Nonafluorobutyl ether enhancing the stability of fluorobenzene-based diluted high-concentration electrolytes in high-voltage lithium metal batteries†
Xinlan
Wang
ab,
Ziqi
Zeng
*a,
Han
Zhang
a,
Yixuan
Dong
a,
Shijie
Cheng
a and
Jia
Xie
 *a
*a
aState Key Laboratory of Advanced Electromagnetic Technology, School of Electrical and Electronic Engineering, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: ziqizeng@hust.edu.cn; xiejia@hust.edu.cn
bState Key Laboratory of Materials Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
First published on 30th April 2024
Abstract
The development of stable electrolytes for high-voltage lithium metal batteries (LMBs) is crucial for advancing battery technology. Diluted high-concentration electrolytes (DHCEs) have shown promise in enhancing interfacial stability, yet challenges persist due to the thermodynamic instability associated with conventional hydrofluoroether diluents and the interphase issues of monofluoroaromatic hydrocarbon diluents under high voltage conditions. In this study, we propose the incorporation of methyl 1,1,2,2,3,3,4,4,4-nonafluorobutyl ether (CNFE) as an additive to fluorobenzene (FB)-based DHCEs for high-voltage LMBs. CNFE with a molecular structure devoid of β-hydrogen guarantees the thermodynamic stability of FB-based DHCEs compared to conventional hydrofluoroether-based DHCEs. Moreover, CNFE promotes the formation of a LiF-rich and stable solid electrolyte interphase (SEI) and cathode electrolyte interphase (CEI). Consequently, a Li‖LiNi0.8Co0.1Mn0.1O2 (NCM811) cell demonstrates a remarkable capacity retention of 84.8% in the 700th cycle at a high cutoff voltage of 4.4 V.
Introduction
The quest for advancements in high energy density batteries has emerged as a critical imperative as conventional lithium-ion batteries (LIBs) approach their theoretical limit.1,2 The augmentation of energy density in LIBs hinges upon the exploitation of electrode materials with superior specific capacities and the elevation of battery operating voltage.3,4 Lithium metal batteries (LMBs), particularly those integrating high-voltage cathodes, hold promise in surpassing 400 W·h kg−1 energy density.5,6 Among the various high-voltage cathodes, the nickel-rich layered material LiNixMnyCo1−x−yO2 (NMC) stands out due to its notable theoretical capacity of approximately 280 mA h g−1 as well as its elevated operating voltage compared to Li/Li+ at around 4 V.7–9 However, challenges persist due to interface instability stemming from aggressive electrolytes,10–12 limiting the longevity of LMBs despite significant efforts aimed at resolving interface issues through electrolyte engineering,13,14 interface engineering,15,16 collector optimization,17,18 separator modification,19,20 and Li metal anode alloying.21–23One of the most effective strategies for addressing interface instability in high-voltage LMBs involves designing novel electrolyte systems to establish a stable solid electrolyte interphase (SEI) as well as cathode electrolyte interphase (CEI).24 Previous studies have explored various approaches, such as high-concentration electrolytes (HCEs),25 diluted high-concentration electrolytes (DHCEs),26,27 fluorinated solvents,28,29 additives,30,31 and salts.32,33 Among them, HCEs and DHCEs have garnered attention because of their ability to enhance interfacial stability by minimizing the presence of free solvent molecules, a departure from conventional dilute electrolytes.34–38 This unique solvation structure inhibits solvent degradation and facilitates preferential decomposition of anions, resulting in the creation of a fluorinated SEI.39,40 Despite their advantages, DHCEs, typically comprising highly fluorinated diluents like 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether (D2) and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE), are susceptible to β-hydrogen (β-H) elimination reactions, yielding hydrogen fluoride (HF) and deteriorating electrolyte.41–45 Our recent study demonstrates that the application of a DHCE with fluorobenzene (FB) as a diluent enhances SEI stability, yet challenges remain in forming a LiF-rich CEI at elevated voltages, necessitating the development of novel DHCEs with improved thermal stability and interphase formation capabilities.46–48 Previously, Arai has reported methyl 1,1,2,2,3,3,4,4,4-nonafluorobutyl ether (CNFE) as a diluent exhibiting characteristics of low viscosity, low melting point, low surface tension, and excellent electrochemical stability for lithium ion battery electrolytes.49 Furthermore, it is noteworthy that CNFE lacks β-H in its molecular structure, suggesting its potential for excellent thermal stability.
Hence, we propose the incorporation of CNFE as an additive (denoted as FB-CNFE-DHCE) to enhance the film-forming performance and antioxidant stability of FB-DHCE. Meanwhile, the unique structure of CNFE lacking β-H enables the electrolyte to exhibit excellent thermal stability and effectively addresses the issue of HF generation in conventional fluorinated ethers. Consequently, Li‖Cu cells using FB-CNFE-DHCE exhibit a superior coulombic efficiency (CE) of 99.1% over 350 cycles. Additionally, the Li‖LiNi0.8Co0.1Mn0.1O2 (NCM811) cell demonstrates an exceptional specific capacity retention of 84.8% after undergoing 700 cycles at 4.4 V. Besides, the cell assembled with NCM811 maintains a favourable specific capacity retention of 76.5% after 400 cycles at 50 °C. We anticipate that these findings will aid in the formulation of successful approaches for creating fluorinated additives or cosolvent in LMB electrolytes.
Experimental section
Materials and reagents
Lithium bis(fluorosulfonyl) imide (LiFSI, 99.5%) was acquired from Dodo Chem. FB (99%), dimethoxyethane (DME, 99.5%), and CNFE (98.0%) were purchased from Aladdin. NCM811 cathodes (loading: 10 and 20 mg cm−2), NCM811 and lithium oxido(oxo)cobalt (LCO) powders were obtained from Guangdong Canrd New Energy Technology Co., Ltd. And the purchased NCM811 cathodes consist of 94.5% active materials, 1.5% CNT, 1.5% SP and 2.5% PVDF. Li metal anodes (Φ14 mm, 200 μm) were acquired from China Energy Lithium Co., Ltd.Preparation of electrolytes and cathodes
The electrolytes were formulated with the formula of LiFSI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME
DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diluents at a 1
diluents at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio. All original solvents are dried with molecular sieves for 1 day before use. The NCM811 (loading: 1.5 mg cm−2) or LCO (loading: 3 mg cm−2) cathode was fabricated using a blend comprising NCM811 or LCO, super P, and polyvinylidene difluoride (8
3 molar ratio. All original solvents are dried with molecular sieves for 1 day before use. The NCM811 (loading: 1.5 mg cm−2) or LCO (loading: 3 mg cm−2) cathode was fabricated using a blend comprising NCM811 or LCO, super P, and polyvinylidene difluoride (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. The obtained slurries were applied to aluminium foil and subjected to vacuum drying for 12 hours.
1), respectively. The obtained slurries were applied to aluminium foil and subjected to vacuum drying for 12 hours.
Electrochemical characterization
The electrochemical performance was tested on a Chenhua electrochemical workstation (CHI660E) and Neware battery test system (CT-4008T-5V10mA-164). Linear sweep voltammetry (LSV) was carried out on Li‖carbon-coated aluminum cells from 3.0 V to 5.0 V at 1 mV s−1. Electrochemical impedance spectrometry (EIS) was conducted from 100 kHz to 0.1 Hz with a voltage amplitude of 10 mV. Li‖Cu and Li‖Li cells were measured with 2 mA cm−2 and 1 mA h cm−2. The Li‖Cu cells used to calculate coulombic efficiency (CE) were initially deposited with Li for 10 h, and then Li was stripped to 1.0 V and subsequently deposited for 10 h. This was further followed by alternating deposition and stripping steps for 1 hour each over a total of 30 cycles, concluding with a final stripping to 1.0 V step. The current density used in all steps was 0.5 mA cm−2. The data obtained from the aforementioned test were inputted into the formula proposed by Aurbach et al.:50In this method, n is the number of cycles (30 is utilized in this work), QC is the charging or discharging capacity of the cell during these 30 cycles, QT is the discharging capacity at initial Li deposition, and QS is the charging capacity at final Li stripping. Li‖NCM811 cells operated at room temperature or 50 °C were cycled between 3 and 4.4 V or 3 and 4.3 V, respectively.
Materials characterisation
All the cycled electrodes used for characterisation were washed in DME and vacuum dried. The Li deposition films were obtained by depositing under 0.5 mA cm−2 for 10 hours. The scanning electron microscopy (SEM) images were collected on a TESCAN MIRA LMS. X-ray photoelectron spectroscopy (XPS) analysis was performed using a Thermo Scientific K-Alpha. Raman spectra were recorded with a LabRAM HR800 to analyse the solvation structures of electrolytes. X-ray diffraction (XRD) was carried out on a MiniFlex600.Results and discussion
To assess the miscibility and properties of FB-CNFE-DHCE, various amounts of CNFE were added to FB-DHCE, considering CNFE's weak polarity and macromolecular structure (Fig. 1a–d). As shown in Fig. 1a–c, electrolytes remain transparent and clear at ratios of FB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNFE = 2.8
CNFE = 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, 2.6
0.2, 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 or 2.4
0.4 or 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 by molar. However, further increased CNFE content leads to electrolyte turbidity and phase separation, indicating limited miscibility (Fig. 1d). Moreover, CNFE introduction improves wetting ability, reducing the contact angle from 43.6° to 26.4° with increasing CNFE proportion (Fig. S1†). Besides, CNFE addition shows minimal impact on ionic conductivity (Fig. S2†). Raman spectroscopy demonstrates the existence of analogous characteristic peaks at 750 and 870 cm−1 in FB-DHCE and FB-CNFE-DHCE, indicating similar solvation structures for coordinated FSI− and DME (Fig. 1e). LSV curves recorded for the carbon-coated aluminum foil/Li cells demonstrate FB-CNFE-DHCE's (4.65 V) higher anodic stability compared to FB-DHCE (4.53 V) (Fig. 1f), attributed to improved decomposition voltage. The reduction potentials of electrolytes were compared by performing LSV on FB-CNFE (3.0
0.6 by molar. However, further increased CNFE content leads to electrolyte turbidity and phase separation, indicating limited miscibility (Fig. 1d). Moreover, CNFE introduction improves wetting ability, reducing the contact angle from 43.6° to 26.4° with increasing CNFE proportion (Fig. S1†). Besides, CNFE addition shows minimal impact on ionic conductivity (Fig. S2†). Raman spectroscopy demonstrates the existence of analogous characteristic peaks at 750 and 870 cm−1 in FB-DHCE and FB-CNFE-DHCE, indicating similar solvation structures for coordinated FSI− and DME (Fig. 1e). LSV curves recorded for the carbon-coated aluminum foil/Li cells demonstrate FB-CNFE-DHCE's (4.65 V) higher anodic stability compared to FB-DHCE (4.53 V) (Fig. 1f), attributed to improved decomposition voltage. The reduction potentials of electrolytes were compared by performing LSV on FB-CNFE (3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0)-DHCE and FB-CNFE (2.4
0)-DHCE and FB-CNFE (2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6)-DHCE (Fig. S3†). The introduction of CNFE results in an increase in the reduction peak potential of the electrolyte from 0.36 V to 0.40 V. However, further experiments are required to discern whether the SEI derived from anions or CNFE, as the reduction potential of FSI− closely approximates this potential.
0.6)-DHCE (Fig. S3†). The introduction of CNFE results in an increase in the reduction peak potential of the electrolyte from 0.36 V to 0.40 V. However, further experiments are required to discern whether the SEI derived from anions or CNFE, as the reduction potential of FSI− closely approximates this potential.
The Li plating and stripping CE was determined by employing Li‖Cu cells with varying electrolyte compositions. As illustrated in Fig. 2a, FB-CNFE (2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4)-DHCE and FB-CNFE (2.4
0.4)-DHCE and FB-CNFE (2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6)-DHCE exhibit higher average CE values of approximately 99.63%, surpassing that of FB-DHCE (99.48%). The long-term cycling tests of Li‖Cu cells show that FB-CNFE (2.6
0.6)-DHCE exhibit higher average CE values of approximately 99.63%, surpassing that of FB-DHCE (99.48%). The long-term cycling tests of Li‖Cu cells show that FB-CNFE (2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4)-DHCE achieves prolonged lifespan and a significantly increased average CE of 99.1% over 350 cycles compared to that of FB-DHCE (98.0% for 200 cycles) (Fig. 2b). This result implies that the introduction of CNFE leads to enhanced reversibility of Li plating/stripping. The time–voltage curve of the cell in FB-DHCE shows an overpotential of 235 mV in the 200th cycle (Fig. S4†), while that in FB-CNFE (2.6
0.4)-DHCE achieves prolonged lifespan and a significantly increased average CE of 99.1% over 350 cycles compared to that of FB-DHCE (98.0% for 200 cycles) (Fig. 2b). This result implies that the introduction of CNFE leads to enhanced reversibility of Li plating/stripping. The time–voltage curve of the cell in FB-DHCE shows an overpotential of 235 mV in the 200th cycle (Fig. S4†), while that in FB-CNFE (2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4)-DHCE is reduced to 115 mV in the 200th cycle, indicating improved plating/stripping kinetics. Similarly, the Li‖Cu cell with FB-CNFE (2.6
0.4)-DHCE is reduced to 115 mV in the 200th cycle, indicating improved plating/stripping kinetics. Similarly, the Li‖Cu cell with FB-CNFE (2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4)-DHCE shows a smaller interphase impedance (≈28 Ω) compared to that of FB-DHCE (≈49 Ω) (Fig. 2c). Thus, FB-CNFE (2.6–0.4)-DHCE, represented as FB-CNFE-DHCE, is used for further investigation. Relevant experiments were conducted on Li‖Li symmetric cells to assess the electrolyte compatibility with Li (Fig. 2d and S5†). The cell with FB-DHCE demonstrates a rapid escalation of overpotential and fails after 340 h because of short circuits caused by Li dendrites. In contrast, with CNFE, the Li‖Li cell exhibits a consistent voltage profile with reduced voltage hysteresis, indicating that CNFE likely creates a stable SEI on the Li metal anode, thereby enhancing interfacial stability. The Li‖Li cell with FB-CNFE-DHCE also exhibits enhanced rate performance compared to FB-DHCE (Fig. 2e). The lower overpotential in the cell containing FB-CNFE-DHCE at different current densities suggests improved electrochemical kinetics of Li plating/stripping, in agreement with the larger exchange current density calculated from the Tafel plot (Fig. S6†).
0.4)-DHCE shows a smaller interphase impedance (≈28 Ω) compared to that of FB-DHCE (≈49 Ω) (Fig. 2c). Thus, FB-CNFE (2.6–0.4)-DHCE, represented as FB-CNFE-DHCE, is used for further investigation. Relevant experiments were conducted on Li‖Li symmetric cells to assess the electrolyte compatibility with Li (Fig. 2d and S5†). The cell with FB-DHCE demonstrates a rapid escalation of overpotential and fails after 340 h because of short circuits caused by Li dendrites. In contrast, with CNFE, the Li‖Li cell exhibits a consistent voltage profile with reduced voltage hysteresis, indicating that CNFE likely creates a stable SEI on the Li metal anode, thereby enhancing interfacial stability. The Li‖Li cell with FB-CNFE-DHCE also exhibits enhanced rate performance compared to FB-DHCE (Fig. 2e). The lower overpotential in the cell containing FB-CNFE-DHCE at different current densities suggests improved electrochemical kinetics of Li plating/stripping, in agreement with the larger exchange current density calculated from the Tafel plot (Fig. S6†).
The SEM images illustrate the surface (Fig. 3a and d) and cross-section (Fig. 3b and e) morphology of Li that was deposited on Cu foil. Although the lithium deposited in FB-DHCE is quite dense, there are still observable gaps present. In contrast, the Li stack formed in FB-CNFE-DHCE exhibits a significantly higher density with almost no interval, corresponding to high CE and excellent cycling stability. Notably, the thickness of the Li film deposited in FB-CNFE-DHCE (27 μm) closely approximates the theoretical thickness (25 μm), suggesting a higher density of Li deposition compared to that in FB-DHCE (30 μm). The above results suggest that CNFE's introduction facilitates the formation of uniform and denser Li deposits, effectively alleviating interfacial reactions with electrolytes. XPS analysis indicates the existence of a prominent peak at 684.5 eV corresponding to LiF, as well as an S–F peak at 688.1 eV in FB-CNFE-DHCE and FB-DHCE samples (Fig. S7,†3c and f).51 Furthermore, the FB-CNFE-DHCE case demonstrates a significantly elevated peak area ratio (10.60) of LiF/S–F bonds in comparison to the FB-DHCE case (0.37). In addition, the F content in FB-CNFE-DHCE (6.11 at%) is higher than that in FB-DHCE (5.08 at%) and the S content in FB-CNFE-DHCE (2.37 at%) is lower than that in FB-DHCE (3.42 at%), demonstrating that CNFE can be preferentially reduced to LiF rather than to FSI− (Table S1†).
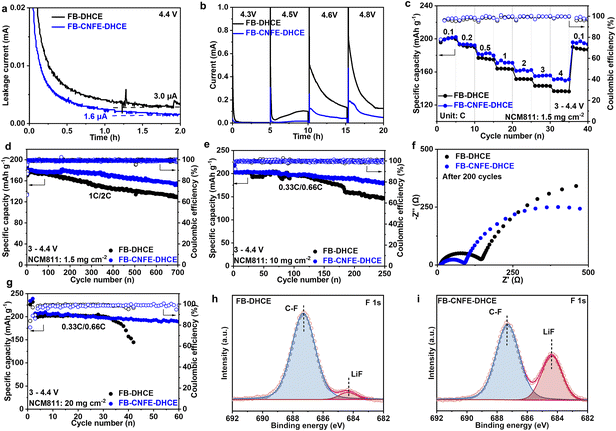
To evaluate FB-CNFE-DHCE's anti-oxidation stability, electrochemical floating tests were conducted on Li‖NCM811 cells at 4.4 V. The leakage current in FB-CNFE-DHCE is about 1.6 μA, which is considerably lower than that in FB-DHCE (3.0 μA) (Fig. 4a). This finding strongly suggests the superior anodic stability of FB-CNFE-DHCE towards high-voltage cathodes, aligning closely with the outcomes obtained from LSV analysis in Fig. 1f. The leakage current at different voltages in Fig. 4b demonstrates that the current of the cell with FB-DHCE becomes chaotic when charged to a constant voltage of 4.5 V and increases sharply at 4.6 V. But the current of FB-CNFE-DHCE keeps stable at 4.3–4.8 V, which is significantly lower than that of FB-DHCE, showing excellent electrochemical stability.
The cyclic voltammetry (CV) test on the Li‖NCM811 cell using FB-CNFE-DHCE demonstrates enhanced reversibility of Li+ extraction/insertion, resulting in a smaller polarization of 50 mV (Fig. S8†). Due to the enhanced interfacial kinetics, the cell in FB-CNFE-DHCE demonstrates an enhanced specific capacity at 4C (144.0 mA h g−1), surpassing that of FB-DHCE (133.7 mA h g−1) (Fig. 4c). The cycling behaviours of Li‖NMC811 cells were compared at 4.4 V to investigate the efficacy of FB-CNFE-DHCE in protecting Ni-rich cathodes (Fig. 4d). The cell using FB-CNFE-DHCE maintains 84.8% of its initial specific capacity after undergoing 700 cycles, whereas that with FB-DHCE maintains only 71.5%. In addition, 4.5 V Li‖ LCO cells using FB-CNFE-DHCE at higher voltages exhibit an excellent specific capacity retention of 87.7% (151.8 mA h g−1) in the 300th cycle compared to that of FB-DHCE (65.1%, 113.6 mA h g−1) (Fig. S9†). This outcome provides further substantiation of the protective properties of the CEI established by FB-CNFE-DHCE at high voltages.
The actual application potential of FB-CNFE-DHCE was demonstrated by operating Li‖NCM811 cells with a higher NCM811 loading (10 mg cm−2) (Fig. 4e). The cell in FB-DHCE only maintains 72.6% of its initial specific capacity after 250 cycles. While the incorporation of CNFE significantly enhances the electrochemical performance, enabling a specific capacity retention of 89.6% (180.9 mA h g−1). The EIS spectra of the corresponding cells are shown in Fig. 4f after the 200th cycle. For the cell with FB-DHCE, the interfacial impedance is as high as 134 Ω, owing to the thick CEI. In comparison, the addition of CNFE facilitates the formation of a CEI with enhanced oxidation resistance, thus effectively hindering the consumption of electrolytes on the cathode surface and preventing the build-up of decomposed by-products with high impedance. As a result, the interphase resistance in FB-CNFE-DHCE is only 77 Ω. High-areal-mass-loading NCM cathodes (20.0 mg cm−2) were also employed in high-energy LMBs to demonstrate the improvement using CNFE (Fig. 4g and S10†). The Li‖NCM811 cell containing FB-CNFE-DHCE achieves stable operation for more than 60 cycles (188.7 mA h g−1, 91.2%), significantly surpassing that of FB-DHCE (rapid capacity degradation).
The resistance evolution in Li‖NCM811 cells using various electrolytes was monitored through in situ EIS during the initial cycle, aiming to further elucidate the cycling lifespan of NCM811 (Fig. S11 and S12†). Throughout the first cycle, Li‖NCM811 cell resistance in FB-CNFE-DHCE consistently remains less than that in FB-DHCE, aligning well with long-term cycling performance, indicating enhanced Li+ transport kinetics and a high-quality CEI under high voltage.
The interfacial chemistry of NCM811 was further analysed using XPS after cycling (Fig. 4h and i). The F 1s XPS spectra display distinctive peaks for LiF (684.5 eV) and C–F bonds (688.1 eV), revealing a significantly higher ratio of peak areas between LiF and C–F bonds in the cathode cycled with FB-CNFE-DHCE (0.47) compared to that cycled with FB-DHCE (0.06). This observation suggests that the incorporation of CNFE facilitates the formation of a LiF-rich CEI. Furthermore, the elevated F/S and F/N ratios in the FB-CNFE-DHCE case provide evidence that the increased LiF content in the CEI originates from CNFE decomposition rather than FSI− (Table S2†). The intact particle morphology of NCM811 after cycling further supports effective cathode protection by FB-CNFE-DHCE, owing to robust CEI formation at high voltage (Fig. S13†).
The thermal stabilities of electrolytes using different diluents (TTE, FEC, FB and FB-CNFE mixtures) were investigated to consider the potential defluorination of fluorinated solvents. All initial solvents underwent a 24 hour desiccation process using molecular sieves prior to utilization. All fresh electrolytes with different diluents are colourless as depicted in Fig. S14†. After 10 days of storage at 50 °C, TTE, FB, and FB-CNFE samples exhibit significant yellowing, while FEC samples display a dark brown colour (Fig. 5a). pH paper tests reveal high acidity in TTE-DHCE and FEC-DHCE (Fig. 5b), indicating defluorination, whereas FB-DHCE and FB-CNFE-DHCE show milder acidity, suggesting superior thermal stability of CNFE compared to TTE and FEC. To assess the impact of electrolyte thermal stability on electrochemical performance, Li‖NCM811 cells were examined at 50 °C (Fig. 5d). The sudden decrease in specific capacity around the 325th cycle in the cell with FB-DHCE or FB-CNFE-DHCE comes from uncontrollable external factors. FB-CNFE-DHCE enables higher specific capacity retention (76.5% after 400 cycles) compared to rapid decline in TTE-DHCE and FEC-DHCE after 200 and 300 cycles, respectively. This result indicates FB-CNFE-DHCE's exceptional high-temperature stability and CEI quality. Meanwhile, the interfacial resistance of FB-CNFE-DHCE (≈144 Ω) is significantly lower than those of TTE-DHCE (≈640 Ω) and FEC-DHCE (≈1016 Ω) after 200 cycles at 50 °C (Fig. 5e). Moreover, FB-CNFE-DHCE demonstrates outstanding rate capability (153.5 mA h g−1, 4C) at 50 °C, surpassing TTE-DHCE (138.8 mA h g−1) and FEC-DHCE (122.3 mA h g−1) (Fig. 5f). The XRD patterns of cycled NCM811 cathodes demonstrate a relatively small shift in the case of FB-CNFE-DHCE, indicating the effective suppression of the detrimental phase transformation (Fig. 5g). In contrast, NCM811 cycled in TTE-DHCE or FEC-DHCE exhibits serious structural deterioration. Moreover, Li‖NCM811 cells in FB-CNFE-DHCE with a NCM811 loading of 10 mg cm−2 at 50 °C also exhibit an excellent capacity retention of 88.8% (177.1 mA h g−1) after 200 cycles, significantly surpassing the TTE-DHCE and FEC-DHCE samples (Fig. 5h). The thermal storage and electrochemical performance at 50 °C highlight the advantages of FB-CNFE-DHCE in high-temperature applications.
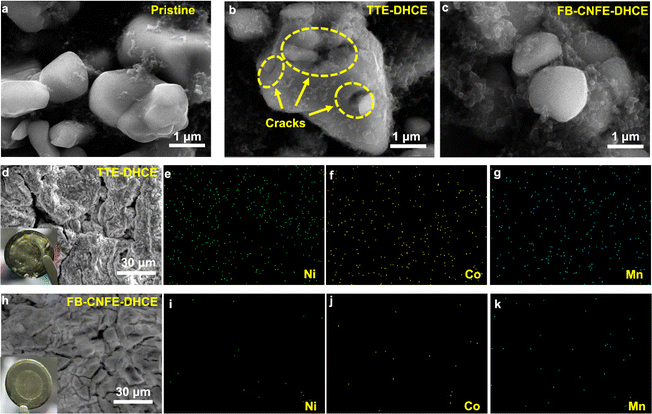
Furthermore, failure analysis of Li‖NCM811 cells cycled at 50 °C reveals particle cracking in NCM811 cycled with TTE-DHCE, attributed to parasitic reactions inducing gas generation and subsequent internal stress (Fig. 6a and b). In contrast, NCM811 particles cycled with FB-CNFE-DHCE remain intact, indicating effective mitigation of internal stress and maintenance of the electrode structure (Fig. 6c). Moreover, unstable CEI induces transition metal (TM) dissolution, observed through significant Ni, Co, and Mn signals on the Li anode cycled with TTE-DHCE (Fig. 6d–g), whereas the Li anode cycled with FB-CNFE-DHCE shows insignificant TM signals, indicating robust CEI suppression of TM dissolution (Fig. 6h–k).
Conclusions
In summary, the utilization of CNFE as an interphase-enhancing additive in FB-DHEC has been demonstrated to facilitate the establishment of a robust CEI and SEI in high-voltage Li‖NCM811 cells. Specifically, the introduction of CNFE results in the creation of a durable LiF-rich CEI and SEI, effectively suppressing Li dendrite growth and solvent decomposition at high voltage. Therefore, the Li‖NCM811 cell demonstrates an impressive cycling performance over 700 cycles at 4.4 V using FB-CNFE-DHCE. Moreover, FB-CNFE-DHCE exhibits significant mitigation of defluorination, resulting in reduced HF formation at high temperatures compared to TTE-DHCE or FEC-DHCE. Consequently, the Li‖NCM811 cell exhibits a stable cycle over 200 cycles at 50 °C. This research presents a novel approach to leverage fluorinated additives with favourable thermal stability and film-forming properties. The findings offer valuable insights into the development of multifunctional electrolytes with enhanced stability and performance for advanced battery technologies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant no. 2022YFB2404800). We gratefully acknowledge the Analytical and Testing Centre of HUST for supporting Raman tests. The authors would like to thank Shiyanjia Lab (https://www.shiyanjia.com/) for the SEM and XPS analyses.Notes and references
- Y. Zhao, T. Zhou, D. Baster, M. El Kazzi, J. W. Choi and A. Coskun, ACS Energy Lett., 2023, 8, 3180–3187 CrossRef CAS
.
- G. Choi, Y. Kim, J. Choi and D. Kim, Adv. Energy Mater., 2023, 13, 2300816 CrossRef CAS
.
- P. Xiao, Y. Zhao, Z. Piao, B. Li, G. Zhou and H.-M. Cheng, Energy Environ. Sci., 2022, 15, 2435–2444 RSC
.
- W. Zhang, Y. Guo, T. Yang, Y. Wang, X. Kong, X. Liao and Y. Zhao, Energy Storage Mater., 2022, 51, 317–326 CrossRef
.
- W. Xue, M. Huang, Y. Li, Y. G. Zhu, R. Gao, X. Xiao, W. Zhang, S. Li, G. Xu, Y. Yu, P. Li, J. Lopez, D. Yu, Y. Dong, W. Fan, Z. Shi, R. Xiong, C.-J. Sun, I. Hwang, W.-K. Lee, Y. Shao-Horn, J. A. Johnson and J. Li, Nat. Energy, 2021, 6, 495–505 CrossRef CAS
.
- Y. Qiao, H. Deng, P. He and H. Zhou, Joule, 2020, 4, 1445–1458 CrossRef CAS
.
- Q. Liu, X. Su, D. Lei, Y. Qin, J. Wen, F. Guo, Y. A. Wu, Y. Rong, R. Kou, X. Xiao, F. Aguesse, J. Bareño, Y. Ren, W. Lu and Y. Li, Nat. Energy, 2018, 3, 936–943 CrossRef CAS
.
- J. Zhang, Q. Li, Y. Zeng, Z. Tang, D. Sun, D. Huang, Y. Tang and H. Wang, ACS Energy Lett., 2023, 8, 1752–1761 CrossRef CAS
.
- Z. Cui, X. Li, X. Bai, X. Ren and X. Ou, Energy Storage Mater., 2023, 57, 14–43 CrossRef
.
- W. Cai, Y. Deng, Z. Deng, Y. Jia, Z. Li, X. Zhang, C. Xu, X.-Q. Zhang, Y. Zhang and Q. Zhang, Adv. Energy Mater., 2023, 13, 2301396 CrossRef CAS
.
- P. Shi, L.-P. Hou, C.-B. Jin, Y. Xiao, Y.-X. Yao, J. Xie, B.-Q. Li, X.-Q. Zhang and Q. Zhang, J. Am. Chem. Soc., 2022, 144, 212–218 CrossRef CAS PubMed
.
- X. Fan and C. Wang, Chem. Soc. Rev., 2021, 50, 10486–10566 RSC
.
- Y. Kim, D. Stepien, H. Moon, K. Schönherr, B. Schumm, M. Kuenzel, H. Althues, D. Bresser and S. Passerini, ACS Appl. Mater. Interfaces, 2023, 15, 20987–20997 CrossRef CAS PubMed
.
- Y.-X. Zhan, P. Shi, C.-B. Jin, Y. Xiao, M.-Y. Zhou, C.-X. Bi, B.-Q. Li, X.-Q. Zhang and J.-Q. Huang, Adv. Funct. Mater., 2022, 32, 2206834 CrossRef CAS
.
- L. Zhang, Q. Jin, K. Zhao, X. Zhang and L. Wu, Dalton Trans., 2022, 51, 16565–16573 RSC
.
- M. Zhao, X. Huang, D. Zhuang, L. Sheng, X. Xie, M. Cao, J. Pan, H. Fan and J. He, J. Energy Storage, 2022, 47, 103665 CrossRef
.
- X. Liu, F. Tang, H. Hu, H. Huang, X. Ji, L. Chen and Z. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 13761–13771 CrossRef CAS PubMed
.
- X. Fu, C. Shang, M. Yang, E. M. Akinoglu, X. Wang and G. Zhou, J. Power Sources, 2020, 475, 228687 CrossRef CAS
.
- S. Liu, X. Ji, J. Yue, S. Hou, P. Wang, C. Cui, J. Chen, B. Shao, J. Li, F. Han, J. Tu and C. Wang, J. Am. Chem. Soc., 2020, 142, 2438–2447 CrossRef CAS PubMed
.
- W. Jing, K. Zou, X. Dai, J. Sun, Q. Tan, Y. Chen and Y. Liu, J. Alloys Compd., 2022, 924, 166517 CrossRef CAS
.
- R. Pan, Z. Cui, M. Yi, Q. Xie and A. Manthiram, Adv. Energy Mater., 2022, 12, 2103806 CrossRef CAS
.
- X. Luo, L. Xing, J. Vatamanu, J. Chen, J. Chen, M. Liu, C. Wang, K. Xu and W. Li, J. Energy Chem., 2022, 65, 1–8 CrossRef CAS
.
- P. Xiao, R. Luo, Z. Piao, C. Li, J. Wang, K. Yu, G. Zhou and H.-M. Cheng, ACS Energy Lett., 2021, 6, 3170–3179 CrossRef CAS
.
- F. Hai, X. Tian, Y. Yi, Z. Wu, S. Zheng, J. Guo, W. Tang, W. Hua and M. Li, Energy Storage Mater., 2023, 54, 641–650 CrossRef
.
- Z. Chang, Y. Qiao, H. Yang, H. Deng, X. Zhu, P. He and H. Zhou, Energy Environ. Sci., 2020, 13, 4122–4131 RSC
.
- H. Zhang, Z. Zeng, S. Wang, Y. Wu, C. Li, M. Liu, X. Wang, S. Cheng and J. Xie, Nano Res., 2023, 17, 2638–2645 CrossRef
.
- P. Lai, B. Huang, X. Deng, J. Li, H. Hua, P. Zhang and J. Zhao, Chem. Eng. J., 2023, 461, 141904 CrossRef CAS
.
- T. Li, Z. Chen, F. Bai, C. Li and Y. Li, J. Energy Chem., 2023, 81, 404–409 CrossRef CAS
.
- Y. Zou, F. Cheng, Y. Lu, Y. Xu, C. Fang and J. Han, Small, 2023, 19, 2203394 CrossRef CAS PubMed
.
- X. Ren, Y. Zhang, M. H. Engelhard, Q. Li, J.-G. Zhang and W. Xu, ACS Energy Lett., 2018, 3, 14–19 CrossRef CAS
.
- S. Tan, Z. Shadike, J. Li, X. Wang, Y. Yang, R. Lin, A. Cresce, J. Hu, A. Hunt, I. Waluyo, L. Ma, F. Monaco, P. Cloetens, J. Xiao, Y. Liu, X.-Q. Yang, K. Xu and E. Hu, Nat. Energy, 2022, 7, 484–494 CrossRef CAS
.
- H. Zheng, H. Xiang, F. Jiang, Y. Liu, Y. Sun, X. Liang, Y. Feng and Y. Yu, Adv. Energy Mater., 2020, 10, 2070127 CrossRef CAS
.
- I. Phiri, J. Kim, D.-H. Oh, M. Ravi, H.-S. Bae, J. Hong, S. Kim, Y.-C. Jeong, Y. M. Lee, Y.-G. Lee and M.-H. Ryou, ACS Appl. Mater. Interfaces, 2021, 13, 31605–31613 CrossRef CAS PubMed
.
- S. Chen, J. Zheng, D. Mei, K. S. Han, M. H. Engelhard, W. Zhao, W. Xu, J. Liu and J.-G. Zhang, Adv. Mater., 2018, 30, 1706102 CrossRef PubMed
.
- Z. Wang, Y. Sun, Y. Mao, F. Zhang, L. Zheng, D. Fu, Y. Shen, J. Hu, H. Dong, J. Xu and X. Wu, Energy Storage Mater., 2020, 30, 228–237 CrossRef
.
- A. Kotronia, H. D. Asfaw, C.-W. Tai, M. Hahlin, D. Brandell and K. Edström, ACS Appl. Mater. Interfaces, 2021, 13, 3867–3880 CrossRef CAS PubMed
.
- T. Li, X.-Q. Zhang, P. Shi and Q. Zhang, Joule, 2019, 3, 2647–2661 CrossRef CAS
.
- L. Le, M. Liao, A. Nguyen and D. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 37497–37503 CrossRef CAS PubMed
.
- T. Li, Y. Li, Y. Sun, Z. Qian and R. Wang, ACS Mater. Lett., 2021, 3, 838–844 CrossRef CAS
.
- S. Lin, H. Hua, Z. Li and J. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 33710–33718 CrossRef CAS PubMed
.
- M. Yoon, Y. Dong, Y. Yoo, S. Myeong, J. Hwang, J. Kim, S.-H. Choi, J. Sung, S. J. Kang, J. Li and J. Cho, Adv. Funct. Mater., 2020, 30, 1907903 CrossRef CAS
.
- K. Kim, I. Park, S.-Y. Ha, Y. Kim, M.-H. Woo, M.-H. Jeong, W. C. Shin, M. Ue, S. Y. Hong and N.-S. Choi, Electrochim. Acta, 2017, 225, 358–368 CrossRef CAS
.
- L. Yu, S. Chen, H. Lee, L. Zhang, M. H. Engelhard, Q. Li, S. Jiao, J. Liu, W. Xu and J.-G. Zhang, ACS Energy Lett., 2018, 3, 2059–2067 CrossRef CAS
.
- K. Park, Y. Jo, B. Koo, H. Lee and H. Lee, Chem. Eng. J., 2022, 427, 131889 CrossRef CAS
.
- W. Li, Y. Jie, Y. Chen, M. Yang, Y. Chen, X. Li, Y. Guo, X. Meng, R. Cao and S. Jiao, Nano Res., 2023, 16, 8417–8424 CrossRef CAS
.
- H. Zhang, Z. Zeng, F. Ma, X. Wang, Y. Wu, M. Liu, R. He, S. Cheng and J. Xie, Adv. Funct. Mater., 2023, 33, 2212000 CrossRef CAS
.
- Z. Jiang, Z. Zeng, X. Liang, L. Yang, W. Hu, C. Zhang, Z. Han, J. Feng and J. Xie, Adv. Funct. Mater., 2021, 31, 2005991 CrossRef CAS
.
- H. Zhang, Z. Zeng, R. He, Y. Wu, W. Hu, S. Lei, M. Liu, S. Cheng and J. Xie, Energy Storage Mater., 2022, 48, 393–402 CrossRef
.
- J. Arai, J. Electrochem. Soc., 2003, 150, A219 CrossRef CAS
.
- Y. Meng, D. Zhou, R. Liu, Y. Tian, Y. Gao, Y. Wang, B. Sun, F. Kang, M. Armand, B. Li, G. Wang and D. Aurbach, Nat. Energy, 2023, 8, 1023–1033 CrossRef CAS
.
- X. Fan, L. Chen, X. Ji, T. Deng, S. Hou, J. Chen, J. Zheng, F. Wang, J. Jiang, K. Xu and C. Wang, Chem, 2018, 4, 174–185 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta01083c |
| This journal is © The Royal Society of Chemistry 2024 |