Chen and Y. Q. Lin, Nano Res., 2022, 15, 8804–8809 CrossRef CAS.
- J. Yang, R. Zhang, H. Zhao, H. Qi, J. Li, J.-F. Li, X. Zhou, A. Wang, K. Fan, X. Yan and T. Zhang, Exploration, 2022, 2, 20210267 CrossRef CAS PubMed.
- N. B. Alsharif, B. Katana, S. Murath and I. Szilagyi, Adv. Colloid Interface Sci., 2021, 294, 102456 CrossRef CAS PubMed.
- N. B. Alsharif, D. Viczián, A. Szczes and I. Szilagyi, J. Phys. Chem. C, 2023, 127, 17201–17212 CrossRef CAS PubMed.
- S. Zhao, Y. X. Li, Q. Y. Liu, S. R. Li, Y. Cheng, C. Q. Cheng, Z. Y. Sun, Y. Du, C. J. Butch and H. Wei, Adv. Funct. Mater., 2020, 30, 2004692 CrossRef CAS.
- Z. Guo, J. Hong, N. Song and M. Liang, Acc. Mater. Res., 2024, 5, 347–357 CrossRef CAS.
- B. Q. Wang, Y. Y. Fang, X. Han, R. T. Jiang, L. Zhao, X. Yang, J. Jin, A. J. Han and J. F. Liu, Angew. Chem., Int. Ed., 2023, 62, e202307133 CrossRef CAS PubMed.
- B. Seong, J. Kim, W. Kim, S. H. Lee, X. H. Pham and B. H. Jun, Int. J. Mol. Sci., 2021, 22, 10382 CrossRef CAS PubMed.
- M. D. Garrido, J. El Haskouri, M. D. Marcos, F. Pérez-Pla, J. V. Ros-Lis and P. Amorós, Nanomaterials, 2022, 12, 3503 CrossRef CAS PubMed.
- F. Y. Cheng, S. Kotha, M. Fu, Q. Yang, H. Wang, W. W. He and X. B. Mao, Nano Today, 2024, 54, 102142 CrossRef CAS.
- M. Zandieh and J. W. Liu, Angew. Chem., Int. Ed., 2022, 61, 202212013 CrossRef PubMed.
- Y. J. Jiang, Z. B. Chen, N. Sui and Z. L. Zhu, J. Am. Chem. Soc., 2024, 146, 7565–7574 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |