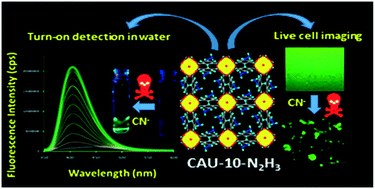
A hydrazine-functionalized, highly stable Al(III) based metal–organic framework (MOF) with CAU-10 (CAU = Christian-Albrechts-University) framework topology namely CAU-10-N2H3 (1) was specifically designed to detect lethal CN− ions in aqueous medium. The MOF was characterized by X-ray powder diffraction, infrared spectroscopy, gas sorption and thermogravimetric analyses. The isophthalate ligand in CAU-10 was functionalized by the hydrazine group to make the acidic –NH protons easily available to the CN− ions. Indeed, the activated compound (1′) showed highly selective and sensitive responses to CN− ions over other common anions with a detection limit of 0.48 μM. A rapid fluorescence enhancement was observed for 1′ with a large spectral shift (Δλ = 30 nm) in the presence of CN− ions in aqueous solution with the development of visible green fluorescence under a UV lamp. Due to the excellent detection performance to CN− by 1′ in aqueous medium, the material was further used for CN− detection in real water samples. The fluorescence increment with a large blue shift is attributed to the cyanide-induced deprotonation of the –NH group, which was confirmed by 1H NMR titration measurements. The initial photo-induced electron transfer (PET) process is prohibited by this deprotonation, which causes the fluorescence enhancement. Time-resolved fluorescence lifetime measurements suggest that 1′ can also act as a lifetime based CN− sensor. Finally, the CN− sensing ability of 1′ inside living RAW 264.7 macrophages was demonstrated through live-cell imaging investigations.