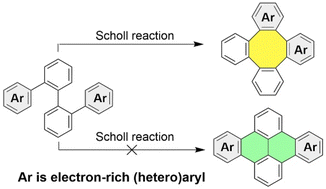
Scholl reactions typically lead to the formation of six-membered rings of sp2 carbon atoms, while the formation of eight-membered rings is uncommon. However, this study reveals that the Scholl reactions of o-quateraryls can form one eight-membered ring in a regioselective manner, resulting in the synthesis of substituted and π-extended tetraphenylenes. The regioselectivity is observed when the two terminal aryl groups in the o-quateraryls are significantly more electron-rich than the internal arylene groups. Furthermore, by adjusting the reaction conditions, the regioselectivity of the Scholl reaction of o-quateraryls with two 3,4-dialkoxyphenyl terminal units and one or two internal 2,3-naphthylene units can be controlled to form either an eight-membered ring or two six-membered rings. Notably, the formation of two six-membered rings leads to the synthesis of novel derivatives of tribenzo[a,e,l]pyrene and tetrabenzo[a,e,h,l]pyrene. In addition, one derivative of tetrabenzo[a,e,h,l]pyrene exhibits π-stacking in the solid state with short C-to-C contacts, enabling it to function as a p-type organic semiconductor in solution-processed field effect transistors.