 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polar-solvent-free colloidal synthesis of highly luminescent alkylammonium lead halide perovskite nanocrystals†
Oleh
Vybornyi
ab,
Sergii
Yakunin
ab and
Maksym V.
Kovalenko
*ab
aETH Zürich – Swiss Federal Institute of Technology Zürich, Vladimir Prelog Weg 1, CH-8093 Zürich, Switzerland. E-mail: mvkovalenko@ethz.ch
bEmpa-Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Thin Films and Photovoltaics, Überlandstrasse 129, CH-8600 Dübendorf, Switzerland
First published on 26th November 2015
Abstract
A novel synthesis of hybrid organic–inorganic lead halide perovskite nanocrystals (CH3NH3PbX3, X = Br or I) that does not involve the use of dimethylformamide or other polar solvents is presented. The reaction between methylamine and PbX2 salts is conducted in a high-boiling nonpolar solvent (1-octadecene) in the presence of oleylamine and oleic acid as coordinating ligands. The resulting nanocrystals are characterized by high photoluminescence quantum efficiencies of 15–50%, outstanding phase purity and tunable shapes (nanocubes, nanowires, and nanoplatelets). Nanoplatelets spontaneously assemble into micrometer-length wires by face-to-face stacking. In addition, we demonstrate amplified spontaneous emission from thin films of green-emitting CH3NH3PbBr3 nanowires with low pumping thresholds of 3 μJ cm−2.
In the past few years, a great deal of attention has been devoted to hybrid lead halide semiconductors with a perovskite crystal structure, generically described by the formula ABX3 (where A is an organic or inorganic cation, B is a metal cation in the octahedral coordination state and X is a halogen anion). Such compounds exhibit outstanding optoelectronic properties in the form of thin films, microcrystals and bulk single-crystals.1–12 Initial motivation to investigate these materials arose from the demonstration of solution-cast, inexpensive solar cells with certified power conversion efficiencies exceeding 20% (NREL efficiency chart, http://www.nrel.gov).1,13–15 Subsequently, other perovskite-based optoelectronic devices were demonstrated – sensitive solution-cast photodetectors operating in the visible,16–18 ultraviolet19,20 and X-ray21 spectral regions and light-emitting diodes22–26 – which all harness the unique defect-tolerant photophysics of these lead halide compounds.
The research landscape with respect to perovskites has also recently extended to the nanoscale, where quantum-size effects and the benefits of the colloidal state and shape engineering can be expected, stimulating efforts on the synthesis of supported and colloidal nanostructures of hybrid11,27–40 and fully inorganic perovskites.41,42 When compared to more conventional CdSe-based nanocrystals (NCs), perovskite NCs exhibit a number of unique properties such as high photoluminescence (PL) quantum yields (up to 90%) without the need for additional electronic passivation by adding a wider-gap shell (e.g. CdSe/CdS and CdSe/ZnS NCs), and novel forms of chemical tunability such as by deliberately partial or complete anion-exchange reactions.36,42,43 Concerning hybrid organic–inorganic perovskite NCs, all reported colloidal procedures to date feature a common strategy, initially outlined by Pérez-Prieto et al.:29 the lead halide source and alkylammonium halide are dissolved in a “good” solvent (e.g. highly polar, that dissolves salts), usually dimethylformamide (DMF), and then injected into a “poor” (nonpolar) solvent such as toluene or 1-octadecene (ODE), containing surfactants, usually oleic acid (OA) and oleylamine (OAm). The subsequent formation of perovskite NCs proceeds primarily due to the lower solubility caused by the large amount of a nonpolar solvent. Highly polar DMF is also commonly used as the solvent for preparing supported, layered perovskite nanostructures.44,45
An alternative synthesis route, that is primarily based on ionic metathesis and does not involve polar solvents, was developed by our group for the synthesis of fully inorganic cesium lead halide NCs (CsPbX3 where X = Cl, Br or I).41,42 Cesium oleate as the Cs+ source is injected into a solution of PbX2 in ODE containing long-chain capping ligands (an OAm/OA mixture). PbX2 thus serves as both the Pb2+ and X− source, releasing Pb-oleate as a by-product. In this communication, we extend this ionic metathesis approach to hybrid perovskites, by replacing Cs-oleate with methylamine. The proton needed to form CH3NH3+ is then provided by OA, and the overall reaction is described as follows (see the ESI,† for experimental details; X = Br or I):
CH3NH3PbI3 NCs
To achieve a high overall reaction yield, a minimal amount of the OAm/OA ligand mixture, just sufficient to solubilize PbI2, must be used. The CH3NH2 precursor solution is injected at 55 °C, immediately producing a stable, dark-brown colloid of cubic ∼10 nm NCs. The UV-Vis absorption and photoluminescence (PL) spectra of CH3NH3PbI3 NCs (Fig. 1) exhibit an absorption band edge at ∼750 nm, which is blue-shifted by 30–50 nm compared to the bulk material,10 due to quantum-size effects originating from the large exciton Bohr radius. PL quantum yields are typically 20–30%. Importantly, the powder X-ray diffraction (XRD) pattern can be indexed as pure tetragonal CH3NH3PbI3, with a line-width consistent with a mean particle size of ∼10 nm (Fig. 1c). Higher reaction temperatures of up to 100 °C lead to platelet-like CH3NH3PbI3 NCs (see Fig. S1†). By varying the ratio of OAm to OA, various other shapes such as smaller dot-like NCs and nanowire morphologies can be produced, but with suboptimal shape- and phase-uniformity (see Fig. S1†).CH3NH3PbBr3 NCs
PbBr2 is observed to react slower, yet with nearly quantitative reaction yield. By adjusting the amount of OAm, this novel synthesis selectively yields either blue-emitting square nanoplatelet-like NCs (Fig. 2a, b and e) or green-luminescent nanowires (Fig. 2c, d and f). The XRD patterns can be indexed as pure orthorhombic CH3NH3PbBr3. These 35 ± 5 nm square-shaped nanoplatelets exhibit an absorption peak at ∼450 nm and a single PL emission peak at 465 nm, with a quantum yield (QY) of 18% and a full width at half maximum (FWHM) of 19 nm. CH3NH3PbBr3 nanowires (10 × 100 nm2) exhibit a narrow PL emission peak at 532 nm, with a QY of 15% and a FWHM of 22 nm. Bright emission is also retained in the solid state, as can be seen for thin films of nanoplatelets and nanowires encapsulated between two plastic films and stored in air for several months (Fig. 2e and f, taken under ultraviolet illumination, λ = 365 nm).The formation of layered lead bromide perovskites, closest analogues to our nanoplatelets, was a subject of numerous recent studies.26,28,32,37,46–49 In such structures, optical properties are largely determined by the quantum confinement caused by small thicknesses of just a few nanometers. XRD experiments confirm that the individual colloidal nanoplatelets comprise the perovskite CH3NH3PbBr3 structure and, from TEM, they are ∼5 nm in thickness. Right after synthesis, CH3NH3PbBr3 nanoplatelets form stable colloidal solutions in toluene. TEM samples obtained from highly dilute samples illustrate separated, flat-lying platelets (see Fig. 3). Upon evaporation or simply by storing concentrated dispersions, a light-yellow precipitate of wire-like superlattices is formed by face-to-face stacking of nanoplatelets (see Fig. 3). Oftentimes this self-organization involves the entire ensemble. We hypothesize that these superlattices are, in fact, analogues of Ruddlesden–Popper perovskite phases. Thin layers of CH3NH3PbBr3 are separated by oleylammonium cations, leading to an overall superlattice composition of (oleyl-NH3)2(CH3NH3)n−1PbnBr3n+1, where n is the number of unit cells in the perovskite-like slab whose limits correspond to a fully 3D lattice of CH3NH3PbBr3 (at n = ∞) and a single-layer 2D lattice of (oleyl-NH3)2PbBr4 (at n = 1). XRD reflections at 2θ values of 11.5° (d = 7.68 Å) and 13.44° (d = 6.58 Å) (Fig. 2g), both corresponding to distances larger than the unit cell (5.93 Å), indicate a superlattice spacing of ∼54 Å, fully consistent with TEM data.
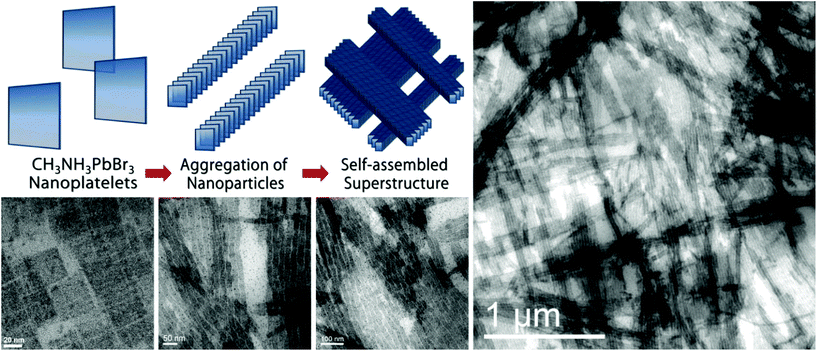 | ||
| Fig. 3 Self-organization of CH3NH3PbBr3 nanoplatelets: from individual platelets to the stacking into wire-like structures, followed by bunching of wire-like superlattices. | ||
From the absorption peak position of ∼450 nm, we estimate a value of n = 3 based on previous studies of layered lead bromide perovskites.27,45 The inter-slab distance of 54 Å can thus be interpreted as the sum of two oleylamine molecules and three unit cells of a perovskite slab. Note that bulk, fully 3-dimensional CH3NH3PbBr3 is luminescent with PL at ∼570 nm,10 while fully 2-dimensional (RNH3)2PbBr4 compounds emit at 400–420 nm.44
Stability and durability of CH3NH3PbX3 NCs
Colloidal lead halide perovskites are much more chemically labile than more conventional colloidal quantum dots of metal chalcogenides.50 The comparison of CH3NH3PbX3 NCs against CsPbX3 NCs (as in our recent study41) demonstrates the much lower stability of the former. All characterization presented in this communication concerning CH3NH3PbX3 NCs was conducted on rather “dirty” samples, as for example those precipitated from the crude solution and redispersed in toluene. Additional purification steps, conducted either in air or under an inert-gas atmosphere, had always led to decomposition of the NCs. Non-purified samples, in contrast, can maintain their bright PL for weeks or months after synthesis.Clearly, a large excess of capping ligands is required to maintain the chemical integrity of CH3NH3PbX3 NCs. Humidity is a factor contributing to the severe instability of CH3NH3PbX3,51 causing the release of CH3NH2 or PbI2, or even the formation of (CH3NH3)4PbI6·2H2O.52 Satisfactory retention of the chemical integrity of MAPbX3 NCs in the presence of an excess of ligands, despite inherently high surface-to-volume ratios, can be ascribed to the highly hydrophobic nature of the capping layer. Another source of instability is the low energy of formation of CH3NH3PbX3 compounds,53 leading to slow irreversible dissociation into CH3NH3X or CH3NH2 + HX, with all volatile products in either case, and PbX2. A ligand-passivated surface may inhibit this decomposition, explaining the satisfactory stability of CH3NH3PbBr3 NCs in a “dirty” state. The low melting point of hybrid and fully inorganic perovskite NCs leads to facile sintering in their solid state films, oftentimes readily observed in TEM images.
Stimulated emission from CH3NH3PbBr3 NCs
Upon pulsed excitation (400 nm, 100 fs), a low-threshold amplified spontaneous emission (ASE) is observed from a film of CH3NH3PbBr3 NCs on a glass substrate (Fig. 4). The ASE band is spectrally different from the PL emission: it has a narrower bandwidth of 7–9 nm (compared to the PL FWHM of 20 nm), and is red-shifted by almost 20 nm with respect to the PL maximum, presumably owing to the bi-excitonic nature of the optical gain.34,37 The threshold for building ASE is ∼3 ± 1 μJ cm−2, a factor of 2–3 lower than for CsPbBr3 NCs,54 when emitting at the nearly same wavelengths. However, an interesting difference is observed with regard to the emission relaxation. The 240 ns PL lifetime of CH3NH3PbBr3 NCs is notably longer than the 5 ns lifetime of CsPbBr3 NCs, both in solution and under similar excitation intensities. For CsPbBr3 NCs, PL lifetime is invariant in solution and fluency-dependent in compact films (Fig. S2†). This can be explained by exciton–exciton interactions, leading to faster relaxation with increasing pumping fluency. For CH3NH3PbBr3 nanowires, such behaviour is already seen in solution and may be explained by the co-existence of multiple excitons within the individual nanowire.In summary, in this report we describe a new solution-phase synthesis of highly-luminescent CH3NH3PbX3 (X = Br and I) nanocrystals with cube, wire and platelet morphologies. We have shown that polar solvents such as DMF can be completely eliminated from the synthesis strategy, thus allowing the nucleation and growth to occur in a fully homogeneous medium. We observed self-organization of CH3NH3PbBr3 nanoplatelets into wire-like superlattices. We also assessed lasing properties of CH3NH3PbBr3 nanowires and observed low thresholds for ASE. On the practical side, we note that hybrid organic–inorganic perovskite nanostructures suffer from severe instability and cannot easily be isolated in a purified state without decomposition. Hence we suggest that future strategies on their synthesis and applications should focus on the development of surface passivation with multi-dentate ligands that do not desorb during purification or on their efficient encapsulation into polymer or inorganic matrices.
Acknowledgements
This work was supported by the European Comission via the Marie-Sklodowska Curie action Phonsi (H2020-MSCA-ITN-642656) and by the ERC Starting Grant NANOSOLID (GA no. 306733). We thank Prof. Manfred Fiebig and his research group for granting us access to their femtosecond laser apparatus and for technical assistance, and Dr Nicholas Stadie for reading the manuscript. We also acknowledge the support of the Scientific Center for Optical and Electron Microscopy (ETH Zürich) and the Empa Electron Microscopy Center.Notes and references
- M. Gratzel, Nat. Mater., 2014, 13, 838–842 CrossRef CAS PubMed.
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS.
- N. G. Park, J. Phys. Chem. Lett., 2013, 4, 2423–2429 CrossRef CAS.
- H. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542–546 CrossRef CAS PubMed.
- I. Chung, B. Lee, J. He, R. P. Chang and M. G. Kanatzidis, Nature, 2012, 485, 486–489 CrossRef CAS PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Gratzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS PubMed.
- W. Nie, H. Tsai, R. Asadpour, J. C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak, M. A. Alam, H. L. Wang and A. D. Mohite, Science, 2015, 347, 522–525 CrossRef CAS PubMed.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Science, 2015, 347, 967–970 CrossRef CAS PubMed.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed.
- F. Zhang, H. Zhong, C. Chen, X. G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. Maculan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nat. Commun., 2015, 6, 7586, DOI:10.1038/ncomms8586.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982 CAS.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- L. Dou, Y. M. Yang, J. You, Z. Hong, W. H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5, 5404 CrossRef CAS PubMed.
- M. I. Saidaminov, V. Adinolfi, R. Comin, A. L. Abdelhady, W. Peng, I. Dursun, M. Yuan, S. Hoogland, E. H. Sargent and O. M. Bakr, Nat. Commun., 2015, 6, 8724, DOI:10.1038/ncomms9724.
- Y. Fang, Q. Dong, Y. Shao, Y. Yuan and J. Huang, Nat. Photonics, 2015, 9, 679–686 CrossRef CAS.
- G. Maculan, A. D. Sheikh, A. L. Abdelhady, M. I. Saidaminov, M. A. Haque, B. Murali, E. Alarousu, O. F. Mohammed, T. Wu and O. M. Bakr, J. Phys. Chem. Lett., 2015, 6, 3781–3786 CrossRef CAS.
- Y. L. Guo, C. Liu, H. Tanaka and E. Nakamura, J. Phys. Chem. Lett., 2015, 6, 535–539 CrossRef CAS PubMed.
- S. Yakunin, M. Sytnyk, D. Kriegner, S. Shrestha, M. Richter, G. J. Matt, H. Azimi, C. J. Brabec, J. Stangl, M. V. Kovalenko and W. Heiss, Nat. Photonics, 2015, 9, 444–449 CrossRef CAS.
- Z. K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687–692 CrossRef CAS PubMed.
- O. A. Jaramillo-Quintero, R. S. Sanchez, M. Rincon and I. Mora-Sero, J. Phys. Chem. Lett., 2015, 6, 1883–1890 CrossRef CAS PubMed.
- Y. H. Kim, H. Cho, J. H. Heo, T. S. Kim, N. Myoung, C. L. Lee, S. H. Im and T. W. Lee, Adv. Mater., 2015, 27, 1248–1254 CrossRef CAS PubMed.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- E. R. Dohner, A. Jaffe, L. R. Bradshaw and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 13154–13157 CrossRef CAS PubMed.
- J. A. Sichert, Y. Tong, N. Mutz, M. Vollmer, S. Fischer, K. Z. Milowska, R. García Cortadella, B. Nickel, C. Cardenas-Daw, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Nano Lett., 2015, 15, 6521–6527 CrossRef CAS PubMed.
- S. T. Ha, X. Liu, Q. Zhang, D. Giovanni, T. C. Sum and Q. Xiong, Adv. Opt. Mater., 2014, 2, 838–844 CrossRef CAS.
- L. C. Schmidt, A. Pertegas, S. Gonzalez-Carrero, O. Malinkiewicz, S. Agouram, G. Minguez Espallargas, H. J. Bolink, R. E. Galian and J. Pérez-Prieto, J. Am. Chem. Soc., 2014, 136, 850–853 CrossRef CAS PubMed.
- D. Di, K. P. Musselman, G. Li, A. Sadhanala, Y. Ievskaya, Q. Song, Z. K. Tan, M. L. Lai, J. L. MacManus-Driscoll, N. C. Greenham and R. H. Friend, J. Phys. Chem. Lett., 2015, 6, 446–450 CrossRef CAS PubMed.
- Y. Fu, F. Meng, M. B. Rowley, B. J. Thompson, M. J. Shearer, D. Ma, R. J. Hamers, J. C. Wright and S. Jin, J. Am. Chem. Soc., 2015, 137, 5810–5818 CrossRef CAS PubMed.
- S. Gonzalez-Carrero, G. M. Espallargas, R. E. Galian and J. Perez-Prieto, J. Mater. Chem. A, 2015, 3, 14039–14045 CAS.
- S. Gonzalez-Carrero, R. E. Galian and J. Pérez-Prieto, J. Mater. Chem. A, 2015, 3, 9187–9193 CAS.
- S. González-Carrero, R. E. Galian and J. Pérez-Prieto, Part. Part. Syst. Charact., 2015, 32, 709–720 CrossRef.
- H. Huang, A. S. Susha, S. V. Kershaw, T. F. Hung and A. L. Rogach, Adv. Sci., 2015, 2, 1500194 Search PubMed.
- D. M. Jang, K. Park, D. H. Kim, J. Park, F. Shojaei, H. S. Kang, J. P. Ahn, J. W. Lee and J. K. Song, Nano Lett., 2015, 15, 5191–5199 CrossRef CAS PubMed.
- P. Tyagi, S. M. Arveson and W. A. Tisdale, J. Phys. Chem. Lett., 2015, 6, 1911–1916 CrossRef CAS PubMed.
- A. B. Wong, M. Lai, S. W. Eaton, Y. Yu, E. Lin, L. Dou, A. Fu and P. Yang, Nano Lett., 2015, 15, 5519–5524 CrossRef CAS PubMed.
- F. Zhu, L. Men, Y. Guo, Q. Zhu, U. Bhattacharjee, P. M. Goodwin, J. W. Petrich, E. A. Smith and J. Vela, ACS Nano, 2015, 9, 2948–2959 CrossRef CAS PubMed.
- S. Zhuo, J. Zhang, Y. Shi, Y. Huang and B. Zhang, Angew. Chem., Int. Ed., 2015, 54, 5693–5696 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- L. Dou, A. B. Wong, Y. Yu, M. Lai, N. Kornienko, S. W. Eaton, A. Fu, C. G. Bischak, J. Ma, T. Ding, N. S. Ginsberg, L. W. Wang, A. P. Alivisatos and P. Yang, Science, 2015, 349, 1518–1521 CrossRef CAS PubMed.
- Y. Tabuchi, K. Asai, M. Rikukawa, K. Sanui and K. Ishigure, J. Phys. Chem. Solids, 2000, 61, 837–845 CrossRef CAS.
- M. Era, S. Morimoto, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1994, 65, 676 CrossRef CAS.
- T. Kondo, T. Azuma, T. Yuasa and R. Ito, Solid State Commun., 1998, 105, 253–255 CrossRef CAS.
- G. Lanty, K. Jemli, Y. Wei, J. Leymarie, J. Even, J. S. Lauret and E. Deleporte, J. Phys. Chem. Lett., 2014, 5, 3958–3963 CrossRef CAS PubMed.
- D. B. Mitzi, K. Chondroudis and C. R. Kagan, IBM J. Res. Dev., 2001, 45, 29–45 CrossRef CAS.
- D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2009, 110, 389–458 CrossRef PubMed.
- G. Niu, X. Guo and L. Wang, J. Mater. Chem. A, 2015, 3, 8970–8980 CAS.
- A. Halder, D. Choudhury, S. Ghosh, A. S. Subbiah and S. K. Sarkar, J. Phys. Chem. Lett., 2015, 6, 3180–3184 CrossRef CAS.
- Y.-Y. Zhang, S. Chen, P. Xu, H. Xiang, X.-G. Gong, A. Walsh and S.-H. Wei, 2015, arXiv preprint arXiv:1506.01301.
- S. Yakunin, L. Protesescu, F. Krieg, M. I. Bodnarchuk, G. Nedelcu, M. Humer, G. De Luca, M. Fiebig, W. Heiss and M. V. Kovalenko, Nat. Commun., 2015, 6, 8056, DOI:10.1038/ncomms9056.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, additional figures. See DOI: 10.1039/c5nr06890h |
| This journal is © The Royal Society of Chemistry 2016 |




