DOI:
10.1039/C1CE06328F
(Paper)
CrystEngComm, 2012,
14, 1345-1353
Framework dimensionality of copper(I) coordination polymers of 4,4′-bipyrimidine controlled by anions and solvents†
Received
7th October 2011
, Accepted 9th November 2011
First published on 2nd December 2011
Abstract
The reaction of [Cu(C2H4)n]NO3 with 4,4′-bipyrimidine (bpm) in Me2CO under C2H4 afforded a polymeric Cu(I)–bpm/C2H4 adduct [Cu2(bpm)(C2H4)(NO3)2]n (1) with an infinite 1-D zigzag chain structure. Similar reactions of [Cu(C2H4)n]ClO4 or [Cu(MeCN)4]BF4 with bpm in Me2CO under C2H4 afforded Cu(I)–bpm/C2H4 adducts {[Cu3(bpm)2(C2H4)2](ClO4)3}n (2) and {[Cu3(bpm)2(C2H4)2]–(BF4)3}n (3), respectively, which have an infinite 1-D zigzag ladder structure. It is interesting that two disordered ClO4− or BF4− anions are accommodated in the inside cavity of the ladder chains. In contrast, the reaction of [Cu(MeCN)4]BF4 with bpm in MeOH under C2H4 afforded a Cu(I)–bpm/C2H4 adduct {[Cu4(bpm)3(C2H4)3(MeOH)](BF4)4·2H2O·3MeOH}n (4). Three Cu atoms are bridged by three bpm ligands to form a metallacalix[3]arene structure with three legs of C2H4. Furthermore, these metallacalix[3]arenes are linked through another Cu atom in the terminal N atom of bpm to produce a chiral 2-D sheet structure with space groupP63. One BF4− anion is accommodated in the small triangular Cu3 cavities, whereas three disordered BF4− anions are encapsulated in the large triangular Cu3 cavities. In contrast to complex 4, [Cu(MeCN)4]BF4 was reacted with bpm in MeOH under Ar, and C2H4 gas was then bubbled into the resultant brown suspensions. The reaction solution was allowed to come to room temperature, and Cu(I)–bpm complex {[Cu3(bpm)3](SiF6)1.5}n (5) was collected. The tetrahedral Cu atom is coordinated by two N atoms in the chelate site of bpm and two N atoms in the terminal sites of two other bpm ligands to form two racemic metallacalix[3]arene structures. It is noteworthy that these metallacalix[3]arenes are joined through the bpm ligands to afford a 3-D cage structure consisting of two right-handed and left-handed helix networks. One disordered SiF62− anion is accommodated in the inside cavity between two opposite metallacalix[3]arene structures. On the basis of these results, it has been concluded that BF4−, PF6−, ClO4− and SiF62− anions can serve as anion templates to self-assemble polymeric Cu(I) C2H4 adducts and a cage compound in complexes 2–5. The NO3− anion is ineffective as an anion template, as indicated by the higher coordination ability of the NO3− anion in complex 1. Additionally, solvent-dependent effects have been observed in the formation process: Me2CO can preferentially induce polymeric 1-D chain and 1-D ladder structures in complexes 1–3, whereas MeOH can produce 2-D sheet and 3-D cage structures by the linkage of metallacalix[3]–arene structures in complexes 4 and 5.
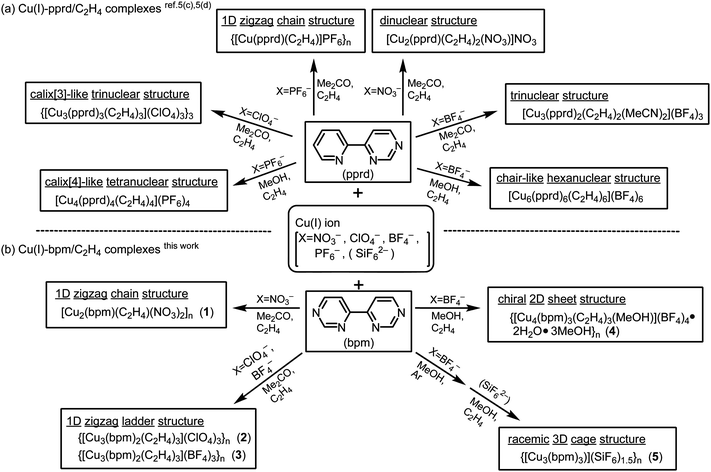
Introduction
The rational design of inorganic artificial receptors for host–guest chemistry is one of the most attractive areas in contemporary supramolecular chemistry.1 In particular, metal-assembled bowl-shaped molecules that are structural analogues of calixarenes and cyclotriveratrylenes have attracted considerable attention,2 in contrast to their versatile behaviours as hosts for inclusion complexation and as efficient ligands for metal ions in classical calixarenes.3 A successful strategy for forming metallamacrocycles closely related to calixarenes has been developed by the combination of cis-protected d8 metal units such as Pd(II) or Pt(II) and an appropriate N-heterocyclic ligand such as pyrimidine or 4,7-phenanthroline derivatives. Recently, a new approach utilizing anion templation to construct metallamacrocycles and cages has been employed with several reported successes.4 However, in contrast to the well-studied templating properties of cationic and neutral species, the use of anionic components to direct the self-assembly process is an area of supramolecular chemistry still in its infancy.
The 4-(2-pyridyl)pyrimidine (pprd) ligand is an attractive nitrogen ligand with a bidentate site for chelation and an exo N-donor site for bridging.5 We have adopted the combination of a Cu(I) or Ag(I) ion and the pprd ligand to construct novel bowl-shaped metallamacrocycles. It has been shown that Cu(I)–pprd metallamacrocycles with C2H4 and CO legs encapsulating ClO4−, PF6− and BF4− anions can be self-assembled by the controls of anion and solvent (Scheme 1(a)),5c,d together with sandwich-shaped Ag(I)–pprd metallamacrocycles encapsulating XF62− (X = Si, Ge and Sn) anions.5b Similar to the pprd ligand, 4,4′-bipyrimidine (bpm) possesses a bidentate site for chelation and two exo N-donor sites for bridging since it can be thought of as a combination of 2,2′-bipyridine and 4,4′-bipyridine. It is expected to produce a greater diversity of finite metallamacrocyclic and infinite polymeric compounds with square/rectangle motifs. However, only a few preliminary reports of the coordination polymers of Cu(I),6Ag(I)6b,7 and Rh(III)8 with bpm and derivatives can be found in the literature. Using 6,6′-diphenyl-4,4′-bipyrimidine (Ph2bpm) as a bpm derivative, we have recently reported that diverse 3-D Cu(I)–Ph2bpm/C2H4 adducts can be self-assembled by the different connectivities of an intermolecular π–π stacking interaction and a C–H⋯N contact.6c
 |
| | Scheme 1
Cu(I)–{pprd, bpm}/C2H4 complexes. | |
As a further investigation, we attempted herein to join discrete Cu(I) metallamacrocycles by the combination of Cu(I) ion and bpm ligand and to synthesize a few polymeric Cu(I) C2H4 adducts by the application of preparative approach in the related Cu(I)–pprd metallamacrocycles.5c,d It was found that a diversity of 1-D chain, 1-D ladder, 2-D sheet Cu(I)–bpm/C2H4 adducts and 3-D Cu(I)–bpm cage compound, in which a ClO4−, BF4− or SiF62− anion was accommodated in the inside cavity, could be self-assembled by the controls of anions and solvents (Scheme 1(b)). Their structures and properties were characterized by X-ray, IR, TG-DTA, SEM and CD analyses. The roles of anion and solvent were determined in their formation processes.
Experimental section
General procedures and reagents
[Cu(MeCN)4]X (X = PF6 and BF4) were prepared according to the literature.94,4′-Bipyrimidine (bpm) was prepared by modifications of the literature method.10 Cu(ClO4)2·6H2O and Cu(NO3)2·3H2O were purchased from Mitsuwa Chemicals and Wako pure Chemicals, respectively. The pure C2H4 gas (>99.9%) was purchased from Sumitomo Seika Chemicals (Japan). All organic solvents were dried and distilled by the usual methods before use. All procedures were carried out using standard Schlenk techniques under Ar and C2H4. IR spectra were recorded with a JASCO FT-IR 430 spectrometer as KBr pellets. Thermogravimetric analysis (TG-DTA) was carried out with a Rigaku Thermo Plus 8120 under flowing N2 gas.
Preparation of Cu(I)–bpm complexes
[Cu2(bpm)(C2H4)(NO3)2]n (1).
The precursor Cu(I) C2H4 complex [Cu(C2H4)n]NO3 was prepared by the reductive reaction of Cu(NO3)2·3H2O (24.2 mg, 0.10 mmol) with Cu wire in Me2CO (5 ml) under C2H4. A 5 ml Me2CO solution of bpm (3.2 mg, 0.02 mmol) was added to the Cu(I) C2H4 solution. The C2H4 gas was then bubbled for 1 hour. The brown suspension was filtered, and the filtrates were sealed in 7 mmϕ glass tubes under C2H4. The reaction solution was allowed to stand at −10 °C for 1 month, and the solution was then allowed to come to room temperature. The brown plate crystals of complex 1 were collected. After complex 1 was dried by flowing C2H4 gas, it was immediately subjected to elementary analysis, IR and TG-DTA. Anal. Calcd for Cu2C10H10N6O6: C, 27.46; H, 2.30; N, 19.22. Found: C, 27.85; H, 3.17; N, 21.77%. IR (KBr, cm−1): 1636(w), 1601(m), 1577(s), 1559(m, C2H4), 1542(w), 1522(m), 1443(m), 1385(s, NO3), 1183(w), 1155(w), 1060(w), 845(w), 746(m), 692(w), 652(m).
{[Cu3(bpm)2(C2H4)2](ClO4)3}n (2).
The precursor Cu(I) C2H4 complex [Cu(C2H4)n]ClO4 was prepared by the reductive reaction of Cu(ClO4)2·6H2O (37.1 mg, 0.10 mmol) with Cu wire in Me2CO (5 ml) under C2H4. A 5 ml Me2CO solution of bpm (3.2 mg, 0.02 mmol) was added to the Cu(I) C2H4 solution. The C2H4 gas was then bubbled for 1 hour. The brown suspension was filtered, and the filtrates were sealed in 7 mmϕ glass tubes under C2H4. The reaction solution was allowed to stand at −10 °C for 1 month, and red plate crystals of complex 2 were collected. After complex 2 was dried by flowing C2H4 gas, it was immediately subjected to elementary analysis and IR. Anal. Calcd for Cu3C20H20N8Cl3O12: C, 27.89; H, 2.34; N, 13.01. Found: C, 26.26; H, 2.50; N, 11.50%. IR (KBr, cm−1) 1598(s), 1568(s), 1548 (m, C2H4), 1536(w), 1474(s), 1396(s), 1335(m, C2H4), 1274(w), 1167(s), 1094–927(s, ClO4), 858(s), 739(s), 684(w), 670(m), 622(s).
Caution
: perchlorate salts of metal complexes with organic compounds are potentially explosive! Only small amounts of materials should be prepared and should be handled with great care.
{[Cu3(bpm)2(C2H4)2](BF4)3}n (3).
[Cu(MeCN)4]BF4 (62.8 mg, 0.20 mmol) and bpm (3.2 mg, 0.02 mmol) were reacted in Me2CO (10 ml) under C2H4. The yellow reaction solution was filtered, and the filtrates were sealed in 7 mmϕ glass tubes under C2H4. The reaction solution was allowed to stand for 2 months at −10 °C, and the solution was then allowed to come to room temperature. The red plate crystals of complex 3 were collected. After complex 3 was dried by flowing C2H4 gas, it was immediately subjected to elementary analysis, IR and TG-DTA. Anal. Calcd for Cu3C20H20B3F12N8: C, 29.17; H, 2.45; N, 13.61. Found: C, 28.95; H, 2.57; N, 13.53%. IR (KBr, cm−1): 1599(s), 1569(s), 1550(m, C2H4), 1476(s), 1409(s), 1338(m, C2H4), 1286(w), 1273(m), 1167(s), 1088–956(s, BF4), 862(m), 740(s), 685(m), 672(m), 520(m).
{[Cu4(bpm)3(C2H4)3(MeOH)](BF4)4·2H2O·3MeOH}n (4).
[Cu(MeCN)4]BF4 (62.8 mg, 0.20 mmol) and bpm (3.2 mg, 0.02 mmol) were reacted in MeOH (10 ml) under C2H4. The yellow reaction solution was filtered, and the filtrates were sealed in 7 mmϕ glass tubes under C2H4. The reaction solution was allowed to stand for 1 week at −10 °C. Two kinds of reddish-brown hexagonal prismatic (4a) and plate crystals (4b) were collected. The preliminary X-ray determinations showed that both crystals had the same crystallographic lattice constants. A single crystal X-ray analysis was carried out using appropriate plate crystals 4b. After complex 4 was dried by flowing C2H4 gas, it was immediately subjected to elementary analysis, IR and TG-DTA. Anal. Calcd for Cu4C34H50N12O6B4F16: C, 30.84; H, 3.81; N, 12.69. Found: C, 30.70; H, 3.75; N, 12.63%. IR (KBr, cm−1): 1596(s), 1568(m), 1545(m, C2H4), 1472(s), 1402(s), 1335(m, C2H4), 1285(m) 1167(s), 1062(s, BF4), 843(m), 742(s), 696(w), 685(w), 666(s), 521(m).
{[Cu3(bpm)3](SiF6)1.5}n (5).
[Cu(MeCN)4]BF4 (9.4 mg, 0.03 mmol) and bpm (1.6 mg, 0.01 mmol) were reacted in MeOH (10 ml) under Ar. The C2H4 gas was bubbled into dark brown suspensions to form a clear yellow solution. The reaction solution was filtered, and the filtrates were sealed in 7 mmϕ glass tubes under C2H4. The reaction solution was allowed to stand for 1 month at −10 °C, and the solution was then allowed to come to room temperature. The black brick crystals of complex 5 were collected after 2 weeks. After complex 5 was dried by flowing C2H4 gas, it was immediately subjected to elementary analysis and IR. Anal. Calcd for Cu3C24H18N12Si1.5F9: C, 32.82; H, 2.07; N, 19.14; Found: C, 32.65; H, 2.03; N, 19.04%. IR (KBr, cm−1): 1637(w), 1577(s), 1523(m), 1444(m), 1384(s), 1277(w), 1184(m), 1084(m), 1061(m), 845(m), 747(s, SiF6), 484(s, SiF6), 450(w).
X-ray crystal structure determinations
All measurements of Cu(I)–bpm complexes 1–5 were made on a Rigaku Mercury CCD diffractometer with graphite monochromated Mo-Kα radiation (λ = 0.71070 Å). The diffraction data were collected at −157(2), −165(2), −153(2), −175(2) and −155(2) °C for complexes 1–5 in the ω scan mode, respectively. Of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327, 11
327, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447, 17
447, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589, 32
589, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 and 35
111 and 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 reflections that were collected, 3258, 3651, 3464, 4171 and 1248 were unique (Rint = 0.0240, 0.0484, 0.0310, 0.0486 and 0.0523) for complexes 1–5, respectively. Data were collected and processed using the Crystal Clear program (Rigaku). The linear absorption coefficient, μ, for Mo-Kα radiation is 29.96, 23.47, 21.65, 16.343 and 21.07 cm−1 for complexes 1–5, respectively. The data were corrected for Lorentz and polarization effects.
743 reflections that were collected, 3258, 3651, 3464, 4171 and 1248 were unique (Rint = 0.0240, 0.0484, 0.0310, 0.0486 and 0.0523) for complexes 1–5, respectively. Data were collected and processed using the Crystal Clear program (Rigaku). The linear absorption coefficient, μ, for Mo-Kα radiation is 29.96, 23.47, 21.65, 16.343 and 21.07 cm−1 for complexes 1–5, respectively. The data were corrected for Lorentz and polarization effects.
The structures were solved by direct methods (SHELXS-97) and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were included and constrained to the ideal position and thermal displacement parameter using the AFIX command on SHELXL-97. In complex 2, one of the disordered ClO4− anions was restrained as the same thermal displacement parameter using the EADP command on SHELXL-97. In complex 3, fluorine atoms of the disordered BF4− anions were restrained for the thermal displacement parameter using the DELU and SIMU commands on SHELXL-97. In complex 4b, each disordered BF4− anion, MeOH, and H2O except boron and hydrogen atoms were restrained to the same thermal displacement parameters using the EADP commands on SHELXL-97, respectively. In complex 5, the disordered F(3) and F(4) atoms of SiF62− anions were restrained for thermal displacement parameters using the DELU and SIMU commands on SHELXL-97. The hydrogen atoms of disordered water molecules were not located. The final cycle of full-matrix least squares refinement was based on {3258, 2814}, {3651, 2606}, {3464, 2955}, {4171, 4033} and {1248, 1247} observed reflections (all data, I > 2σ(I)) for complexes 1–5, respectively. The unweighted and weighted agreement factors of R = Σ‖Fo| − |Fc‖/Σ|Fo|, R1 = Σ‖Fo| − |Fc‖/Σ|Fo| (Fo > 4σ(Fo)) and wR2= [Σw(Fo2 − Fc2)2/Σw(F02)2]1/2 were used. The R, R1 and wR2 values were {0.0434, 0.0317 and 0.0686}, {0.0703, 0.0685 and 0.1941}, {0.0703, 0.0685 and 0.1941}, {0.0703, 0.0685 and 0.1941} and {0.0727, 0.0726 and 0.1729} for complexes 1–5, respectively. All calculations were performed using the WinGX 1.80. Crystal data and details of the structure determination are summarized in Table 1.
| |
[Cu2(bpm)(C2H4)–(NO3)2]n (1) |
{[Cu3(bpm)2(C2H4)2]–(ClO4)3}n (2) |
{[Cu3(bpm)2(C2H4)2]–(BF4)3}n (3) |
{[Cu4(bpm)3(C2H4)3(MeOH)]–(BF4)4·2H2O·3MeOH}n (4b) |
{[Cu3(bpm)3](SiF6)1.5}n (5) |
|
R = Σ∣∣Fo∣ − ∣Fc∣∣/Σ∣Fo∣. R1 = Σ∣∣Fo∣ − ∣Fc∣∣/Σ∣Fo∣ (Fo > 4σ(Fo)). wR2=[Σw(Fo2 − Fc2)2/Σw(Fo2)2]1/2.
|
| Formula |
C10H10Cu2N6O6 |
C20H20Cl3Cu3N8O12 |
C20H20B3Cu3F12N8 |
C34H49Cu4N12B4F16O6 |
C48H36Cu6N24Si3F18 |
| Formula weight |
437.32 |
861.44 |
823.49 |
1323.23 |
1756.48 |
| Crystal system |
Monoclinic |
Monoclinic |
Monoclinic |
Hexagonal |
Cubic |
| Space group |
P21/a (no. 14) |
C2/c (no. 15) |
C2/c (no. 15) |
P63 (no. 173) |
Ia3(−) (no. 206) |
|
a/Å |
14.897(9) |
20.8073(9) |
20.8286(7) |
14.384(5) |
18.673(5) |
|
b/Å |
5.819(3) |
13.2133(18) |
12.9965(5) |
14.384(5) |
18.673(5) |
|
c/Å |
17.441(10) |
15.3486(11) |
15.3593(5) |
15.215(5) |
18.673(5) |
|
α/° |
90.0 |
90.0 |
90.0 |
90.0 |
90.0 |
|
β/° |
107.581(6) |
132.126(2) |
132.5660(10) |
90.0 |
90.0 |
|
γ/° |
90.0 |
90.0 |
90.0 |
120.0 |
90.0 |
|
V/Å3 |
1441.4(14) |
3129.7(5) |
3062.17(19) |
2726.2(16) |
6510.9(18) |
|
Z
|
4 |
4 |
4 |
2 |
4 |
|
D
calc/g cm−3 |
2.015 |
1.828 |
1.786 |
1.498 |
1.820 |
|
F(000) |
872.0 |
1720.0 |
1624.0 |
1316.0 |
3536.0 |
|
μ (Mo-Kα)/cm−1 |
29.96 |
23.47 |
21.65 |
16.343 |
21.07 |
| Temperature/K |
116(2) |
108(2) |
120(2) |
98(2) |
118(2) |
| Observed reflections |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 (Rint = 0.0237) 327 (Rint = 0.0237) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447 (Rint = 0.0484) 447 (Rint = 0.0484) |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589 (Rint = 0.0310) 589 (Rint = 0.0310) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 (Rint = 0.0486) 111 (Rint = 0.0486) |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 (Rint = 0.0523) 743 (Rint = 0.0523) |
| Refined reflections |
3258 (all data); 2814 (I > 2σ(I)) |
3651 (all data); 2606 (I > 2σ(I)) |
3464 (all data); 2955 (I > 2σ(I)) |
4171 (all data); 4033 (I > 2σ(I)) |
1248 (all data); 1247 (I > 2σ(I)) |
|
R
|
0.0434 (all data) |
0.0870 (all data) |
0.0418 (all data) |
0.0703 (all data) |
0.0727 (all data) |
|
R
1
|
0.0317 (I > 2σ(I)) |
0.0570 (I > 2σ(I)) |
0.0326 (I > 2σ(I)) |
0.0685 (I > 2σ(I)) |
0.0726 (I > 2σ(I)) |
|
wR2 |
0.0686 (all data) |
0.1475 (all data) |
0.0761 (all data) |
0.1941 (all data) |
0.1729 |
| GOF |
1.099 |
1.067 |
1.057 |
1.074 |
1.422 |
| Flack parameter |
— |
— |
— |
0.07(3) |
— |
Results and discussion
Crystal structures of Cu(I)–bpm complexes
[Cu2(bpm)(C2H4)(NO3)2]n (1).
The reaction of [Cu(C2H4)n]–NO3 with bpm in Me2CO under C2H4 afforded brown plate crystals of [Cu2(bpm)(C2H4)(NO3)2]n (1). The crystal structure of complex 1 is presented in Fig. 1. The Cu atom is coordinated by two N atoms of bpm in the chelate site and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in a trigonal-planar geometry. The other Cu atom is coordinated by two N atoms of the different bpm ligands in the exo bridging site and two O atoms of the different NO3− anions in a distorted tetragonal geometry to form an infinite 1-D zigzag chain structure. Although there have been several Cu(I) complexes with a NO3− anion in the chelating bidentate mode11 and the bridging bidentate manner,12 there are few Cu(I) complexes with a NO3− anion in the unidentate coordination mode.5d,11b,d,f Additionally, Cu(I) C2H4 adducts have been poorly characterized due to the extremely labile nature of the Cu(I)–C2H4 interaction.5c,d,6c,13 In particular, preparative and structural reports of polynuclear and polymeric Cu(I) C2H4 complexes are few.5c,d,6c,13e,f Therefore, polymeric Cu(I)–bpm/C2H4 adduct 1 with the η1-NO3− anion is of significance. In our related Cu(I)–pprd complexes (Scheme 1(a)), we have proved that the conformation of metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts can be controlled by the choice of anion: the self-assemblies by anion templation of BF4−, PF6− and ClO4− under C2H4 preferentially can induce metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts.5c,d In contrast, the NO3− anion is ineffective as an anion template in the formation of expected metallamacrocyclic Cu(I)–pprd/C2H4 adducts due to the higher coordination ability of the NO3− anion. In fact, the reaction of [Cu(C2H4)n]NO3 with pprd in Me2CO under C2H4 afforded dinuclear Cu(I)–pprd/C2H4 complex, [Cu2(pprd)(C2H4)2(NO3)]NO3.5d In this study, two NO3− anions were similarly coordinated to the Cu atom in the terminal bridging sites of bpm in the η1-fashion. This finding suggests that the NO3− anion is ineffective as an anion template in the construction of expected metallamacrocyclic Cu(I)–bpm/C2H4 adducts, although the combination of [Cu(C2H4)n]NO3 and bpm under C2H4 can produce a rare polymeric 1-D zigzag chain Cu(I) C2H4 adduct. In the coordinated C2H4, the C
C bond of C2H4 in a trigonal-planar geometry. The other Cu atom is coordinated by two N atoms of the different bpm ligands in the exo bridging site and two O atoms of the different NO3− anions in a distorted tetragonal geometry to form an infinite 1-D zigzag chain structure. Although there have been several Cu(I) complexes with a NO3− anion in the chelating bidentate mode11 and the bridging bidentate manner,12 there are few Cu(I) complexes with a NO3− anion in the unidentate coordination mode.5d,11b,d,f Additionally, Cu(I) C2H4 adducts have been poorly characterized due to the extremely labile nature of the Cu(I)–C2H4 interaction.5c,d,6c,13 In particular, preparative and structural reports of polynuclear and polymeric Cu(I) C2H4 complexes are few.5c,d,6c,13e,f Therefore, polymeric Cu(I)–bpm/C2H4 adduct 1 with the η1-NO3− anion is of significance. In our related Cu(I)–pprd complexes (Scheme 1(a)), we have proved that the conformation of metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts can be controlled by the choice of anion: the self-assemblies by anion templation of BF4−, PF6− and ClO4− under C2H4 preferentially can induce metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts.5c,d In contrast, the NO3− anion is ineffective as an anion template in the formation of expected metallamacrocyclic Cu(I)–pprd/C2H4 adducts due to the higher coordination ability of the NO3− anion. In fact, the reaction of [Cu(C2H4)n]NO3 with pprd in Me2CO under C2H4 afforded dinuclear Cu(I)–pprd/C2H4 complex, [Cu2(pprd)(C2H4)2(NO3)]NO3.5d In this study, two NO3− anions were similarly coordinated to the Cu atom in the terminal bridging sites of bpm in the η1-fashion. This finding suggests that the NO3− anion is ineffective as an anion template in the construction of expected metallamacrocyclic Cu(I)–bpm/C2H4 adducts, although the combination of [Cu(C2H4)n]NO3 and bpm under C2H4 can produce a rare polymeric 1-D zigzag chain Cu(I) C2H4 adduct. In the coordinated C2H4, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance of 1.373(4) Å is slightly longer than those [1.30(1)–1.366(6) Å] in the reported trigonal-planar Cu(I) C2H4 complexes5d,13c–i and that [1.313 (exptl) and 1.333 (calc.) Å] of metal-free C2H4.14 The average Cu–N distance of 2.011 Å in the terminal bridging site is slightly shorter than that (2.006 Å) in the chelate site.
C distance of 1.373(4) Å is slightly longer than those [1.30(1)–1.366(6) Å] in the reported trigonal-planar Cu(I) C2H4 complexes5d,13c–i and that [1.313 (exptl) and 1.333 (calc.) Å] of metal-free C2H4.14 The average Cu–N distance of 2.011 Å in the terminal bridging site is slightly shorter than that (2.006 Å) in the chelate site.
 |
| | Fig. 1 X-Ray crystal structure of complex 1. | |
{[Cu3(bpm)2(C2H4)2](ClO4)3}n (2) and {[Cu3(bpm)2(C2H4)2]–(BF4)3}n (3).
The reaction of [Cu(C2H4)n]ClO4 with bpm in Me2CO under C2H4 afforded red plate crystals of {[Cu3(bpm)2–(C2H4)2](ClO4)3}n (2). In contrast to complex 1, this result indicates a significant anion-dependent effect in the formation process of Cu(I)–bpm/C2H4 adducts. The crystal structure of complex 2 is shown in Fig. 2. The Cu atom is coordinated by four N atoms in the chelate site of two different bpm ligands in the distorted tetrahedral geometry. The other Cu atom is coordinated by two terminal N atoms in the bridging sites of two different bpm ligands and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the distorted trigonal geometry to form an infinite 1-D zigzag ladder structure. It is interesting that two disordered ClO4− anions are accommodated in the inside cavity of the ladder chains. Although there have been several reports of the encapsulation of NO3−,15BF4−,5d,15a,b,16PF6−,15a,b,17Cl218 and I22–19 anions, less is known about the encapsulation of a ClO4− anion into macrocycles and cages.15a,16h,20
C bond of C2H4 in the distorted trigonal geometry to form an infinite 1-D zigzag ladder structure. It is interesting that two disordered ClO4− anions are accommodated in the inside cavity of the ladder chains. Although there have been several reports of the encapsulation of NO3−,15BF4−,5d,15a,b,16PF6−,15a,b,17Cl218 and I22–19 anions, less is known about the encapsulation of a ClO4− anion into macrocycles and cages.15a,16h,20
 |
| | Fig. 2 X-Ray crystal structure of complex 2 encapsulating ClO4− anions. | |
A similar reaction of [Cu(MeCN)4]BF4 with bpm in Me2CO under C2H4 gave red plate crystals of {[Cu3(bpm)2(C2H4)2]–(BF4)3}n (3). The crystal structure of complex 3 is shown in Fig. 3. The infinite 1-D zigzag ladder structure of complex 3 essentially resembles that of complex 2, in which two disordered BF4− anions are accommodated in the inside cavity of the ladder chains. These findings indicate that BF4− and ClO4− anions can serve as anion templates in the formation of polymeric 1-D ladder Cu(I) C2H4 adducts, although BF4− and ClO4− anions contribute to the formation of metallamacrocyclic Cu(I) C2H4 adducts in the related Cu(I)–pprd complexes.5c,d
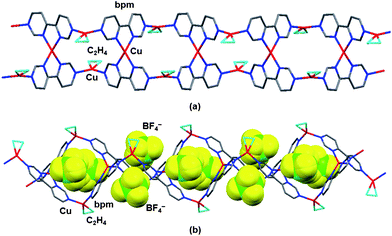 |
| | Fig. 3 X-Ray crystal structure of complex 3 encapsulating BF4− anions. | |
In the coordinated C2H4, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distances of 1.385(8) and 1.370(9) Å in complexes 2 and 3 are slightly longer than that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d
C distances of 1.385(8) and 1.370(9) Å in complexes 2 and 3 are slightly longer than that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d
{[Cu4(bpm)3(C2H4)3(MeOH)](BF4)4·2H2O·3MeOH}n (4).
Further attempts to react [Cu(MeCN)4]BF4 with bpm in MeOH under C2H4 afforded reddish-brown hexagonal prismatic (4a) and plate crystals (4b) of {[Cu4(bpm)3(C2H4)3(MeOH)]–(BF4)4·2H2O·3MeOH}n, with the crystals of both having the same crystallographic lattice constants. In contrast to complex 3, this result indicates a remarkable solvent-dependent effect in the formation process of Cu(I)–bpm/C2H4 adducts. The SEM images of crystals 4a and 4b are shown in Fig. 4. In particular, it is interesting that the shape of crystals 4a showed a hexagonal prismatic structure with a hollow hole, in which the outside diameter is about 250 ± 25 μm and the inside diameter is about 130 ± 10 μm. Although it is difficult to decide the crystal growth process at this time, it is considered that a horn-like shape should be formed by the aggregations of the torus-shape plates according to the enlarged SEM images (ESI, Fig. S1†). For instance, it has been known that the hexagonal ZnP(Py)4 nanorods with a hollow hole could encapsulate fullerene (C60), which showed photo-induced electron transfer and light energy conversion properties.21 Thus coordination polymers with a hollow hole are expected to develop into structurally and functionally interesting host compounds.
 |
| | Fig. 4 The SEM images of hexagonal prismatic crystals for 4a (a) and plate crystals for 4b (b). | |
A single crystal X-ray analysis was carried out using appropriate plate crystals 4b. The crystal structure of complex 4b is shown in Fig. 5. No inversion center was found, confirming P63 as the correct space group. Each Cu atom is coordinated by three N atoms in chelate and bridging sites of bpm and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the distorted tetrahedral geometry. Three Cu atoms are bridged by three bpm ligands to form a metallacalix[3]arene structure with three legs of C2H4. Furthermore, these metallacalix[3]arenes are linked through another Cu atom with MeOH in the terminal N atom of bpm to produce a 2-D sheet structure with small and large Cu3 cavities (Fig. 5). Although C2H4 adducts to the 2-D surfaces of CuMCl4 (M = Al and Ga) have been determined by powder X-ray diffraction analysis,22 complex 4b is the first 2-D sheet Cu(I) C2H4 adduct. It should be noted that each 2-D sheet structure is arranged in parallel along the c-axis, resulting in the formation of a chiral 2-D sheet structure (Fig. 6 and 7). The presence of chirality could be confirmed by circular dichroic (CD) spectroscopy in the solid state (ESI, Fig. S2†). The positive cotton effect was observed. More noteworthy are one BF4− anion is functionally accommodated in the small triangular Cu3 cavities with neighbouring Cu⋯Cu distances of 6.06 Å and three disordered BF4− anions are encapsulated in the large triangular Cu3 cavities with corresponding distances of 14.38 Å. It was shown that the BF4− anion can play a role as an anion template to build a chiral 2-D sheet structure consisting of the linkage of [Cu3(bpm)3] frameworks with a metallacalix[3]arene structure. To the best of our knowledge, the encapsulations of BF4− anion into macrocycles and cage compounds have been limited.5d,15a,b,16 As such, this compound is expected to develop as an unprecedented inorganic anion receptor with chirality. In the coordinated C2H4, the C
C bond of C2H4 in the distorted tetrahedral geometry. Three Cu atoms are bridged by three bpm ligands to form a metallacalix[3]arene structure with three legs of C2H4. Furthermore, these metallacalix[3]arenes are linked through another Cu atom with MeOH in the terminal N atom of bpm to produce a 2-D sheet structure with small and large Cu3 cavities (Fig. 5). Although C2H4 adducts to the 2-D surfaces of CuMCl4 (M = Al and Ga) have been determined by powder X-ray diffraction analysis,22 complex 4b is the first 2-D sheet Cu(I) C2H4 adduct. It should be noted that each 2-D sheet structure is arranged in parallel along the c-axis, resulting in the formation of a chiral 2-D sheet structure (Fig. 6 and 7). The presence of chirality could be confirmed by circular dichroic (CD) spectroscopy in the solid state (ESI, Fig. S2†). The positive cotton effect was observed. More noteworthy are one BF4− anion is functionally accommodated in the small triangular Cu3 cavities with neighbouring Cu⋯Cu distances of 6.06 Å and three disordered BF4− anions are encapsulated in the large triangular Cu3 cavities with corresponding distances of 14.38 Å. It was shown that the BF4− anion can play a role as an anion template to build a chiral 2-D sheet structure consisting of the linkage of [Cu3(bpm)3] frameworks with a metallacalix[3]arene structure. To the best of our knowledge, the encapsulations of BF4− anion into macrocycles and cage compounds have been limited.5d,15a,b,16 As such, this compound is expected to develop as an unprecedented inorganic anion receptor with chirality. In the coordinated C2H4, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance of 1.31(2) Å is similar to that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d The average Cu–N distance of 2.025 Å in the terminal bridging site is slightly shorter than that (2.144 Å) in the chelate site.
C distance of 1.31(2) Å is similar to that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d The average Cu–N distance of 2.025 Å in the terminal bridging site is slightly shorter than that (2.144 Å) in the chelate site.
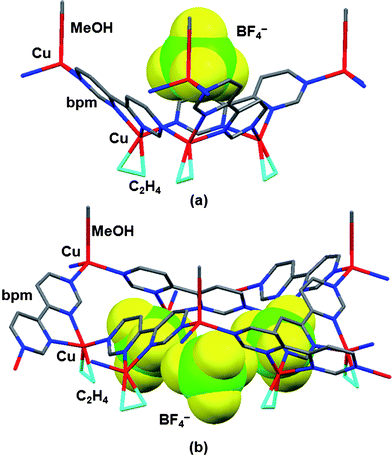 |
| | Fig. 5 X-Ray crystal structure of complex 4b encapsulating BF4− anions in small (a) and large Cu3 cavities (b). | |
 |
| | Fig. 6 2-D sheet structure of complex 4b encapsulating BF4− anions. The solvated MeOH and H2O molecules are omitted for clarity. | |
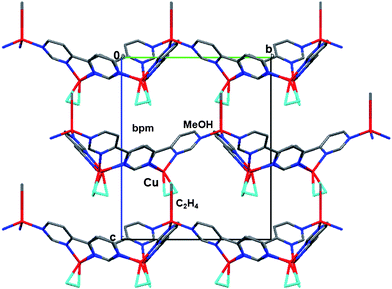 |
| | Fig. 7 X-Ray crystal packing structure of complex 4b along the a-axis. The solvated MeOH and H2O molecules are omitted for clarity. | |
{[Cu3(bpm)3](SiF6)1.5}n (5).
In contrast to Cu(I) C2H4 adduct 4, [Cu(MeCN)4]BF4 was reacted with bpm in MeOH under Ar, and the C2H4 gas was then bubbled into the resultant brown suspensions to afford a clear yellow solution. The reaction solution was allowed to stand for 2 months at −10 °C, but we were not able to obtain any crystals and precipitates due to the higher solubility. The reaction solution was then allowed to come to room temperature, and black brick crystals of {[Cu3(bpm)3]–(SiF6)1.5}n (5) were collected after 2 weeks. The crystal structure of complex 5 is shown in Fig. 8. The tetrahedral Cu atom is coordinated by two N atoms in the chelate site of bpm and two N atoms in the terminal sites of two other bpm ligands to form two racemic metallacalix[3]arene structures, in which the neighbouring triangular Cu⋯Cu distances are 5.73 Å. It is noteworthy that these metallacalix[3]arenes are joined through the bpm ligands to afford a 3-D cage structure consisting of two right-handed (blue) and left-handed (red) helix networks. One disordered SiF62− anion is accommodated in the inside cavity between two opposite metallacalix[3]arene structures (Fig. 9). Although there have been only a few reports regarding the encapsulation of a SiF62− anion to create sandwich-shaped Ag(I) metallamacrocycles5b and an organic anion complex,23 the encapsulation of a SiF62− anion into a racemic 3-D Cu(I) cage compound is quite unique. It would be interesting to elucidate the formation process of this 3-D Cu(I) cage compound. Presumably, a chopped Cu(I)–bpm/C2H4 complex should be induced from a polymeric Cu(I)–bpm complex by the addition of C2H4 since a dark brown suspension under Ar was changed to a clear yellowish-brown solution upon bubbling of C2H4. Similar behaviors have been observed in the formation process of our related Cu(I)–pprd/C2H4 adducts.5c Subsequently, a coordinatively unsaturated Cu(I)–bpm complex would be produced as an intermediate in the dissociation equilibrium of C2H4 upon the reaction solution standing at room temperature. Finally, the SiF62− anion could act as an anion template to form a 3-D Cu(I) cage compound 5.5b These results also suggest that a source of SiF62− anion can be introduced by the reaction of a BF4− anion and SiO2 component from a glass-made reactor and tube.5b,23,24
![X-Ray crystal structures of complex 5 encapsulating disordered SiF62− anions. Top {(a) and (b)} and side views (c) in two racemic metallacalix[3]arene structures.](/image/article/2012/CE/c1ce06328f/c1ce06328f-f8.gif) |
| | Fig. 8 X-Ray crystal structures of complex 5 encapsulating disordered SiF62− anions. Top {(a) and (b)} and side views (c) in two racemic metallacalix[3]arene structures. | |
 |
| | Fig. 9 3-D Cu(I) cage structure of complex 5 encapsulating disordered SiF62− anions, which consist of two right-handed (blue) and left-handed (red) helix networks. | |
TG-DTA curves and IR spectra of Cu(I)–bpm/C2H4 adducts
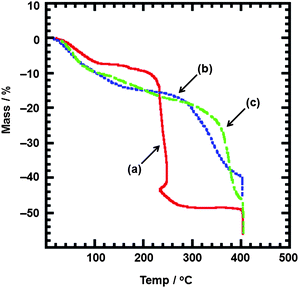
Thermogravimetric analysis (TG-DTA) was carried out under 20 ml min−1 flowing N2 gas for Cu(I)–bpm/C2H4 adducts 1, 3 and 4 except for explosive Cu(I)–bpm/C2H4–ClO4 complex 2. The temperature was ramped at a rate of 5° min−1 from 20 to 400 °C. As shown in Fig. 10, 1-D chain Cu(I)–bpm/C2H4 adduct 1 displayed a mass loss of three-step curves at 20–115 (sharp, 7.2%), 115–185 (gentle, 1.6%) and 185–250 °C (sharp, 36.8%). The thermal decomposition of complex 1 was determined near 250 °C with the rapid mass loss by the elimination of bpm ligands in accordance with exothermic DTA behaviors. The mass loss of 7.2% at the first step is correlated with the elimination of one C2H4 molecule (calcd 6.4%). The total mass loss of 38.4% (calcd 36.1%) at the sum of first and second steps was roughly identical to the elimination of one bpm molecule. Similarly, 1-D ladder Cu(I)–bpm/C2H4 adducts 3 showed a mass loss of three-step curves at 20–70 (gentle, 6.6%), 70–170 (gentle, 7.5%) and 170–330 °C (sharp, 12.9%). The mass loss of 6.6% at the first step is correlated with the elimination of two C2H4 molecules (calcd 6.8%). The total mass loss of 20.4% (calcd 19.2%) at the sum of the first and second steps was roughly assigned to the elimination of one bpm molecule. In contrast, 2-D sheet Cu(I)–bpm/C2H4 adducts 4 showed a total mass loss of 20.3% (calcd 18.8%) with gentle relatively unclear three-step curves at 20–120 (10.0%) and 120–320 °C (10.3%) corresponding to three C2H4, two H2O and four MeOH molecules, and the curve subsequently showed a sharp decline from around 320 °C in response to the elimination of the bpm ligand. At the present time, gentle two-step curves are unidentified due to simultaneous desorptions of the coordinated C2H4 and MeOH molecules and the solvated H2O and MeOH molecules, although endothermic DTA behaviour was clearly observed at the first step (20–120 °C).
 |
| | Fig. 10
TG-DTA curves of Cu(I)–bpm/C2H4 adducts 1, 3 and 4 under flowing N2 gas. Solid line (a) for 1, broken line (b) for 3 and dashed-dotted line (c) for 4. | |
The νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bands of Cu(I)–bpm/C2H4 adducts 1–4 are observed at 1559 (1), 1548 (2), 1550 (3) and 1545 (4) cm−1, respectively [metal-free C2H4, 1623 cm−1]. These νC
C bands of Cu(I)–bpm/C2H4 adducts 1–4 are observed at 1559 (1), 1548 (2), 1550 (3) and 1545 (4) cm−1, respectively [metal-free C2H4, 1623 cm−1]. These νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C wavenumbers are slightly larger than those (1537–1543 cm−1) of the related Cu(I)–pprd/C2H4 metallamacrocycles encapsulating ClO4−, PF6− and BF4− anions,5c,5d indicative of the contribution of poor Cu → C2H4 π back-bonding.
C wavenumbers are slightly larger than those (1537–1543 cm−1) of the related Cu(I)–pprd/C2H4 metallamacrocycles encapsulating ClO4−, PF6− and BF4− anions,5c,5d indicative of the contribution of poor Cu → C2H4 π back-bonding.
Conclusion
As summarized in Scheme 1, the reactions of Cu(I) ion with NO3−, ClO4− and BF4− anions and bpm under C2H4 in Me2CO afforded Cu(I)–bpm/C2H4 adducts 1–3 with infinite 1-D chain and 1-D ladder structures, whereas similar reactions of Cu(I) ion with BF4− and SiF62− anions and bpm under C2H4 in MeOH gave Cu(I) coordination polymers 4 and 5 with 2-D sheet and 3-D cage structures by the linkage of [Cu3(bpm)3] frameworks with a metallacalix[3]arene structure. It has been shown that BF4−, PF6−, ClO4− and SiF62− anions can serve as anion templates to self-assemble polymeric Cu(I) C2H4 adducts and a cage compound in complexes 2–5. The NO3− anion is ineffective as an anion template, as indicated by the higher coordination ability of the NO3− anion in complex 1. Additionally, the solvent-dependent effects are clarified in the formation process: Me2CO preferentially can induce polymeric 1-D chain and 1-D ladder structures in complexes 1–3, whereas MeOH can produce 2-D sheet and 3-D cage structures by the linkage of metallacalix[3]arene structures in complexes 4 and 5. These results are expected to contribute to the design and architecture of structurally and functionally new inorganic anion receptors, in combination with previous results regarding the related Cu(I) and Ag(I) pprd complexes.5b–d
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (no. 20550069 and 23550085) from the Ministry of Education, Science, Sports, Culture and Technology in Japan.
Notes and references
-
(a)
J. W. Steed and J. L. Atwood, Supramolecular Chemistry, Wiley & Sons, Chichester, 2nd edn, 2009 Search PubMed
 ;
(b)
L. R. MacGillivray, Metal–Organic Frameworks, ed. L. R. MacGillivray, Wiley & Sons, New Jersey, 2010 Search PubMed
;
(b)
L. R. MacGillivray, Metal–Organic Frameworks, ed. L. R. MacGillivray, Wiley & Sons, New Jersey, 2010 Search PubMed  ;
(c)
J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspective, Wiley-VCH: Weinheim, 1995 Search PubMed
;
(c)
J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspective, Wiley-VCH: Weinheim, 1995 Search PubMed  ;
(d) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS
;
(d) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS  ;
(e) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS
;
(e) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS  ;
(f) L. Cronin, Annu. Rep. Prog. Chem., Sect. A, 2003, 99, 289 RSC
;
(f) L. Cronin, Annu. Rep. Prog. Chem., Sect. A, 2003, 99, 289 RSC  ;
(g) F. Würthner, C.-C. You, C. R. Saha and Moller, Chem. Soc. Rev., 2004, 33, 133 RSC
;
(g) F. Würthner, C.-C. You, C. R. Saha and Moller, Chem. Soc. Rev., 2004, 33, 133 RSC  ;
(h) P. Thanasekaran, R.-T. Liao, Y.-H. Liu, T. Rajendran, S. Rajagopal and K.-L. Lu, Coord. Chem. Rev., 2005, 249, 1085 CrossRef CAS
;
(h) P. Thanasekaran, R.-T. Liao, Y.-H. Liu, T. Rajendran, S. Rajagopal and K.-L. Lu, Coord. Chem. Rev., 2005, 249, 1085 CrossRef CAS  .
.
- S. Kumar, N. Kaur and H. Singh, Adv. Heterocycl. Chem., 2008, 96, 123 CrossRef CAS
 .
.
-
(a)
D. Gutsche, Calixarenes, Royal Society of Chemistry, Cambridge, 1998 Search PubMed
 ;
(b)
Z. Asfari, V. Böhmer, J. Harrowfield and J. Vicens, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, 2001 Search PubMed
;
(b)
Z. Asfari, V. Böhmer, J. Harrowfield and J. Vicens, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, 2001 Search PubMed  ;
(c)
D. J. Cram and J. M. Cram, Container Molecules and their Guests, Royal Society of Chemistry, Cambridge, 1994 Search PubMed
;
(c)
D. J. Cram and J. M. Cram, Container Molecules and their Guests, Royal Society of Chemistry, Cambridge, 1994 Search PubMed  .
.
-
(a)
A. Bianchi, K. Bowman-James and E. García-España, Supramolecular Chemistry of Anions; Wiely-VCH: Weinheim, 1997 Search PubMed
 ;
(b) P. A. Gale, J. L. Sessler and V. Král, Chem. Commun., 1998, 1 RSC
;
(b) P. A. Gale, J. L. Sessler and V. Král, Chem. Commun., 1998, 1 RSC  ;
(c) P. A. Gale, Coord. Chem. Rev., 2000, 199, 181 CrossRef CAS
;
(c) P. A. Gale, Coord. Chem. Rev., 2000, 199, 181 CrossRef CAS  ;
(d) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989 CrossRef CAS
;
(d) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989 CrossRef CAS  ;
(e) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS
;
(e) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS  ;
(f) P. A. Gale, Coord. Chem. Rev., 2001, 213, 79 CrossRef CAS
;
(f) P. A. Gale, Coord. Chem. Rev., 2001, 213, 79 CrossRef CAS  ;
(g) P. A. Gale, Coord. Chem. Rev., 2003, 240, 191 CrossRef CAS
;
(g) P. A. Gale, Coord. Chem. Rev., 2003, 240, 191 CrossRef CAS  ;
(h) R. Vilar, Angew. Chem., Int. Ed., 2003, 42, 1460 CrossRef CAS
;
(h) R. Vilar, Angew. Chem., Int. Ed., 2003, 42, 1460 CrossRef CAS  ;
(i) P. D. Beer, M. R. Sambrook and D. Curiel, Chem. Commun., 2006, 2105 RSC
;
(i) P. D. Beer, M. R. Sambrook and D. Curiel, Chem. Commun., 2006, 2105 RSC  ;
(j) N. Gimeno and R. Vilar, Coord. Chem. Rev., 2006, 250, 3161 CrossRef CAS
;
(j) N. Gimeno and R. Vilar, Coord. Chem. Rev., 2006, 250, 3161 CrossRef CAS  ;
(k) R. Vilar, Struct. Bonding, 2008, 129, 175 CrossRef CAS
;
(k) R. Vilar, Struct. Bonding, 2008, 129, 175 CrossRef CAS  ;
(l) R. Vilar, Eur. J. Inorg. Chem., 2008, 357 CrossRef CAS
;
(l) R. Vilar, Eur. J. Inorg. Chem., 2008, 357 CrossRef CAS  ;
(m) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC
;
(m) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC  .
.
-
(a) D. A. Beauchamp and S. J. Loeb, Chem. Commun., 2002, 2484 RSC
 ;
(b) M. Maekawa, S. Kitagawa, T. Kuroda, Sowa and M. Munakata, Chem. Commun., 2006, 2161 RSC
;
(b) M. Maekawa, S. Kitagawa, T. Kuroda, Sowa and M. Munakata, Chem. Commun., 2006, 2161 RSC  ;
(c) M. Maekawa, H. Konaka, T. Minemastu, T. Kuroda, Sowa, M. Munakata and S. Kitagawa, Chem. Commun., 2007, 5179 RSC
;
(c) M. Maekawa, H. Konaka, T. Minemastu, T. Kuroda, Sowa, M. Munakata and S. Kitagawa, Chem. Commun., 2007, 5179 RSC  ;
(d) M. Maekawa, A. Nabei, T. Tominaga, K. Sugimoto, T. Minematsu, T. Okubo, T. Kuroda, Sowa, M. Munakata and S. Kitagawa, Dalton Trans., 2009, 415 RSC
;
(d) M. Maekawa, A. Nabei, T. Tominaga, K. Sugimoto, T. Minematsu, T. Okubo, T. Kuroda, Sowa, M. Munakata and S. Kitagawa, Dalton Trans., 2009, 415 RSC  .
.
-
(a) F. Bodar, Houillon, T. Humbert, A. Marsura, J.-B. R. de Vains, O. Dusausoy, N. Bouhmaida, N. E. Ghermani and Y. Dusausoy, Inorg. Chem., 1995, 34, 5205 CrossRef
 ;
(b) C. Janiak, L. Uehlin, H.-P. Wu, P. Kliifers, H. Piotrowski and T. G. Scharmann, J. Chem. Soc., Dalton Trans., 1999, 3121 RSC
;
(b) C. Janiak, L. Uehlin, H.-P. Wu, P. Kliifers, H. Piotrowski and T. G. Scharmann, J. Chem. Soc., Dalton Trans., 1999, 3121 RSC  ;
(c) M. Maekawa, T. Tominaga, T. Okubo, T. Kuroda, Sowa and M. Munakata, Eur. J. Inorg. Chem., 2009, 4225 CrossRef CAS
;
(c) M. Maekawa, T. Tominaga, T. Okubo, T. Kuroda, Sowa and M. Munakata, Eur. J. Inorg. Chem., 2009, 4225 CrossRef CAS  .
.
- D. A. Beauchamp and S. J. Loeb, Dalton Trans., 2007, 4760 RSC
 .
.
- J. E. Redman, N. Feeder, S. J. Teat and J. K. M. Sanders, Inorg. Chem., 2001, 40, 2486 CrossRef CAS
 .
.
-
G. J. Gubus, Inorganic Syntheses, ed by D. F. Shriver, Wiley-Interscience, New York, 1979, 19, 90 Search PubMed
 .
.
- E. Ioachim, E. A. Medlycott, M. I. J. Polson and G. S. Hanan, Eur. J. Org. Chem., 2005, 3775 CrossRef CAS
 .
.
-
(a) M. Munakata, S. Kitagawa, N. Ujimaru, M. Nakamura, M. Maekawa and H. Matsuda, Inorg. Chem., 1993, 32, 826 CrossRef CAS
 ;
(b) T. Kuroda, Sowa, M. Munakata, H. Matsuda, S. Akiyama and M. Maekawa, J. Chem. Soc., Dalton Trans., 1995, 2201 RSC
;
(b) T. Kuroda, Sowa, M. Munakata, H. Matsuda, S. Akiyama and M. Maekawa, J. Chem. Soc., Dalton Trans., 1995, 2201 RSC  ;
(c) C. Pettinari, G. G. Lobbia, G. Sclavi and D. Leonesi, Polyhedron, 1995, 14, 1709 CrossRef CAS
;
(c) C. Pettinari, G. G. Lobbia, G. Sclavi and D. Leonesi, Polyhedron, 1995, 14, 1709 CrossRef CAS  ;
(d) V. Olijnyk, T. Glowiak and M. Mys'kiv, J. Chem. Crystallogr., 1995, 25, 621 CrossRef CAS
;
(d) V. Olijnyk, T. Glowiak and M. Mys'kiv, J. Chem. Crystallogr., 1995, 25, 621 CrossRef CAS  ;
(e) H. Lang, A. del Villar, B. Walfort and G. Rheinwald, J. Organomet. Chem., 2004, 689, 1464 CrossRef CAS
;
(e) H. Lang, A. del Villar, B. Walfort and G. Rheinwald, J. Organomet. Chem., 2004, 689, 1464 CrossRef CAS  ;
(f) C. Sivasankar, M. Nethaji and A. G. Samuelson, Inorg. Chem. Commun., 2004, 7, 238 CrossRef CAS
;
(f) C. Sivasankar, M. Nethaji and A. G. Samuelson, Inorg. Chem. Commun., 2004, 7, 238 CrossRef CAS  ;
(g) S.-M. Chen, C.-Z. Lu, C.-K. Xia, X.-J. Xu and Q.-G. Zhai, Cryst. Growth Des., 2005, 5, 1485 CrossRef CAS
;
(g) S.-M. Chen, C.-Z. Lu, C.-K. Xia, X.-J. Xu and Q.-G. Zhai, Cryst. Growth Des., 2005, 5, 1485 CrossRef CAS  .
.
-
(a) S. Kitagawa, M. Kondo, S. Kawata, S. Wada, M. Maekawa and M. Munakata, Inorg. Chem., 1995, 34, 1455 CrossRef CAS
 ;
(b) R. Yang, K. Lin, Y. Hou, D. Wang and D. Jin, Polyhedron, 1997, 16, 4033 CrossRef
;
(b) R. Yang, K. Lin, Y. Hou, D. Wang and D. Jin, Polyhedron, 1997, 16, 4033 CrossRef  ;
(c) J.-P. Lang, H. Kawaguchi and K. Tatsumi, J. Organomet. Chem., 1998, 569, 109 CrossRef CAS
;
(c) J.-P. Lang, H. Kawaguchi and K. Tatsumi, J. Organomet. Chem., 1998, 569, 109 CrossRef CAS  ;
(d) R. Yang, Y. Sun, D. Zhao and L. Guo, J. Coord. Chem., 2003, 56, 1169 CrossRef CAS
;
(d) R. Yang, Y. Sun, D. Zhao and L. Guo, J. Coord. Chem., 2003, 56, 1169 CrossRef CAS  .
.
-
(a) H. V. R. Dias and J. Wu, Eur. J. Inorg. Chem., 2008, 509 CrossRef CAS
 ;
(b) J. S. Thompson, R. L. Harlow and J. F. Whitney, J. Am. Chem. Soc., 1983, 105, 3522 CrossRef CAS
;
(b) J. S. Thompson, R. L. Harlow and J. F. Whitney, J. Am. Chem. Soc., 1983, 105, 3522 CrossRef CAS  ;
(c) J. S. Thompson and J. F. Whitney, Inorg. Chem., 1984, 23, 2813 CrossRef CAS
;
(c) J. S. Thompson and J. F. Whitney, Inorg. Chem., 1984, 23, 2813 CrossRef CAS  ;
(d) H. Masuda, N. Yamamoto, T. Taga, K. Machida, S. Kitagawa and M. Munakata, J. Organomet. Chem., 1987, 322, 121 CrossRef CAS
;
(d) H. Masuda, N. Yamamoto, T. Taga, K. Machida, S. Kitagawa and M. Munakata, J. Organomet. Chem., 1987, 322, 121 CrossRef CAS  ;
(e) M. Munakata, T. Kuroda, Sowa, M. Maekawa and M. Nakamura, Inorg. Chem., 1994, 33, 1284 CrossRef CAS
;
(e) M. Munakata, T. Kuroda, Sowa, M. Maekawa and M. Nakamura, Inorg. Chem., 1994, 33, 1284 CrossRef CAS  ;
(f) J. Dai, M. Yamamoto, T. Kuroda, Sowa, M. Maekawa, Y. Suenaga and M. Munakata, Inorg. Chem., 1997, 36, 2688 CrossRef CAS
;
(f) J. Dai, M. Yamamoto, T. Kuroda, Sowa, M. Maekawa, Y. Suenaga and M. Munakata, Inorg. Chem., 1997, 36, 2688 CrossRef CAS  ;
(g) Y. Suenaga, L.-P. Wu, T. Kuroda, Sowa, M. Munakata and M. Maekawa, Polyhedron, 1997, 16, 67 CrossRef CAS
;
(g) Y. Suenaga, L.-P. Wu, T. Kuroda, Sowa, M. Munakata and M. Maekawa, Polyhedron, 1997, 16, 67 CrossRef CAS  ;
(h) B. F. Straub, F. Eisenträger and P. Hofmann, Chem. Commun., 1999, 2507 RSC
;
(h) B. F. Straub, F. Eisenträger and P. Hofmann, Chem. Commun., 1999, 2507 RSC  ;
(i) X. Dai and T. H. Warren, Chem. Commun., 2001, 1998 RSC
;
(i) X. Dai and T. H. Warren, Chem. Commun., 2001, 1998 RSC  ;
(j) H. V. R. Dias, H.-L. Lu, H.-J. Kim, S. A. Polach, T. K. H. H. Goh, R. G. Browning and C. J. Lovely, Organometallics, 2002, 21, 1466 CrossRef CAS
;
(j) H. V. R. Dias, H.-L. Lu, H.-J. Kim, S. A. Polach, T. K. H. H. Goh, R. G. Browning and C. J. Lovely, Organometallics, 2002, 21, 1466 CrossRef CAS  ;
(k) H. V. R. Dias, X. Wang and H. V. K. Diyabalanage, Inorg. Chem., 2005, 44, 7322 CrossRef CAS
;
(k) H. V. R. Dias, X. Wang and H. V. K. Diyabalanage, Inorg. Chem., 2005, 44, 7322 CrossRef CAS  ;
(l) H. V. R. Dias, S. Singh and J. A. Flores, Inorg. Chem., 2006, 45, 8859 CrossRef CAS
;
(l) H. V. R. Dias, S. Singh and J. A. Flores, Inorg. Chem., 2006, 45, 8859 CrossRef CAS  ;
(m) G. Santiso, Quinones, A. Reisinger, J. Slattery and I. Krossing, Chem. Commun., 2007, 5046 RSC
;
(m) G. Santiso, Quinones, A. Reisinger, J. Slattery and I. Krossing, Chem. Commun., 2007, 5046 RSC  ;
(n) J. A. Flores and H. V. R. Dias, Inorg. Chem., 2008, 47, 4448 CrossRef CAS
;
(n) J. A. Flores and H. V. R. Dias, Inorg. Chem., 2008, 47, 4448 CrossRef CAS  ;
(o) M. J. Bainbridge, J. R. L. Smith and P. H. Walton, Dalton Trans., 2009, 3143 RSC
;
(o) M. J. Bainbridge, J. R. L. Smith and P. H. Walton, Dalton Trans., 2009, 3143 RSC  ;
(p) J. A. Flores, V. Badarinarayana, S. Singh, C. J. Lovely and H. V. R. Dias, Dalton Trans., 2009, 7648 RSC
;
(p) J. A. Flores, V. Badarinarayana, S. Singh, C. J. Lovely and H. V. R. Dias, Dalton Trans., 2009, 7648 RSC  ;
(q) X. Kou and H. V. R. Dias, Dalton Trans., 2009, 7529 RSC
;
(q) X. Kou and H. V. R. Dias, Dalton Trans., 2009, 7529 RSC  .
.
-
(a) G. J. H. van Nes and A. Vos, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1979, 35, 2593 CrossRef CAS
 ;
(b) I. Krossing and A. Reisinger, Angew. Chem., Int. Ed., 2003, 42, 5725 CrossRef CAS
;
(b) I. Krossing and A. Reisinger, Angew. Chem., Int. Ed., 2003, 42, 5725 CrossRef CAS  , and references cited therein.
, and references cited therein.
-
(a) R.-D. Schnebeck, E. Freisinger and B. Lippert, Angew. Chem., Int. Ed., 1999, 38, 168 CrossRef CAS
 ;
(b) R.-D. Schnebeck, E. Freisinger, F. Glahé and B. Lippert, J. Am. Chem. Soc., 2000, 122, 1381 CrossRef CAS
;
(b) R.-D. Schnebeck, E. Freisinger, F. Glahé and B. Lippert, J. Am. Chem. Soc., 2000, 122, 1381 CrossRef CAS  ;
(c) S.-H. Li, H.-P. Huang, S.-Y. Yu, Y.-Z. Li, H. Huang, Y. Sei and K. Yamaguchi, Dalton Trans., 2005, 2346 RSC
;
(c) S.-H. Li, H.-P. Huang, S.-Y. Yu, Y.-Z. Li, H. Huang, Y. Sei and K. Yamaguchi, Dalton Trans., 2005, 2346 RSC  .
.
-
(a) S. Mann, G. Huttner, L. Zsolnai and K. Heinze, Angew. Chem., Int. Ed. Engl., 1996, 35, 2808 CrossRef CAS
 ;
(b) M. Staffilani, K. S. B. Hancock, J. W. Steed, K. T. Holman, J. L. Atwood, R. K. Juneja and R. S. Burkhalter, J. Am. Chem. Soc., 1997, 119, 6324 CrossRef CAS
;
(b) M. Staffilani, K. S. B. Hancock, J. W. Steed, K. T. Holman, J. L. Atwood, R. K. Juneja and R. S. Burkhalter, J. Am. Chem. Soc., 1997, 119, 6324 CrossRef CAS  ;
(c) J. S. Fleming, K. L. V. Mann, C.-A. Carraz, E. Psillakis, J. C. Jeffery, J. A. McCleverty and M. D. Ward, Angew. Chem., Int. Ed., 1998, 37, 1279 CrossRef CAS
;
(c) J. S. Fleming, K. L. V. Mann, C.-A. Carraz, E. Psillakis, J. C. Jeffery, J. A. McCleverty and M. D. Ward, Angew. Chem., Int. Ed., 1998, 37, 1279 CrossRef CAS  ;
(d) C. S. Campos, Fernández, R. Clerac and K. R. Dunbar, Angew. Chem., Int. Ed., 1999, 38, 3477 CrossRef
;
(d) C. S. Campos, Fernández, R. Clerac and K. R. Dunbar, Angew. Chem., Int. Ed., 1999, 38, 3477 CrossRef  ;
(e) H. Amouri, M. N. Rager, F. Cagnol and J. Vaissermann, Angew. Chem., Int. Ed., 2001, 40, 3636 CrossRef CAS
;
(e) H. Amouri, M. N. Rager, F. Cagnol and J. Vaissermann, Angew. Chem., Int. Ed., 2001, 40, 3636 CrossRef CAS  ;
(f) H. Amouri, L. Mimassi, M. N. Rager, B. E. Mann, C. Guyard, Duhayon and L. Raehm, Angew. Chem., Int. Ed., 2005, 44, 4543 CrossRef CAS
;
(f) H. Amouri, L. Mimassi, M. N. Rager, B. E. Mann, C. Guyard, Duhayon and L. Raehm, Angew. Chem., Int. Ed., 2005, 44, 4543 CrossRef CAS  ;
(g) S. P. Argent, H. Adams, T. Riis, Johannessen, J. C. Jeffery, L. P. Harding, O. Mamula and M. D. Ward, Inorg. Chem., 2006, 45, 3905 CrossRef CAS
;
(g) S. P. Argent, H. Adams, T. Riis, Johannessen, J. C. Jeffery, L. P. Harding, O. Mamula and M. D. Ward, Inorg. Chem., 2006, 45, 3905 CrossRef CAS  ;
(h) X.-T. Meng, Q.-S. Li, F.-B. Xu, H.-B. Song, C. E. Anson and Z.-Z. Zhang, Inorg. Chem., 2006, 45, 7986 CrossRef CAS
;
(h) X.-T. Meng, Q.-S. Li, F.-B. Xu, H.-B. Song, C. E. Anson and Z.-Z. Zhang, Inorg. Chem., 2006, 45, 7986 CrossRef CAS  .
.
- D. A. McMorran and P. J. Steel, Angew. Chem., Int. Ed., 1998, 37, 3295 CrossRef CAS
 .
.
-
(a) R. Vilar, D. M. P. Mingos, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 1998, 37, 1258 CrossRef CAS
 ;
(b) S.-T. Cheng, E. Doxiadi, R. Vilar, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 2001, 2239 RSC
;
(b) S.-T. Cheng, E. Doxiadi, R. Vilar, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 2001, 2239 RSC  ;
(c) J. A. Wisner, P. D. Beer, M. G. B. Drew and M. R. Sambrook, J. Am. Chem. Soc., 2002, 124, 12469 CrossRef CAS
;
(c) J. A. Wisner, P. D. Beer, M. G. B. Drew and M. R. Sambrook, J. Am. Chem. Soc., 2002, 124, 12469 CrossRef CAS  .
.
-
(a) Z. Zheng, C. B. Knobler, M. D. Mortimer, G. Kong and M. F. Hawthorne, J. Am. Chem. Soc., 1996, 35, 1235 CAS
 ;
(b) M. F. Hawthorne and Z. Zheng, Acc. Chem. Res., 1997, 30, 267 CrossRef CAS
;
(b) M. F. Hawthorne and Z. Zheng, Acc. Chem. Res., 1997, 30, 267 CrossRef CAS  .
.
- C. S. Campos, Fernández, B. L. Schottel, H. T. Chifotides, J. K. Bera, J. Bacsa, J. M. Koomen, D. H. Russell and K. R. Dunbar, J. Am. Chem. Soc., 2005, 127, 12909 CrossRef
 .
.
- T. Hasobe, A. S. D. Sandanayaka, T. Wada and Y. Araki, Chem. Commun., 2008, 3372 RSC
 .
.
-
(a) R. M. Sullivan, H. Liu, D. S. Smith, J. C. Hanson, D. Osterhout, M. Ciraolo, C. P. Grey and J. D. Martin, J. Am. Chem. Soc., 2003, 125, 11065 CrossRef CAS
 ;
(b) M. D. Capracotta, R. M. Sullivan and J. D. Martin, J. Am. Chem. Soc., 2006, 128, 13463 CrossRef CAS
;
(b) M. D. Capracotta, R. M. Sullivan and J. D. Martin, J. Am. Chem. Soc., 2006, 128, 13463 CrossRef CAS  .
.
-
(a) S. J. Coles, J. G. Frey, P. A. Gale, M. B. Hursthouse, M. E. Light, K. Navakhun and G. L. Thomas, Chem. Commun., 2003, 568 RSC
 ;
(b) S. J. Coles, J. G. Frey, P. A. Gale, M. B. Hursthouse, M. E. Light, K. Navakhun and G. L. Thomas, Chem. Commun., 2003, 1462 CAS
;
(b) S. J. Coles, J. G. Frey, P. A. Gale, M. B. Hursthouse, M. E. Light, K. Navakhun and G. L. Thomas, Chem. Commun., 2003, 1462 CAS  , addition and correction to ref. 22a.
, addition and correction to ref. 22a.
-
(a) J. Reedijk, Comments Inorg. Chem., 1982, 1, 379 CrossRef CAS
 ;
(b) P. J. van Koningsbruggen, J. G. Haasnoot, R. A. G. de Graaff and J. Reedijk, J. Chem. Soc., Dalton Trans., 1993, 483 RSC
;
(b) P. J. van Koningsbruggen, J. G. Haasnoot, R. A. G. de Graaff and J. Reedijk, J. Chem. Soc., Dalton Trans., 1993, 483 RSC  ;
(c) G. A. van Albada, O. Roubeau, I. Mutikainen, U. Turpeinen and J. Reedijk, New J. Chem., 2003, 27, 1693 RSC
;
(c) G. A. van Albada, O. Roubeau, I. Mutikainen, U. Turpeinen and J. Reedijk, New J. Chem., 2003, 27, 1693 RSC  ;
(d) H. Casellas, A. Pevec, B. Kozlevcar, P. Gamez and J. Reedijk, Polyhedron, 2005, 24, 1549 CrossRef CAS
;
(d) H. Casellas, A. Pevec, B. Kozlevcar, P. Gamez and J. Reedijk, Polyhedron, 2005, 24, 1549 CrossRef CAS  .
.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327, 11
327, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447, 17
447, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589, 32
589, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 and 35
111 and 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 reflections that were collected, 3258, 3651, 3464, 4171 and 1248 were unique (Rint = 0.0240, 0.0484, 0.0310, 0.0486 and 0.0523) for complexes 1–5, respectively. Data were collected and processed using the Crystal Clear program (Rigaku). The linear absorption coefficient, μ, for Mo-Kα radiation is 29.96, 23.47, 21.65, 16.343 and 21.07 cm−1 for complexes 1–5, respectively. The data were corrected for Lorentz and polarization effects.
743 reflections that were collected, 3258, 3651, 3464, 4171 and 1248 were unique (Rint = 0.0240, 0.0484, 0.0310, 0.0486 and 0.0523) for complexes 1–5, respectively. Data were collected and processed using the Crystal Clear program (Rigaku). The linear absorption coefficient, μ, for Mo-Kα radiation is 29.96, 23.47, 21.65, 16.343 and 21.07 cm−1 for complexes 1–5, respectively. The data were corrected for Lorentz and polarization effects.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 (Rint = 0.0237)
327 (Rint = 0.0237)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447 (Rint = 0.0484)
447 (Rint = 0.0484)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589 (Rint = 0.0310)
589 (Rint = 0.0310)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 (Rint = 0.0486)
111 (Rint = 0.0486)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743 (Rint = 0.0523)
743 (Rint = 0.0523)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in a trigonal-planar geometry. The other Cu atom is coordinated by two N atoms of the different bpm ligands in the exo bridging site and two O atoms of the different NO3− anions in a distorted tetragonal geometry to form an infinite 1-D zigzag chain structure. Although there have been several Cu(I) complexes with a NO3− anion in the chelating bidentate mode11 and the bridging bidentate manner,12 there are few Cu(I) complexes with a NO3− anion in the unidentate coordination mode.5d,11b,d,f Additionally, Cu(I) C2H4 adducts have been poorly characterized due to the extremely labile nature of the Cu(I)–C2H4 interaction.5c,d,6c,13 In particular, preparative and structural reports of polynuclear and polymeric Cu(I) C2H4 complexes are few.5c,d,6c,13e,f Therefore, polymeric Cu(I)–bpm/C2H4 adduct 1 with the η1-NO3− anion is of significance. In our related Cu(I)–pprd complexes (Scheme 1(a)), we have proved that the conformation of metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts can be controlled by the choice of anion: the self-assemblies by anion templation of BF4−, PF6− and ClO4− under C2H4 preferentially can induce metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts.5c,d In contrast, the NO3− anion is ineffective as an anion template in the formation of expected metallamacrocyclic Cu(I)–pprd/C2H4 adducts due to the higher coordination ability of the NO3− anion. In fact, the reaction of [Cu(C2H4)n]NO3 with pprd in Me2CO under C2H4 afforded dinuclear Cu(I)–pprd/C2H4 complex, [Cu2(pprd)(C2H4)2(NO3)]NO3.5d In this study, two NO3− anions were similarly coordinated to the Cu atom in the terminal bridging sites of bpm in the η1-fashion. This finding suggests that the NO3− anion is ineffective as an anion template in the construction of expected metallamacrocyclic Cu(I)–bpm/C2H4 adducts, although the combination of [Cu(C2H4)n]NO3 and bpm under C2H4 can produce a rare polymeric 1-D zigzag chain Cu(I) C2H4 adduct. In the coordinated C2H4, the C
C bond of C2H4 in a trigonal-planar geometry. The other Cu atom is coordinated by two N atoms of the different bpm ligands in the exo bridging site and two O atoms of the different NO3− anions in a distorted tetragonal geometry to form an infinite 1-D zigzag chain structure. Although there have been several Cu(I) complexes with a NO3− anion in the chelating bidentate mode11 and the bridging bidentate manner,12 there are few Cu(I) complexes with a NO3− anion in the unidentate coordination mode.5d,11b,d,f Additionally, Cu(I) C2H4 adducts have been poorly characterized due to the extremely labile nature of the Cu(I)–C2H4 interaction.5c,d,6c,13 In particular, preparative and structural reports of polynuclear and polymeric Cu(I) C2H4 complexes are few.5c,d,6c,13e,f Therefore, polymeric Cu(I)–bpm/C2H4 adduct 1 with the η1-NO3− anion is of significance. In our related Cu(I)–pprd complexes (Scheme 1(a)), we have proved that the conformation of metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts can be controlled by the choice of anion: the self-assemblies by anion templation of BF4−, PF6− and ClO4− under C2H4 preferentially can induce metallamacrocyclic Cu(I)–pprd/C2H4, CO adducts.5c,d In contrast, the NO3− anion is ineffective as an anion template in the formation of expected metallamacrocyclic Cu(I)–pprd/C2H4 adducts due to the higher coordination ability of the NO3− anion. In fact, the reaction of [Cu(C2H4)n]NO3 with pprd in Me2CO under C2H4 afforded dinuclear Cu(I)–pprd/C2H4 complex, [Cu2(pprd)(C2H4)2(NO3)]NO3.5d In this study, two NO3− anions were similarly coordinated to the Cu atom in the terminal bridging sites of bpm in the η1-fashion. This finding suggests that the NO3− anion is ineffective as an anion template in the construction of expected metallamacrocyclic Cu(I)–bpm/C2H4 adducts, although the combination of [Cu(C2H4)n]NO3 and bpm under C2H4 can produce a rare polymeric 1-D zigzag chain Cu(I) C2H4 adduct. In the coordinated C2H4, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance of 1.373(4) Å is slightly longer than those [1.30(1)–1.366(6) Å] in the reported trigonal-planar Cu(I) C2H4 complexes5d,13c–i and that [1.313 (exptl) and 1.333 (calc.) Å] of metal-free C2H4.14 The average Cu–N distance of 2.011 Å in the terminal bridging site is slightly shorter than that (2.006 Å) in the chelate site.
C distance of 1.373(4) Å is slightly longer than those [1.30(1)–1.366(6) Å] in the reported trigonal-planar Cu(I) C2H4 complexes5d,13c–i and that [1.313 (exptl) and 1.333 (calc.) Å] of metal-free C2H4.14 The average Cu–N distance of 2.011 Å in the terminal bridging site is slightly shorter than that (2.006 Å) in the chelate site.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the distorted trigonal geometry to form an infinite 1-D zigzag ladder structure. It is interesting that two disordered ClO4− anions are accommodated in the inside cavity of the ladder chains. Although there have been several reports of the encapsulation of NO3−,15BF4−,5d,15a,b,16PF6−,15a,b,17Cl218 and I22–19 anions, less is known about the encapsulation of a ClO4− anion into macrocycles and cages.15a,16h,20
C bond of C2H4 in the distorted trigonal geometry to form an infinite 1-D zigzag ladder structure. It is interesting that two disordered ClO4− anions are accommodated in the inside cavity of the ladder chains. Although there have been several reports of the encapsulation of NO3−,15BF4−,5d,15a,b,16PF6−,15a,b,17Cl218 and I22–19 anions, less is known about the encapsulation of a ClO4− anion into macrocycles and cages.15a,16h,20


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distances of 1.385(8) and 1.370(9) Å in complexes 2 and 3 are slightly longer than that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d
C distances of 1.385(8) and 1.370(9) Å in complexes 2 and 3 are slightly longer than that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the distorted tetrahedral geometry. Three Cu atoms are bridged by three bpm ligands to form a metallacalix[3]arene structure with three legs of C2H4. Furthermore, these metallacalix[3]arenes are linked through another Cu atom with MeOH in the terminal N atom of bpm to produce a 2-D sheet structure with small and large Cu3 cavities (Fig. 5). Although C2H4 adducts to the 2-D surfaces of CuMCl4 (M = Al and Ga) have been determined by powder X-ray diffraction analysis,22 complex 4b is the first 2-D sheet Cu(I) C2H4 adduct. It should be noted that each 2-D sheet structure is arranged in parallel along the c-axis, resulting in the formation of a chiral 2-D sheet structure (Fig. 6 and 7). The presence of chirality could be confirmed by circular dichroic (CD) spectroscopy in the solid state (ESI, Fig. S2†). The positive cotton effect was observed. More noteworthy are one BF4− anion is functionally accommodated in the small triangular Cu3 cavities with neighbouring Cu⋯Cu distances of 6.06 Å and three disordered BF4− anions are encapsulated in the large triangular Cu3 cavities with corresponding distances of 14.38 Å. It was shown that the BF4− anion can play a role as an anion template to build a chiral 2-D sheet structure consisting of the linkage of [Cu3(bpm)3] frameworks with a metallacalix[3]arene structure. To the best of our knowledge, the encapsulations of BF4− anion into macrocycles and cage compounds have been limited.5d,15a,b,16 As such, this compound is expected to develop as an unprecedented inorganic anion receptor with chirality. In the coordinated C2H4, the C
C bond of C2H4 in the distorted tetrahedral geometry. Three Cu atoms are bridged by three bpm ligands to form a metallacalix[3]arene structure with three legs of C2H4. Furthermore, these metallacalix[3]arenes are linked through another Cu atom with MeOH in the terminal N atom of bpm to produce a 2-D sheet structure with small and large Cu3 cavities (Fig. 5). Although C2H4 adducts to the 2-D surfaces of CuMCl4 (M = Al and Ga) have been determined by powder X-ray diffraction analysis,22 complex 4b is the first 2-D sheet Cu(I) C2H4 adduct. It should be noted that each 2-D sheet structure is arranged in parallel along the c-axis, resulting in the formation of a chiral 2-D sheet structure (Fig. 6 and 7). The presence of chirality could be confirmed by circular dichroic (CD) spectroscopy in the solid state (ESI, Fig. S2†). The positive cotton effect was observed. More noteworthy are one BF4− anion is functionally accommodated in the small triangular Cu3 cavities with neighbouring Cu⋯Cu distances of 6.06 Å and three disordered BF4− anions are encapsulated in the large triangular Cu3 cavities with corresponding distances of 14.38 Å. It was shown that the BF4− anion can play a role as an anion template to build a chiral 2-D sheet structure consisting of the linkage of [Cu3(bpm)3] frameworks with a metallacalix[3]arene structure. To the best of our knowledge, the encapsulations of BF4− anion into macrocycles and cage compounds have been limited.5d,15a,b,16 As such, this compound is expected to develop as an unprecedented inorganic anion receptor with chirality. In the coordinated C2H4, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance of 1.31(2) Å is similar to that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d The average Cu–N distance of 2.025 Å in the terminal bridging site is slightly shorter than that (2.144 Å) in the chelate site.
C distance of 1.31(2) Å is similar to that [1.313 (exptl) and 1.333 (calc.) Å] of the metal-free C2H414 and those (1.30(1)–1.366(6) Å) in tetrahedral Cu(I) C2H4 complexes13b,j and related Cu(I)–pprd/C2H4 metallamacrocycles.5c,d The average Cu–N distance of 2.025 Å in the terminal bridging site is slightly shorter than that (2.144 Å) in the chelate site.


![X-Ray crystal structures of complex 5 encapsulating disordered SiF62− anions. Top {(a) and (b)} and side views (c) in two racemic metallacalix[3]arene structures.](/image/article/2012/CE/c1ce06328f/c1ce06328f-f8.gif)


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bands of Cu(I)–bpm/C2H4 adducts 1–4 are observed at 1559 (1), 1548 (2), 1550 (3) and 1545 (4) cm−1, respectively [metal-free C2H4, 1623 cm−1]. These νC
C bands of Cu(I)–bpm/C2H4 adducts 1–4 are observed at 1559 (1), 1548 (2), 1550 (3) and 1545 (4) cm−1, respectively [metal-free C2H4, 1623 cm−1]. These νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C wavenumbers are slightly larger than those (1537–1543 cm−1) of the related Cu(I)–pprd/C2H4 metallamacrocycles encapsulating ClO4−, PF6− and BF4− anions,5c,5d indicative of the contribution of poor Cu → C2H4 π back-bonding.
C wavenumbers are slightly larger than those (1537–1543 cm−1) of the related Cu(I)–pprd/C2H4 metallamacrocycles encapsulating ClO4−, PF6− and BF4− anions,5c,5d indicative of the contribution of poor Cu → C2H4 π back-bonding.; (b) L. R. MacGillivray, Metal–Organic Frameworks, ed. L. R. MacGillivray, Wiley & Sons, New Jersey, 2010 Search PubMed
; (c) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspective, Wiley-VCH: Weinheim, 1995 Search PubMed
; (d) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS
; (e) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS
; (f) L. Cronin, Annu. Rep. Prog. Chem., Sect. A, 2003, 99, 289 RSC
; (g) F. Würthner, C.-C. You, C. R. Saha and Moller, Chem. Soc. Rev., 2004, 33, 133 RSC
; (h) P. Thanasekaran, R.-T. Liao, Y.-H. Liu, T. Rajendran, S. Rajagopal and K.-L. Lu, Coord. Chem. Rev., 2005, 249, 1085 CrossRef CAS
.
.
; (b) Z. Asfari, V. Böhmer, J. Harrowfield and J. Vicens, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, 2001 Search PubMed
; (c) D. J. Cram and J. M. Cram, Container Molecules and their Guests, Royal Society of Chemistry, Cambridge, 1994 Search PubMed
.
; (b) P. A. Gale, J. L. Sessler and V. Král, Chem. Commun., 1998, 1 RSC
; (c) P. A. Gale, Coord. Chem. Rev., 2000, 199, 181 CrossRef CAS
; (d) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989 CrossRef CAS
; (e) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS
; (f) P. A. Gale, Coord. Chem. Rev., 2001, 213, 79 CrossRef CAS
; (g) P. A. Gale, Coord. Chem. Rev., 2003, 240, 191 CrossRef CAS
; (h) R. Vilar, Angew. Chem., Int. Ed., 2003, 42, 1460 CrossRef CAS
; (i) P. D. Beer, M. R. Sambrook and D. Curiel, Chem. Commun., 2006, 2105 RSC
; (j) N. Gimeno and R. Vilar, Coord. Chem. Rev., 2006, 250, 3161 CrossRef CAS
; (k) R. Vilar, Struct. Bonding, 2008, 129, 175 CrossRef CAS
; (l) R. Vilar, Eur. J. Inorg. Chem., 2008, 357 CrossRef CAS
; (m) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC
.
; (b) M. Maekawa, S. Kitagawa, T. Kuroda, Sowa and M. Munakata, Chem. Commun., 2006, 2161 RSC
; (c) M. Maekawa, H. Konaka, T. Minemastu, T. Kuroda, Sowa, M. Munakata and S. Kitagawa, Chem. Commun., 2007, 5179 RSC
; (d) M. Maekawa, A. Nabei, T. Tominaga, K. Sugimoto, T. Minematsu, T. Okubo, T. Kuroda, Sowa, M. Munakata and S. Kitagawa, Dalton Trans., 2009, 415 RSC
.
; (b) C. Janiak, L. Uehlin, H.-P. Wu, P. Kliifers, H. Piotrowski and T. G. Scharmann, J. Chem. Soc., Dalton Trans., 1999, 3121 RSC
; (c) M. Maekawa, T. Tominaga, T. Okubo, T. Kuroda, Sowa and M. Munakata, Eur. J. Inorg. Chem., 2009, 4225 CrossRef CAS
.
.
.
.
.
; (b) T. Kuroda, Sowa, M. Munakata, H. Matsuda, S. Akiyama and M. Maekawa, J. Chem. Soc., Dalton Trans., 1995, 2201 RSC
; (c) C. Pettinari, G. G. Lobbia, G. Sclavi and D. Leonesi, Polyhedron, 1995, 14, 1709 CrossRef CAS
; (d) V. Olijnyk, T. Glowiak and M. Mys'kiv, J. Chem. Crystallogr., 1995, 25, 621 CrossRef CAS
; (e) H. Lang, A. del Villar, B. Walfort and G. Rheinwald, J. Organomet. Chem., 2004, 689, 1464 CrossRef CAS
; (f) C. Sivasankar, M. Nethaji and A. G. Samuelson, Inorg. Chem. Commun., 2004, 7, 238 CrossRef CAS
; (g) S.-M. Chen, C.-Z. Lu, C.-K. Xia, X.-J. Xu and Q.-G. Zhai, Cryst. Growth Des., 2005, 5, 1485 CrossRef CAS
.
; (b) R. Yang, K. Lin, Y. Hou, D. Wang and D. Jin, Polyhedron, 1997, 16, 4033 CrossRef
; (c) J.-P. Lang, H. Kawaguchi and K. Tatsumi, J. Organomet. Chem., 1998, 569, 109 CrossRef CAS
; (d) R. Yang, Y. Sun, D. Zhao and L. Guo, J. Coord. Chem., 2003, 56, 1169 CrossRef CAS
.
; (b) J. S. Thompson, R. L. Harlow and J. F. Whitney, J. Am. Chem. Soc., 1983, 105, 3522 CrossRef CAS
; (c) J. S. Thompson and J. F. Whitney, Inorg. Chem., 1984, 23, 2813 CrossRef CAS
; (d) H. Masuda, N. Yamamoto, T. Taga, K. Machida, S. Kitagawa and M. Munakata, J. Organomet. Chem., 1987, 322, 121 CrossRef CAS
; (e) M. Munakata, T. Kuroda, Sowa, M. Maekawa and M. Nakamura, Inorg. Chem., 1994, 33, 1284 CrossRef CAS
; (f) J. Dai, M. Yamamoto, T. Kuroda, Sowa, M. Maekawa, Y. Suenaga and M. Munakata, Inorg. Chem., 1997, 36, 2688 CrossRef CAS
; (g) Y. Suenaga, L.-P. Wu, T. Kuroda, Sowa, M. Munakata and M. Maekawa, Polyhedron, 1997, 16, 67 CrossRef CAS
; (h) B. F. Straub, F. Eisenträger and P. Hofmann, Chem. Commun., 1999, 2507 RSC
; (i) X. Dai and T. H. Warren, Chem. Commun., 2001, 1998 RSC
; (j) H. V. R. Dias, H.-L. Lu, H.-J. Kim, S. A. Polach, T. K. H. H. Goh, R. G. Browning and C. J. Lovely, Organometallics, 2002, 21, 1466 CrossRef CAS
; (k) H. V. R. Dias, X. Wang and H. V. K. Diyabalanage, Inorg. Chem., 2005, 44, 7322 CrossRef CAS
; (l) H. V. R. Dias, S. Singh and J. A. Flores, Inorg. Chem., 2006, 45, 8859 CrossRef CAS
; (m) G. Santiso, Quinones, A. Reisinger, J. Slattery and I. Krossing, Chem. Commun., 2007, 5046 RSC
; (n) J. A. Flores and H. V. R. Dias, Inorg. Chem., 2008, 47, 4448 CrossRef CAS
; (o) M. J. Bainbridge, J. R. L. Smith and P. H. Walton, Dalton Trans., 2009, 3143 RSC
; (p) J. A. Flores, V. Badarinarayana, S. Singh, C. J. Lovely and H. V. R. Dias, Dalton Trans., 2009, 7648 RSC
; (q) X. Kou and H. V. R. Dias, Dalton Trans., 2009, 7529 RSC
.
; (b) I. Krossing and A. Reisinger, Angew. Chem., Int. Ed., 2003, 42, 5725 CrossRef CAS
, and references cited therein.
; (b) R.-D. Schnebeck, E. Freisinger, F. Glahé and B. Lippert, J. Am. Chem. Soc., 2000, 122, 1381 CrossRef CAS
; (c) S.-H. Li, H.-P. Huang, S.-Y. Yu, Y.-Z. Li, H. Huang, Y. Sei and K. Yamaguchi, Dalton Trans., 2005, 2346 RSC
.
; (b) M. Staffilani, K. S. B. Hancock, J. W. Steed, K. T. Holman, J. L. Atwood, R. K. Juneja and R. S. Burkhalter, J. Am. Chem. Soc., 1997, 119, 6324 CrossRef CAS
; (c) J. S. Fleming, K. L. V. Mann, C.-A. Carraz, E. Psillakis, J. C. Jeffery, J. A. McCleverty and M. D. Ward, Angew. Chem., Int. Ed., 1998, 37, 1279 CrossRef CAS
; (d) C. S. Campos, Fernández, R. Clerac and K. R. Dunbar, Angew. Chem., Int. Ed., 1999, 38, 3477 CrossRef
; (e) H. Amouri, M. N. Rager, F. Cagnol and J. Vaissermann, Angew. Chem., Int. Ed., 2001, 40, 3636 CrossRef CAS
; (f) H. Amouri, L. Mimassi, M. N. Rager, B. E. Mann, C. Guyard, Duhayon and L. Raehm, Angew. Chem., Int. Ed., 2005, 44, 4543 CrossRef CAS
; (g) S. P. Argent, H. Adams, T. Riis, Johannessen, J. C. Jeffery, L. P. Harding, O. Mamula and M. D. Ward, Inorg. Chem., 2006, 45, 3905 CrossRef CAS
; (h) X.-T. Meng, Q.-S. Li, F.-B. Xu, H.-B. Song, C. E. Anson and Z.-Z. Zhang, Inorg. Chem., 2006, 45, 7986 CrossRef CAS
.
.
; (b) S.-T. Cheng, E. Doxiadi, R. Vilar, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 2001, 2239 RSC
; (c) J. A. Wisner, P. D. Beer, M. G. B. Drew and M. R. Sambrook, J. Am. Chem. Soc., 2002, 124, 12469 CrossRef CAS
.
; (b) M. F. Hawthorne and Z. Zheng, Acc. Chem. Res., 1997, 30, 267 CrossRef CAS
.
.
.
; (b) M. D. Capracotta, R. M. Sullivan and J. D. Martin, J. Am. Chem. Soc., 2006, 128, 13463 CrossRef CAS
.
; (b) S. J. Coles, J. G. Frey, P. A. Gale, M. B. Hursthouse, M. E. Light, K. Navakhun and G. L. Thomas, Chem. Commun., 2003, 1462 CAS
, addition and correction to ref. 22a.
; (b) P. J. van Koningsbruggen, J. G. Haasnoot, R. A. G. de Graaff and J. Reedijk, J. Chem. Soc., Dalton Trans., 1993, 483 RSC
; (c) G. A. van Albada, O. Roubeau, I. Mutikainen, U. Turpeinen and J. Reedijk, New J. Chem., 2003, 27, 1693 RSC
; (d) H. Casellas, A. Pevec, B. Kozlevcar, P. Gamez and J. Reedijk, Polyhedron, 2005, 24, 1549 CrossRef CAS
.
