A unimolecular artificial cation channel based on cascaded hydrated acid groups†
Pengyang
Xin
 *,
Hailong
Yuan
,
Long
Zhang
,
Qiuhui
Zhu
,
Xunpeng
Ning
,
Yufei
Song
,
Yuqing
Shu
and
Yonghui
Sun
*
*,
Hailong
Yuan
,
Long
Zhang
,
Qiuhui
Zhu
,
Xunpeng
Ning
,
Yufei
Song
,
Yuqing
Shu
and
Yonghui
Sun
*
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, NMPA Key Laboratory for Research and Evaluation of Innovative Drug, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007, China. E-mail: pyxin27@163.com; syonghui1994@163.com
First published on 7th October 2024
Abstract
A cation channel possessing cascaded hydrated acid groups has been successfully constructed using pillar[5]arene integrated with dual cyclodextrins. As a proof-of-concept, the secondary side of cyclodextrin substituted by 24 –CO2H groups presents high coordination sites, which helps hydrated cations to quickly dehydrate and accelerates efficient cation transport (Rb+ > Cs+ > K+ > Na+ > Li+). Notably, benefitted by the protonation and deprotonation of –CO2H groups, ion permeation activity of the channel molecules under acidic condition (pH = 6.0) is 2.8 times higher than that under alkaline conditions (pH = 8.0), exhibiting pH-modulated property and promising potential in building intelligent artificial ion channels with customized features.
Introduction
A balance of intracellular and extracellular ion homeostasis is crucial for achieving various vital physiological functions, most of which are exquisitely regulated by channel proteins on the cell membrane.1–3 A profound understanding of the structural conformation and function of these biomolecules enable us to elucidate the detailed mechanism of transport and structure–activity relationships. Generally, ion selectivity of the channel proteins is determined by the selectivity filter located in the central and narrow region of channel protein subunits.4 For example, KcsA (K+ channel) has a four-layered tetrameric structure lined by eight backbone carbonyl ligands at each site. These closely spaced carbonyl sites enable the cascade dehydration of K+.5 Similarly, acidic side chains of the highly conserved amino acid residues at the upper and lower ports of the Nav channel exhibit strong electronegativity, and this unique structure contributes to the cascading dehydration of hydrated cations and accelerates efficient cation transport.6 Recently, inspired by natural channel protein structures, chemists are devoted to developing biomimetic artificial ion transporters to simulate the biological functions of natural ion channels.7–15 More recently, Talukdar and colleagues presented a barrel-rosette ion channel containing hydrogen-bond and lone-pair donating groups within the channel cavity, showing prominent ion permeation activity across the bilayer.16 Barboiu and co-workers reported transmembrane ion channels made up of amphiphilic peptide-oligourea chimeric helices, which can yield unique bioactivity due to ion binding within the channels.17 The above example shows cascaded multi-binding sites that seem to be the key to ion permeation. Significant progress has been made in the field of synthetic transporters. Nevertheless, devising artificial transporters with high ion permeation activity, similar to natural channel proteins undergoing cascaded dehydration, still represents a daunting task, which not only deepens our understanding of ion transmembrane transport but also opens up a path for the application of artificial transporters in medicine, materials, and catalysis.In recent years, our group has become interested in the unimolecular artificial channels constructed using disulfide-bridged cyclodextrin dimer, cyclodextrin-grafted functional peptides and cyclodextrin-pillar[5]arene hybrid molecule, which could achieve high channel structure stability and ion transport function, and this approach has been extended to redox-regulated channels.18–20 Actually, previous studies have proved that a unimolecular channel with cyclodextrin or pillararene motif would be better choices for the design and fabrication of an artificial transporter.21–23 Taking inspiration from the cyclodextrin-pillar[5]arene hybrid structure, the modifiability of the secondary side of cyclodextrin provides the possibility for constructing a functional ion transporter. We envision that the introduction of multiple hydrated acid groups could simulate the cascade dehydration properties of the natural channels. Herein, we proposed a new ion transporter, defined as a cascaded hydrated acid groups molecular tube (Scheme 1), which carries 24 –CO2H-modified α-cyclodextrins on pillar[5]arene periphery through a click reaction and exhibits excellent selectivity for alkali metal ions and pH regulation properties. As a proof-of-concept, molecular tube design has the following characteristics: (i) the carboxyl group at the secondary side of cyclodextrin can amplify the cation binding affinity in the solution; (ii) numerous carboxyl groups are distributed at the upper and lower ends of the channel, which can enhance the dehydration ability during the ion permeation stage, resulting in the high selectivity for cations, which we call cascade dehydration; (iii) the pillararene portion of the channel can endow cations with high permeability.
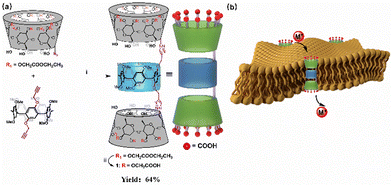 | ||
| Scheme 1 (a) The synthesis route of molecule 1: (i) CuSO4·5H2O, sodium ascorbate, DMSO, r.t.; (ii) NaOH, MeOH/H2O, r.t. (b) Schematic diagram of molecule 1 inserted into the lipid bilayer. | ||
Results and discussion
The synthesis of channel molecule 1 is presented in Scheme 1. Bialkynyl-pillar[5]arene was prepared according to the literature20 and then 1 was obtained by the click reaction with ester-functionalized mono-6-azido-6-deoxy-α-cyclodextrin and further deprotecting the ester group. All compounds were characterized by NMR spectroscopy, mass spectroscopy and HPLC (Fig. S1–S20, ESI†). The ion-transport activity of channel molecule 1 was assessed using the pH-sensitive fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS), which was encapsulated within the egg yolk phosphatidylcholine (EYPC) liposomes (pH = 7.0) (Fig. 1a). As the concentration of channel molecule 1 in the liposome suspension increased, the concentration-dependent increase in the fluorescence intensity of HPTS established under the pH gradient (the extravesicular pH = 6.0) of unilamellar vesicles was observed (Fig. 1b), indicating that they were inserted into the lipid bilayer and mediated ion transmembrane transport. Hill analysis of the dose–response curve showed that the EC50 (effective concentration of 1 to obtain 50% activity) of 1 was determined to be 0.76 ± 0.08%, revealing a nonlinear concentration–activity relationship with a Hill coefficient of 0.87 (Fig. 1c). The Hill coefficient (n), n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
< ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, represents that 1 form a unimolecular channel. These assays were also evaluated using EYPC in the buffer solution with the intravesicular and extravesicular environment at pH = 7.0 and pH = 8.0, respectively (Fig. S21 and S22, ESI†). The results showed 2.8 times higher EC50 value of 1 (EC50 = 1.89
1, represents that 1 form a unimolecular channel. These assays were also evaluated using EYPC in the buffer solution with the intravesicular and extravesicular environment at pH = 7.0 and pH = 8.0, respectively (Fig. S21 and S22, ESI†). The results showed 2.8 times higher EC50 value of 1 (EC50 = 1.89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.48%, n
0.48%, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.61) than that under the acidic condition (the extravesicular pH = 6.0), implying that 1 probably has pH-modulated property because of its –CO2H groups.
0.61) than that under the acidic condition (the extravesicular pH = 6.0), implying that 1 probably has pH-modulated property because of its –CO2H groups.
The pH-modulated properties of molecule 1 inspire us to further explore its ion-transport activity at different pH values. HPTS assays demonstrated the ion-transport activity of 1 in EYPC liposome suspension (the intravesicular pH = 7.0, the extravesicular pH = 4.0, pH = 5.0, pH = 6.0, pH = 8.5, pH = 10, respectively) under different pH gradients by continuously monitoring the fluorescence intensity for 6.5 minutes. As shown in Fig. 2, the fluorescence intensity in the acidic environment (pH = 6.0, pH = 5.0, pH = 4.0) is much stronger than that in an alkaline environment (pH = 8.5, pH = 10), indicating that molecule 1 has excellent ionophoric capacity in acidic milieu and higher ion transmembrane transport activities under acidic conditions rather than alkaline conditions, which is probably because the deprotonation of molecule 1 changes its amphiphilicity under alkaline conditions and thus the inability to form transmembrane ion channels.
 | ||
| Fig. 2 (a) Schematic representation of HPTS assay at different pH values (pHin = 7.0, pHout = 4.0, 5.0, 6.0, 8.5, 10.0). (b) The ion transport activity of molecule 1 at different pH gradients. | ||
Then, the alkali-metal ions transport ability of 1 was evaluated by the fluorescent assay based on HPTS (Fig. S23 ESI†). In this assay, large unilamellar vesicles (LUVs) were fabricated by trapping HEPES buffer (10 mM, pH = 7), 100 mM MCl (M+ = Li+, Na+, K+, Rb+, Cs+), and HPTS. The extravesicular environment was set using 100 mM NaCl and HEPES buffer (10 mM, pH = 6). As shown in Fig. 3, the HPTS assay revealed that molecule 1 is able to transport alkali-metal ions across the LUV membrane. The transport activity of 1 decreased in the order Rb+ > Cs+ > K+ > Na+ > Li+, corresponding to the Eisenman sequence II. The above results indicate that molecule 1 allows the carboxyl terminal groups of the channel to directly interact with and coordinate the permeating ions, which realize the cascaded dehydration of cations similar to natural channels, promoting the efficient transport of cations.
In order to elucidate the transport mechanisms of molecule 1, we employed three strategies for validation, including SPQ (Cl− sensitive dye) assay, HPTS assay based on FCCP ((carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, an H+ transport carrier) and VA (Valinomycin, a K+ selective carrier). As shown in Fig. 4a, establishing a concentration gradient of chloride ions inside and outside the LUVs, the addition of molecule 1 did not cause significant fluorescence changes in SPQ (9%) compared to the blank (5%), indicating that Cl− cannot be transported through molecule 1, thus excluding the possibility of H+/Cl− symport and Cl−/OH− antiport serving as the ion permeation mechanism. As depicted in Fig. 4b, the simultaneous introduction of both 1 and FCCP into the LUVs suspension resulted in a significant increase of 82% in the fluorescence intensity of HPTS, which was 23% and 71% higher than using molecule 1 (59%) and FCCP (11%) independently, indicating that cations as the main transport ion are higher than that of H+ or OH− transport rates. The VA-HPTS assay scheme is described in Fig. 4c. The fluorescence intensity of HPTS in the presence or absence of VA after the addition of molecule 1 was not significantly different, only 3%, which is nearly the same as the 4% difference between VA alone (7%) and the blank (3%). This result indicates that the transmembrane transport rate of K+ is much faster than the transport rate of H+ or OH−.
Planar bilayer conductance was also conducted to verify the transport mechanism and membrane behaviour (Fig. S24 and S25 ESI†). A planar lipid bilayer composed of diphytanoylphosphatidylcholine separates two chambers containing a 1.0 M KCl solution. The addition of molecule 1 to the cis chamber, followed by multiple voltage levels (+100, +80, −80 and −100 mV), were applied to the membrane, and regular square-like single channel currents were observed (Fig. 5a–d). As shown in Fig. 5e, the current voltage (I–V) curves display a linear relationship under different voltages within the range from −100 to +100 mV in the presence of molecule 1, and the corresponding conductance (γ) of 1 was calculated as 16.1 ± 0.2 pS, indicating that molecule 1 can be incorporated into the lipid bilayers and mediated effective cation transport through the channel mechanism. Furthermore, we tested the K+/Cl− selectivity of molecule 1 in asymmetrical KCl solutions (Fig. S26 in ESI†). Based on the corresponding I–V curve, we used the Goldman–Hodgkin–Katz equation to determine the permeability ratio (P) for PK+/PCl−, which was 3, demonstrating that molecule 1 can serve as the cation channel in the lipid bilayer, consistent with the HPTS assay results. The cation transport selectivity of molecule 1 is attributed to the cascaded hydrated acid groups on both sides of molecule 1; multi carboxyl side chains help the cations to quickly cascade dehydrate and promote efficient cation transport.
Conclusions
In summary, we designed an amphiphilic unimolecular cation channel 1 based on the idea of cascaded dehydration by introducing cascaded hydrated acid groups on both sides of the hybrid molecules. Through vesicle-based HPTS assay and patch-clamp experiments, it was found that molecule 1 not only exhibits excellent membrane binding ability but also can mediate effective cation transmembrane transport. More importantly, the transmembrane transport ability of molecule 1 has pH-modulated property due to the presence of multi carboxyl groups. This strategy of simulating natural channel cascade dehydration at the molecular level provides a good explanation for the cation permeation pathway, providing inspiration for the development of artificial cation channels and has potential applications in the treatment of channelopathies.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from the National Natural Science Foundation of China (22271079, 82130103), Research Project from Pingyuan Laboratory (2023PY-ZZ-0201), Special Project for Fundamental Research in the University of Henan Province (21ZX005), Central Plains Scholars and Scientists Studio Fund (2018002) and Henan Normal University Research Foundation for the Doctoral Program (QD2023058). We also acknowledge financial support from the Henan Key Laboratory of Organic Functional Molecules and Drug Innovation.References
- J. Eggermont, D. Trouet, I. Carton and B. Nilius, Cell Biochem. Biophys., 2001, 35, 263 CrossRef.
- A. Thiemann, S. Gründer, M. Pusch and T. J. Jentsch, Nature, 1992, 356, 57 CrossRef.
- H. E. Kato, F. Zhang, O. Yizhar, C. Ramakrishnan, T. Nishizawa, K. Hirata, J. Ito, Y. Aita, T. Tsukazaki, S. Hayashi, P. Hegemann, A. D. Maturana, R. Ishitani, K. Deisseroth and O. Nureki, Nature, 2012, 482, 369 CrossRef.
- M. J. Ryan, L. Gao, F. I. Valiyaveetil, A. A. Kananenka and M. T. Zanni, J. Am. Chem. Soc., 2024, 146, 1543 CrossRef.
- J. Li, L. Du, X. Kong, J. Wu, D. Lu, L. Jiang and W. Guo, Natl. Sci. Rev., 2023, 10, nwad260 CrossRef.
- T. Dudev and C. Lim, Acc. Chem. Res., 2014, 47, 3580 CrossRef.
- N. Li, F. Chen, J. Shen, H. Zhang, T. Wang, R. Ye, T. Li, T. P. Loh, Y. Y. Yang and H. Zeng, J. Am. Chem. Soc., 2020, 142, 21082 CrossRef PubMed.
- Y. Lin, B. Wu, Y. Zeng, H. Yuan, C. Ji, Z. Liu, X. Kong, Y. Sui, T. Yin, Y. Zhu, J. Chen and C. Lang, Angew. Chem., Int. Ed., 2024, 63, e202408558 CrossRef.
- M. Ahmad, T. G. Johnson, M. Flerin, F. Duarte and M. J. Langton, Angew. Chem., Int. Ed., 2024, 63, e202403314 CrossRef.
- I. A. Rather, M. Ahmad, P. Talukdar and R. Ali, J. Mater. Chem. B, 2024, 12, 5950 RSC.
- N. Shao, L. Yuan, L. Liu, Z. Cong, J. Wang, Y. Wu and R. Liu, J. Am. Chem. Soc., 2024, 146, 11254 Search PubMed.
- I. M. Andrei, A. Chaix, B. T. Benkhaled, R. Dupuis, C. Gomri, E. Petit, M. Polentarutti, A. van der Lee, M. Semsarilar and M. Barboiu, J. Am. Chem. Soc., 2023, 145, 21213 CrossRef PubMed.
- A. V. Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791 CrossRef PubMed.
- M. Barboiu and A. Gilles, Acc. Chem. Res., 2013, 46, 2814 CrossRef PubMed.
- R. D. Mukhopadhyay, Y. Kim, J. Koo and K. Kim, Acc. Chem. Res., 2018, 51, 2730 CrossRef PubMed.
- S. Chattopadhayay, A. Ghosh, T. K. Mukhopadhyay, R. Sharma, A. Datta and P. Talukdar, Angew. Chem., Int. Ed., 2023, 62, e202313712 CrossRef PubMed.
- C. Dutta, P. Krishnamurthy, D. Su, J. Li, S. H. Yoo, G. W. Collie, M. Pasco, J. Fan, M. Luo, M. Barboiu, G. Guichard, R. M. Kini and P. Kumar, Small Sci., 2024, 2300352 CrossRef CAS.
- P. Xin, L. Xu, W. Dong, L. Mao, J. Guo, J. Bi, S. Zhang, Y. Pei and C. P. Chen, Angew. Chem., Int. Ed., 2023, 62, e202217859 CrossRef CAS PubMed.
- L. Shi, W. Zhao, Z. Jiu, J. Guo, Q. Zhu, Y. Sun, B. Zhu, J. Chang and P. Xin, Angew. Chem., Int. Ed., 2024, 63, e202403667 CrossRef CAS PubMed.
- P. Xin, H. Kong, Y. Sun, L. Zhao, H. Fang, H. Zhu, T. Jiang, J. Guo, Q. Zhang, W. Dong and C. P. Chen, Angew. Chem., Int. Ed., 2019, 58, 2779 CrossRef CAS.
- Y. H. Fu, Y. F. Hu, T. Lin, G. W. Zhuang, Y. L. Wang, W. X. Chen, Z. T. Li and J. L. Hou, Nat. Chem., 2024, 16, 1418 CrossRef CAS.
- P. Zhu, L. Kong, Y. Zhang, Q. Liu, X. Liao, Y. Song and B. Yang, Chin. Chem. Lett., 2023, 372, 121198 CAS.
- L. Chen, Y. Ren, F. Fan, T. Wu, Z. Wang, Y. Zhang, J. Zhao and G. Cui, Chin. Chem. Lett., 2023, 574, 233081 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01508h |
| This journal is © The Royal Society of Chemistry 2024 |




