Tuning the LiF content in the SEI by engineering the molecular structures of porous organic polymers for solid-state lithium metal batteries†
Shi
Zhou
a,
Yiting
Zhu
a,
Haoran
Hu
a,
Chenghan
Li
a,
Jie
Jiang
 *ab,
Jianyu
Huang
*ab,
Jianyu
Huang
 *ac and
Biao
Zhang
*ac and
Biao
Zhang
 *ab
*ab
aSchool of Materials Science and Engineering, Xiangtan University, Xiangtan 411105, China. E-mail: jiangjie@xtu.edu; jyhuang8@hotmail.com; xiaobiao_zhang@outlook.com
bKey Laboratory of Low Dimensional Materials and Application Technology, Ministry of Education, Xiangtan University, Hunan 411105, China
cClean Nano Energy Center, State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao 066004, China
First published on 16th February 2023
Abstract
LiF, which is considered as a key component of the solid electrolyte interface (SEI), can promote the uniform deposition of lithium. However, excessive LiF will limit the transport of lithium ions (Li+) in the SEI, resulting in low coulombic efficiency and lithium dendrite growth. Regulating the LiF concentration in the SEI is challenging but critical to the development of solid-state lithium metal batteries (SSLMBs). Herein, we synthesized porous organic polymers (POPs) that can be flexibly modified at the molecular level. These modified POPs (denoted as POPs-X-FP, X = 2, 3, 4) are added into a PEO electrolyte as fillers to form composite solid electrolytes (denoted as CSE-X-FP, X = 2, 3, 4, respectively). CSE-X-FP have different catalytic decomposition capabilities of TFSI− anions in the electrolyte due to the different fluorine atom substitution positions on the benzene ring of the POPs. Consequently, the LiF concentration in the SEI can be optimized by tuning the fluorine atom substitution positions on the benzene rings of the POPs to boost the performance of PEO based ASSLMBs. Using the LiF optimized SEI, the LiFePO4(LFP)//CSE-4-FP//Li full cell delivered a long cycle life of 1200 cycles with an initial capacity of 160.7 mA h g−1 at 0.5C. This study provides a new strategy to regulate the LiF content in the SEI layer to boost the performance of SSLMBs.
1. Introduction
Solid-state lithium metal batteries (SSLMBs) are considered as next-generation energy storage devices due to their high energy density and excellent safety, among which PEO-based polymeric SSLMBs are of great interest because of their excellent processing performance, good flexibility and outstanding lithium salt solubility.1–4 However, the problem of poor cycle life due to uncontrolled lithium dendrite growth has severely limited the large-scale application of PEO-based SSLMBs.5–9 The mechanical strength of PEO-based electrolytes is insufficient to inhibit lithium dendrite growth because the yield stress of lithium dendrites is as high as 244 MPa.10–13 The promotion of the uniform deposition of lithium and effectively delaying the growth of lithium dendrites are the grand challenges in SSLMBs. Strategies to construct artificial SEI layers with excellent performance can promote the uniform deposition of lithium and inhibit the growth of lithium dendrites, thus significantly improving the performance of SSLMBs.14–16Numerous studies have shown that LiF in the SEI can effectively promote the planar growth of lithium metal on the lithium surface and inhibit the formation of lithium dendrites; therefore, LiF is one of the indispensable components in the SEI.17–21 For this reason, a large amount of work has been devoted to promoting LiF formation in the SEI. Zhao22et al. developed a simple surface fluorination reaction by heating the fluoropolymer CYTOP, and the LiF-containing coating has excellent cycling stability compared to the LiF-free lithium metal coating. Besides the direct addition of fluorine-containing substances, there are also additives that can promote the decomposition of anions in lithium salts to produce LiF. For example, Tao23et al. accelerated TFSI− decomposition by using Li2S to construct LiF-rich SEI layers, and the as-formed SEI effectively prevented the continuous interfacial reaction between PEO and lithium metal, thus enhancing its cycling performance. Han24et al. induced the decomposition of TFSI− by introducing LiNO3 into a PEO-based electrolyte, thus forming a Li3N–LiF interface between the solid electrolyte and the lithium, which promoted uniform lithium deposition, and improved the critical current density, cycling performance and coulombic efficiency. Therefore, the presence of LiF greatly improves the performance of the SEI and inhibits the growth of lithium dendrites.
However, excessive LiF does not improve the SEI performance due to its low Li+ conductivity, which can even lead to SEI failure.25 Ma's team found that the addition of TPFPP− to liquid electrolytes results in the formation of excessive LiF in the SEI, which impedes the Li+ diffusion kinetics of the SEI, causing large polarization potential and interface impedance that lead to deteriorating cell cycling performance.26 In addition, Wang's group found that cells with MCMB-F3 showed low coulombic efficiency and lithium dendrite growth, which was caused by too much LiF in the SEI.27 Therefore, the amount of LiF in the SEI is particularly important, but there are few reports in the literature on how to regulate the content of LiF in the SEI of SSLMBs and how much LiF in the SEI is best for improving the performance of SSLMBs.
Porous organic polymers (POPs) are lightweight polymer networks with rich structures and functional groups that can be flexibly modified at the molecular level and are widely used in catalysis. In this work, POPs were used as PEO-based solid electrolyte fillers to catalyze the formation of LiF in SEI layers.28–31 Porous organic polyacetal amines (POPs-X-FPs, X = 2, 3, 4 represent three different fluorine positions in the benzene rings of POPs, Fig. 1a) were prepared by polymerization of 2-fluorobenzaldehyde, 3-fluorobenzaldehyde, and 4-fluorobenzaldehyde with melamine, respectively, and as fillers for the preparation of PEO-based composite solid-state electrolytes (CSE-X-FPs, X = 2, 3, 4). We show that the catalytic efficiency of POPs-X-FP can be tuned by adjusting the position of the fluorine atom substitution on the benzene ring of POPs-X-FP, thus achieving control of the LiF content in the CSE-X-FP//Li SEI layer. Based on this finding, the optimal content of LiF in the SEI layer was investigated by testing the electrochemical performance of CSE-X-FP. The results show that CSE-4-FP forms a SEI with a suitable LiF content (8.72%), which reduces the nucleation potential barrier and promotes the uniform deposition of lithium, and the LiFePO4(LFP)//CSE//Li cells achieved high capacity retention and long cycle life.
 | ||
| Fig. 1 Illustration of the design strategy for the composite solid electrolyte (CSE). (a) Filler synthesis. (b) CSE membrane preparation process. (c) POPs-X-FP catalyzes the formation of LiF-SEI. | ||
2. Experimental section
2.1 Materials
PEO (Mv = 1 × 106), bis(trifluoromethane)sulfonimide lithium salt (LiTFSI), N-methyl pyrrolidone (NMP), 2-fluorobenzaldehyde, 3-fluorobenzaldehyde, 4-fluorobenzaldehyde, melamine, acetonitrile and dimethyl sulfoxide (DMSO) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Dichloromethane (DCM), tetrahydrofuran (THF), N,N-dimethylformamide (DMF) and other reagents were purchased from Shanghai Chemical Reagent Company. DMSO was purified by vacuum distillation. Other reagents are reagent grade.The formation of aminal linkages in the polymer networks was confirmed by Fourier transform infrared spectroscopy (FTIR) (Fig. S1†). The FTIR spectra show that after polymerizations the absorption of aldehyde groups at 1690 cm−1 disappeared, and instead, the stretching vibrations of N–H at 3410 cm−1 and 1195 cm−1, and C–H at 2917 cm−1 belonging to the aminal linkages appeared, confirming the formation of aminal linkages. In addition, the characteristic absorptions of triazine rings were observed at 1540 cm−1.
Using nitrogen as the probe, the comparisons of pore sizes and distributions for POPs-X-FP were conducted through the analyses of the adsorption isotherms by nonlocal density functional theory (NLDFT). As shown in Fig. S2 and Table S1,† the pore size of POPs-X-FP is mainly distributed at 1.27 nm and the specific surface areas of POP-2-FP, POP-3-FP, and POP-4-FP were 199 m2 g−1, 141 m2 g−1, and 241 m2 g−1, respectively, which can prove that POPs-X-FP has a porous structure.
2.2 Preparation of the composite polymer solid electrolyte
CSE-X-FP: as shown in Fig. 1b, PEO and LiTFSI were vacuum dried, and then mixed with an EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li+ molar ratio of 10
Li+ molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then 1 wt% POPs-X-FP was added to the PEO and LiTFSI mixture, which were dissolved in acetonitrile and stirred until evenly dispersed. The slurry was poured into a mold and naturally dried for 12 h, followed by drying for 24 h at 60 °C to completely vaporize the residual solvent. The as-received membrane has a thickness of 150–180 μm and was punched into 19 mm diameter disks, which were stored in an argon-filled glove box for future use.
1. Then 1 wt% POPs-X-FP was added to the PEO and LiTFSI mixture, which were dissolved in acetonitrile and stirred until evenly dispersed. The slurry was poured into a mold and naturally dried for 12 h, followed by drying for 24 h at 60 °C to completely vaporize the residual solvent. The as-received membrane has a thickness of 150–180 μm and was punched into 19 mm diameter disks, which were stored in an argon-filled glove box for future use.
3. Results and discussion
3.1 Electrolyte performance tests
For PEO-based all-solid composite electrolytes, ionic conductivity is an important parameter affecting their application in energy storage devices. Ionic conductivity tests were conducted on CSE-X-FP. As shown in Fig. S4,† CSE-X-FP has a lower impedance than PEO-LiTFSI. The ionic conductivity is greatly enhanced compared to PEO-LiTFSI. The XRD patterns of the CSE-X-FP and PEO-LiTFSI electrolytes are shown in Fig. 2b. It can be observed that PEO-LiTFSI exhibits very sharp characteristic diffraction peaks at 19° and 23°. In contrast, the characteristic diffraction peaks of CSE-X-FP are weaker and broader with the addition of POPs-X-FP, indicating that the addition of polyamines reduced the crystallinity of PEO, which led to better transport of Li+ in the electrolyte. Furthermore, DSC was used to characterise the phase change behaviour of CSE-X-FP. As shown in Fig. S3,† the glass transition temperature and melting transition point of CSE-X-FP were reduced compared to PEO + LiTFSI, suggesting that the addition of POPs inhibits chain recombination in the PEO matrix and increases the amorphous phase, which facilitates ion transport. In addition, Raman spectroscopy was performed to study the dissociation of lithium salts in CSE-X-FP. As shown in Fig. 2c, the peaks at 725–755 cm−1 can be divided into two different dissociation states: free TFSI− at 740 cm−1 and contact ion pair at 744 cm−1.33 From Fig. 2c, it can be seen that the peak intensity at 740 cm−1 is lower than that at 744 cm−1 in PEO-LiTFSI, while the peak intensity at 740 cm−1 is higher than that at 744 cm−1 in CSE-X-FP, indicating that the large amount of N-polyamine rich structure on the surface of POPs-X-FP promotes the dissociation of LiTFSI through Lewis acid–base interactions, as shown in Fig. 2e.31,34 Furthermore, C–F on POPs-X-FP weakens the interaction between Li+ and ether oxygen in PEO, resulting in a higher ionic conductivity compared to PEO-LiTFSI. Additionally, the Li+ transfer number (tLi+) of CSE-2-FP, CSE-3-FP, and CSE-4-FP was measured to be 0.18, 0.169, and 0.155, respectively. In contrast, the tLi+ of PEO-LiTFSI was only 0.095 (Fig. S5†). The electrochemical window was tested by linear scanning voltammetry (LSV) as shown in Fig. 2d. PEO decomposition started at 4.1 V, while CSE-2-FP, CSE-3-FP, and CSE-4-FP were stable at 4.5 V, 5.1 V and 5 V, respectively. The elevated electrochemical window implies that it can be matched to a high voltage cathode.3.2 Characterization of the catalytic capacity of POPs-X-FP
To study the effect of POPs-X-FP on SEI composition, XPS was used to analyze the chemical composition of the lithium metal surfaces after constant current polarization at 0.1 mA cm−2 (Fig. S6–S8†). The atomic percentages of the different elements on the lithium metal surface were calculated according to the elemental sensitivity factor method, as shown in Table S2.† Combining the F 1s spectra of the circulating Li surfaces in different electrolytes (Fig. 3a) and Table S3† the LiF concentrations were calculated to be 6.68%, 16.28% and 8.72% for CSE-2-FP, CSE-3-FP and CSE-4-FP, respectively. LiF was derived from the combination of fluorine ions generated by the decomposition of TFSI− with Li+.3,35,36 The different LiF content was attributed to the different catalytic effect on TFSI− decomposition caused by the change in the substitution position of fluorine atoms in the POPs.36 To investigate the effect of the POPs on TFSI− decomposition, we assembled Li//CSE//SS (stainless steel) cells for cyclic voltammetry (CV) tests (Fig. 3b and c). The peak in the 0.8–1.4 V region is associated with TFSI− reduction decomposition,36,37 and the reduction currents of TFSI− in the electrolytes of CSE-2-FP, CSE-3-FP, and CSE-4-FP were 1.24 × 10−5 A, 1.40 × 10−5 A, and 1.29 × 10−5 A in the first scan, respectively. The different current arose from the different strength of catalytic ability of POPs for TFSI− decomposition, which can be regulated by changing the substitution position to achieve the control of the content of LiF in the SEI. After that, the cells were subjected to a second round of the CV test, and the disappearance of the peak in this region indicated that TFSI− was no longer decomposed. This indicates that the LiF content in the SEI did not change, which means that the SEI with a different LiF content has been successfully constructed.To reveal the effect of the difference of POPs on the catalytic ability of TFSI− decomposition (Fig. 3d), we used density functional theory (DFT) to calculate the effect of POPs on the C–F bond length of TFSI−. As shown in Table S4,† the bond lengths of C–F in TFSI− interacting with fluorine atoms on POPs-2-FP, POPs-3-FP, and POPs-4-FP are 1.359 Å, 1.377 Å, and 1.372 Å, respectively. The substitution position of the fluorine atom on POPs resulted in the variation of the electron cloud distribution between the benzene ring and the fluorine atom, which leads to variation of the C–F bond length. A longer C–F bond means that less activation energy is required for the formation of LiF which results in a higher content of LiF in the SEI layer. The bond length results show that POPs-3-FP has the strongest catalytic ability and therefore the highest amount of LiF in the SEI layer. Therefore, by regulating the chemical structure of the filler surface, the bond length of the C–F bond in TFSI− can be controlled, and thus the LiF content in the SEI layer can be regulated.38
3.3 Effect of different components of the SEI on lithium deposition
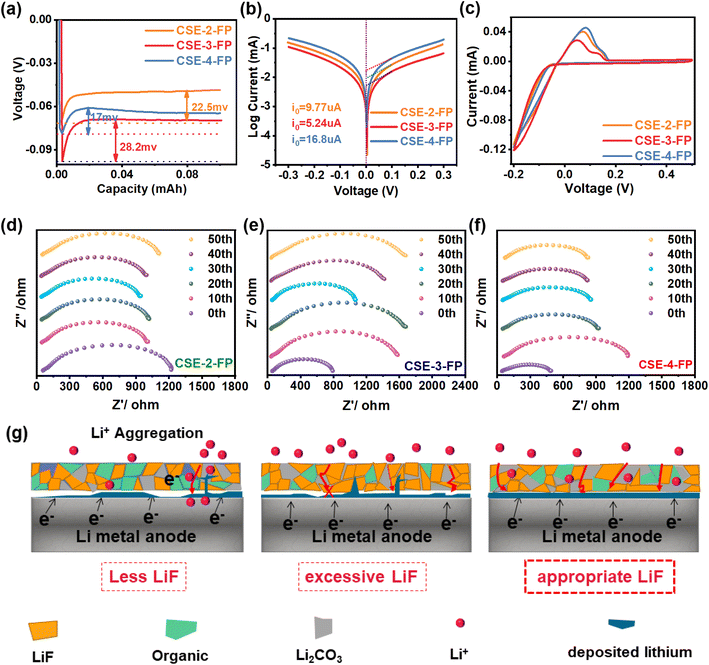
To evaluate the lithium dendrite inhibition effect of the SEI with different contents of LiF, we investigated the interfacial stability of CSE and lithium metal in Li//CSE//Li half-cells at 0.1 mA cm−2 current density. As shown in Fig. 4a, the initial polarization voltages of CSE-2-FP, CSE-3-FP, and CSE-4-FP were 65 mV, 100 mV, and 46 mV, respectively. Apparently CSE-3-FP exhibited the highest initial polarization voltage, and the voltage dropped to 0 V at 297 h suddenly, indicating the failure of the symmetrical cell due to a short circuit. The polarization voltage of CSE-2-FP was larger than that of CSE-4-FP, and when the cycle time increased to 1052 h, the short circuit of CSE-2-FP occurred as manifested by a voltage drop. Meanwhile CES-4-FP could be cycled stably for more than 1200 h (Fig. 4b). Therefore, SEI layers with different LiF contents have great impact on the cycling performance of the Li symmetric cells. Also, cycling tests at different current densities (0.2–0.4 mA cm−2) were performed at 60 °C to investigate the effect of SEI layers with different LiF contents on cycling stability at higher current densities. As shown in Fig. S9,† the polarization voltage of CSE-4-FP was smaller and more stable than that of CSE-2-FP and CSE-3-FP. This indicates that the SEI constructed with CSE-4-FP facilitated lithium deposition and delayed the growth of lithium dendrites to improve cell stability.To further investigate the effect of the SEI with different LiF contents on lithium deposition, the surface of lithium metal in the Li//CSE//Li cell after 100 and 350 cycles at a current density of 0.1 mA cm−2 was studied using scanning electron microscopy (SEM). As shown in Fig. 4c, after 100 cycles, the lithium surface of CSE-2-FP shows many ripples and a small amount of mossy lithium. In contrast, CSE-3-FP showed a relatively rough lithium surface, forming many small bumps and mossy lithium (Fig. 4d). In stark contrast, CSE-4-FP exhibited a smooth and flat lithium surface (Fig. 4e). After 350 cycles, it can be observed that the lithium surface of the CSE-2-FP cell was very rough and covered with compact spherical lithium (Fig. 4f). In the CSE-3-FP cell, the lithium surface was uneven with protrusions and deep pits (Fig. 4g), which might be caused by the excessive LiF in the SEI that hindered uniform lithium deposition. The lithium surface of CSE-2-FP is covered with dense spherical lithium, which means that fewer dendrites were formed on the lithium surface. The lithium surface of CSE-3-FP is very uneven, and there are many sites for the growth of lithium dendrites, so it is difficult to return many dendrites to normal after short circuit. In contrast, the lithium surface of the CSE-4-FP cell was flat and covered with dense, fine spherical lithium (Fig. 4h). Compared with CSE-3-FP and CSE-2-FP, CSE-4-FP can effectively facilitate uniform lithium deposition on the lithium surface, indicating its better stability to lithium and enhanced ability to inhibit dendrite growth, which is attributed to POPs-4-FP catalyzing the formation of an appropriate amount of LiF in the SEI and thus improving SEI performance.24
3.4 Performance and kinetics of Li plating/stripping
In order to reveal the differences in the SEI formed from different POPs, Li‖Cu cells and Li‖Li symmetric cells were tested to evaluate the lithium plating/stripping behavior and kinetics in various electrolytes. As shown in Fig. 5a, the nucleation overpotentials of CSE-2-FP, CSE-3-FP, and CSE-4-FP measured from the Li//CSE//Li cells at the initial stage of lithium deposition were 22.5 mV, 28.2 mV, and 17 mV, respectively,39,40 indicating that CSE-4-FP regulated lithium deposition with the lowest potential barrier. The exchange current density (I0) measured for the Li//CSE//Li cells (Fig. 5b) is related to the ion transfer kinetics at the SEI,41 with I0 being 16.8 μA for CSE-4-FP, which is 3.2 and 1.7 times higher than that of CSE-3-FP and CSE-2-FP, respectively, demonstrating that the SEI formed by CSE-4-FP has faster Li+ transfer kinetics than that formed by CSE-2-FP and CSE-3-FP. This is because POPs-4-FP catalyzes the formation of a moderate amount of LiF in the SEI, which results in better Li+ transfer kinetics of the SEI. Furthermore, the lithium plating/stripping of the Li//CSE//Cu cell was tested by CV (Fig. 5c), and CSE-4-FP showed a strong lithium stripping peak, suggesting that it was more reversible than CSE-2-FP and CSE-3-FP. Meanwhile, the cycling impedance of the Li//CSEs//Li cell was tested, as shown in Fig. 5d–f. The impedance of CSE-3-FP increased sharply with the increase in cycling time. After activation, the impedance of CSE-4-FP was more stable than those of CSE-2-FP and CSE-3-FP, indicating that the SEI formed by POPs-4-FP catalysis prevented massive electrolyte decomposition and facilitated uniform lithium deposition, thus reducing the interfacial impedance.23,26,31These results demonstrate that the content of LiF has a great impact on the performance of the SEI and in turn the performance of the battery. As shown in Fig. 5g, the SEI layer formed by CSE-2-FP has the least amount of LiF, and the poor electron-insulating ability of the SEI may cause electron tunneling through the SEI layer, thus inducing massive decomposition of the electrolyte.26 This is manifested by the formation of a large amount of Li2CO3, as indicated by the highest peaks of C–O in the C 1s of CSE-2-FP (Fig. S10†). In contrast, the SEI formed by the CSE-3-FP electrolyte has the highest LiF content, which affords a SEI with good electronic insulation against electrolyte decomposition. However, LiF has a low Li+ conductivity and a high Li+ diffusion energy barrier (0.729 eV), which increases the lithium plating/stripping overpotential, thus inducing lithium dendrite growth and high interfacial impedance.25,42–45 The SEI layer of CSE-4-FP has an appropriate LiF content, which endows the SEI with good electron insulation and Li+ transport, thus promoting uniform lithium deposition and resulting in good performance.
3.5 Electrochemical performance of the Li‖LFP full cells
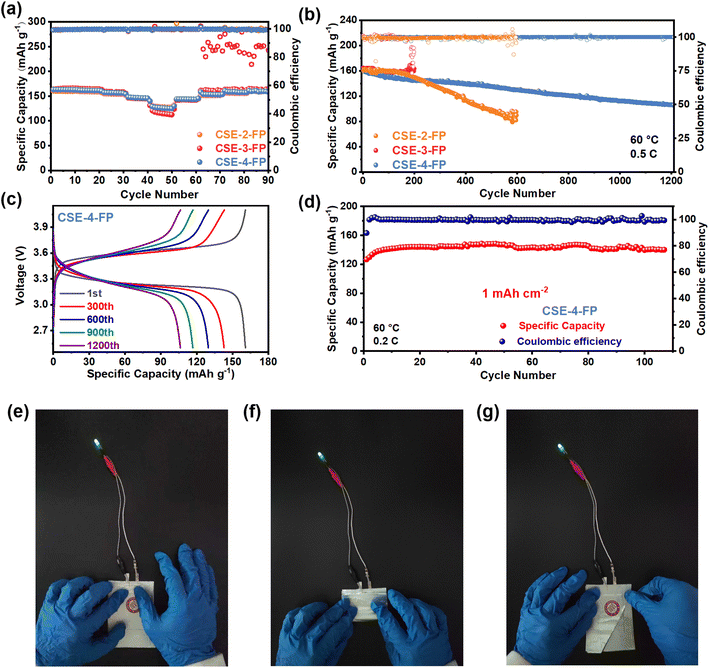
In order to investigate the effect of different SEIs on the performance of full cells, the rate performance of the LFP//CSE//Li cell was tested at 60 °C (Fig. 6a). The capacities of CSE-2-FP, CSE-3-FP, and CSE-4-FP at 0.1C were 160.8 mA h g−1, 163.9 mA h g−1, and 161.4 mA h g−1, respectively. At 2C, the capacities of CSE-2-FP and CSE-4-FP remained relatively stable. But the capacity of CSE-3-FP continued to decrease when the rate increased to 2C. When the rate gradually returned to 0.1C, the coulombic efficiency of CSE-3-FP decreased, and the charging curve jittered and eventually the cell failed. When resumed to 0.1C, the capacities for the CSE-2-FP and CSE-4-FP cells were 159.5 mA h g−1 and 159.7 mA h g−1, with a capacity retention of 99.2% and 98.9%, respectively. The LFP//CSE//Li cells were tested at 60 °C and 0.5C for long cycles (Fig. 6b). The initial capacities of the CSE-2-FP, CSE-3-FP, and CSE-4-FP cells were 160.8 mA h g−1, 164.4 mA h g−1, and 160.7 mA h g−1, respectively. CSE-2-FP failed after 590 cycles with a remaining specific capacity of 84 mA h g−1. During the initial cycling, the capacity decreased rapidly with increasing cycle time, while the polarization voltage continued to increase (Fig. S11†). The failure of CSE-2-FP was attributed to the low LiF content (6.68%) in the SEI, which made the SEI electrically poorly insulated, resulting in continuous electrolyte decomposition that increased the interfacial impedance. CSE-3-FP failed after 190 cycles with a noisy charging voltage curve (Fig. S12†), and the voltage could not rise normally to the cutoff voltage, suggesting that the growth of lithium dendrites punctured the solid electrolyte film, causing a large number of micro-shorts and eventually the battery failure. It is shown that excessive LiF (16.28%) in the SEI increased the migration potential of Li+ and thus led to the growth of lithium dendrites. As shown in Fig. 6c, CSE-4-FP cycled over 1200 cycles with a remaining specific capacity of 108 mA h g−1 and with no significant change in polarization voltage, which was attributed to the formation of a SEI with an appropriate amount of LiF (8.72%) in CSE-4-FP that promoted uniform lithium deposition, reduced the side reactions between the electrolyte and lithium, and improved the cycling performance. These results demonstrate that the content of LiF in the SEI impacts the battery performance significantly. Fig. S13† summarizes the cycle life and LiF ratios. It can be found that a too high (16.28%) or too low (6.68%) LiF content in the SEI leads to bad battery performance, and a LiF content of 8.72% exhibits the best battery performance.To evaluate the practical application of CSE-4-FP, the active substance loading was increased to 1 mA h cm−2 (6 mg cm−2), and the LFP//CSE-4-FP//Li cell had a remaining specific capacity of 140 mA h g−1 after more than 100 cycles (Fig. 6d). A 1C cycling test was also carried out to further discuss the cycling stability of CSE-4-FP (Fig. S14†). After 100 cycles, the specific capacity was 133 mA h g−1 and the capacity retention rate was 92%. In addition, LiNi0.8Co0.1Mn0.1O2 (NMC811) was selected as the cathode to explore the compatibility of CSE-4-FP with a high voltage cathode. As shown in Fig. S15,† at a discharge rate of 1C, the initial discharge specific capacity is 162.5 mA h g−1 and after 100 cycles the discharge specific capacity is 145.1 mA h g−1. The low temperature application is an important indicator for evaluating the application of polymer electrolytes, and after 0.2C cycles at 30 °C (Fig. S16†), the discharge capacity reached 151 mA h g−1 and remained stable after activation. These results demonstrate that CSE-4-FP can meet the needs of practical applications. Furthermore, safety is an important indicator for the practical application of batteries. As shown in Fig. 6e–g, the pouch cell of CSE-4-FP still light up LED bulbs after being folded and cut, which proves the fantastic safety performance of SSLMBs based on CSE-4-FP. In the previous test, a lithium sheet with a thickness of several hundred microns was used, which would reduce the effect of side reactions between the lithium and CSE, so a 50 μm thin lithium sheet was also tested. The LFP//CSE-4-FP//Li cell exhibited an initial capacity of 144 mA h g−1, and after 200 cycles, the capacity was still 125 mA h g−1 with an average coulombic efficiency of 99.16% (Fig. S17†), which indicates that the SEI formed by POPs-4-FP effectively promoted the uniform deposition of lithium and inhibited the electrolyte decomposition, thus improving the cycling performance of the battery.
4. Conclusions
In this work, we prepared composite solid-state electrolytes (CSE-X-FP, X = 2, 3, 4) by using porous organic polymers (POPs-X-FP, X = 2, 3, 4) with different structures as fillers and compounding them with PEO. We found that the change in the substitution position of fluorine atoms on POPs resulted in different performances of POPs in extending the C–F bond length on TFSI−, which endowed POPs with different catalytic abilities of TFSI− decomposition, and thus successfully constructed SEI layers with different LiF contents. Based on the SEI layers with different LiF contents, we performed a series of electrochemical performance tests on CSE-X-FP. The exchange current density of CSE-4-FP (LiF content of 8.72%) is 1.7 times and 3.2 times higher than that of CSE-2-FP (LiF content of 6.68%) and CSE-3-FP (LiF content of 16.28%), respectively. Benefiting from the good performance of the SEI, the full cell of LFP//CSE-4-FP//Li reaches a capacity of 108 mA h g−1 after 1200 cycles, while LFP//CSE-2-FP//Li and LFP//CSE-3-FP//Li full cells fail after 590 and 190 cycles, respectively. The high loading active material full cells and the thin lithium cells of POPs-4-FP still show good performance. The above results show that the content of LiF at around 8.7% in the SEI exhibits the best performance of the SSLMBs. This work provides an important strategy to control the LiF content in the SEI layer to boost the performance of SSLMBs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province, China (No. 2020JJ5530); National Natural Science Foundation of China (No. 51772262, U20A20336, and 21935009); Natural Science Foundation of Hebei Province (No. B2020203037).References
- S. K. Jin and W. Y. Yoon, Electrochim. Acta, 2004, 50, 531–534 CrossRef.
- B. Liu, J. G. Zhang and W. Xu, Joule, 2018, S2542435118300977 Search PubMed.
- L. Yue, J. Ma, J. Zhang, J. Zhao, S. Dong, Z. Liu, G. Cui and L. Chen, Energy Storage Mater., 2016, 5, 139–164 CrossRef.
- Q. Zhang, K. Liu, F. Ding and X. Liu, Nano Res., 2017, 10, 36 Search PubMed.
- Z. Li, J. Fu, S. Zheng, D. Li and X. Guo, Small, 2022, 18, 2200891 CrossRef CAS PubMed.
- D. Cao, X. Sun, Q. Li, A. Natan, P. Xiang and H. Zhu, Matter, 2020, 3, 57–94 CrossRef.
- H. Shen, K. Chen, J. Kou, Z. Jia, N. Tamura, W. Hua, W. Tang, H. Ehrenberg and M. Doeff, Mater. Today, 2022, 57, 181–190 CrossRef.
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef CAS PubMed.
- Z. Xue, D. He and X. Xie, J. Mater. Chem. A, 2015, 3, 19218–19253 RSC.
- Y. Ji, K. Yang, M. Liu, S. Chen, X. Liu, B. Yang, Z. Wang, W. Huang, Z. Song and S. Xue, Adv. Funct. Mater., 2021, 31, 2104830 CrossRef CAS.
- Z. Yu, X. Zhang, C. Fu, H. Wang, M. Chen, G. Yin, H. Huo and J. Wang, Adv. Energy Mater., 2021, 11, 2003250 CrossRef CAS.
- M. Yan, J. Y. Liang, T. T. Zuo, Y. X. Yin, S. Xin, S. J. Tan, Y. G. Guo and L. J. Wan, Adv. Funct. Mater., 2020, 30, 1908047 CrossRef CAS.
- L. Zhang, T. Yang, C. Du, Q. Liu, Y. Tang, J. Zhao, B. Wang, T. Chen, Y. Sun and P. Jia, Nat. Nanotechnol., 2020, 15, 94–98 CrossRef CAS PubMed.
- Z. Ju, J. Nai, Y. Wang, T. Liu, J. Zheng, H. Yuan, O. Sheng, C. Jin, W. Zhang and Z. Jin, Nat. Commun., 2020, 11, 1–10 CrossRef PubMed.
- B. Wu, J. Lochala, T. Taverne and J. Xiao, Nano Energy, 2017, 40, 34–41 CrossRef CAS.
- Y. Gao, Z. Yan, J. L. Gray, X. He, D. Wang, T. Chen, Q. Huang, Y. C. Li, H. Wang and S. H. Kim, Nat. Mater., 2019, 18, 384–389 CrossRef CAS PubMed.
- J. Tan, J. Matz, P. Dong, J. Shen and M. Ye, Adv. Energy Mater., 2021, 11, 2100046 CrossRef CAS.
- Y. Yuan, F. Wu, Y. Bai, Y. Li, G. Chen, Z. Wang and C. Wu, Energy Storage Mater., 2019, 16, 411–418 CrossRef.
- X. Yang, M. Jiang, X. Gao, D. Bao, Q. Sun, N. Holmes, H. Duan, S. Mukherjee, K. Adair and C. Zhao, Energy Environ. Sci., 2020, 13, 1318–1325 RSC.
- Y. Liu, R. Hu, D. Zhang, J. Liu, F. Liu, J. Cui, Z. Lin, J. Wu and M. Zhu, Adv. Mater., 2021, 33, 2004711 CrossRef CAS PubMed.
- C. Gong, S. D. Pu, X. Gao, S. Yang, J. Liu, Z. Ning, G. J. Rees, I. Capone, L. Pi and B. Liu, Adv. Energy Mater., 2021, 11, 2003118 CrossRef CAS.
- J. Zhao, L. Liao, F. Shi, T. Lei, G. Chen, A. Pei, J. Sun, K. Yan, G. Zhou and J. Xie, J. Am. Chem. Soc., 2017, 139, 11550–11558 CrossRef CAS PubMed.
- O. Sheng, J. Zheng, Z. Ju, C. Jin, Y. Wang, M. Chen, J. Nai, T. Liu, W. Zhang and Y. Liu, Adv. Mater., 2020, 32, 2000223 CrossRef CAS PubMed.
- Z. A. Zhao, A. Jw, A. Sz, A. Hy, A. Zz, M. B. Fei, A. Ph, A. Ty, A. Gh and A. Wqh, Energy Storage Mater., 2021, 43, 229–237 CrossRef.
- Z. Zhu, Y. Tang, Z. Lv, J. Wei, Y. Zhang, R. Wang, W. Zhang, H. Xia, M. Ge and X. Chen, Angew. Chem., Int. Ed., 2018, 130, 3718–3722 CrossRef.
- D. Wu, J. He, J. Liu, M. Wu, S. Qi, H. Wang, J. Huang, F. Li, D. Tang and J. Ma, Adv. Energy Mater., 2022, 2200337 CrossRef CAS.
- C. Cui, C. Yang, N. Eidson, J. Chen, F. Han, L. Chen, C. Luo, P. F. Wang, X. Fan and C. Wang, Adv. Mater., 2020, 32, 1906427 CrossRef CAS PubMed.
- X. Zou, H. Ren and G. Zhu, Chem. Commun., 2013, 49, 3925–3936 RSC.
- N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef CAS.
- B. Zhang, B. Li and Z. Wang, ACS Sens., 2020, 5, 162–170 CrossRef CAS PubMed.
- C. Li, S. Zhou, L. Dai, X. Zhou, B. Zhang, L. Chen, T. Zeng, Y. Liu, Y. Tang and J. Jiang, J. Mater. Chem. A, 2021, 9, 24661–24669 RSC.
- G. Li, B. Zhang and Z. Wang, Macromolecules, 2016, 49, 2575–2581 CrossRef CAS.
- W. Liu, C. Yi, L. Li, S. Liu, Q. Gui, D. Ba, Y. Li, D. Peng and J. Liu, Angew. Chem., Int. Ed., 2021, 133, 13041–13050 CrossRef.
- L. Gao, J. Li, J. Ju, B. Cheng, W. Kang and N. Deng, J. Energy Chem., 2021, 54, 644–654 CrossRef CAS.
- G. G. Eshetu, X. Judez, C. Li, M. Martinez-Ibañez, I. Gracia, O. Bondarchuk, J. Carrasco, L. M. Rodriguez-Martinez, H. Zhang and M. Armand, J. Am. Chem. Soc., 2018, 140, 9921–9933 CrossRef CAS PubMed.
- Y. Liu, X. Tao, Y. Wang, C. Jiang, C. Ma, O. Sheng, G. Lu and X. W. Lou, Science, 2022, 375, 739–745 CrossRef CAS PubMed.
- Z. Zhao, W. Chen, S. Impeng, M. Li, R. Wang, Y. Liu, L. Zhang, L. Dong, J. Unruangsri and C. Peng, J. Mater. Chem. A, 2020, 8, 3459–3467 RSC.
- X. Zhou, C. Li, B. Zhang, F. Huang, P. Zhou, X. Wang and Z. Ma, J. Mater. Sci. Technol., 2023, 136, 140–148 CrossRef.
- R. Zhang, X. R. Chen, X. Chen, X. B. Cheng, X. Q. Zhang, C. Yan and Q. Zhang, Angew. Chem., Int. Ed., 2017, 129, 7872–7876 CrossRef.
- K. Yan, Z. Lu, H.-W. Lee, F. Xiong, P.-C. Hsu, Y. Li, J. Zhao, S. Chu and Y. Cui, Nat. Energy, 2016, 1, 1–8 Search PubMed.
- X. Wang, S. Wang, H. Wang, W. Tu, Y. Zhao, S. Li, Q. Liu, J. Wu, Y. Fu and C. Han, Adv. Mater., 2021, 33, 2007945 CrossRef CAS PubMed.
- X. Ji, S. Hou, P. Wang, X. He, N. Piao, J. Chen, X. Fan and C. Wang, Adv. Mater., 2020, 32, 2002741 CrossRef CAS PubMed.
- Y. Liu, X. Xu, O. O. Kapitanova, P. V. Evdokimov, Z. Song, A. Matic and S. Xiong, Adv. Energy Mater., 2022, 12, 2103589 CrossRef CAS.
- Y. Zhang, T.-T. Zuo, J. Popovic, K. Lim, Y.-X. Yin, J. Maier and Y.-G. Guo, Mater. Today, 2020, 33, 56–74 CrossRef CAS.
- R. M. Gao, H. Yang, C. Y. Wang, H. Ye, F. F. Cao and Z. P. Guo, Angew. Chem., Int. Ed., 2021, 60, 25508–25513 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09896b |
| This journal is © The Royal Society of Chemistry 2023 |