 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Sodium cluster-driven safety concerns of sodium-ion batteries
Jiaping
Niu
ab,
Junyuan
Dong
ab,
Xiaohu
Zhang
a,
Lang
Huang
*ac,
Guoli
Lu
a,
Xiaolei
Han
a,
Jinzhi
Wang
a,
Tianyu
Gong
a,
Zheng
Chen
ac,
Jingwen
Zhao
*ac and
Guanglei
Cui
*abc
aQingdao New Energy Shandong Laboratory, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China. E-mail: huanglang@qibebt.ac.cn; zhaojw@qibebt.ac.cn; cuigl@qibebt.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
cShandong Energy Institute, Qingdao 266101, China
First published on 11th March 2025
Abstract
Correction for ‘Sodium cluster-driven safety concerns of sodium-ion batteries’ by Jiaping Niu et al., Energy Environ. Sci., 2025, 18, 2474–2484, https://doi.org/10.1039/D4EE05509H.
An error with the display of Fig. 3b caused the y-axis of this plot to be missing. Fig. 3 should appear as follows.
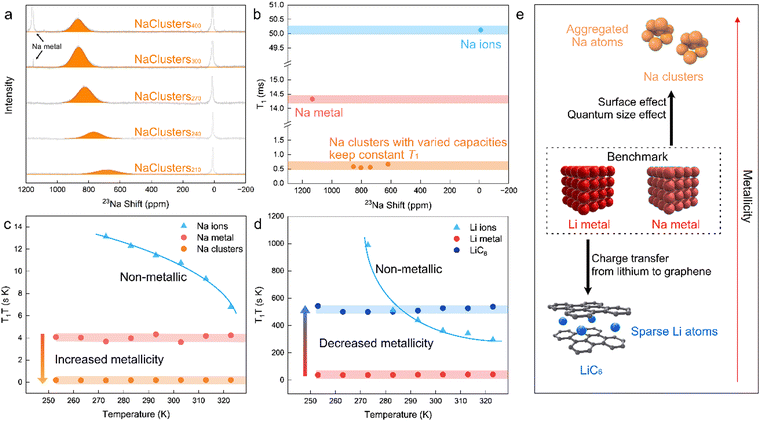 | ||
| Fig. 1 Metallicity of sodium clusters in HC compared to sodium metal. (a) ssNMR curves of four sodiated HC materials with capacities of 210, 240, 270 and 300 mA h g−1. (b) Spin–lattice relaxation rates of sodium metal, sodium clusters (sodiated HC with varying capacities in Fig. 3a) and sodium ions. Comparison of the Korringa product of the spin–lattice relaxation time with the temperature as a function of temperature for (c) sodium metal, sodium clusters, sodium ions as well as (d) lithium metal, LiC6, and lithium ions. (e) Schematic of Li and Na metallicity trends with distinct microenvironments. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2025 |
