Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools
A. Prasanna
de Silva
*a,
Thomas S.
Moody
b and
Glenn D.
Wright
a
aSchool of Chemistry and Chemical Engineering, Queen's University, Belfast, Northern Ireland BT9 5AG, Northern Ireland. E-mail: a.desilva@qub.ac.uk; gwright04@qub.ac.uk
bBiocatalysis Group, Almac Sciences, Almac House, 20 Seagoe Industrial Estate, Craigavon, Northern Ireland BT63 5QD. E-mail: tom.moody@almacgroup.com
First published on 6th October 2009
Abstract
Fluorescent sensors are an important part of the analytical scientist's toolbox. The use of fluorescent PET (Photoinduced Electron Transfer) sensors has seen particular growth in recent times. This Critical Review discusses recent growth areas in fluorescent PET sensors by emphasizing the modular features of the ‘fluorophore–spacer–receptor’ design. The occurrence of the dipicolylamine receptor in PET sensor designs is critically examined as a case in point.
 Glenn Wright, Tom Moody and A. P. de Silva (left to right) | A. P. de Silva received his early education in chemistry at the University of Colombo, Sri Lanka, followed by PhD and postdoctoral research in organic photochemistry at the Queen's University of Belfast. After spending a few years lecturing in chemistry at Colombo, he returned to Belfast where he is a professor and a percussionist. He has been visiting professor in Louvain-La-Neuve, Cachan, Bordeaux, Strasbourg, Peradeniya, Kandy, Nara, Bangkok and Shanghai. With his co-workers, he published the first experimental molecular logic gates in the primary literature and established the generality of one of the main principles underlying luminescent sensors. Thomas S. Moody received his 1st Class BSc (Hons) in chemistry and PhD in physical organic chemistry from the Queen's University of Belfast focusing on the synthesis and application of fluorescent sensors. He has completed a Masters Degree with distinction in Business, specialising in business strategy. Tom has broad industrial experience leading to his current position of leading a multi-disciplinary team developing and implementing commercially valuable bioprocesses from milligram to tonne manufacture. Tom continues to keep a keen interest in the area of optical sensors and is applying this technology to the discovery of new biocatalysts. Glenn D. Wright was born in Belfast, Northern Ireland, and grew up in Carrickfergus on the County Antrim coast. He received his 1st Class BSc (Hons) in chemistry at the Queen's University of Belfast in 2007 after having taken a year out to work in a pharmaceutical company in Germany. He stayed at Queen's and is currently working towards his PhD with A. P. de Silva. When not doing research, he has a passion for travel, rock-climbing and the outdoors. |
Introduction
Analytical science continues to be enriched by the principles of supramolecular chemistry.1,2 Hardly a week goes by without a new fluorescent PET (Photoinduced Electron Transfer) sensor being announced. What are these sensors and how do they operate? How did they evolve? What are the reasons for their widespread development? This Critical Review briefly addresses these questions before tracing the recent lineage of a single sub-field of fluorescent PET sensors and presenting some highlights in the field from the past year.Molecular engineering design
A single simple picture (Fig. 1) encapsulates the design of fluorescent PET sensors. The ‘fluorophore–spacer–receptor’ format is a rational combination of three components. The rationale is contained in Fig. 2a and 2b. In its ‘off’ state, excitation of the fluorophore component of the sensor produces an electron transfer from the receptor to the fluorophore as one possibility. In other words, the excited state energy of the fluorophore needs to be sufficient to provide both the reduction potential of the fluorophore and the oxidation potential of the receptor. This is a thermodynamic condition first derived by Weller.3 In its ‘on’ state, excitation of the fluorophore results in fluorescence only because the PET process is arrested by the arrival of the analyte at the receptor site. The arrest can be easily comprehended by considering H+ as the analyte. H+ electrostatically attracts the electron which increases the oxidation potential of the analyte-bound receptor to the point that the thermodynamics for PET are no longer favourable.4,5 | ||
| Fig. 1 The ‘fluorophore–spacer–receptor’ format of fluorescent PET sensors. | ||
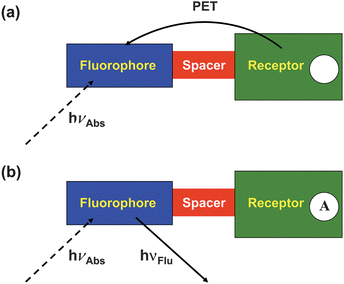 | ||
| Fig. 2 (a) An electron transfer from the analyte-free receptor to the photo-excited fluorophore creates the ‘off’ state of the sensor. (b) The electron transfer from the analyte-bound receptor is blocked resulting in the ‘on’ state of the sensor. | ||
These ideas can also be expressed with the aid of molecular orbital energy diagrams (Fig. 3a and 3b). We note the bracketing of the receptor HOMO by the frontier orbitals of the fluorophore in the ‘off’ state of the sensor and the stabilization of the analyte-bound receptor's HOMO to lie below the fluorophore's HOMO in the ‘on’ state. Fig. 3a and 3b allow us to deduce an even simpler criterion for PET sensor design: PET occurs if the oxidation potential of the receptor is smaller in magnitude than that of the fluorophore. The opposite applies in the ‘on’ state of the sensor. This rule of thumb is very useful practically, even though several approximations are involved. More accurate treatment of PET processes are available for the interested reader.3,6–8 MO energies and related redox potentials are increasingly used by PET sensor designers.9–12
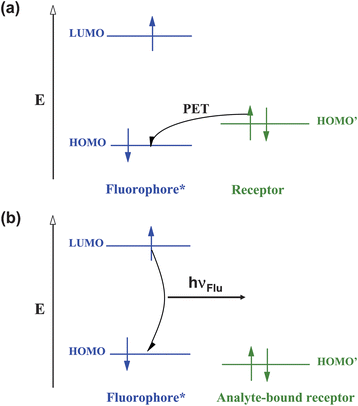 | ||
| Fig. 3 Molecular orbital energy diagrams which show the relative energetic dispositions of the frontier orbitals of the fluorophore and the receptor in (a) the analyte-free situation and (b) the analyte-bound situation. | ||
The availability of a quantitative design criterion is common in engineering but rare in chemistry. The case of fluorescent PET sensors is a rare example of molecular engineering design. Just like houses and cars, molecular PET sensors can now be designed and built for a variety of individual purposes.
Each of the three components within the ‘fluorophore–spacer–receptor’ format deserves the designer's attention. The analyte to be sensed determines the choice of receptor. The reciprocal of the binding constant for the receptor–analyte interaction determines the median analyte concentration to be sensed. Consideration needs to be given at this stage to the selectivity of the receptor towards the analyte and against anticipated levels of potential interferents.
Desired colours for excitation and emission help in the choice of fluorophore. For instance, intracellular studies using glass microscopy optics will preclude the use of excitation wavelengths below 340 nm. Tissue experiments will prefer these wavelengths to be in the red region.
The ease of sensor synthesis dictates the choice of spacer, but the more fundamental determinant is that the spacer must be short enough to permit reasonably fast PET rates in the ‘off’ state of the sensor.13–15 Even virtual spacers can be used provided that other means, such as sterically-induced orthogonalization,16 maintains the separation between the fluorophore and the receptor.
Let us consider an example of simple pH sensing which will serve as a foundation for a case study (see below). Fig. 4 shows two of the main options available to the excited PET sensor 1.4 In order to apply the approximate Weller equation, we note that the excited state energy of the anthracene fluorophore is 3.0 eV.17 Its reduction potential is −2.0 V (vs. sce). The oxidation potential of the receptor can be estimated from that of triethylamine (+1.0 V).17 As the transiting electron falls through these potentials, the corresponding energies are 2.0 eV and 1.0 eV respectively (Fig. 5a). So the approximate ΔG for PET is 0.0 eV.17 This gives a sufficiently fast PET rate to overcome fluorescence (kPET ≫ kFlu). The same result can be obtained by the rule of thumb when we note that the oxidation potential of the receptor is +1.0 V as above and that the oxidation potential of the anthracene fluorophore is +1.0 V. So ΔGPET is 0.0 eV again. When we consider the H+-bound amine receptor of 1, its oxidation potential rises to an immeasurably high value. ΔGPET becomes a large positive number and fluorescence dominates.
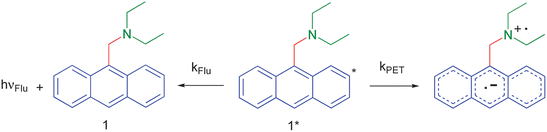 | ||
| Fig. 4 De-excitation pathways open to the photo-excited fluorescent PET sensor 1. | ||
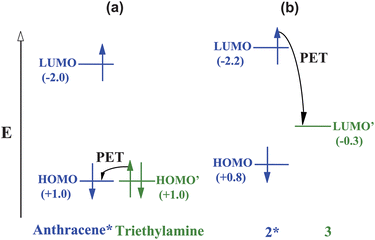 | ||
| Fig. 5 The relative energetic dispositions of the frontier orbitals of the fluorophore and the receptor in the analyte-free situation for PET sensor systems (a) 1 and (b) 2·3. Redox potentials (V vs. sce) are given in parentheses as a measure of molecular orbital energies. The energies are not to scale. | ||
Another more recent example shows how these thermodynamic arguments help even when fluorescent PET sensors are constructed without a covalent linkage between the fluorophore and the receptor. Self-assembly of the trianionic fluorophore 2 within the cavity of the tetracationic receptor 3 produces the ‘off’ state of the sensor system.18 The reduction potential of the receptor can be estimated from that of dimethyl viologen (−0.3 V).17 The oxidation potential of 2 is 0.8 V and its excited state energy is 3.0 eV (Fig. 5b).19 Fast PET is made possible by significantly negative ΔGPET value (−1.9 eV). Displacement of 2 from its complex with 3 by guanosine triphosphate (GTP) occurs due to increased synergistic effects, such as electrostatics and π-stacking inside the cavity. Since the fluorophore 2 is now distanced from the receptor 3 (besides somewhat less favourable thermodynamics for PET) fluorescence is switched ‘on’. Sensing of GTP is thus enabled.
The validity of such molecular engineering makes PET sensors20 an important segment of research in molecular devices.21
Early history
The first case fitting the above description of a fluorescent PET sensor was Wang and Morawetz's dibenzylamine compound (4; as seen later in Fig. 7b).22 It contained a small fluorophore requiring excitation in the deep ultraviolet, spaced with a methylene group from an aliphatic amine receptor. Naturally, H+ was the chosen target, though it was also engaged with Zn2+ and also reacted with acetic anhydride. Several cases23–28 followed with spacers ranging from trimethylene to none at all. The latter situation occurred due to the sterically-enforced twisting of an aniline receptor from an anthracene fluorophore. The generality of the sensing principle was established with a set of related cases28–36 carrying different fluorophores (or phosphors), spacers and receptors. Reviews of this phase are available.4,5,37,38Current uptake
As may be expected, a principle that is general, flexible and extensible tends to be put to use by people seeking solutions to various analytical problems. A sensing principle would naturally be applied to target various analytes in various situations. A commercially successful example which measures blood components like H+, Na+, K+ and Ca2+ deserves a special mention.39 There is a growing body of work where PET sensors are operating within living cells.40,41 The current situation is perhaps best shown graphically. Fig. 6a and 6b show the sources for fluorescent PET sensors and switches around the world as deduced from the literature. Some of these laboratories may have produced a single publication in this field or several dozen. It is clear that research in fluorescent PET sensors is now a delocalized activity.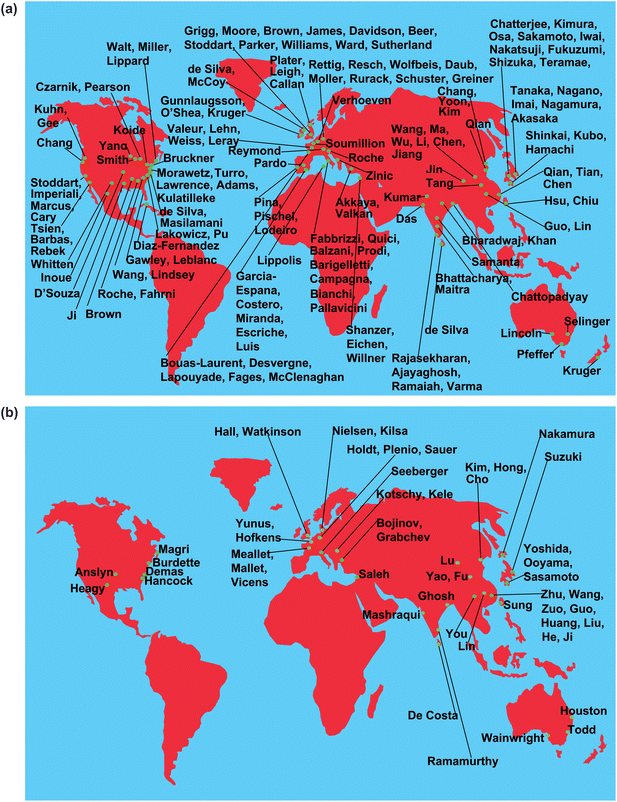 | ||
| Fig. 6 (a,b) Sources of fluorescent PET sensors. Only the names of corresponding authors from the literature are given. The corresponding authors will be happy to receive evidence of errors and omissions so that future versions of the maps can be improved. | ||
A case study: dipicolylamine-based sensors
It is educational to track how a single avenue of fluorescent PET sensors has evolved. It illustrates how different people considering different problems can exploit a single structural motif. Consider di(2-picolyl)amine {IUPAC name: 2-pyridinemethanamine, N-(2-pyridinylmethyl)-} which is a popular receptor42 for d-block cations among coordination chemists. All of the structures discussed are contained within Fig. 7a and 7b.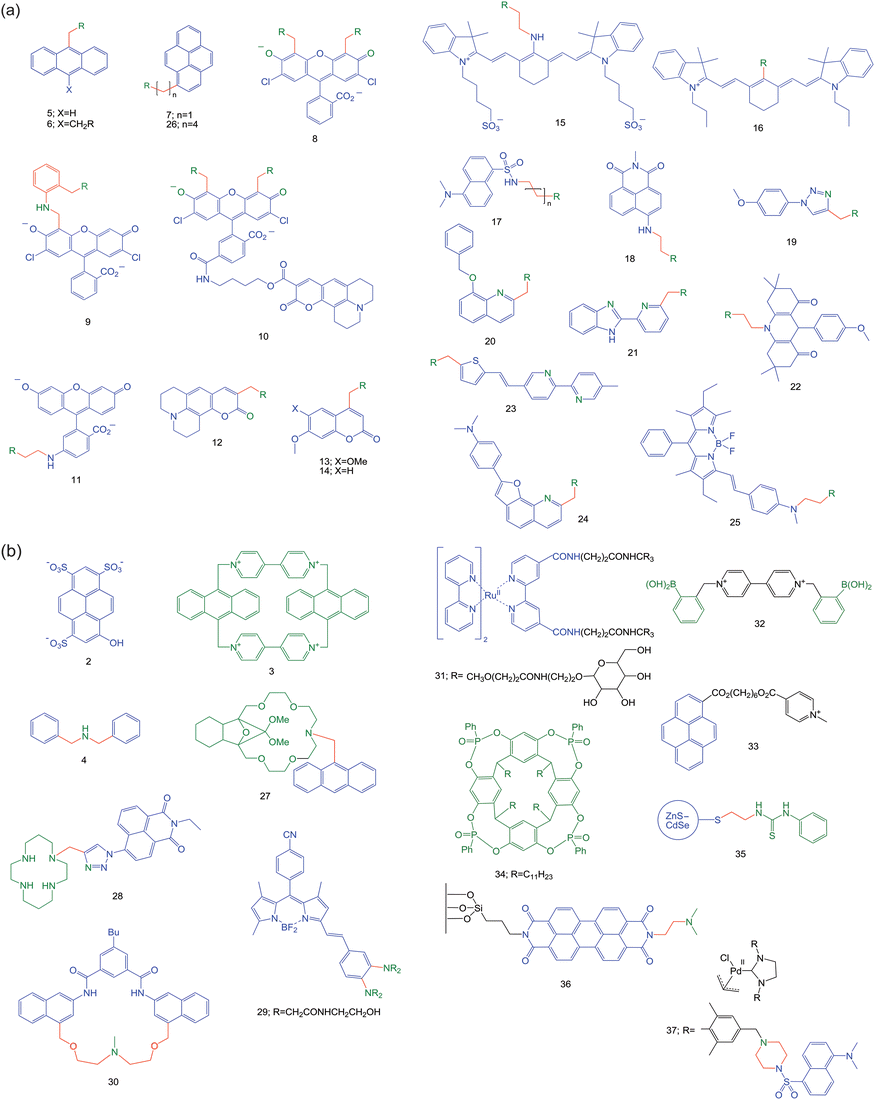 | ||
| Fig. 7 (a) Structural formulae of the dipicolylamine-based sensors discussed in the case study. In all these cases, the R group represents the di(2-pyridylmethyl)amino moiety. (b) Structural formulae for the compounds highlighted from the past year. Following on from Fig. 2a and 2b, the following colour scheme is employed in both Fig. 7a and 7b: fluorophores in blue, spacers in red and receptors in green. When atoms in the fluorophore can also ligate to the analyte, these are shown in green. | ||
Though 5 (Fig. 7a) was a previously known compound,43 S. A. de Silva et al.44 were the first to recognize its ‘fluorophore–spacer–receptor’ format, its PET potential and its di(2-picolyl)amine receptor42 for Zn2+. Indeed a strong Zn2+-induced switching ‘on’ of fluorescence (fluorescence enhancement factor, FE = 77) is seen in acetonitrile. No wavelength shift of the emission band is seen as befits a PET system. The replacement of the terminal methyl groups of 1 by 2-pyridyl units will make the ΔGPET only slightly more positive. The thermodynamic conditions for cation sensing discussed in a previous paragraph are maintained. More recent crystallographic and computational studies have also backed up the Zn2+-binding of 5.45
The modularity of the PET sensing system 5 can now be exploited in many ways and we track structural mutations where the dipicolylamine unit is conserved. The number of laboratories that have followed this path is remarkable, spurred on by the need for monitoring the neurophysiology of Zn2+ and its role in degenerative disease.40,41,46
The extension of PET systems by adding an extra ‘spacer–receptor’ component can lead to improvements in FE values.4,47 This is seen when we go from 5 to 6,48,49 with the requirement that each receptor captures Zn2+. However, ethanol:water (1:1) was needed as the solvent.
The hydrophobicity of the anthracene fluorophore would hamper the use of 5 for monitoring Zn2+ in the cytosol. Hydrophobic PET sensors can localize in intracellular membranes and lose their ion-sensing ability.12 Therefore, replacement of anthracene by hydrophilic heterocyclic fluorophores is logical, and is discussed in the very next paragraph. On the other hand, the hydrophobicity of 5 or its cousin 750 can be exploited for Zn2+ measurement in nanospaces adjoining membranes. The fluorophore is expected to embed in detergent micelles with the more hydrophilic receptor being accessible to water neighbouring the membrane51,52 and any Zn2+ therein. In the event, a strong Zn2+-induced FE value of 7 is found for 7 in neutral Tween 20 micelles.50
The mutation of 5 is easiest to see in 8,53 where the tricyclic anthracene has become the tricyclic fluorescein. The chloro substituents serve to reduce 8's response to H+ in physiological conditions.54 Sensor 8 gives a Zn2+-induced FE value of 2 in physiological media and permits fluorescence microscopy in living cells. However, the residual fluorescence response to H+ is still quite large, since scavenging of Zn2+ does not noticeably reduce the fluorescence. Nevertheless, 8 was the vanguard of a strong programme55 which included cases like 9 and 10. Sensor 956 locates the dipicolylamine unit somewhat remote from the fluorophore. However, the aniline NH group is just one methylene unit away from the fluorophore so that PET would occur at a reasonably rapid rate.57 The NH unit joins in Zn2+-binding along with the dipicolylamine receptor. Interestingly, another strong program begun in 200058 has a similar juxtaposition of an aniline NH and a dipicolylamine, as well as a fluorescein fluorophore, e.g. sensor 11.59
Sensor 1060 contains 8 as the Zn2+-responsive component with green emission of dichlorofluorescein as the output. It also carries an aminocoumarin fluorophore whose blue emission is Zn2+-independent, which is released by hydrolysis of the ester bond of 10 by intracellular esterases. Virtually the same aminocoumarin fluorophore is present in 1261,62 (though closer to the receptor) and so the Zn2+-independent emission intensity might be expected. However, the positioning of the dipicolylamine near the coumarin carbonyl oxygen allows the latter to participate in Zn2+-binding. Since this push-pull fluorophore has an internal charge transfer (ICT) excited state whose δ− pole lies at the carbonyl oxygen, Zn2+ causes an emission red-shift due to the electrostatic attraction. Alkoxycoumarin fluorophores when coupled to amine receptors have suitable PET thermodynamics.63,64 Cases like 1361,62 and 1465 show Zn2+-induced FE values of 22 (in methanol) and 8 (in acetonitrile) respectively.
While blue (e.g.coumarin) and green (e.g.fluorescein) emissions remain the workhorses of fluorescent sensor research, red-emitting fluorophores are sought after for intracellular and tissue studies due to easy transmission of light. Zn2+ sensors 1566 and 1667 address this need. PET has been achieved with cyanine fluorophores when coupled with electron-rich anilinic receptors,68 but is harder to produce with the dipicolylamine receptor. Sensor 16 succeeds at this with a Zn2+-induced FE value of 7 (in water) whereas 15 only shows wavelength shifts in fluorescence excitation spectra typical of ICT excited states, e.g. Tsien's classical Ca2+ sensors.69
The blue-green region is also represented by several more Zn2+ sensors 17–21.70–75 All of them show significant Zn2+-induced FE values, though some of them involve the fluorophore's participation in the Zn2+-coordination sphere. System 2276 is clearly of the ‘fluorophore–spacer–dipicolylamine’ format but PET appears to be thermodynamically unfavourable. So, 22 is highly emissive to begin with and only the quenching metal ion Cu2+ signals its presence. Zn2+ has no effect.
Another blue-green-emitting Zn2+ sensor 2377 is distinguished by operating over a large dynamic range. Besides the dipicolylamine receptor, 23 also contains a 2,2′-bipyridine as a low-affinity binding site which is engaged at high Zn2+ concentrations. This incurs positive charging and planarization of the bipyridine rings and so the ICT character of the excited push-pull fluorophore is enhanced with a significant red-shift. The switching ‘on’ of the blue emission and that of the green emission occur at two different Zn2+ concentration ranges.
The conjugation of an electron-rich aminophenyl substituent with a furoquinoline unit increases its reduction potential to weaken PET activity of 24.78 However, this produces a push-pull fluorophore with considerable ICT character in its excited state. The quinoline nitrogen atom of the fluorophore clearly participates in the Zn2+-coordination sphere, so that the ICT character of the excited state increases, resulting in a Zn2+-induced red-shift from green to orange. This allows ratiometric measurement of Zn2+ in living cells.
The story doesn't end here. Some of these Zn2+ sensors have led to another valuable line of research. It started with the filling of the unsaturated coordination sphere of dipicolylamine-Zn2+ (mentioned above) with anionic ligands such as phosphates. When di-receptor cases such as 6 are studied in neat water, binding of two Zn2+ ions cannot be achieved under experimental conditions unless the mutual repulsion between the two metal centres is reduced by inserting a bridging anion. Therefore, PET suppression and fluorescence switching ‘on’ requires the presence of Zn2+ as well as the phosphate anion. Phosphorylation of tyrosines in peptides can be neatly signalled in this way. Of course, dephosphorylation can be followed fluorimetrically too.79,80 Sensor 6 with Zn2+ also switches ‘on’ when uridine 5′-diphosphate is made available from uridine 5′-diphosphate glycoside during glycosyl transfer to sugar derivatives catalyzed by glycosyltransferases.81 The latter enzymes are measurable in a label-free manner. Sensor 6 with Zn2+ also lights up when phosphatidylserine is brought to the outer face of cell membranes when the cell is ready to die. Thus, apoptosis can be detected in a simple way.82
Phosphates can also be detected (though in acetonitrile solution) by the quenching of the emission of the Zn2+ complex of 25 by a factor of 70.83 Sensor 25 itself is poorly emissive and Zn2+ causes an FE value of 5. A PET mechanism is probable. The coordination sphere of the single dipicolylamine-Zn2+ is made up by the aniline nitrogen nearby and, importantly, also by phosphate which reduces the positive charge density so that the Zn2+-induced PET suppression is weakened. The ICT nature of the push-pull fluorophore is clearly signalled by the Zn2+-induced blue-shift.
Compound 853 is re-incarnated in the form of its di-Zn2+ complex for pyrophosphate sensing.84 An FE value of 3 is achieved. It appears that, as seen perhaps more strongly for 6,79 the full binding of both dipicolylamine sites and the full PET suppression is only achieved when the pyrophosphate bridges the two Zn2+ centres. In this case, pyrophosphate is of just the right length. Pyrophosphate bridging two Zn2+-dipicolylamine units is also found in the ground state dimer of 2685 (indicated by red-shifts in the absorption spectrum) which leads to a corresponding red-shifted emission compared to the pyrene monomer.
It is clear that the first PET sensing experiment on 544 keeps on putting out new shoots – a testament to the flexibilities arising from the modular nature of PET sensors.
Some highlights from the past year
Besides the sustained success of Zn2+ sensing with PET sensors carrying bis(picolyl)amine receptors, recent cases also include macrocycles. An oxa-bridged version of older azacrown ether-based PET sensors,2927 (Fig. 7b),86 contains an amine PET donor and an anthracene fluorophore. It produces a Zn2+-induced FE of 100 but does not respond to alkali cations. Interestingly, Na+ in sufficient concentration displaces Zn2+ and decreases the FE value of 27, as the smaller ion coordinates to the oxygen atoms rather than the nitrogen.Cases based on tetraazamacrocycle receptors are also useful for Zn2+ sensing.87 A new example is 28,88 where the triazole group also plays a receptor role to cause selective binding of Zn2+ even over Cd2+, with a Zn2+-induced FE value of 6. Interestingly, it detects the intracellular flux of Zn2+ during cell apoptosis.
In spite of the successes concerning selective Zn2+ sensing, Cd2+ was an interferent in many of these cases. The rarity of intracellular Cd2+ has been the saviour in this regard. Selective Cd2+ sensing, which would be useful in studies of Cd2+ toxicity, has been a harder nut to crack.89–91 PET sensor 29 with a polyamide receptor provides a neat solution even within HeLa cells.92 A Cd2+-induced FE value of 100 is attained. Steric hindrance between the receptor arms can produce a virtual spacer so that the usual PET behaviour can occur.
Organic chemical reactions rather than ion-coordination can also lead to PET-based fluorescence switching ‘on’, as seen in 30.93 When solubilized in aqueous Tween 20 micellar solution, its amine group serves as the PET donor so that the fluorophore's emission is ‘off’. The dangerous alkylating agent chloromethyl methyl ether forms a quaternary ammonium product which prevents PET and fluorescence is switched ‘on’. Related cases94 are known.
As we saw with the 2·3 system,18 covalent linking of a fluorophore and a PET-active receptor is not necessary for a working PET sensing system. There is no PET within 31, a tris(2,2′-bipyridyl)Ru(II) lumophore which is connected to a mannose-capped dendrimer.95 Lectins, those exquisite sugar-binding proteins, rely on polyvalency. Therefore, lectins such as Concanavalin A associate strongly with 31. However, this does not produce a luminescence switch unless an extra component is provided. The extra component 32 contains arylboronic acid groups for sugar binding and a 4,4′-bipyridinium unit as a PET acceptor. In the absence of lectin, 31 and 32 associate so that PET takes place and the luminescence is ‘off’. The addition of Concanavalin A displaces 32 from the mannose caps and a resultant increase in luminescence occurs.
Though perhaps not as grand as lectin sensing, synthetic macrocycles can also be sensed via PET schemes. A pyrene fluorophore and a pyridinium PET acceptor can be discerned within 33.96 The phosphonate-bridged resorcinarene 34 can engulf the pyridinium unit just like simpler resorcinarenes do.97 Upon complexation, PET becomes energetically unfavourable, causing an increase in fluorescence.
As seen in the previous pages, PET sensors usually consist of molecular lumophores. An important extension to this idea has been reported98 where the lumophore component represents a quantum dot, the darling of nanotechnology. This particular quantum dot is a ZnS–CdSe core–shell structure. Sensor 35 possesses a thiourea receptor which binds to CH3CO2− with two hydrogen bonds and this increases the reduction potential of the receptor, thus enhancing PET to the lumophore.
We finish off with two cases where ‘fluorophore–spacer–receptor’ systems are aimed at monitoring intermediates in catalytic cycles in solution. For instance, a transient Lewis acid centre could bind a receptor to switch ‘on’ the fluorescence of a PET sensor. This would be a valuable analytical tool. Example 3699 attacks this problem at the single molecule level since the required sensitivity of similar sensors based on perylenediimide fluorophores has been demonstrated.100 Though this ambitious goal is yet to be achieved, the H+-induced switching ‘on’ of fluorescence is demonstrated by negating the PET donor amine by protonation. The single molecule studies show this pH dependence, though the presence of relatively long-lived dark states at low pH leading to ‘blinking’ is a complication.
The dimethylaminonaphthalenesulfonylamide fluorophore undergoes PET from neighbouring amines as seen in the case of 17.101 A similar ‘fluorophore–spacer–receptor’ motif can be found within 37. This motif is tagged to an N-heterocyclic carbene Pd(II) complex with the intent of monitoring its catalytic activity in a Suzuki coupling reaction between an aryl bromide and a boronic acid. As the reaction progresses, the halide ion product quenches 37's fluorescence due to the heavy atom effect. Such monitoring of a product formation is not the main point in the present context of monitoring of catalytic intermediates. However, the smaller fluorescence loss observed upon addition of base to prepare the active Pd(0) catalytic species before the addition of the aryl halide is potentially more interesting. If the mopping up of trace Brønsted acids can be ruled out, a ‘stepping-stone’ mechanism could be imagined, where an electron is transferred from Pd(0) to the fluorophore via the amine receptor to quench fluorescence, but not from Pd(II).
Conclusion
The preceding pages have summarized a few of the current growth areas in fluorescent PET sensors where problems in analytical science are being attacked. We hope the quantitative design basis of PET sensors where molecules can be viewed as engineering objects will appeal to bright analytical minds so that more of them will join in this venture. With such a combination of forces, even more analytical solutions will emerge from the versatile fluorescent PET system as the days go on.Acknowledgements
We thank the Allen McClay trust for support.References
- J.-M. Lehn, Supramolecular Chemistry, VCH, Weinheim, 1995 Search PubMed.
- E. V. Anslyn, J. Org. Chem., 2007, 72, 687–699 CrossRef.
- A. Weller, Pure Appl. Chem., 1968, 16, 115–123 CrossRef CAS.
- R. A. Bissell, A. P. de Silva, H. Q. N. Gunaratne, P. L. M. Lynch, G. E. M. Maguire and K. R. A. S. Sandanayake, Chem. Soc. Rev., 1992, 21, 187–195 RSC.
- R. A. Bissell, A. P. de Silva, H. Q. N. Gunaratne, P. L. M. Lynch, G. E. M. Maguire, C. P. McCoy and K. R. A. S. Sandanayake, Top. Curr. Chem., 1993, 168, 223–264.
- Photoinduced electron transfer, ed. M. A. Fox and M. Chanon, Elsevier, Amsterdam, 1988 Search PubMed.
- G. J. Kavarnos, Fundamentals of photoinduced electron transfer, VCH, Weinheim, New York, 1993 Search PubMed.
- Electron Transfer, ed. V. Balzani, Wiley-VCH, Weinheim, 2003 Search PubMed.
- A. Chatterjee, T. M. Suzuki, Y. Takahashi and D. A. P. Tanaka, Chem.–Eur. J., 2003, 9, 3920–3929 CrossRef CAS.
- S. Uchiyama, T. Santa and K. Imai, Analyst, 2000, 125, 1839–1845 RSC.
- T. Ueno, Y. Urano, K. Setsukinai, H. Takakusa, H. Kojima, K. Kikuchi, K. Ohkubo, S. Fukuzumi and T. Nagano, J. Am. Chem. Soc., 2004, 126, 14079–14085 CrossRef CAS.
- C. J. Fahrni, L. C. Yang and D. G. VanDerveer, J. Am. Chem. Soc., 2003, 125, 3799–3812 CrossRef CAS.
- G. L. Closs and J. R. Miller, Science, 1988, 240, 440–447 CrossRef CAS.
- J. C. Beeson, M. A. Huston, D. A. Pollard, T. K. Venkatachalam and A. W. Czarnik, J. Fluoresc., 1993, 3, 65–69 CAS.
- M. Onoda, S. Uchiyama, T. Santa and K. Imai, Luminescence, 2002, 17, 11–14 CrossRef CAS.
- S. A. Jonker, F. Ariese and J. W. Verhoeven, Rec. Trav. Chem. Pays Bas, 1989, 108, 109–115 Search PubMed.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of photochemistry, CRC Press, Boca Raton, 3rd edn, 2006 Search PubMed.
- P. P. Neelakandan, M. Hariharan and D. Ramaiah, J. Am. Chem. Soc., 2006, 128, 11334–11335 CrossRef CAS.
- N. Tarumoto, N. Miyagawa, S. Takahara and T. Yamaoka, Polym. J., 2005, 37, 545–549 CrossRef CAS.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef.
- V. Balzani, A. Credi and M. Venturi, Molecular devices and machines, VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- Y. C. Wang and H. Morawetz, J. Am. Chem. Soc., 1976, 98, 3611–3615 CrossRef CAS.
- B. K. Selinger, Aust. J. Chem., 1977, 30, 2087–2094.
- G. S. Beddard, R. S. Davidson and T. D. Whelan, Chem. Phys. Lett., 1978, 56, 54–58 CrossRef CAS.
- H. Shizuka, M. Nakamura and T. Morita, J. Phys. Chem., 1979, 83, 2019–2024 CrossRef CAS.
- H. Shizuka, T. Ogiwara and E. Kimura, J. Phys. Chem., 1985, 89, 4302–4306 CrossRef CAS.
- J. P. Konopelski, F. Kotzyba-Hibert, J.-M. Lehn, J.-P. Desvergne, F. Fages, A. Castellan and H. Bouas-Laurent, J. Chem. Soc., Chem. Commun., 1985, 433–436 RSC.
- A. P. de Silva and R. A. D. D. Rupasinghe, J. Chem. Soc., Chem. Commun., 1985, 1669–1670 RSC.
- A. P. de Silva and S. A. de Silva, J. Chem. Soc., Chem. Commun., 1986, 1709–1710 RSC.
- M. E. Huston, K. W. Haider and A. W. Czarnik, J. Am. Chem. Soc., 1988, 110, 4460–4462 CrossRef CAS.
- A. P. de Silva, S. A. de Silva, A. S. Dissanayake and K. R. A. S. Sandanayake, J. Chem. Soc., Chem. Commun., 1989, 1054–1056 RSC.
- A. P. de Silva and K. R. A. S. Sandanayake, J. Chem. Soc., Chem. Commun., 1989, 1183–1185 RSC.
- A. P. de Silva and H. Q. N. Gunaratne, J. Chem. Soc., Chem. Commun., 1990, 186–187 RSC.
- A. P. de Silva and K. R. A. S. Sandanayake, Angew. Chem., Int. Ed. Engl., 1990, 29, 1173–1175 CrossRef.
- E. U. Akkaya, M. E. Huston and A. W. Czarnik, J. Am. Chem. Soc., 1990, 112, 3590–3593 CrossRef CAS.
- R. A. Bissell and A. P. de Silva, J. Chem. Soc., Chem. Commun., 1991, 1148–1150 RSC.
- A. J. Bryan, A. P. de Silva, S. A. de Silva, R. A. D. D. Rupasinghe and K. R. A. S. Sandanayake, Biosensors, 1989, 4, 169–179 CrossRef CAS.
- Fluorescent Chemosensors of Ion and Molecule Recognition, ACS Symp. Ser. 538, ed. A. W. Czarnik, American Chemical Society, Washington DC, 1993 Search PubMed.
- J. K. Tusa and H. He, J. Mater. Chem., 2005, 15, 2640–2647 RSC.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS.
- R. M. Smith and A. E. Martell, in Critical Stability Constants, Plenum, New York, 1975, vol. 2, p. 246 Search PubMed.
- S. Bhattacharya and S. S. Mandal, Chem. Commun., 1996, 1515–1516 RSC.
- S. A. de Silva, A. Zavaleta, D. E. Baron, O. Allam, E. V. Isidor, N. Kashimura and J. M. Percarpio, Tetrahedron Lett., 1997, 38, 2237–2240 CrossRef CAS.
- S. A. de Silva, M. L. Kasner, M. A. Whitener and S. L. Pathirana, Int. J. Quantum Chem., 2004, 100, 753–757 CrossRef CAS.
- C. J. Frederickson, Int. Rev. Neurobiol., 1989, 31, 145–238 CAS.
- A. P. de Silva, T. P. Vance, M. E. S. West and G. D. Wright, Org. Biomol. Chem., 2008, 6, 2468–2480 RSC.
- K. Kubo and A. Mori, Chem. Lett., 2003, 32, 926–927 CrossRef CAS.
- K. Kubo and A. Mori, J. Mater. Chem., 2005, 15, 2902–2907 RSC.
- S. Bhattacharya and A. Gulyani, Chem. Commun., 2003, 1158–1159 RSC.
- R. A. Bissell, A. J. Bryan, A. P. de Silva and C. P. McCoy, J. Chem. Soc., Chem. Commun., 1994, 405–407 RSC.
- S. Uchiyama, K. Iwai and A. P. de Silva, Angew. Chem., Int. Ed., 2008, 47, 4667–4669 CrossRef CAS.
- S. C. Burdette, G. K. Walkup, B. Spingler, R. Y. Tsien and S. J. Lippard, J. Am. Chem. Soc., 2001, 123, 7831–7841 CrossRef CAS.
- R. Y. Tsien, Am. J. Physiol. Cell Physiol., 1992, 263, C723–C728 CAS.
- E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193–203 CrossRef CAS.
- S. C. Burdette, C. J. Frederickson, W. M. Bu and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 1778–1787 CrossRef CAS.
- R. A. Bissell, A. P. de Silva, W. T. M. L. Fernando, S. T. Patuwathavithana and T. K. S. D. Samarasinghe, Tetrahedron Lett., 1991, 32, 425–428 CrossRef CAS.
- T. Hirano, K. Kikuchi, Y. Urano, T. Higuchi and T. Nagano, J. Am. Chem. Soc., 2000, 122, 12399–12400 CrossRef CAS.
- K. Komatsu, K. Kikuchi, H. Kojima, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2005, 127, 10197–10204 CrossRef CAS.
- C. C. Woodroofe and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 11458–11459 CrossRef CAS.
- N. C. Lim and C. Bruckner, Chem. Commun., 2004, 1094–1096 RSC.
- N. C. Lim, J. V. Schuster, M. C. Porto, M. A. Tanudra, L. Yao, H. C. Freake and C. Bruckner, Inorg. Chem., 2005, 44, 2018–2030 CrossRef CAS.
- K. Sasamoto, T. Ushijima, M. Saito and Y. Ohkura, Anal. Sci., 1996, 12, 189–193 CAS.
- A. P. de Silva, H. Q. N. Gunaratne, P. L. M. Lynch, A. L. Patty and G. L. Spence, J. Chem. Soc., Perkin Trans. 2, 1993, 1611–1616 RSC.
- C. P. Kulatilleke, S. A. de Silva and Y. Eliav, Polyhedron, 2006, 25, 2593–2596 CrossRef CAS.
- K. Kiyose, H. Kojima, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2006, 128, 6548–6549 CrossRef CAS.
- B. Tang, H. Huang, K. H. Xu, L. L. Tong, G. W. Yang, X. Liu and L. G. An, Chem. Commun., 2006, 3609–3611 RSC.
- B. Ozmen and E. U. Akkaya, Tetrahedron Lett., 2000, 41, 9185–9188 CrossRef CAS.
- R. Y. Tsien, Biochemistry, 1980, 19, 2396–2404 CrossRef CAS.
- T. W. Kim, J. H. Park and J. I. Hong, J. Chem. Soc., Perkin Trans. 2, 2002, 923–927 RSC.
- J. L. Fan, Y. K. Wu and X. J. Peng, Chem. Lett., 2004, 33, 1392–1393 CrossRef CAS.
- J. L. Fan, X. J. Peng, Y. K. Wu, E. H. Lu, J. Hou, H. B. Zhang, R. Zhang and X. M. Fu, J. Lumin., 2005, 114, 125–130 CrossRef CAS.
- S. Huang, R. J. Clark and L. Zhu, Org. Lett., 2007, 9, 4999–5002 CrossRef CAS.
- L. Xue, H. H. Wang, X. J. Wang and H. Jiang, Inorg. Chem., 2008, 47, 4310–4318 CrossRef CAS.
- Z. P. Liu, C. L. Zhang, Y. L. Li, Z. Y. Wu, F. Qian, X. L. Yang, W. J. He, X. Gao and Z. J. Guo, Org. Lett., 2009, 11, 795–798 CrossRef CAS.
- L. B. Li, S. J. Ji and Y. Liu, Chin. J. Chem., 2008, 26, 979–982 CrossRef CAS.
- L. Zhang, R. J. Clark and L. Zhu, Chem.–Eur. J., 2008, 14, 2894–2903 CrossRef CAS.
- L. Xue, C. Liu and H. Jiang, Chem. Commun., 2009, 1061–1063 RSC.
- A. Ojida, Y. Mito-oka, K. Sada and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 2454–2463 CrossRef CAS.
- A. Ojida, Y. Mito-oka, M. Inoue and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 6256–6258 CrossRef CAS.
- J. Wongkongkatep, Y. Miyahara, A. Ojida and I. Hamachi, Angew. Chem., Int. Ed., 2006, 45, 665–668 CrossRef CAS.
- A. V. Koulov, K. A. Stucker, C. Lakshmi, J. P. Robinson and B. D. Smith, Cell Death Differ., 2003, 10, 1357–1359 CrossRef CAS.
- A. Coskun, E. Deniz and E. U. Akkaya, Tetrahedron Lett., 2007, 48, 5359–5361 CrossRef CAS.
- Y. J. Jang, E. J. Jun, Y. J. Lee, Y. S. Kim, J. S. Kim and J. Yoon, J. Org. Chem., 2005, 70, 9603–9606 CrossRef CAS.
- H. K. Cho, D. H. Lee and J. I. Hong, Chem. Commun., 2005, 1690–1692 RSC.
- F. A. Khan, K. Parasuraman and K. K. Sadhu, Chem. Commun., 2009, 2399–2401 RSC.
- T. Hirano, K. Kikuchi, Y. Urano, T. Higuchi and T. Nagano, Angew. Chem., Int. Ed., 2000, 39, 1052–1054 CrossRef CAS.
- E. Tamanini, A. Katewa, L. M. Sedger, M. H. Todd and M. Watkinson, Inorg. Chem., 2009, 48, 319–324 CrossRef CAS.
- M. E. Huston, C. Engleman and A. W. Czarnik, J. Am. Chem. Soc., 1990, 112, 7054–7056 CrossRef CAS.
- T. Gunnlaugsson, T. C. Lee and R. Parkesh, Tetrahedron, 2004, 60, 11239–11249 CrossRef CAS.
- X. J. Peng, J. J. Du, J. L. Fan, J. Y. Wang, Y. K. Wu, J. Z. Zhao, S. G. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500–1501 CrossRef CAS.
- T. Y. Cheng, Y. F. Xu, S. Y. Zhang, W. P. Zhu, X. H. Qian and L. P. Duan, J. Am. Chem. Soc., 2008, 130, 16160–16161 CrossRef CAS.
- J. J. Lee and B. D. Smith, Chem. Commun., 2009, 1962–1963 RSC.
- S. Tal, H. Salman, Y. Abraham, M. Botoshansky and Y. Eichen, Chem.–Eur. J., 2006, 12, 4858–4864 CrossRef CAS.
- R. Kikkeri, I. Garcia-Rubio and P. H. Seeberger, Chem. Commun., 2009, 235–237 RSC.
- E. Biavardi, G. Battistini, M. Montalti, R. M. Yebeutchou, L. Prodi and E. Dalcanale, Chem. Commun., 2008, 1638–1640 RSC.
- M. Inouye, K. Hashimoto and K. Isagawa, J. Am. Chem. Soc., 1994, 116, 5517–5518 CrossRef CAS.
- J. F. Callan, R. C. Mulrooney, S. Kamila and B. McCaughan, J. Fluoresc., 2008, 18, 527–532 CrossRef CAS.
- R. Ameloot, M. Roeffaers, M. Baruah, G. De Cremer, B. Sels, D. De Vos and J. Hofkens, Photochem. Photobiol. Sci., 2009, 8, 453–456 RSC.
- L. Zang, R. C. Liu, M. W. Holman, K. T. Nguyen and D. M. Adams, J. Am. Chem. Soc., 2002, 124, 10640–10641 CrossRef CAS.
- V. Sashuk, D. Schoeps and H. Plenio, Chem. Commun., 2009, 770–772 RSC.
| This journal is © The Royal Society of Chemistry 2009 |
