Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two-component supramolecular hydrogel for controlled drug release†
Anna K.
Patterson
and
David K.
Smith
 *
*
Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: david.smith@york.ac.uk
First published on 18th August 2020
Abstract
A hybrid gel has been developed by combining two supramolecular gelators. Each gelator endows the hybrid gel with its own characteristics. One gelator enables pH-mediated controlled release of the active pharmaceutical ingredient naproxen, while the other new gelator enhances mechanical stability. Self-assembly thus gives multi-functional gels with potential applications.
Supramolecular hydrogels, soft materials typically comprising ca. 99% water immobilised by a network of entangled fibres, are of increasing interest in biomedical applications.1 The fibres self-assemble into a ‘solid-like’ network from a low-molecular-weight gelator (LMWG).2 Self-assembled hydrogels have many attractive properties, particularly their responsive nature and generally high biocompatibility.3 There is also the potential to program desired functionality into the bulk gel, through simple molecular-scale modifications of the LMWG.4 This has led to increasing interest in their use in biomedical settings, including drug delivery.5 However, supramolecular gels often have low mechanical strength, making them difficult to manipulate.
One way of enhancing the mechanical strength of supramolecular hydrogels is to combine them with polymer gelators, which are generally more mechanically robust, albeit less responsive to external stimuli.6 Increasingly, hybrid gels comprising two or more supramolecular gelators are also being studied, with the hope that the properties of the overall gel can be modified by tuning the ratio of the components, although applications of this approach remain rare.7 Combining multiple gelators is potentially a simple way of accessing gels with different properties.8 When two LMWGs are combined, self-assembly can occur in different ways giving different types of self-organised material. The individual gelators may preferentially interact with other gelators of the same type, leading to ‘self-sorted’ fibres.9 Alternatively, each gelator may preferentially interact with the other gelator, and fibrils will assemble with alternating gelators.10 If the gelators have no strong preference for either interaction, then random mixing can occur.11 Finally, one gelator may disrupt the assembly of the other through non-productive interactions, causing loss of gelation. Self-sorting gelators are desirable because orthogonal networks can be exploited to control the properties of the gel (Fig. 1).12
In recent years, we have investigated hydrogels based on the industrially-relevant 1,3:2,4-dibenzylidenesorbitol (DBS) framework.13 The parent molecule (DBS) and simple derivatives, have long been exploited for thickening properties, but were not previously capable of forming hydrogels as they are too insoluble in water. By modifying the aromatic ‘wings’ of DBS, we developed hydrogelators.14 One of these is DBS-CONHNH2, with hydrophilic acyl hydrazide groups.15 It forms a hydrogel using a simple heat/cool cycle, and is stable across a range of pH values. Hydrogels formed from DBS-CONHNH2 have been used for environmental remediation,15 drug delivery,16 and cell culture.17 Controlled release of active pharmaceutical ingredients (APIs) like naproxen (NPX) can be achieved as a result of interactions between the DBS-CONHNH2 fibres and the API carboxylic acid. However, the gels are mechanically weak and difficult to manipulate. We have previously combined DBS-CONHNH2 with polymer gelators to enhance rheological performance.18 Here, we wanted to combine DBS-CONHNH2 with a LMWG to produce a new tuneable, responsive gel.
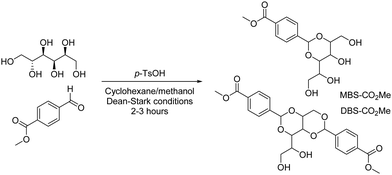
In the hunt for suitable LMWGs to combine with DBS-CONHNH2, we decided to develop a new gelator, having noticed interesting properties of a by-product from its synthesis (Scheme 1). DBS-CO2Me is made by condensation of D-sorbitol and 4-methylcarboxy benzaldehyde, giving mono-, di- and tri-substituted sorbitol derivatives (MBS, DBS and TBS derivatives). This reaction can be optimised for either DBS (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aldehyde
1 aldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbitol) or MBS product (1
sorbitol) or MBS product (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 aldehyde
4 aldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbitol) (for full method and characterisation see ESI,† Fig. S1 and S2). We noticed MBS-CO2Me formed effective gels in water. The ability of simpler MBS derivatives to form hydrogels is known in the literature.19DBS-CO2Me does not form hydrogels because it is insufficiently soluble, but for MBS-CO2Me, the lack of one aromatic ring, and the presence of two additional OH groups increases hydrophilicity, making it sufficiently water-soluble. The effect of solubility on gelation is increasingly understood.20
sorbitol) (for full method and characterisation see ESI,† Fig. S1 and S2). We noticed MBS-CO2Me formed effective gels in water. The ability of simpler MBS derivatives to form hydrogels is known in the literature.19DBS-CO2Me does not form hydrogels because it is insufficiently soluble, but for MBS-CO2Me, the lack of one aromatic ring, and the presence of two additional OH groups increases hydrophilicity, making it sufficiently water-soluble. The effect of solubility on gelation is increasingly understood.20
We investigated the properties of this new hydrogelator. Gelation was achieved via a heat/cool cycle, giving transparent gels (Fig. S4, ESI†). The minimum gelation concentration (MGC) was 0.75% wt/vol, with lower loadings giving solutions. SEM and TEM indicated a fibrous network. TEM shows relatively homogeneous helical fibres, with a diameter of ca. 8 nm (Fig. 2, left). Drying gels can cause morphological change21 – in an attempt to limit this, we cryo-dried samples for SEM. The small nanofibres and relative lack of aggregation are consistent with the observation that gels formed by MBS-CO2Me in water are transparent. This is similar to gels formed by DBS-CONHNH2, which also have nanofibres ca. 10 nm in diameter.15
 | ||
| Fig. 2 Left: TEM image of an MBS-CO2Me hydrogel; scale bar 100 nm. Right: SEM image of an MBS-CO2Me hydrogel; scale bar 1 μm. | ||
Rheology was carried out to explore the mechanical properties of the MBS-CO2Me hydrogel. At a loading of 0.85% wt/vol, G′ > G′′ in the linear viscoelastic region (LVR), with G′ being ca. 2800 Pa (Fig. S6a, ESI†). This is a significantly stiffer gel than that formed by DBS-CONHNH2 (G′ = 1200 Pa). The two gels had similar elasticity, with G′/G′′ crossover points of ca. 12%. Interestingly, MBS-CO2Me was much more robust and easier to physically manipulate than DBS-CONHNH2, with gel discs being easily transferred for rheology, with no breakage.
Variable temperature (VT) circular dichroism (CD) spectroscopy of MBS-CO2Me had a maximum ellipticity at ca. 280 nm, showing the aromatic ring of MBS-CO2Me experiences a chiral environment (Fig. S10, ESI†). This CD band was temperature-dependent, proving the chiral environment is generated by self-assembly. The most significant change in ellipticity was at 45–55 °C, supportive of a gel–sol transition at this temperature.
Simple tube inversion methodology confirmed MBS-CO2Me hydrogels indeed had Tgel values at ca. 55 °C, significantly lower than DBS-CONHNH2 (ca. 80 °C), despite the higher concentration (Table S1, ESI†). We reason the greater hydrophilicity of MBS-CO2Me makes it more temperature-sensitive, i.e., it dissolves more easily on heating. When the resulting hot solutions were left to stand overnight, gels re-formed. However, rather than being transparent, they were white, with some precipitate, indicative of greater aggregation and less controlled assembly. When Tgel values for these re-formed gels were determined, those at lower concentrations had lower Tgel values, while there was little impact on the Tgel values at higher concentrations. These gels at higher loadings also re-formed opaque gels for a second time. Rheology indicated a slight increase in G′ values.
MBS-CO2Me gels were then tested for their ability to encapsulate and release the API naproxen (NPX, Fig. 3). This was achieved by mixing the API and gelator as solids in advance of the heat/cool cycle. Hydrogels were still formed (Table S2 and Fig. S8, ESI†), but when API release was investigated, the MBS-CO2Me hydrogel broke-down rapidly (within 3 h) once the supernatant was placed on top of the gel (Fig. 3). This occurred in the presence of any supernatant, including water and a range of buffers. This was not observed with DBS-CONHNH2, and was initially surprising given the MBS-CO2Me gel is more robust. We reason that the higher solubility of MBS-CO2Me makes the gel less stable to the presence of excess aqueous medium. NPX has a maximum absorbance at 329 nm, allowing API release to be quantified via UV-vis spectroscopy. When incubated at 37 °C with pH 7 buffer, around 85% of NPX was released within 3 h, roughly the point at which the gel broke down completely – we thus propose an ‘erosion’ mode of API release for MBS-CO2Me.
 | ||
| Fig. 3 Release of naproxen into pH 7 buffer from MBS-CO2Me hydrogels showing rapid release driven by erosion of the gel. | ||
We combined MBS-CO2Me and DBS-CONHNH2, hoping their different properties would be synergistic. Our goal was a hybrid gel with mechanical properties enhanced by MBS-CO2Me, but maintaining the controlled release and water stability of DBS-CONHNH2. Initially, gelation was optimised. DBS-CONHNH2 and MBS-CO2Me hydrogels have very different MGCs (0.20% and 0.75% wt/vol respectively). Varying concentrations were explored, with the gelators mixed as solids. The total MGC for the hybrid gel was 0.3% wt/vol (DBS-CONHNH2 at 0.2% wt/vol, and MBS-CO2Me at 0.1% wt/vol). Hybrid hydrogels formed even with both gelators below their individual MGCs (DBSCONHNH2 at 0.16% wt/vol and MBS-CO2Me at 0.2% wt/vol).
Hydrogels with a range of LMWG concentrations were prepared, and their thermal properties investigated. Given that MBS-CO2Me hydrogels have lower Tgel values (ca. 55 °C) than DBS-CONHNH2 hydrogels (ca. 80 °C), it was predicted that increasing the proportion of MBS-CO2Me in the hybrid gel may lower Tgel, providing access to a range of Tgel values. This was indeed the case, with Tgel values from 27 °C to >100 °C being obtained (Tables S3 and S4, ESI†). In general, a greater proportion of MBS-CO2Me gave a lower Tgel, and a greater proportion of DBS-CONHNH2 gave a higher Tgel. Total gelator loading was also important – more gelator gave higher Tgel values.
SEM and TEM indicated nanofibres of ca. 10 nm diameter (Fig. S4 and S5, ESI†). It was not possible to differentiate nanofibres for the individual gelators, as they are similar in morphology, but it was clear self-assembly in the mixed system was not disrupted, nor did different types of assembly arise as a result of mixing.
VT 1H NMR studies investigated gelator immobilisation in the hybrid gel, and understand how the LMWGs respond to an increase in temperature (Fig. 4). Hybrid gels (0.80% wt/vol MBS-CO2Me and 0.22% wt/vol DBS-CONHNH2) were prepared in an NMR tube using D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50
H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
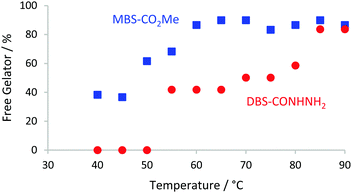
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, D2O alone leads to reduced solubility of MBS-CO2Me), with DMSO (2 μl) as an internal standard. This allows determination of the gelator assembled in the ‘solid-like’ network, because mobile gelator in the ‘liquid-like’ phase is visible in the 1H NMR, and can be quantified by relative integration. At room temperature, neither gelator was observed in the spectrum, indicating both are assembled into ‘solid-like’ networks. As temperature increased, the signals for MBS-CO2Me became visible first, from around 40 °C with around 90% being free at 65 °C. This reflects the higher solubility of MBS-CO2Me and its lower Tgel value (see above). The signal corresponding to mobile DBS-CONHNH2 first appears at around 55 °C, and 90% of the gelator is free at 85 °C, in-line with its higher Tgel value. A small amount of the DBS-CONHNH2 disassembles at ca. 55 °C before MBS-CO2Me disassembly is complete. It is therefore possible that self-sorting in this system is not perfect, but nonetheless, the networks do still largely disassemble in a sequential way and thus with a good degree of self-sorting.
50, D2O alone leads to reduced solubility of MBS-CO2Me), with DMSO (2 μl) as an internal standard. This allows determination of the gelator assembled in the ‘solid-like’ network, because mobile gelator in the ‘liquid-like’ phase is visible in the 1H NMR, and can be quantified by relative integration. At room temperature, neither gelator was observed in the spectrum, indicating both are assembled into ‘solid-like’ networks. As temperature increased, the signals for MBS-CO2Me became visible first, from around 40 °C with around 90% being free at 65 °C. This reflects the higher solubility of MBS-CO2Me and its lower Tgel value (see above). The signal corresponding to mobile DBS-CONHNH2 first appears at around 55 °C, and 90% of the gelator is free at 85 °C, in-line with its higher Tgel value. A small amount of the DBS-CONHNH2 disassembles at ca. 55 °C before MBS-CO2Me disassembly is complete. It is therefore possible that self-sorting in this system is not perfect, but nonetheless, the networks do still largely disassemble in a sequential way and thus with a good degree of self-sorting.
It was hoped the stiffer MBS-CO2Me would reinforce the more interactive, but mechanically weak, DBS-CONHNH2, giving a gel that was easier to manipulate. Hybrid hydrogels with varying loadings of MBS-CO2Me (‘high’: 0.80% wt/vol, ‘low’: 0.10% wt/vol) and DBS-CONHNH2 (‘high’: 0.28% wt/vol, ‘low’: 0.24% wt/vol) were tested by rheology (Table 1 and Fig. S6, S7, ESI†). Pleasingly, with higher proportions of MBS-CO2Me, the hybrid gel was stiffer than either DBS-CONHNH2 or MBS-CO2Me alone (G′ = 9600 Pa). At higher MBS-CO2Me concentrations, increased loading of DBS-CONHNH2 leads to a slightly weaker gel, perhaps as a result of the more extensive weaker DBS-CONHNH2 network being more dominant. However, at the lowest concentrations of MBS-CO2Me, where the concentration of MBS-CO2Me here is below the MGC, the gels are slightly weaker than DBS-CONHNH2 alone, suggesting a small amount of MBS-CO2Me network can disrupt the DBS-CONHNH2 network. Therefore, there is scope to tune the rheological properties of the hybrid hydrogel, to give properties suitable for the desired application.
| Gelator | G′/Pa | G′′/Pa |
|---|---|---|
| DBS-CONHNH2 | 1200 | 60 |
| MBS-CO2Me | 2800 | 550 |
| High MBS, low DBS | 9600 | 700 |
| High MBS, high DBS | 6300 | 900 |
| Low MBS, low DBS | 300 | 25 |
| Low MBS, high DBS | 500 | 30 |
Given DBS-CONHNH2 can achieve pH-mediated naproxen release,18 we wanted to determine if this ability was retained by the hybrid gel, and thus investigated encapsulation and release of NPX. Stable gels were still formed in the presence of NPX, and the addition did not have a significant impact on the rheological properties of the hydrogels – there was perhaps a slight stiffening reflected by a small increase in G′ (Fig. S8 and S9, ESI†). We studied the interaction of the hybrid gels with NPX by 1H NMR. A known mass of each gelator and NPX, were dissolved in D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50
H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), and the hydrogel formed in an NMR tube. An internal standard (DMSO) was added to allow quantification of any ‘mobile’ NPX. These studies indicated that, like DBS-CONHNH2 gels, over 90% of the NPX was not visible in the NMR spectrum, and is therefore likely bound to the gel network as a result of non-covalent interactions between the acid on NPX and the hydrazide. The interactive properties of DBS-CONHNH2 thus appear to translate into the mechanically-robust hybrid gel.
50), and the hydrogel formed in an NMR tube. An internal standard (DMSO) was added to allow quantification of any ‘mobile’ NPX. These studies indicated that, like DBS-CONHNH2 gels, over 90% of the NPX was not visible in the NMR spectrum, and is therefore likely bound to the gel network as a result of non-covalent interactions between the acid on NPX and the hydrazide. The interactive properties of DBS-CONHNH2 thus appear to translate into the mechanically-robust hybrid gel.
The release of NPX from the hybrid gels, into buffers with different pH values, was then investigated by monitoring the UV-vis absorbance at 329 nm. Pleasingly, the supramolecular hybrid gel showed pH-dependent release (Fig. 5). The NPX release profile at pH 7 is similar to that observed previously for DBS-CONHNH2,16 with rapid initial release, and total release of 85–95%. In contrast, release at pH 5.5 is slower and much less favoured, reaching only ca. 35% in the first 3 h, and a maximum of just 55%. This is a result of the interactions between DBS-CONHNH2 fibres and NPX, which occur when NPX is protonated. The pKa of NPX is 4.15,22 and at pH 5.5 a greater proportion of NPX will be protonated than at higher pH values, and thus able to interact with gel nanofibres, limiting release. This controlled release is very different to the erosion release observed when using MBS-CO2Me alone, and is attractive for oral delivery. It has the potential to reduce uptake in the acidic stomach, facilitating drug protection, and may assist controlled release in the small intestine. This is important for NSAIDs such as NPX, which have known side effects in the upper gastrointestinal tract23 Changing LMWG concentrations had little effect on the release process (Fig. S11 and S12, ESI†).
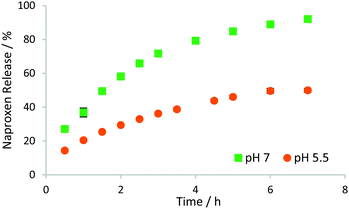 | ||
| Fig. 5 Release of NPX, from hybrid hydrogels based on DBS-CONHNH2 (0.20% wt/vol) and MBS-CO2Me (0.70% wt/vol) at varying pH values. | ||
In summary, a two-component supramolecular hydrogel, formed from two gelators (DBS-CONHNH2 and novel MBS-CO2M), has been used to encapsulate and release NPX. NMR studies indicate that the gel has sequentially assembled networks. Each gelator has its own unique characteristics programmed into the performance of the gel. Specifically, the hybrid gel retains the interactive nature of DBS-CONHNH2, facilitating controlled drug release, while having improved mechanical strength as a result of MBS-CO2Me. NPX release is pH-mediated, lower pH leading to less release, with potential for controlled release in the small intestine. Further work will focus on tuning multi-component hydrogels to give different release profiles, enabling other drug delivery applications, as well as investigating such gels in wider biomedical applications like tissue engineering or wound healing.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) B. Hu, C. Owh, P. L. Chee, W. R. Leow, X. Liu, Y.-L. Wu, P. Guo, X. J. Loh and X. Chen, Chem. Soc. Rev., 2018, 47, 6917–6929 RSC; (b) L. Saunders and P. X. Ma, Macromol. Biosci., 2019, 19, 1800313 CrossRef PubMed.
- (a) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed; (b) E. R. Draper and D. J. Adams, Chem, 2017, 3, 390–410 CrossRef CAS.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed.
- Molecular Gels: Structure and Dynamics, ed. R. G. Weiss, Royal Society of Chemistry, Cambridge, 2018 Search PubMed.
- (a) K. J. Skilling, F. Citossi, T. D. Bradshaw, M. Ashford, B. Kellam and M. Marlow, Soft Matter, 2014, 10, 237–256 RSC; (b) A. Vashist, A. Kaushik, K. Alexis, R. D. Jayant, V. Sagar, A. Vashist and M. Nair, Curr. Pharm. Des., 2017, 23, 3595–3602 CrossRef CAS PubMed; (c) J. Mayr, C. Saldías and D. Díaz Díaz, Chem. Soc. Rev., 2018, 47, 1484–1515 RSC.
- D. J. Cornwell and D. K. Smith, Mater. Horiz., 2015, 2, 279–293 RSC.
- (a) L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089–6102 RSC; (b) J. Raeburn and D. J. Adams, Chem. Commun., 2015, 51, 5170–5180 RSC.
- E. R. Draper and D. J. Adams, Chem. Soc. Rev., 2018, 47, 3395–3405 RSC.
- (a) J. R. Moffat and D. K. Smith, Chem. Commun., 2009, 316–318 RSC; (b) E. R. Draper, E. G. B. Eden, T. O. McDonald and D. J. Adams, Nat. Chem., 2015, 7, 848–852 CrossRef CAS PubMed; (c) N. Singh, K. Zhang, C. A. Angulo-Pachón, E. Mendes, J. H. van Esch and B. Escuder, Chem. Sci., 2016, 7, 5568–5572 RSC; (d) S. Onogi, H. Shigemitsu, T. Yoshii, T. Tanida, M. Ikeda, R. Kubota and I. Hamachi, Nat. Chem., 2016, 8, 743–752 CrossRef CAS PubMed; (e) E. R. Draper, B. Dietrich and D. J. Adams, Chem. Commun., 2017, 53, 1864–1867 RSC; (f) R. Kubota, S. Liu, H. Shigemitsu, K. Nakamura, W. Tanaka, M. Ikeda and I. Hamachi, Bioconjugate Chem., 2018, 29, 2058–2067 CrossRef CAS PubMed; (g) C. C. Piras and D. K. Smith, Chem. – Eur. J., 2019, 25, 11318–11326 CAS; (h) B. O. Okesola, Y. Wu, B. Derkus, S. Gani, D. Wu, D. Knani, D. K. Smith, D. J. Adams and A. Mata, Chem. Mater., 2019, 31, 7883–7897 CrossRef CAS PubMed.
- (a) M. Tena-Solsona, S. Alonso-de Castro, J. F. Miravet and B. Escuder, J. Mater. Chem. B, 2014, 2, 6192–6197 RSC; (b) G.-F. Liu, W. Ji, W.-L. Wang and C.-L. Feng, ACS Appl. Mater. Interfaces, 2015, 7, 301–307 CrossRef CAS PubMed.
- (a) D. M. Ryan, T. M. Doran and B. L. Nilsson, Chem. Commun., 2011, 47, 475–477 RSC; (b) H. A. M. Ardoña, E. R. Draper, F. Citossi, M. Wallace, L. C. Serpell, D. J. Adams and J. D. Tovar, J. Am. Chem. Soc., 2017, 25, 8685–8692 CrossRef PubMed.
- C. Colquhoun, E. R. Draper, E. G. B. Eden, B. N. Cattoz, K. L. Morris, L. Chen, T. O. McDonald, A. E. Terry, P. C. Griffiths, L. C. Serpell and D. J. Adams, Nanoscale, 2014, 6, 13719–13725 RSC.
- B. O. Okesola, V. M. P. Vieira, D. J. Cornwell, N. K. Whitelaw and D. K. Smith, Soft Matter, 2015, 11, 4768–4787 RSC.
- D. K. Smith, Chem. Commun., 2018, 54, 4743–4760 RSC.
- B. O. Okesola and D. K. Smith, Chem. Commun., 2013, 49, 11164–11166 RSC.
- E. J. Howe, B. O. Okesola and D. K. Smith, Chem. Commun., 2015, 51, 7451–7454 RSC.
- V. M. P. Vieira, A. C. Lima, M. de Jong and D. K. Smith, Chem. – Eur. J., 2018, 24, 15112–15118 CrossRef CAS.
- (a) P. R. A. Chivers and D. K. Smith, Chem. Sci., 2017, 8, 7218–7227 RSC; (b) C. C. Piras, P. Slavik and D. K. Smith, Angew. Chem., Int. Ed., 2020, 59, 853–859 CrossRef CAS; (c) P. R. A. Chivers, J. A. Kelly, M. J. S. Hill and D. K. Smith, React. Chem. Eng., 2020, 5, 1112–1117 RSC.
- (a) J. Song, H. Sun, S. Sun and R. Feng, Trans. Tianjin Univ., 2013, 19, 319–325 CrossRef CAS; (b) K. Fan, H. Kong, X. Yang and J. Song, RSC Adv., 2016, 6, 80934–80938 RSC; (c) G. C. Dizon, G. Atkinson, S. P. Argent, L. T. Santu and D. B. Amabilino, Soft Matter, 2020, 16, 4640–4654 RSC.
- Y. Lan, M. G. Corradini, R. G. Weiss, S. R. Raghavan and M. A. Rogers, Chem. Soc. Rev., 2015, 44, 6035–6058 RSC.
- D. J. Adams, Gels, 2018, 4, 32–37 CrossRef.
- F. Barbato, M. I. La Rotonda and F. Quaglia, J. Pharm. Sci., 1997, 86, 225–229 CrossRef CAS.
- N. Bhala and J. Emberson, et al. , Lancet, 2013, 382, 769–779 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, characterisation and assay data. See DOI: 10.1039/d0cc03962d |
| This journal is © The Royal Society of Chemistry 2020 |