Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sulfate-induced large amplitude conformational change in a Solomon link†
Cuong Dat
Do
 a,
Dávid
Pál
a,
Dávid
Pál
 a,
Andrey
Belyaev
a,
Andrey
Belyaev
 b,
Marion
Pupier
a,
Anniina
Kiesilä
b,
Elina
Kalenius
b,
Marion
Pupier
a,
Anniina
Kiesilä
b,
Elina
Kalenius
 b,
Bartomeu
Galmés
b,
Bartomeu
Galmés
 c,
Antonio
Frontera
c,
Antonio
Frontera
 c,
Amalia
Poblador-Bahamonde
c,
Amalia
Poblador-Bahamonde
 a and
Fabien B. L.
Cougnon‡
a and
Fabien B. L.
Cougnon‡
 *ab
*ab
aDepartment of Organic Chemistry, University of Geneva, 30 Quai Ernest-Ansermet, 1211 Geneva 4, Switzerland
bDepartment of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, FI-40014 JYU, Finland. E-mail: fabien.b.l.cougnon@jyu.fi
cDepartment de Química, Universitat de les Illes Balears, Carretera de Valldemossa km 7.5, 07122 Palma de Mallorca, Baleares, Spain
First published on 6th October 2023
Abstract
A doubly-interlocked [2]catenane – or Solomon link – undergoes a complex conformational change upon addition of sulfate in methanol. This transformation generates a single pocket where two SO42− anions bind through multiple hydrogen bonds and electrostatic interactions. Despite the close proximity of the two anions, binding is highly cooperative.
Biopolymer conformational changes are essential to many biological processes such as the cooperative binding of four molecules of dioxygen to haemoglobin,1 and the remote transfer of information mediated by G protein-coupled receptors.2,3 Taking inspiration from nature, chemists have designed a broad range of synthetic folded molecules, or foldamers,4–11 able to change conformation.12–16 However, the propensity of foldamers to unfold in response to external stimuli limits both the diversity and the amplitude of the accessible transformations.17,18 Non-biomimetic folded molecules such as multiply entangled macrocycles19–25 may provide a means to solve this issue. Multiply entangled macrocycles can be considered to be folded because the presence of entanglements reduces their conformational freedom and can block them in specific conformational states. Unlike foldamers, these structures cannot unfold unless a covalent bond is broken but can be deformed in the three dimensions of space by moving the individual threads relative to one another. This process, referred to as “Reidemeister moves”26,27 in mathematics, should in principle allow transitions between multiple conformational states of different shapes and symmetries. Harnessing such complex conformational behaviour would provide unprecedented opportunities to precisely control the position of functional groups in space and time. Yet, Reidemeister moves have been rarely observed in macromolecules.28,29
Our group has recently reported the synthesis of a doubly-interlocked [2]catenane, or Solomon link (SL),30 able to undergo a relatively modest conformational change between two C4-symmetric states in acetonitrile/water mixtures.31 We show now that the addition of sulfate to the same Solomon link in methanol triggers a conformational change of greater amplitude (Fig. 1 and Fig. S1, ESI†), which transforms the C4-symmetric conformer 1 previously observed in pure water31 into a new D2-symmetric conformer (2) different from any of the conformers previously reported. This discovery demonstrates the rich conformational behaviour of multiply entangled macrocycles and establishes their potential as a platform for developing sophisticated multi-responsive systems.
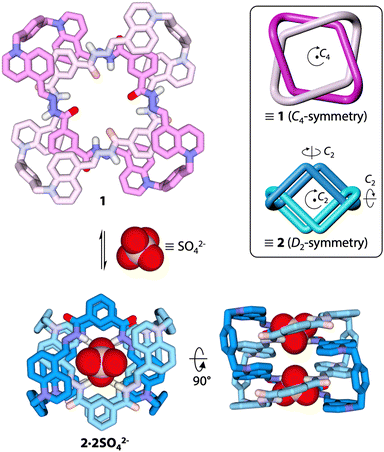 | ||
| Fig. 1 Sulfate-induced conformational change of a Solomon link in methanol (BP86-D3/def2-TZVP optimized geometries). The insert shows a cartoon representation of conformers 1 and 2. | ||
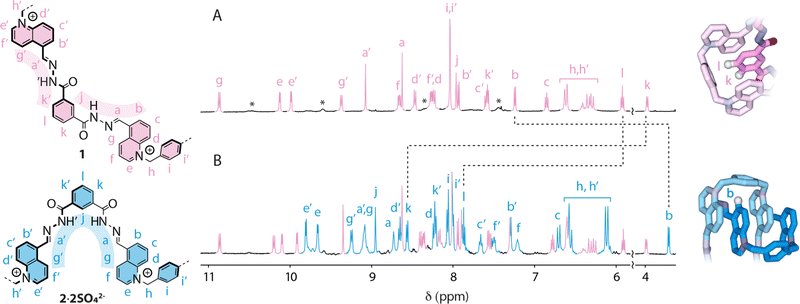
The 1H NMR spectrum of Solomon link SL8+·8CF3CO2− in CD3OD (Fig. 2A) is consistent with conformer 1. The full NMR characterization is presented in the ESI† (Fig. S2–S5). An optimum packing of the aromatic units results in well-dispersed resonances spreading over ca. 7 ppm, from 3.97 ppm to 10.87 ppm. The isophthalic units, each surrounded by two quinoliniums and a xylyl unit, experience a substantial upfield shift. Isophthalic protons k (3.97 ppm) and l (5.91 ppm) are particularly shielded because they are directly oriented towards the xylyl units in a T-shaped relationship. NOE correlations j ↔ NH ↔ a ↔ b and k′ ↔ NH′ ↔ a′ ↔ g′ recorded in partially non-deuterated CD3OH (Fig. S3, ESI†) show that acylhydrazone protons NH and NH′ point in divergent directions.
The presence of lower intensity, broad signals (labelled with a star in Fig. 2A) indicates the existence of other minor conformers that may be amplified in response to an external stimulus. We reasoned that the addition of an appropriate anion could trigger the amplification of a conformer with a suitable pocket for anion binding, i.e., with all the NH and NH′ hydrogen-bond donors converging towards the centre of the cavity. This hypothesis was tested by carrying out 1H NMR titrations with a range of common anions (Cl−, Br−, I−, CF3SO3−, PF6−, SCN−, NO3−, ReO4−, ClO4−, and SO42− as tetra-n-butylammonium salts, Fig. S6, ESI†).
None of the anions tested induced any response with the notable exception of SO42−. The addition of sulfate led to the appearance of a new set of signals corresponding to D2-symmetric conformer 2 (Fig. 2B), which was fully characterized in solution by NMR (Fig. S9–S12, ESI†) and modeled using dispersion-corrected DFT (BP86-D3/def2-TZVP) methodology.32,33 Upon binding to sulfate, the Solomon link undergoes a large amplitude conformational change that carries atoms across up to 1.6 nm (Fig. S1, ESI†). In conformation 2, the quinolinium units stack on each side of the Solomon link. The protons of the quinoliniums buried in the stack (b–g) are significantly shielded compared to those of the quinoliniums located on the outer surface (b′–g′). Quinolinium proton b is the most upfield shifted signal (δ = 3.74 ppm) because it is facing the xylyl unit in a T-shape relationship. The isophthalic units are entirely exposed to the solvent and no longer experience the characteristic shielding observed in conformation 1. Finally, NOE correlations g ↔ a ↔ NH ↔ j ↔ NH′ ↔ a′ ↔ g′ (Fig. S10, ESI†) confirm that both acylhydrazone NH and NH′ hydrogen bond donors are oriented towards the centre of the cavity of 2, providing an ideal binding site where sulfate can nest.
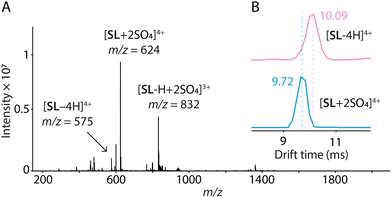
Electrospray ionization mass spectrometry (ESI-HRMS) corroborates that the Solomon link preferentially binds SO42− over the other anions tested (Fig. S13–S15, ESI†), as observed by NMR. More importantly, peaks at m/z 624.2026 and m/z 831.9337, corresponding to [SL + 2SO4]4+ and [SL-H + 2SO4]3+, respectively, disclose that the Solomon link binds two equivalents of sulfate (Fig. 3). The central cavity of conformer 2 displays two identical sites where SO42− can bind via the formation of four N–H⋯O–S and ten C–H⋯O–S hydrogen bonds (Fig. S1, ESI†). The tetrahedral disposition of the four acylhydrazone NH hydrogen bond donors of each binding site nicely matches the tetrahedral geometry of the dianion. The preferential binding of SO42− may thus be explained, at least partially, by the high complementarity between the dianion and the binding sites, in terms of size and geometry. The absence of response with the tetrahedral monoanions ReO4− and ClO4− is likely explained by a weaker electrostatic interaction.
Conformers 1 and 2 slowly exchange on the NMR timescale. The fraction θ of sulfate-bound conformer 2 can therefore be easily measured from the relative integration of the NMR signals as a function of the total concentration of sulfate [SO42−]. The Hill coefficient nH ≈ 2 obtained from the plot log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/(1 − θ) = f(log[SO42−]) indicates strong positive cooperativity34 (Fig. 4A). The narrow concentration window in which the transition occurs (Fig. 4A, insert) supports this conclusion. This result is unexpected as the second binding event creates unfavourable electrostatic interactions between the two anions, constrained in close proximity within a single pocket.35,36 The occurrence of positive cooperativity may be rationalised by considering that the conformational change triggered by the first molecule of sulfate brings all the hydrogen bond donor components of the second binding site (CHs and NHs) in the correct position to bind the second molecule of sulfate, considerably enhancing affinity for the subsequent binding event (K1 ≪ K2). The electrostatic repulsion between the two anions is presumably offset by both the formation of multiple hydrogen bonds and attractive electrostatic interactions between the anions and the polycationic Solomon link.
θ/(1 − θ) = f(log[SO42−]) indicates strong positive cooperativity34 (Fig. 4A). The narrow concentration window in which the transition occurs (Fig. 4A, insert) supports this conclusion. This result is unexpected as the second binding event creates unfavourable electrostatic interactions between the two anions, constrained in close proximity within a single pocket.35,36 The occurrence of positive cooperativity may be rationalised by considering that the conformational change triggered by the first molecule of sulfate brings all the hydrogen bond donor components of the second binding site (CHs and NHs) in the correct position to bind the second molecule of sulfate, considerably enhancing affinity for the subsequent binding event (K1 ≪ K2). The electrostatic repulsion between the two anions is presumably offset by both the formation of multiple hydrogen bonds and attractive electrostatic interactions between the anions and the polycationic Solomon link.
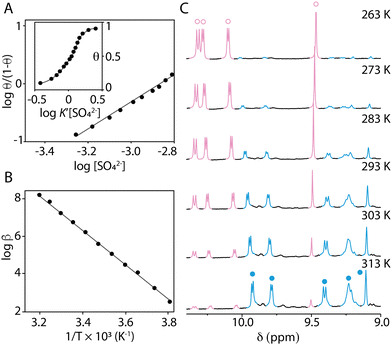 | ||
| Fig. 4 (A) Hill plot obtained from a titration of SO42− into a CD3OD solution of the Solomon link at 313 K. The insert shows the corresponding speciation curve.34 In this plot, [SO42−] is normalized by the apparent equilibrium constant K′ corresponding to the concentration of SO42− producing half-occupation. (B) Van’t Hoff plot. (C) Variable temperature 1H NMR spectra of the Solomon link after addition of 2.6 equiv. of sulfate (CD3OD, 500 MHz). The signals corresponding to conformers 1 and 2 are coloured in pink (○) and blue (•), respectively. | ||
The affinity of the Solomon link for sulfate, extrapolated from the Hill plot, is highly dependent on temperature. The overall association constant β = K1·K2 is weak at 263 K (β = 13 M−2) and increases with temperature (β = 6.8 × 103 M−2 at 313 K). Van’t Hoff analysis (Fig. 4B) shows that binding is entropically driven (ΔS° = +348 ± 10 J mol−1 K−1, ΔH° = +86 ± 4 kJ mol−1). The switch can thus be efficiently operated by changing temperature between 263 K and 313 K in the presence of a small excess of SO42− (2.6 equiv., Fig. 4C). Sulfate-bound conformer 2 is favoured at high temperature. The host–guest complex dissociates when the temperature decreases, and the Solomon link switches back to conformer 1.
Sulfate-bound conformer 2 was only observed in methanol and not in other solvents such as acetonitrile and water (Fig. S21–S22, ESI†), which stabilise better the other conformers.31 Even the presence of small amounts of water in methanol interferes with the formation of the host–guest complex (Fig. S23, ESI†). It is also interesting to note that the transformation 1 → 2 results in a contraction of the Solomon link detectable by both diffusion spectroscopy (DOSY) and ion-mobility mass spectrometry (IM-MS). During the conformational change, an increase in diffusion coefficient indicates a decrease in ca. 30% of the Solomon link bulk volume consistent with the values measured from the molecular models (Fig. S24, ESI†). The contraction is also evidenced by IM-MS. A clear decrease in experimental collision cross-section values measured upon binding of the two equiv. of sulfate confirms that sulfate-bound conformer 2 is more compact (DTCCSHe = 395.4 Å2 for [SL-4H]4+vs. 378.8 Å2 for [SL + 2SO4]4+, see Table S2 for details, ESI†). Here again, the experimental DTCCSHe measured for [SL + 2SO4]4+ closely corresponds to the theoretical TMLJCCSHe value of 373.5 Å2 calculated from the optimized DFT structure. The host–guest complexes formed with other anions are observed with lower abundance and display larger DTCCSHe values compared to [SL-4H]4+, indicating exclusion complexation. The conformational change thus appears to be specific to SO42− both in solution and in the gas phase.
The Solomon link is a remarkably polymorphic molecule, able to undergo either medium (as previously reported)31 or large amplitude deformations, depending on the experimental conditions. We have now identified three different conformers, whose NMR features are compared in Fig. S25 (ESI†). The protean behaviour of the Solomon link, reminiscent of that of biomolecules, is considerably more complex than that of topologically simpler macromolecules. Such findings may thus lay the groundwork for the design of increasingly sophisticated entangled macrocycles that can change their three-dimensional shape, and perhaps their function, in response to multiple stimuli.
The authors thank the Department of Chemistry at the University of Jyväskylä, the Department of Organic Chemistry at the University of Geneva, the MICIU/AEI of Spain (projects ID2020-115637GB-I00, TED2021-130946B-100 FEDER funds) and the Otto A. Malm Foundation for financial support.
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- M. F. Perutz, Nature, 1972, 237, 495–499 CrossRef CAS PubMed.
- D. M. Rosenbaum, S. G. Rasmussen and B. K. Kobilka, Nature, 2009, 459, 356–363 CrossRef CAS PubMed.
- D. M. Thal, A. Glukhova, P. M. Sexton and A. Christopoulos, Nature, 2018, 559, 45–53 CrossRef CAS PubMed.
- E. A. John, C. J. Massena and O. B. Berryman, Chem. Rev., 2020, 120, 2759–2782 CrossRef CAS PubMed.
- S. Rinaldi, Molecules, 2020, 25, 3276 CrossRef CAS PubMed.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed.
- C. M. Goodman, S. Choi, S. Shandler and W. F. DeGrado, Nat. Chem. Biol., 2007, 3, 252–262 CrossRef CAS PubMed.
- Y. Ferrand and I. Huc, Acc. Chem. Res., 2018, 51, 970–977 CrossRef CAS PubMed.
- D. Mazzier, S. De, B. Wicher, V. Maurizot and I. Huc, Angew. Chem., Int. Ed., 2020, 59, 1606–1610 CrossRef CAS PubMed.
- C. G. Pappas, P. K. Mandal, B. Liu, B. Kauffmann, X. M. Miao, D. Komaromy, W. Hoffmann, C. Manz, R. Chang, K. Liu, K. Pagel, I. Huc and S. Otto, Nat. Chem., 2020, 12, 1180–1186 CrossRef CAS PubMed.
- Y. Jin, P. K. Mandal, J. Wu, N. Bocher, I. Huc and S. Otto, J. Am. Chem. Soc., 2023, 145, 2822–2829 CrossRef CAS PubMed.
- R. Rodriguez, E. Suarez-Picado, E. Quinoa, R. Riguera and F. Freire, Angew. Chem., Int. Ed., 2020, 59, 8616–8622 CrossRef CAS PubMed.
- J. Atcher, P. Mateus, B. Kauffmann, F. Rosu, V. Maurizot and I. Huc, Angew. Chem., Int. Ed., 2021, 60, 2574–2577 CrossRef CAS PubMed.
- D. Pijper and B. L. Feringa, Angew. Chem., Int. Ed., 2007, 46, 3693–3696 CrossRef CAS PubMed.
- T. Miyagawa, A. Furuko, K. Maeda, H. Katagiri, Y. Furusho and E. Yashima, J. Am. Chem. Soc., 2005, 127, 5018–5019 CrossRef CAS PubMed.
- B. A. Le Bailly and J. Clayden, Chem. Commun., 2016, 52, 4852–4863 RSC.
- Z. Yu and S. Hecht, Angew. Chem., Int. Ed., 2011, 50, 1640–1643 CrossRef CAS PubMed.
- F. C. Parks, Y. Liu, S. Debnath, S. R. Stutsman, K. Raghavachari and A. H. Flood, J. Am. Chem. Soc., 2018, 140, 17711–17723 CrossRef CAS PubMed.
- S. R. Beeren, C. T. McTernan and F. Schaufelberger, Chem, 2023, 9, 1378–1412 CAS.
- W.-X. Gao, H.-J. Feng, B.-B. Guo, Y. Lu and G.-X. Jin, Chem. Rev., 2020, 120, 6288–6325 CrossRef CAS PubMed.
- S. D. P. Fielden, D. A. Leigh and S. L. Woltering, Angew. Chem., Int. Ed., 2017, 56, 11166–11194 CrossRef CAS PubMed.
- R. S. Forgan, J. P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434–5464 CrossRef CAS PubMed.
- Z. Ashbridge, E. Kreidt, L. Pirvu, F. Schaufelberger, J. H. Stenlid, F. Abild-Pedersen and D. A. Leigh, Science, 2022, 375, 1035–1041 CrossRef CAS PubMed.
- Y. Inomata, T. Sawada and M. Fujita, J. Am. Chem. Soc., 2021, 143, 16734–16739 CrossRef CAS PubMed.
- H.-N. Zhang, H.-J. Feng, Y.-J. Lin and G.-X. Jin, J. Am. Chem. Soc., 2023, 145, 4746–4756 CrossRef CAS PubMed.
- K. Reidemeister, Abh. Math. Semin. Univ. Hambg., 1927, 5, 24–32 CrossRef.
- J. W. Alexander and G. B. Briggs, Ann. Math., 1926, 28, 562–586 CrossRef.
- J. P. Carpenter, C. T. McTernan, J. L. Greenfield, R. Lavendomme, T. K. Ronson and J. R. Nitschke, Chem, 2021, 7, 1534–1543 CAS.
- V. Marcos, A. J. Stephens, J. Jaramillo-Garcia, A. L. Nussbaumer, S. L. Woltering, A. Valero, J. F. Lemonnier, I. J. Vitorica-Yrezabal and D. A. Leigh, Science, 2016, 352, 1555–1559 CrossRef CAS PubMed.
- F. B. L. Cougnon, K. Caprice, M. Pupier, A. Bauzá and A. Frontera, J. Am. Chem. Soc., 2018, 140, 12442–12450 CrossRef CAS PubMed.
- K. Caprice, M. Pupier, A. Bauzá, A. Frontera and F. B. L. Cougnon, Angew. Chem., Int. Ed., 2019, 58, 8053–8057 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS PubMed.
- A. Schäfer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- C. A. Hunter and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 7488–7499 CrossRef CAS PubMed.
- W. Zhao, A. H. Flood and N. G. White, Chem. Soc. Rev., 2020, 49, 7893–7906 RSC.
- Q. He, P. Tu and J. L. Sessler, Chem, 2018, 4, 46–93 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc04555b |
| ‡ Current address: Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, FI-40014 JYU, Finland. |
| This journal is © The Royal Society of Chemistry 2023 |